Promising Abilities of Fungal Lipases of Aspergilli Strains in the Production of Biodiesel from Plant Oil Wastes
Abstract
1. Introduction
2. Materials and Methods
2.1. The Source of Fungal Strains Isolates
2.2. Lipase Production by Submerged Fermentation
2.3. Lipase Production Assay
2.4. Enzyme Characterization
2.5. Lipase-Catalyzed Esterification and Transesterification Reactions
3. Results
3.1. Utilizing Lipase Enzyme as a Catalyst in an Esterification Reaction
3.2. Lipase Enzyme on Catalyzing Biodiesel Production
3.3. Mass Spectrometry
4. Discussion
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerrand, D. Economics of food and feed enzymes: Status and prospectives. In Enzymes in Human and Animal Nutrition: Principles and Perspectives; Nunes, C.S., Kumar, V., Eds.; Academic Press: London, UK, 2018; pp. 487–514. [Google Scholar]
- Singh, R.; Singh, T.; Pandey, A. Microbial enzymes-An overview. In Advances in Enzyme Technology; Singh, R.S., Singhania, R.R., Pandey, A., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–40. [Google Scholar]
- Kango, N.; Jana, U.K.; Choukade, R. Fungal Enzymes: Sources and Biotechnological Applications. In Advancing Frontiers in Mycology & Mycotechnology: Basic and Applied Aspects of Fungi; Satyanarayana, T., Deshmukh, S.K., Deshpande, M.V., Eds.; Springer: Singapore, 2019; pp. 515–538. [Google Scholar]
- Sharma, R.; Chisti, Y.; Banerjee, U. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Wang, I.; Ann, P. A simple method for detection of lipolytic microorganisms in soils. Soil Biol. Biochem. 2005, 37, 597–599. [Google Scholar] [CrossRef]
- Toscano, L.; Gochev, V.; Montero, G.; Stoytcheva, M. Enhanced production of extracellular lipase by novel mutant strain of Aspergillus niger. Biotechnol. Biotechnol. Equip. 2011, 25, 2243–2247. [Google Scholar] [CrossRef]
- Durrett, T.P.; Benning, C.; Ohlrogge, J. Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 2008, 54, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Fehr, W.R. Breeding for modified fatty acid composition in soybean. Crop. Sci. 2007, 47, S72–S87. [Google Scholar] [CrossRef]
- Koria, L.; Nithya, G. Analysis of Datura stramonium Linn. Biodiesel by gas chromatography—Mass spectrometry (gc-ms) and influence of fatty acid composition on the fuel related characteristics. J. Phytol. 2012, 4, 6–9. [Google Scholar]
- Frankel, E.N. Lipid oxidation. Prog. Lipid Res. 1980, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Canakci, M.; Monyem, A.; Van Gerpen, J. Accelerated oxidation processes in biodiesel. Trans. ASAE 1999, 42, 1565–1572. [Google Scholar] [CrossRef]
- Duffield, J.A. Biodiesel: Production and economic issues. Inhal. Toxicol. 2007, 19, 1029–1031. [Google Scholar] [CrossRef]
- Gashaw, A.; Getachew, T.; Teshita, A. A Review on biodiesel production as alternative fuel. J. For. Prod. Ind. 2015, 4, 80–85. [Google Scholar]
- Kato, M.; Fuchimoto, J.; Tanino, T.; Kondo, A. Preparation of a whole cell biocatalyst of mutated Candida antarctica Lipase B (mCALB) by a yeast molecular display system and its practical properties. Appl. Microbiol. Biotechnol. 2007, 75, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, T.; Kadir, M.; Cesur, C.; Gokdogan, O. Biodiesel production potential from oil seeds in Turkey. Renew. Sustain. Energy Rev. 2016, 58, 842–851. [Google Scholar]
- Ghaly, E.; Dave, D.; Brooks, M.; Budge, S. Production of biodiesel by enzymatic transesterification: Review. Am. J. Biochem. Biotechnol. 2010, 6, 54–76. [Google Scholar] [CrossRef]
- Haki, G.; Rakshit, S. Developments in industrially important thermostable enzymes: A review. Bioresour. Technol. 2003, 89, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Fidel, T.R.; Leticia, M.; Fidel, T. Developments in the Use of Lipase Transesterification for Biodiesel Production from Animal Fat Waste. Appl. Sci. 2020, 10, 5085. [Google Scholar] [CrossRef]
- Hwang, H.; Qi, F.; Yuan, C. Lipase-catalyzed process for biodiesel production: Protein engineering and lipase production. Biotechnol. Bioeng. 2014, 111, 639–653. [Google Scholar] [CrossRef]
- Du, W.; Liu, D. Biodiesel from conventional feedstocks. Adv. Biochem. Eng. Biotechnol. 2012, 128, 53–68. [Google Scholar]
- Fan, X.; Niehus, X.; Sandoval, G. Lipases as biocatalyst for biodiesel production. Method Mol. Biol. 2012, 861, 471–483. [Google Scholar]
- Ha, S.; Lanb, M.; Lee, S. Lipase-catalyzed biodiesel production from soybean oil in ionic liquids. Enzym. Microb. Technol. 2007, 41, 481–483. [Google Scholar] [CrossRef]
- Winayanuwattikun, P.; Kaewpiboon, C.; Piriyakananon, K.; Chulalaksananukul, W.; Yongvanich, T.; Svasti, J. Immobilized lipase from potential lipolytic microbes for catalyzing biodiesel production using palm oil as feedstock. Afr. J. Biotechnol. 2011, 10, 1666–1673. [Google Scholar] [CrossRef]
- Su, F.; Peng, C.; Guan-Lin, L. Biodiesel production from woody oil catalyzed by Candida rugosa lipase in ionic liquid. Renew. Energy 2016, 90, 325–329. [Google Scholar] [CrossRef]
- Jong, B.; Liew, P.; Lebai, J. Performance and microbial diversity of palm oil mill effluent microbial fuel cell. Lett. Appl. Microbiol. 2011, 53, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Mustar, S. Single Cell Protein from Palm Oil Mill Effluent (POME); University of Malaya: Bankook, Thailand, 2002. [Google Scholar]
- Alabdalall, A.H.; Al-Anazi, N.A.; Aldakeel, S.A.; AbdulAzeez, S.; Borgio, J.F. Molecular, physiological, and biochemical characterization of extracellular lipase production by Aspergillus niger using submerged fermentation. PeerJ 2020, 8, e9425. [Google Scholar] [CrossRef] [PubMed]
- Ayinla, Z.A.; Ademakinwa, A.N.; Agboola, F.K. Studies on the optimization of lipase production by Rhizopus sp. ZAC3 isolated from the contaminated soil of a palm oil processing shed. J. Appl. Biol. Biotechnol. 2017, 5, 30–37. [Google Scholar]
- Oliveira, A.C.D.; Fernandes, M.L.; Mariano, A.B. Production and characterization of an extracellular lipase from Candida guilliermondii. Braz. J. Microbiol. 2014, 45, 1503–1511. [Google Scholar] [CrossRef]
- Sethi, B.K.; Nanda, P.K.; Sahoo, S. Characterization of biotechnologically relevant extracellular lipase produced by Aspergillus terreus NCFT 4269.10. Briazilian J. Microbiol. 2016, 47, 143–149. [Google Scholar] [CrossRef]
- Shangguan, J.J.; Liu, Y.Q.; Wang, F.J. Expression and Characterization of a Novel Lipase from Aspergillus fumigatus with High Specific Activity. Appl. Biochem. Biotechnol. 2011, 165, 949–962. [Google Scholar] [CrossRef]
- Souza, L.T.A.; Oliveira, J.S.; Santos VLd Regis, W.C.B.; Santoro, M.M.; Resende, R.R. Lipolytic Potential of Aspergillus japonicus LAB01: Production, Partial Purification, and Characterisation of an Extracellular Lipase. Biomed. Res. Int. 2014, 2, 118–129. [Google Scholar] [CrossRef]
- Malekabadia, S.; Badoei-dalfarda, A.; Karami, Z. Biochemical characterization of a novel cold-active, halophilic andorganic solvent-tolerant lipase from B. licheniformis KM12 withpotential application for biodiesel production. Int. J. Biol. Macromol. 2018, 109, 389–398. [Google Scholar] [CrossRef]
- Alabdalall, A.H.; Al-Anazi, N.A.; Aldakheel, L.A.; Amer, F.H.I.; Aldakheel, F.A.; Ababutain, I.M.; Alghamdi, A.I.; Al-Khaldi, E.M. Application and characterization of crude fungal lipases used to degrade fat and oil wastes. Sci. Rep. 2021, 11, 19670. [Google Scholar] [CrossRef]
- Ji, Q.C.; Xiao, S.J.; He, B.F.; Liu, X.N. Purification and characterization of an organic solvent-tolerant lipase from Pseudomonas aeruginosa LX1 and its application for biodiesel production. J. Mol. Catal. B Enzym. 2010, 66, 264–269. [Google Scholar] [CrossRef]
- Oliveria, F.; Souza, C.E.; Peclat, V.R.; Salgado, J.M. Optimization of lipase production by Aspergillus ibericus from oil cakes and its application in esterification reactions. FBP 2018, 102, 268–277. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Li, D.; Yang, B.; Wang, Y. One-step synthesis of high-yield biodiesel from waste cooking oils by a novel and highly methanol-tolerant immobilized lipase. Bioresour. Technol. 2017, 235, 18. [Google Scholar] [CrossRef]
- Technol, C.K. Enzymatic Oil-Degumming by a Novel Microbial Phospholipase. Eur. J. Lipid Sci. 2001, 103, 333–340. [Google Scholar] [CrossRef]
- Singh, A.; Mukhopadhyay, M. Overview of fungal lipase: A review. Appl. Biochem. Biotechnol. 2012, 166, 486–520. [Google Scholar] [CrossRef]
- Sande, D.; Souza, L.; Oliveira, J. Colletotrichum gloeosporioides lipase: Characterization and use in hydrolysis and esterifications. Afr. J. Microbiol. Res. 2015, 9, 1322–1330. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Sudyoadsuk, T.; Promarak, V. Rubber seed oil as potential non-edible feedstock for biodiesel production usingheterogeneous catalyst in Thailand. Renew. Energy 2017, 101, 937–944. [Google Scholar] [CrossRef]
- Bajaj, A.; Lohan, P.; Jha, P.N.; Mehrotra, R. Biodiesel production through lipasecatalyzed transesterification: An overview. J. Mol. Catal. B Enzym. 2010, 62, 9–14. [Google Scholar] [CrossRef]
- Mander, P.; Cho, S.; Simkhada, J.R.; Choi, Y.H.; Park, D.J.; Yoo, J.C. An organic solvent-tolerant lipase from Streptomyces sp. CS133 for enzymatic transesterification of vegetable oils in organic media. Process. Biochem. 2012, 47, 635–642. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Kumar, M.; Sukla, L.B.; Subudhi, E. Bioprospecting hot spring metagenome: Lipase for the production of biodiesel. Environ. Sci. Pollut. Res. 2017, 24, 3802–3809. [Google Scholar] [CrossRef]
- Kuo, T.C.; Shaw, J.F.; Lee, G.C. Conversion of crude Jatropha curcas seed oil into biodiesel using liquid recombinant Candida rugosa lipase isozymes. Bioresour. Technol. 2015, 192, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Jumina, J.; Kurniawan, Y.S.; Lubis, A.B.; Larasati, E.I.; Purwono, B.; Triono, S. Utilization of vanillin to prepare sulfated Calix [4]resorcinarene as efficient organocatalyst for biodiesel production based on methylation of palmitic acid and oleic acid. Heliyon 2023, 9, e16100. [Google Scholar] [CrossRef] [PubMed]
- Eloka-Eboka, A.C.; Igbum, G.O.; Inambao, F.L. Biodiesel methyl ester production and testing from selscted African tropical seed oil feedstocks. Energy Procedia 2017, 142, 755–767. [Google Scholar] [CrossRef]
- Murad, A.M.; Vianna, G.R.; Machado, A.M.; da Cunha, N.B.; Coelho, C.M.; Lacerda, V.A.; Coelho, M.C.; Rech, E.L. Mass spectrometry characterisation of fatty acids from metabolically engineered soybean seeds. Anal. Bioanal. Chem. 2014, 406, 2873–2883. [Google Scholar] [CrossRef]



| Organic Acid | Isolates | ||||
|---|---|---|---|---|---|
| Organic Solvent | A. niger MH078571 | A. niger MH079049 | |||
| FFA % | Ester Percentage % | FFA % | Ester Percentage % | ||
| Butyric acid | n-Butanol | 0.755 | 99.25 | 0.812 | 99.19 |
| Iso-butanol | 0.861 | 99.14 | 0.919 | 99.08 | |
| Benzyl alcohol | 0.986 | 99.01 | 1.016 | 98.99 | |
| Methanol | 0.845 | 99.16 | 0.871 | 99.13 | |
| Propionic acid | n-Butanol | 0.666 | 99.33 | 0.707 | 99.3 |
| Iso-butanol | 0.721 | 99.28 | 0.721 | 99.28 | |
| Benzyl alcohol | 0.664 | 99.34 | 0.699 | 99.30 | |
| Methanol | 0.673 | 99.33 | 0.704 | 99.3 | |
| Lactic acid | n-Butanol | 0.624 | 99.38 | 0.944 | 99.06 |
| Iso-butanol | 0.681 | 99.32 | 0.632 | 99.37 | |
| Benzyl alcohol | 0.774 | 99.23 | 0.678 | 99.32 | |
| Methanol | 0.798 | 99.20 | 0.816 | 99.18 | |
| Oleic acid | n-Butanol | 0.214 | 99.79 | 0.165 | 99.83 |
| Iso-butanol | 0.365 | 99.64 | 0.391 | 99.61 | |
| Benzyl alcohol | 0.252 | 99.75 | 0.248 | 99.75 | |
| Methanol | 0.192 | 99.80 | 0.143 | 99.86 | |
| Time Conversion | A. niger MH078571 | A. niger MH079049 | ||||
|---|---|---|---|---|---|---|
| Oliva Oil | Jojoba Oil | Palm Oil | Oilva Oil | Jojoba Oil | Palm Oil | |
| 6 h | 8.95 | 8.18 | 14.54 | 10.80 | 9.89 | 12.36 |
| 10 h | 20.97 | 21.52 | 20.37 | 34.61 | 26.35 | 21.36 |
| 14 h | 47.68 | 49.73 | 42.98 | 58.70 | 51.16 | 45.53 |
| 18 h | 81.18 | 87.44 | 52.01 | 87.77 | 74.96 | 50.71 |
| 22 h | 61.58 | 68.18 | 74.51 | 70.50 | 68.34 | 71.94 |
| 26 h | 54.65 | 57.62 | 70.56 | 54.34 | 63.72 | 67.66 |
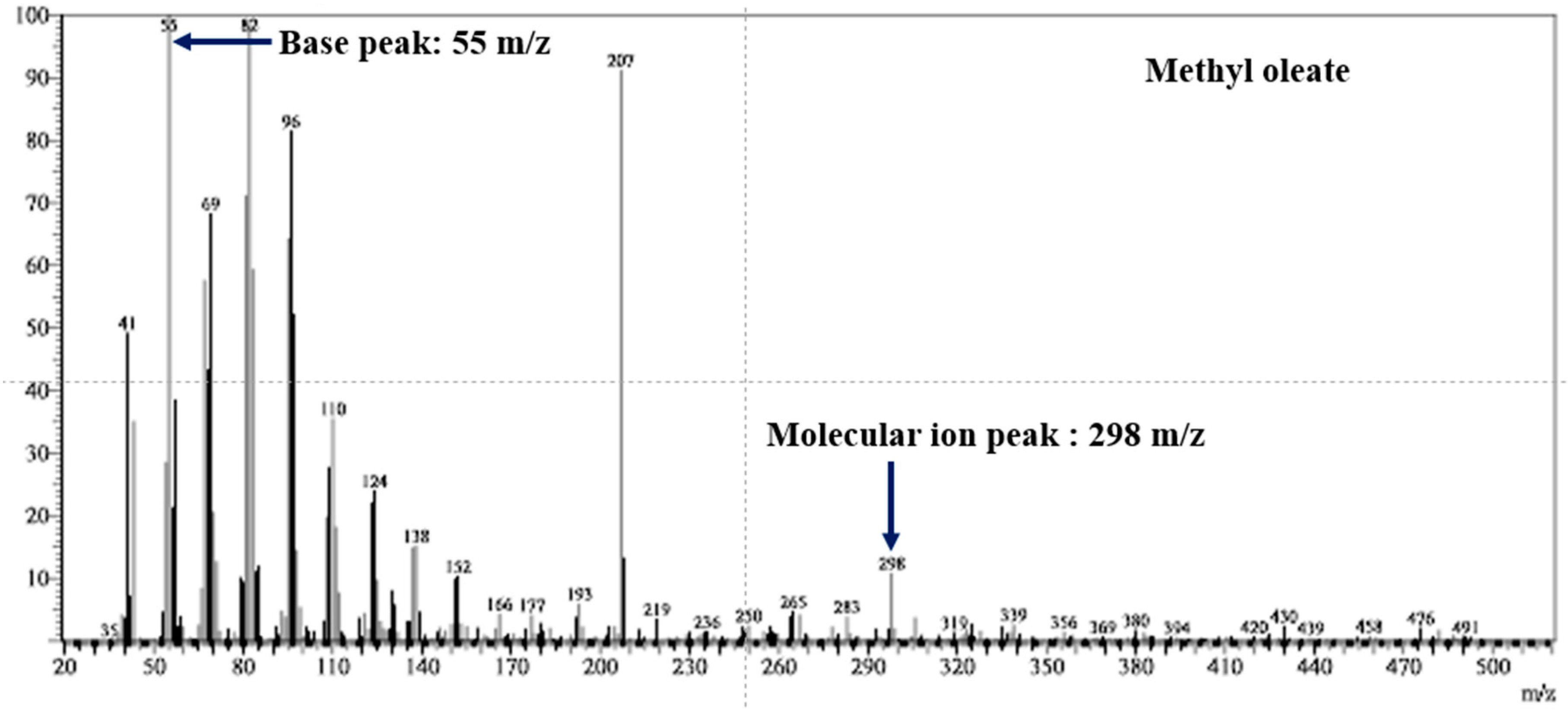
| Name of Fatty Acid Ester | Molecular Formula | Chemical Structures of the Parent Acids | Molecular Ion Peak (m/z) (Found) | Molecular Weight (Calc.) | Base Peak (m/z) |
|---|---|---|---|---|---|
| Methyl palmitate | C16H34O2 | 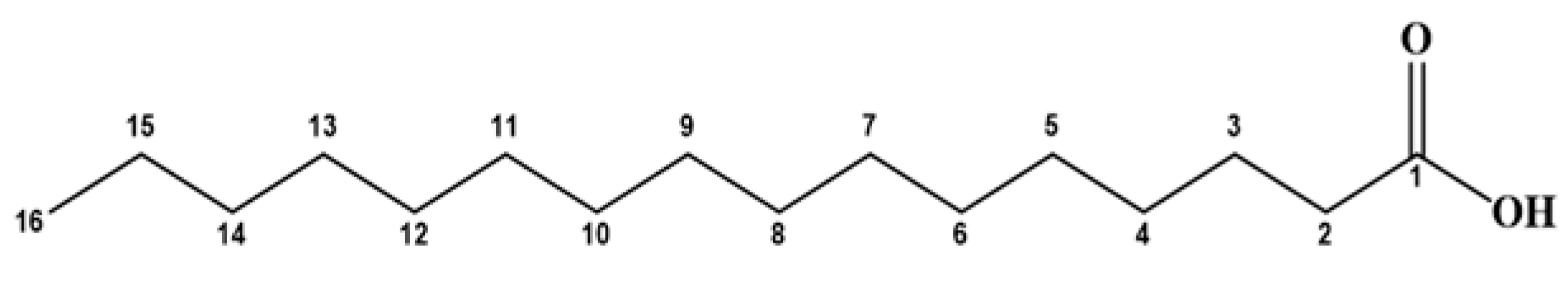 | 272 | 270 | 57 |
| Methyl linoleate | C18H34O2 |  | 294 | 298 | 57 |
| Methyl oleate | C18H36O2 | 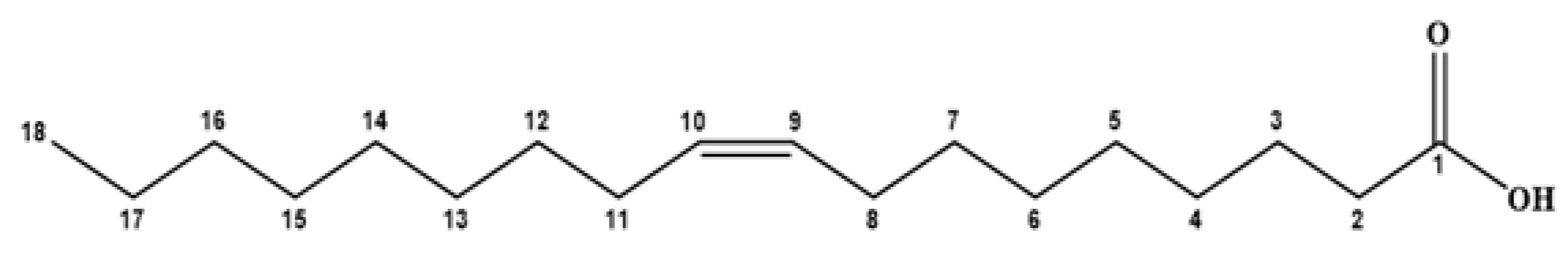 | 298 | 297 | 55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Anazi, N.A.; Alabdalall, A.H.; Alsoufi, M.H.; Al-Ghamdi, A.; Aldakheel, F.A. Promising Abilities of Fungal Lipases of Aspergilli Strains in the Production of Biodiesel from Plant Oil Wastes. Energies 2024, 17, 381. https://doi.org/10.3390/en17020381
Al-Anazi NA, Alabdalall AH, Alsoufi MH, Al-Ghamdi A, Aldakheel FA. Promising Abilities of Fungal Lipases of Aspergilli Strains in the Production of Biodiesel from Plant Oil Wastes. Energies. 2024; 17(2):381. https://doi.org/10.3390/en17020381
Chicago/Turabian StyleAl-Anazi, Norah A., Amira H. Alabdalall, Maryam H. Alsoufi, Azza Al-Ghamdi, and Fatimah A. Aldakheel. 2024. "Promising Abilities of Fungal Lipases of Aspergilli Strains in the Production of Biodiesel from Plant Oil Wastes" Energies 17, no. 2: 381. https://doi.org/10.3390/en17020381
APA StyleAl-Anazi, N. A., Alabdalall, A. H., Alsoufi, M. H., Al-Ghamdi, A., & Aldakheel, F. A. (2024). Promising Abilities of Fungal Lipases of Aspergilli Strains in the Production of Biodiesel from Plant Oil Wastes. Energies, 17(2), 381. https://doi.org/10.3390/en17020381







