CO2-Free Hydrogen Production by Methane Pyrolysis Utilizing a Portion of the Produced Hydrogen for Combustion
Abstract
1. Introduction
2. Numerical Simulation Methods
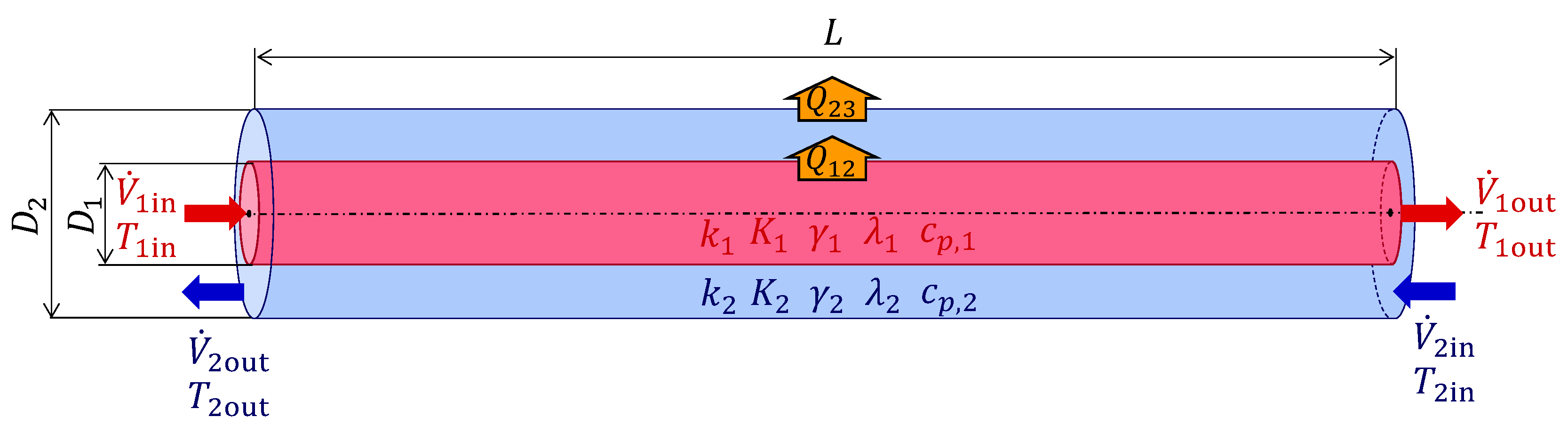
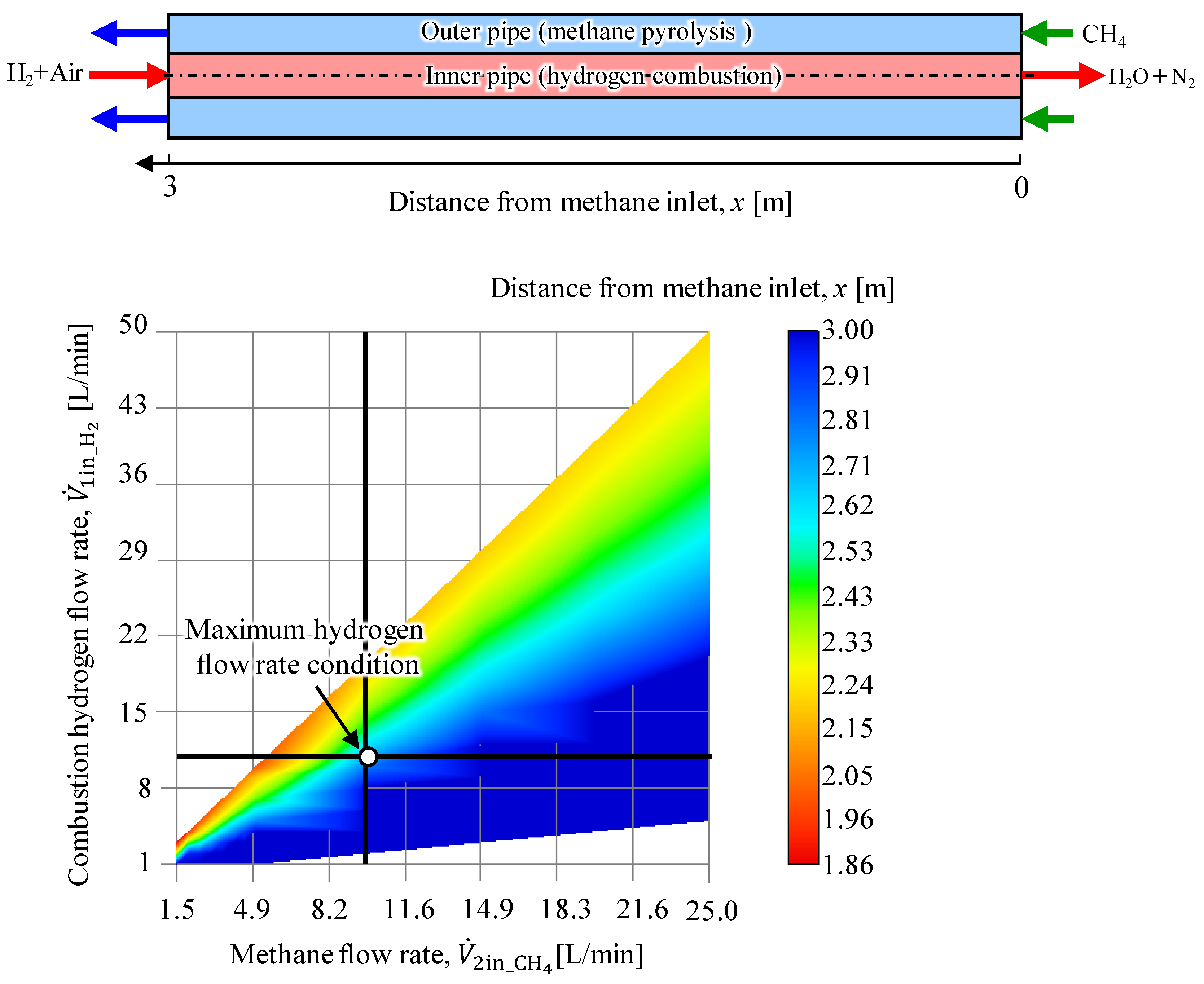
2.1. Proposed System and Simulation Model
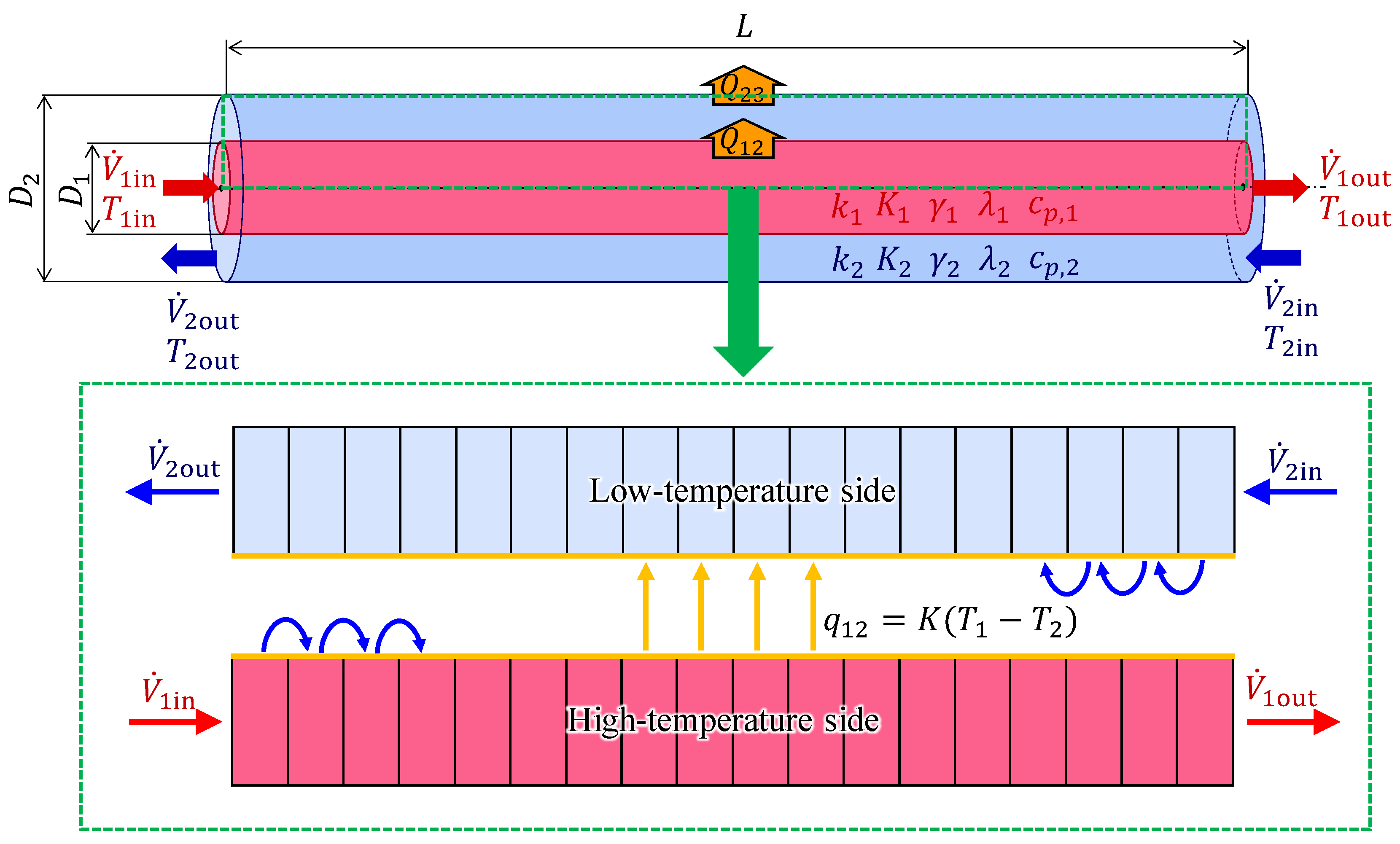
2.2. Plug-Flow Reactor Model
2.3. Theory of the Counterflow Double-Pipe Heat Exchanger
2.4. Programmatic Association
- Calculate the temperature in the PFR on the high-temperature side while maintaining a constant temperature in the PFR on the low-temperature side.
- Calculate the temperature in the PFR on the low-temperature side while maintaining the temperature in the PFR on the high-temperature side at the value obtained in Step 1, and compute the heat transfer.
- Recalculate the temperature in the PFR on the high-temperature side while maintaining the temperature in the PFR on the low-temperature side at the value obtained in Step 2, and compute the heat transfer.
- Recalculate the temperature in the PFR on the low-temperature side while maintaining the temperature in the PFR on the high-temperature side at the value obtained in Step 3, and compute the heat transfer.
- Repeat Steps 3 and 4 until the convergence criteria presented in Section 2.5 are satisfied.
2.5. Convergence Criteria
3. Numerical Analysis
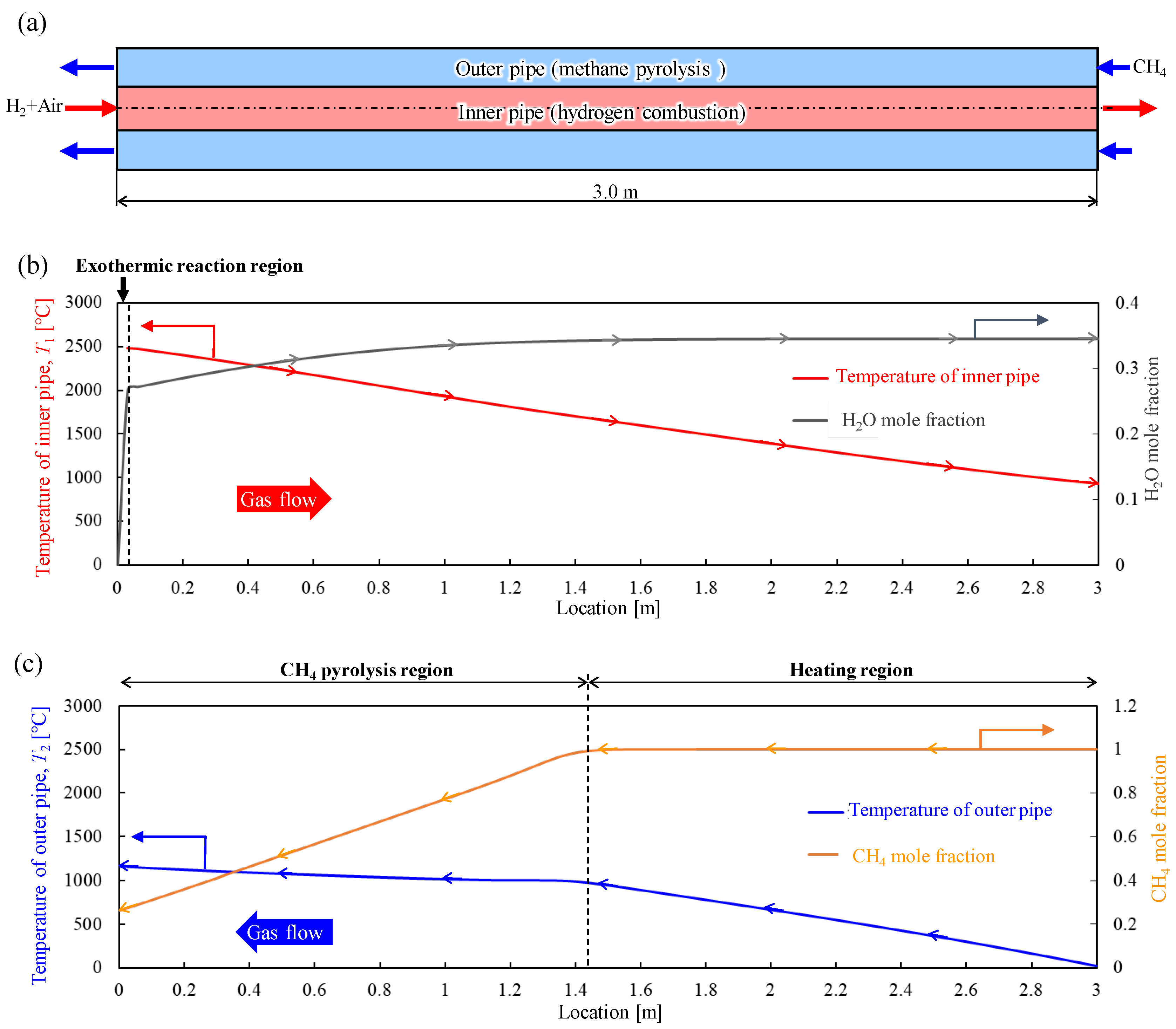
3.1. Determining Basic Model Configuration
3.2. System Investigations
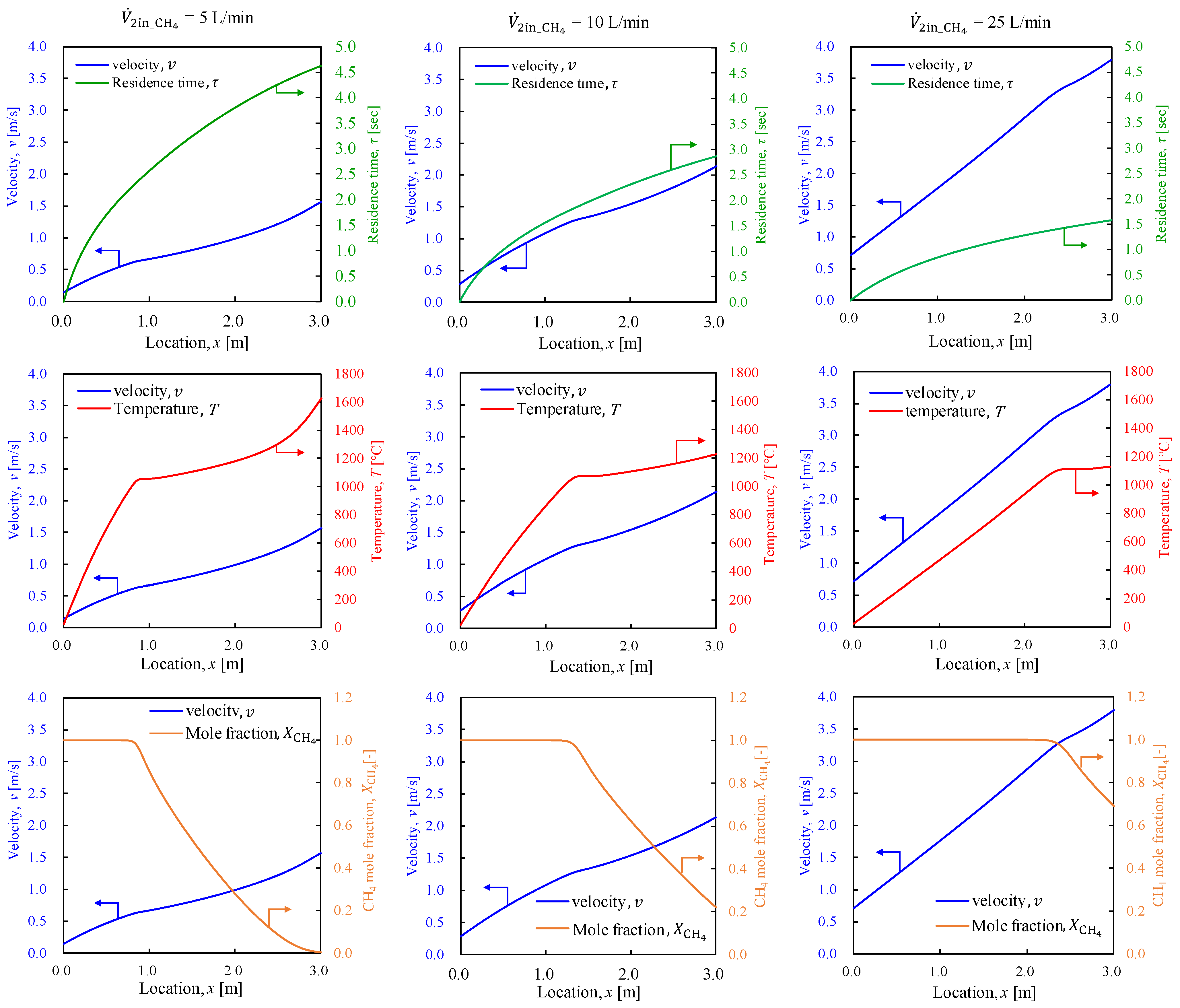
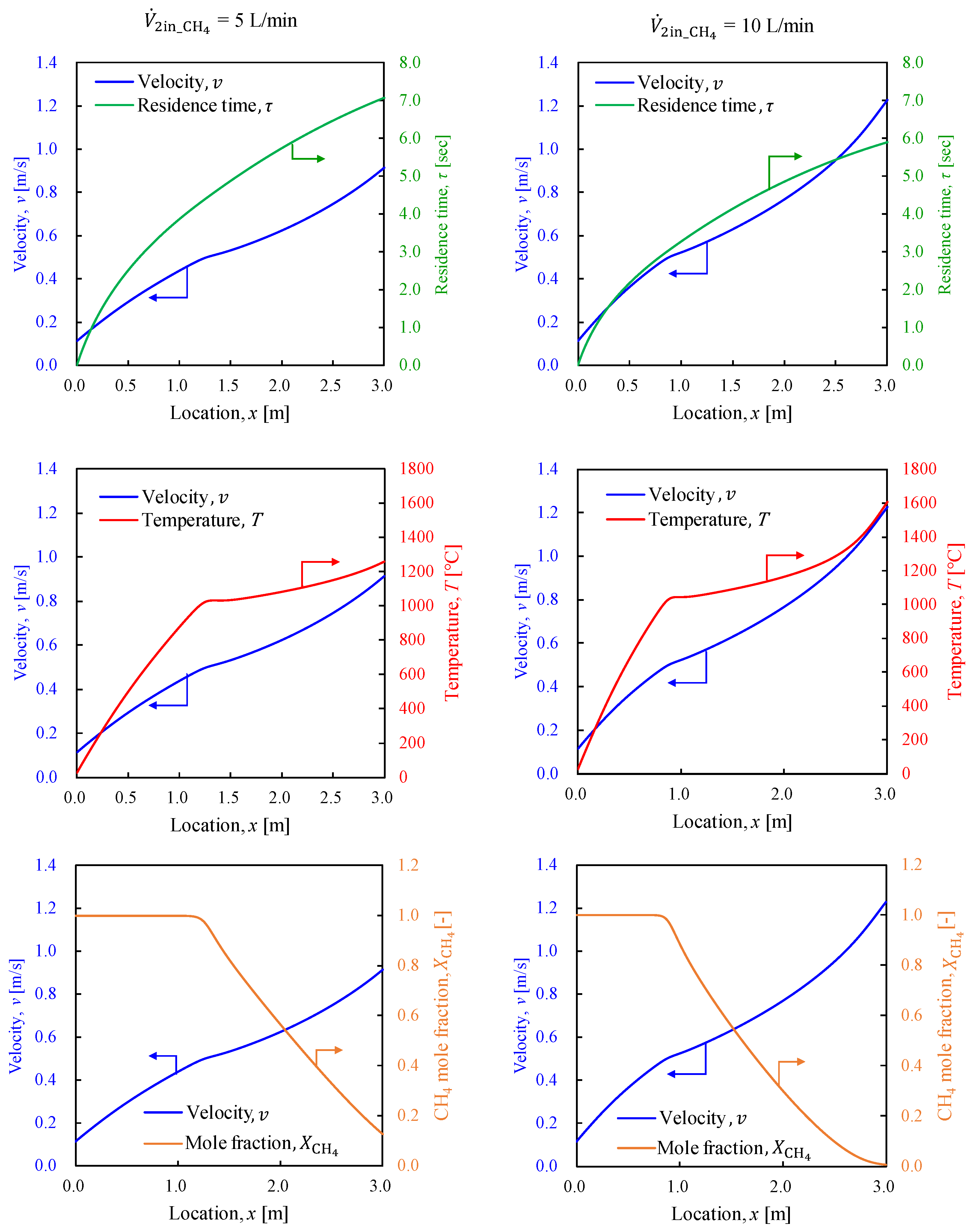
3.2.1. General System Performance
3.2.2. Outer Pipe Temperature
3.2.3. System Efficiency
4. Conclusions
- An investigation of the fundamental geometry of a counterflow double-pipe heat exchanger was conducted using the PFR-based model. The results indicate that under the conditions of this study, a length of approximately 3.0 m is required to achieve the desired target heating temperature of 1200 °C and achieve methane pyrolysis without the use of a catalyst. Furthermore, this configuration met the feasibility criteria for the proposed system under the considered conditions.
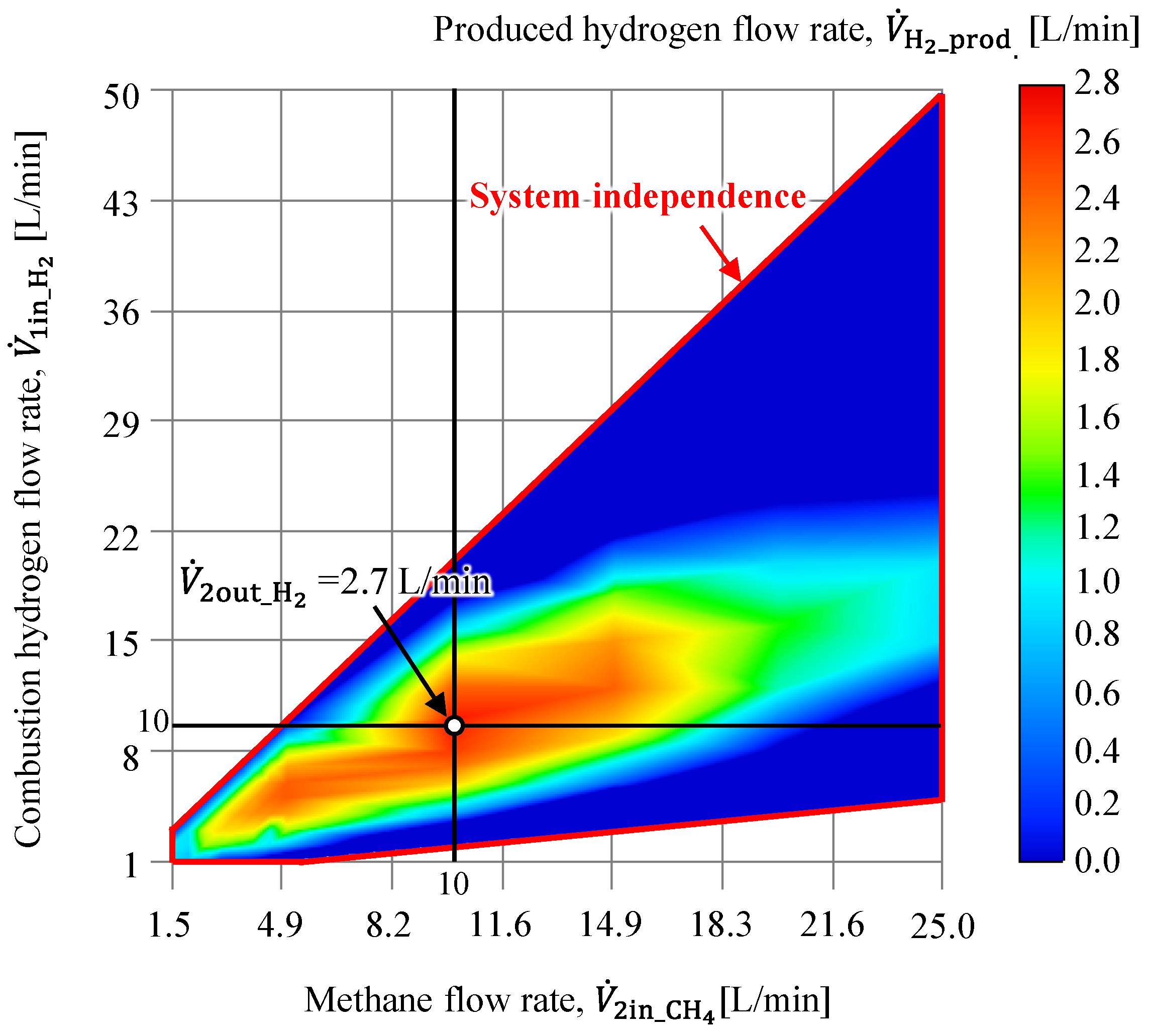
- The base configuration applied in this study was applied to explore the system viability by varying the methane and combustion hydrogen flow rates to determine the conditions for achieving the maximum produced hydrogen flow rate. The results indicate that given the considered study configuration, a minimum methane residence time of at least 3.0 s is required.
- The methane conversion rate map revealed a methane conversion rate of approximately 60% at the point where hydrogen production was maximized. The methane conversion rate increased as the methane flow rate decreased and the combustion hydrogen flow rate increased. Thus, the residence time and conditions that allow greater heat transfer from the inner pipe to the outer pipe were identified as significant contributors to higher methane conversion rates.
- Methane conversion and hydrogen production maps were obtained to provide an index for estimating the system conditions according to the desired hydrogen generation application.
- The flow conditions investigated in this study confirmed that the optimal conditions were a methane flow rate of 10 L/min and a combustion hydrogen flow rate of 10 L/min (β = 1.0), at which the methane conversion was α = 0.64 and the product hydrogen flow rate was 2.7 L/min.
- By focusing on the temperature of the methane gas in the outer pipe, an optimal configuration was observed for each set of conditions, suggesting that increasing the residence time, enhancing the heat exchange distance, and improving the heat exchange efficiency through modifications in the pipe geometry, such as increasing the inner tube diameter and attaching fins to the inner tube to enlarge the heat transfer surface area, can improve hydrogen production.
- An investigation of system efficiency produced a map indicating higher efficiency at lower methane flow rates. Furthermore, system efficiency improved when carbon was considered as a product, suggesting that carbon also has a significant impact on system efficiency and indicating the necessity of further investigations into the production of carbon by methane pyrolysis.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kabeyi, M.J.B.; Olanrewaju, O.A. Sustainable energy transition for renewable and low carbon grid electricity generation and supply. Front. Energy Res. 2022, 9, 743114. [Google Scholar] [CrossRef]
- Abbasi, K.R.; Shahbaz, M.; Zhang, J.; Irfan, M.; Alvarado, R. Analyze the environmental sustainability factors of China: The role of fossil fuel energy and renewable energy. Renew. Energy 2022, 187, 390–402. [Google Scholar] [CrossRef]
- Xiong, J.; Xu, D. Relationship between energy consumption, economic growth and environmental pollution in China. Environ. Res. 2021, 194, 110718. [Google Scholar] [CrossRef]
- Xia, W.; Apergis, N.; Bashir, M.F.; Ghosh, S.; Doğan, B.; Shahzad, U. Investigating the role of globalization, and energy consumption for environmental externalities: Empirical evidence from developed and developing economies. Renew. Energy 2022, 183, 219–228. [Google Scholar] [CrossRef]
- Yusaf, T.; Laimon, M.; Alrefae, W.; Kadirgama, K.; Dhahad, H.A.; Ramasamy, D.; Kamarulzaman, M.K.; Yousif, B. Hydrogen energy demand growth prediction and assessment (2021–2050) using a system thinking and system dynamics approach. Appl. Sci. 2022, 12, 781. [Google Scholar] [CrossRef]
- Wijayasekera, S.C.; Hewage, K.; Siddiqui, O.; Hettiaratchi, P.; Sadiq, R. Waste-to-hydrogen technologies: A critical review of techno-economic and socio-environmental sustainability. Int. J. Hydrogen Energy 2022, 47, 5842–5870. [Google Scholar] [CrossRef]
- Janoszek, T.; Masny, W. CFD simulations of allothermal steam gasification process for hydrogen production. Energies 2021, 14, 1532. [Google Scholar] [CrossRef]
- Muradov, N.; Smith, F.; Huang, C.; T-Raissi, A. Autothermal catalytic pyrolysis of methane as a new route to hydrogen production with reduced CO2 emissions. Catal. Today 2006, 116, 281–288. [Google Scholar] [CrossRef]
- Takenaka, S.; Shigeta, Y.; Tanabe, E.; Otsuka, K. Methane decomposition into hydrogen and carbon nanofibers over supported Pd-Ni catalysts. J. Catal. 2003, 220, 468–477. [Google Scholar] [CrossRef]
- Zhou, L.; Basset, J.M. Unsupported Ni–Pt alloy metal catalysts prepared by water-in-oil (W/O) microemulsion method for methane cracking. Fuel 2016, 181, 805–810. [Google Scholar] [CrossRef]
- Mei, I.L.S.; Lock, S.S.M.; Vo, D.V.N.; Abdullah, B. Thermo-catalytic methane decomposition for hydrogen production: Effect of palladium promoter on Ni-based catalysts. Bull. Chem. React. Eng. Catal. 2016, 11, 191–199. [Google Scholar] [CrossRef]
- Ouyang, M.; Boldrin, P.; Maher, R.C.; Chen, X.; Liu, X.; Cohen, L.F.; Brandon, N.P. A mechanistic study of the interactions between methane and nickel supported on doped ceria. Appl. Catal. B Environ. 2019, 248, 332–340. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, Z.; Zhang, X. Production of COx-free hydrogen and nanocarbon by direct decomposition of undiluted methane on Ni–Cu–alumina catalysts. Appl. Catal. A Gen. 2004, 269, 179–186. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y.; Kuvshinov, G.G. Effective catalysts for direct cracking of methane to produce hydrogen and filamentous carbon. Part I. Nickel catalysts. Appl. Catal. A Gen. 2000, 201, 61–70. [Google Scholar] [CrossRef]
- Bayat, N.; Rezaei, M.; Meshkani, F. Methane decomposition over Ni–Fe/Al2O3 catalysts for production of COx-free hydrogen and carbon nanofiber. Int. J. Hydrogen Energy 2016, 41, 1574–1584. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Wan Daud, W.M.A.; Abbas, H.F. Production of greenhouse gas free hydrogen by thermocatalytic decomposition of methane—A review. Renew. Sustain. Energy Rev. 2015, 44, 221–256. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, G. Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 2011, 162, 1–48. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Drewett, N.E.; Wang, X.; Yoo, S.J.; Wang, H.; Deng, T.; Kim, J.G.; Chen, H.; Huang, K.; et al. Integrating catalysis of methane decomposition and electrocatalytic hydrogen evolution with Ni/CeO2 for improved hydrogen production efficiency. ChemSusChem 2019, 12, 1000–1010. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Jia, Q.; Takriff, M.S. Catalytic decomposition of methane over rare earth metal (Ce and La) oxides supported iron catalysts. Appl. Surf. Sci. 2019, 467–468, 236–248. [Google Scholar] [CrossRef]
- Urdiana, G.; Valdez, R.; Lastra, G.; Valenzuela, M.Á.; Olivas, A. Production of hydrogen and carbon nanomaterials using transition metal catalysts through methane decomposition. Mater. Lett. 2018, 217, 9–12. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, Q.; Ma, D. Nanostructured catalyst for Fischer–Tropsch synthesis. Chin. J. Chem. 2018, 36, 798–808. [Google Scholar] [CrossRef]
- Awadallah, A.E.; El-Desouki, D.S.; Abdel-Azim, S.M.; Aboul-Gheit, N.A.K.; Abdel-Hamid, S.M.; Aboul-Gheit, A.K. Effect of La, Ce and Nd oxides addition on the activity and stability of Co/MgO catalyst for methane decomposition into COx-free H2 production and carbon nanotubes. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 525–534. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Aboul-Enein, A.A.; Mahmoud, A.H.; Abd El Rehim, S.S.; El-Ziaty, A.K.; Aboul-Gheit, A.K. ZrxMg1-xO supported cobalt catalysts for methane decomposition into COx-free hydrogen and carbon nanotubes. Int. J. Green Energy 2018, 15, 568–576. [Google Scholar] [CrossRef]
- Zhou, L.; Enakonda, L.R.; Saih, Y.; Loptain, S.; Gary, D.; Del-Gallo, P.; Basset, J.M. Catalytic methane decomposition over Fe–Al2O3. ChemSusChem 2016, 9, 1243–1248. [Google Scholar] [CrossRef]
- Qian, J.X.; Chen, T.W.; Enakonda, L.R.; Liu, D.B.; Mignani, G.; Basset, J.M.; Zhou, L. Methane decomposition to produce COx-free hydrogen and nano-carbon over metal catalysts: A review. Int. J. Hydrogen Energy 2020, 45, 7981–8001. [Google Scholar] [CrossRef]
- Cunha, A.F.; Órfão, J.J.M.; Figueiredo, J.L. Methane decomposition on Ni–Cu alloyed Raney-type catalysts. Int. J. Hydrogen Energy 2009, 34, 4763–4772. [Google Scholar] [CrossRef]
- Chambers, A.; Nemes, T.; Rodriguez, N.M.; Baker, R.T.K. Catalytic behavior of graphite nanofiber supported nickel particles. 1. Comparison with other support media. J. Phys. Chem. B 1998, 102, 2251–2258. [Google Scholar] [CrossRef]
- Cunha, A.F.; Órfão, J.J.M.; Figueiredo, J.L. Methane decomposition on Fe–Cu Raney-type catalysts. Fuel Process. Technol. 2009, 90, 1234–1240. [Google Scholar] [CrossRef]
- Lua, A.C.; Wang, H.Y. Hydrogen production by catalytic decomposition of methane over Ni–Cu–Co alloy particles. Appl. Catal. B Environ. 2014, 156–157, 84–93. [Google Scholar] [CrossRef]
- Billaud, F.; Gueret, C.; Weill, J. Thermal decomposition of pure methane at 1263 K. Experiments and mechanistic modelling. Thermochim. Acta 1992, 211, 303–322. [Google Scholar] [CrossRef]
- Wang, S.; Wen, Y.; Shi, Z.; Zaini, I.N.; Jonsson, P.G.; Yang, W. Novel carbon-negative methane production via integrating anaerobic digestion and pyrolysis of organic fraction of municipal solid waste. Energy Convers. Manag. 2022, 252, 115042. [Google Scholar] [CrossRef]
- Sun, E.; Zhai, S.; Kim, D.; Gigantino, M.; Haribal, V.; Dewey, O.S.; Williams, S.M.; Wan, G.; Nelson, A.; Marin-Quiros, S.; et al. A semi-continuous process for co-production of CO2-free hydrogen and carbon nanotubes via methane pyrolysis. Cell Rep. Phys. Sci. 2023, 4, 101338. [Google Scholar] [CrossRef]
- Patel, S.; Kundu, S.; Halder, P.; Marzbali, M.H.; Chiang, K.; Surapaneni, A.; Shah, K. Production of hydrogen by catalytic methane decomposition using biochar and activated char produced from biosolids pyrolysis. Int. J. Hydrogen Energy 2020, 45, 29978–29992. [Google Scholar] [CrossRef]
- Yousefi, M.; Donne, S. Experimental study for thermal methane cracking reaction to generate very pure hydrogen in small or medium scales by using regenerative reactor. Front. Energy Res. 2022, 10, 971383. [Google Scholar] [CrossRef]
- New Energy and Industrial Technology Development Organization (NEDO). NEDO Hydrogen and Fuel Cell Project Review 2021, Hydrogen Technology in General (Leading R&D); NEDO Final Report; New Energy and Industrial Technology Development Organization (NEDO): Kawasaki, Japan, 2021. [Google Scholar]
- Muradov, N.; Veziroğlu, T. “Green” path from fossil-based to hydrogen economy: An overview of carbon-neutral technologies. Int. J. Hydrogen Energy 2008, 33, 6804–6839. [Google Scholar] [CrossRef]
- Cheon, S.; Byun, M.; Lim, D.; Lee, H.; Lim, H. Parametric study for thermal and catalytic methane pyrolysis for hydrogen production: Techno-economic and scenario analysis. Energies 2021, 14, 6102. [Google Scholar] [CrossRef]
- Cantera Developers. Reactors and Reactor Networks. Available online: https://cantera.org/science/reactors/reactors.html (accessed on 20 October 2023).
- Shimizu, K.; Hibi, A.; Koshi, M.; Morii, Y.; Tsuboi, N. Updated kinetic mechanism for high-pressure hydrogen combustion. J. Propuls. Power 2011, 27, 383–395. [Google Scholar] [CrossRef]
- Ranzi, E.; Frassoldati, A.; Grana, R.; Cuoci, A.; Faravelli, T.; Kelley, A.P.; Law, C.K. Hierarchical and comparative kinetic modeling of laminar flame speeds of hydrocarbon and oxygenated fuels. Prog. Energy Combust. Sci. 2012, 38, 468–501. [Google Scholar] [CrossRef]
- Muto, T.; Asahara, M.; Miyasaka, T.; Asato, K.; Uehara, T.; Koshi, M. Methane pyrolysis characteristics for the practical application of hydrogen production system using permalloy plate catalyst. Chem. Eng. Sci. 2023, 274, 117931. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen production by methane decomposition: A review. Int. J. Hydrogen Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Shirsath, A.B.; Mokashi, M.; Lott, P.; Muller, H.; Pashminehazar, R.; Sheppard, T.; Tischer, S.; Maier, L.; Grunwaldt, J.D.; Deutschmann, O. Soot formation in methane pyrolysis reactor: Modeling soot growth and particle characterization. J. Phys. Chem. A 2023, 127, 2136–2147. [Google Scholar] [CrossRef]
- Abanades, A.; Ruiz, E.; Ferruelo, E.M.; Hernández, F.; Cabanillas, A.; Martínez, J.M.; Rubio, J.A.; López, C.; Gavela, R.; Barrera, G.; et al. Experimental analysis of direct thermal methane cracking. Int. J. Hydrogen Energy 2011, 36, 12877–12886. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroğlu, T.N. From hydrocarbon to hydrogen–carbon to hydrogen economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Holmen, A.; Olsvik, O.; Rokstad, O.A. Pyrolysis of natural gas: Chemistry and process concepts. Fuel Process. Technol. 1995, 42, 249–267. [Google Scholar] [CrossRef]
- Arutyunov, V.S.; Vedeneev, V.I. Pyrolysis of methane in the temperature range 1000–1700 K. Russ. Chem. Rev. 1991, 60, 1384. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Aksoyiu, E.; Goodman, D.W. Nonoxidative activation of methane. Catal. Rev. 2003, 45, 151–203. [Google Scholar] [CrossRef]
- Geißler, T.; Abánades, A.; Heinzel, A.; Mehravaran, K.; Müller, G.; Rathnam, R.K.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; et al. Hydrogen production via methane pyrolysis in a liquid metal bubble column reactor with a packed bed. Chem. Eng. J. 2016, 299, 192–200. [Google Scholar] [CrossRef]
- Pérez, B.J.L.; Jiménez, J.A.M.; Bhardwaj, R.; Goetheer, E.; van Sint Annaland, M.; Gallucci, F. Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment. Int. J. Hydrogen Energy 2021, 46, 4917–4935. [Google Scholar] [CrossRef]
- Murata, K.; Fujita, K.; Uchida, K. Production of hydrogen by pyrolysis of methane over Ni/Ca/carbon catalysts. J. Japan Pet. Inst. 1997, 40, 129–133. [Google Scholar] [CrossRef]
- Kushch, S.D.; Muradyan, V.E.; Fursikov, P.V.; Knerelman, E.I.; Kuznetsov, V.L.; Butenko, Y.V. Methane pyrolysis over carbon catalysts. Eurasian Chem. Technol. J. 2001, 3, 67–72. [Google Scholar] [CrossRef]






| Conditions | Parameters | Set Value |
|---|---|---|
| Geometry conditions | Inner pipe diameter, D1 | 20 mm |
| Outer pipe diameter, D2 | 40 mm | |
| Double-pipe length, L | 3 m | |
| Flow rate conditions | Methane flow rate, | 2.55 L/min |
| Combustion hydrogen flow rate, | 1.7 L/min | |
| Equivalence ratio, | 1.0 | |
| Oxidizing agent | Air | |
| Pressure, | 101,325 Pa |
| Conditions | Parameters | Set Value |
|---|---|---|
| Geometry conditions | Inner pipe diameter, D1 | 20 mm |
| Outer pipe diameter, D2 | 40 mm | |
| Double-pipe length, L | 3.0 m | |
| Flow rate conditions | Methane flow rate, | 1.5–5.0 L/min |
| Combustion hydrogen flow rate, | 1.0–10.0 L/min | |
| Equivalence ratio, φ | 1.0 | |
| Oxidizing agent | Air | |
| Pressure, p | 101,325 Pa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uehara, T.; Asahara, M.; Miyasaka, T. CO2-Free Hydrogen Production by Methane Pyrolysis Utilizing a Portion of the Produced Hydrogen for Combustion. Energies 2024, 17, 367. https://doi.org/10.3390/en17020367
Uehara T, Asahara M, Miyasaka T. CO2-Free Hydrogen Production by Methane Pyrolysis Utilizing a Portion of the Produced Hydrogen for Combustion. Energies. 2024; 17(2):367. https://doi.org/10.3390/en17020367
Chicago/Turabian StyleUehara, Takuma, Makoto Asahara, and Takeshi Miyasaka. 2024. "CO2-Free Hydrogen Production by Methane Pyrolysis Utilizing a Portion of the Produced Hydrogen for Combustion" Energies 17, no. 2: 367. https://doi.org/10.3390/en17020367
APA StyleUehara, T., Asahara, M., & Miyasaka, T. (2024). CO2-Free Hydrogen Production by Methane Pyrolysis Utilizing a Portion of the Produced Hydrogen for Combustion. Energies, 17(2), 367. https://doi.org/10.3390/en17020367





