Feasibility of Nutrient Removal and Recovery from Abattoir Wastewater Using Microalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. Process Description
2.2. Economic Evaluation
2.3. Environmental Assessment
3. Results and Discussion
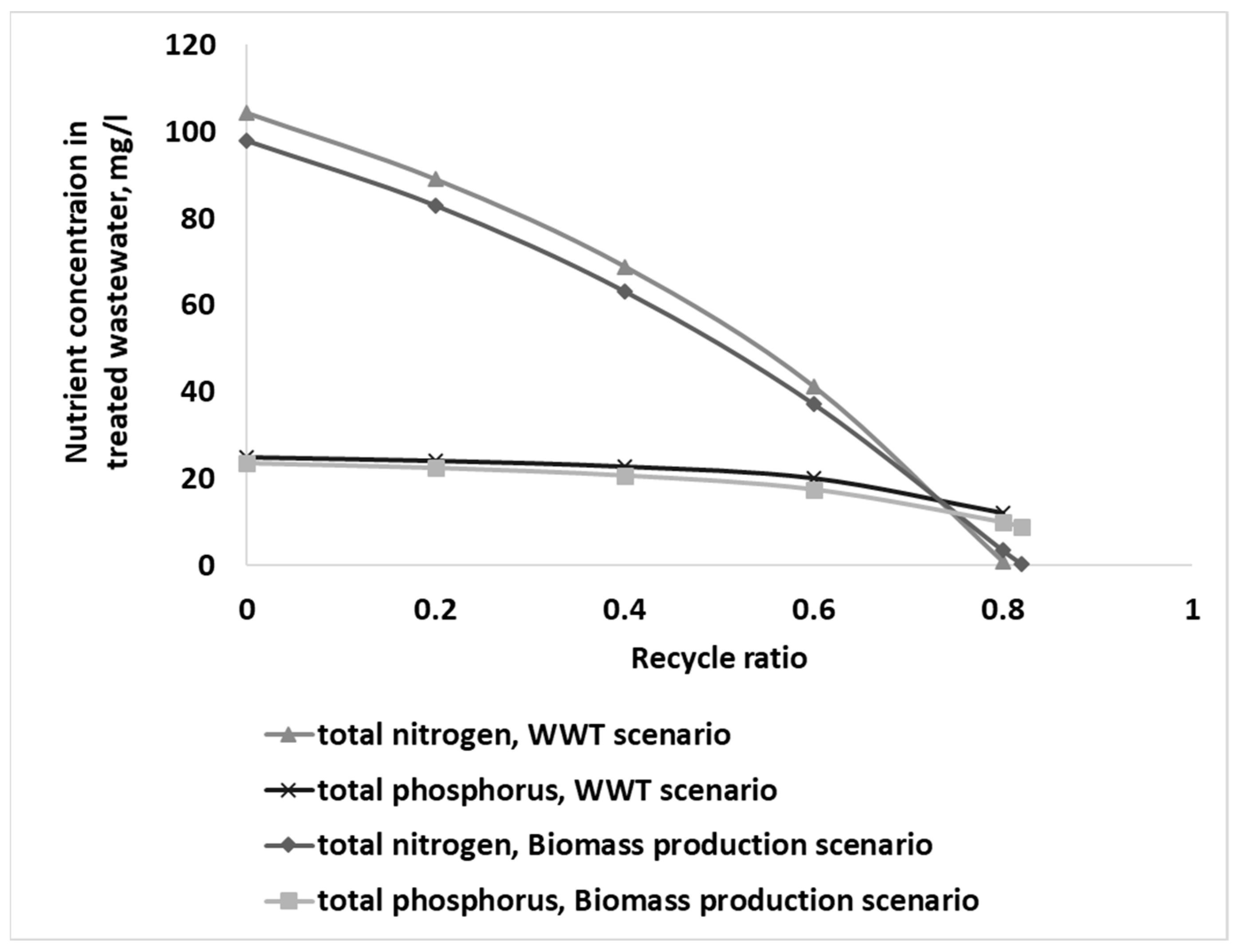
3.1. Nutrient Removal and Biomass Production
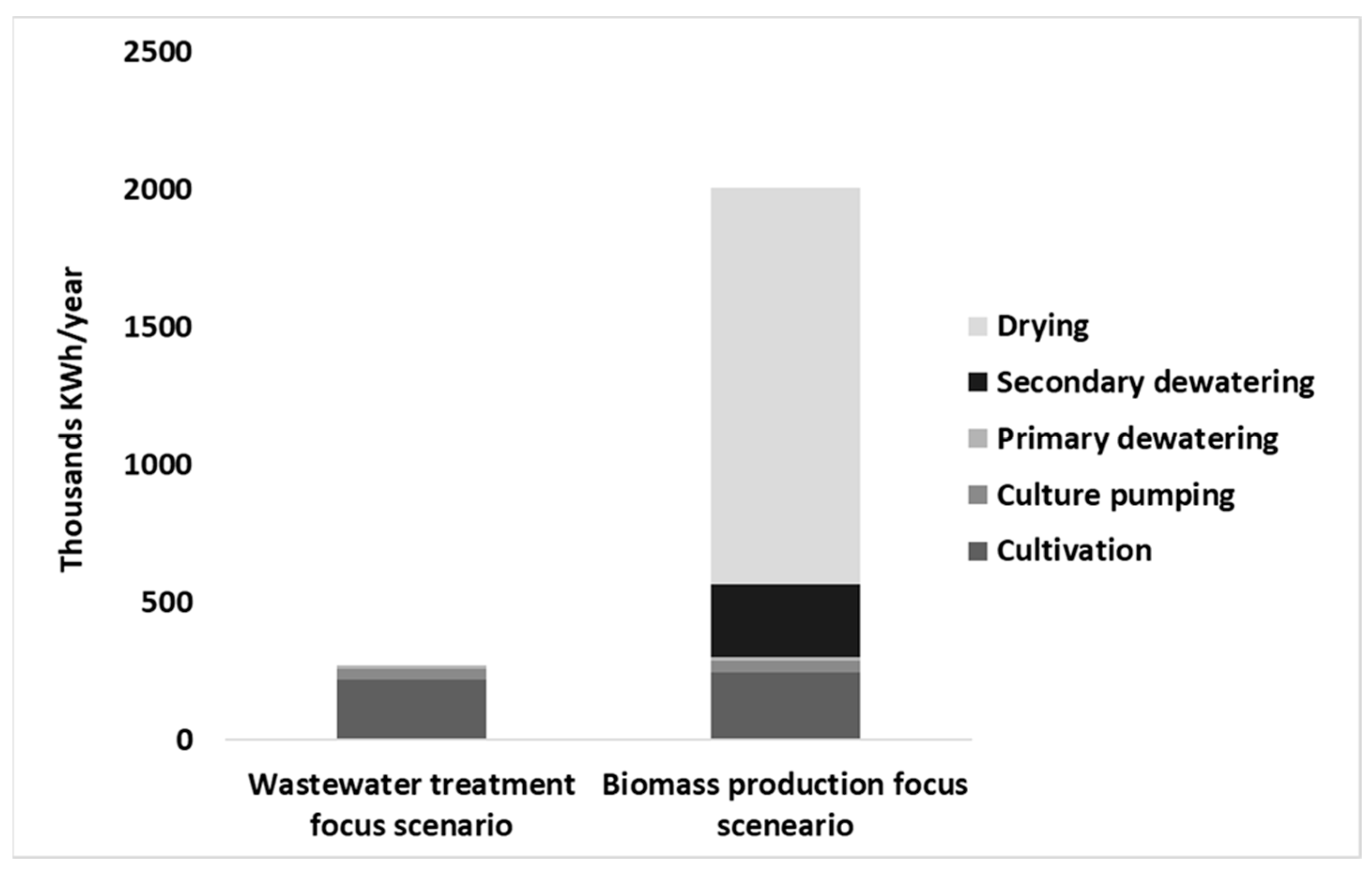
3.2. Economics
3.3. Feasiblity Analysis
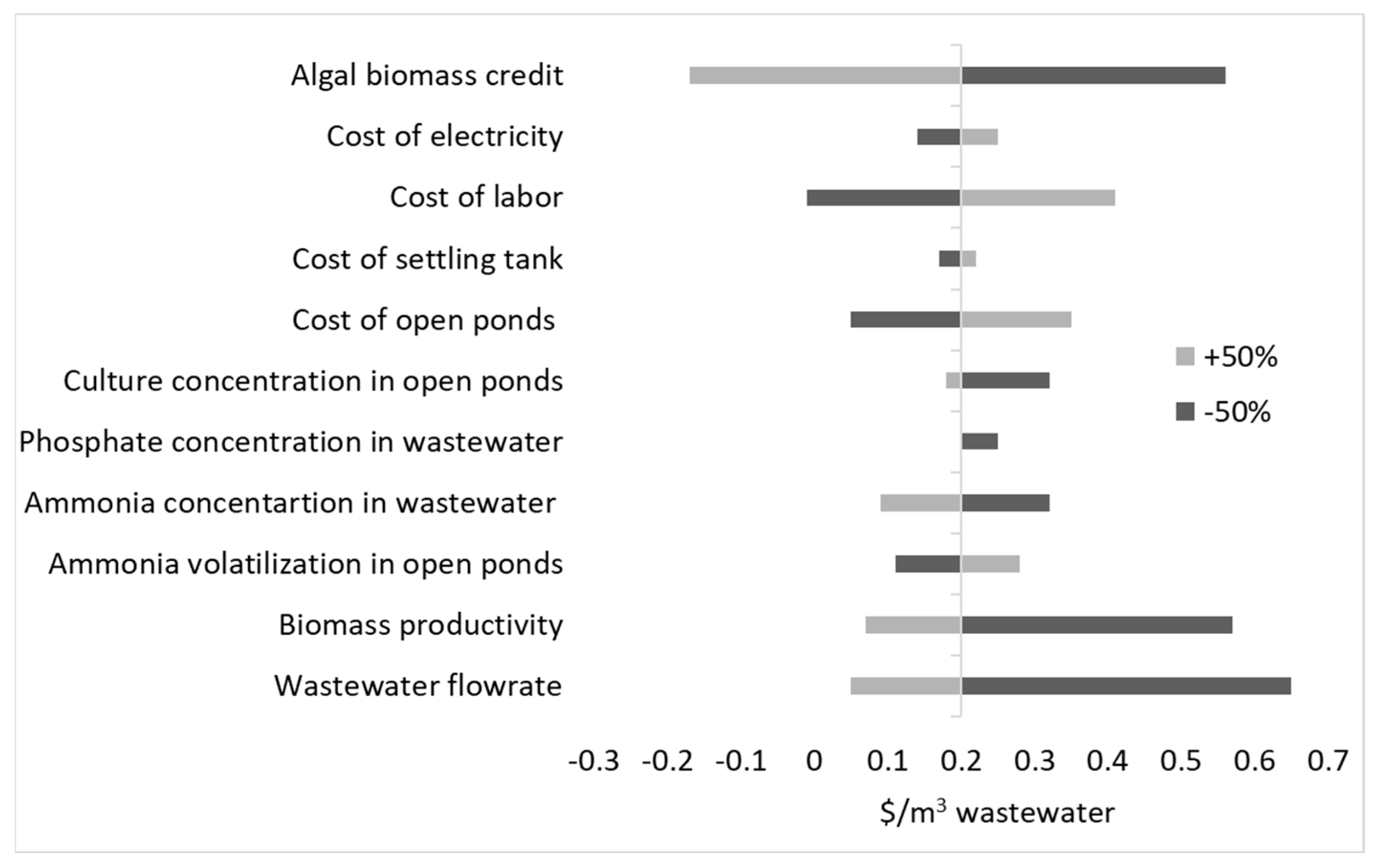
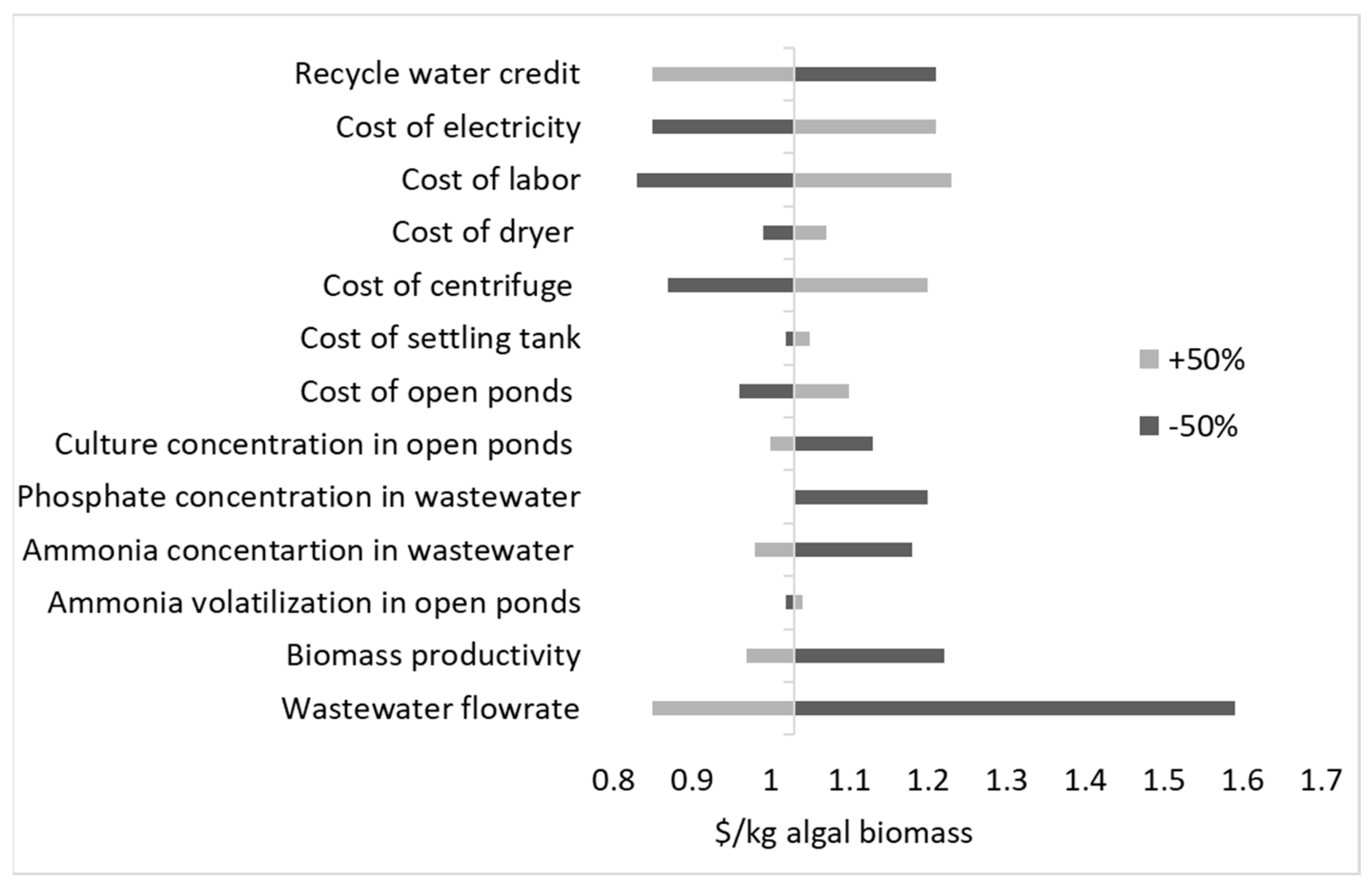
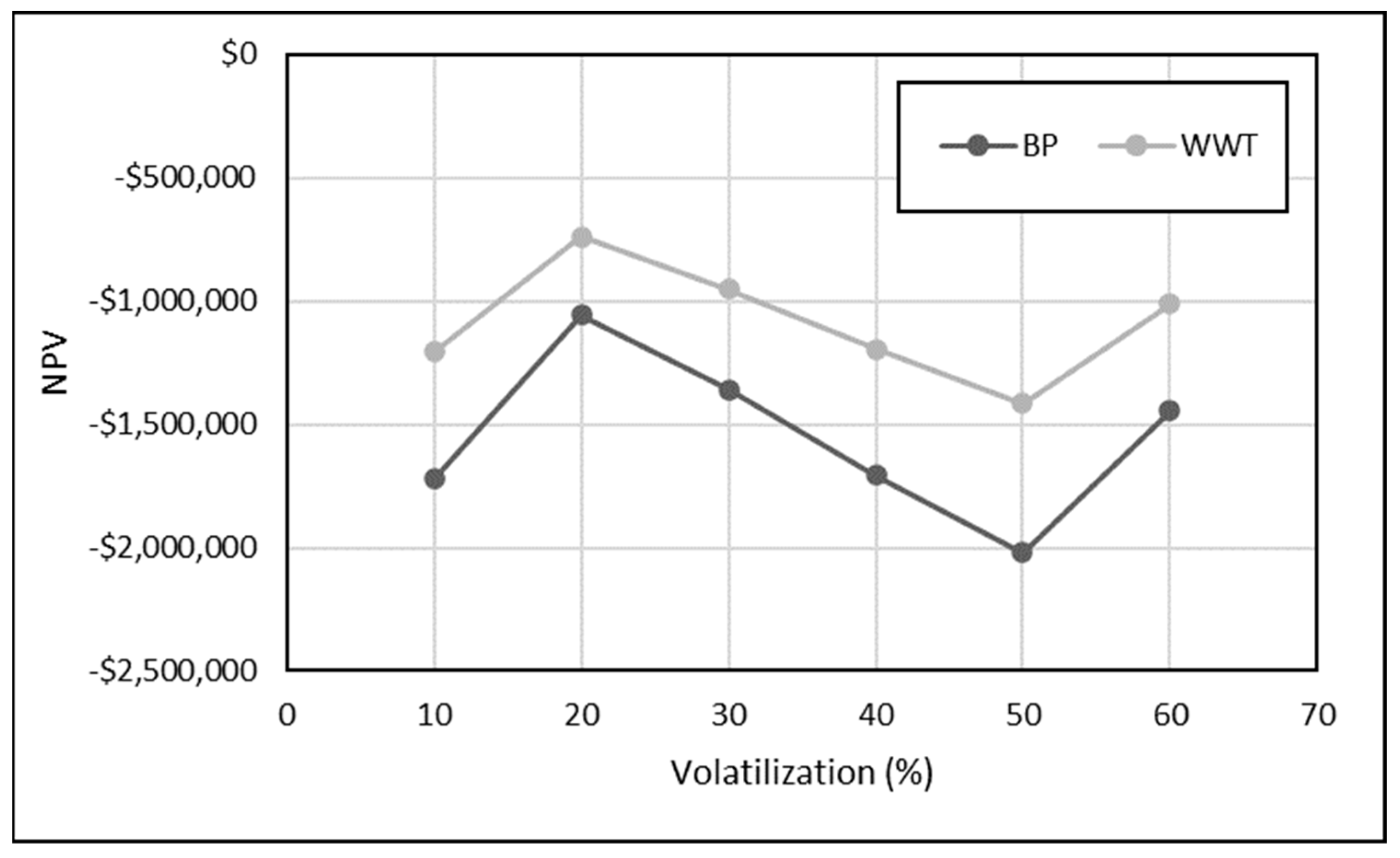
3.3.1. Impact of Ammonia Loss on Profitability
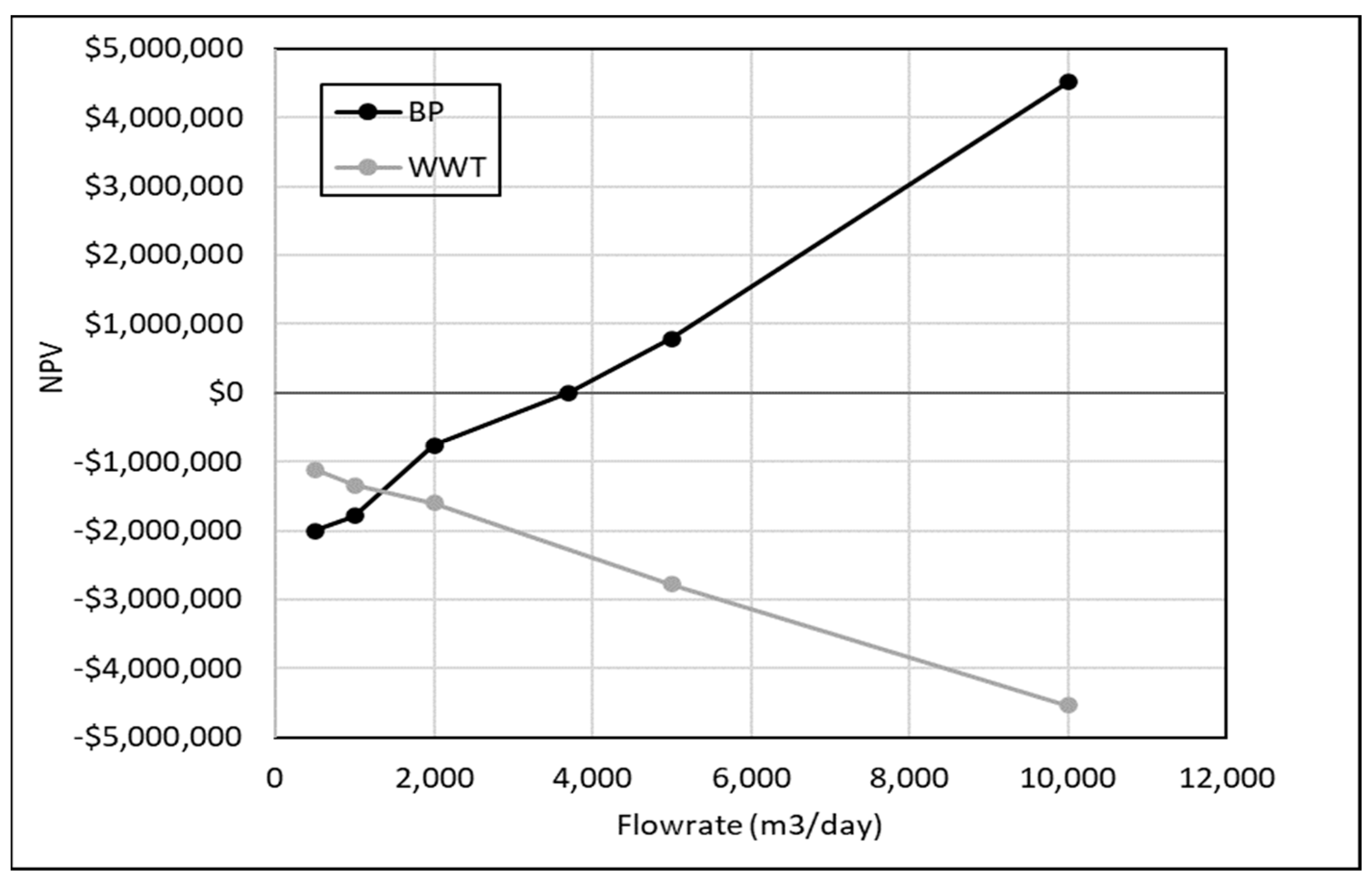
3.3.2. Impact of Wastewater Flowrate on Profitability
3.4. GHG Emission
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bustillo-Lecompte, C.F.; Mehrvar, M. Slaughterhouse wastewater characteristics, treatment, and management in the meat processing industry: A review on trends and advances. J. Environ. Manag. 2015, 161, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Djekic, I. Environmental Impact of Meat Industry—Current Status and Future Perspectives. Procedia Food Sci. 2015, 5, 61–64. [Google Scholar] [CrossRef]
- Warnecke, M.; Farrugia, T.; Ferguson, C. Review of Abattoir Water Usage Reduction, Recycling and Reuse; Meat & Livestock Australia Limited: Sidney, Australia, 2008. [Google Scholar]
- Li, W.-W.; Yu, H.-Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- Min, B.; Kim, J.; Oh, S.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P. Anaerobic Co-Digestion of Paunch and DAF Sludge; Meat & Livestock Australia Limited: Sidney, Australia, 2013. [Google Scholar]
- Raeisossadati, M.; Vadiveloo, A.; Bahri, P.A.; Parlevliet, D.; Moheimani, N.R. Treating anaerobically digested piggery effluent (ADPE) using microalgae in thin layer reactor and raceway pond. J. Appl. Phycol. 2019, 31, 2311–2319. [Google Scholar] [CrossRef]
- Vadiveloo, A.; Nwoba, E.G.; Moheimani, N.R. Viability of combining microalgae and macroalgae cultures for treating anaerobically digested piggery effluent. J. Environ. Sci. 2019, 82, 132–144. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Ayre, J.M.; Moheimani, N.R.; Ubi, B.E.; Ogbonna, J.C. Growth comparison of microalgae in tubular photobioreactor and open pond for treating anaerobic digestion piggery effluent. Algal Res. 2016, 17, 268–276. [Google Scholar] [CrossRef]
- Hamawand, I.; Ghadouani, A.; Bundschuh, J.; Hamawand, S.; Al Juboori, R.A.; Chakrabarty, S.; Yusaf, T. A critical review on processes and energy profile of the Australian meat processing industry. Energies 2017, 10, 731. [Google Scholar] [CrossRef]
- Whitton, R.; Le Mével, A.; Pidou, M.; Ometto, F.; Villa, R.; Jefferson, B. Influence of microalgal N and P composition on wastewater nutrient remediation. Water Res. 2016, 91, 371–378. [Google Scholar] [CrossRef]
- Olguín, E.J. Dual purpose microalgae–bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a Biorefinery. Biotechnol. Adv. 2012, 30, 1031–1046. [Google Scholar] [CrossRef]
- Chaudry, S.; Bahri, P.A.; Moheimani, N.R. Pathways of processing of wet microalgae for liquid fuel production: A critical review. Renew. Sustain. Energy Rev. 2015, 52, 1240–1250. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of Nutrients From Wastewaters Using Microalgae. Front. Sustain. Food Syst. 2018, 2, 59. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Vadiveloo, A.; Ayre, J.M.; Pluske, J.R. Nutritional profile and in vitro digestibility of microalgae grown in anaerobically digested piggery effluent. Algal Res. 2018, 35, 362–369. [Google Scholar] [CrossRef]
- Kose, A.; Ozen, M.O.; Elibol, M.; Oncel, S.S. Investigation of in vitro digestibility of dietary microalga Chlorella vulgaris and cyanobacterium Spirulina platensis as a nutritional supplement. 3 Biotech 2017, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Safafar, H.; Uldall Nørregaard, P.; Ljubic, A.; Møller, P.; Løvstad Holdt, S.; Jacobsen, C. Enhancement of protein and pigment content in two Chlorella species cultivated on industrial process water. J. Mar. Sci. Eng. 2016, 4, 84. [Google Scholar] [CrossRef]
- Maizatul, A.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Hashim, M.A. An overview of the utilisation of microalgae biomass derived from nutrient recycling of wet market wastewater and slaughterhouse wastewater. Int. Aquat. Res. 2017, 9, 177–193. [Google Scholar] [CrossRef]
- Mayberry, D.; Bartlett, H.; Moss, J.; Wiedemann, S.; Herrero, M. Greenhouse Gas Mitigation Potential of the Australian Red Meat Production and Processing Sectors; Meat & Livestock Australia Limited: Sidney, Australia, 2018. [Google Scholar]
- McCabe, B.K.; Harris, P.; Antille, D.L.; Schmidt, T.; Lee, S.; Hill, A.; Baillie, C. Toward profitable and sustainable bioresource management in the Australian red meat processing industry: A critical review and illustrative case study. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2415–2439. [Google Scholar] [CrossRef]
- Shayesteh, H.; Vadiveloo, A.; Bahri, P.A.; Moheimani, N.R. Can CO2 addition improve the tertiary treatment of anaerobically digested abattoir effluent (ADAE) by Scenedesmus sp.(Chlorophyta)? Algal Res. 2021, 58, 102379. [Google Scholar] [CrossRef]
- Shayesteh, H.; Vadiveloo, A.; Bahri, P.A.; Moheimani, N.R. Long term outdoor microalgal phycoremediation of anaerobically digested abattoir effluent. J. Environ. Manag. 2022, 323, 116322. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment With CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Wang, L.; Li, Y.; Mohr, M.J.; Hu, B.; Zhou, W.; Chen, P.; Ruan, R. Cultivating Chlorella sp. in a Pilot-Scale Photobioreactor Using Centrate Wastewater for Microalgae Biomass Production and Wastewater Nutrient Removal. Appl. Biochem. Biotechnol. 2011, 165, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Martinez, A.; Martin Garcia, N.; Romero, I.; Seco, A.; Ferrer, J. Microalgae cultivation in wastewater: Nutrient removal from anaerobic membrane bioreactor effluent. Bioresour. Technol. 2012, 126, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Ayre, J.M.; Moheimani, N.R.; Borowitzka, M.A. Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Res. 2017, 24, 218–226. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.; Shu, Q.; Takala, J.; Hiltunen, E.; Feng, P.; Yuan, Z. Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res. 2013, 47, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiong, H.; Hui, Z.; Zeng, X. Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Bioresour. Technol. 2012, 104, 215–220. [Google Scholar] [CrossRef]
- Jia, H.; Yuan, Q. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Fraga, F. Phytoplanktonic biomass synthesis: Application to deviations from Redfield stoichiometry. Instituto de Ciencias del Mar (ICM) 2001, 65 (Suppl. S2), 153–169. [Google Scholar] [CrossRef][Green Version]
- Chaudry, S.; Bahri, P.A.; Moheimani, N.R. Superstructure optimization and energetic feasibility analysis of process of repetitive extraction of hydrocarbons from Botryococcus braunii—A species of microalgae. Comput. Chem. Eng. 2017, 97, 36–46. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Cord-Ruwisch, R.; Raes, E.; Borowitzka, M.A. Non-destructive oil extraction from Botryococcus braunii (Chlorophyta). J. Appl. Phycol. 2013, 25, 1653–1661. [Google Scholar] [CrossRef]
- Lundquist, T.J.; Woertz, I.C.; Quinn, N.W.T.; Benemann, J.R. A Realistic Technology and Engineering Assessment of Algae Biofuel Production; Energy Biosciences Institute: Berkeley, CA, USA, 2010. [Google Scholar]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- COWI. Cleaner Production Assessment in Meat Processing; Consulting Engineers and Planners AS: Lyngby, Denmark, 2017. [Google Scholar]
- García, J.; Mujeriego, R.; Hernández-Mariné, M. High rate algal pond operating strategies for urban wastewater nitrogen removal. J. Appl. Phycol. 2000, 12, 331–339. [Google Scholar] [CrossRef]
- Kinidi, L.; Tan, I.A.W.; Abdul Wahab, N.B.; Tamrin, K.F.B.; Hipolito, C.N.; Salleh, S.F. Recent Development in Ammonia Stripping Process for Industrial Wastewater Treatment. Int. J. Chem. Eng. 2018, 2018, 14. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis and Design of Chemical Processes; Pearson Education: London, UK, 2008. [Google Scholar]
- Ridoutt, B.; Sanguansri, P.; Alexander, D. Environmental Performance Review: Red Meat Processing Sector 2015; Australian Meat Processor Corporation: Sydney, Australia, 2015. [Google Scholar]
- Chaudry, S.; Bahri, P.A.; Moheimani, N.R. Life cycle analysis of milking of microalgae for renewable hydrocarbon production. Comput. Chem. Eng. 2019, 121, 510–522. [Google Scholar] [CrossRef]
- Robb, D.H.F.; Macleod, M.; Hasan, M.R.; Stoto, D. Greenhouse gas emissions from aquaculture, A life cycle assessment of three Asian systems. FAO Fish. Aquac. Tech. Pap. 2017, 609. [Google Scholar]
- Fornarelli, R.; Bahri, P.A.; Moheimani, N. Utilization of Microalgae to Purify Waste Streams and Production of Value Added Products; Australian Meat Processor Corporation: Sydney, Australia, 2017. [Google Scholar]
- Watts, P.J.; Davis, R.J.; Keane, O.B.; Luttrell, M.M.; Tucker, R.W.; Stafford, R.; Janke, S. Beef Cattle Feedlots: Design and Construction; Meat & Livestock Australia Limited: Sidney, Australia, 2016. [Google Scholar]
- Fatone, F.; Baeza, J.A.; Batstone, D.; Cema, G.; Crutchik, D.; Diez-Montero, R.; Huelsen, T.; Lyberatos, G.; Mcleod, A.; Mosquera-Corral, A.; et al. Nutrient removal. In Innovative Wastewater Treatment & Resource Recovery Technologies, Impacts on Energy, Economy and Environment; Lema, J.M., Suarez, S., Eds.; IWA Publishing: London, UK, 2017. [Google Scholar]
- Barone, R.S.C.; Sonoda, D.Y.; Lorenz, E.K.; Cyrino, J.E.P. Digestibility and pricing of Chlorella sorokiniana meal for use in tilapia feeds. Sci. Agric. 2018, 75, 184–190. [Google Scholar] [CrossRef]
- Ruiz, J.; Olivieri, G.; De Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.; Kleinegris, D.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Raja, R.; Coelho, A.; Hemaiswarya, S.; Kumar, P.; Carvalho, I.S.; Alagarsamy, A. Applications of microalgal paste and powder as food and feed: An update using text mining tool. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 740–747. [Google Scholar] [CrossRef]
- Fernández, F.A.; Sevilla, J.M.F.; Grima, E.M. Costs analysis of microalgae production. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 551–566. [Google Scholar]
- Hone, S.; Gooday, P.; Hafi, A.; Greenville, J. Discount Rates and Risk in the Economic Analysis of Agricultural Projects; ABARES: Canberra, Australia, 2022. [Google Scholar]
- Buonocore, E.; Mellino, S.; De Angelis, G.; Liu, G.; Ulgiati, S. Life cycle assessment indicators of urban wastewater and sewage sludge treatment. Ecol. Indic. 2018, 94, 13–23. [Google Scholar] [CrossRef]





| Parameter | Wastewater Treatment (WWT) Focus | Biomass Production (BP) Focus |
|---|---|---|
| Paddle-wheel-driven raceway pond area, hectare (ha) * | 10 | 11 |
| Capital cost, million $ | 1.58 | 4.64 |
| Operating cost, million $/year | 0.27 | 0.85 |
| By product credit | - | - |
| Algae biomass credit (as soybean meal) for WWT focus scenario, million $/year | 0.21 | - |
| Recycle water credit (class B) for biomass production focus scenario, million $/year | - | 0.22 |
| Net operating cost after by-product credit, million $/year | 0.06 | 0.63 |
| Wastewater treatment cost, $/m3 of treated wastewater | 0.2 | |
| Biomass production cost, $/kg of algal biomass | - | 1.03 |
| Section | Cultivation and Harvesting | Secondary Dewatering | Drying |
|---|---|---|---|
| Cost, $/kg biomass | 0.46 | 0.48 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudry, S.; Alavianghavanini, A.; Darvehei, P.; Moheimani, N.R.; Bahri, P.A. Feasibility of Nutrient Removal and Recovery from Abattoir Wastewater Using Microalgae. Energies 2024, 17, 308. https://doi.org/10.3390/en17020308
Chaudry S, Alavianghavanini A, Darvehei P, Moheimani NR, Bahri PA. Feasibility of Nutrient Removal and Recovery from Abattoir Wastewater Using Microalgae. Energies. 2024; 17(2):308. https://doi.org/10.3390/en17020308
Chicago/Turabian StyleChaudry, Sofia, Arsalan Alavianghavanini, Pooya Darvehei, Navid R. Moheimani, and Parisa A. Bahri. 2024. "Feasibility of Nutrient Removal and Recovery from Abattoir Wastewater Using Microalgae" Energies 17, no. 2: 308. https://doi.org/10.3390/en17020308
APA StyleChaudry, S., Alavianghavanini, A., Darvehei, P., Moheimani, N. R., & Bahri, P. A. (2024). Feasibility of Nutrient Removal and Recovery from Abattoir Wastewater Using Microalgae. Energies, 17(2), 308. https://doi.org/10.3390/en17020308





