Biodiesel Production Processes with Yeast: A Sustainable Approach
Abstract
1. Introduction
2. Evaluation of Sustainable Indicators in Each Stage
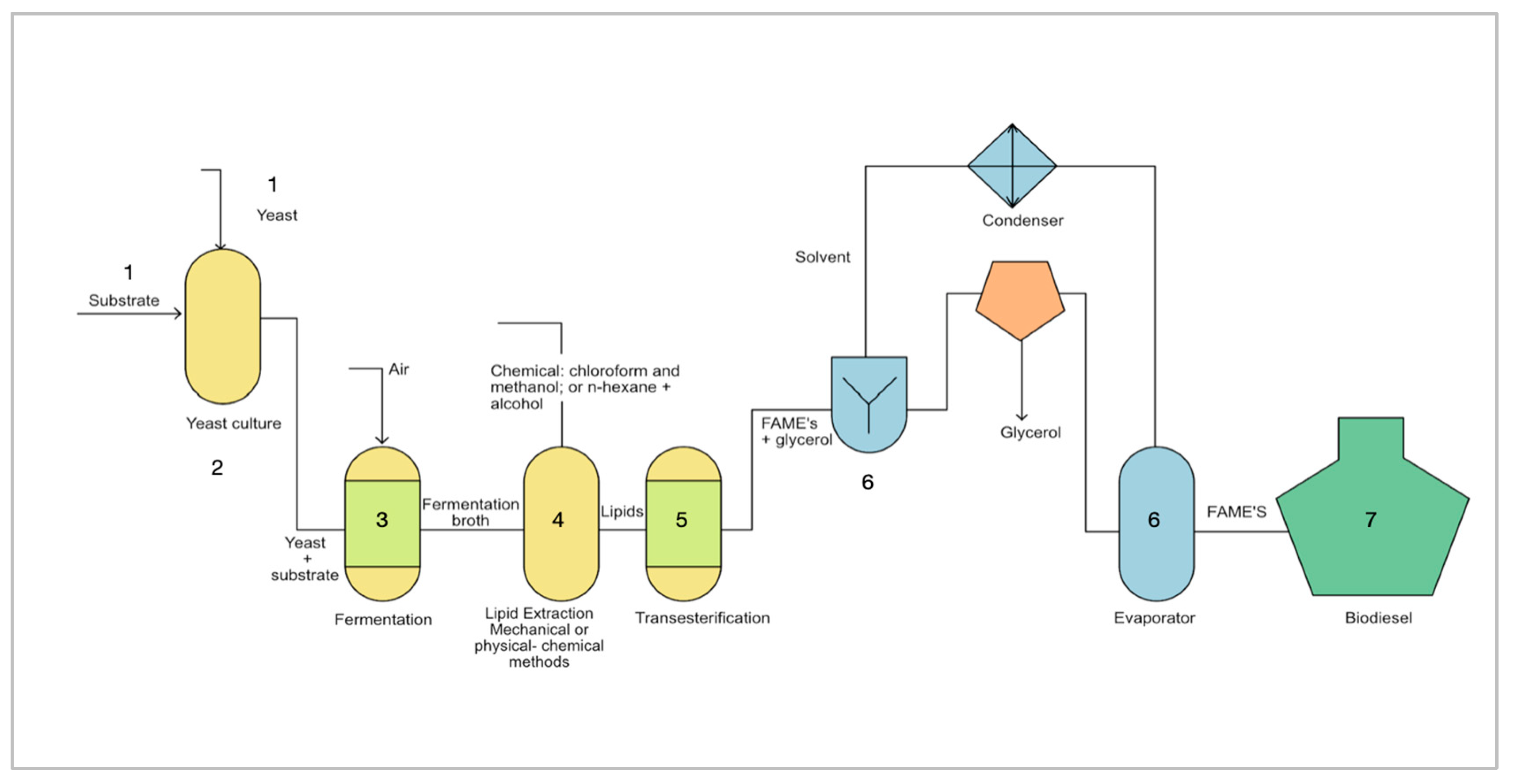
3. Biodiesel Production Process from Yeast Lipids
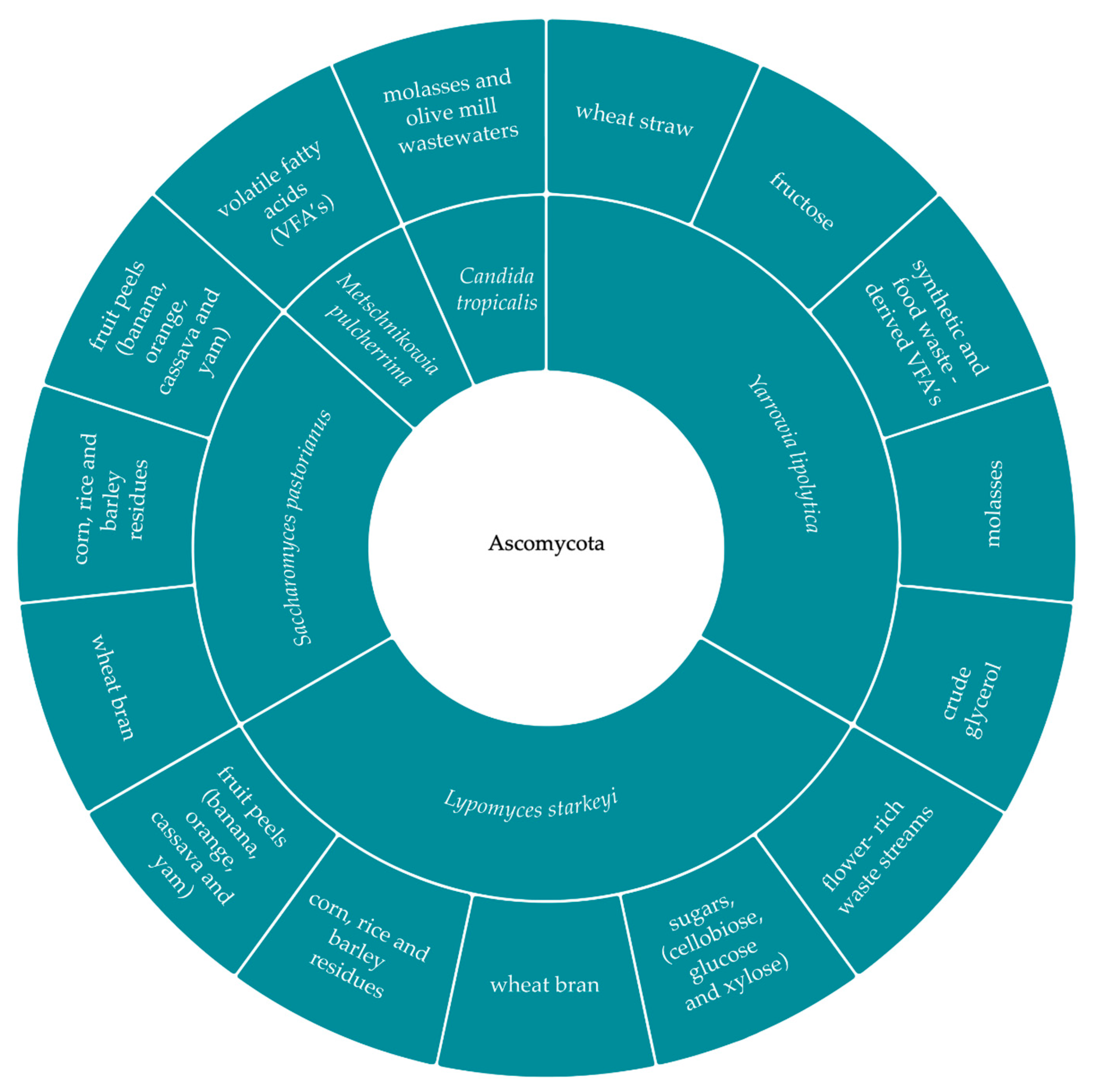
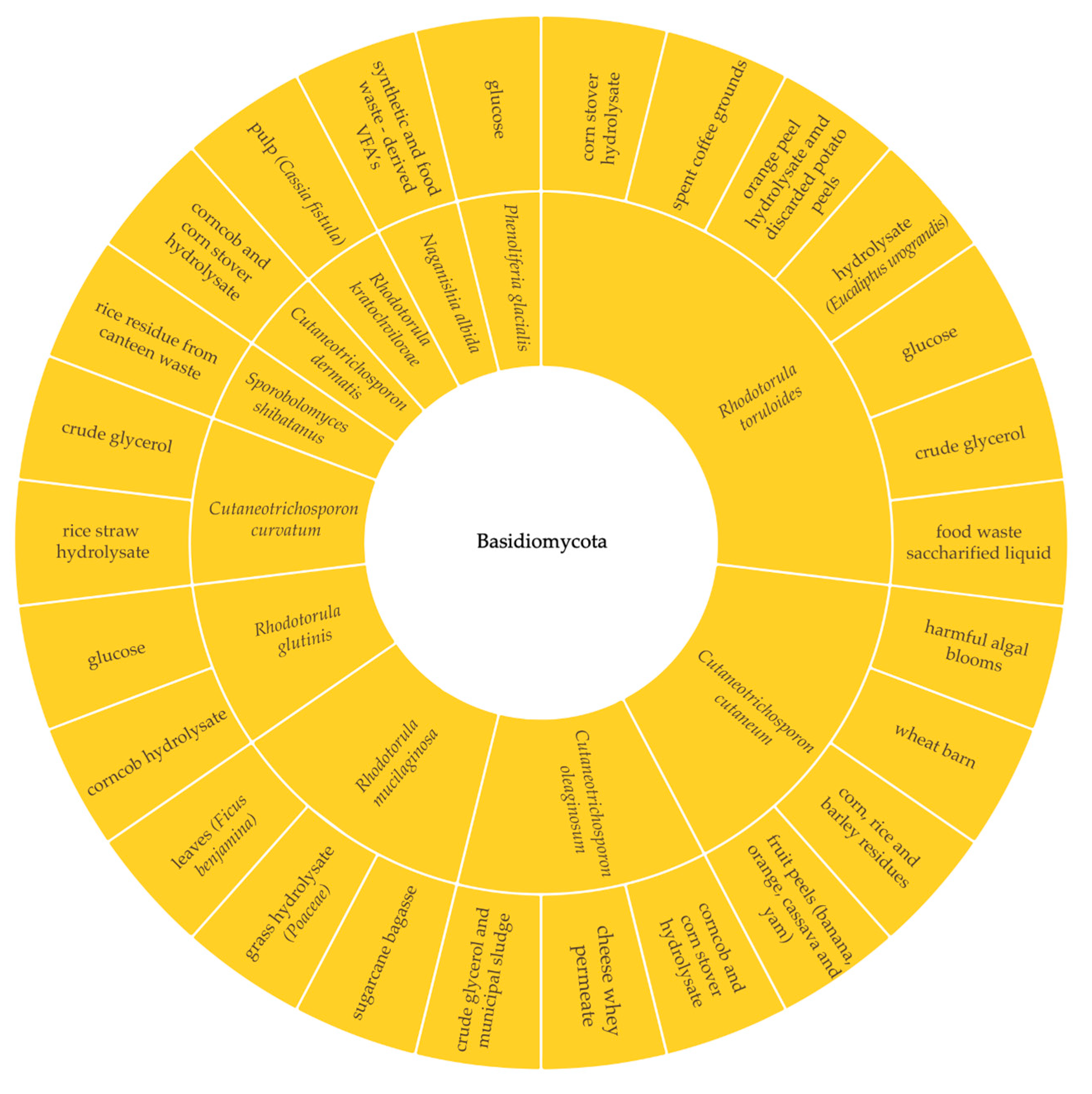
3.1. Selection of the Yeast Strain and Substrate
3.1.1. Sugars
3.1.2. Pure and Crude Glycerol Combined with Other Carbon Sources
3.1.3. Volatile Fatty Acids (VFAs)
3.1.4. Industrial Effluents
3.1.5. Food Loss and Waste (FLW)
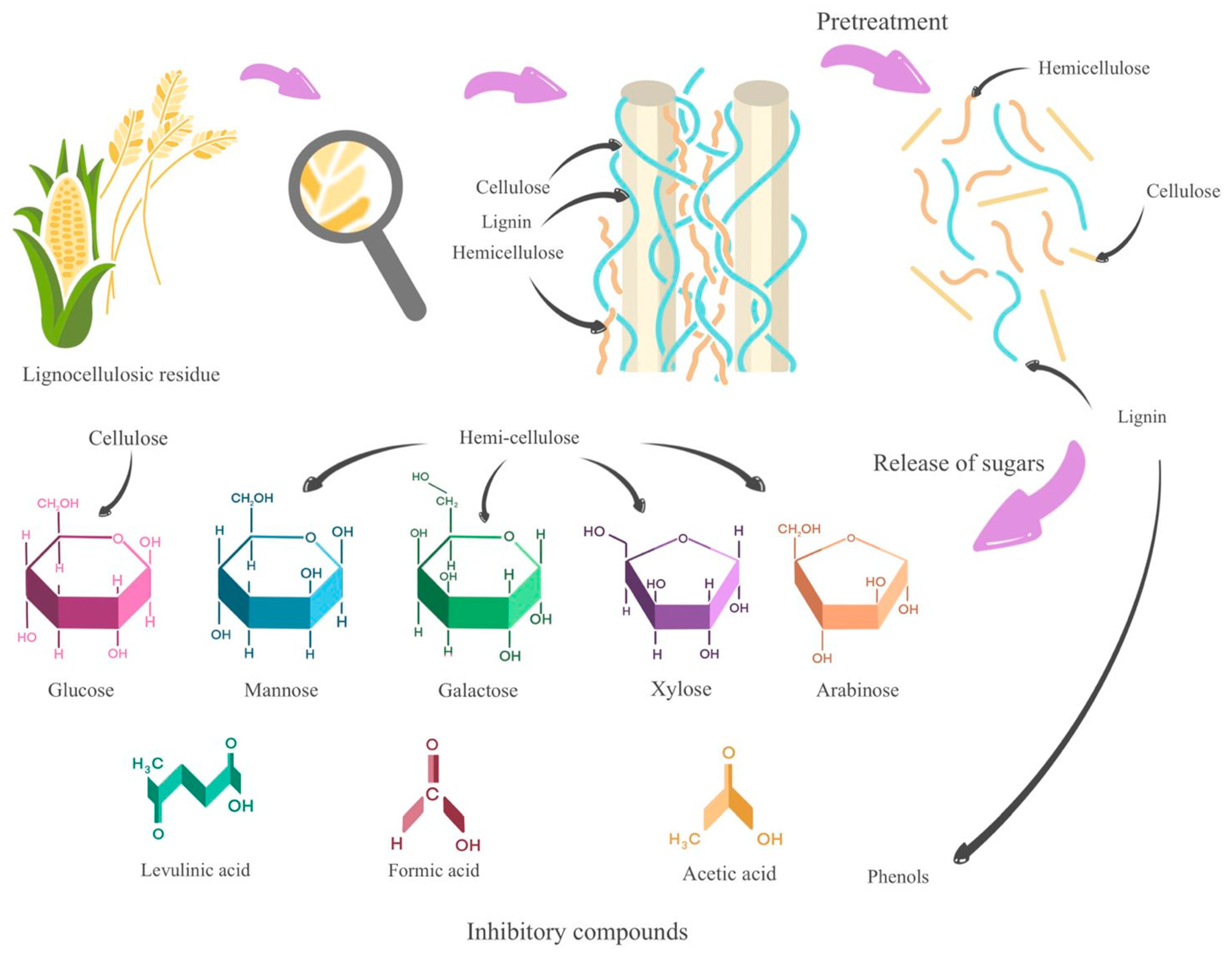
3.1.6. Lignocellulosic Residues
3.2. Selection Criteria for Substrate and Yeast Strain
3.3. Cultivation of the Selected Yeast Strain under Optimal Conditions
3.4. Lipid Production
- (a)
- (b)
- Fed batch: in this modality, the carbon source is administered throughout the process, and when it reaches a minimum concentration, it is fed back into the system, repeating this process each time the concentration level decreases to such a level that the growth of the yeast is inhibited by the scarcity of the carbon source. This modality allows mitigating the inhibitory effect of a high initial concentration of the carbon source, from 35% to 80%, which makes the process more efficient [25,33,108,117,137].
- (c)
- Continuous: in this mode, the carbon source is fed continuously at a specific dilution rate, which is generally equivalent to one third of the growth rate of the microorganism [140].
3.5. Extraction of Lipids
3.6. Transesterification Reaction
3.7. Refining Process of Fatty Acid Methyl Ester (FAME) to Obtain Biodiesel
4. Evaluation of Greenhouse Gas Emissions and Efficiency in the Use of Second-Generation Biodiesel in Engines
5. Conclusions
- In the first stage, which is the selection of yeast strain and substrate, yeasts such as Y. lipolytica, R. toruloides, R. glutinis, R. mucilaginosa, L. starkeyi, and C. curvatum have proven to be excellent lipid producers. This is attributed to their capability to grow on a wide variety of low-cost substrates. The most sustainable include used cooking oil, glucose, and sugars derived from lignocellulosic residues.
- In the substage of lignocellulosic residue pretreatments, the most sustainable pretreatment in terms of efficiency involves sulfuric acid (2% v/v). However, this pretreatment emits a significant amount of CO2, which can be reduced if the solid load is doubled.
- In the stage of cultivating the selected yeast strain, it has been observed that the growth pH range of most yeast strains is between 5 and 6, the temperature is between 25 and 32 °C, the stirring speed is between 100 and 200 rpm, and the inoculum size is between cells/mL. Yeast growth at a pH below 6 inhibits bacterial contamination, and growth at room temperature benefits economic and environmental aspects as there is energy savings.
- In the lipid production stage, the most efficient fermentation modes are batch and fed-batch. This stage has been optimized using a two-stage fermentation system, managing non-aseptic conditions, and reusing lysed yeasts from one batch to another, resulting in energy and cost savings. However, this stage produces twice the amount of CO2 emissions compared to the wastewater produced by the reaction itself. Additionally, for scaling up to be potential and profitable, it is necessary to simultaneously produce other high-value nutritional and pharmaceutical products, such as docosahexaenoic acid (DHA).
- In the lipid extraction stage, the most sustainable technique in terms of cost and efficiency is ultrasound because it is fast and does not require a large amount of solvent. This technique can be twice as efficient as solvent extraction but is currently only used on a laboratory scale.
- In the transesterification reaction stage, both acid and alkali catalysts are more economical and efficient due to their shorter times, and the conversion of FAME exceeds 100%. Yet, heterogeneous catalysts proved to be a promising alternative because they can be reused many times in the process without significantly affecting their high conversions. This results in a reduction in the overall processing cost.
- In the FAME refining stage to obtain biodiesel, magnesium silicates are the most sustainable, as they have the advantage of being more efficient regardless of the moisture content in the biodiesel.
- Yeast-derived biodiesel is a good alternative for use in engines, mixed in proportions with diesel (10 and 20%), because its composition is very similar to vegetable oils, it reduces the emission of greenhouse gases, and it has good performance in engines. However, the technology is not yet fully developed so that it can fully replace fossil fuels and meet the required demand.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U. S. E. P. Agency. Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 3 November 2023).
- Uprety, B.K.; Venkatesagowda, B.; Rakshit, S.K. Current Prospects on Production of Microbial Lipid and Other Value-Added Products Using Crude Glycerol Obtained from Biodiesel Industries. BioEnergy Res. 2017, 10, 1117–1137. [Google Scholar] [CrossRef]
- Vargas, F.; Pérez, A.; Delgado, R.; Hernández, E.; Suástegui, J.A. Performance Analysis of a Compression Ignition Engine Using Mixture Biodiesel Palm and Diesel. Sustainability 2019, 11, 4918. [Google Scholar] [CrossRef]
- Buyukkaya, E. Effects of biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel 2010, 89, 3099–3105. [Google Scholar] [CrossRef]
- Solís, J.; Sheinbaum, C. Energy Consumption and CO2 Emission from Road Transport in Mexico and Mitigation Scenarios. Obtenido De. Available online: http://www.scielo.org.mx/pdf/rica/v32n1/0188-4999-rica-32-01-00007.pdf (accessed on 14 November 2019).
- Knothe, G.; Krahl, J.; Van Gerpen, J. The Biodiesel Handbook, 2nd ed.; AOCS American Oil Chemists Society Publishing: Champaign, IL, USA, 2010; pp. 1–501. [Google Scholar]
- Kuan, I.-C.; Kao, W.-C.; Chen, C.-L.; Yu, C.-Y. Microbial Biodiesel Production by Direct Transesterification of Rhodotorula glutinis Biomass. Energies 2018, 11, 1036. [Google Scholar] [CrossRef]
- Vignesh, P.; Kumar, A.R.P.; Ganesh, N.S.; Jayaseelan, V.; Sudhakar, K. Biodiesel and green diesel generation: An overview. Oil Gas Sci. Technol.–Rev. d’IFP Energies Nouv. 2021, 76, 6. [Google Scholar] [CrossRef]
- Ozsezen, A.N.; Canakci, M.; Turkcan, A.; Sayin, C. Performance and combustion characteristics of a DI diesel engine fueled with waste palm oil and canola oil methyl esters. Fuel 2009, 88, 629–636. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous yeasts for biodiesel: Current and future trends in biology and production. Biotechnol. Adv. 2014, 32, 1336–1360. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, H.; Lin, L. Biodiesel: An Alternative to Conventional Fuel. Energy Procedia 2012, 16, 1874–1885. [Google Scholar] [CrossRef]
- Meena, A.; Singh, A.; Kholiya, F.; Meena, R. Synthesis of biodiesel from non-edible Jatropha curcas oil using potassium hydrogen sulphate-graphene oxide based composite (KHS-GO(cat)) catalyst. Indian J. Chem. Technol. 2022, 29, 181–187. [Google Scholar]
- Sheinbaum, C.; Balam, M.V.; Robles, G.; de Larrea, S.L.; Mendoza, R. Biodiesel from waste cooking oil in Mexico City. Waste Manag. Res. 2015, 33, 730–739. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Alves, J.M.; Pereira, A.S.; Belo, I. Waste Cooking Oils as Feedstock for Lipase and Lipid-Rich Biomass Production. Eur. J. Lipid Sci. Technol. 2018, 121, 1800188. [Google Scholar] [CrossRef]
- Lopes, H.J.S.; Bonturi, N.; Miranda, E.A. Rhodotorula toruloides Single Cell Oil Production Using Eucalyptus urograndis Hemicellulose Hydrolysate as a Carbon Source. Energies 2020, 13, 795. [Google Scholar] [CrossRef]
- Ayadi, I.; Belghith, H.; Gargouri, A.; Guerfali, M. Utilization of Wheat Bran Acid Hydrolysate by Rhodotorula mucilaginosa Y-MG1 for Microbial Lipid Production as Feedstock for Biodiesel Synthesis. BioMed Res. Int. 2019, 2019, 3213521. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, S.; Go, A.W.; Chen, K.-H.; Nguyen, P.L.T.; Ismadji, S.; Ju, Y.-H. Release of sugar by acid hydrolysis from rice bran for single cell oil production and subsequent in-situ transesterification for biodiesel preparation. Fuel Process. Technol. 2017, 167, 281–291. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Z.; Chen, S.; Jin, M. Microbial lipid production from dilute acid and dilute alkali pretreated corn stover via Trichosporon dermatis. Bioresour. Technol. 2019, 295, 122253. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Chevalot, I.; Aggelis, G. Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mohan, S.V. Microbial lipid production by Cryptococcus curvatus from vegetable waste hydrolysate. Bioresour. Technol. 2018, 254, 284–289. [Google Scholar] [CrossRef]
- Morales-Palomo, S.; Tomás-Pejó, E.; González-Fernández, C. Phosphate limitation as crucial factor to enhance yeast lipid production from short-chain fatty acids. Microb. Biotechnol. 2023, 16, 372–380. [Google Scholar] [CrossRef]
- Chebbi, H.; Leiva-Candia, D.; Carmona-Cabello, M.; Jaouani, A.; Dorado, M.P. Biodiesel production from microbial oil provided by oleaginous yeasts from olive oil mill wastewater growing on industrial glycerol. Ind. Crop. Prod. 2019, 139, 111535. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Sala, M.; Roncaglia, L.; De Lucia, M.; Leonardi, A.; Rossi, M. Single cell oils of the cold-adapted oleaginous yeast Rhodotorula glacialis DBVPG 4785. Microb. Cell Factories 2010, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, J.; Tsakraklides, V.; Kamineni, A.; Greenhagen, E.H.; Consiglio, A.L.; MacEwen, K.; Crabtree, D.V.; Afshar, J.; Nugent, R.L.; Hamilton, M.A.; et al. Engineering of a high lipid producing Yarrowia lipolytica strain. Biotechnol. Biofuels 2016, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Wang, Q.; Shen, H.; Hu, C.; Jin, G.; Zhao, Z.K. Co-fermentation of cellobiose and xylose by Lipomyces starkeyi for lipid production. Bioresour. Technol. 2012, 117, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Lazar, Z.; Dulermo, T.; Neuvéglise, C.; Coq, A.-M.C.-L.; Nicaud, J.-M. Hexokinase—A limiting factor in lipid production from fructose in Yarrowia lipolytica. Metab. Eng. 2014, 26, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Maina, S.; Pateraki, C.; Kopsahelis, N.; Paramithiotis, S.; Drosinos, E.H.; Papanikolaou, S.; Koutinas, A. Microbial oil production from various carbon sources by newly isolated oleaginous yeasts. Eng. Life Sci. 2017, 17, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; French, W.T.; Hernandez, R.; Alley, E.; Paraschivescu, M. Effects of furfural and acetic acid on growth and lipid production from glucose and xylose by Rhodotorula glutinis. Biomass Bioenergy 2011, 35, 734–740. [Google Scholar] [CrossRef]
- Chi, Z.; Zheng, Y.; Ma, J.; Chen, S. Oleaginous yeast Cryptococcus curvatus culture with dark fermentation hydrogen production effluent as feedstock for microbial lipid production. Int. J. Hydrogen Energy 2011, 36, 9542–9550. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhou, X.; Bao, W.; Cheng, S.; Zheng, L. Enhanced lipid production by Yarrowia lipolytica cultured with synthetic and waste-derived high-content volatile fatty acids under alkaline conditions. Biotechnol. Biofuels 2020, 13, 3. [Google Scholar] [CrossRef]
- Vajpeyi, S.; Chandran, K. Microbial conversion of synthetic and food waste-derived volatile fatty acids to lipids. Bioresour. Technol. 2015, 188, 49–55. [Google Scholar] [CrossRef]
- Xu, X.; Kim, J.Y.; Cho, H.U.; Park, H.R.; Park, J.M. Bioconversion of volatile fatty acids from macroalgae fermentation into microbial lipids by oleaginous yeast. Chem. Eng. J. 2015, 264, 735–743. [Google Scholar] [CrossRef]
- Chaiyaso, T.; Srisuwan, W.; Techapun, C.; Watanabe, M.; Takenaka, S. Direct bioconversion of rice residue from canteen waste into lipids by new amylolytic oleaginous yeast Sporidiobolus pararoseus KX709872. Prep. Biochem. Biotechnol. 2018, 48, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gao, Z.; Gao, M.; Ma, Y.; Ma, H.; Zhang, M.; Liu, Y.; Wang, Q. Microbial lipid production from food waste saccharified liquid and the effects of compositions. Energy Convers. Manag. 2018, 172, 306–315. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Karatay, S.E.; Dönmez, G. Improving the lipid accumulation properties of the yeast cells for biodiesel production using molasses. Bioresour. Technol. 2010, 101, 7988–7990. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.; Lopes, M.; Ramôa, R.; Pereira, A.S.; Belo, I. Candida tropicalis as a Promising Oleaginous Yeast for Olive Mill Wastewater Bioconversion. Energies 2021, 14, 640. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Bhattacharya, A.; Nain, L.; Prasanna, R.; Khare, S.K. Valorization of agro-starchy wastes as substrates for oleaginous microbes. Biomass Bioenergy 2019, 127, 105294. [Google Scholar] [CrossRef]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of fermentation media from flour-rich waste streams for microbial lipid production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, A.; Mituła, P.; Rymowicz, W.; Mirończuk, A.M. Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Li, Z.; Zhou, X.; Cheng, S.; Zheng, L. Oleaginous yeast Yarrowia lipolytica culture with synthetic and food waste-derived volatile fatty acids for lipid production. Biotechnol. Biofuels 2017, 10, 247. [Google Scholar] [CrossRef]
- Pereira, A.S.; Belo, I.; Lopes, M. Enhancing Microbial Lipids Synthesis for Biodiesel Production by Y. lipolytica W29 from Volatile Fatty Acids: Two-Stage Batch Strategies. Appl. Sci. 2022, 12, 8614. [Google Scholar] [CrossRef]
- Rakicka, M.; Lazar, Z.; Dulermo, T.; Fickers, P.; Nicaud, J.M. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol. Biofuels 2015, 8, 104. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, D.; Liu, X.; Li, A.; Chandran, K. Enhanced lipid accumulation in Metschnikowia pulcherrima using volatile fatty acids under non-sterile repeated batch cultivation. Int. Biodeterior. Biodegrad. 2021, 163, 105256. [Google Scholar] [CrossRef]
- Gallego-García, M.; Susmozas, A.; Moreno, A.D.; Negro, M.J. Evaluation and Identification of Key Economic Bottlenecks for Cost-Effective Microbial Oil Production from Fruit and Vegetable Residues. Fermentation 2022, 8, 334. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [Google Scholar] [CrossRef] [PubMed]
- Park, G.W.; Chang, H.N.; Jung, K.; Seo, C.; Kim, Y.-C.; Choi, J.H.; Woo, H.C.; Hwang, I.-J. Production of microbial lipid by Cryptococcus curvatus on rice straw hydrolysates. Process Biochem. 2017, 56, 147–153. [Google Scholar] [CrossRef]
- Thiru, M.; Sankh, S.; Rangaswamy, V. Process for biodiesel production from Cryptococcus curvatus. Bioresour. Technol. 2011, 102, 10436–10440. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S.; French, W.T.; Hernandez, R.; Holmes, W.E.; Claupein, W. Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Rane, D.V.; Pawar, P.P.; Odaneth, A.A.; Lali, A.M. Microbial oil production by the oleaginous red yeast, Rhodotorula glutinis NCIM 3168, using corncob hydrolysate. Biomass Convers. Biorefinery 2021, 13, 1987–1997. [Google Scholar] [CrossRef]
- Patel, A.; Sindhu, D.K.; Arora, N.; Singh, R.P.; Pruthi, V.; Pruthi, P.A. Biodiesel production from non-edible lignocellulosic biomass of Cassia fistula L. fruit pulp using oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour. Technol. 2015, 197, 91–98. [Google Scholar] [CrossRef]
- Enshaeieh, M.; Abdoli, A.; Madani, M. Single Cell Oil (SCO) Production by Rhodotorula mucilaginosa and Its Environmental Benefits. J. Agric. Sci. Technol. 2015, 17, 387–400. [Google Scholar]
- Madani, M.; Rezahasani, R.; Hoveida, L.; Ghojavand, S.; Enshaeieh, M. Two-step optimization process for grass hydrolysate application as biodiesel feedstock with novel quality characteristics. Environ. Sci. Pollut. Res. 2020, 27, 39354–39364. [Google Scholar] [CrossRef] [PubMed]
- Bandhu, S.; Khot, M.B.; Sharma, T.; Sharma, O.P.; Dasgupta, D.; Mohapatra, S.; Hazra, S.; Khatri, O.P.; Ghosh, D. Single Cell Oil from Oleaginous Yeast Grown on Sugarcane Bagasse-Derived Xylose: An Approach toward Novel Biolubricant for Low Friction and Wear. ACS Sustain. Chem. Eng. 2018, 6, 275–283. [Google Scholar] [CrossRef]
- Ma, X.; Gao, Z.; Gao, M.; Wu, C.; Wang, Q. Microbial lipid production from food waste saccharified liquid under two-stage process. Bioresour. Technol. 2019, 289, 121626. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhao, X.; Shen, H.; Wang, Q.; Zhao, Z.K. Microbial lipid production by Rhodosporidium toruloides under sulfate-limited conditions. Bioresour. Technol. 2011, 102, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, X.; Wang, W.; Du, W.; Liu, D. Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem. Eng. J. 2012, 65, 30–36. [Google Scholar] [CrossRef]
- Carota, E.; Petruccioli, M.; D’annibale, A.; Gallo, A.M.; Crognale, S. Orange peel waste–based liquid medium for biodiesel production by oleaginous yeasts. Appl. Microbiol. Biotechnol. 2020, 104, 4617–4628. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; García, I.; Papadaki, A.; Tsouko, E.; Koutinas, A.; Dorado, M. Biodiesel production using microbial lipids derived from food waste discarded by catering services. Bioresour. Technol. 2021, 323, 124597. [Google Scholar] [CrossRef]
- Liu, Z.; Natalizio, F.; Dragone, G.; Mussatto, S.I. Maximizing the simultaneous production of lipids and carotenoids by Rhodosporidium toruloides from wheat straw hydrolysate and perspectives for large-scale implementation. Bioresour. Technol. 2021, 340, 125598. [Google Scholar] [CrossRef]
- Kumar, V.; Arora, N.; Pandey, S.; Jaiswal, K.K.; Nanda, M.; Vlaskin, M.S.; Chauhan, P.K. Microwave-assisted pretreatment of harmful algal blooms for microbial oil-centered biorefinery approach. Biomass Convers. Biorefinery 2020, 12, 3097–3105. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.-F.; Xiong, L.; Chen, X.-D.; Ma, L.-L. Oil production by the yeast Trichosporon dermatis cultured in enzymatic hydrolysates of corncobs. Bioresour. Technol. 2012, 110, 711–714. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y.; Li, J. Lipid Production for Biodiesel from Sludge and Crude Glycerol. Water Environ. Res. 2017, 89, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Donzella, S.; Fumagalli, A.; Arioli, S.; Pellegrino, L.; D’incecco, P.; Molinari, F.; Speranza, G.; Ubiali, D.; Robescu, M.S.; Compagno, C. Recycling Food Waste and Saving Water: Optimization of the Fermentation Processes from Cheese Whey Permeate to Yeast Oil. Fermentation 2022, 8, 341. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; He, Q.; Liu, Y.; Zhao, M.; Liu, Y.; Zhou, W.; Gong, Z. Highly efficient fed-batch modes for enzymatic hydrolysis and microbial lipogenesis from alkaline organosolv pretreated corn stover for biodiesel production. Renew. Energy 2022, 197, 1133–1143. [Google Scholar] [CrossRef]
- Huang, Q.; Kamal, R.; Shen, H.; Lu, H.; Song, J.; Chu, Y.; Xue, C.; Zhao, Z.K. Pilot-scale conversion of corn stover into lipids by the red yeast Rhodosporidium toruloides. J. Environ. Chem. Eng. 2022, 10, 108858. [Google Scholar] [CrossRef]
- Giannakis, N.; Carmona-Cabello, M.; Makri, A.; Leiva-Candia, D.; Filippi, K.; Argeiti, C.; Pateraki, C.; Dorado, M.; Koutinas, A.; Stylianou, E. Spent coffee grounds and orange peel residues based biorefinery for microbial oil and biodiesel conversion estimation. Renew. Energy 2023, 209, 382–392. [Google Scholar] [CrossRef]
- Shin, S.; Go, J.H.; Moon, M.; Park, G.W. Automatic Fed-Batch Cultivation Enhances Microbial Lipid Production from Volatile Fatty Acids. Energies 2023, 16, 1996. [Google Scholar] [CrossRef]
- Sundaramahalingam, M.; Sivashanmugam, P.; Rajeshbanu, J.; Ashokkumar, M. A review on contemporary approaches in enhancing the innate lipid content of yeast cell. Chemosphere 2022, 293, 133616. [Google Scholar] [CrossRef]
- Samavi, M.; Uprety, B.K.; Rakshit, S. Bioconversion of Poplar Wood Hemicellulose Prehydrolysate to Microbial Oil Using Cryptococcus curvatus. Appl. Biochem. Biotechnol. 2019, 189, 626–637. [Google Scholar] [CrossRef]
- Castellini, M.; Ubertini, S.; Barletta, D.; Baffo, I.; Buzzini, P.; Barbanera, M. Techno-Economic Analysis of Biodiesel Production from Microbial Oil Using Cardoon Stalks as Carbon Source. Energies 2021, 14, 1473. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Parsons, S.; Abeln, F.; McManus, M.C.; Chuck, C.J. Techno-economic analysis (TEA) of microbial oil production from waste resources as part of a biorefinery concept: Assessment at multiple scales under uncertainty. J. Chem. Technol. Biotechnol. 2019, 94, 701–711. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Sartaj, K.; Pruthi, V.; Pruthi, P.A. Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew. Sustain. Energy Rev. 2016, 62, 836–855. [Google Scholar] [CrossRef]
- Huang, C.; Chen, X.-F.; Xiong, L.; Yang, X.-Y.; Chen, X.-D.; Ma, L.-L.; Chen, Y. Microbial oil production from corncob acid hydrolysate by oleaginous yeast Trichosporon coremiiforme. Biomass Bioenergy 2013, 49, 273–278. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N.; Oleskowicz-Popiel, P. Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus. Fuel 2018, 217, 420–426. [Google Scholar] [CrossRef]
- Chen, Z.; Reznicek, W.D.; Wan, C. Deep eutectic solvent pretreatment enabling full utilization of switchgrass. Bioresour. Technol. 2018, 263, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Okafor, N.; Okeke, B.C. Modern Industrial Microbiology and Biotechnology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Leiva-Candia, D.; Pinzi, S.; Macías, R.; Koutinas, A.; Webb, C.; Dorado, M. The potential for agro-industrial waste utilization using oleaginous yeast for the production of biodiesel. Fuel 2014, 123, 33–42. [Google Scholar] [CrossRef]
- Milessi, T.S.S.; Antunes, F.A.F.; Chandel, A.K.; Silva, S.S. Rice bran extract: An inexpensive nitrogen source for the production of 2G ethanol from sugarcane bagasse hydrolysate. 3 Biotech 2013, 3, 373–379. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L. A comparative study on de novo and ex novo lipid fermentation by oleaginous yeast using glucose and sonicated waste cooking oil. Ultrason. Sonochem. 2019, 52, 364–374. [Google Scholar] [CrossRef]
- Donot, F.; Fontana, A.; Baccou, J.; Strub, C.; Schorr-Galindo, S. Single cell oils (SCOs) from oleaginous yeasts and moulds: Production and genetics. Biomass Bioenergy 2014, 68, 135–150. [Google Scholar] [CrossRef]
- Khot, M.; Raut, G.; Ghosh, D.; Alarcón-Vivero, M.; Contreras, D.; Ravikumar, A. Lipid recovery from oleaginous yeasts: Perspectives and challenges for industrial applications. Fuel 2020, 259, 116292. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Louhasakul, Y. Industrial wastes as a promising renewable source for production of microbial lipid and direct transesterification of the lipid into biodiesel. Bioresour. Technol. 2013, 142, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, Z. Biodiesel production by direct methanolysis of oleaginous microbial biomass. J. Chem. Technol. Biotechnol. 2007, 82, 775–780. [Google Scholar] [CrossRef]
- Enshaeieh, M.; Abdoli, A.; Madani, M.; Bayat, M. Recycling of lignocellulosic waste materials to produce high-value products: Single cell oil and xylitol. Int. J. Environ. Sci. Technol. 2015, 12, 837–846. [Google Scholar] [CrossRef][Green Version]
- Mast, B.; Zöhrens, N.; Schmidl, F.; Hernandez, R.; French, W.T.; Merkt, N.; Claupein, W.; Graeff-Hönninger, S. Lipid Production for Microbial Biodiesel by the Oleagenious Yeast Rhodotorula glutinis Using Hydrolysates of Wheat Straw and Miscanthus as Carbon Sources. Waste Biomass Valorization 2014, 5, 955–962. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, F.; Du, W.; Liu, C.; Liu, D. Effects of some inhibitors on the growth and lipid accumulation of oleaginous yeast Rhodosporidium toruloides and preparation of biodiesel by enzymatic transesterification of the lipid. Bioprocess Biosyst. Eng. 2012, 35, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, H.; Yan, Q.; Wu, X.; Zhang, H. Superparamagnetic nanospheres with efficient bifunctional acidic sites enable sustainable production of biodiesel from budget non-edible oils. Energy Convers. Manag. 2023, 297, 117758. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, K.; Jiang, Y.; Chen, L.; Zhang, H.; Li, H.; Yang, S. Biomass-derived hydrophobic metal-organic frameworks solid acid for green efficient catalytic esterification of oleic acid at low temperatures. Fuel Process Technol. 2023, 239, 107558. [Google Scholar] [CrossRef]
- Chen, L.; He, L.; Zheng, B.; Wei, G.; Li, H.; Zhang, H.; Yang, S. Bifunctional acid-activated montmorillonite catalyzed biodiesel production from non-food oil: Characterization, optimization, kinetic and thermodynamic studies. Fuel Process Technol. 2023, 250, 107903. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Ficha de Datos de Seguridad:Hexano. Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/MX/es/sds/sial/296090 (accessed on 26 September 2022).
- Merck. Safety Data Sheet for Isooctano 104727. Supelco. Available online: https://www.merckmillipore.com/MX/es/product/msds/MDA_CHEM-104727?Origin=SERP (accessed on 26 September 2022).
- Mahmudul, H.; Hagos, F.; Mamat, R.; Adam, A.A.; Ishak, W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Tamilalagan, A.; Singaram, J.; Rajamohan, S. Generation of biodiesel from industrial wastewater using oleaginous yeast: Performance and emission characteristics of microbial biodiesel and its blends on a compression injection diesel engine. Environ. Sci. Pollut. Res. 2019, 26, 11371–11386. [Google Scholar] [CrossRef]
- Wahlen, B.D.; McCurdy, A.T.; Willis, R.M.; Morgan, M.D.; Dye, D.J.; Bugbee, B.; Wood, B.D.; Seefeldt, L.C. Biodiesel from Microalgae, Yeast, and Bacteria: Engine Performance and Exhaust Emissions. Energy Fuels 2013, 27, 220–228. [Google Scholar] [CrossRef]
- Sayin, C.; Gumus, M.; Canakci, M. Effect of fuel injection pressure on the injection, combustion and performance characteristics of a DI diesel engine fueled with canola oil methyl esters-diesel fuel blends. Biomass Bioenergy 2012, 46, 435–446. [Google Scholar] [CrossRef]
- Rajak, U.; Verma, T.N. Influence of combustion and emission characteristics on a compression ignition engine from a different generation of biodiesel. Eng. Sci. Technol. Int. J. 2020, 23, 10–20. [Google Scholar] [CrossRef]
- Rajak, U.; Nashïne, P.; Verma, T.N. Comparative assessment of the emission characteristics of first, second and third generation biodiesels as fuel in a diesel engine. J. Therm. Eng. 2020, 6, 211–225. [Google Scholar] [CrossRef]
- Kumar, A.; Subramanian, K. Control of greenhouse gas emissions (CO2, CH4 and N2O) of a biodiesel (B100) fueled automotive diesel engine using increased compression ratio. Appl. Therm. Eng. 2017, 127, 95–105. [Google Scholar] [CrossRef]
- Niculescu, R.; Clenci, A.; Iorga-Siman, V. Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines. Energies 2019, 12, 1194. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, L.; Babu, M.; Arora, P.; Singh, V.; Kumar, N.; Varyani, T. Comparative evaluation of performance and emission characteristics of jatropha, karanja and polanga based biodiesel as fuel in a tractor engine. Fuel 2009, 88, 1698–1707. [Google Scholar] [CrossRef]
- van der Pol, E.C.; Bakker, R.R.; Baets, P.; Eggink, G. By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Appl. Microbiol. Biotechnol. 2014, 98, 9579–9593. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Xu, X.; Zhang, L.; Nie, Q.; Xian, M. Biodiesel production from oleaginous microorganisms. Renew. Energy 2009, 34, 1–5. [Google Scholar] [CrossRef]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; da Costa Sousa, L.; Balan, V. Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar] [CrossRef]
- Raimondi, S.; Rossi, M.; Leonardi, A.; Bianchi, M.M.; Rinaldi, T.; Amaretti, A. Getting lipids from glycerol: New perspectives on biotechnological exploitation of Candida freyschussii. Microb. Cell Factories 2014, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Carota, E.; Crognale, S.; D’Annibale, A.; Gallo, A.M.; Stazi, S.R.; Petruccioli, M. A sustainable use of Ricotta Cheese Whey for microbial biodiesel production. Sci. Total. Environ. 2017, 584-585, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Johnravindar, D.; Karthikeyan, O.P.; Selvam, A.; Murugesan, K.; Wong, J.W. Lipid accumulation potential of oleaginous yeasts: A comparative evaluation using food waste leachate as a substrate. Bioresour. Technol. 2018, 248, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, Z.; Wang, Q.; Liu, Y. Biodiesels from microbial oils: Opportunity and challenges. Bioresour. Technol. 2018, 263, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Chang, K.-S.; Lee, C.-F.; Hsu, C.-L.; Huang, C.-W.; Jang, H.-D. Microbial lipid production by oleaginous yeast Cryptococcus sp. in the batch cultures using corncob hydrolysate as carbon source. Biomass Bioenergy 2015, 72, 95–103. [Google Scholar] [CrossRef]
- Merck. Dextrose. Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/MX/es/search/dextrosa?focus=products&page=1&perpage=30&sort=relevance&term=dextrosa&type=product (accessed on 26 September 2022).
- Merck. D-(+)-Xylose. Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/MX/es/search/xilose?focus=products&page=1&perpage=30&sort=relevance&term=xilose&type=product (accessed on 26 September 2022).
- Merck. D-(+)-Cellobiose. Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/MX/es/product/sial/22150?gclid=EAIaIQobChMIs5OY3uK4_QIVHBatBh1XIAEuEAAYASAAEgKoZvD_BwE&gclsrc=aw.ds (accessed on 28 February 2023).
- U. D. o. Agriculture and E. R. Service. Production Volume of Glucose Syrup in the United States from 2007 to 2019. Statista. Available online: https://statista.ibero.elogim.com/statistics/496485/glucose-production-in-the-us/ (accessed on 26 September 2022).
- Soccol, C.R.; Neto, C.J.D.; Soccol, V.T.; Sydney, E.B.; da Costa, E.S.F.; Medeiros, A.B.P.; Vandenberghe, L.P.d.S. Pilot scale biodiesel production from microbial oil of Rhodosporidium toruloides DEBB 5533 using sugarcane juice: Performance in diesel engine and preliminary economic study. Bioresour. Technol. 2017, 223, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, Y.; Hernandez, R.; Zhang, G.; Escalante, F.M.; Holmes, W.; French, W.T. Lipids accumulation in Rhodotorula glutinis and Cryptococcus curvatus growing on distillery wastewater as culture medium. Environ. Prog. Sustain. Energy 2013, 32, 69–74. [Google Scholar] [CrossRef]
- Peng, W.-F.; Huang, C.; Chen, X.-F.; Xiong, L.; Chen, X.-D.; Chen, Y.; Ma, L.-L. Microbial conversion of wastewater from butanol fermentation to microbial oil by oleaginous yeast Trichosporon dermatis. Renew. Energy 2013, 55, 31–34. [Google Scholar] [CrossRef]
- Seo, Y.H.; Lee, I.; Jeon, S.H.; Han, J.-I. Efficient conversion from cheese whey to lipid using Cryptococcus curvatus. Biochem. Eng. J. 2014, 90, 149–153. [Google Scholar] [CrossRef]
- Mena, M. 931 Millones de Toneladas de Alimentos Terminan en la Basura Cada Año. Statista. Available online: https://es.statista.com/grafico/24368/volumen-anual-estimado-de-alimentos-desperdiciados-en-los-hogares/ (accessed on 2 February 2022).
- CEC. Characterization and Management of Food Loss and Waste in North America; Comission for Environmental Cooperation: Montreal, QC, Canada, 2017. [Google Scholar]
- Sundaram, P.V.; Singaram, J.; Ashokan, T. Detoxification of food-waste hydrolysate to enhance lipid production in M. pulcherrima—An alternative feedstock for biodiesel. Int. J. Environ. Sustain. Dev. 2018, 17, 151–161. [Google Scholar] [CrossRef]
- Bhatt, S.M.; Shilpa. Lignocellulosic feedstock conversion, inhibitor detoxification and cellulosic hydrolysis—A review. Biofuels 2014, 5, 633–649. [Google Scholar] [CrossRef]
- Poontawee, R.; Yongmanitchai, W.; Limtong, S. Efficient oleaginous yeasts for lipid production from lignocellulosic sugars and effects of lignocellulose degradation compounds on growth and lipid production. Process Biochem. 2017, 53, 44–60. [Google Scholar] [CrossRef]
- Passoth, V.; Sandgren, M. Biofuel production from straw hydrolysates: Current achievements and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5105–5116. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Zheng, Y.; Jiang, A.; Chen, S. Lipid Production by Culturing Oleaginous Yeast and Algae with Food Waste and Municipal Wastewater in an Integrated Process. Appl. Biochem. Biotechnol. 2011, 165, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Merck. Safety Data Sheet for Ácido sulfúrico 96% 108131. Available online: https://www.merckmillipore.com/MX/es/product/msds/MDA_CHEM-108131?Origin=PDP (accessed on 18 October 2022).
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid pretreatment of lignocellulosic biomass for energy vectors production: A review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Canilha, L.; Kumar Chandel, A.; dos Santos Milessi, T.S.; Fernandes Antunes, F.A.; da Costa Freitas, W.L.; das Gracas Almeida Felipe, M.; da Silva, S.S. Bioconversion of Sugarcane Biomass into Ethanol: An Overview about Composition, Pretreatment Methods, Detoxification of Hydrolysates, Enzymatic Saccharification, and Ethanol Fermentation. J. Biomed. Biotechnol. 2012, 2012, 989572. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Wang, Q.; Yang, X.; Xie, H.; Zhao, Z.K. Efficient conversion of biomass into lipids by using the simultaneous saccharification and enhanced lipid production process. Biotechnol. Biofuels 2013, 6, 36. [Google Scholar] [CrossRef]
- Saini, R.; Hegde, K.; Osorio-Gonzalez, C.S.; Brar, S.K.; Vezina, P. Evaluating the Potential of Rhodosporidium toruloides-1588 for High Lipid Production Using Undetoxified Wood Hydrolysate as a Carbon Source. Energies 2020, 13, 5960. [Google Scholar] [CrossRef]
- Caporusso, A.; Capece, A.; De Bari, I. Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes. Fermentation 2021, 7, 50. [Google Scholar] [CrossRef]
- Grubišić, M.; Perečinec, M.G.; Peremin, I.; Mihajlovski, K.; Beluhan, S.; Šantek, B.; Šantek, M.I. Optimization of Pretreatment Conditions and Enzymatic Hydrolysis of Corn Cobs for Production of Microbial Lipids by Trichosporon oleaginosus. Energies 2022, 15, 3208. [Google Scholar] [CrossRef]
- Liu, W.; Mao, W.; Zhang, C.; Liu, L.; Zhang, Z.; Guo, C.; Lin, J. Effective and economic microbial lipid biosynthesis for biodiesel production by two-phase whole-cell biocatalytic process. J. Clean. Prod. 2021, 298, 126798. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, S.; Hu, C.; Wang, Q.; Hua, Y.; Zhao, Z.K. Lipid production from Jerusalem artichoke by Rhodosporidium toruloides Y4. J. Ind. Microbiol. Biotechnol. 2010, 37, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, S.; Leiva, D.; López-García, I.; Redel-Macías, M.D.; Dorado, M.P. Latest trends in feedstocks for biodiesel production. Biofuels Bioprod. Biorefining 2014, 8, 126–143. [Google Scholar] [CrossRef]
- Bhuiya, M.; Rasul, M.; Khan, M.; Ashwath, N.; Azad, A. Prospects of 2nd generation biodiesel as a sustainable fuel—Part: 1 selection of feedstocks, oil extraction techniques and conversion technologies. Renew. Sustain. Energy Rev. 2016, 55, 1109–1128. [Google Scholar] [CrossRef]
- Anschau, A.; Xavier, M.C.; Hernalsteens, S.; Franco, T.T. Effect of feeding strategies on lipid production by Lipomyces starkeyi. Bioresour. Technol. 2014, 157, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Taskin, M.; Ortucu, S.; Aydogan, M.N.; Arslan, N.P. Lipid production from sugar beet molasses under non-aseptic culture conditions using the oleaginous yeast Rhodotorula glutinis TR29. Renew. Energy 2016, 99, 198–204. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, Y.; Ma, X.; Wang, Q.; Liu, Y. A novel variable pH control strategy for enhancing lipid production from food waste: Biodiesel versus docosahexaenoic acid. Energy Convers. Manag. 2019, 189, 60–66. [Google Scholar] [CrossRef]
- Liang, Y.; Tang, T.; Umagiliyage, A.L.; Siddaramu, T.; McCarroll, M.; Choudhary, R. Utilization of sorghum bagasse hydrolysates for producing microbial lipids. Appl. Energy 2012, 91, 451–458. [Google Scholar] [CrossRef]
- Karamerou, E.E.; Webb, C. Cultivation modes for microbial oil production using oleaginous yeasts—A review. Biochem. Eng. J. 2019, 151, 107322. [Google Scholar] [CrossRef]
- Qin, L.; Liu, L.; Zeng, A.-P.; Wei, D. From low-cost substrates to Single Cell Oils synthesized by oleaginous yeasts. Bioresour. Technol. 2017, 245, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Sreeharsha, R.V.; Mohan, S.V. Obscure yet Promising Oleaginous Yeasts for Fuel and Chemical Production. Trends Biotechnol. 2020, 38, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R. Microbial oils: A customizable feedstock through metabolic engineering. Eur. J. Lipid Sci. Technol. 2015, 117, 141–144. [Google Scholar] [CrossRef]
- Probst, K.V.; Schulte, L.R.; Durrett, T.P.; Rezac, M.E.; Vadlani, P.V. Oleaginous yeast: A value-added platform for renewable oils. Crit. Rev. Biotechnol. 2016, 36, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-J.; Jiang, J.-G. Characterization of malic enzyme and the regulation of its activity and metabolic engineering on lipid production. RSC Adv. 2015, 5, 45558–45570. [Google Scholar] [CrossRef]
- Demuez, M.; Mahdy, A.; Tomás-Pejó, E.; González-Fernández, C.; Ballesteros, M. Enzymatic cell disruption of microalgae biomass in biorefinery processes. Biotechnol. Bioeng. 2015, 112, 1955–1966. [Google Scholar] [CrossRef]
- Cong, W.-J.; Nanda, S.; Li, H.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Metal–organic framework-based functional catalytic materials for biodiesel production: A review. Green Chem. 2021, 23, 2595–2618. [Google Scholar] [CrossRef]
- Ramírez, I.E.M.; Vela, N.A.C.; Rincón, J.J. Biodiesel, un Combustible Renovable; Investigación y Ciencia de la Universidad Autónoma de Aguascalientes: Aguascalientes, Mexico, 2011; Volume 55, pp. 62–70. [Google Scholar]
- Atadashi, I.; Aroua, M.; Aziz, A.A. Biodiesel separation and purification: A review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Bateni, H.; Saraeian, A.; Able, C. A comprehensive review on biodiesel purification and upgrading. Biofuel Res. J. 2017, 4, 668–690. [Google Scholar] [CrossRef]
- Suthar, K.; Dwivedi, A.; Joshipura, M. A review on separation and purification techniques for biodiesel production with special emphasis on Jatropha oil as a feedstock. Asia-Pacific J. Chem. Eng. 2019, 14, e2361. [Google Scholar] [CrossRef]
- Shen, X.; Shi, J.; Cao, X.; Zhang, X.; Zhang, W.; Wu, H.; Yao, Z. Real-world exhaust emissions and fuel consumption for diesel vehicles fueled by waste cooking oil biodiesel blends. Atmospheric Environ. 2018, 191, 249–257. [Google Scholar] [CrossRef]
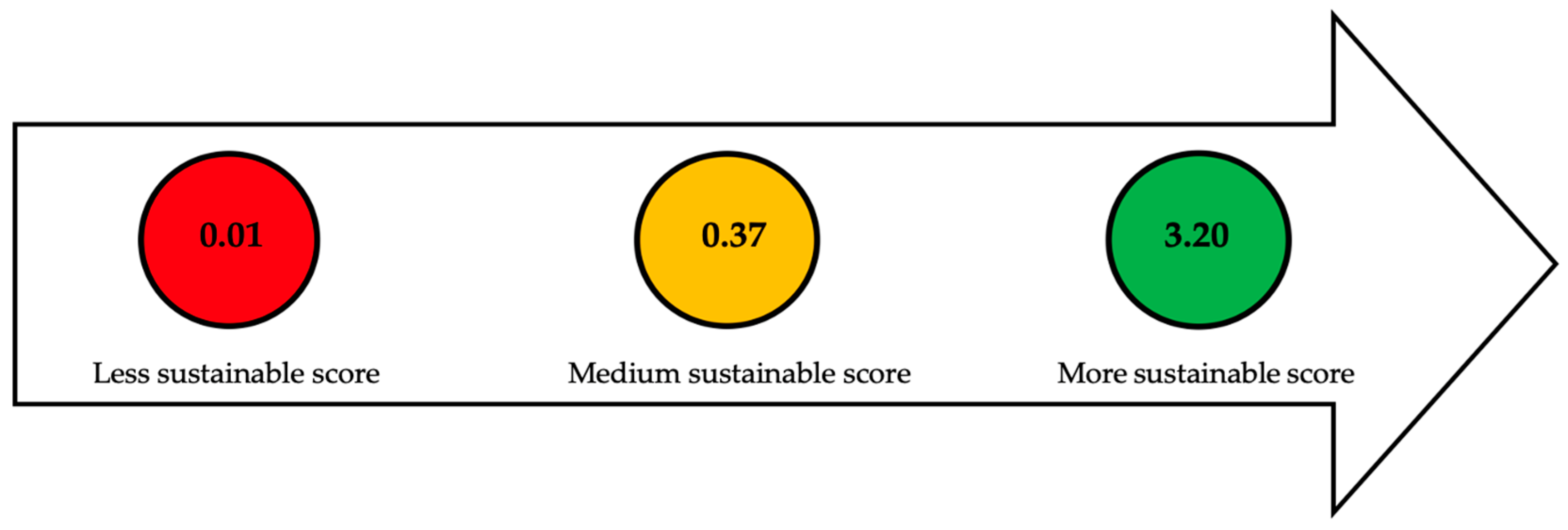
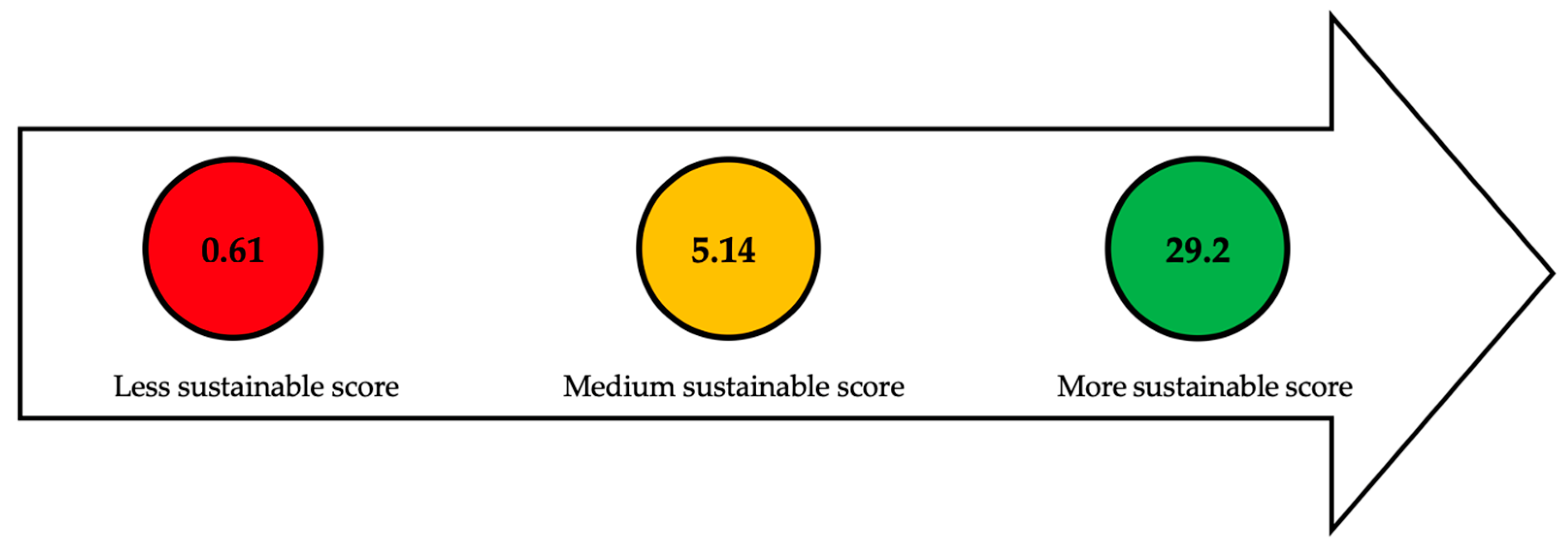
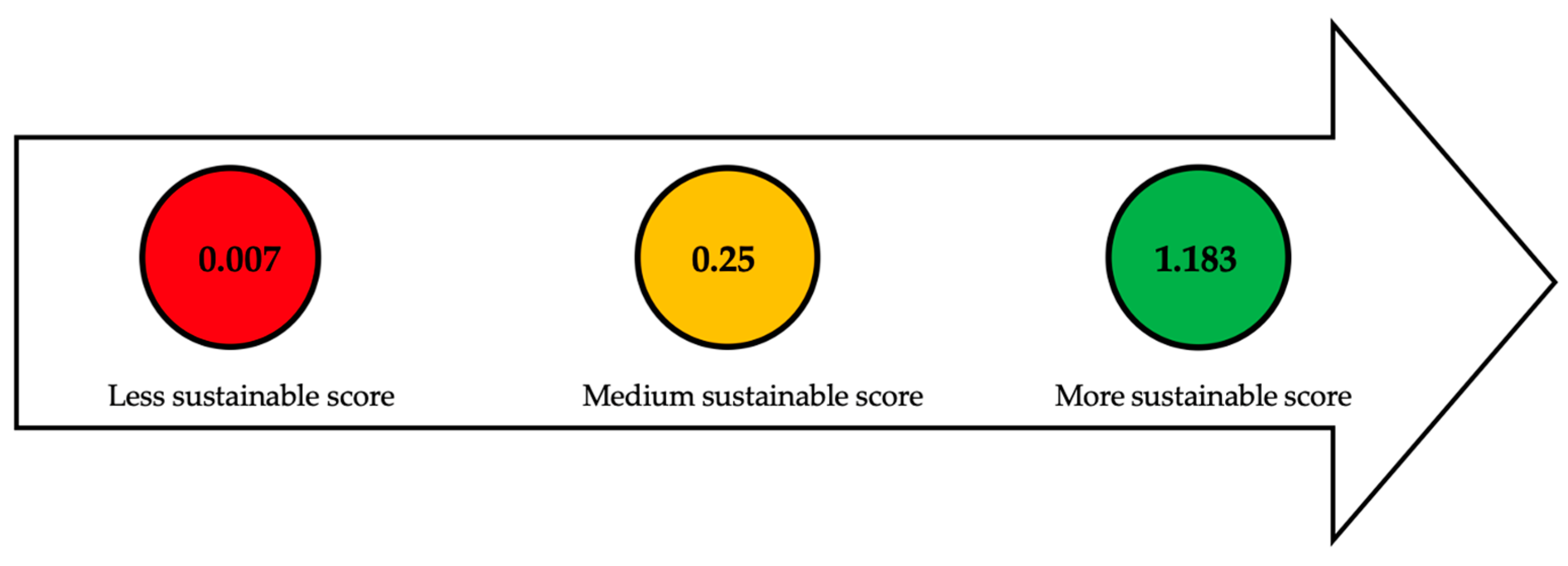
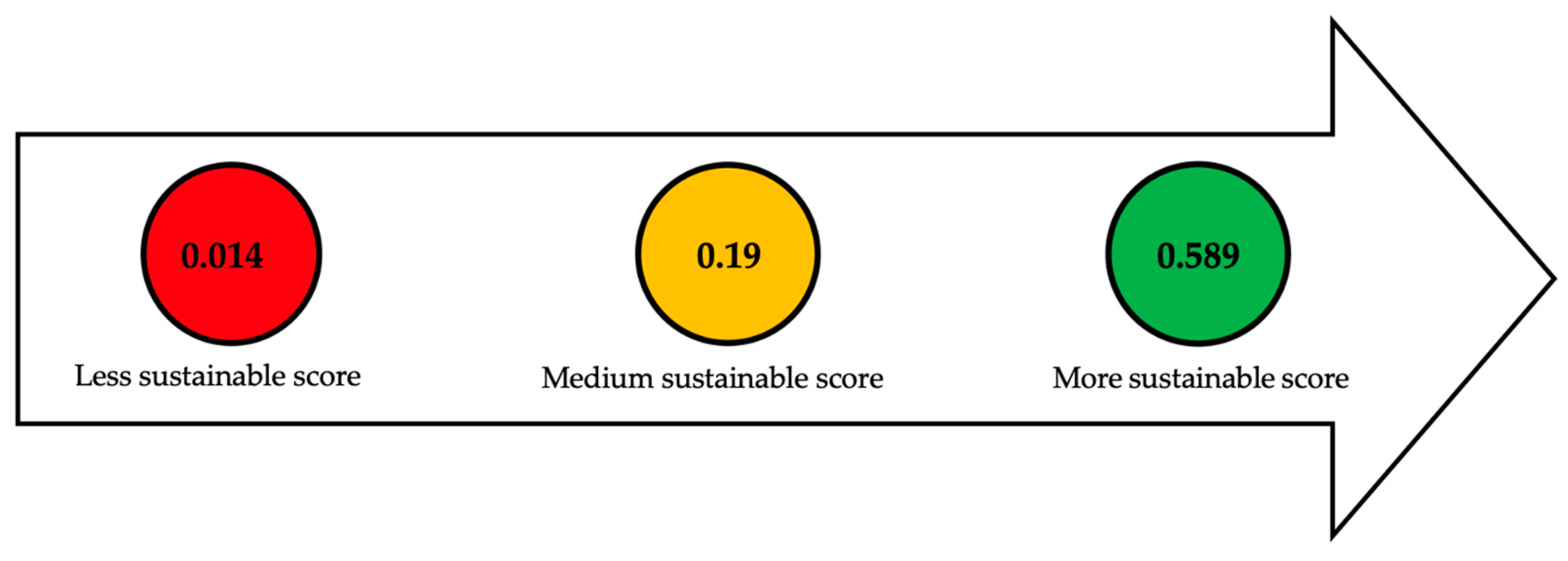
| Section or Stage | Function | Efficiency | Carbon Source Yield | Cost | Qualitative Indicators | Global Sustainable Score |
|---|---|---|---|---|---|---|
| Selection of the yeast strain and substrate | Provide a carbon source | No | Average of the indicators evaluated previously | |||
| Lignocellulosic residues pretreatments | Generate reducing sugars that serve as a carbon source | No | No | -Environmental impact -Economic impact -Efficiency | Advantages - disadvantages | |
| Selection criteria for substrate | Produce biodiesel | No | No | Qualitative (nominal) | -Availability of the substrate -Level of production of substrate -Accessibility -Composition -Production of high amount of lipids | No |
| Selection criteria for yeast strain | Produce biodiesel | No | No | No | -Based on the substrate -optimal growth conditions (pH, temperature, pressure, agitation speed and oxygenation level | |
| Cultivation of the selected yeast strain under optimal conditions | Prepare the inoculum | No | No | No | -composition of the culture medium -optimal values of operating conditions (pH, temperature and stirring speed). | No |
| Lipid production | Obtain lipids | No | No | -carbon/nitrogen (C/N) ratio -temperature -pH -agitation speed -inoculum size -environmental impact (CO2 emissions and wastewater produced) | No | |
| Extraction of lipids | Breaking the cell wall of the yeast to release the intracellular lipids | No | -environmental impact -economic impact -efficiency | Average of efficiency and cost indicators | ||
| Transesterification reaction | Convert lipids to biodiesel | Lipid bioconversion to FAME (%) | No | No | -temperature of reaction -catalyst concentration -methanol ratio used -moisture content present in the lipid | No |
| Component | Sugar Cane Bagasse | Wheat Straw | Corn Straw | Rice Straw | Barley Straw | Oat Straw | Jerusalem Artichoke | Leaves | Nutshell | Perennial Grass |
|---|---|---|---|---|---|---|---|---|---|---|
| Cellulose | 33 | 38–48 | 36–41 | 33 | 43.3 | 41 | 20.95–25.99 | 15–20 | 25–30 | 31–37 |
| hemicellulose | 30 | 23–29 | 26–30 | 26 | 29.6 | 16 | 4.5–5.4 | 80–85 | 25–30 | 20.4–29 |
| Lignin | 29 | 13–19 | 16–21 | 7 | 7.7 | 11 | 5–5.7 | 0 | 30–40 | 17.6–19 |
| Pretreatments | Description | Environmental Impact | Economic Impact | Efficiency | Sustainable Score Advantages - Disadvantages | References |
|---|---|---|---|---|---|---|
| Chemical | Treatment with dilute or concentrated acids or alkalis at room temperature. | -No ecological at 100% | -Economical -Large-scale use | -Improve hemicellulose solubility -Salt formation, which affects the composition of hydrolysates, -Very efficient | 2 | [76] |
| Biological | Treatment with white rot fungus enzymes | -Ecological | -No economical -Reusable | -Fast and efficient | 2 | [77] [76] |
| Green solvents | Treatment with mixtures composed of a hydrogen acceptor species and a hydrogen donor species, such as choline chloride with glycerol | -Process at room temperature | -It can be reused many times in the process -The solvent formulation can be more expensive | -Reduction of inhibitors formation | 2 | [79] |
| Mechanical | Application of shear force to decrease particle size in biomass | -High energy consumption | -No economical | -Decrease crystallinity -No inhibitory compounds | 0 | [76] |
| Oxidative | Biomass treatment with oxidizing agents such as H2O2 and peracetic acid. | -Require high temperatures | -No economical | -Very selective action on polymeric chain functional groups -It can produce inhibitory compounds that affect microbial growth. | −1 | [78] [76] |
| Thermal | Biomass heating at T > 150 °C that solubilizes hemicellulose | -Require high temperatures | -No economical | -It produces inhibitory compounds that affect other phases of the process | -2 | [76] |

| Cell Disruption Techniques | Description | Environmental Impact | Economic Impact | Efficiency | Sustainable Score Advantages - Disadvantages | References |
|---|---|---|---|---|---|---|
| Thermal | High temperatures and pressure to break down the cell wall. | -High energy consumption | -Low solvent consumption -Scale up potential | -Less time required | 2 | [85] |
| Mechanical | Shear force applications such as bead-milling, abrasive beads, high-pressure homogenization, and ultrasound | -High energy consumption | -High solvent consumption -Scale up potential | -Fast -Effective -Minimize lipid degradation | 1 | [85] |
| Chemical | hydrochloric and sulfuric acid applications, which react with the cell wall to release glucose, mannose, and glucosamine monomers. | -Corrosion problems in reactors for long time -No eco-friendly | -No sophisticated equipment is required -Economical | No reported | 0 | [85] |
| Biological | They use enzymes such as glucomanases and proteases, which act on the -glucan, mannan, and protein layers, solubilizing the cell wall. | -Eco-friendly | -Low solvent consumption -High cost -Hard to scale up | -High selectivity -High time consumption | 0 | [85] |
| Transesterification in situ | Breaking cells, lipid extraction, and transesterification happen in one single step. | No reported | -High solvents comsumption -Hard to scale up | -Fast -Impurities can be extracted during the process | −2 | [85] |

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Solís, A.; Lobato-Calleros, O.; Moreno-Terrazas, R.; Lappe-Oliveras, P.; Neri-Torres, E. Biodiesel Production Processes with Yeast: A Sustainable Approach. Energies 2024, 17, 302. https://doi.org/10.3390/en17020302
Sánchez-Solís A, Lobato-Calleros O, Moreno-Terrazas R, Lappe-Oliveras P, Neri-Torres E. Biodiesel Production Processes with Yeast: A Sustainable Approach. Energies. 2024; 17(2):302. https://doi.org/10.3390/en17020302
Chicago/Turabian StyleSánchez-Solís, Alejandra, Odette Lobato-Calleros, Rubén Moreno-Terrazas, Patricia Lappe-Oliveras, and Elier Neri-Torres. 2024. "Biodiesel Production Processes with Yeast: A Sustainable Approach" Energies 17, no. 2: 302. https://doi.org/10.3390/en17020302
APA StyleSánchez-Solís, A., Lobato-Calleros, O., Moreno-Terrazas, R., Lappe-Oliveras, P., & Neri-Torres, E. (2024). Biodiesel Production Processes with Yeast: A Sustainable Approach. Energies, 17(2), 302. https://doi.org/10.3390/en17020302








