Abstract
Supercritical multicomponent thermal fluid (scMCTF) is a novel medium with great potential for heavy oil thermal recovery. The production rate of scMCTF will affect the injection efficiency of thermal fluid, and then affect the development effect of thermal recovery. However, at present, there are few reports on the production rate of each component of scMCTF, and their understanding is not clear. According to the existing production rate data of supercritical water (scH2O) gasification products, based on the generation mechanism of scMCTF, the production rate of thermal fluid generation products under different generation conditions was calculated, and its influencing factors were identified. The results show the following: (1) The factors affecting the production rate of scMCTF generation products can be divided into three categories: reaction raw material factors, reaction condition factors, and catalytic factors. (2) The hydrocarbon number of raw material, reaction temperature, reaction time, and catalyst concentration were positively correlated with the production rate of the product. (3) The concentration of the reaction raw material is negatively correlated with the production rate of the product. The higher the concentration of the raw material is, the lower the concentration of H2O is, and the steam reforming reaction is inhibited, which leads to the decrease in the production rate. (4) The effect of reaction pressure and catalyst load on the product is not significant. (5) The reaction product production rate increased first and then decreased with the ratio of H2O to oil in the raw material emulsion and the ratio of preheated H2O to raw material discharge. (6) The effect of metal salt catalysts is relatively stable, and the catalytic effect of simple metal catalysts is significantly different under the action of different types of accelerators, so it is necessary to study the degree of synergization of different accelerators on the catalytic effect. The results can lay a foundation for the subsequent experimental and theoretical research design.
1. Introduction
The development of unconventional oil and gas such as heavy oil, shale gas, and oil require technological innovation [1,2]. scMCTF injection is a novel thermal recovery technology for heavy oil proposed in recent years [3,4]. scMCTF is composed of supercritical water (scH2O), supercritical nitrogen (scN2), and supercritical carbon dioxide (scCO2), and it possesses high solubility, high diffusivity, and high reactivity [5]. Compared to traditional steam mediums, it can significantly enhance heavy oil recovery [6,7].
scMCTF is produced by gasification reaction and oxidation reaction of organic matter under high temperature and high pressure. The production rate of scMCTF is influenced by various reaction conditions such as feedstock, temperature, and pressure [8,9,10], and affects the thermal fluids’ injection rate and injection volume. The injection rate and volume are key parameters in the thermal fluids injection process for heavy oil recovery, and different injection rate and volume will bring disparate oil recovery. Precisely controlling these parameters according to the pre-set plan is fundamental to ensuring the effectiveness of thermal recovery [11,12]. Therefore, accurately understanding the influence of different reaction conditions on the production rate of scMCTF is crucial for the efficient development of heavy oil resources using this technology.
However, there is currently a lack of research on the production rate of scMCTF, leading to insufficient understanding in this area. In light of this, based on existing research data on the production rate of hydrocarbon organics commonly used in oil fields under scH2O conditions, and grounded in the theory of scMCTF generation, this study calculates the production rate of scMCTF under different reaction conditions, analyzes the variation patterns of production rate with different factors, and identifies the key reaction conditions influencing the production rate. The research findings can provide a reference for future research approaches and the design of methodologies for studying the production rate of scMCTF.
2. scMCTF Yield Calculation
The typical generation process of scMCTF includes two stages: organic matter gasification and oxidation of gasification products [13]. The reaction equations for the gasification process in the first stage (Equations (1)–(3)) and the oxidation process in the second stage (Equations (4)–(6)) are as follows. Through a literature search, 367 data samples of gasification production rates under scH2O conditions for commonly used hydrocarbon organics in oil fields under different reaction conditions were obtained (Table 1). These samples show the production rate of gasification products in a scH2O environment, which corresponds to the production rate of the first-stage products in the generation process of scMCTF.

Table 1.
Sample data on the production rate of gasification products from scH2O treatment of commonly used hydrocarbon organics in oilfields.
Based on the production rate data of the first-stage products and using the theory of scMCTF generation along with the principle of mass conservation, the production rate of each component in the scMCTF can be calculated. The calculation is based on the following assumptions: ① The organic feedstock and H2O introduced into the gasification reactor react completely. ② The reverse reactions of the water-gas shift reaction and the methanation reaction can be neglected [32,33]. ③ The oxygen (O2) injected into the oxidation reactor reacts completely with the gasification products from the first stage. ④ The small amounts of C2Hn gases in the products are ignored. ⑤ The mass fraction of O2 in the air is assumed to be 76.7%, and nitrogen (N2) is 23.3%. The calculation process is shown in Figure 1.
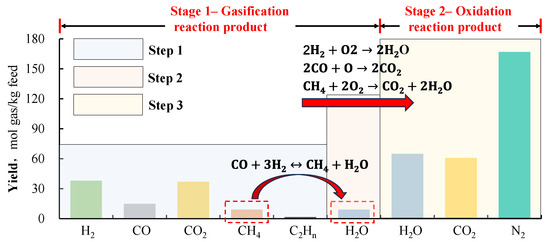
Figure 1.
Schematic diagram for production rate of calculation in the scMCTF (data sourced from [15]).
Step 1: Obtain and organize the production rate data of the first-stage products from previous literature.
Step 2: Since previous studies cooled the products before analysis, the reaction-generated H2O was not included in the product analysis. However, in the methanation reaction, the molar ratio of methane to H2O in the products is 1:1, which is used to determine the production rate of H2O in the first-stage gasification reaction.
Step 3: Based on the production rate of the first-stage products and using the oxidation reaction equations from the second stage, the production rate of scH2O and scCO2 in the second-stage reaction products can be determined. The N2 production rate is then determined based on the O2 consumption in the second-stage reaction and the O2–N2 ratio in the air.
By following these steps, the production rate of the scMCTF can be established.
3. Influencing Factors of scMCTF Yield
The factors influencing the production rate of scMCTF components can be categorized into three types: ① Feedstock factors, including feedstock type, feedstock concentration, and water-to-oil ratio in the feedstock emulsion. ② Reaction condition factors, including reaction pressure, reaction temperature, reaction time, and flow rate ratio of preheated H2O to feedstock during the reaction process. ③ Catalytic factors, including catalyst type, concentration, and loading.
3.1. Reaction Material Factor
As can be seen from Figure 2, each component’s yield of scMCTF generally increases with the number of carbon atoms in the reaction raw material. Hydrocarbons are composed of carbon and hydrogen atoms, and the greater the number of carbon and hydrogen atoms, the larger the molecular weight of the feedstock, resulting in higher scH2O and scCO2 content in the products after the complete reaction. Additionally, since feedstocks with larger molecular weights generate more hydrogen and methane during the gasification stage, more O2 is consumed during the oxidation stage, leading to an increase in the amount of N2 remaining in the air.
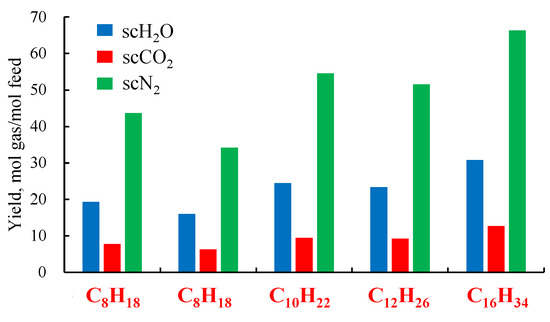
Figure 2.
Production rates of scMCTF from different feedstocks (data from [14]).
Figure 3 shows the negative impact of feedstock concentration on the production rate of each component in the scMCTF. The higher the feedstock concentration, the lower the production rate. This is because the steam reforming reaction is sensitive to H2O concentration [34] and a decrease in H2O concentration inhibits the steam reforming reaction, leading to incomplete reactions when the feedstock concentration is high [32]. As observed from the overall data distribution in Figure 3, within the low concentration range, the production rate of components decreases as the concentration increases. As the concentration continues to rise, the production rate gradually stabilizes, suggesting the existence of a threshold concentration. Once the feedstock concentration exceeds this threshold, the impact of concentration on production rate becomes less significant. Therefore, an appropriate feedstock concentration is crucial: if the concentration is too low, the amount of each component in the scMCTF may be insufficient, while an excessively high concentration can inhibit the reaction.
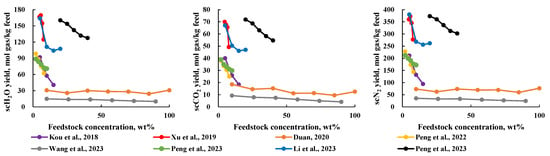
Figure 3.
Relationship between reaction feedstock concentration and production rate of various components [16,17,19,24,27,28,29,30].
Catalysts are widely used in reactions to enhance production rate [16,17,21,22,25]. Commonly used metal salt catalysts and elemental metal catalysts are generally difficult to dissolve in hydrocarbons. Therefore, the feedstock, H2O, and catalysts are often prepared into an emulsion to improve catalyst dispersion and thus increase catalytic efficiency. Xu [17], using a continuous reaction system (where the feedstock and products are continuously input and output), studied the effect of the water-to-oil ratio in the emulsion on the component production rate. They found that the component production rate first increased and then decreased as the water-to-oil ratio of the emulsion increased (Figure 4). They attributed this to the dual effects of reaction temperature changes and the micro-explosion phenomenon. On one hand, a higher water-to-oil ratio means a higher H2O content in the emulsion, which can lower the local temperature in the reaction zone, leading to an increase in side reactions and negatively affecting the reaction outcome. On the other hand, since H2O has a lower boiling point compared to hydrocarbon feedstocks, it reaches a superheated state more quickly when heated in the reactor. This causes micro-explosions, breaking the feedstock into smaller droplets, thereby enhancing the reaction efficiency. Therefore, optimizing the water-to-oil ratio in the emulsion can improve the component production rate. Additionally, since the water-to-oil ratio in the emulsion affects the feedstock concentration, these two factors should be considered together in the optimization process.
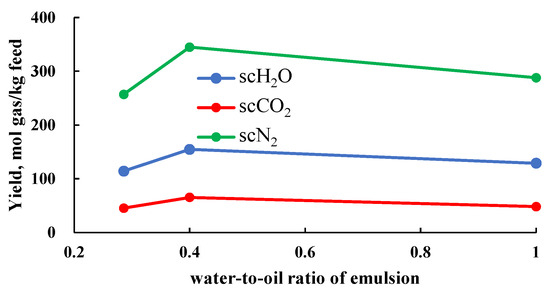
Figure 4.
Relationship between water-to-oil ratio and production rate of various components (data from [17]).
3.2. Reaction Conditioning Factor
Researchers believe that reaction pressure has an insignificant impact on product production rate, which has led to the adoption of a fixed reaction pressure in studies [14,15,16,17,18,23,24,25,26]. Some researchers have verified this view by studying the effect of reaction pressure on production rate [19,27,28]. As shown in Figure 5, the production rate of products shows almost no change with increasing pressure, with only a slight effect on scCO2 production rate [19]. This effect has not been deeply analyzed, but considering the findings and viewpoints of most researchers, it is likely due to experimental error.
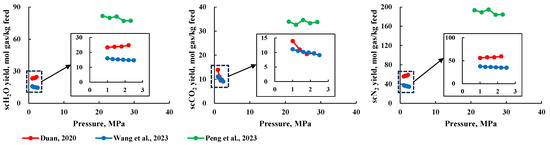
Figure 5.
Relationship between pressure and production rate of various components [19,27,28].
Reaction temperature is one of the most important factors affecting product production rate. During the generation of scMCTF, the production rate of all components increases with rising temperature (Figure 6), and the trend of production rate growth with temperature is generally consistent across components. Higher temperatures favor the reaction process and product formation. However, higher temperatures also mean increased temperature requirements for the supercritical thermal fluid equipment and higher heating fuel costs. Therefore, it is necessary to lower the reaction temperature as much as possible while still ensuring adequate product production rate, to reduce equipment demands and fuel costs.
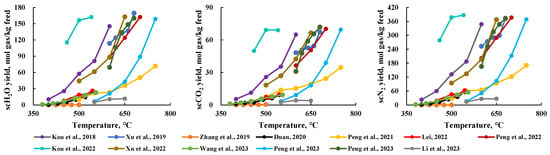
Figure 6.
Relationship between temperature and production rate of various components [16,17,18,19,20,23,24,25,26,27,28,30,31].
In a batch reaction system (where feedstock input, reaction, and product output occur in intermittent cycles), the reaction time is usually sufficient to ensure a complete reaction of the feedstock. However, in a continuous reaction system, the time during which the feedstock comes into contact with scH2O determines the extent of the reaction and the production rate of the products. As shown in Figure 7, the product production rate increases with a longer reaction time, but after sufficient reaction time, the rate of production rate increase slows down and eventually levels off. Generally, a reaction time of over 30 min is sufficient to ensure a complete reaction of the products. However, in continuous systems, where feedstock is continuously introduced and scMCTF is produced, reaction time for the feedstock is typically shorter [14,26]. Therefore, to achieve a complete reaction within a shorter time, it is necessary to increase the reaction temperature or add catalysts to reduce the time required for the feedstock to fully react.
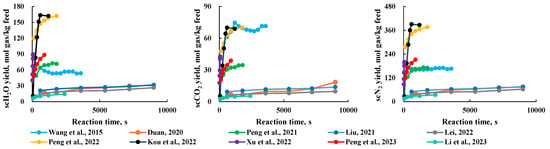
Figure 7.
Relationship between reaction time and production rate of various components [15,19,20,21,23,24,25,26,28,31].
In a continuous reaction system, preheated H2O in a supercritical state and the feedstock are injected into the reactor through different nozzles at a specific ratio to react. Therefore, the “preheated water-to-feedstock flow rate ratio” factor applies only to continuous reaction systems and is not relevant to batch systems. As shown in Figure 8, as the preheated water-to-feedstock flow rate ratio increases, the product production rate first increases and then decreases, indicating the existence of an “optimal flow rate ratio” during this process. This pattern is primarily due to two reasons. First, a larger flow of preheated H2O enhances heat and mass transfer within the reactor, allowing the feedstock to quickly absorb heat and react under high flow conditions. However, an excessively high flow rate shortens the residence time of the reactants in the reactor, which may cause the feedstock to exit the reactor before fully reacting. Additionally, Figure 8 shows that the “optimal flow rate ratio” varies across different studies, which is due to differences in experimental conditions. Therefore, determining the specific “optimal flow rate ratio” under given conditions is crucial for optimizing the production rate.
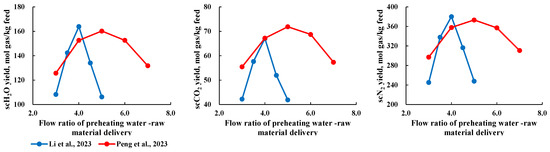
Figure 8.
Relationship between flow ratio of preheating water and raw material delivery, and components production [29,30].
3.3. Catalytic Factor
Metal salt catalysts and elemental metal catalysts are the two most commonly used types of catalysts in scH2O gasification reactions. Among them, elemental metal catalysts can be combined with various promoters to enhance their activity. As shown in Figure 9, all types of catalysts effectively increase production rates. Metal salt catalysts exhibit a significant catalytic effect, with relatively small differences in performance between different types of metal salts [21,30], making their catalytic effects quite stable. On the other hand, the catalytic performance of elemental metal catalysts varies greatly depending on the type of promoter used. For example, in the study by [16], the catalytic effect of a Ni catalyst supported on ZrO2 showed significant variation when different promoters were added. Compared to the catalyst without a promoter, the catalyst with Co as a promoter nearly doubled the product production rate, while using Y as a promoter resulted in a catalytic effect that was even worse than that without any promoter.
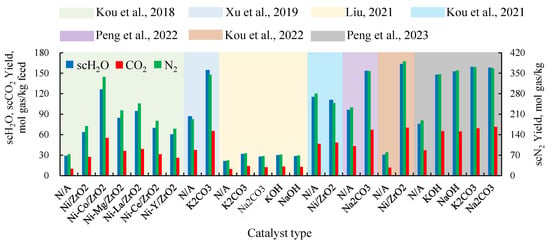
Figure 9.
Production rate of various components under different catalysts [16,17,21,22,24,25,30].
Catalysts can increase product production rate by promoting steam reforming reactions and water-gas shift reactions. The higher the catalyst mass concentration, the greater the amount of active catalytic material, which in turn enhances the reaction. As shown in Figure 10, the production rate of each component in the scMCTF increases with the rise in catalyst mass concentration. However, the extent of the production rate increase varies among different studies. For instance, in study [21], raising the catalyst mass concentration from 1 wt% to 3 wt% led to increases in component production rates of 15.7%, 20.5%, and 15.7%, respectively. In another study [23], increasing the catalyst mass concentration from 1 wt% to 5 wt% resulted in production rate increases of approximately 7.0% for scH2O, 8.2% for scCO2, and 6.5% for scN2. In a third study [30], increasing the catalyst mass concentration from 4 wt% to 8 wt% resulted in production rate increases of 30.3% for scH2O, 28.9% for scCO2, and 28.1% for scN2.
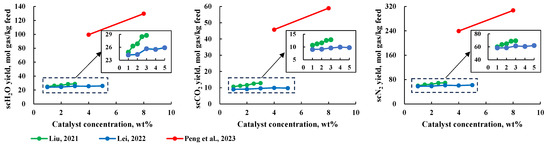
Figure 10.
Relationship between catalyst mass concentration and production tare of various components [21,23,30].
Some elemental metal catalysts tend to deactivate at high temperatures [35]. To retain the catalytic activity of elemental metals and mitigate deactivation under high-temperature conditions, combining elemental metals with metal oxide supports to create supported catalysts is effective. The catalyst loading refers to the mass fraction of the active phase in the supported catalyst. Lei [23] prepared a supported catalyst with Ni as the active phase and Al2O3 as the support, studying the impact of catalyst loading on production rate. The study revealed that catalyst loading has a minimal effect on product rate, which is consistent with Lei’s findings on catalyst concentration (Figure 11). This may be due to a phenomenon similar to the catalyst concentration threshold, where catalyst loading also has a threshold. In the study [23], the loading likely exceeded this threshold, resulting in an insignificant change in production rate with varying loading levels.
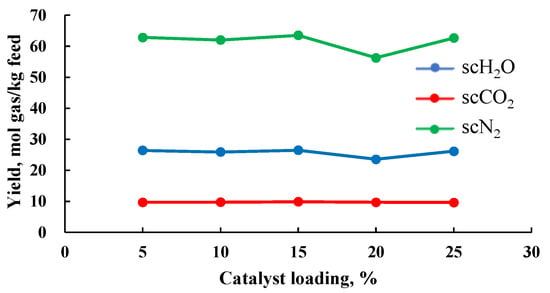
Figure 11.
Relationship between the amount of catalyst load and components production rate (data from [23]).
4. Discussion
We mentioned above that the previous work focused on the yield of organic gasification products, but did not pay attention to the yield of scMCTF [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31], because the concept of scMCTF has been put forward in the past five years [3,4,5,6,7], and there are very few studies on it. Meanwhile, there is no relevant report on the yield of scMCTF so far. Therefore, it is a novel work which provides a first view of the factors affecting the scMCTF yield in generation processes. This view can help scholars understand the factors that affect the yield of scMCTF and how the yield changes with them, which is the basis for conducting further experimental or simulation research.
The reference provided by this work is also limited because it has some irremediable defects. The thermal fluid yield obtained in this work is calculated theoretically based on the yield of organic matter gasification products in previous works, which means that research conditions are not uniform. In other words, the data for each of the researchers we cite may have been obtained under very different experimental conditions [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. For example, in Section 3.2, the temperature is shown as an important factor (Figure 6). If the effect of temperature on yield is to be studied, factors other than temperature such as pressure, catalyst, etc., should be consistent. However, we cannot guarantee that other factors are the same, which leads to the possibility that other factors may have an additional effect on the temperature effect. Therefore, this work can only provide a qualitative rule, but cannot give specific and quantitative suggestions.
5. Conclusions and Prospects
The reaction conditions during the formation of scMCTF significantly affect product production rate. However, there is currently a lack of research specifically focused on the production rate of components in scMCTF, leading to an incomplete understanding of the subject. This paper, drawing on similar studies of scH2O gasification product production rates and based on the theory of scMCTF formation, calculates the product production rate under different reaction conditions and investigates the factors influencing the production rate of each component. The following conclusions were drawn:
- (1)
- The factors influencing the production rate of scMCTF components can be classified into three categories: ① feedstock factors, including feedstock type, feedstock concentration, and the water-to-oil rate of the feedstock emulsion; ② reaction conditions, including reaction pressure, reaction temperature, reaction time, and preheated water-to-feedstock flow rate ratio during the reaction process; ③ catalytic factors, including catalyst type, catalyst concentration, and catalyst loading.
- (2)
- There is a positive correlation between product production rate and the number of carbon-hydrogen atoms in the feedstock, reaction temperature, reaction time, and catalyst concentration. When the reaction is complete, the higher the number of carbon-hydrogen atoms in the feedstock, the higher the production rate; higher reaction temperatures lead to higher product production rates; as reaction time extends, the product production rate initially increases and then stabilizes; and higher catalyst concentration results in higher product production rates.
- (3)
- There is a negative correlation between feedstock concentration and product production rate. Higher feedstock concentrations result in lower H2O concentrations, which inhibit the steam reforming reaction and subsequently reduce the production rate.
- (4)
- Reaction pressure and catalyst loading have a minimal impact on product production rate, with production rates remaining largely unchanged as pressure and catalyst loading vary.
- (5)
- Product production rate exhibits an initial increase followed by a decrease with changes in both the water-to-oil ratio of the feedstock emulsion and the preheated water-to-feedstock flow rate ratio. The change in product production rate with the water-to-oil ratio is driven by both temperature variation and micro-explosion effects, while the change in production rate with the preheated water-to-feedstock flow rate ratio is due to differences in local reactor temperatures and the residence time of reactants.
- (6)
- Metal salt catalysts and elemental metal catalysts are the two most commonly used catalysts in scH2O gasification reactions. Metal salt catalysts generally exhibit stable performance. The catalytic effectiveness of elemental metal catalysts, however, varies significantly depending on the type of promoter used. Therefore, it is essential to study the enhancement effects of different promoters on catalytic activity.
Author Contributions
Methodology, J.T. and M.D.; Formal analysis, F.X. and D.K.; Investigation, S.Y.; Writing – original draft, W.Z.; Funding acquisition, Z.Q. and W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by National Natural Science Foundation of China (Grant number 52004048, U22B2074, 52104025), the Natural Science Foundation of Chongqing Municipality, China (Grant number cstc2020jcyj msxmX0856), Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN202001508), Natural Science Foundation of Chongqing, China (CSTB2022NSCQ-MSX0858), the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJZD-M202301501), Talent Plan project of Chongqing Municipal (cstc2022ycjh-bgzxm0055).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
Authors Xu, F. and Kong, D. were employed by the company China National Petroleum Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Li, Q.C.; Li, Y.D.; Cheng, Y.F.; Li, Q.; Wang, F.; Wei, J.; Liu, Y.; Zhang, C.; Song, B.; Yan, C.; et al. Numerical simulation of fracture reorientation during hydraulic fracturing in perforated horizontal well in shale reservoirs. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1807–1813. [Google Scholar] [CrossRef]
- Li, Q.C.; Cheng, Y.F.; Li, Q.; Zhang, C. Establishment and evaluation of strength criterion for clayey silt hydrate-bearing sediments. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 742–750. [Google Scholar] [CrossRef]
- College of Petroleum and Natural Gas Engineering, Chongqing University of Science and Technology. The Key Project Launch and Program Review Meeting of the National Self-Science Fund Enterprise Innovation and Development Joint Fund of Our School Was Held [EB/OL]. 2023. Available online: https://sgy.cqust.edu.cn/info/1221/2142.htm (accessed on 6 December 2023).
- State Key Laboratory of Dynamic Engineering Multiphase Flow, Xi’an Jiaotong University. Academician Guo Liejin, Director of the Laboratory, and Young Teacher Zhao Qiuyang, Have Made Important Progress in the Research on the Mechanism of Supercritical Fluid Development of Unconventional Oil, and Their Results Have Been Selected as a Journal Cover Article [EB/OL]. 2023. Available online: http://mfpe.xjtu.edu.cn/info/1077/6897.htm (accessed on 6 December 2023).
- Zhao, Q.Y.; Jin, H.; Xu, J.L.; Peng, Z.Y.; Wang, H.; Guo, L. Theory and Technology of Multi-Component Supercritical Thermal Fluid Generation and In-Situ Conversion Technique for Heavy Crude Oil Resources Exploitation. J. Xi’an Jiaotong Univ. 2023, 57, 31–45. [Google Scholar]
- Li, X.Y.; Sun, X.F.; Cai, J.M.; Zhang, Q.; Pan, X.; Zhang, Y. Experimental investigation on supercritical multi-thermal fluid flooding using a novel 2-dimensional model. Energy 2023, 283, 129136. [Google Scholar] [CrossRef]
- Huang, Z.J.; Zhao, Q.Y.; Chen, L.; Miao, Y.; Wang, H.; Jin, H.; Guo, L. Fundamentals of Enhanced Heavy Oil Recovery by Supercritical Multi-Component Thermal Fluid Flooding. J. Eng. Thermophys. 2022, 43, 974–981. [Google Scholar]
- Yan, Z.Y.; Tan, X.Y. Hydrogen generation from oily wastewater via supercritical water gasification (SCWG). J. Ind. Eng. Chem. 2015, 23, 44–49. [Google Scholar]
- Sanchez-Hernandez, A.M.; Martin-Sanchez, N.; Sanchez-Montero, M.J.; Izquierdo, C.; Salvador, F. Effect of pressure on the gasification of dodecane with steam and supercritical water and consequences for H2 production. J. Mater. Chem. A 2018, 6, 1671. [Google Scholar] [CrossRef]
- Wang, G.Y.; Li, J.S.L.; Li, X.J.; Kou, J.; Ge, Z.; Li, L.; Peng, P.; Guo, L. Experimental study on supercritical water gasification of oily sludge using a continuous two-step method. J. Hazard. Mater. 2023, 455, 131619. [Google Scholar] [CrossRef]
- Yang, G.L.; Li, Y.H.; He, H.Z. Research and Application of Multi-Component Thermal Fluid Stimulation in the Extra-Heavy Oil Reservoir. Spec. Oil Gas Reserv. 2020, 27, 103–107. [Google Scholar]
- Zhong, L.G.; Jiang, Y.; Ma, S. Physical and numerical simulation of multi-component-thermal-fluid-assisted gravity drainage in deep and extra-heavy oil reservoirs offshore. China Offshore Oil Gas 2015, 27, 68–73. [Google Scholar]
- Xu, J.; Peng, Z.; Rong, S.; Zhao, Q.; Jin, H.; Guo, L.; Zhou, T.; Zhang, X. Optimal retrofit of a novel multi-component supercritical thermal fluid generation system via thermodynamic analysis. Appl. Therm. Eng. 2023, 219, 119511. [Google Scholar] [CrossRef]
- Susanti, R.F.; Dianningrum, L.W.; Yum, T.; Kim, Y.; Lee, Y.-W.; Kim, J. High-production rate hydrogen production by supercritical water gasification of various feedstocks: Alcohols, glucose, glycerol and long-chain alkanes. Chem. Eng. Res. Des. 2014, 92, 1834–1844. [Google Scholar] [CrossRef]
- Wang, F.Q.; Zhu, S.J.; Gong, X.L. Gasification of Oily Sludge in Supercritical Water. Oxid. Commun. 2015, 38, 1391–1400. [Google Scholar]
- Kou, J.J.; Xu, J.J.; Jin, H.; Guo, L.; Zhang, D.; Cao, W. Evaluation of modified Ni/ZrO2 catalysts for hydrogen production by supercritical water gasification of oil-containing wastewater. Int. J. Hydrogen Energy 2018, 43, 13896–13903. [Google Scholar] [CrossRef]
- Xu, J.; Kou, J.; Guo, L.; Jin, H.; Peng, Z.; Ren, C. Experimental study on oil-containing wastewater gasification in supercritical water in a continuous system. Int. J. Hydrogen Energy 2019, 44, 15871–15881. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.Y.; Cheng, C.; Zhong, J. Study on the Reaction of Saturated Hydrocarbons of Oily Sludge in Supercritical Water. Environ. Sci. Technol. 2019, 32, 20–23+29. [Google Scholar]
- Duan, Y.W. Experimental Study on Hydrogen Production by Supercritical Water Gasification of Oily Sludge. Master’s Thesis, Xi’an Shiyou University, Xi’an, China, 2020. [Google Scholar]
- Peng, P.; Guo, S.; Li, L.; Jin, H.; Ge, Z.; Guo, L. Supercritical water gasification mechanism of polymer-containing oily sludge. Int. J. Hydrogen Energy 2021, 46, 26834–26847. [Google Scholar] [CrossRef]
- Liu, X.B. Study on Mechanism of Hydrogen Production by Supercritical Water Gasification of Oily Sludge under Alkaline. Master’s Thesis, Xi’an Shiyou University, Xi’an, China, 2021. [Google Scholar]
- Kou, J.J.; Lei, Y.; Li, G.L.; Cheng, K.; Wang, R.; Zhang, D.; Jin, H.; Guo, L. Structural effect of ZrO2 on supported Ni-based catalysts for supercritical water gasification of oil-containing wastewater. Int. J. Hydrogen Energy 2021, 46, 12874–12885. [Google Scholar] [CrossRef]
- Lei, D.L. Study on Heterogeneous Gasification Law of Oily Sludge in Supercritical Water System. Master’s Thesis, Xi’an Shiyou University, Xi’an, China, 2022. [Google Scholar]
- Peng, Z.Y.; Rong, S.Q.; Xu, J.J.; Jin, H.; Zhang, J.; Shang, F.; Guo, L. Reaction pathways and kinetics for hydrogen production by oilfield wastewater gasification in supercritical water. Fuel 2022, 314, 123135. [Google Scholar] [CrossRef]
- Kou, J.J.; Feng, H.F.; Wei, W.W.; Wang, G.; Sun, J.; Jin, H.; Guo, L. Study on the detailed reaction pathway and catalytic mechanism of a Ni/ZrO2 catalyst for supercritical water gasification of diesel oil. Fuel 2022, 312, 122849. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, Z.; Ren, C.; Yi, L.; Wei, W.; Jin, H.; Guo, L. Supercritical water gasification of oil-containing wastewater with a homogeneous catalyst: Detailed reaction kinetic study. Int. J. Hydrogen Energy 2022, 47, 25541–25554. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, S.Z.; Qi, H.Y.; Jiang, H.; Duan, Y. Characteristics and prediction model of hydrogen production of oily sludge by supercritical water gasification. Int. J. Hydrogen Energy 2023, 48, 11191–11204. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Xu, J.J.; Rong, S.Q.; Zhang, M.; Wang, L.; Jin, H.; Guo, L. Clean treatment and resource utilization of oilfield wastewater using supercritical water gasification. J. Clean. Prod. 2023, 411, 137239. [Google Scholar] [CrossRef]
- Li, L.H.; Wang, G.Y.; Li, X.J.; Wang, L.; Zhang, J.; Cheng, K.; Peng, P.; Cao, W.; Jin, H.; Guo, L. Experimental study on alkali catalytic gasification of oily sludge in supercritical water with a continuous reactor. J. Environ. Manag. 2023, 327, 116957. [Google Scholar] [CrossRef]
- Peng, Z.Y.; Rong, S.Q.; Xu, J.J.; Luo, K.; Zhang, J.; Jin, H.; Guo, L. Hydrogen production from oilfield wastewater by gasification in supercritical water with a continuous system. Fuel 2023, 344, 128094. [Google Scholar] [CrossRef]
- Li, L.H.; Li, X.J.; Cao, W. Reaction pathway and kinetics study on supercritical water gasification of oily sludge. J. Anal. Appl. Pyrolysis 2023, 170, 105920. [Google Scholar] [CrossRef]
- Jin, H.; Lu, Y.; Liao, B.; Guo, L.; Zhang, X. Hydrogen production by coal gasification in supercritical water with a fluidized bed reactor. Int. J. Hydrogen Energy 2010, 35, 7151–7160. [Google Scholar] [CrossRef]
- Lan, R.H.; Jin, H.; Guo, L.J.; Ge, Z.; Guo, S.; Zhang, X. Hydrogen Production by Catalytic Gasification of Coal in Supercritical Water. Energy Fuels 2014, 28, 6911–6917. [Google Scholar] [CrossRef]
- Guo, S.M.; Guo, L.J.; Cao, C.Q.; Yin, J.; Lu, Y.; Zhang, X. Hydrogen production from glycerol by supercritical water gasification in a continuous flow tubular reactor. Int. J. Hydrogen Energy 2012, 37, 5559–5568. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Lu, H.; Kobayashi, E.; Ishikawa, T.; Komiyama, M. Raney-Nickel Catalyst Deactivation in Supercritical Water Gasification of Ethanol Fermentation Stillage and its Mitigation. Top. Catal. 2014, 57, 1078–1084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).