1. Introduction
The global quest for sustainable and clean carbon sources for material and energy usages has intensified in recent years, with a particular focus on developing new and more efficient conversion processes. In particular, lignocelluloses feedstocks such as wood chips, derived from renewable forestry resources, have emerged as promising fuels in this context [
1]. While a large variety of conversion processes are well known for these feedstocks producing electricity, heat, or biochar (e.g., biomass-fired power plants, pyrolysis, etc.), the production of a usable synthesis gas (a gas with neglectable concentrations of nitrogen and major amounts of H
2 and CO) via gasification has not yet been able to fully establish itself on the market. This might seem surprising as gasification processes offer the potential to generate a synthesis gas—a versatile precursor for the production of various chemicals and energy carriers—but can be explained by higher operation costs due to their technical complexity as well as the limited capacities of existing plants [
2,
3,
4,
5]. With rising prices for CO
2 emissions and constant demands for carbon-based precursors in (chemical) industries, sustainable gasification processes might become a crucial part of future, carbon-based industries [
6,
7], possibly providing a nitrogen-free synthesis gas. In the following, the word “synthesis gas” describes a product gas derived from gasification using nitrogen-free gasification agents, resulting in a nitrogen-free product gas (minor amounts of N
2 are inevitable due to nitrogen contents from the fuel). Gases derived from gasification processes using air are called “product gas”.
Among the evolving strategies to optimize gasification performance, the use of oxygen-enriched gasification agents stands out as a key advancement in enhancing process efficiency, syngas quality, and overall system sustainability [
8]. Especially when focusing on the usage of the produced gas, the nitrogen content within can be challenging as it lowers the heating value of the gas [
9]. However, the most outstanding disadvantage is the limited upgrading of this gas in order to improve the energetic or material usage [
10].
A typical product gas from air-blown biomass gasification consists of around 40–50 Vol.-% of nitrogen; further processing of this gas means that only 50–60 Vol.-% of the gas is actually used [
3]. This results in lower efficiencies compared to conventional processes using anthropogenic feedstocks and therefore higher costs. While most gas components can be separated from each other easily on a technical scale, this process becomes more complicated with lower boiling points of the gasses and the increasing complexity of the gas mixture itself. It is uneconomical to separate nitrogen from product gasses derived from biomass gasification. Therefore, the usage of nitrogen-free gasification agents is of high interest giving the possibility of creating an almost nitrogen-free gas [
8]. While this approach can be used for all kinds of gasification reactors, its main challenge is temperature control within the gasifier. Using pure oxygen as the gasification agent can result in exceeding specific temperature limits; therefore, mixtures of different gasification agents such as oxygen, steam, and CO
2 are usually applied, allowing for the possible adjustment of reactor temperatures.
While the usage of mixtures of steam and oxygen as gasification agents has been widely studied [
8,
11,
12,
13,
14,
15,
16,
17,
18], the usage of CO
2 and O
2 is relatively new, especially when using fixed-bed gasifiers. As high amounts of steam can lead to an increase in tar formation due to lowering the reactor temperatures [
9], partially substituting the steam with CO
2 could result in a better product gas quality with decreased tar contents, solving this problem. Furthermore, CO
2 could potentially be converted into CO via the Boudouard reaction, increasing the benefit of the process while providing an additional, environmentally friendly CO source. Especially when combining this approach with upcoming aspirations of transitioning the economy into a carbon-neutral hydrogen-based economy, large quantities of O
2 and CO
2 will be available as a by-product of electrolysis and carbon-capture processes. Coupling these sectors provides us with the opportunity to direct several current problems at once:
In recent years, the focus has mainly been on fluidized bed or entrained flow gasification systems [
3,
4,
19]. This is primarily justified due to their scalability combined with a great range of feedstock to be used, giving these technologies certain advantages when compared to fixed-bed gasifiers. On the other hand, modern fixed-bed gasifiers can potentially yield higher carbon conversion rates as they offer (spatially) separated reaction zones, which increases the controllability of the system [
20]. Furthermore, downdraft gasifiers have the advantage of passing the produced pyrolysis gas through a reduction zone consisting of a hot coal bed. This results in a thermal treatment of these gasses, significantly reducing their tar content due to tar-cracking in that zone.
This scientific paper delves into the critical exploration of gasifying wood chips under the influence of oxygen-enriched, nitrogen-free gasification agents, unraveling the intricate interplay between operating parameters, product yields, and overall system performance. The unique advantages of oxygen-enriched gasification agents hold the promise of addressing longstanding challenges associated with conventional gasification methods. By promoting higher reaction rates and enabling precise control over the gasification environment, oxygen-enriched gasification agents emerge as a transformative element in the pursuit of cleaner and more energy-efficient fuel conversion processes. In this study, we aimed to systematically investigate the impact of varying O2 and CO2 concentrations in the gasification agent stream as well as varying water contents in the feedstock used on key gasification performance metrics, such as syngas composition, tar content, and overall process efficiency.
2. Materials and Methods
2.1. Feedstock
The feedstock used in the experiments consisted of commercially available coniferous wood chips with size category P 45. The wood chips were dried in actively vented drying containers for at least 6 weeks prior to their use. While the dried wood chips were used in the first two experiments, a batch of the wood chips was rewetted in order to investigate the influence of a higher water content of the feedstock on the process. Ultimate and proximate analyses of the feedstocks used are shown in
Table 1.
As seen in
Figure 1, both samples only contain minor amounts of bark, resulting in low ash contents, minimizing the risk of technical problems due to sintering or slagging.
2.2. Gasifier
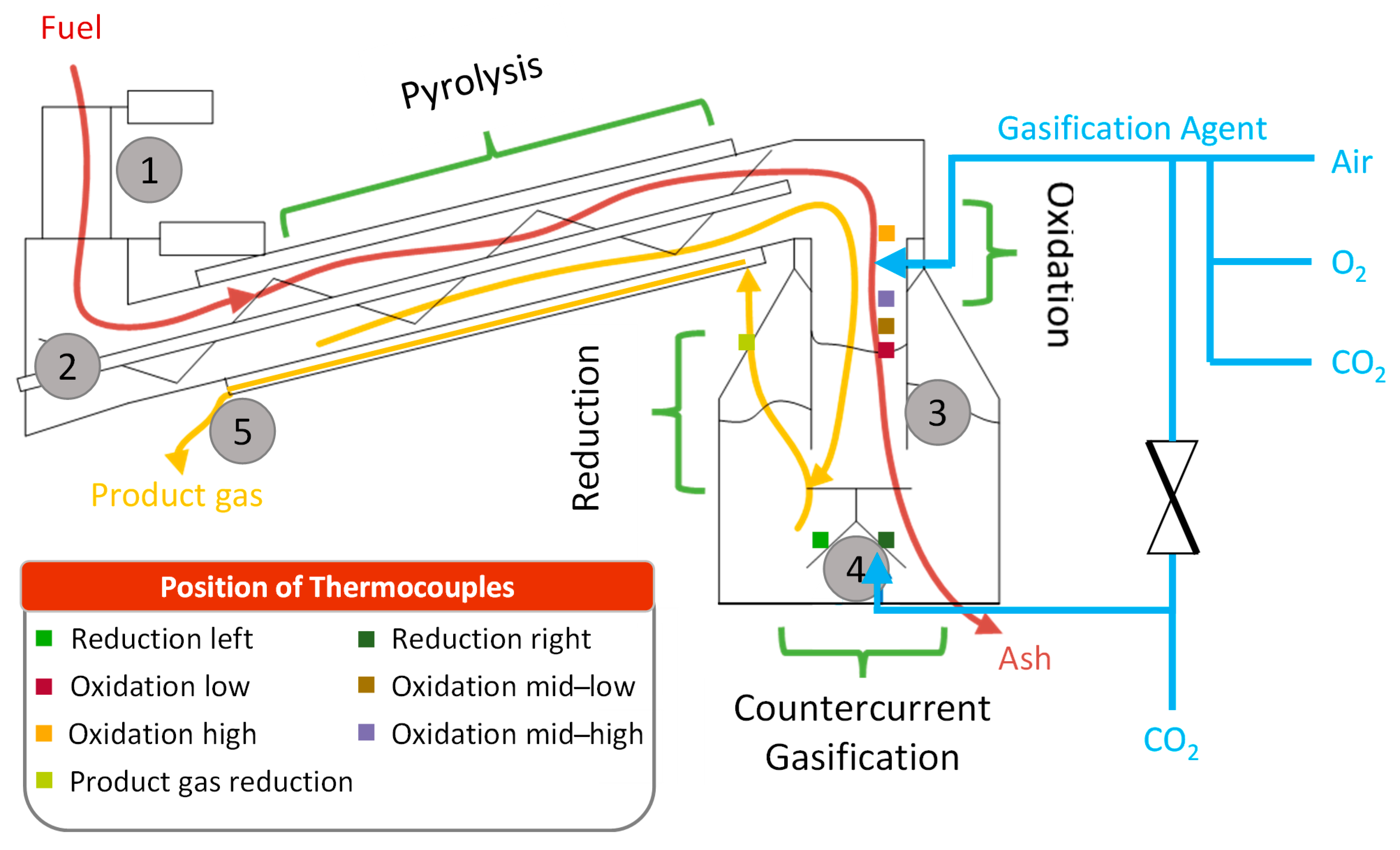
A double-fired downdraft gasifier was used in this study. It was provided by LiPRO Energy GmbH &Co. KG (26203 Wardenburg, Germany) and consisted of their standard HKW 50 gasifier model. The system is rated at 100 kW
th and relies on an autothermal process which uses part of the feedstock to produce the heat for the gasification process. It is designed to convert wood chips and roadside green into a product gas that is usually converted into electricity and heat using a gas engine. A schematic representation of the system is shown in
Figure 2. The feedstock is transported from a bunker into the reactor via a screw conveyor, in which it is pyrolyzed by heating it with the produced gas (double-pipe design). The pyrolyzed feedstock is then transported through the oxidation zone of the gasification reactor where most of the gasification agent is added to convert the emerging pyrolysis gas into the product gas. This zone is followed by a reduction zone that consists of the pyrolyzed feedstock formed in the process. In this zone, the charcoal is partially oxidized while the produced tars are cracked. Furthermore, CO
2 is partially converted to CO via the Boudouard reaction. The remaining charcoal at the bottom of the gasification reactor is treated with a small portion of the gasification agent to fully convert the carbon into a product gas while additionally heating the reduction zone. Residues like ashes and remaining charcoal are removed by a grate. During normal operations, the amount of gasification agent entering the reactor via the oxidation and reduction zone depends on the temperatures within the reactor. A central process management system monitors a variety of process parameters such as the temperatures and pressures of the gasifier and adjusts the addition of the gasification agent based on these. If temperatures reach preset limits within a certain area of the reactor, a larger part of the gasification agent is directed towards the opposite reactor zone, cooling down the desired area. This is achieved by the automated settings of a control valve in the gasification agent feed piping.
For this study, the gasification agent supply (usually air) of the gasifier was equipped with an experimental setup to gradually substitute the gasification agent air for a mixture of O2 and CO2. As the required volumes of these gasses were relatively large (approx. 40–50 m3/h), two racks (450 L per rack, 300 bar) per gas type were connected to the gasification agent supply pipes. The gas racks were electrically heated to prevent freezing. Additionally, an electrically heated pressure reducer and heated piping were used. To adjust the gas flow, several needle valves with volume flow measurements were used. Furthermore, pressure sensors and thermocouples were also fitted to the setup in order to obtain the gas flow at standard conditions.
2.3. Characterization of Syngas
The produced synthesis gas was characterized by a variety of different analyzers and methods regarding its composition and tar content. All measurements were carried out in the cooled product gas (approx. 50 °C) after particulate removal to minimize any interference of particulates with the measurement equipment. Permanent gasses such as O2, H2, CO, and CH4 were measured using a multi-gas measurement device (VISIT 03H, Messtechnik Eheim GmbH, 74193 Schwaigern, Germany) equipped with nondispersive infrared sensors (NDIRs), thermal conductivity detectors (TCDs), and electrochemical sensors. In order to protect the sensors of the device, sample gas preparation was used. First, the sample gas was washed using a washing oil and water and then it was passed through silica gel, activated carbon, and a series of filters. The measurement of CO2 and selected components like hydrocarbons (C1–C6), acid gasses, and nitrogen oxides was taken using Fourier-transform infrared spectroscopy (FTIR, CX4000, Gasmet Technologies GmbH, 76131 Karlsruhe, Germany). As the total concentration of infrared (IR) active sample gas components was expected to be too high, the sample gas was diluted using pure nitrogen (grade: 5.0). This was carried out to prevent a phenomenon called total absorption, where the gas is so IR-active that it absorbs all the light from the IR source in the FTIR, making it impossible to characterize the gas. Additionally, gas components that occur in smaller concentrations cannot be detected in the resulting spectra of undiluted sample gasses due to broadening of the CO, CO2, and H2O peak at high concentrations due to overlapping. Therefore, a volumetric dilution of 1:10 (sample gas to nitrogen) was used. The exact dilution was measured by an internal diluting device via pressure sensors. The sample was taken via a heated sample line (180 °C) that was connected to a series of heated quartz wool filters which were used to protect the device from dust or condense tars from the sample gas.
In order to calculate the volume flow of the product gas, the static and dynamic pressure within the product gas pipe was measured using an S-Pitot-Tube. The pressures obtained were logged by the gasifier central process management system with a frequency of 30 s. Based on the measured gas composition, the density of the gas was calculated. In combination with the recorded gas pressures, the volume flow was calculated according to Bernoulli’s equation.
All experiments were further characterized regarding their tar content. Sample preparation was carried out based on CEN/TS 15439 (Tar Protocol) [
21]. Therefore, a sample port was installed behind the particulate removal unit of the gasifier and a stainless steel canula (inner diameter: 6 mm) was placed in the central gas flow. From there, a tube (Noroprene, G-60-A) was connected to a row of 7 impinger bottles. While the first and the last bottles were empty and functioned as a condenser and safety bottle, the remaining 5 bottles were filled with 2-propanol. Bottle 1–3 were placed in a cold bath at around 0 °C while bottles 4–7 were cooled to −20 °C. Gas sampling was examined as long as possible with a constant flow of approx. 0.5 m
3/h. The obtained liquid from the impinger bottles was collected, all equipment was flushed with 2-propanol, and the resulting liquid was unified with the sample. To prevent any degradation of the samples, they were stored in brown glass bottles with PTFE seals and stored at −10 °C until they were analyzed via gas chromatography–mass spectrometry (GC-MS). The samples were screened for Benzene, Toluene, Xyloles (BTX-fraction), 16-EPA-PAK, and other aromatic and non-aromatic hydrocarbons. Detection limits observed in the analysis depended on the overall sample condition and ranged from 0.05 to 0.18 mg/m
3i.N.dry. A schematic overview of the measurement positions is displayed in
Figure 3.
3. Experimental Procedure
All experiments were conducted with a “hot” gasifier, meaning that it was operated conventionally with air as a gasification agent for at least 3 h before switching to the desired mixture of oxygen and carbon dioxide. This was achieved to ensure that the gasifier was in stable operation conditions before conducting experiments and to avoid any inhomogeneities within the reactor. In order to achieve a smooth transition in operation conditions form air to synthetic mixtures of gasification agents, the air compressor was turned off and the piping towards the compressor closed via a valve. The gasification agent mixture of O2 and CO2 was then gradually increased until it reached the desired setpoint. We aimed for the highest amount of oxygen possible for all experiments, while the limiting factor was seen in the reactor temperature in the oxidation zone. A maximum temperature of 1150 °C was set to prevent damages in the reactor, but it was intended to not exceed temperatures above 1100 °C to ensure operations within the defined temperature limit. After reaching the desired operation conditions using the gasification agent mixture, the gasifier was operated until stable operation conditions were obtained. The criteria used were constant reactor temperatures as well as a steady gas composition regarding the permanent gasses. After reaching stable operations, measurements were taken to assess the operating status.
The first experiment was conducted to investigate the overall influence of using O
2 and CO
2 as gasification agents on the gasification performance. In the second experiment, the influence of increased CO
2 dosage in the reduction zone was studied. The last experiment focused on the influence of a feedstock with a higher water content on the system performance. The obtained experimental conditions are depicted in
Table 2.
4. Results and Discussion
The implementation of O
2 and CO
2 as gasification agents did not result in any technical problems regarding the used gasifier equipment. The temperatures within the reactor were higher when using oxygen-enriched gasification agents instead of air, limiting the oxygen concentration in the gasification agent. Nonetheless, stable operation conditions were easily obtained. For the first experiment, the temperatures showed a similar behavior as in regular operations with air, as the oxygen concentration in the gasification agent was approx. 18.4 Vol.-%. When increasing the amount of CO
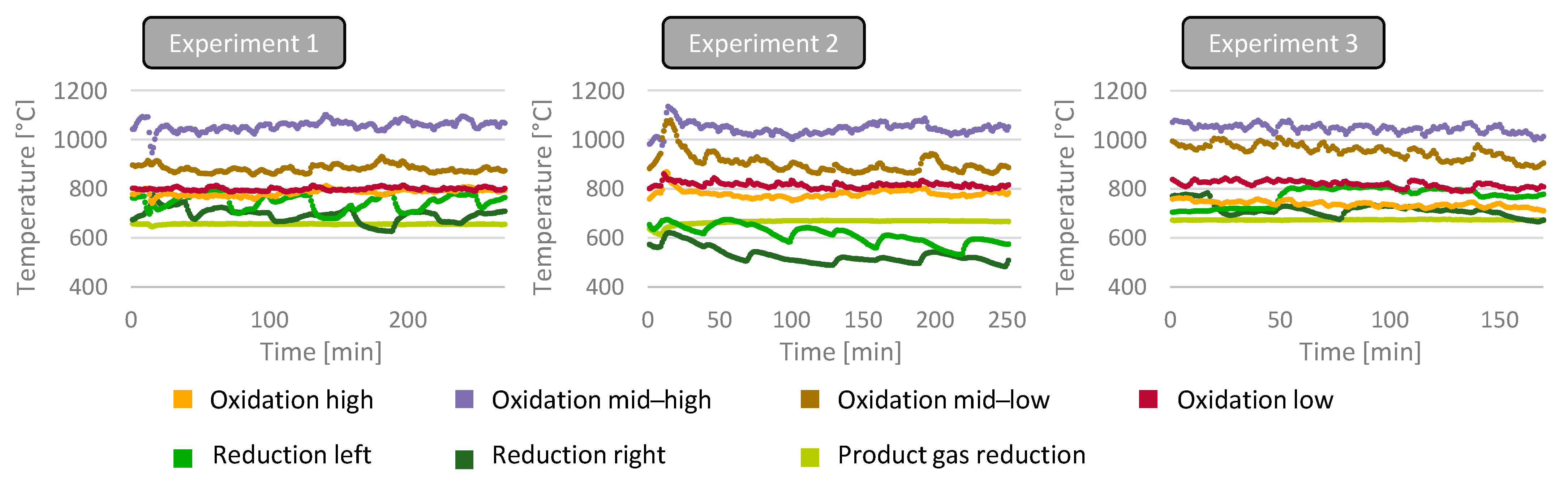
2 in the gasification agent in the reduction zone, a significant drop in the temperatures in the reduction zone was observed while all other temperatures remained on similar levels compared to the first experiment. The usage of a feedstock with a higher water content, as investigated in experiment 3, showed a slight decrease in overall temperatures but the temperatures in the reduction zone. The time course of the most important reactor temperatures is illustrated in
Figure 4.
4.1. Influence of Gasification Agent
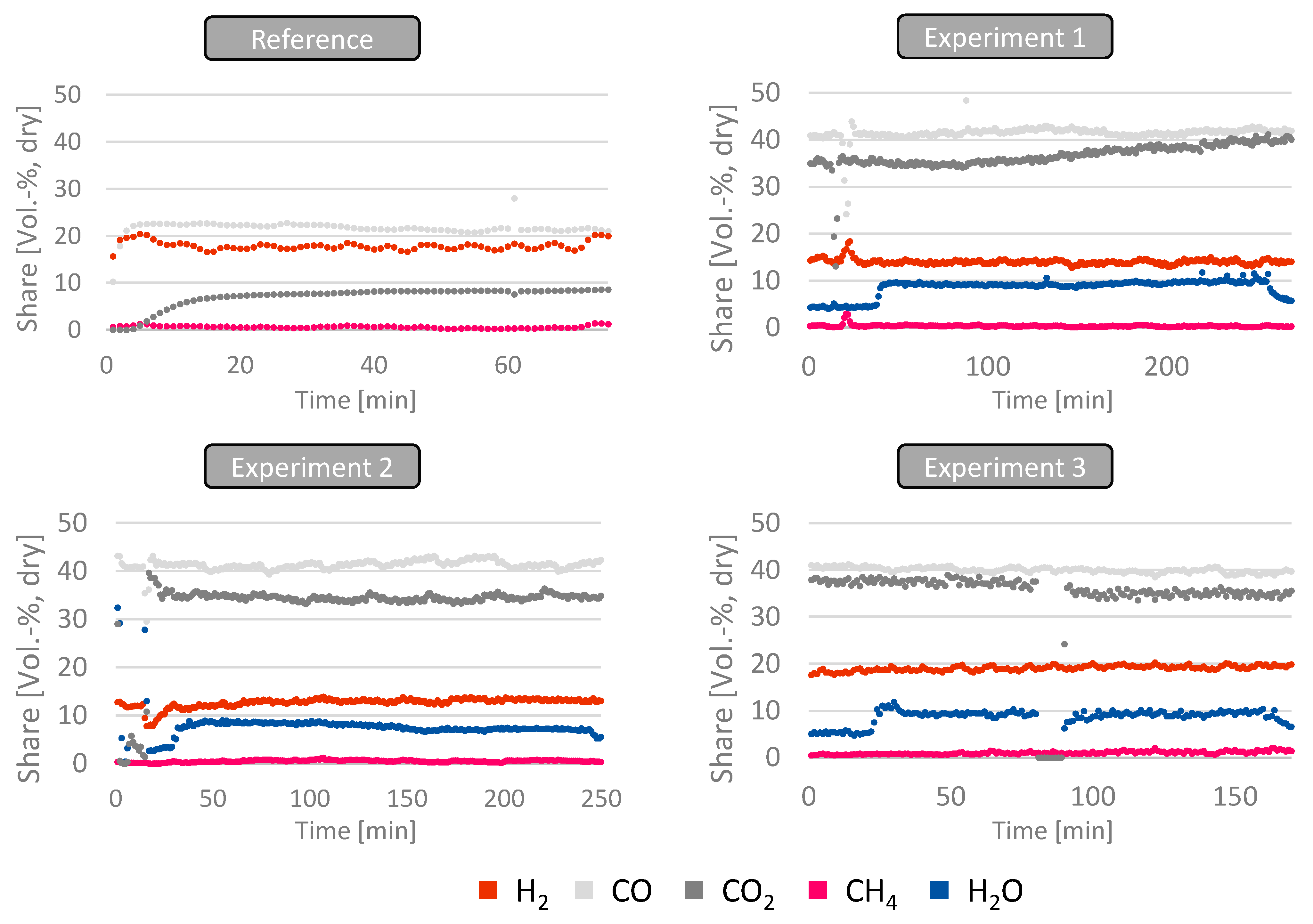
When focusing on the gas composition for the experiments, it was found that the overall concentration of CO and CO
2 almost double compared to the conventional operation with air. This can be explained by the exchange of N
2 by CO
2 for those experiments and a partial conversion of the CO
2 towards CO via the Boudouard reaction. Furthermore, the amount of CH
4 remained at a comparably low level of about 0.4–1.0 Vol.-% for all experiments conducted, being in a similar range as in air-blown operations. For the first two experiments, the amount of hydrogen in the product gas significantly decreased (12–14 Vol.-%) compared to conventional operations (17–19 Vol.-%), while an increase in water intake by using wet woodchips resulted in similar hydrogen contents (18–20 Vol.-%). It is assumed that this phenomenon can be explained by limiting water gas shift reactions due to higher CO
2 concentrations in the synthesis gas and limited water for the first two experiments, while increasing the water content in the gas increases the formation of H
2. A detailed view of the product gas composition for all experiments conducted is illustrated in
Figure 5.
When compared to the operation with air as a gasification agent, a higher heating value of the gas was obtained as a result of the replacement of nitrogen in the product gas. While the typical heating value of the air-blown product gas is around 5.5 MJ/m3dry, an increase to 6.9 to 7.6 MJ/m3dry was observed by using the oxygen–carbon dioxide mixture as a gasification agent. This increase is mainly due to an increase in CO concentrations in the product gas, almost doubling when compared to normal operations.
In order to calculate the cold gas efficiency, it was estimated that about 8 kg of feedstock is fed into the gasifier with each movement of the double slide lock of the fuel feed. This estimation was based on measurements in previous tests and can vary depending on the density of the feedstock as well as its overall geometry. As it was impossible to weigh each feedstock batch, this estimation was used for all experiments. Based on these assumptions, the cold gas efficiency of the gasifier was calculated with 91.8% for the first experiment. About 19.7% of the added CO
2 was converted in the reactor as only 30.0 m
3dry/h of CO
2 remained in the product gas while 37.38 m
3dry/h was added. Key performance parameters for the experiments conducted are depicted in
Table 3.
4.2. Influence of CO2 Enrichment in Reduction Zone
The influence of CO2 enrichment in the gasification agent entering the reactor in the reduction zone was investigated in an experiment where an additional 4.5 m3dry/h of CO2 (approx. 10% of the overall CO2) was added to the gasification agent in the reduction zone of the reactor. It was expected that the additional CO2 would increase the amount of CO in the product gas due to increased Boudouard reactions and reverse water gas shift reactions. Surprisingly, the addition of CO2 did not show any significant changes within the volumetric product gas’s composition; only minor decreases in H2 and CO2 concentrations were observed, while the CO and CH4 concentrations remained almost the same. The ratio of CO:CO2 increased from 1.13 for the first experiment to 1.26 for the CO2 enrichment, while the CO:H2 ratio also increased from 2.95 to 3.25. A slight increase in product gas formation (approx. 15%) was observed in the experiment. It is uncertain if the increase in gas production is due to the change in operating parameters or due to a slight deviation in the gasification agent mixture, compromising slightly more oxygen compared to the first experiment. The conversion rate of CO2 increased compared to the first experiment from about 19.7% to about 27.2%, proving that this experimental setup is capable of converting large amounts of CO2 towards CO.
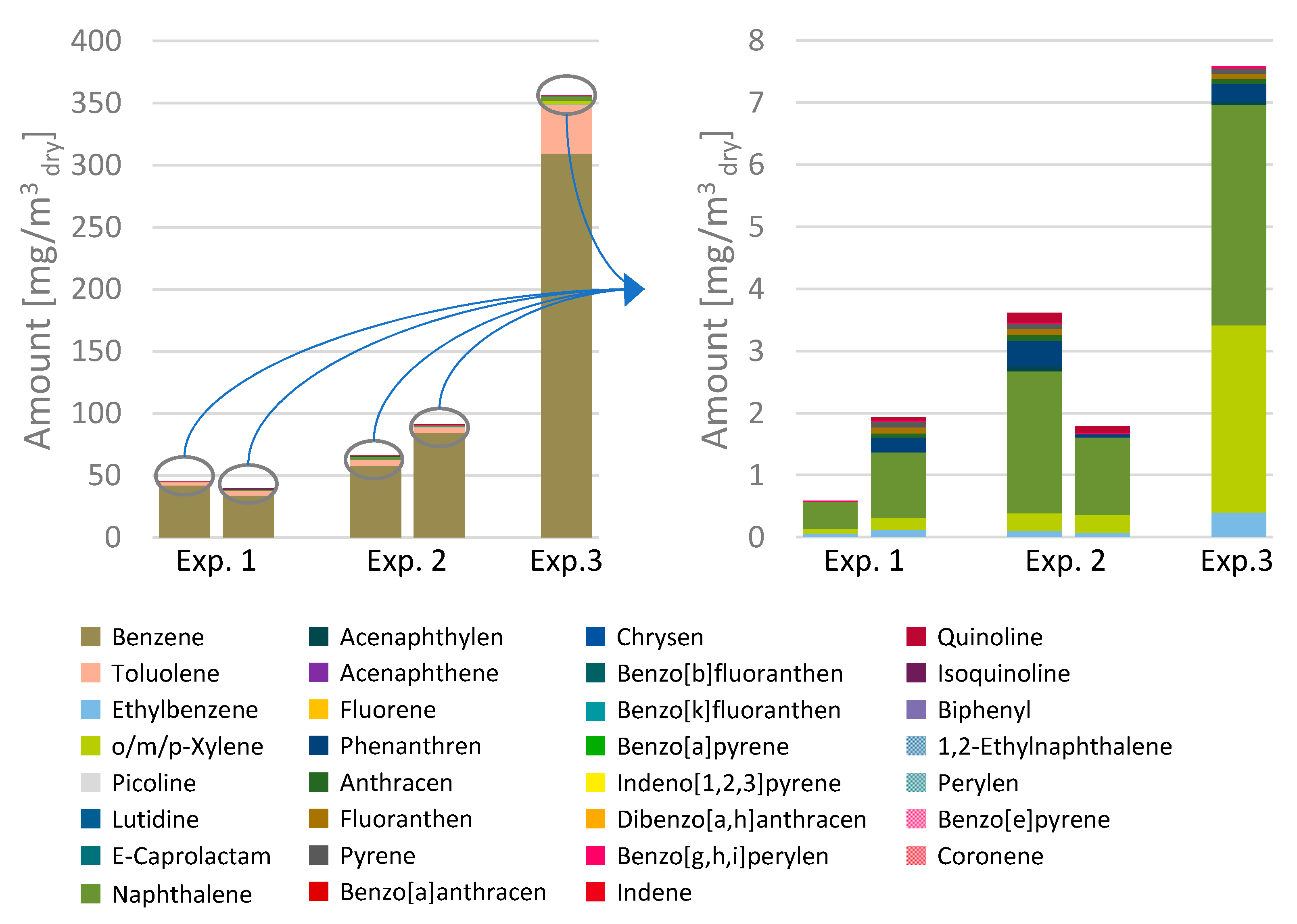
When focusing on the tar content within the product gas as displayed in
Figure 6, it was observed that the overall concentration was around 38–45 mg/m
3dry for the first experiment and increased slightly to about 62–90 mg/m
3dry with an increased amount of CO
2 in the reduction zone.
It is assumed that this is a result of lower temperatures in the reduction zone (approx. 500–600 °C) compared to the first experiment (approx. 650–800 °C), resulting in less thermal cracking of tars. The majority of the tars detected were Benzene and Toluene, making up over 95% (grav.) of the tars for both experiments. In comparison to the previous experiment, the cold gas efficiency increased from 91.8% to 95.5%. It is uncertain if this increase is due to measuring errors or the more effective conversion of CO2 to CO.
4.3. Influence of Water Content in Fuel on Process
As the previous experiments were successfully conducted, it was of interest to examine the influence of the water content of the feedstock on the overall process. It was expected to result in higher amounts of hydrogen due to the increased presence of water for water gas shift reactions. Furthermore, it was anticipated to allow higher oxygen concentrations in the gasification agent as more energy, provided by additional exothermal oxidation processes, would be needed to evaporate the increased amount of water in the wetter fuel. For the experiment, an oxygen concentration of 23.1 Vol.-% was achieved in the gasification agent. As expected, the hydrogen content in the product gas increased to an average of 19.1 Vol.-%, while the concentration of CO slightly decreased to 40.0 Vol.-%. The concentration of CO2 remained in a similar range compared to the first experiment, but the concentration of CH4 increased slightly to an average of about 0.6 Vol.-%. The amount of tars detected in the product gas significantly increased to approx. 350 mg/m3dry while, as previously noted, the majority of those tars were found to be Benzene and Toluene. The ratio of CO:H2 decreased to 2.09 compared to the first experiment, while the CO:CO2 ratio almost remained static (1.11). As the higher water content of the gas requires more energy to evaporate, the cold gas efficiency of this experiment dropped significantly to approx. 83.5%. Equally, the conversion rate of CO2 within the reactor decreased to −7.5%, producing more CO2 than was added.