The Impact of Hydrogen on Flame Characteristics and Pollutant Emissions in Natural Gas Industrial Combustion Systems
Abstract
1. Introduction
2. Materials and Methods
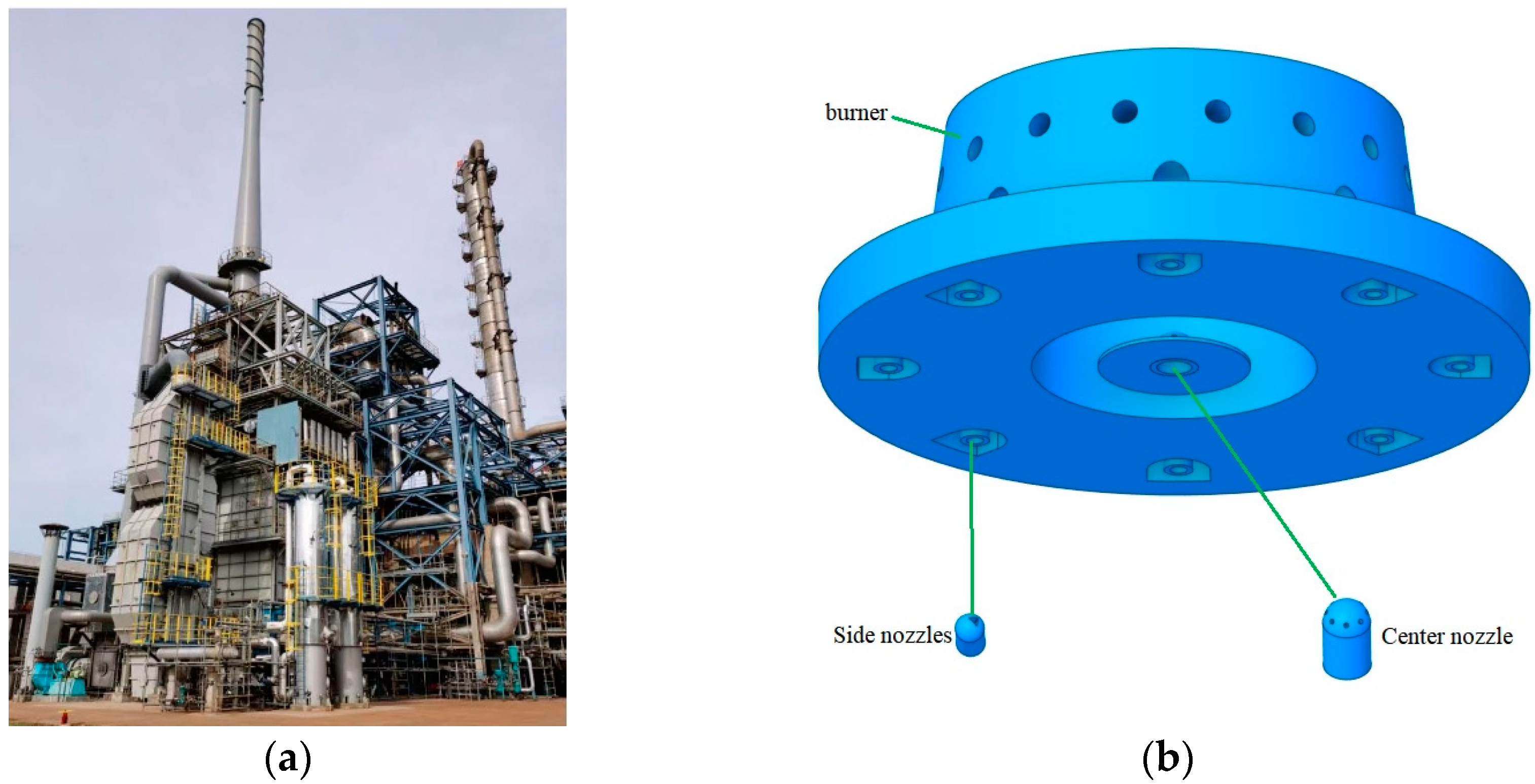
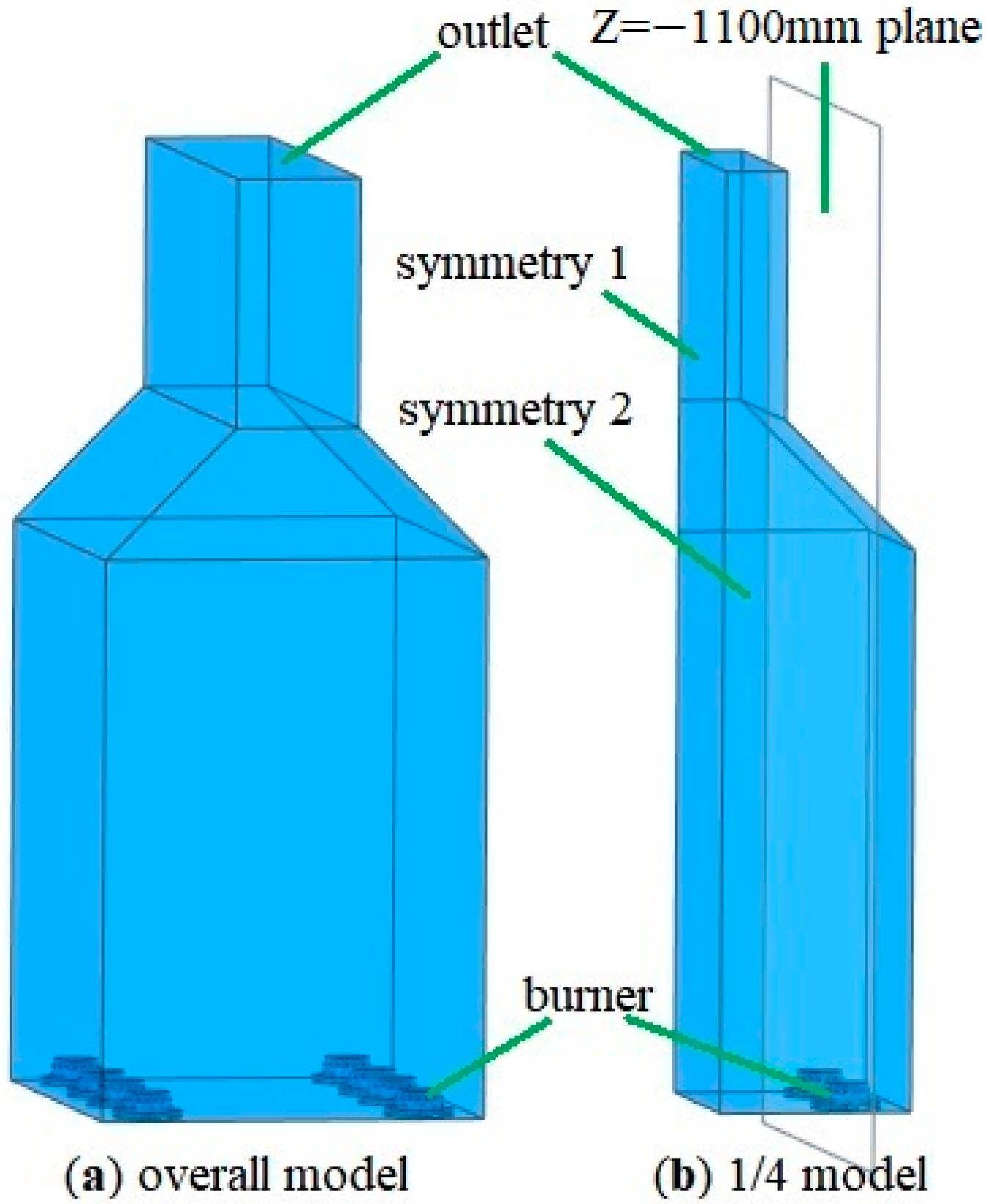
2.1. Geometric Model
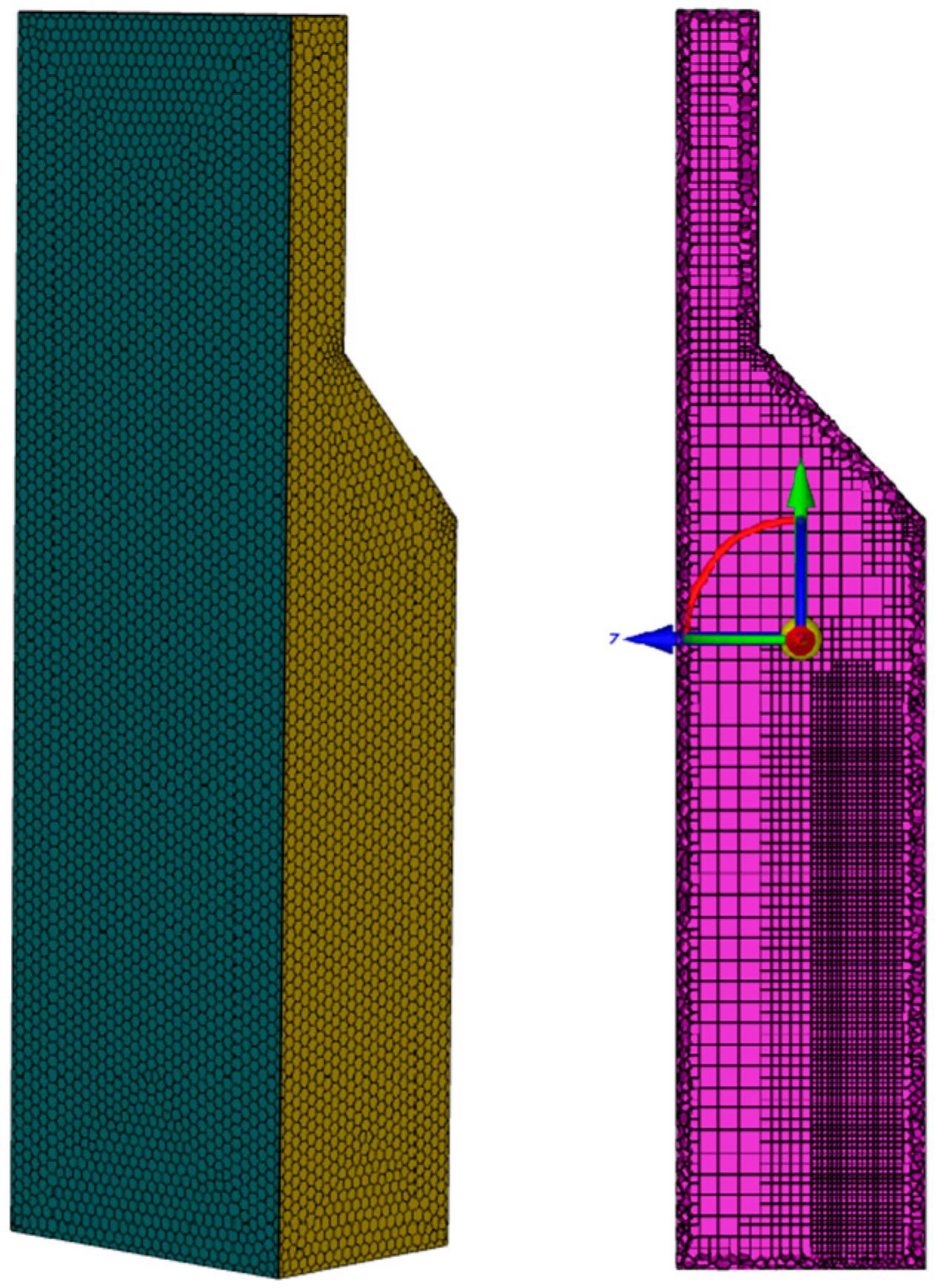
2.2. Numerical Methods and Boundary Condition
2.3. Turbulence Model
2.4. Combustion Model
2.5. NO Formation Model
2.5.1. Thermal NO
2.5.2. Prompt NO
2.5.3. N2O Intermediate NO
3. Results and Discussion
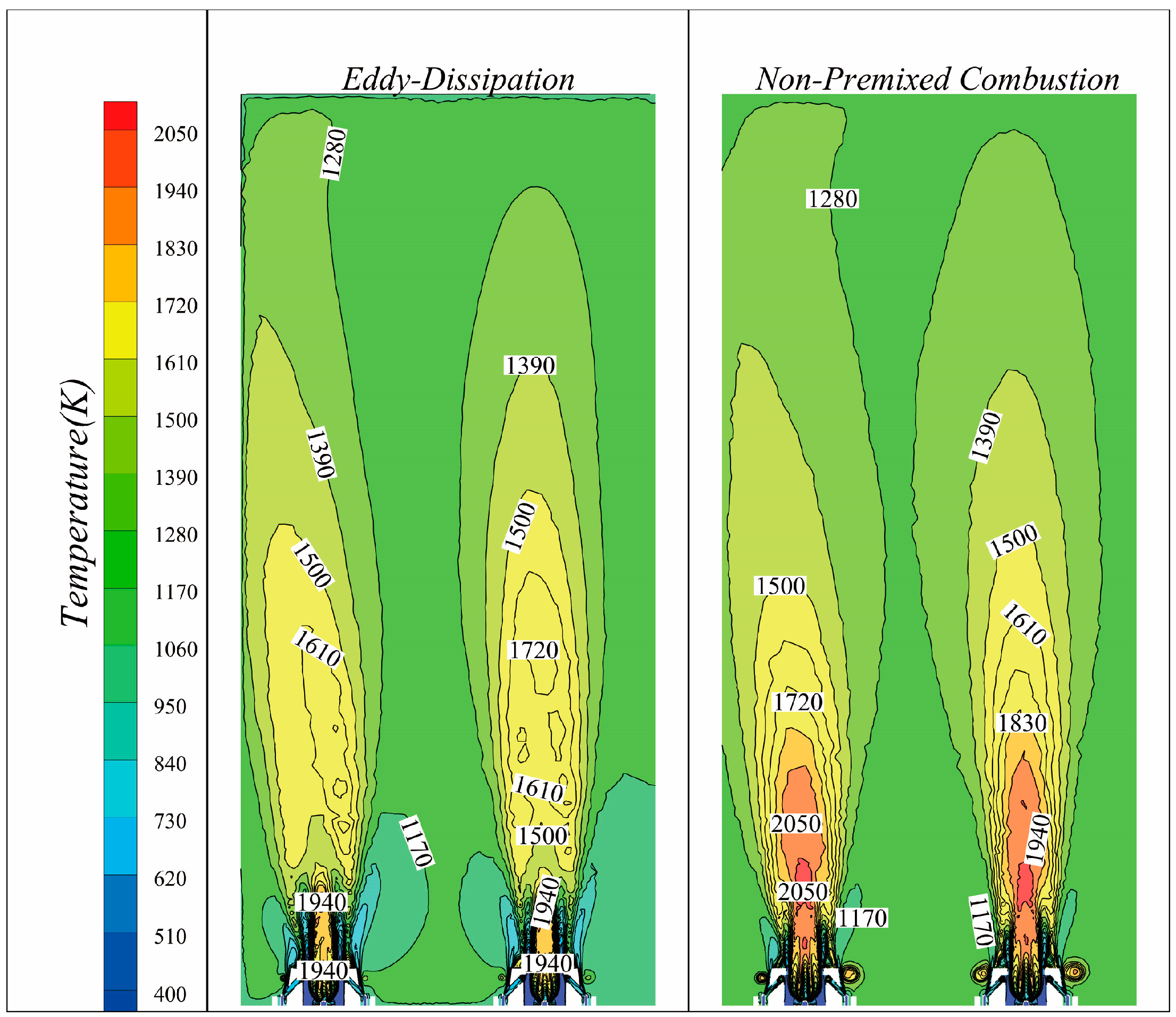
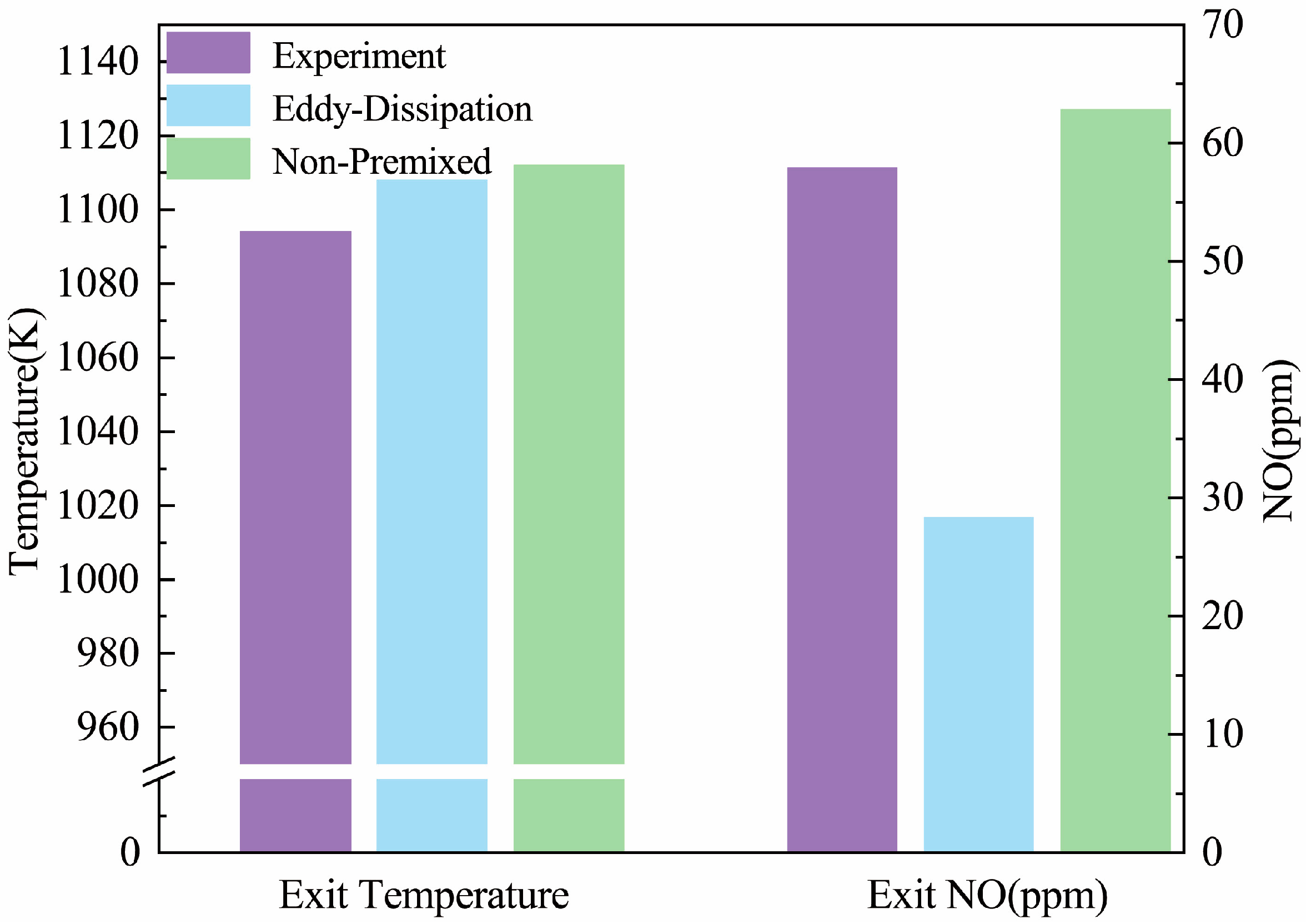
3.1. Comparison of Natural Gas Combustion Experimental and Numerical Simulation Errors
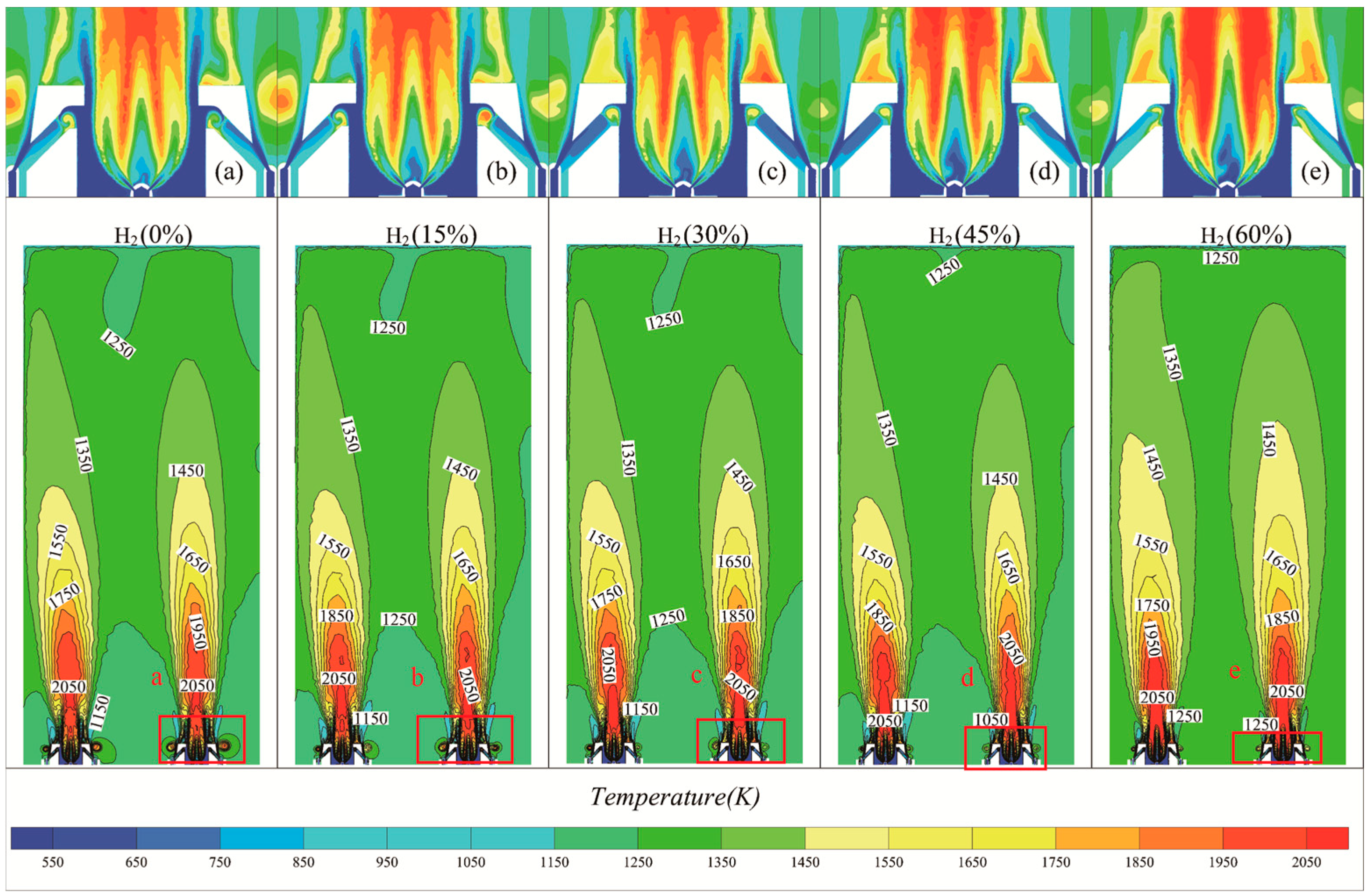
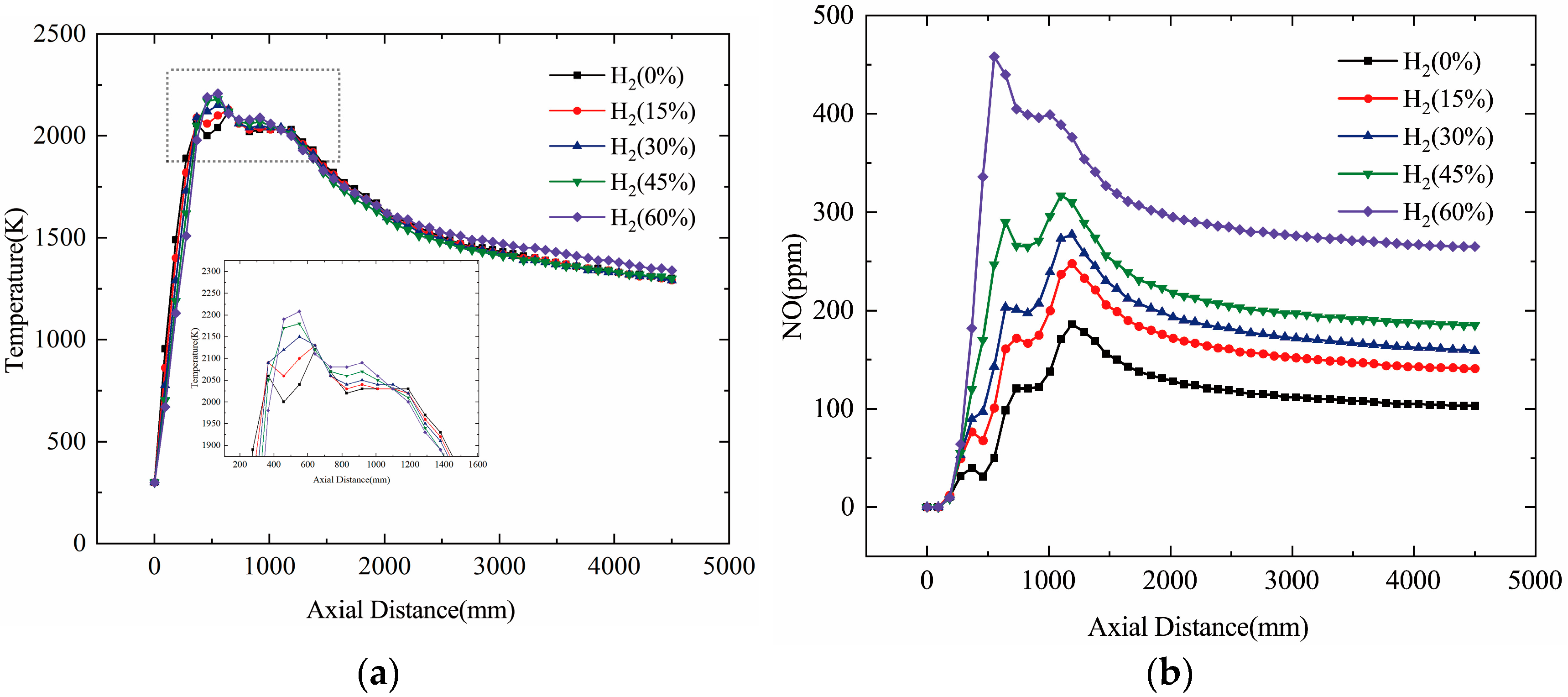
3.2. Effect of Hydrogen on Combustion Temperature
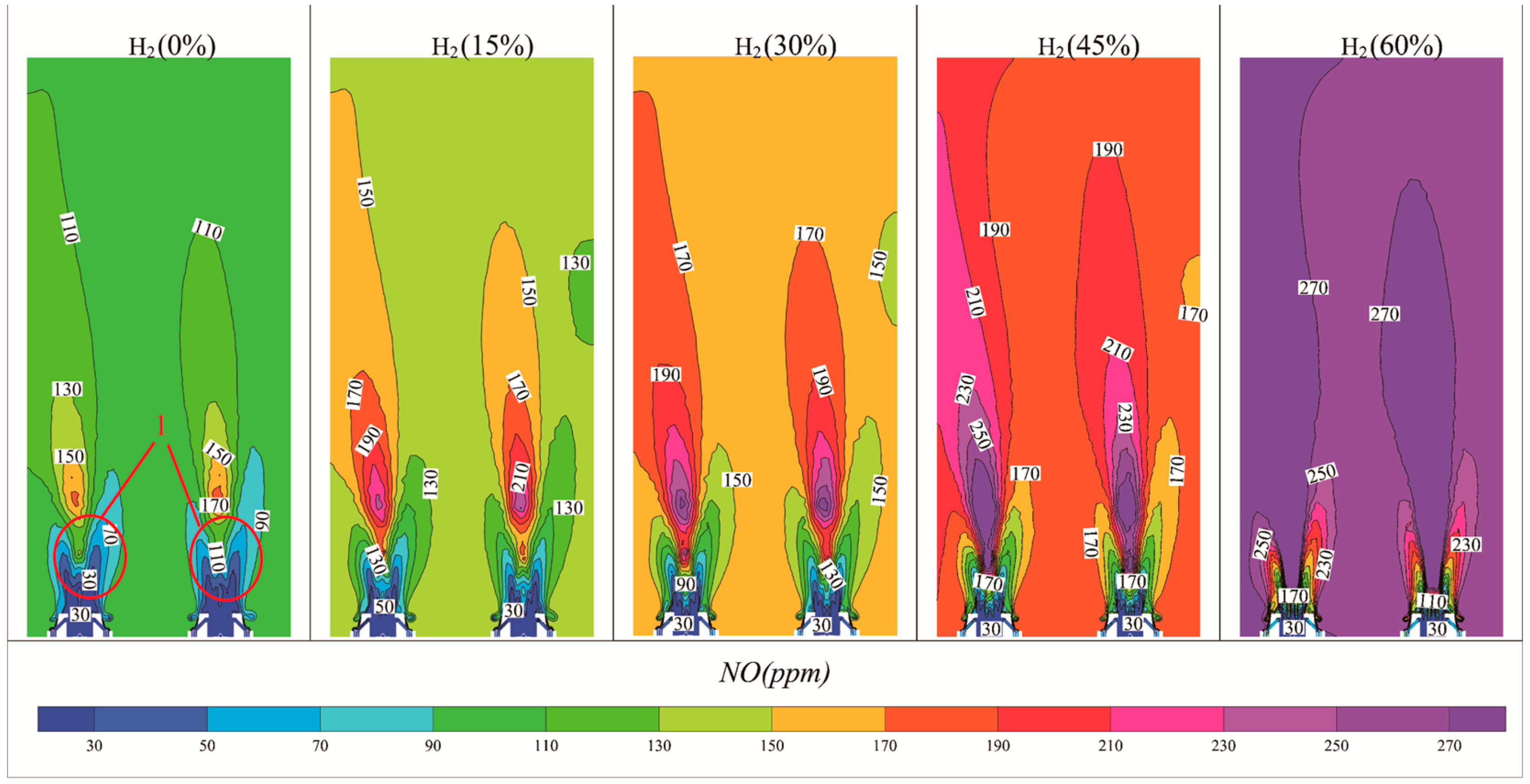
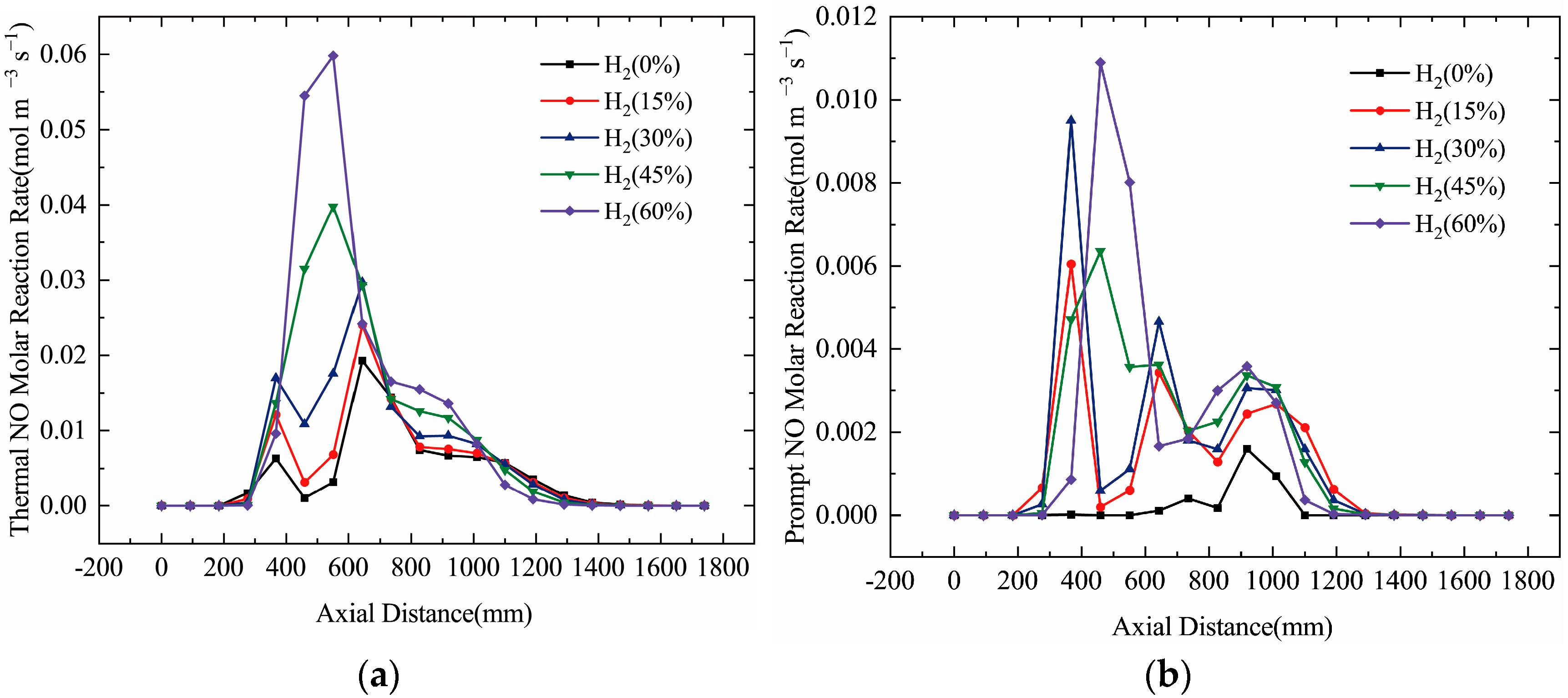
3.3. Effect of Hydrogen on NO Production
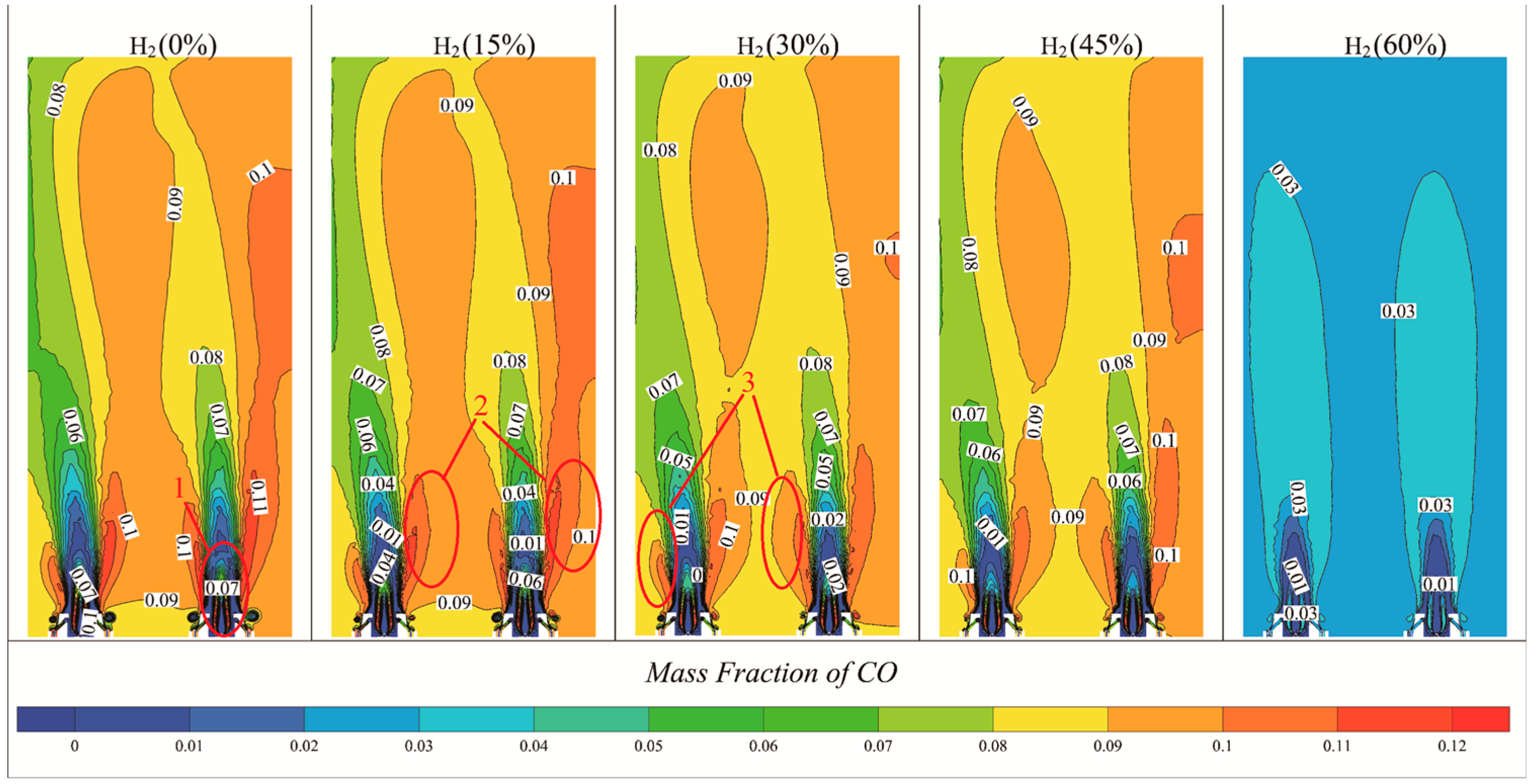
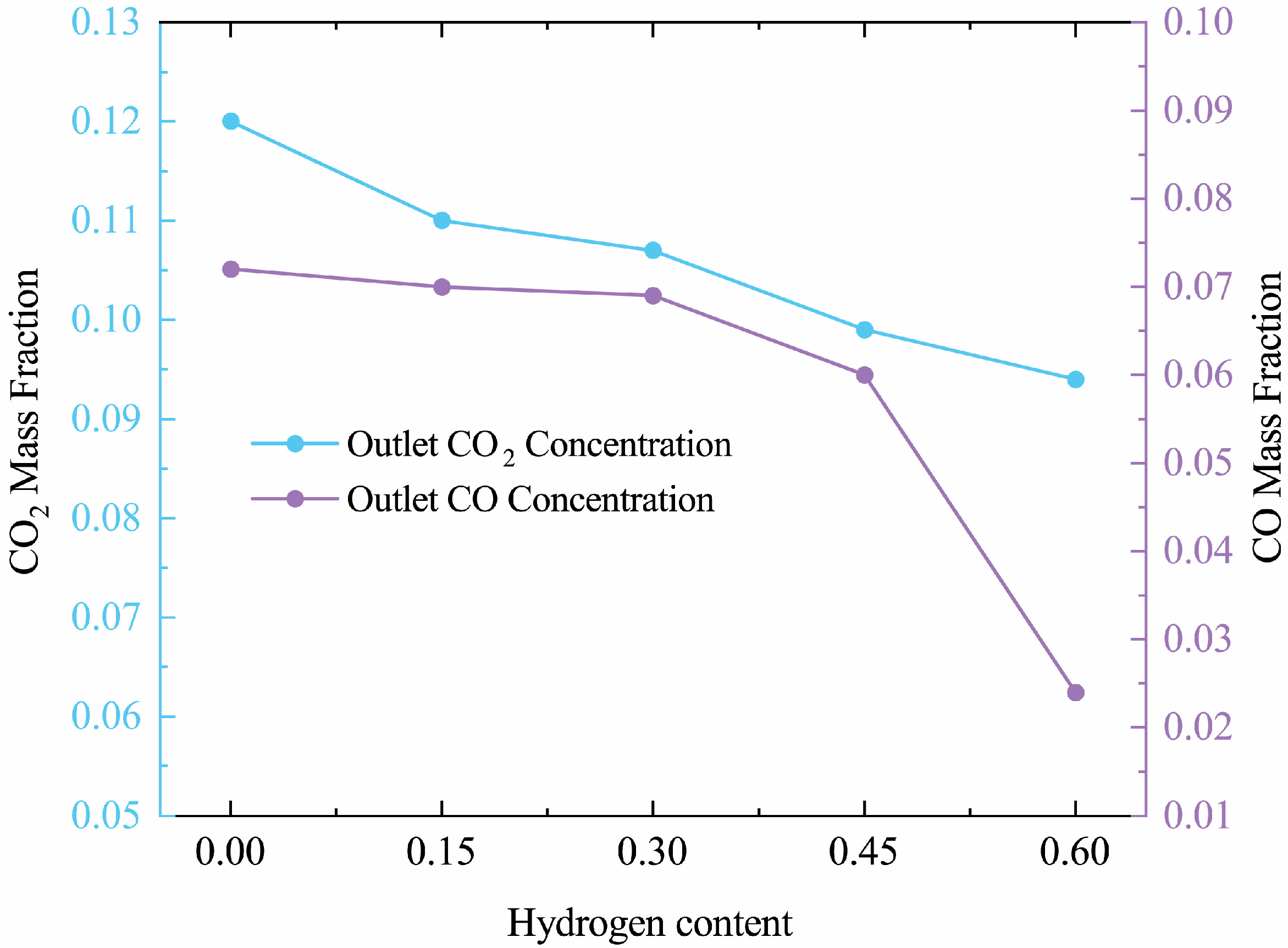
3.4. Effect of Hydrogen on CO2, CO Production
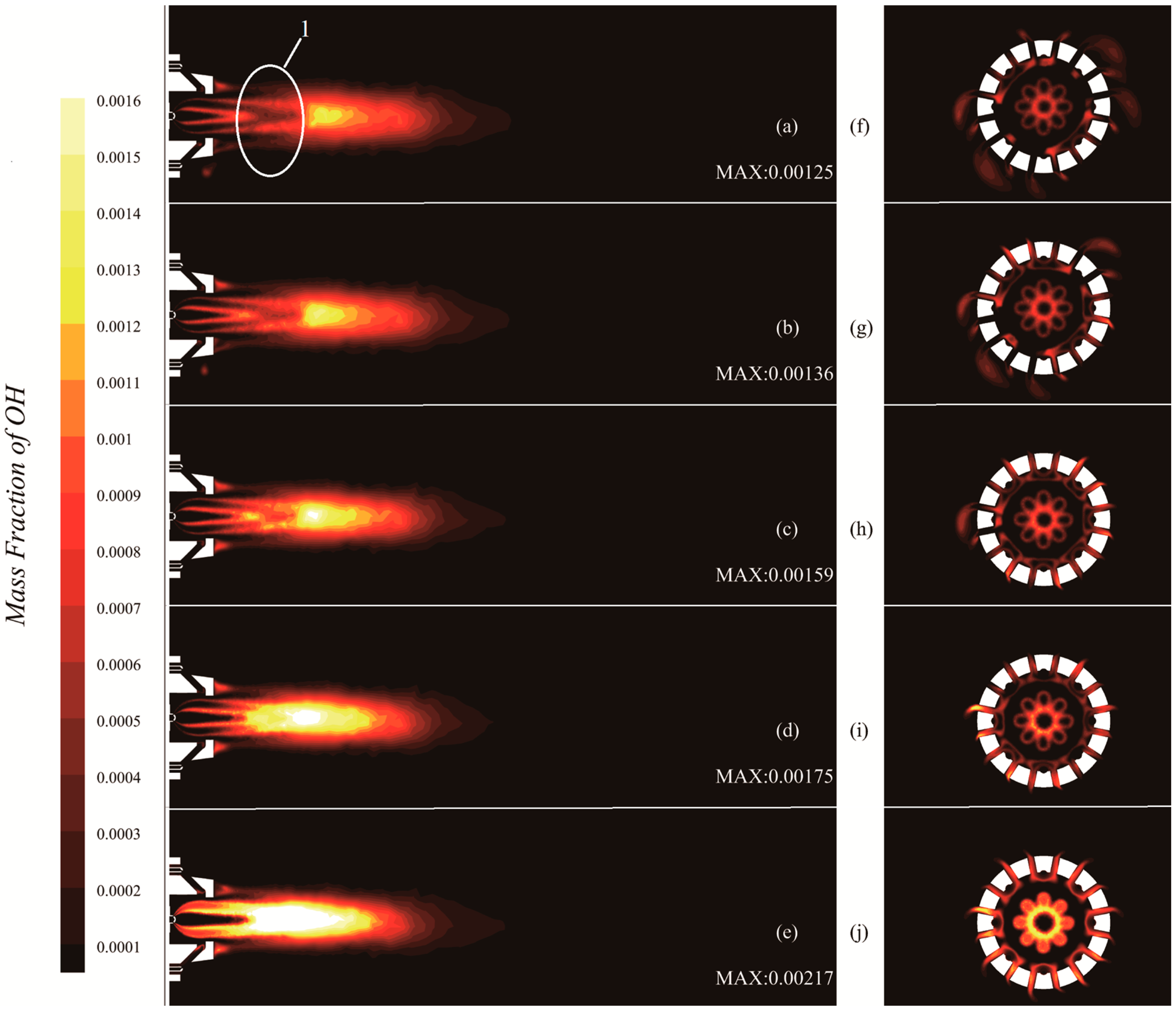
3.5. The Impact of Hydrogen on the Intermediate Product Hydroxyl (OH)
4. Conclusions
- (1)
- Numerical Simulation Validation: The CFD numerical simulation results for natural gas combustion were validated against experimental measurements, confirming the reliability of the numerical model. It was observed that the flame temperature increased by 47 K, 52 K, 72 K, and 91 K with the addition of 15%, 30%, 45%, and 60% molar mass hydrogen, respectively. Although the flame temperature showed a noticeable increase, the change in the outlet temperature of the heater was minimal.
- (2)
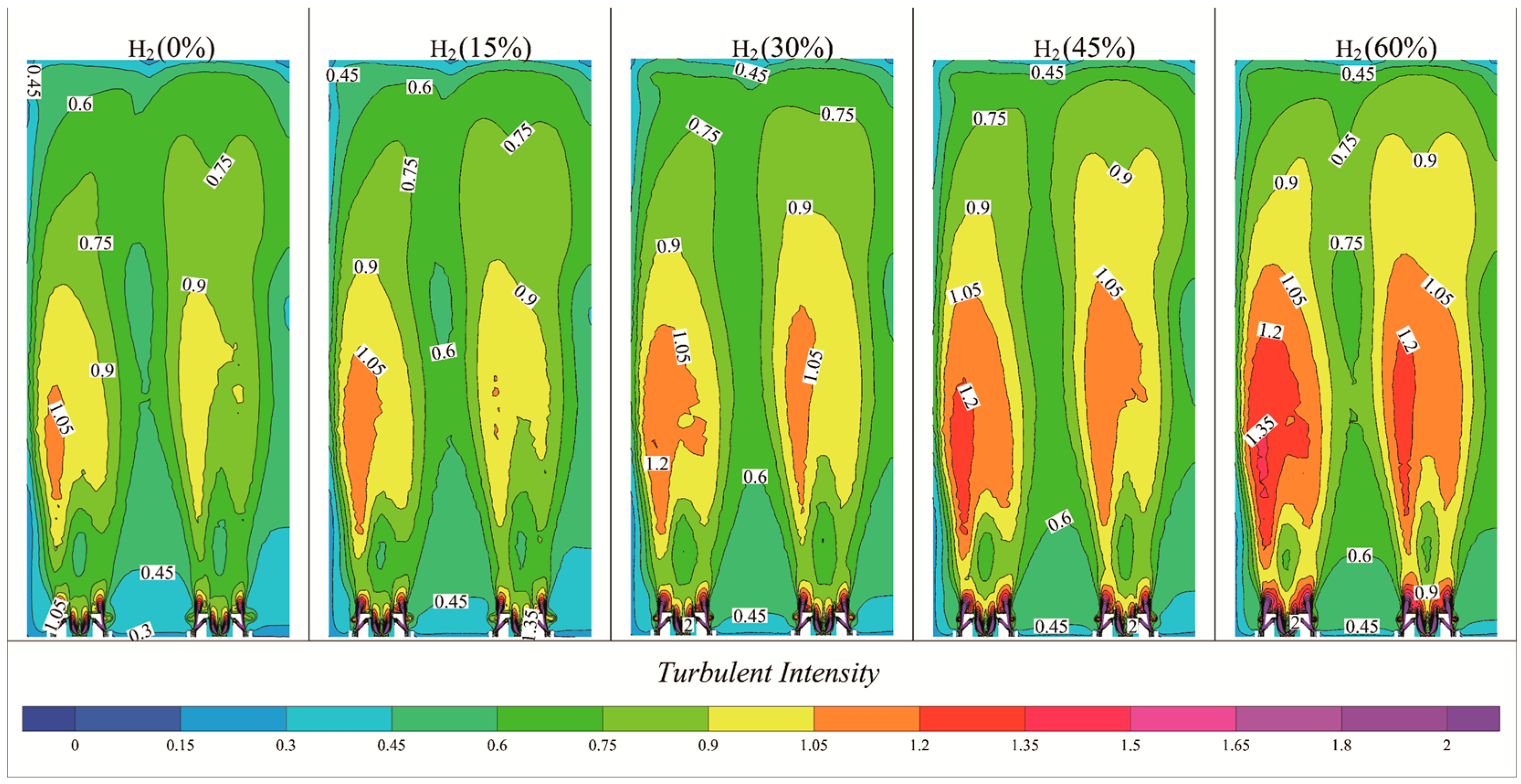
- Turbulence Characteristics: The intensity and velocity of turbulence within the furnace chamber increased with higher hydrogen content, demonstrating a more pronounced effect compared to pure natural gas combustion. This increase in turbulence led to observable changes in both the height and width of the flame.
- (3)
- NO Concentration: As the hydrogen content in natural gas increased, both the flame temperature and NO concentration rose. Specifically, at 60% hydrogen content, the NO concentration in the furnace chamber increased by 155% compared to pure natural gas combustion, and the NO concentration at the furnace outlet rose by 146%. Thus, higher hydrogen content correlates with an increase in NO emissions.
- (4)
- CO2 and CO Emissions: The introduction of hydrogen into the natural gas mixture led to a reduction in carbon content, resulting in significant decreases in CO2 and CO emissions. At 60% hydrogen content, CO2 and CO levels decreased by 37.5% and 70%, respectively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Xie, D.; Cao, Q.; Hu, J.; Tang, Y.; Shi, B.; Wang, N. Characteristics of Oxy-Methane Flame in an Axial/Tangential Swirl Jet Burner. Exp. Therm. Fluid Sci. 2022, 139, 110732. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, B.; Zhu, T. Characterizing Combustion Instability in Non-Premixed Methane Combustion Using Internal Flue Gas Recirculation. Appl. Energy 2024, 370, 123602. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Pan, H.; Yue, Y. Effect of Structure Parameters on Low Nitrogen Performance of Burner Based on Orthogonal Experiment Method. Case Stud. Therm. Eng. 2022, 39, 102404. [Google Scholar] [CrossRef]
- Mu, L.; Wang, S.; Lu, J.; Liu, G.; Zhao, L.; Lan, Y. Effect of Flue Gas Condensing Waste Heat Recovery and Its Pressure Drop on Energy Saving and Carbon Reduction for Refinery Heating Furnace. Energy 2023, 279, 128081. [Google Scholar] [CrossRef]
- Bowman, C.T. Control of Combustion-Generated Nitrogen Oxide Emissions: Technology Driven by Regulation. Symp. (Int.) Combust. 1992, 24, 859–878. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, D.; Dong, X. Investigation on NO Emissions and Thermal Performance of an Ammonia/Methane-Fuelled Micro-Combustor. Int. J. Hydrogen Energy 2024, 58, 912–923. [Google Scholar] [CrossRef]
- Nhan, H.K.; Kwon, M.; Kim, S.; Park, J.H. CFD Investigation of NOx Reduction with a Flue-Gas Internal Recirculation Burner in a Mid-Sized Boiler. J. Mech. Sci. Technol. 2019, 33, 2967–2978. [Google Scholar] [CrossRef]
- Nada, Y.; Kidoguchi, Y.; Matsumoto, M.; Sugiyama, K.; Oono, T.; Fujii, Y.; Horikawa, R. Effects of Spacing between Fuel and Oxidizer Nozzles on NOx Emission from Spray Combustion Furnace Operating under Various Oxidizer Temperatures. Fuel 2024, 366, 131398. [Google Scholar] [CrossRef]
- Swaminathan, S.; Spijker, C.; Raonic, Z.; Koller, M.; Kofler, I.; Raupenstrauch, H. Numerical Study of an Industrial Burner to Optimise NOx Emissions and to Evaluate the Feasibility of Hydrogen-Enriched Fuel. Int. J. Hydrogen Energy 2024, 49, 1210–1220. [Google Scholar] [CrossRef]
- Ortolani, A.; Yeadon, J.; Ruane, B.; Paul, M.C.; Campobasso, M.S. Numerical and Experimental Analysis of the Formation of Nitrogen Oxides in a Non-Premixed Industrial Gas Burner. Results Eng. 2024, 23, 102392. [Google Scholar] [CrossRef]
- Chen, J.; Fan, W.; Feng, G.; Guo, H.; Zhang, H. NO Emission Characteristics of Air Coflowed Non-Premixed Ammonia Jet Flame at Elevated Ambient Temperatures and with N2 Dilution. J. Clean. Prod. 2024, 435, 140463. [Google Scholar] [CrossRef]
- Shan, X.; Yu, W.; Hu, B.; Wen, K.; Ren, S.; Men, Y.; Li, M.; Gong, J.; Zheng, H.; Hong, B. A Methodology to Determine Target Gas Supply Reliability of Natural Gas Pipeline System Based on Cost-Benefit Analysis. Reliab. Eng. Syst. Saf. 2024, 251, 110364. [Google Scholar] [CrossRef]
- Di, T.; Sun, X.; Chen, P.; Huang, Q.; Liu, X. A Novel Static Mixer for Blending Hydrogen into Natural Gas Pipelines. Int. J. Hydrogen Energy 2024, 86, 1118–1128. [Google Scholar] [CrossRef]
- Huang, Z.; Jin, H.; Ma, H.; Yu, X.; Li, Y.; Wang, C. Experimental Study on Flame Behaviors and Dynamic Parameters Induced by Hydrogen Jet Flame in an Enclosed Compartment. Therm. Sci. Eng. Prog. 2024, 54, 102851. [Google Scholar] [CrossRef]
- Sekar, M.; Alahmadi, T.A.; Nithya, S. Numerical Simulation of Industrial Gas Burners Fueled with Hydrogen-Methane Mixtures for Enhanced Combustion Efficiency and Reduced Greenhouse Gas Emissions. Fuel 2024, 370, 131807. [Google Scholar] [CrossRef]
- Cellek, M.S.; Pınarbaşı, A. Investigations on Performance and Emission Characteristics of an Industrial Low Swirl Burner While Burning Natural Gas, Methane, Hydrogen-Enriched Natural Gas and Hydrogen as Fuels. Int. J. Hydrogen Energy 2018, 43, 1194–1207. [Google Scholar] [CrossRef]
- Yang, H.; Jin, S.; He, Y.; Liu, Y. Experimental Study on Flame Temperature Distribution and Pollutant Emission Characteristics of CH4 Mixed with CO2/N2/H2 Combustion. Int. J. Hydrogen Energy 2024, 71, 750–762. [Google Scholar] [CrossRef]
- Xu, S.; Chen, Y.; Tian, Z.; Liu, H. NO Emission Reduction Characteristics of CH4/H2 Staged MILD Combustion over a Wide Range of Hydrogen-Blending Ratios. Fuel 2024, 372, 132239. [Google Scholar] [CrossRef]
- Gan, Z.; Yang, S.; Wang, H. Investigation of the Heating Characteristics of Turbulent Non-Premixed Gas Combustion in the Industrial-Scale Walking Beam Type Reheating Furnace. Appl. Therm. Eng. 2024, 257, 124212. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, Y.; Wang, X.; Tian, Z.; Hu, G.; Du, W. Investigation of NOx Emission under Different Burner Structures with the Optimized Combustion Model. Neurocomputing 2022, 482, 224–235. [Google Scholar] [CrossRef]
- Bose, D.; Kumar, I.; Hens, A. Enhancing Fuel-Air Mixing in COG-BOG Non-Premixed Combustion: A CFD Analysis with Different Turbulent Models. J. Indian Chem. Soc. 2024, 101, 101222. [Google Scholar] [CrossRef]
- Wu, X.; Guo, Q.; Gong, Y.; Liu, J.; Luo, X.; Wu, T.; Yu, G. Influence of Burner Geometry on Atomization of Coal Water Slurry in an Entrained-Flow Gasifier. Chem. Eng. Sci. 2022, 247, 117088. [Google Scholar] [CrossRef]
- Roy, S.; Shuai, R.; Wang, P.; Zhang, Z.; Qian, W.; Ferrante, A.; Dai, K. Combustor Shape Optimization and NO Emission Characteristics for Premixed NH3-CH4 Turbulent Swirling Flame towards Sustainable Combustion. Aerosp. Sci. Technol. 2024, 150, 109216. [Google Scholar] [CrossRef]
- Zeldovich, Y.B.; Sadovnikov, P.Y.; Frank-Kamenetskii, D.A. Oxidation of Nitrogen in Combustion; Academy of Sciences: Moscow, Russia, 1947. [Google Scholar]
- Masum, B.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Palash, S.M.; Abedin, M.J. Effect of Ethanol–Gasoline Blend on NOx Emission in SI Engine. Renew. Sustain. Energy Rev. 2013, 24, 209–222. [Google Scholar] [CrossRef]
- Irace, P.H.; Gopan, A.; Axelbaum, R.L. An Investigation of Thermal Radiation from Laminar Diffusion Flames in a Tri-Coflow Burner with Central Oxygen. Combust. Flame 2022, 242, 112158. [Google Scholar] [CrossRef]
- Hanson, R.K.; Salimian, S. Survey of Rate Constants in the N/H/O System. In Combustion Chemistry; Gardiner, W.C., Ed.; Springer: New York, NY, USA, 1984; pp. 361–421. ISBN 978-1-4684-0188-2. [Google Scholar]
- Glarborg, P.; Miller, J.A.; Kee, R.J. Kinetic Modeling and Sensitivity Analysis of Nitrogen Oxide Formation in Well-Stirred Reactors. Combust. Flame 1986, 65, 177–202. [Google Scholar] [CrossRef]
- De Soete, G.G. Overall Reaction Rates of NO and N2 Formation from Fuel Nitrogen. Symp. (Int.) Combust. 1975, 15, 1093–1102. [Google Scholar] [CrossRef]
- Dupont, V.; Pourkashanian, M.; Williams, A.; Woolley, R. The Reduction of NOx Formation in Natural Gas Burner Flames. Fuel 1993, 72, 497–503. [Google Scholar] [CrossRef]
- Shang, X.; Xie, J.; Chen, J.; Gu, Y. Numerical Study on Combustion Characteristics of Biogas Cocombustion in a 300 MW Coal-Fired Boiler Furnace. ACS Omega 2024, 9, 20378–20387. [Google Scholar] [CrossRef]
- Lu, L.; Jiang, H. Study of NO and CO Formation Pathways in Jet Flames with CH4/H2 Fuel Blends. Energies 2024, 17, 4382. [Google Scholar] [CrossRef]
- Wang, D.; Tan, Z.; Xu, J.; Meng, H. Quantitative Studies of NO Emissions from Various Reaction Pathways in Swirling Combustion of Hydrogen-Enriched Methane. Int. J. Hydrogen Energy 2024, 53, 409–421. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, L.; Cao, C.; Chen, X.; Xing, C.; Shi, H.; Liu, L.; Qiu, P. The Effects of N2 and Steam Dilution on NO Emission for a H2/Air Micromix Flame. Int. J. Hydrogen Energy 2022, 47, 27266–27278. [Google Scholar] [CrossRef]
- Ding, H.; Ouyang, Z.; Wang, W.; Zhang, X.; Zhu, S. Experimental Study on the Influence of O2/CO2 Ratios on NO Conversion and Emission during Combustion and Gasification of High-Temperature Coal Char. Fuel 2022, 310, 122311. [Google Scholar] [CrossRef]
- Zheng, S.; He, Y.; Hu, B.; Zhu, J.; Zhou, B.; Lu, Q. Effects of Radiation Reabsorption on the Flame Speed and NO Emission of NH3/H2/Air Flames at Various Hydrogen Ratios. Fuel 2022, 327, 125176. [Google Scholar] [CrossRef]
- Rrustemi, D.N.; Ganippa, L.C.; Axon, C.J. Investigation of Boost Pressure and Spark Timing on Combustion and NO Emissions under Lean Mixture Operation in Hydrogen Engines. Fuel 2023, 353, 129192. [Google Scholar] [CrossRef]
- Nicol, D.G.; Steele, R.C.; Marinov, N.M.; Malte, P.C. The Importance of the Nitrous Oxide Pathway to NOx in Lean-Premixed Combustion. J. Eng. Gas Turbines Power 1995, 117, 100–111. [Google Scholar] [CrossRef]
- Wang, J.; Cong, B.; Zhang, K.; Zheng, B. Investigation of the Temperature Field of Turbulent Non-Premixed Flame for the Variable Angles of Inclination of NexGen Burner. Case Stud. Therm. Eng. 2023, 49, 103353. [Google Scholar] [CrossRef]


| Cell Count | Turbulence Model | NOx (ppm) |
|---|---|---|
| 4,574,814 | 62.73 | |
| 2,723,223 | 62.77 | |
| 1,884,301 | 62.84 | |
| 1,308,430 | 75.83 | |
| 943,477 | 83.64 |
| Species | Natural Gas | 15% H2 | 30% H2 | 45% H2 | 60% H2 | Air |
|---|---|---|---|---|---|---|
| CH4 | 0.9506 | 0.8006 | 0.65006 | 0.5006 | 0.35006 | - |
| C2H6 | 0.029 | 0.029 | 0.029 | 0.029 | 0.029 | - |
| C3H8 | 0.0095 | 0.0095 | 0.0095 | 0.0095 | 0.0095 | - |
| C4H10 | 0.0026 | 0.0026 | 0.0026 | 0.0026 | 0.0026 | - |
| N2 | 0.0058 | 0.0058 | 0.0058 | 0.0058 | 0.0058 | 0.79 |
| CO2 | 0.0025 | 0.0025 | 0.0025 | 0.0025 | 0.0025 | - |
| O2 | - | - | - | - | - | 0.21 |
| H2 | - | 0.15 | 0.3 | 0.45 | 0.6 | - |
| No. | Reaction Name | Chemical Reaction Equation |
|---|---|---|
| 1 | Hydrogen–oxygen reaction | |
| 2 | Carbon monoxide oxidation | |
| 3 | Methane oxidation | |
| 4 | Water–gas shift reaction | |
| 5 | Reverse water–gas shift reaction | |
| 6 | Hydroxyl production and consumption | |
| 7 | Hydrogen dissociation | |
| 8 | Oxygen dissociation | |
| 9 | Hydroxyl oxidation | |
| 10 | Intermediate reactions in hydrogen–oxygen combustion |
| No. | Reaction Name | Chemical Reaction Equation |
|---|---|---|
| 1 | Complete methane combustion | |
| 2 | Partial methane oxidation | |
| 3 | Carbon monoxide oxidation | |
| 4 | Water–gas shift reaction | |
| 5 | Reverse water–gas shift reaction | |
| 6 | Hydrogen combustion | |
| 7 | Hydrogen dissociation | |
| 8 | Oxygen dissociation | |
| 9 | Hydroxyl production and consumption | |
| 10 | Hydroxyl oxidation |
| Outlet Temperature (K) | Error | Outlet NO Concentration (ppm) | Error | |
|---|---|---|---|---|
| Experimental measurement data | 1094 | - | 57.92 | - |
| Eddy Dissipation | 1108 | 1.28% | 28.35 | 51.05% |
| Non-Premixed | 1112 | 1.65% | 62.84 | 8.49% |
| Past Study | 1125 | 2.37% | 67.38 | 9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.; Wang, Z.; Xu, J.; Yi, W. The Impact of Hydrogen on Flame Characteristics and Pollutant Emissions in Natural Gas Industrial Combustion Systems. Energies 2024, 17, 4959. https://doi.org/10.3390/en17194959
Lan Y, Wang Z, Xu J, Yi W. The Impact of Hydrogen on Flame Characteristics and Pollutant Emissions in Natural Gas Industrial Combustion Systems. Energies. 2024; 17(19):4959. https://doi.org/10.3390/en17194959
Chicago/Turabian StyleLan, Yamei, Zheng Wang, Jingxiang Xu, and Wulang Yi. 2024. "The Impact of Hydrogen on Flame Characteristics and Pollutant Emissions in Natural Gas Industrial Combustion Systems" Energies 17, no. 19: 4959. https://doi.org/10.3390/en17194959
APA StyleLan, Y., Wang, Z., Xu, J., & Yi, W. (2024). The Impact of Hydrogen on Flame Characteristics and Pollutant Emissions in Natural Gas Industrial Combustion Systems. Energies, 17(19), 4959. https://doi.org/10.3390/en17194959




