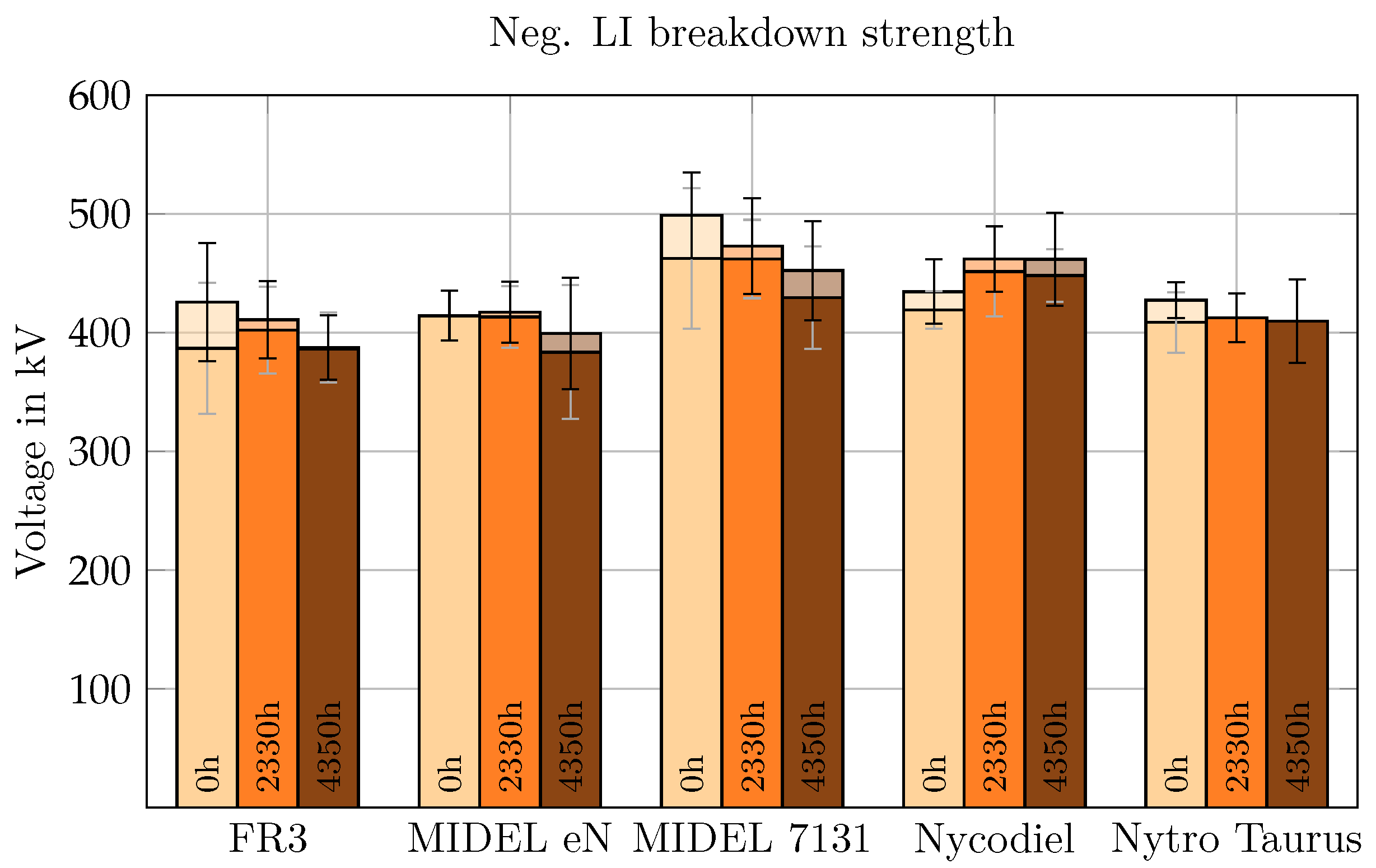1. Introduction
With increasing demand for environmentally sustainable insulating fluids, natural and synthetic esters have recently been adopted in distribution transformers due to their eco-friendly nature and enhanced fire safety [
1,
2]. However, for large power transformers, mineral oil remains the preferred insulating fluid because the breakdown behavior of esters under high voltage conditions is not yet fully understood [
3]. Recent research has centered on streamer propagation in non-uniform fields at lower voltage levels [
4,
5,
6]. Nevertheless, point–plane configurations do not reflect actual transformer designs, reducing the relevance of these findings for power transformers.
Avoiding downtime and unexpected failures is critical, especially for essential and expensive equipment like power transformers. It is therefore necessary to assess whether insulation has degraded due to aging and to develop reliable methods for evaluating the condition of the insulating liquid. Therefore, key aging markers of the insulating liquid are constantly being monitored during the operating lifetime of the transformer. Standardized AC breakdown tests conducted on regularly collected oil samples are typically part of the transformer monitoring strategy. However, since lightning impulse (LI) testing represents a worst-case scenario for stress on insulating liquids during transformer design, it is essential that the insulation can withstand this type of stress. However, LI tests are complex, particularly at high voltages in semi-uniform fields, compared to AC tests, which require simpler set-ups and smaller sample sizes. Nevertheless, standard AC testing provides no insights into how the liquids behave during switching faults or lightning-induced overvoltage conditions.
This paper aims, first, to evaluate the aging characteristics of ester liquids in comparison to mineral oil through long-term aging experiments. Second, it assesses the breakdown behavior of both new and aged liquids by comparing LI breakdown voltage (BDV) in realistic semi-uniform field geometries with standard-compliant AC BDV. This serves the purpose of being able to evaluate streamer behavior under realistic conditions during LI voltage tests. On the other hand, it needs to be compared whether investigations in realistic field geometries under LI voltage on aged liquids represent a necessary aging marker that must be taken into account or whether investigations in standard compliant AC testings are sufficient to determine the degradation of the liquids.
2. Experimental Description
2.1. Sample Preparation
This study involves five different insulating liquids: two natural esters (NEs) (FR3 and MIDEL eN), two synthetic esters (SEs) (MIDEL 7131 and Nycodiel), and one mineral oil (MO) (Nytro Taurus). To establish the initial condition for the new (non-aged) samples and initiate the aging process, all liquids undergo drying and degassing under vacuum and controlled temperature. Therefore, the samples were heated to 55 °C and then kept in the oven at this constant temperature for at least 36 h under vacuum of less than 50 mbar. This was followed by a cooling period of at least 24 h under vacuum to reach ambient temperature until the sample was taken for testing. This procedure ensured a similar starting condition for all liquids with a relative water content below 10%.
A thermal catalytic method was employed to accelerate the aging of the insulating liquids. Temperatures of 105 °C for MO and 125 °C for esters were selected to simulate transformer aging, equivalent to 27 years over 2330 h (3 months) and 50 years over 4350 h (6 months), based on the principle that transformer lifespan decreases by half with every 6 K to 8 K temperature increase [
7]. Copper and iron core materials from transformers were introduced into the liquids at a fixed catalyst ratio of 152
/10 L
insulating.liquid and 153.6
/10 L
insulating.liquid [
8]. The glass containers were not sealed airtight. A quantity of 20 L was aged per liquid. The samples for the different aging stages were dried and degassed separately.
2.2. Measurement Set-Up
2.2.1. Breakdown Voltage
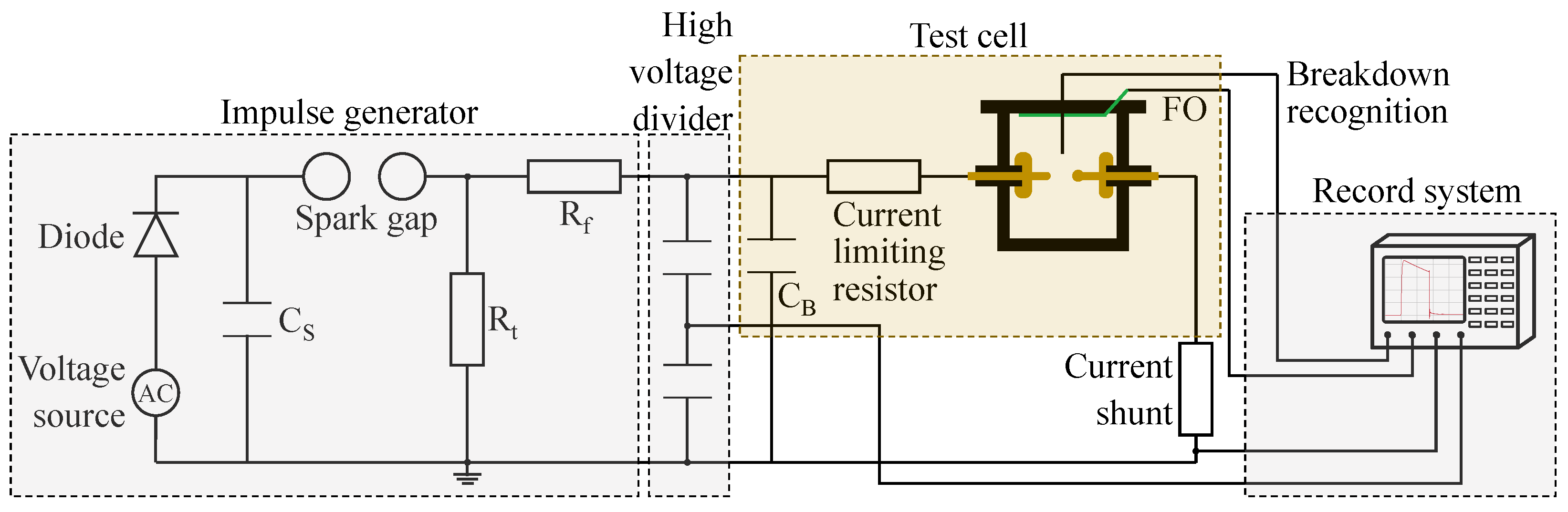
The configuration for measuring the BDV under LI conditions is depicted in
Figure 1. The system employed a 7-stage impulse generator capable of delivering a 1.2/50 standard LI voltage up to 560 kV, in accordance with IEC 60060-1:2010 [
9]. Voltage magnitude and waveform were monitored using a LeCroy HDC6104 digital oscilloscope, which offers a sample rate of 2.5 GS/s and an analog bandwidth of 1 GHz, with data obtained via a high-voltage divider. In order to keep the volume of the test oil minimal but at the same time applying high voltages, the test vessel was situated within an outer grounded oil tank containing 1860 L of synthetic ester, thus preventing external flashover, as illustrated in
Figure 2. The voltage was introduced into the oil tank through a 220 kV transformer bushing and directed into the test vessel using a 3.5 kΩ damping resistor, which limited the energy input to the oil during breakdown testing.
The parameters for the measurement set-up are defined within the constraints of the external conditions. The voltage limit of the generator is 560 kV, and thus, a breakdown should occur within this voltage threshold. Additionally, a realistic electrode arrangement, reflecting the homogeneity characteristic of a tap changer [
2], should be selected. The volume of the test cell should be minimized to reduce the amount of liquid subject to aging. This results in the following combination of test parameters: The blunt point–sphere electrode configuration within the test vessel was set with a gap of 20 mm. The blunt point electrode had a tip radius of 2 mm, while the sphere electrode had a radius of 5 mm. The test vessel contained 7.2 L of insulating oil to be tested. Being opaque, the test cell facilitated the detection of pre-breakdown light emissions using two fiber optics (FOs): one for detecting breakdown and another side illumination FO to observe pre-breakdown phenomena.
To determine the AC BDV, a set-up was employed in accordance with the standard of DIN EN IEC 60156:2024-90 05 [
10], utilizing partially spherical electrodes; compare
Figure 3. The diameter of the electrodes amounted to 36 mm and the electrodes were placed with a gap distance of 2 mm to ensure breakdown within the voltage limits of the measuring device.
2.2.2. Light and Current Measurement
Light and current measurements were conducted exclusively during the LI voltage tests to determine the inception voltage and breakdown characteristics. A 0.84Ω non-inductive shunt resistor was incorporated in series within the grounding path of the electrode arrangement for current measurement. Pre-breakdown light phenomena were captured using a side illumination FO, which converted the light emissions into an electrical signal through an optoelectronic transducer featuring an avalanche photodiode, and this signal was recorded with an oscilloscope.
2.2.3. Measurement Methodology
For both AC and the LI testing, the test cells were thoroughly cleaned and rinsed with the testing liquid. Following a gradual filling of the test cell, a resting period of 30 min was allowed to ensure that no gas bubbles formed, which could compromise the breakdown strength of the oil. Conditioning shots were conducted to establish the starting voltage for the LI tests (approximately 70% of BDV) and to eliminate any residual gas formation and particles on the electrode surfaces. The rising step method outlined in ASTM D3300-12 [
11] was employed for the LI voltage testing. In concrete terms, this means that the impulse voltage is increased at a constant rate of 10 kV/step from starting voltage with three impulses/step until the breakdown occurs. Between each impulse a waiting time of 1 min was ensured and after each breakdown 5 min of resting was implemented. In this way, 10 breakdowns of each liquid were determined. The determination of the AC BDV was conducted according to DIN EN IEC 60156:2024-05 [
10], utilizing a constant voltage increase of 2 kV/s and a waiting period of 5 min between breakdown events. The gap distance between the electrodes was chosen at 2 mm instead of the 2.5 mm specified in the standard, as for some liquids a breakdown in the new condition could not be achieved within the limits of the measuring device. A rest period of 30 min after filling the test vessel with the liquid again ensured the absence of gas bubbles. However, in deviation from the standard, the insulating liquid was only stirred for one minute after each breakdown and not during the voltage increase. For both voltage types, the BDV was assessed using Weibull distribution analysis, with a minimum of 10 breakdown events.
2.3. Chemical Parameter
2.3.1. Water Content
As a crucial indicator of the liquid’s condition, the water content in transformers is closely monitored. Elevated water levels contribute to the degradation of the transformer’s paper insulation [
12,
13] and lead to a reduction in BDV. This relationship is particularly significant for relative water content, as BDV decreases notably when water content exceeds 20% [
14]. Consequently, a higher absolute water content in polar liquids, such as esters, does not necessarily result in lower BDV, due to their greater solubility of water.
Absolute water content was determined using coulometric Karl–Fischer titration, while relative water content was assessed by measuring water activity with a capacitive thin-film polymer sensor.
The water saturation of each liquid was calculated by forming the relationship between the absolute and the relative water content. The water saturation of the liquids depends on the liquid’s ability to dissolve water by forming various bonding. The water absorption capacity is therefore directly dependent on the amount of polar components in the liquids [
15].
2.3.2. Total Acid Number
The total acid number (TAN) quantifies the presence of free acids in the insulating medium, making it a valuable indicator of the quality of an insulating liquid. During the oxidation process associated with aging, various products are generated that contribute to acidity. However, these products differ significantly in their corrosiveness, and TAN alone cannot accurately predict corrosivity under operational conditions [
16]. Low-molecular-weight acids can damage the transformer’s paper insulation, whereas high-molecular-weight acids, such as those produced in esters, may even enhance the service life of the insulating paper [
12,
13].
The TAN was measured potentiometrically in accordance with IEC 62021:2003 [
16], using a fixed endpoint of pH = 11.5. The quantity of potassium hydroxide (KOH) needed to neutralize one gram of the oil sample to this endpoint is reported as TAN in mg
KOH/g.
2.3.3. Viscosity
Since polymerization is a by-product of the aging process [
17,
18] and leads to increased viscosity, which directly impacts the cooling capacity of the insulating liquid, viscosity is monitored throughout the aging period. Measurements were taken using capillary viscometry at 40 °C.
2.3.4. Color
The color of the insulating liquid undergoes significant changes throughout the lifespan of a transformer. Color variations were observed and recorded visually following sampling.
3. Degree of Homogeneity
A numerical field simulation utilizing COMSOL Multiphysics was conducted to assess the degree of homogeneity for the chosen electrode configuration and gap distance. The degree of homogeneity, denoted as
, is defined in Equation (
1), where
represents the average electric field strength and
indicates the maximum field strength.
For the chosen blunt point–sphere electrode arrangement, as illustrated in
Figure 4, the degree of homogeneity was computed to be
= 0.207.
The asymmetry of the electrode configuration is expected to produce a slight polarity effect under LI voltage.
4. Aging of Insulating Liquids
In general, degradation could be seen in all investigated liquids. In esters, the degradation appears mainly in form of hydrolysis, where water is consumed and ester molecules are split into fatty acids and alcohol [
19]. A higher water content accelerates hydrolysis, which increases the TAN [
19]. Free fatty acids can again polymerize, which leads to longer molecular chains that contribute to increasing viscosity [
20,
21]. In MO, oxidation processes of double bonds of olefins and aromatic compounds are the main degradation factor [
22]. The cross-linking of oxidized oil molecules leads to the formation of X-wax and sludge [
23].
In
Table 1 the aging parameters of the absolute and relative water content and water saturation, as well as the TAN and the viscosity, are summarized. When analyzing the measured aging parameters (see
Table 1 and
Figure 5,
Figure 6,
Figure 7 and
Figure 8), the relative water content for non-aged liquids reached a maximum of 10%. Aged NEs displayed similar relative water levels, ranging from 7% to 8%, with FR3 having a higher absolute water content. This indicates that FR3 has greater water solubility, potentially due to its higher concentration of polar molecules, as suggested by the elevated TANv. The aged SEs showed a relative water content of approximately 4%, with both having comparable absolute water levels. Only in MO did the relative water content increase beyond 20% in the aged sample.
Figure 5 illustrates the progression of the absolute water content. After aging, the NEs and SEs exhibited comparable absolute water levels. The highest water content, across both aging stages, was observed in the NE FR3, while the lowest was detected in the MO Nytro Taurus. However, Nytro Taurus had the highest relative water content due to its significantly lower water solubility. Throughout the aging process, the water saturation in Nytro Taurus approximately doubled, increasing from 50ppm in the non-aged state to 100ppm after 3 or 6 months of aging. As stated before a high absolute water content accelerates hydrolysis [
19], leading to a higher amount of acids. This correlation can be most clearly seen for the NE FR3 with a high water content during aging and simultaneously the highest TAN; compare
Figure 5 and
Figure 6.
Figure 6 shows the evolution of the TAN. In the non-aged state, TAN levels for the NEs and SE MIDEL 7131 were below 0.04 mg
KOH/g. For Nycodiel and Nytro Taurus, TAN values were below the titrator’s detection limit of 0.01 mg
KOH/g. With extended aging, TAN increased markedly for all liquids. After 2330 h, MIDEL 7131 had the highest TAN at 0.64 mg
KOH/g, while Nycodiel exhibited the lowest, with only one-tenth of
MIDEL 7131’s value despite their similar composition and structure. After 6 months, FR3 showed the highest TAN at 1.36 mg
KOH/g, and Nytro Taurus the lowest. However, a higher TAN does not necessarily indicate faster deterioration of the insulation, as TAN alone does not account to the corrosiveness of the acids. After 6 months of aging, the TAN of NEs surpassed the 0.5 mg
KOH/g safety threshold, according to [
24]. TAN limits for SEs during the service life time of operational transformers have yet to be clearly defined.
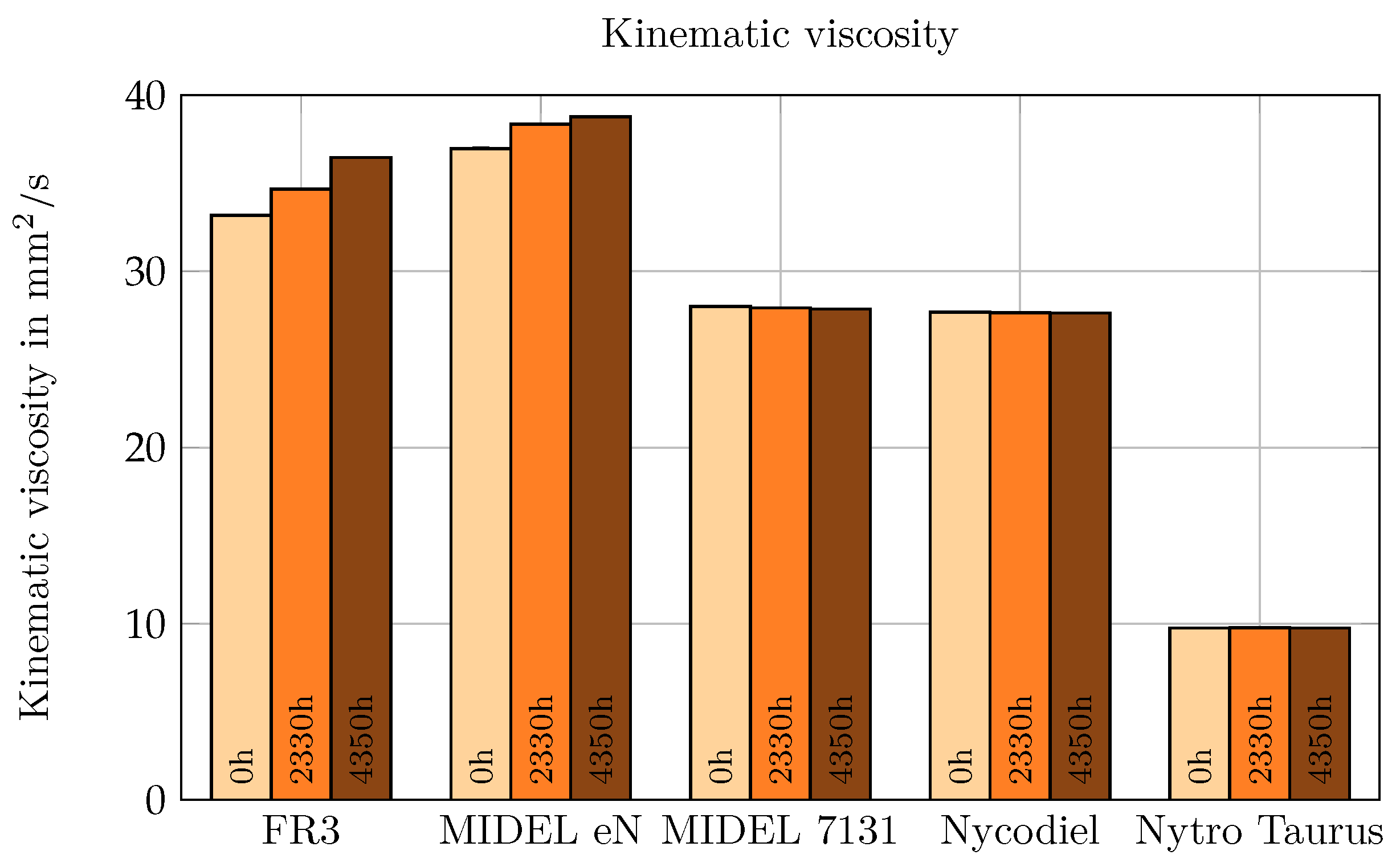
As shown in
Figure 7, viscosity increase during aging was observed only in the NEs. The most significant rise occurred in FR3, with a 10% increase in viscosity. MIDEL eN exhibited a 5% rise after 4350 h of aging. In contrast, the viscosity of the remaining oils showed no change. Viscosity is closely linked to chain length, the degree of unsaturated bonds, and the structure of hydrocarbon chains [
25]. Natural esters, with chain lengths of up to 18 carbon atoms, have the longest carbon chains and the highest proportion of unsaturated fatty acids [
26]. In synthetic esters, chain lengths are deliberately kept short, resulting in lower viscosity [
27]. Mineral oil exhibits the lowest viscosity due to its high content of cyclic alkanes [
23,
28]. Among these liquids, only natural esters show a noticeable increase in viscosity during aging. It is suggested that oxidation leads to the formation of acids, which undergo secondary reactions to polymerize into longer-chain molecules, thus increasing viscosity [
29].
The color change during aging is illustrated in
Figure 8. Initially, all liquids were transparent, with FR3 showing a slight green hue, and the other liquids having a faint yellow tint. Over time, after 3 months of aging, the esters developed a strong yellow color, while the MO turned dark brown. By the end of 6 months, FR3 and MIDEL 7131 had taken on a golden-brown appearance. The color of MIDEL eN, Nycodiel, and Nytro Taurus showed little to no change throughout the aging process. Soot formation was observed only in the MO. Within the ester liquids, FR3 and MIDEL 7131 showed the most significant change in color after 6 months, as well as the highest formation of acids during the aging, as seen in
Figure 6 and described before. It is therefore assumed that the change in color is linked to the formation of acids within the liquids.
5. Breakdown Voltage
To determine the BDV under both LI and AC, at least 10 breakdowns were recorded for each set-up. The results were evaluated using Weibull distribution, where the reported voltage represents the 50%-BDV.
5.1. Lightning Impulse Voltage
BDV was determined following the rising voltage method as outlined in ASTM [
11]. Current and light measurements were employed to assess the inception voltage, with streamers considered to initiate once current and light were detected.
Figure 9 displays the LI BDV for positive polarity. For the SE MIDEL 7131, breakdown was only achieved in three out of ten events before reaching the limit of the LI generator of 560 kV, indicating that the BDV exceeded 560 kV, and the graph is open-ended. For the remaining liquids, BDV was successfully determined. MIDEL 7131 exhibited the highest BDV in both non-aged and aged (2330 h) conditions, with the SEs showing the greatest breakdown resistance when non-aged. Both the NEs and MO exhibited a BDV between 430 kV and 440 kV. When comparing the aged conditions, SEs demonstrated consistent performance, displaying a superior resistance to breakdown in all cases. The lowest BDV, 385 kV, was found in the NE MIDEL eN after 4350 h of aging. Breakdown under positive LI for MO was comparable to that of the NEs.
Despite the high absolute BDV under positive LI, the SEs exhibited the most pronounced degradation during aging. Nycodiel experienced an 11% BDV decrease, while MIDEL 7131 showed an average reduction of at least 24%. In contrast, the MO Nytro Taurus showed negligible BDV change despite significant visual and measured chemical and physical aging, with an average BDV reduction of just 3%.
Streamer inception was observed in the NE FR3 when new, slightly below the BDV. In cases where streamer inception voltage differed from BDV, a slight opaque offset is depicted in
Figure 9, with inception voltage consistently lower than BDV. Streamer inception was noted in aged NEs, and most notably in aged MO, while it was barely detected in SEs under positive LI voltage. In aged MIDEL 7131, inception voltage closely aligned with the BDV.
In general, it was found across all liquids that there was no difference between inception voltage and BDV for new liquids. Therefore, the streamer tend to be initiation driven [
30]. That means that once a streamer is initiated it immediately leads to breakdown [
31]. However, in the cause of aging for NEs and MO the gap between initiation voltage and BDV increases, it leads to the assumption that for these aged liquids propagation driven streamer mechanisms [
30] are becoming more important for the breakdown processes.
The strongest resistance to breakdown under negative LI voltage in a semi-uniform field was observed in the SEs, irrespective of aging, as shown in
Figure 10. The NEs and MO displayed similar BDV values, ranging from 414 kV to 427 kV in new liquids, 410 kV to 417 kV after 3 months of aging, and 388 kV to 409 kV after 6 months aging period. In comparison, aged SEs exhibited BDVs between 462 kV and 473 kV (3 months aging time) and 452 KV to 461 kV (6 months aging time), with the BDV of Nycodiel even increasing during aging. The negative LI BDV in both new and moderately aged liquids was lower than for positive LI voltage.
Aging-related BDV reduction under negative LI was less than 10% across all insulating media, with Nycodiel showing an increase. This suggests that negative LI voltage in a semi-uniform, asymmetric field is less suitable than positive LI for identifying aging-related deterioration in insulating liquids.
The inception or initiation of streamer prior to breakdown under negative LI voltage, also depicted in
Figure 10, was observed in nearly all new, non-aged liquids, unlike under positive LI voltage. For aged esters, streamer inception could be observed in almost every case, however only to a very slight extent. The difference between BDV and inception voltage became smaller with aging, especially for FR3,
MIDEL 7131, and Nytro Taurus. Therefore, the shift in propagation mechanisms is seen to be from rather propagation driven to a more initiation driven process [
30] under negative polarity in semi-uniform field. This is the case for all three kinds of liquids.
This might imply that degradation by-products such as water or acids have a smaller impact on breakdown of the insulating liquids under negative LI voltage. However, in aged esters, streamer inception occurred at lower voltages under negative LI voltage.
Despite the BDV not changing significantly in the cause of aging for each liquid, different streamer mechanisms seem to play a role as the relationship between initiation and the propagation-driven streamer mechanism [
30] is shifting throughout the aging time. The degradation of the liquids has different influences under the opposing polarities. The consumption of aromatic compounds in the MO Nytro Taurus, and thus the degradation of compounds with electron-scavenging and electron-releasing properties [
32], leads to different mechanisms of streamer propagation at positive and negative polarity. The same can observed for the degradation of double bonds, polymerization, and increase in absolute water content, mainly in NEs. It could be clearly shown that the aging processes have an effect on the propagation mechanisms of the various liquids investigated under LI voltage in a semi-uniform field. For a deeper understanding of the liquids in operation, these realistic conditions should be investigated and considered. However, a major effect of aging on the BDV of the respective liquids could not be demonstrated.
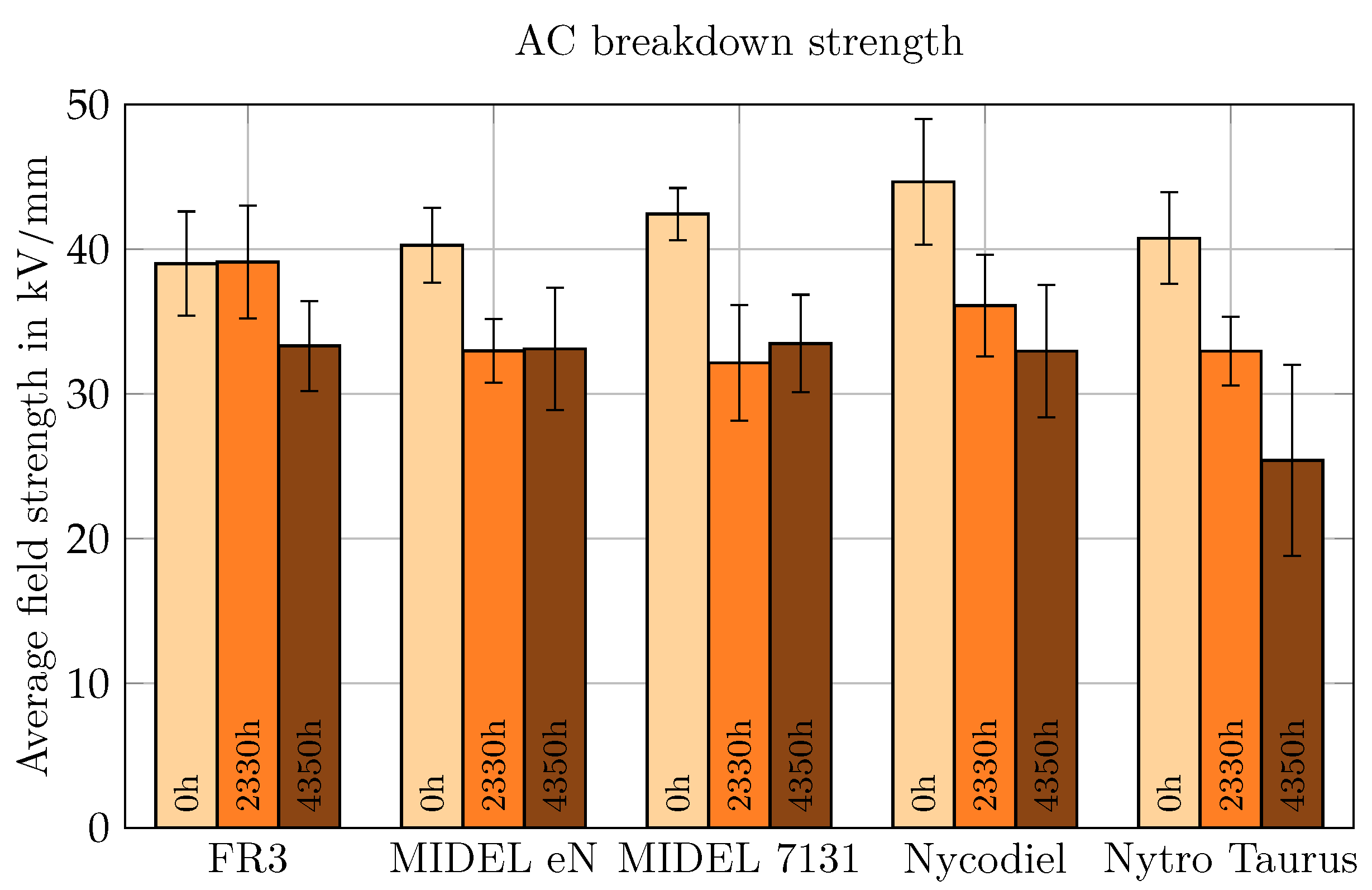
5.2. Alternating Current Voltage
For AC voltage testing, the average electric field strength was selected for comparison of breakdown values. The AC measurement results are shown in
Figure 11. The breakdown performance of the non-aged NEs and MO was similar, with electric field strengths between 39 kV/mm and 40.8 kV/mm. SEs demonstrated greater resistance to breakdown. Unlike the behavior under LI voltage, Nycodiel exhibited the highest AC BDV, with an average field strength of 44.7 kV/mm.
After aging, all liquids showed a reduction in breakdown field strength, except for FR3 aged for 2330 h or 3 months. The breakdown strength of aged esters did not significantly differ from one another, while the MO experienced the most substantial decline. The presence of water and soot in MO, as illustrated in
Figure 8, significantly affected its AC breakdown performance. After 4350 h of aging, the average field strength of MO was 38% lower than in its non-aged state. In comparison, the reduction in field strength for the other liquids after aging was 15% for FR3 and 18% for MIDEL eN. For the SEs, a 21% decrease was observed for MIDEL 7131 and a 26% decline for Nycodiel.
5.3. Comparison of Breakdown Behavior
Each liquid displayed varying tolerances to different types of voltage stress after aging-related degradation. For most, the AC voltage testing resulted in greater stress when comparing aged conditions to their respective non-aged states. The here presented findings indicate that negative LI voltage is the least effective for detecting fluid degradation. In asymmetric semi-homogeneous fields under positive LI voltage, aging is more apparent through a decrease in BDV. When comparing AC and LI breakdown results for MIDEL 7131, aging effects were more pronounced under LI voltage (>24%) than for AC (21%). Conversely, for Nytro Taurus, the BDV under positive LI voltage only dropped by 3% after aging compared to a 38% reduction under AC voltage.
6. Time to Breakdown
The time to breakdown was used to compare the breakdown behavior for the different polarities under LI voltage and examine the influence of the aging procedure on the ability to withstand high voltages. It has to be noted that the time to breakdown differs from the propagation time of a streamer. As it was not possible with the existing measurement set-up to distinguish between propagation current and noise due to the trigger event at the very beginning of the LI, the point of inception could not be determined exactly. Therefore, time to breakdown states the time from the voltage rise of the LI to the point of time when the voltage collapses and current rapidly increases. It can be assumed that a later time to breakdown also accounts for slower streamer propagation as breakdown occurs at lower voltages at the tail of the LI.
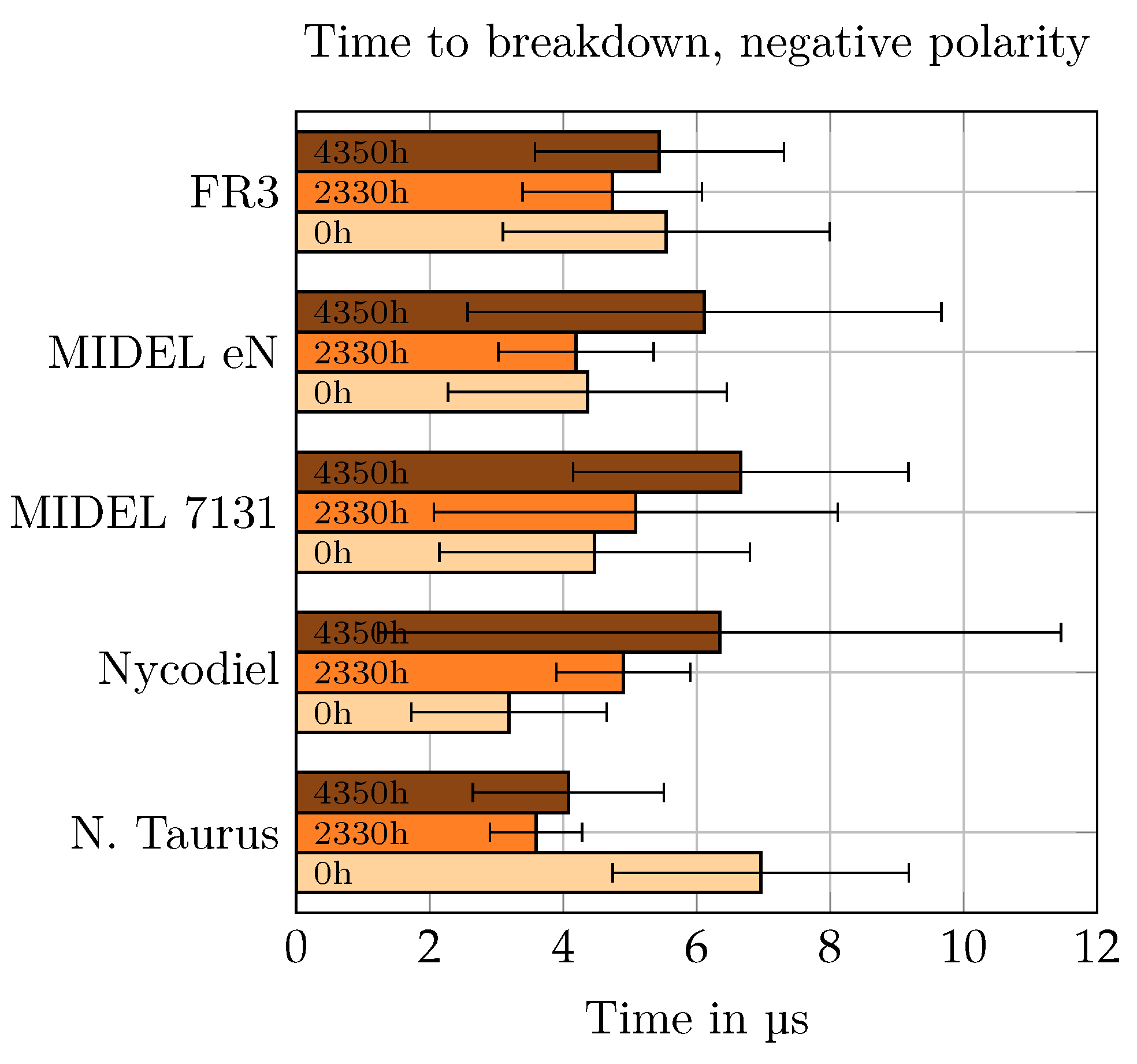
In
Figure 12 and
Figure 13, the average time to breakdown for each oil, aging condition, and polarity is displayed.
It could be observed that isolated breakdowns up to about 20 μs have taken place. However, most breakdowns occurred within the first 10 μs of the LI, except for breakdowns in esters aged for 4350 h under negative polarity. There, the scattering of the data was largest and more breakdowns appeared after 10 μs of the LI. In general, the scattering of the time to breakdown was high, but in most cases it was higher under negative polarity. The scattering in MO seemed to be lower than for the ester liquids. When in the following comparison of the time to breakdown, it is referred to the average value.
For positive polarity, no clear trend could be examined when observing the influence of aging or differences between the liquids. A slight correlation could be seen in the data when analyzing new oils and the longest aged oils. For the SEs, time to breakdown decreased with longer aging period, whereas the data suggested that for the NEs time to breakdown increased with longer aging period. For negative polarity, as described before, scattering was highest in esters aged for 4350 h. Late breakdowns happened regardless of the voltage level, so the breakdown became more unpredictable. Due to late breakdowns, the average time to breakdown increased with longer aging period for the ester liquids, whereas the MO has shorter time to breakdown for the aged oil.
As stated in [
4,
33], streamers under positive polarity are growing more rapidly due to their higher conductivity. For new oils, this is only the case for the NEs and the MO. For SEs, average time to breakdown is higher for positive polarity. When aged, breakdowns are achieved slightly faster for positive polarity for the ester liquids, as this would have been the case in divergent fields. For the MO, this trend could not be observed. Due to their change in molecular structure by cause of aging-related degradation, breakdown under negative polarity in esters happened later during the LI than in new esters. Again, this was not the case for MO.
7. Conclusions
After catalytically and thermally aging the insulating liquids for 2330 h (3 months) and 4350 h (6 months), degradation of the liquids was visually evident, particularly in the color change, which was most remarkably observed in MO. The absolute water content increased over time, though the relative water content of NEs and SEs remained low, below 10%, after aging. However, MO showed a significant increase in water content, exceeding 20%, which is a level of water saturation that negatively impacts the AC breakdown strength [
14]. In contrast, the higher water content in MO did not similarly affect LI breakdown strength. The TAN increased significantly for all oils during aging, with esters showing higher TAN values due to their chemical structure. FR3 and MIDEL 7131 had the highest TAN, though this did not correlate with a substantial decline in breakdown values. MO had the lowest TAN, remaining below the critical threshold of 0.3 mg
KOH/g [
34]. Viscosity increased by up to 10% in NEs only, while the other liquids showed no significant change in viscosity during aging.
Testing aged insulating liquids under LI voltage is recommended as this simulates the stress caused by switching faults or lightning overvoltage, which are worst case scenarios for power transformers. Especially in power transformers, failure caused by impulse overvoltage must be completely avoided. It is essential that the electrode and field geometry replicate a realistic semi-uniform field to ensure reliable results. The breakdown performance of an insulating liquid under LI voltage can degrade more compared to its new condition than under AC voltage stress over the operating lifetime, as seen with MIDEL 7131. However, when time and resources are limited, this study indicates that a standard AC breakdown test provides a reasonable estimate of the liquid’s degradation. Negative LI voltage, especially in asymmetric semi-uniform fields, as used in this study, proved less effective for detecting aging-related reductions in breakdown voltage.
Due to the high scattering of the data, findings from the time to breakdown are limited. Scattering for negative polarity LI voltage and longest-aged oil samples is highest, meaning that breakdown is least predictable. SEs have higher average time to breakdown in positive polarity in new oils, whereas it is less under positive polarity for the remaining oils. In esters under negative polarity, breakdown occurred later along the impulse when the liquid deteriorated due to aging. In mineral oil, it was reverse.
With the increasing introduction of ester-based insulating liquids for power transformers, the understanding of prebreakdown and breakdown phenomena are still yet to be completely understood. Therefore, reliable testing methods are essential, particularly those applicable to real transformer conditions, to assess the performance and condition of esters. Both new and aged insulating media must be evaluated and rated based on their breakdown performance under testing conditions that replicate worst-case stresses and realistic field geometries.
As in power transformers, the oil gaps are mostly wider than the 20 mm for LI testing used here; more research needs to be carried out for longer gap distances, however, while keeping the degree of homogeneity (
) above 0.19 [
2]. Higher voltage levels will be required for this. Yet, it is not predictable how the breakdown voltage and the time to breakdown will be affected when up-scaling the gap distance and the electrode size due to the influence of the volume effect or the surface effect. Further investigations must be conducted in this field to obtain as complete a representation of realistic electrode geometries as possible to ensure their applicability to power transformers.
For the design and dimensioning of transformers, the measurement of insulating liquids under LI voltage in a semi-uniform field is extremely important, especially in comparison to mineral oil. In addition, it serves to gain essential knowledge for different liquids with regard to the breakdown processes and their influencing factors in the semi-uniform field. Regarding the assessment of the progress of aging, the degradation of the fluid under AC has a much greater influence than with LI and can therefore be considered as a marker for aging.