An Overview of Different Water Electrolyzer Types for Hydrogen Production
Abstract
1. Introduction
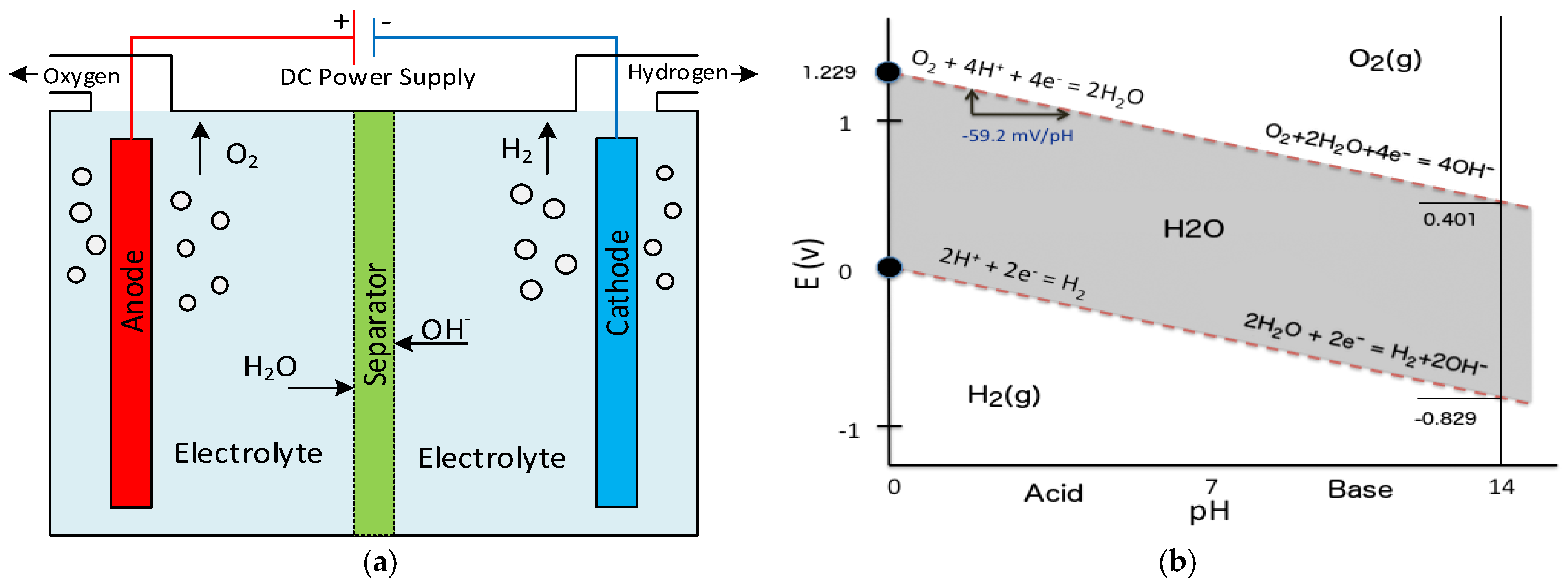
2. The Fundamentals of Electrolysis Processes
Cathode (reduction): 2 H + (aq) + 2e− → H2(g) Eo = 0.00 V
Overall reaction: 2 H2O(L) → 2 H2(g) + O2(g) Eocell = −1.23 V
3. Main Electrolysis Types

4. Electrolysis Materials
4.1. Electrolyte
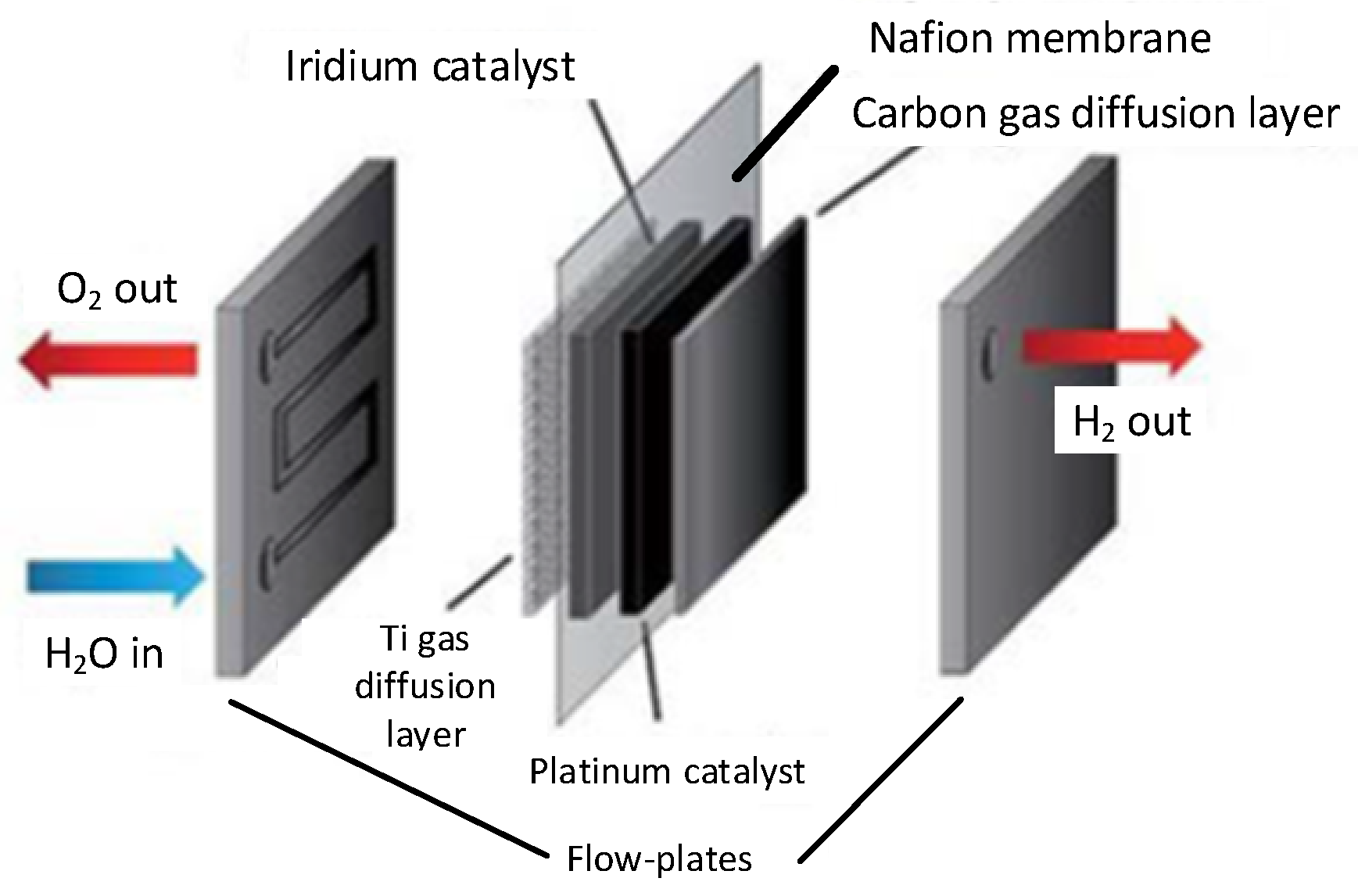
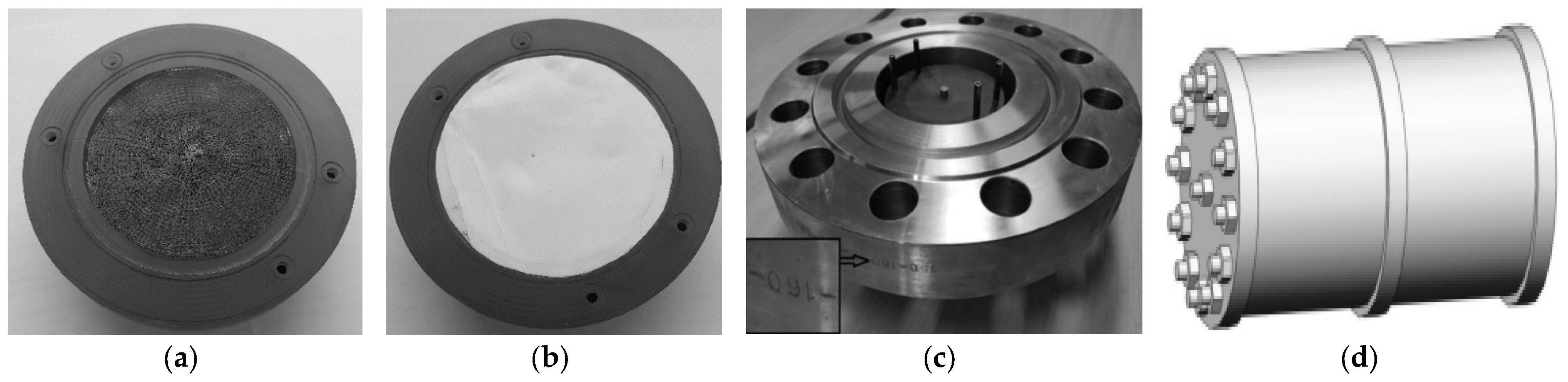
4.2. Electrode
- Minimizes ohmic drops, for high conductivity of electrons and ions.
- The catalyst is in contact with the aqueous phase, known as high wettability.
- The catalyst has a high surface.
- Low amount of bubble-blocked pores and coverage of the catalyst.
- Gases and electrolytes have high permeability to ease mass transport.
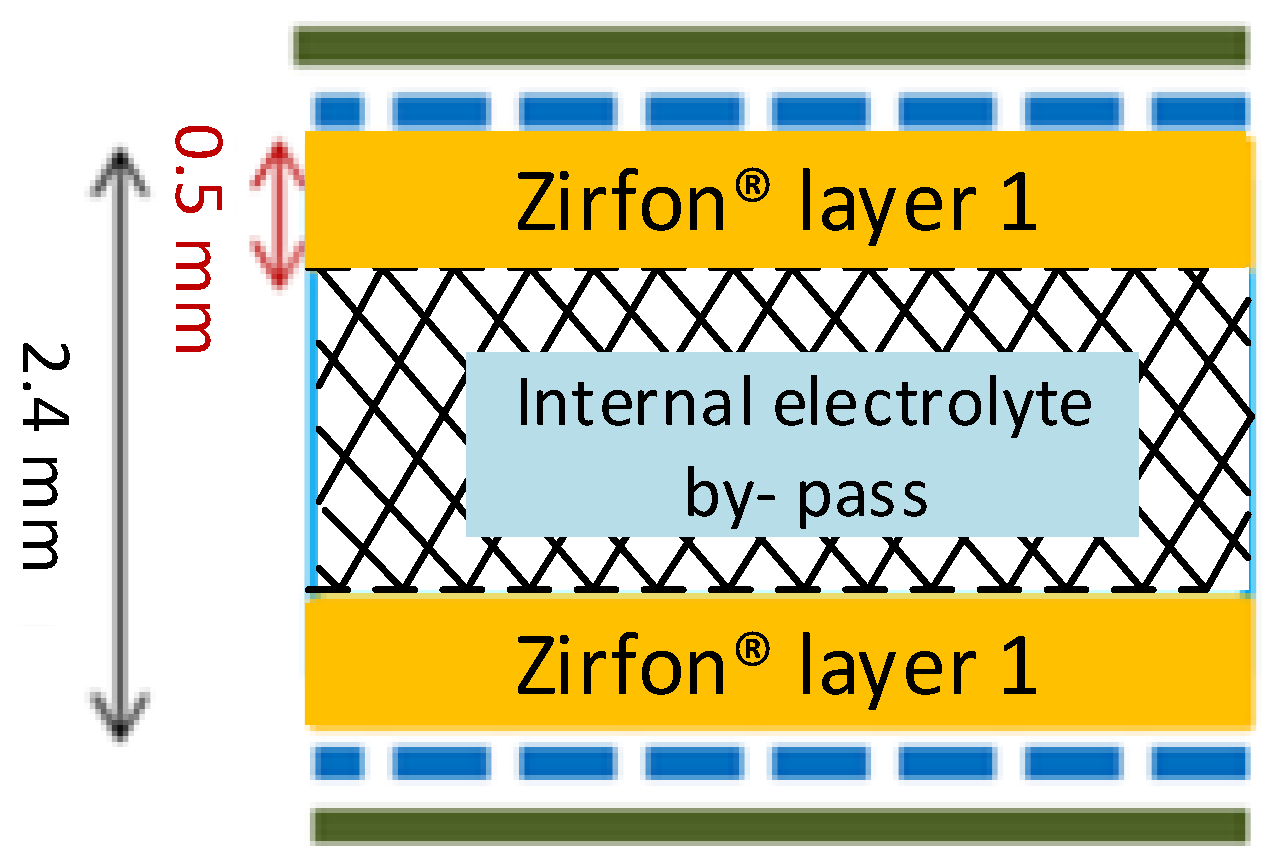
4.3. Separator
5. Industrial Applications for Electrolyzers
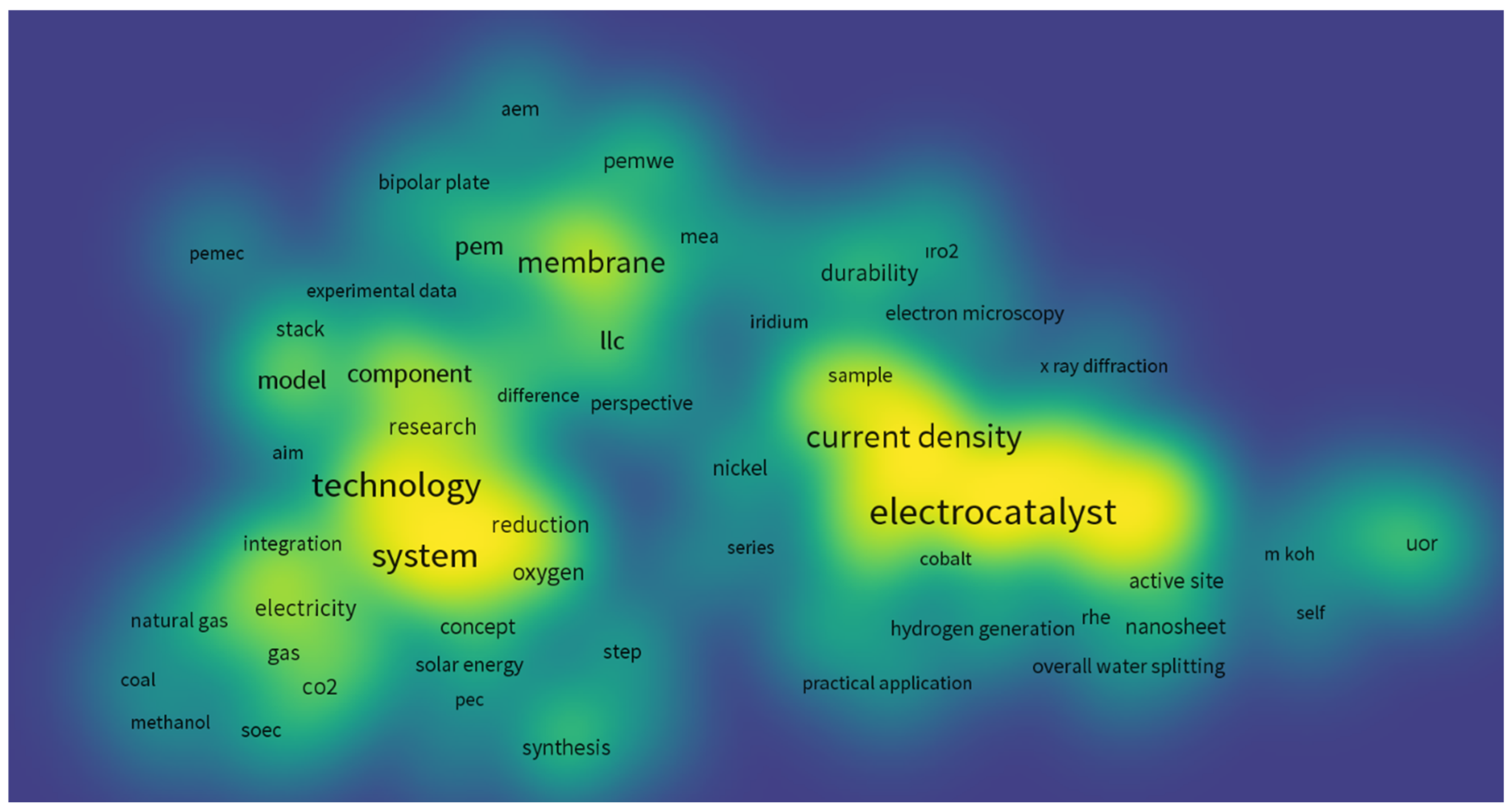
6. Bibliometric WOS Analyses with VOSviewer Software
7. Conclusions
Funding
Conflicts of Interest
References
- Kundu, A.; Sahu, J.N.; Redzwan, G.; Hashim, M.A. An overview of cathode material and catalysts suitable for generating hydrogen in microbial electrolysis cell. Int. J. Hydrogen Energy 2013, 38, 1745–1757. [Google Scholar] [CrossRef]
- Stojić, D.L.; Marčeta, M.P.; Sovilj, S.P.; Miljanić, Š.S. Hydrogen generation from water electrolysis possibilities of energy-saving. J. Power Sources 2003, 118, 315–319. [Google Scholar] [CrossRef]
- Şahin, M.E. An Efficient Solar-Hydrogen DC-DC Buck Converter System with Sliding Mode Control. El-Cezeri 2019, 6, 558–570. [Google Scholar] [CrossRef]
- Vartiainen, E.; Oy, F.G.; Breyer, C.; Moser, D.; Medina, E.R.; Busto, C.; Jäger-Waldau, A. True Cost of Solar Hydrogen–Levelised Cost of Hydrogen in Europe 2021–2050. In Proceedings of the 38th European Photovoltaic Solar Energy Conference and Exhibition, Online, 6–10 September 2021; pp. 1601–1607. [Google Scholar]
- Backhus, A.O. Hydrogen Energy; Nova Science Publications: New York, NY, USA, 2006. [Google Scholar]
- Scott, K. Introduction to Electrolysis, Electrolysers and Hydrogen Production. 2019. Available online: https://books.rsc.org/books/edited-volume/789/chapter/525233/Introduction-to-Electrolysis-Electrolysers-and (accessed on 26 October 2021).
- Pletcher, D.; Li, X. Prospects for alkaline zero gap water electrolysers for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 15089. [Google Scholar] [CrossRef]
- Rashid, M.D.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen production by water electrolysis: A review of alkaline water electrolysis, PEM water electrolysis and high-temperature water electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 2249–8958. [Google Scholar]
- Eroğlu, İ. Hidrojen çağının getirdiği yenilikler. FİGES Arge Derg. 2021, 9, 5–13. [Google Scholar]
- Lessing, P.A. Materials for hydrogen generation via water electrolysis. J. Mater. Sci. 2007, 42, 3477–3487. [Google Scholar] [CrossRef]
- Dos Santos, K.G.; Eckert, C.T.; De Rossi, E.; Bariccatti, R.A.; Frigo, E.P.; Lindino, C.A.; Alves, H.J. Hydrogen production in the electrolysis of water in Brazil, a review. Renew. Sustain. Energy Rev. 2017, 68, 563–571. [Google Scholar] [CrossRef]
- Naimi, Y.; Antar, A. Hydrogen generation by water electrolysis. In Advances in Hydrogen Generation Technologies; InTechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Yünkül, F. Yeni bir Elektrod Hazırlayarak Alternatif Akımla Suyun Elektrolizi. Master’s Thesis, Yıldız Technical University, İstanbul, Turkey, 2011. [Google Scholar]
- Yuzer, B.; Selçuk, H.; Chehade, G.; Demir, M.E.; Dincer, I. Evaluation of hydrogen production via electrolysis with ion-exchange membranes. Energy 2020, 190, 116420. [Google Scholar] [CrossRef]
- Marangio, F.; Santarelli, M.; Calì, M. Theoretical model and experimental analysis of a high-pressure PEM water electrolyser for hydrogen production. Int. J. Hydrogen Energy 2009, 34, 1143–1158. [Google Scholar] [CrossRef]
- Marangio, F.; Pagani, M.; Santarelli, M.; Cali, M. Concept of a high pressure PEM electrolyser prototype. Int. J. Hydrogen Energy 2011, 36, 7807–7815. [Google Scholar] [CrossRef]
- Mutlu, R.N.; Kucukkara, I.; Gizir, A.M. Hydrogen generation by electrolysis under subcritical water condition and the effect of the aluminium anode. Int. J. Hydrogen Energy 2020, 45, 12641–12652. [Google Scholar] [CrossRef]
- Yan, Z.C.; Li, C.; Lin, W.H. Hydrogen generation by glow discharge plasma electrolysis of methanol solutions. Int. J. Hydrogen Energy 2009, 34, 48–55. [Google Scholar] [CrossRef]
- Schalenbach, M.; Zeradjanin, A.R.; Kassian, O.; Cherevko, S.; Mayrhofer, K.J. A perspective on low-temperature water electrolysis–challenges in alkaline and acidic technology. Int. J. Electrochem. Sci. 2018, 13, 1173–1226. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, B.; Wang, P.; Liu, S. Hydrogen generation with acid/alkaline amphoteric water electrolysis. J. Energy Chem. 2019, 38, 162–169. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Avcıoğlu, A.O. Renewable Energy Sources and Technologies; Course Presentations; Ankara University: Ankara, Turkey, 2017. [Google Scholar]
- Olivier, P.; Bourasseau, C.; Bouamama, P.B. Low-temperature electrolysis system modelling: A review. Renew. Sustain. Energy Rev. 2017, 78, 280–300. [Google Scholar] [CrossRef]
- Bender, G.; Dinh, H.N.; Danilovic, N.; Weber, A. HydroGEN: Low-Temperature Electrolysis; 2018 DOE Annual Merit Review. 2018. Available online: https://www.nrel.gov/docs/fy19osti/73769.pdf (accessed on 1 September 2024).
- Zumdahl, S.S.; Zumdahl, S.A. Chemistry, 9th ed.; Cengage Learning: Belmont, CA, USA, 2013; p. 30. [Google Scholar]
- Şahin, M.E. A photovoltaic powered electrolysis converter system with maximum power point tracking control. Int. J. Hydrogen Energy 2020, 45, 9293–9304. [Google Scholar] [CrossRef]
- Şahin, M.E.; Okumuş, H.İ.; Aydemir, M.T. Implementation of an electrolysis system with DC/DC synchronous buck converter. Int. J. Hydrogen Energy 2014, 39, 6802–6812. [Google Scholar] [CrossRef]
- Anonime. Pourbaix Diagram. Available online: https://en.wikipedia.org/wiki/Pourbaix_diagram (accessed on 1 February 2022).
- Stolten, D. Hydrogen Science and Engineering: Materials, Processes, Systems and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 898. ISBN 9783527674299. [Google Scholar]
- Gallandat, N.; Romanowicz, K.; Züttel, A. An analytical model for the electrolyser performance derived from materials parameters. J. Power Energy Eng. 2017, 5, 34–49. [Google Scholar] [CrossRef]
- Anonime. High-Temperature Electrolysis. Available online: https://en.wikipedia.org/wiki/High-temperature_electrolysis (accessed on 1 February 2022).
- Leng, Y.; Chen, G.; Mendoza, A.J.; Tighe, T.B.; Hickner, M.A.; Wang, C.Y. Solid-state water electrolysis with an alkaline membrane. J. Am. Chem. Soc. 2012, 134, 9054–9057. [Google Scholar] [CrossRef] [PubMed]
- Raoof, J.B.; Ojani, R.; Esfeden, S.A.; Nadimi, S.R. Fabrication of bimetallic Cu/Pt nanoparticles modified glassy carbon electrode and its catalytic activity toward hydrogen evolution reaction. Int. J. Hydrogen Energy 2010, 35, 3937–3944. [Google Scholar] [CrossRef]
- IRENA. Green Hydrogen Cost Reduction: Scaling Up Electrolysers to Meet the 1.5 °C Climate Goal; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- Bouzek, K. Anion Selective Membranes for Alkaline Water Electrolysis. Presentation, University of Chemistiry and Technology. Prageua. Available online: https://elyntegration.eu/wp-content/uploads/polymer-electrolytes-for-ael-bouzek.pdf (accessed on 1 February 2022).
- Holladay, J.D.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Li, C.; Baek, J.B. The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy 2021, 87, 106162. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low-cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Anderson, E. Green Hydrogen Production Using Low-Temperature Water Electrolysis, Greening High Temperature Manufacturing BU-ISE/ITIF Workshop. Nel. 27 January 2021. Available online: https://www.bu.edu/igs/files/2021/01/E-Anderson_Nel-LT-ELY-Overview_BU-ISE_ITIF-Wkshp_20210127.pdf (accessed on 1 September 2024).
- Anonime. Water Electrolyses. Available online: https://en.wikipedia.org/wiki/Electrolysis_of_water (accessed on 1 February 2022).
- Haynes, W.M. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Badwal, S.P.S.; Giddey, S.; Munnings, C. Hydrogen production via solid electrolytic routes. WIREs Energy Environ. 2012, 2, 473–487. [Google Scholar] [CrossRef]
- Wang, Y.; Narayanan, S.R.; Wu, W. Field-Assisted Splitting of Pure Water Based on Deep-Sub-Debye-Length Nanogap Electrochemical Cells. ACS Nano 2017, 11, 8421–8428. [Google Scholar] [CrossRef]
- Alternative Electrochemical Systems for Ozonation of Water. NASA Tech Briefs (Technical Report); NASA. 20 March 2007; MSC-23045. Available online: https://ntrs.nasa.gov/api/citations/20110023852/downloads/20110023852.pdf (accessed on 1 September 2024).
- Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J.; Guan, M.; Lin, M.-C.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Goni-Urtiaga, A.; Presvytes, D.; Scott, K. Solid acids as electrolyte materials for proton exchange membrane (PEM) electrolysis. Int. J. Hydrogen Energy 2012, 37, 3358–3372. [Google Scholar] [CrossRef]
- Haverkort, J.W.; Rajaei, H. Voltage losses in zero-gap alkaline water electrolysis. J. Power Sources 2021, 497, 229864. [Google Scholar] [CrossRef]
- Jiang, T. Development of Alkaline Electrolyzer Electrodes and Their Characterization in Overall Water Splitting. Ph.D. Thesis, Université Bourgogne Franche-Comté, Dijon, France, 2020. [Google Scholar]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.-J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, L.; Nordlund, D.; Chen, H.; Fan, L.; Zhang, B.; Sheng, X.; Daniel, Q.; Sun, L. Dendritic core-shell nickel-iron-copper metal/metal oxide electrode for efficient electrocatalytic water oxidation. Nat. Commun. 2018, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Brauns, J.; Schönebeck, J.; Kraglund, M.R.; Aili, D.; Hnát, J.; Žitka, J.; Mues, W.; Jensen, J.O.; Bouzek, K.; Turek, T. Evaluation of diaphragms and membranes as separators for alkaline water electrolysis. J. Electrochem. Soc. 2021, 168, 014510. [Google Scholar] [CrossRef]
- Şahin, M.E.; Okumuş, H.İ. Hydrogen production system design with synchronous buck converter. In Proceedings of the National Conference on Electrical, Electronics and Computer Engineering, Bursa, Turkey, 2–5 December 2010; pp. 58–61. [Google Scholar]
- Şahin, M.E. Designing an electrolysis system with DC/DC buck converter. Master’s Thesis, Gazi University, Ankara, Turkey, 2006. [Google Scholar]
- Vermeiren, P.; Adriansens, W.; Moreels, J.P.; Leysen, R. Evaluation of the Zirfon® separator for use in alkaline water electrolysis and Ni-H2 batteries. Int. J. Hydrogen Energy 1998, 23, 321–324. [Google Scholar] [CrossRef]
- Rajput, A.; Kundu, A.; Chakraborty, B. Recent Progress on Copper-Based Electrode Materials for Overall Water-Splitting. ChemElectroChem 2021, 8, 1698–1722. [Google Scholar] [CrossRef]
- Liu, C.; Colón, B.C.; Ziesack, M.; Silver, P.A.; Nocera, D.G. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 2016, 352, 1210–1213. [Google Scholar] [CrossRef]
- Kale, S.B.; Babar, P.T.; Kim, J.-H.; Lokhande, C.D. Synthesis of one dimensional Cu2S nanorods using a self-grown sacrificial template for the electrocatalytic oxygen evolution reaction (OER). New J. Chem. 2020, 44, 8771–8777. [Google Scholar] [CrossRef]
- Wendt, H.; Hofmann, H. Ceramic diaphragms for advanced alkaline water electrolysis. J. Appl. Electrochem. 1989, 19, 605–610. [Google Scholar] [CrossRef]
- NHT Corp. Nickel Mesh for Water Electrolysis Hydrogen Production. Available online: https://nickelgreen.com/nickel-mesh-for-water-electrolysis-hydrogen-production/ (accessed on 24 September 2024).
- Yeo, R.S.; McBreen, J.; Kissel, G.; Kulesa, F.; Srinivasan, S. Perfluorosulphonic acid (Nafion) membrane as a separator for an advanced alkaline water electrolyser. J. Appl. Electrochem. 1980, 10, 741–747. [Google Scholar] [CrossRef]
- Sodaye, H.S.; Bindal, R.C.; Dey, T.K.; Misra, B.M. Membrane separator for water electrolysis: Characterization. Int. J. Polym. Mater. 2005, 54, 63–70. [Google Scholar] [CrossRef]
- Ahmad Kamaroddin, M.F.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Membrane-based electrolysis for hydrogen production: A review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.M.; Sequeira, C.A.; Figueiredo, J.L. Hydrogen production by alkaline water electrolysis. Química Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Conway, B.E.; Yeager, E.; White, R.E. Comprehensive Treatise of Electrochemistry; Plenum Press: New York, NY, USA, 1981. [Google Scholar]
- Kim, S.; Han, J.H.; Yuk, J.; Kim, S.; Song, Y.; So, S.; Lee, K.T.; Kim, T.-H. Highly selective porous separator with a thin skin layer for alkaline water electrolysis. J. Power Sources 2022, 524, 231059. [Google Scholar] [CrossRef]
- Schalenbach, M.; Lueke, W.; Stolten, D. Hydrogen diffusivity and electrolyte permeability of the Zirfon PERL separator for alkaline water electrolysis. J. Electrochem. Soc. 2016, 163, F1480. [Google Scholar] [CrossRef]
- Shiva, K.S.; Ramakrishna, S.U.B.; Srinivasulu, R.D.; Bhagawan, D.; Himabindu, V. Synthesis of Polysulfone and Zirconium Oxide Coated Asbestos Composite Separators for Alkaline Water Electrolysis. Chem. Eng. Process Technol. 2017, 3, 1035. [Google Scholar]
- Reissner, R.; Schiller, G.; Guelzow, E.; Gallego, Y.A.; Doyen, W.; Funke, A.; Vaes, J.; Bowen, J.R. Hydrogen from Regenerative Energy Power Sources: Pressurised alkaline electrolyser with high efficiency and wide operating range The Project “RESelyser”. In Proceedings of the 19th World Hydrogen Energy Conference 2012, Toronto, ON, Canada, 3–7 June 2012. [Google Scholar]
- Çelik, C. Hydrogen Energy. Available online: https://slideplayer.biz.tr/slide/17036615/ (accessed on 5 September 2023).
- Ruth, M.; Mayyas, A.; Mann, M. Manufacturing Competitiveness Analysis for PEM and Alkaline Water Electrolysis Systems, Fuel Cell Seminar and Energy Expo, 8 November 2017. Available online: https://www.nrel.gov/docs/fy19osti/70380.pdf (accessed on 14 September 2024).
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Leroy, R.L. Industrial water electrolysis—Present and future. Int. J. Hydrogen Energy 1983, 8, 401–417. [Google Scholar] [CrossRef]
- Kinoshita, K. Electrochemical Oxygen Technology, 1st ed.; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Xia, Y.; Cheng, H.; He, H.; Wei, W. Efficiency and consistency enhancement for alkaline electrolyzers driven by renewable energy sources. Commun. Eng. 2023, 2, 22. [Google Scholar] [CrossRef]
- Kuleshov, V.N.; Kuleshov, N.V.; Kurochkin, S.V.; Fedotov, A.A.; Sleptsova, E.E.; Blinov, D.V.; Gavriluk, A.A.; Zhmurko, I.E. Water electrolyzer for renewable energy systems. E3S Web Conf. 2021, 289, 05004. [Google Scholar] [CrossRef]
- Lipman, T.; Edwards, J.L.; Brooks, C. Renewable Hydrogen: Technology Review and Policy Recommendations for State-Level Sustainable Energy Futures. 2006. Available online: https://escholarship.org/uc/item/48w7f7z2 (accessed on 14 September 2024).
- Glatzmaier, G.; Blake, D.; Showalter, S. Assessment of Methods for Hydrogen Production Using Concentrated Solar Energy; NREL/TP-570-23629; National Renewable Energy Laboratory: Golden, CO, USA, 1998. [Google Scholar]
- Mann, M.K.; Spath, P.L.; Amos, W.A. Techno-economic Analysis of Different Options for the Production of Hydrogen from Sunlight, Wind, and Biomass. In Proceedings of the 1998 U.S. DOE Hydrogen Program Review, Alexandria, VA, USA, 28–30 April 1998. NREL/CP-570-25315. [Google Scholar]
- Nel ASA Corporation. Water Electrolysers/Hydrogen Generators. Available online: https://nelhydrogen.com/water-electrolysers-hydrogen-generators/ (accessed on 24 September 2023).
- ASPILSAN Corporation. Energy Storage Systems. Available online: https://www.aspilsan.com/en/cozum-kategori/enerji-depoloma/ (accessed on 24 September 2023).
- Electric Hydrogen Corporation. Our Timeline. Available online: https://eh2.com/about/#company (accessed on 14 September 2024).
- Angstrom Advanced Inc. Hydrogen Generation Products. Available online: https://www.angstrom-advanced.com/pro6-HGbyWE.html# (accessed on 14 September 2024).
- IRENA. Hydrogen: A Renewable Energy Perspective; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019. [Google Scholar]
- HFK. 3000 mL/min PEM Fuel Cell Hydrogen Generator Hydrogen Fuel Cell Hydrogen Electrolyzer. Available online: https://www.alibaba.com/product-detail/wholesale-3000ml-min-Pem-Fuel-Cell_1600888062721.html (accessed on 17 September 2024).
- Electric Hydrogen. The 100 MW Plant, Technical Specification Sheet. August 2024. Available online: https://eh2.com/wp-content/uploads/2024/08/Pre-NDA-Spec-Sheet-August-2023-1-3.pdf (accessed on 14 September 2024).
- Incer-Valverde, J.; Korayem, A.; Tsatsaronis, G.; Morosuk, T. “Colors” of hydrogen: Definitions and carbon intensity. Energy Convers. Manag. 2023, 291, 117294. [Google Scholar] [CrossRef]
- Sebbagh, T.; Şahin, M.E.; Beldjaatit, C. Green hydrogen revolution for a sustainable energy future. Clean Technol. Environ. Policy, 2024; 1–24, early access. [Google Scholar]
- Omid, M.A.; Şahin, M.E.; Cora, Ö.N. Challenges and future perspectives on production, storage technologies, and transportation of hydrogen: A review. Energy Technol. 2024, 12, 2300997. [Google Scholar] [CrossRef]






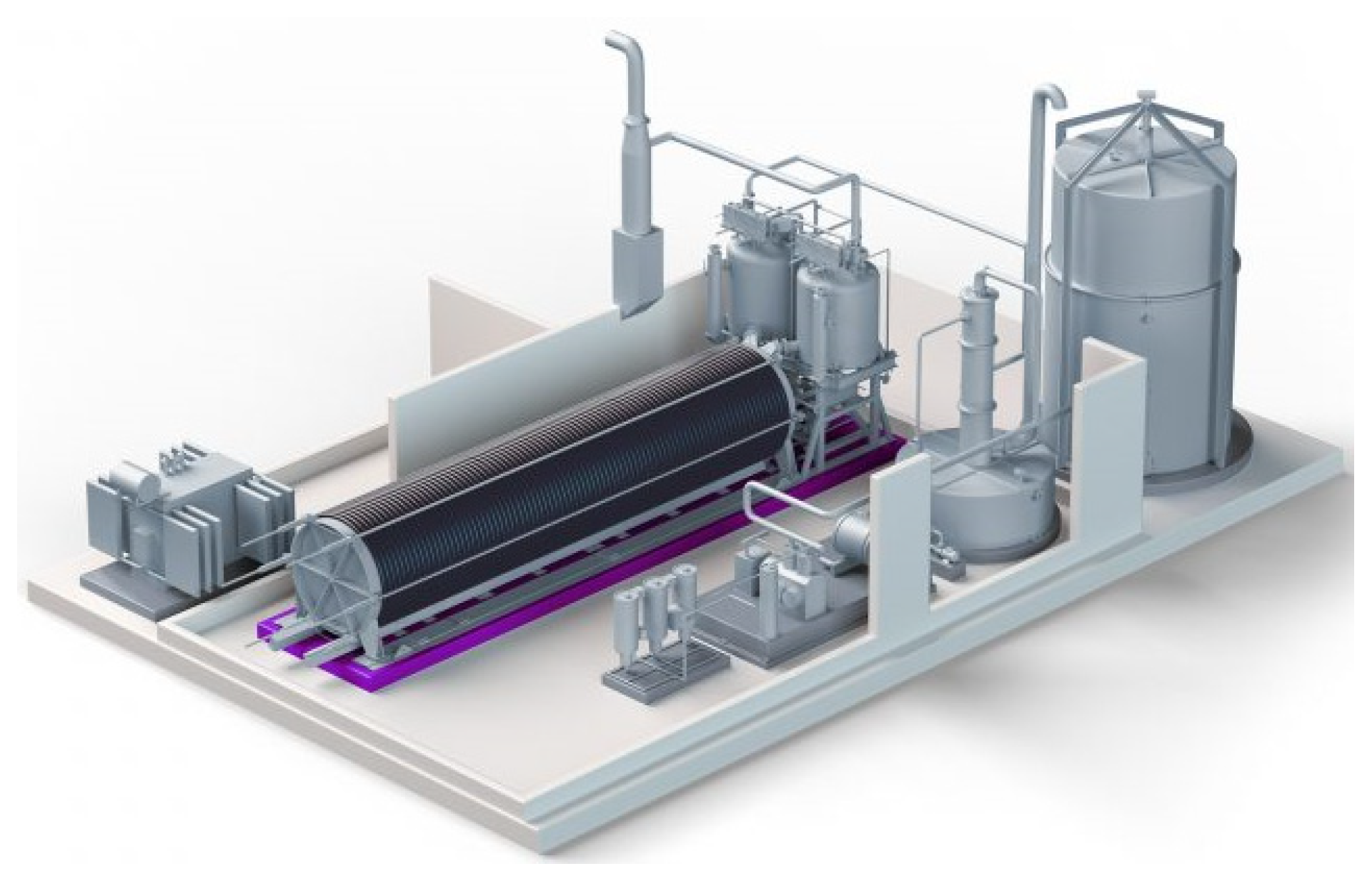
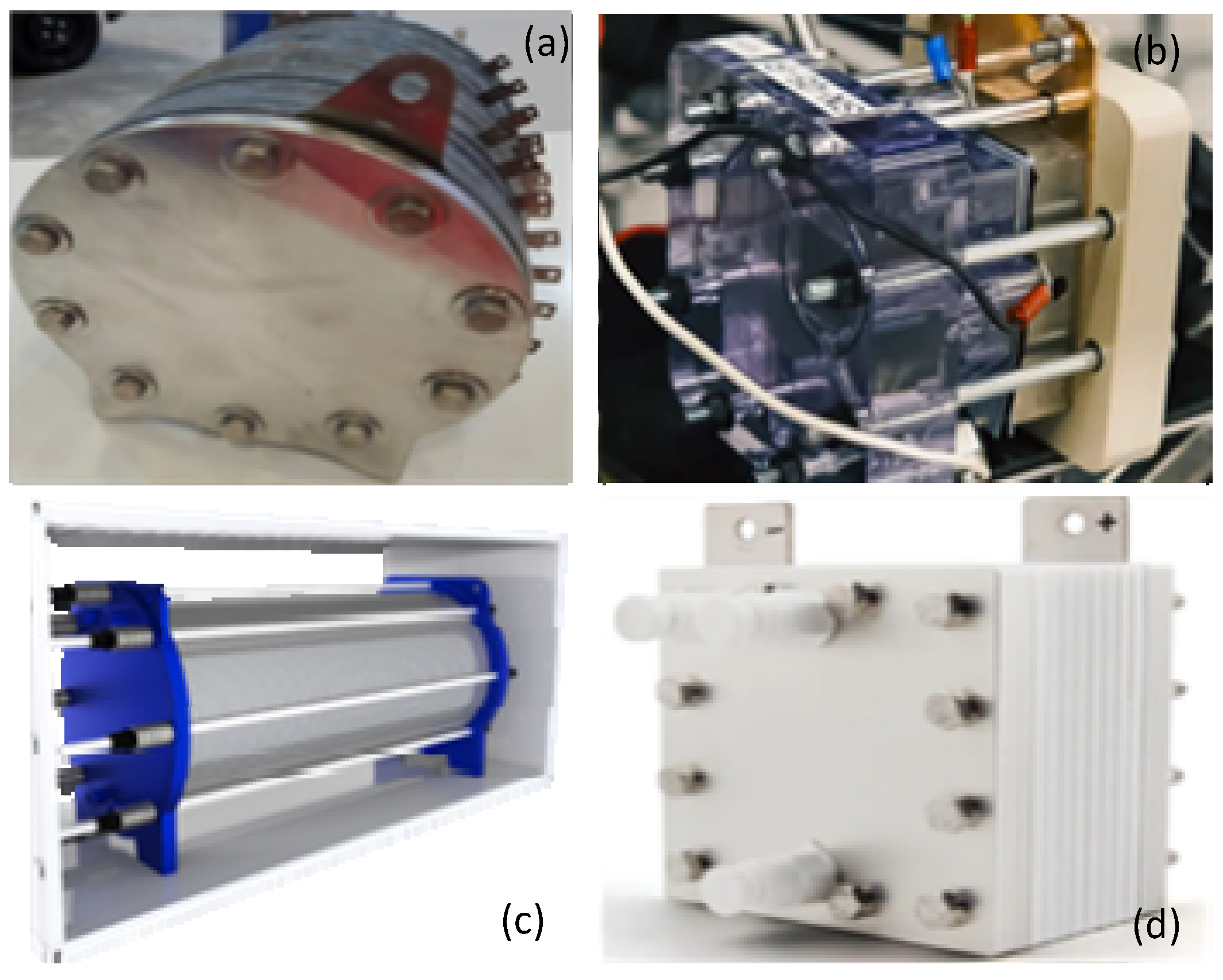




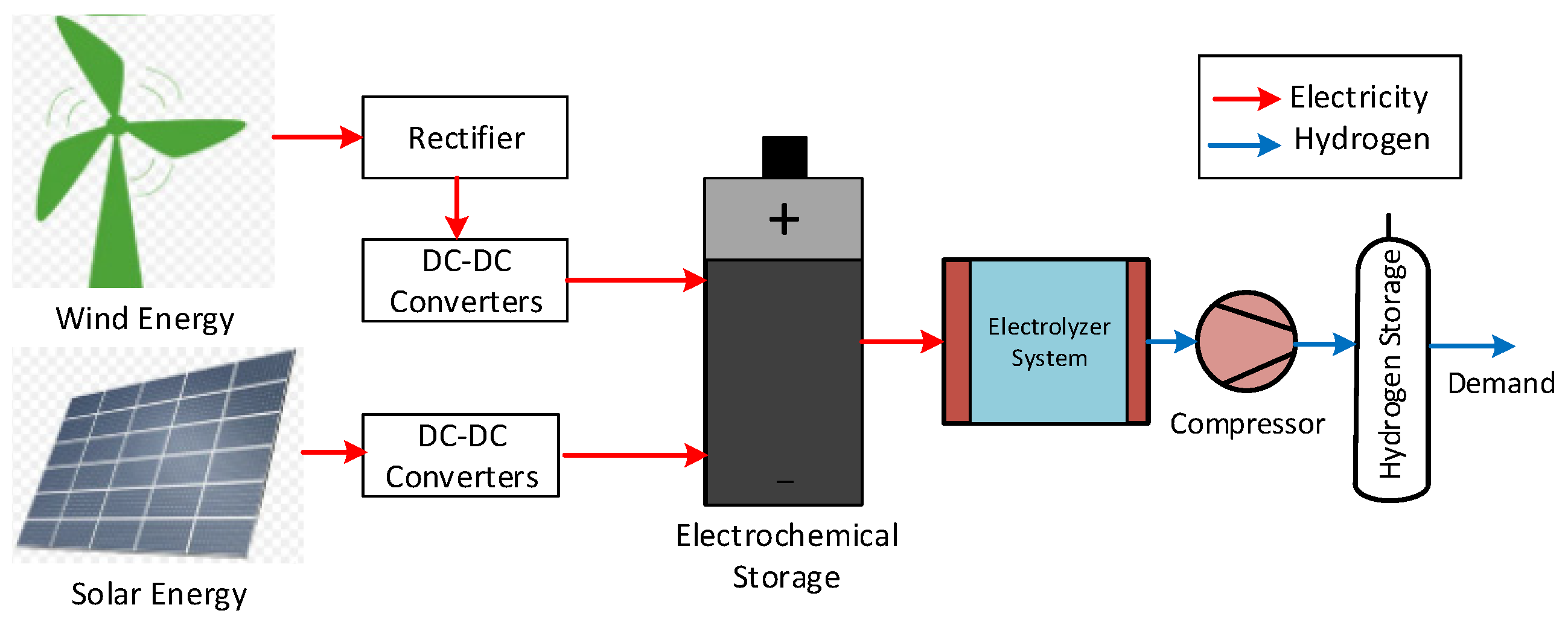

| Electrolysis Type | Anode Reaction | Cathode Reaction | Charge Carrier | Temperature Range |
|---|---|---|---|---|
| Alkaline | 40–90 °C | |||
| Proton-exchange membranes | 20–100 °C | |||
| High-temperature (solid oxide) | 700–1000 °C | |||
| Anion-exchange membranes | 40–60 °C |
| Electrolysis Types | Advantages and Technologies | Disadvantages and Technologies |
|---|---|---|
| Alkaline Electrolysis |
|
|
| PEM Electrolysis |
|
|
| High-Temperature Electrolysis |
|
|
| Anion Exchange Membranes |
|
|
| Company Names | |||||
|---|---|---|---|---|---|
| Parameters | De Nora S.A.P | Norsk Hydro | Electrolyzer Corp. | Teledyne Energy System | General Electric |
| Type of cell | B-FB | B-FP | M-T | B-FP | B-FB |
| Anode | Expanded Ni-plated Mild steel | Activated Ni-coated Steel | Ni-coated Steel | Ni screen | PTFE-bonded Noble metal |
| Cathode | Activated Ni-plated Steel | Activate Ni-coated Steel | Steel | Ni screen | PTFE-bonded Noble metal |
| Electro. pressure (MPa) | Ambient | Ambient | Ambient | 0.2 | 0.4 |
| Electro. temperature (°C) | 80 | 80 | 70 | 82 | 80 |
| Percentage of electrolyte | 29% KOH | 25% KOH | 28% KOH | 35% | Nafion |
| Density of current (Am−2) | 1500 | 1750 | 1340 | 2000 | 5000 |
| Voltage of cell (V) | 1.85 | 1.75 | 1.9 | 1.9 | 1.7 |
| Efficiency of current (%) | 98.5 | 98.5 | >99.9 | NR | NR |
| Purity of oxygen (%) | 99.6 | 99.3–99.7 | 99.7 | >98.0 | >98.0 |
| Purity of hydrogen (%) | 99.9 | 98.9–99.9 | 99.9 | 99.99 | >99.0 |
| Condition and Component | Alkaline | PEM | AEM | Solid Oxide |
|---|---|---|---|---|
| Operating temperature | 70–90 °C | 50–80 °C | 40–60 °C | 700–850 °C |
| Operating pressure | 1–30 bar | <70 bar | <35 bar | 1 bar |
| Electrolyte type | Potassium hydroxide (KOH) 5–7 molL−1 | PFSA membranes | DVB polymer support with KOH or NaHCO3 1 molL−1 | Yttria-stabilized zirconia (YSZ) |
| Separator solid electrolyte (above) | ZrO2 stabilized with PPS mesh | Solid electrolyte (above) | Solid electrolyte (above) | Solid electrolyte (above) |
| Electrode/catalyst (oxygen side) | Nickel-coated perforated stainless steel | Iridium oxide | High surface area Nickel or NiFeCo alloys | Perovskite-type (e.g., LSCF, LSM) |
| Electrode/catalyst (hydrogen side) | Nickel-coated perforated stainless steel | Platinum nanoparticles on carbon black | High surface area Nickel or NiFeCo alloys | Ni/YSZ |
| Porous transport layer (anode) | Nickel mesh (not always present) | Platinum-coated sintered porous titanium | Nickel foam | Coarse nickel-mesh or foam |
| Porous transport layer (cathode) | Nickel mesh | Sintered porous titanium or carbon cloth | Nickel foam or carbon cloth | None |
| Bipolar plate anode | Nickel-coated stainless steel | Platinum-coated titanium | Nickel-coated stainless steel | None |
| Bipolar plate cathode | Nickel-coated stainless steel | Gold-coated titanium | Nickel-coated stainless steel | Cobalt-coated stainless steel |
| Sealing and frames | PSU, PTFE, EPDM | PTFE, PSU, ETFE | PTFE, silicon | Ceramic glass |
| Efficiency (%) | 62–82 | 67–82 | 48–60 | 50–60 |
| Power unit (kW) | 1000 | 1000 | 70 | 05–100 |
| Min. stack cost | 270 USD/kWh | 400 USD/kWh | Unknown | >2000 USD/kwh |
| Lifetime stack | 50,000–80,000 h | 60,000 h | >5000 h | <20,000 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin, M.E. An Overview of Different Water Electrolyzer Types for Hydrogen Production. Energies 2024, 17, 4944. https://doi.org/10.3390/en17194944
Şahin ME. An Overview of Different Water Electrolyzer Types for Hydrogen Production. Energies. 2024; 17(19):4944. https://doi.org/10.3390/en17194944
Chicago/Turabian StyleŞahin, Mustafa Ergin. 2024. "An Overview of Different Water Electrolyzer Types for Hydrogen Production" Energies 17, no. 19: 4944. https://doi.org/10.3390/en17194944
APA StyleŞahin, M. E. (2024). An Overview of Different Water Electrolyzer Types for Hydrogen Production. Energies, 17(19), 4944. https://doi.org/10.3390/en17194944






