Abstract
The electrolysis of water is one of the most promising ways of producing green hydrogen. This produces hydrogen using electricity and does not generate additional carbon dioxide like the more conventional reforming of fossil fuels. However, making electrolysis competitive with conventional methods for hydrogen production is a challenge because of the cost of electricity and because of inefficiencies and costs in electrolysis systems. Initially this review looks at the basic design of water electrolysis and asks where energy is lost. Then, a selection of the latest results in the area of magnetic field-enhanced water electrolysis are examined and discussed, in particular focusing on the empirical results of magnetic field-assisted electrolysis with the aim of comparing findings and identifying limitations of current studies such that recommendations can be made for advanced design of hydrogen producing electrolysis systems.
1. Introduction
The production of green hydrogen can be defined as a process which generates hydrogen based on some renewable source such as wind, solar, or biomass energy [1]. In this way, green hydrogen can be considered a clean method of producing hydrogen which will emit less carbon dioxide than grey hydrogen, which is based on using fossil fuels [1]. One promising method for producing green hydrogen is through electrolysis, where water is split to generate hydrogen and oxygen [2]. In particular, this is an attractive option if the energy used to drive electrolysis is obtained from renewable sources. For example, Hosseini et al. consider electrolysis driven by either wind or solar power as a clean method for producing hydrogen [3]. However, they also mention that one of the limitations of electrolysis is the high costs of the rare metals used in the electrodes [3]. A comparison of the costs of producing hydrogen showed that green hydrogen combined with renewable energy might cost between 2.28 and 7.39 $/kg while grey hydrogen from methane or coal might cost 0.67–1.31 $/kg [4]. Hence, there is a drive to make electrolysis systems cheaper and more efficient at producing hydrogen to make them more competitive. Discussion and comparisons of the different methods for producing hydrogen can also be found in the reviews of Acar and Dincer as well as Dash et al. [5,6]. Furthermore, life cycle analysis has also been carried out for different hydrogen production methods [7,8]. In both these studies, the environmental impacts of hydrogen production through electrolysis are compared with different energy sources, and both find electrolysis powered by renewable sources such as wind and solar are the most beneficial [7,8]. Recently, there has also been growing interest in using algae to produce hydrogen [9].
A large number of studies and reviews have investigated how to improve hydrogen production through electrolysis of water. The review of Wang et al. looks in detail at different amorphous materials that can be used as electro-catalysts for water splitting [10]. Olivier et al. review and discuss the modelling and model equations required, including different analytical and empirical relations used [11]. Hu et al. also reviews modelling approaches focusing on alkaline water electrolysis [12]. In addition to giving model equations, they also discuss the importance of different operating conditions such as temperature, pressure, electrolyte flow, and concentration [12]. They also list a range of different ways by which water electrolysis can be enhanced, including modifying operating conditions, changing the design/geometry of the electrolyzer, using different catalysts, and applying external fields (gravity, magnetic, ultrasound, and pulsing electric fields) [12].
The review of Li et al. goes into great depth discussing the theory and mechanisms by which magnetic fields can enhance water electrolysis [13]. They mention the following three main effects [13]:
- Magneto-hydro-dynamic (due to the Lorentz force)
- Magneto-thermal effect (in alternating magnetic fields)
- Interactions with spin states of molecules (in the electrode reactions)
Most recently, Sun et al. have also discussed these effects and some of the latest results that have been obtained from applying magnetic fields to water electrolysis [14]. Both of these reviews provide detailed discussion of the theory and provided examples from the literature where magnetic fields have provided some enhancement [13,14]. However, they have offered only limited comparisons of the different enhancements obtained by these methods [13,14]. In most cases, the enhancements are discussed separately for each existing study and attributed to one of the three main effects mentioned above. In addition, both Sun et al. and Li et al. point out that there may be multiple effects in play when applying a magnetic field, so it is difficult to assess the combined effects, requiring further study [13,14].
This lack of a complete and unified theory for explaining the combined effects under magnetic fields makes it difficult to predict the resulting overall enhancement. Rather than relying on theory, this review looks at the empirical results and findings of different studies which investigated the influence of magnetic fields on water electrolysis. Here, the aim is to compare the different enhancement effects obtained with different types and configurations of magnetic fields. In particular, the two types of magnetic field considered are static (typically uniform) and alternating magnetic fields. For each type, the enhancement effects are compared such that limitations can be identified, and recommendations can be made about future directions of research.
Section 2 gives basic details about the design and modelling of electrolysis systems, highlighting inefficiencies/limitations. Section 3 examines the empirical performance of enhancements through the application of external magnetic fields, and Section 4 discusses the limitations of existing work in this field and gives recommendations for future research in the area of magnetic field-assisted water electrolysis.
2. Water Electrolysis Systems Design and Limitations
Water electrolysis systems are all based on the water splitting reaction as shown in Equation (1). This overall reaction relies on electrons being transferred through an electric circuit from the anode to the cathode. However, the reactions which occur at the anode and cathode depend on the type of electrolysis system, with one of the common designs and the associated reactions shown in Figure 1. This type of electrolysis is defined as one of the “mature” methods in terms of technological readiness [15]. In this review, the focus is on enhancing the most mature existing technology, which is alkaline electrolysis.
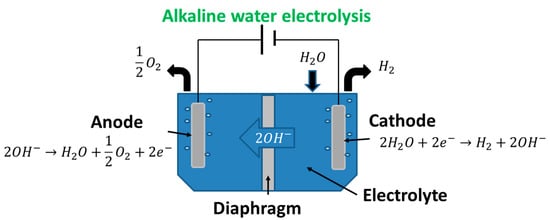
Figure 1.
Basic diagram of an alkaline electrolysis system [12,16].
The energy for water splitting (shown in Equation (1)) is provided by the voltage applied. The Gibbs free energy of this overall reaction is related to the reversible voltage as defined by Equation (2) [16]:
where z is the number of electrons transferred and F is the Faraday constant [16]. The Gibbs free energy depends on the operating temperature and pressure [17]. Correlations are available for predicting this where it can be seen that reduces with increasing temperature [12]. However, the actual voltage used for the electrolysis cell must be higher than this for a number of reasons. Firstly, there will be an irreversible change in entropy such that voltage is increased to
where is the change in enthalpy related to Gibbs free energy through and is the thermoneutral cell voltage [16]. In addition to this, the voltage must be increased further to account for various resistances in the system. These resistances translate to increases in the voltage called overpotentials. Figure 2 shows a typical voltage–current density diagram which shows how voltage changes increase current density.
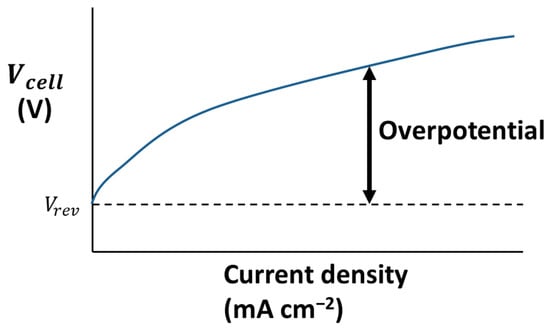
Figure 2.
A typical voltage–current density diagram, based on graphs from Ulleberg [16].
The overpotential can be broken down into ohmic overpotentials (related to factors such as electrolyte concentration, electrode gap, bubble coverage on electrodes, temperature, and pressure) and activation overpotentials (related to factors such as electrode materials, bubble coverage, and current density) as well as diffusion overpotentials [12]. Combining all these terms, the overall cell voltage can be calculated by Equation (4) [12]:
where and are the activation overpotentials of the cathode and anode, which may have different electro catalyst materials, is the ohmic overpotential, and is the diffusion overpotential. While detailed analytical models are available for estimating the separate parts of the overpotential, there are also empirical models for estimating the overall overpotential [11].
The hydrogen production rate can be obtained using Faraday’s law as shown in Equation (5) [11]. This says that the hydrogen production is proportional to the current flowing through the system .
However, due to current losses in the system, sometimes called parasitic losses, it may not be possible to convert 100% of the current supplied into hydrogen production [10]. To account for this difference, the Faraday efficiency can be inserted into Equation (5) to give Equation (6) [16]. Hence, Equation (6) can be used to calculate the hydrogen production from a single electrolysis cell (multiplying by the number of cells to give the total hydrogen production [16]. For alkaline electrolysers the Faraday efficiency can be calculated through empirical correlations [11].
The total power consumption of the electrolyser can be calculated by multiplying the cell voltage and the current as shown in Equation (7). Looking at the above discussion and equations, it can be seen that there are two main sources of inefficiency in these systems:
- Increased energy consumption due to overpotentials
- Reduced hydrogen production due to current losses
Both of these affect the overall energy efficiency of hydrogen production through electrolysis. Typically, when attempting to improve efficiency, research to reduce overpotentials focuses either on overpotentials associated with the hydrogen evolution reaction (HER) at the cathode or the oxygen evolution reaction (OER) at the anode [18].
3. Enhancing the Efficiency of Water Electrolysis with Applied Magnetic Fields
There are various ways to improve the efficiency of an electrolysis system. Here, we exclude switching to other types of less mature electrolysis and focus mostly on alkaline electrolysis, where most of the magnetic-assisted enhancements have been targeted.
3.1. Applying an Alternating Magnetic Field
Applying an alternating magnetic field is expected to have a heating effect (through the magneto thermal effect) [19]. An alternating magnetic field provides “local” heating in the vicinity of conductive objects and, in particular, where the reactions are taking place near the electrodes [20].
A typical method of generating a magnetic field is to use a coil of wire called a solenoid connected to an alternating current (AC), which generates an alternating field [21]. The strength of the magnetic field depends on the current and the number of loops in the solenoid.
The enhancement effect of a selection of cases where alternating magnetic fields are used to reduce overpotentials are shown in Table 1 and Table 2. Table 1 shows reductions obtained based on the oxygen evolution reaction (OER) and Table 2 shows reductions related to the hydrogen evolution reaction (HER).

Table 1.
Enhancement of electrolysis through applying alternating magnetic fields at the oxygen evolution side (OER).

Table 2.
Enhancement of electrolysis through applying alternating magnetic fields at the hydrogen evolution side.
Most of these studies have used potassium hydroxide and it seems to be standard to compare different electro catalyst materials at 1 M KOH using a current density of 10 mA cm−2. Using these conditions, it is also possible to compare the enhancement that can be achieved through applying different strengths of magnetic field.
3.1.1. The Effects of Different Electrode Materials
The catalyst materials used consist of mostly ferromagnetic materials, although it is interesting to note that enhancement can also be achieved using a bare carbon paper as shown by Zheng et al. [23]. This shows that some enhancement through applying an alternating magnetic field can be achieved regardless of the material used.
Considering the OER catalysts shown in Table 1 it can be seen that cobalt- or iron-based materials appear to give the highest enhancements. Through this magnetic field-based enhancement, the performance of these cheaper and more abundant materials can be made competitive with expensive and rare platinum electrodes [18]. A similar story is found when looking at HER electrode materials in Table 2. Electrodes containing nickel, iron, and cobalt have been shown to give the highest enhancement thus far. However, smaller enhancements have also been observed based on MoS2 and gadolinium [27,29]. Even without a magnetic field, nickel-based electro-catalytic materials inside a molybdenum matrix have been shown to be highly effective for HER performance and stability [30]. So, the addition of magnetic fields should further enhance performance with these materials.
3.1.2. The Effects of Magnetic Field Strength and Frequency
For most of the studies considering the use of alternating magnetic fields, a magnetic flux density of between 3 and 5 mT has been applied. However, it is important to note that the values in Table 1 and Table 2 are typically the optimal enhancements achieved after testing ranges of different magnetic field strengths. For example, Zheng et al. tested ranges of magnetic flux densities from 0 to 4.752 mT and found the optimal enhancement at 4.32 mT [22].
It is particularly interesting that El-Nowihy et al. managed to achieve very significant enhancement using much weaker magnetic fields (0.72 mT) for both OER and HER [18]. It is not clear how these significant enhancements were achieved while Zheng et al. found that no significant enhancements are observed with magnetic fields below 2 mT [23]. One possibility could be due to a different frequency used for the alternating magnetic field which is not reported by El-Nowihy et al. [18].
The only study found which has reported different frequency values is the study of Gong et al., who found that a reduced enhancement was observed when reducing the frequency from 300 kHz to 200 kHz [25].
3.1.3. The Effects of Current Density and Magnetic Field Direction
The results in Table 1 and Table 2 are mostly measured at 10 mA cm−2, but for a commercial/industrial water electrolysis system the operating current density of around 400 mA cm−2 [31]. So, it is important that magnetic enhancement is achievable at higher current densities than those typically tested.
While the reported values are at 10 mA cm−2, most of these studies also show trends in values up to 100 mA cm−2; for example, El-Nowihy et al. report significant reductions of up to 78.2% in HER overpotential at 100 mA cm−2 [18]. Reduced overpotentials observed at high current densities are also shown in most of the studies in Table 1 and Table 2, with the exception of the two studies by Zheng et al. [22,23]. In both those studies, it was found that the magnetic field leads to an increased overpotential when current densities increase beyond approximately 30 mA cm−2 [22,23]. The exact theoretical reason for this result is not clear, but the authors suggest that it could be due to some combination of spin-conservation, magneto-hydro-dynamic, and some induced electric field [23]. However, when comparing these two studies with the others which have not found this negative effect, it can be seen that they both use a relatively lower frequency of alternating magnetic field (150 kHz) and they also use a horizontally oriented magnetic field. The direction of the applied magnetic field is rarely mentioned in these studies, perhaps suggesting that it is not relevant. This would make sense if the magneto-thermal affect was the only effect in play, but taking into account other effects, the direction could play a significant role in determining whether the magnetic field has a positive or negative enhancement effect at higher current densities.
3.2. Applying a Static Magnetic Field
The application of a static magnetic field can be achieved either with a permanent magnet or using a solenoid with a direct current (DC) power supply. Many studies have considered applying static magnetic fields in different directions. For this reason, we have defined twelve different options, as shown in Figure 3 and Figure 4 (based on the directions of the Lorentz force [32]). For alternating magnetic fields, this direction is less important because the heating effect is resulting from the rapidly changing field. However, for a static field, the direction is significant and has been investigated.
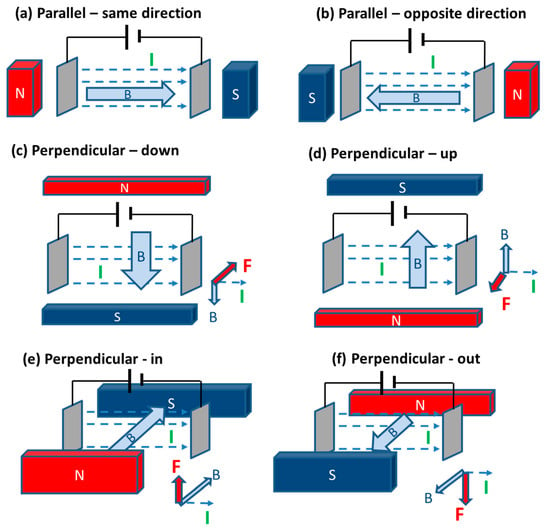
Figure 3.
Different possible configurations using vertical electrodes with magnetic fields.
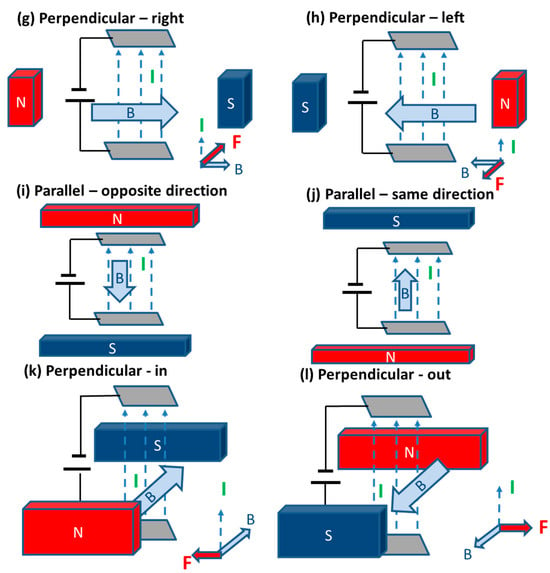
Figure 4.
Different possible configuration using horizontal electrodes.
The enhancement effects of a select number of studies utilising static magnetics fields to enhance water electrolysis are shown in Table 3 and Table 4. Table 3 shows results where the overpotentials have been modified and Table 4 shows cases where the change in current density has been reported. It can be seen from comparing these with values in Table 1 and Table 2 that in many cases static fields have been used with much stronger magnetic field strengths. In many cases, the static fields have also been generated using permanent magnets.

Table 3.
Enhancement of electrolysis overpotentials through applying static magnetic fields.

Table 4.
Enhancement of electrolysis current densities through applying static magnetic fields.
3.2.1. The Effects Electrode Materials with a Static Magnetic Field
As with the use of alternating magnetic fields, the enhancements achieved with static magnetic fields are typically demonstrated using ferromagnetic materials such as nickel, iron, and cobalt. This is not surprising, because previous studies have shown that nickel gave a greater enhancement in the presence of a magnetic field compared to platinum, and a much greater effect than basic graphite [41].
3.2.2. The Effects Using an Upward/Downward Lorentz Force
In many studies, the magnetic field is arranged such that the resulting Lorentz force is upward or downward (configurations (e) or (f) in Figure 3) with respect to parallel vertical electrodes [33,34,35,36,38,40,41,42]. Among these results, it has generally been observed that an upward force gives a more beneficial enhancement [34,36,40,41]. In these studies, it is generally suggested that the combined upward Lorentz force and buoyancy forces lead to smaller bubbles and more effective bubble release, while a downward force acts in opposition to buoyancy and leads to more sluggish bubble release. However, two studies have also found that a downward force gives a more beneficial effect [33,42]. The exact reasons why a downward Lorentz force could give a greater benefit are not clear, but this may be due to the “u” shape of the electrode used by Fogaca et al., where they demonstrate bubbles being released under the vertically aligned u-shape electrode [33].
3.2.3. The Effects Using a Magnetic Field Parallel to the Electric Current
The effect of the Lorentz force is usually strongest when the magnetic field is perpendicular to the electric current. However, it has been shown that having the magnetic field parallel can lead to a circulating force/motion near the electrodes [34,37]. This effect has been shown to have a beneficial enhancement effect when the electrodes are aligned horizontally in configurations (i) or (j), reducing overpotentials and increasing current density [34,43], However, Chen et al. show that this can have the opposite effect if the distance between electrodes is increased to 5 cm at lower current densities [43]. Li et al. also tested using a 5 cm gap between vertical electrodes with configurations (a) or (b) and found this led to a negative effect (increased overpotentials) at higher current densities [34]. So, the gap between electrodes must be smaller (for example 1 cm) to achieve some enhancement with one of these parallel configurations ((a), (b), (i) or (j)).
3.2.4. The Effects Using a Lorentz Force Horizontal and Perpendicular to the Electric Current
A number of configurations in Figure 3 and Figure 4 lead to a horizontal Lorentz force perpendicular to the electric current ((c), (d), (g), (h), (k) and (l)). Compared to the other configuration options discussed in Section 3.2.2 and Section 3.2.3, the effect of applying magnetic fields in these configurations has been shown to only have a positive effect in all the studies considered in Table 3 and Table 4 [18,34,39]. In particular, Li et al. reported a very large reduction in potentials when using horizontally oriented electrodes and a horizontal Lorentz force [34]. Notably, this reduction of 3295 mV is larger than the reductions obtained with other configurations. The Lorentz force in these cases is perpendicular to gravity and buoyancy forces, so the enhancement could be a result of this lack of competition with these other forces.
3.2.5. The Effects of Different Electrode Gaps
For alkaline electrolysis systems similar to the diagram in Figure 1, the locations of the electrodes can be moved to improve efficiency. Pushing them together so they touch the diaphragm is known as the zero-gap design, which is a common design adopted by manufacturers [16], although Haverkort and Rajaei have shown that an almost zero-gap of 0.2 mm gives superior performance compared to zero gap [44]. Furthermore, Lin et al. tested different electrode gaps in combination with an external magnetic field and found that a gap of 2 mm gave the best performance with higher current density [41]. Furthermore, Chen et al. have shown that magnetic fields do not affect performance if the distance between electrodes is greater than 5 cm [43]. It can be seen from Table 3 and Table 4 that the electrode gap is up to 5 cm in all cases where this value has been reported. Although previous research has shown very small gaps of 2 mm or less, significant enhancements have been demonstrated using larger gaps with applied static magnetic fields. It may be that larger gaps up to 5 cm have been favoured so that the phenomena and bubble behaviour can be captured more easily by cameras.
3.2.6. The Effects of Different Current Densities and Magnetic Field Strengths
From the data in Table 3 and Table 4 it appears there is no clear correlation between the enhancement effect and the strength of the magnetic field applied. In most cases, a fixed permanent magnet has been used, although El-Nowihy et al. demonstrate that some enhancement can be realised using a very weak magnetic field [18]. So, there is potentially room for the optimisation of magnetic field strength to improve enhancement effects further. Additionally, it can be seen that the enhancement effect appears to be relative to the operating current density. So, the enhancement effect could potentially be summarised as a percentage increase rather than an absolute increase in current density or a percentage reduction in the overpotentials.
4. Limitations of Existing Studies and Recommendations for Magnetic Enhanced Electrolysis Systems
4.1. Limitations and Recommendations Using an Alternating Magnetic Field
The enhancement of water electrolysis through alternating magnetic fields is a relatively new area of interest. For this reason, only a small number of studies have looked at this method for enhancing water electrolysis, although alternating magnetic fields have been suggested in earlier studies for reducing scale formation in water treatment [45].
Based on the empirical results shown in Table 1 and Table 2 and the discussion in Section 3.1.1, Section 3.1.2 and Section 3.1.3, the following limitations/gaps in the existing work can be identified:
- Enhancement effects have not been investigated for higher current densities (e.g., 400 mA cm−2 and higher)
- The direction of the applied magnetic field has not been considered
- The effects of different frequencies of alternating magnetic fields have not been considered (except for a single study)
It has been shown in two studies that at higher current densities the enhancement from the magnetic field becomes negative at higher current densities of around 40 mA cm−2 [22,23]. So, it is important to look at the effects at higher current densities to ensure that beneficial effects can be achieved using commercial/ industrial water electrolysis which might operate at 400 mA cm−2 [31]. Those negative effects have only been reported in these two studies, which both used horizontally oriented magnetic fields with a frequency of 150 kHz [22,23]. Other studies mentioned in Table 1 and Table 2 have generally used vertically oriented magnetic fields with higher frequencies up to 300 kHz and they have demonstrated beneficial enhancements at higher current densities. For example, Peng et al. and Gong et al. have both shown positive enhancements up to current densities of 100 mA cm−2 [24,25]. In fact, Gong et al. also show that the positive enhancement is reduced when they switch to a lower frequency of alternating magnetic field [25].
So, my recommendations for future work in this field are to conduct further experiments investigating the effects of and optimising the following parameters:
- Frequency of the magnetic field
- Direction of the magnetic field
- Current densities up to 400 mA cm−2
- Strength of the magnetic field
A thorough investigation of these four parameters would show the potential for enhancement using an alternating magnetic field. In addition to these parameters, the electrode materials should also be considered, although I think it is relatively well-known that ferromagnetic materials should be used to obtain the biggest enhancement from applied magnetic fields. Beyond this, further development in the shape of electrode materials as can be considered as shown in the study of Su et al. [27].
4.2. Limitations and Recommendations Using an Static Magnetic Field
The effects of applying a static magnetic field have been studied in greater depth and for wider ranges of current densities. They have also been investigated in terms of applying the magnetic field in various directions relative to the orientation of the electrodes. Based on the empirical evidence shown in Table 3 and Table 4 and the discussion in Section 3.2.1, Section 3.2.2, Section 3.2.3, Section 3.2.4, Section 3.2.5 and Section 3.2.6, the following limitations/gaps in the existing work can be identified:
- There is a lack of studies looking at enhancement using smaller electrode gaps (2 mm or smaller)
- In most cases only 1–2 configurations have been considered.
- It is still not clear exactly which configuration will be the best when other parameters are optimised (electrode gap, magnetic field strength, etc.)
The only study to date which has considered many different configurations (shown in Figure 3 and Figure 4) is that of Li et al. [34]. The results of that study and evidence from other studies in Table 3 and Table 4 show that a configuration with the Lorentz force horizontal and perpendicular to the electric current appears to always give a positive enhancement. In other configurations, the enhancement can be either positive or negative. However, Li et al. used an electrode gap estimated at 5 cm [34], which is known to be less effective compared to smaller gaps [41,43].
So, my recommendations for future work in this field are that experiments should be optimised considering modification of the following parameters:
- Gap between electrodes (using values such as 2 mm or smaller)
- Direction of the resulting Lorentz force
- Strength of magnetic fields
- Horizontal vs. vertical oriented electrodes
As mentioned already, it appears that a horizontal Lorentz force gives the most beneficial enhancement. This could be realised through any of the six configurations (c), (d), (g), (h), (k), or (l), shown in Figure 3 and Figure 4. However, this result must be confirmed using smaller electrode gaps to ensure these configurations are more efficient than the other six possible configurations (a), (b), (e), (f), (i), and (j). Adjustment of the strength of the magnetic field and a comparison of horizontally and vertically oriented electrodes should also be considered to further enhance performance.
Overall, magnetic field-enhanced electrolysis offers the potential for significant energy savings, but there is a need for further research to optimise the configuration, geometry, and operating conditions. Care should be taken to avoid conditions where the magnetic field has a negative effect on the efficiency of water electrolysis.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Zhou, Y.; Li, R.; Lv, Z.; Liu, J.; Zhou, H.; Xu, C. Green hydrogen: A promising way to the carbon-free society. Chin. J. Chem. Eng. 2022, 43, 2–13. [Google Scholar] [CrossRef]
- Horri, B.A.; Ozcan, H. Green hydrogen production by water electrolysis: Current status and challenges. Curr. Opin. Green Sustain. Chem. 2024, 47, 100932. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Afroze, S.; Sofri, A.N.S.B.; Reza, M.S.; Iskakova, Z.B.; Kabyshev, A.; Kuterbekov, K.A.; Bekmyrza, K.Z.; Taimuratova, L.; Uddin, M.R.; Azad, A.K. Solar-powered water electrolysis using hybrid solid oxide electrolyser cell (soec) for green hydrogen—A review. Energies 2023, 16, 7794. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A brief review of hydrogen production methods and their challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Gerloff, N. Comparative life-cycle-assessment analysis of three major water electrolysis technologies while applying various energy scenarios for a greener ydrogen production. J. Energy Storage 2021, 43, 102759. [Google Scholar] [CrossRef]
- Ayub, H.M.U.; Nizami, M.; Qyyum, M.A.; Iqbal, N.; Al-Mutaseb, A.H.; Hasan, M. Sustainable hydrogen production via microalgae: Technological advancements, economic indicators, environmental aspects, challenges, and policy implications. Environ. Res. 2024, 244, 117815. [Google Scholar] [CrossRef]
- Wang, X.; Tian, H.; Yu, X.; Chen, L.; Cui, X.; Shi, J. Advances and insights in amorphous electrocatalyst towards water splitting. Chin. J. Catal. 2023, 51, 5–48. [Google Scholar] [CrossRef]
- Olivier, P.; Bourasseau, C.; Bouamama, P.B. Low-temperature electrolysis system modelling: A review. Renew. Sustain. Energy Rev. 2017, 78, 280–300. [Google Scholar] [CrossRef]
- Hu, S.; Guo, B.; Ding, S.; Yang, F.; Dang, J.; Liu, B.; Gu, J.; Ma, J.; Ouyang, M. A comprehensive review of alkaline water electrolysis mathematical modeling. Appl. Energy 2022, 327, 120099. [Google Scholar] [CrossRef]
- Li, W.; Tian, H.; Ma, L.; Wang, Y.; Liu, X.; Gao, X. Low-temperature water electrolysis: Fundamentals, progress, and new strategies. Mater. Adv. 2022, 3, 5598–5644. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, H.; Yao, H.; Gao, Y.; Zhang, C. Magnetic field-assisted electrocatalysis: Mechanisms and design strategies. Carbon Energy 2024, e575. [Google Scholar] [CrossRef]
- UK Environment Agency. Review of Emerging Techniques for Hydrogen Production from Electrolysis of Water. Available online: https://www.gov.uk/government/publications/review-of-emerging-techniques-for-hydrogen-production-from-electrolysis-of-water (accessed on 27 July 2024).
- Ulleberg, O. Modeling of advanced alkaline electrolyzers: A system simulation approach. Int. J. Hydrogen Energy 2003, 28, 21–33. [Google Scholar] [CrossRef]
- Sakas, G.; Ibanez-rioja, A.; Ruuskanen, V.; Kosonen, A.; Ahola, J.; Bergmann, O. Dynamic energy and mass balance model for an industrial alkaline water electrolyzer plant process. Int. J. Hydrogen Energy 2022, 47, 4328–4345. [Google Scholar] [CrossRef]
- El-Nowihy, G.H.; Abdellatif, M.M.; El-Deab, M.S. Magnetic field-assisted water splitting at ternary nicofe magnetic nanocatalysts: Optimization study. Renew. Energy 2024, 226, 120395. [Google Scholar] [CrossRef]
- Gatard, V.; Deseure, J.; Chatenet, M. Use of magnetic fields in electrochemistry: A selected review. Curr. Opin. Electrochem. 2020, 23, 96–105. [Google Scholar] [CrossRef]
- Xiong, G.; Chen, Y.; Zhou, Z.; Liu, F.; Liu, X.; Yang, L.; Liu, Q.; Sang, Y.; Liu, H.; Zhang, X.; et al. Rapid synthesis of various electrocatalysts on ni foam using a universal and facile induction heating method for efficient water splitting. Adv. Funct. Mater. 2021, 31, 2009580. [Google Scholar] [CrossRef]
- Rouina, M.; Kariminia, H.-R.; Mousavi, S.A.; Shahryari, E. Effect of electromagnetic field on membrane fouling in reverse osmosis process. Desalination 2016, 395, 41–45. [Google Scholar] [CrossRef]
- Zheng, H.-B.; Wang, Y.-L.; Xie, J.-W.; Gao, P.-Z.; Li, D.-Y.; Rebrov, E.V.; Qin, H.; Liu, X.-P.; Xiao, H.-N. Enhanced alkaline oxygen evolution using spin polarization and magnetic heating effects under an AC magnetic field. ACS Appl. Mater. Interfaces 2022, 14, 34627–34636. [Google Scholar] [CrossRef]
- Zheng, H.-B.; Wang, Y.-L.; Zhang, P.; Ma, F.; Gao, P.-Z.; Guo, W.-M.; Qin, H.; Liu, X.-P.; Xiao, H.-N. Multiple effects driven by ac magnetic field for enhanced electrocatalytic oxygen evolution in alkaline electrolyte. Chem. Eng. J. 2021, 426, 130785. [Google Scholar] [CrossRef]
- Peng, D.; Hu, C.; Luo, X.; Huang, J.; Ding, Y.; Zhou, W.; Zhou, H.; Yang, Y.; Yu, T.; Lei, W.; et al. Electrochemical reconstruction of nife/nifeooh superparamagnetic core/catalytic shell heterostructure for magnetic heating enhancement of oxygen evolution reaction. Small 2023, 19, 2205665. [Google Scholar] [CrossRef]
- Gong, X.; Jiang, Z.; Zeng, W.; Hu, C.; Luo, X.; Lei, W.; Yuan, C. Alternating magnetic field induced magnetic heating in ferromagnetic cobalt single-atom catalysts for efficient oxygen evolution reaction. Nano Lett. 2022, 22, 9411–9417. [Google Scholar] [CrossRef]
- Zheng, H.-B.; Chen, H.-H.; Wang, Y.-L.; Gao, P.-Z.; Liu, X.-P.; Rebrov, E.V. Fabrication of magnetic superstructure nife04@mof74 and its derivative for electrocatalytic hydrogen evolution with ac magnetic field. ACS Appl. Mater. Interfaces 2020, 12, 45987–45996. [Google Scholar] [CrossRef]
- Su, M.; Zhou, W.; Liu, L.; Chen, M.; Jiang, Z.; Luo, X.; Yang, Y.; Yu, T.; Lei, W.; Yuan, C. Micro eddy current facilitated by screwed mos2 structure for enhanced hydrogen evolution reaction. Adv. Funct. Mater. 2022, 32, 2111067. [Google Scholar] [CrossRef]
- Cai, L.; Huo, J.; Zou, P.; Li, G.; Liu, J.; Xu, W.; Gao, M.; Zhang, S. Key role of Lorentz excitation in the electromagnetic-enhanced hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2022, 14, 15243–15249. [Google Scholar] [CrossRef]
- Zeng, W.; Jiang, Z.; Gong, X.; Hu, C.; Luo, X.; Lei, W.; Yuan, C. Atomic magnetic heating effect enhanced hydrogen reaction of gd@mos2 single-atom catalysts. Small 2023, 19, 2206155. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Luo, W.; Yu, X.; Chang, Z.; Kong, F.; Zhu, L.; Huang, Y.; Tian, H.; Cui, X.; et al. In situ active site refreshing of electro-catalytic materials for ultra-durable hydrogen evolution at elevated current densities. Adv. Energy Mater. 2024, 14, 2304099. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Ma, J.; Deng, X.; Gu, J.; Yang, F.; Ouyang, M. Study the effect of lye flow rate, temperature, system pressure and different current density on energy consumption in catalyst test and 500W commercial alkaline water electrolysis. Mater. Today Phys. 2022, 22, 100606. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Hsu, W.-N.; Hourng, L.-W.; Shih, T.-S.; Hung, C.-M. Effect of Lorentz force on hydrogen production in water electrolysis employing multielectrodes. J. Mar. Sci. Technol. 2016, 24, 511–518. [Google Scholar] [CrossRef]
- Fogaca, W.; Ikeda, H.; Misumi, R.; Kuroda, Y.; Mitsushima, S. Enhancement of oxygen evolution reaction in alkaline water electrolysis by Lorentz forces generated by an external magnetic field. Int. J. Hydrogen Energy 2024, 61, 1274–1281. [Google Scholar] [CrossRef]
- Li, Y.-H.; Chen, Y.-J. The effect of magnetic field on the dynamics of gas bubbles in water electrolysis. Sci. Rep. 2021, 11, 9346. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Peng, W.; Zhang, W.; Peng, K. Magnetic field enhancing electrocatalysis of co3o4/nf for oxygen evolution reaction. J. Power Sources 2019, 433, 226704. [Google Scholar] [CrossRef]
- Liu, H.-B.; Pan, L.-M.; Qin, Q.-J.; Li, P.-F. Experimental and numerical investigation of gas-liquid flow in water electrolysis under magnetic field. J. Electroanal. Chem. 2019, 832, 293–302. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Li, S.; Sun, J.; Wang, W.; Song, B.; Yang, X.; Wang, X.; Jiang, Z.; Wu, G.; et al. Magnetic field assisted electrocatalytic oxygen evolution reaction of nickel-based materials. J. Mater. Chem. A 2022, 10, 1760. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Zhong, W.; Liu, G.; Zhang, L.; Du, Y. Magnetic field improvement of hydrogen evolution reaction in mof-derived nico2s4 nanostructure. Ceram. Int. 2023, 49, 16836–16841. [Google Scholar] [CrossRef]
- Matsushima, H.; Kiuchi, D.; Fukunaka, Y. Measurement of dissolved hydrogen supersaturation during water electrolysis in a magnetic field. Electrochim. Acta 2009, 54, 5858–5862. [Google Scholar] [CrossRef]
- Kaya, M.F.; Demir, N.; Albawabiji, M.S.; Tas, M. Investigation of alkaline water electrolysis performance for different cost effective electrodes under magnetic field. Int. J. Hydrogen Energy 2017, 42, 17583–17592. [Google Scholar] [CrossRef]
- Lin, M.-Y.; Hourng, L.-W.; Kuo, C.-W. The effect of magnetic force on hydrogen production efficiency in water electrolysis. Int. J. Hydrogen Energy 2012, 37, 1311–1320. [Google Scholar] [CrossRef]
- Li, X.; Hao, C.; Du, Y.; Lu, Y.; Fan, Y.; Wang, M.; Wang, N.; Meng, R.; Wang, X.; Xu, Z.J.; et al. Harnessing magnetic fields to accelerate oxygen evolution reaction. Chin. J. Catal. 2023, 55, 191–199. [Google Scholar] [CrossRef]
- Chen, Y.J.; Li, Y.-H.; Chen, C.-Y. Studying the effect of electrode material and magnetic field on hydrogen production efficiency. Magnetochemistry 2022, 8, 53. [Google Scholar] [CrossRef]
- Haverkort, J.W.; Rajaei, H. Voltage losses in zero-gap alkaline water electrolysis. J. Power Sources 2021, 497, 229864. [Google Scholar] [CrossRef]
- Alabi, A.; Chiesa, M.; Garlisi, C.; Palmisano, G. Advances in anti-scale magnetic water treatment. Environ. Sci. Water Res. Technol. 2015, 1, 408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).