Harnessing Switchgrass for Sustainable Energy: Bioethanol Production Processes and Pretreatment Technologies
Abstract
1. Introduction
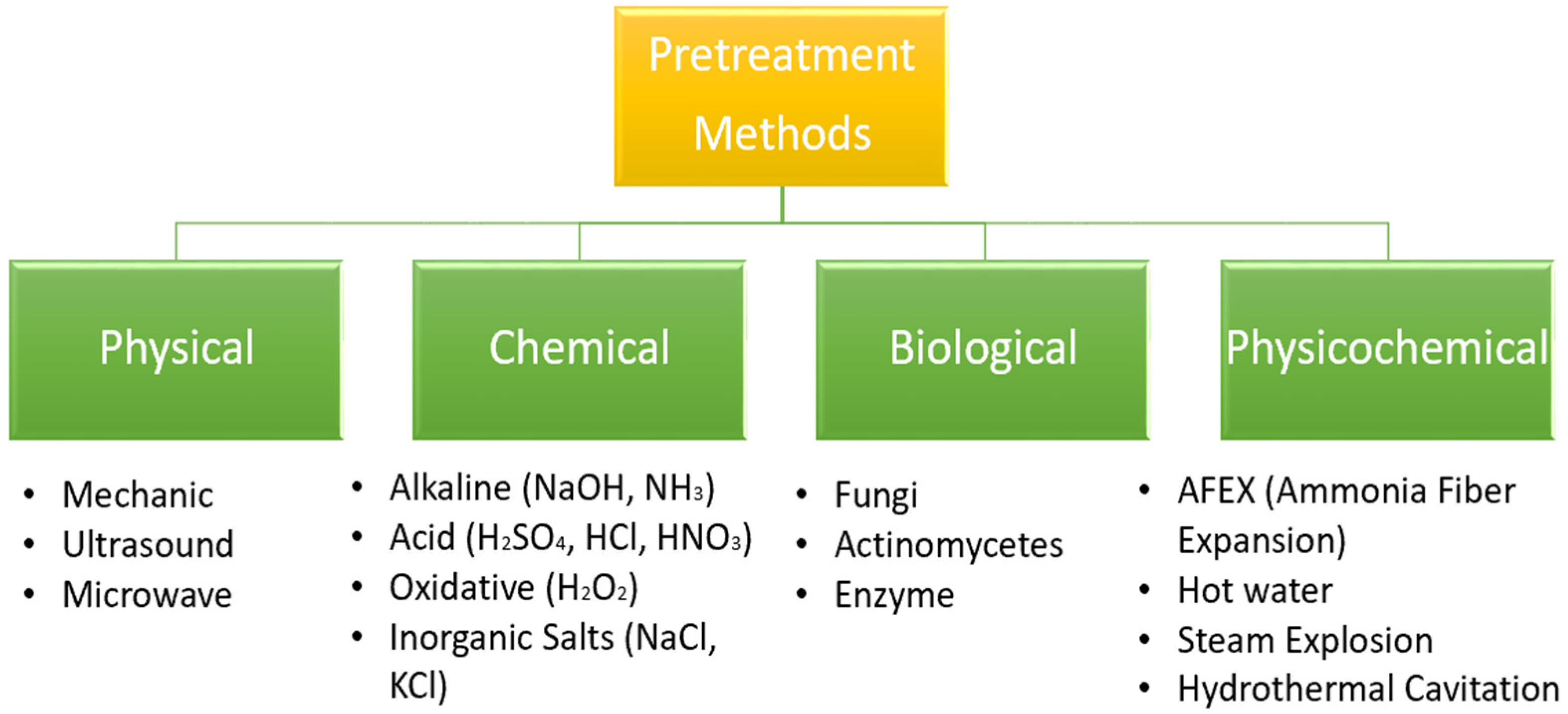
2. Pretreatment Methods
3. Influence of Pretreatments on Switchgrass
3.1. Structural Deformations
3.2. Toxin Release during Pretreatment
3.3. Detoxification Processes
4. Bioethanol Production Process
4.1. Hydrolysis
4.2. Fermentation
4.2.1. Simultaneous Saccharification and Fermentation (SSF)
4.2.2. Separate Hydrolysis and Fermentation (SHF)
5. Brief Comparison of Pretreatment Methods Combined with the Fermentation Process
6. Prospects of Future Study
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, H.; Yadav, V.; Bilal, M.; Iqbal, H.M.N. Bioprospecting Microbial Hosts to Valorize Lignocellulose Biomass—Environmental Perspectives and Value-Added Bioproducts. Chemosphere 2022, 288, 132574. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Wang, C.; Tan, J.K.; Zhang, C.; He, J. Enhanced Butanol Production from Dough and Okara Waste through Co-Fermentation. Renew. Energy 2024, 234, 121157. [Google Scholar] [CrossRef]
- Başar, İ.A.; Perendeci, N.A. Optimization of Zero-Waste Hydrogen Peroxide—Acetic Acid Pretreatment for Sequential Ethanol and Methane Production. Energy 2021, 225, 120324. [Google Scholar] [CrossRef]
- Günerhan, Ü.; Us, E.; Dumlu, L.; Yılmaz, V.; Carrère, H.; Perendeci, A.N. Impacts of Chemical-Assisted Thermal Pretreatments on Methane Production from Fruit and Vegetable Harvesting Wastes: Process Optimization. Molecules 2020, 25, 500. [Google Scholar] [CrossRef] [PubMed]
- Ntaikou, I.; Antonopoulou, G.; Lyberatos, G. Biohydrogen Production from Biomass and Wastes via Dark Fermentation: A Review. Waste Biomass Valorization 2010, 1, 21–39. [Google Scholar] [CrossRef]
- Thanapornsin, T.; Laopaiboon, L.; Laopaiboon, P. Novel Batch and Repeated-Batch Butanol Fermentation from Sweet Sorghum Stem Juice by Co-Culture of Arthrobacter and Immobilized Clostridium in Scaled-Up Bioreactors. Energies 2024, 17, 1009. [Google Scholar] [CrossRef]
- Su, G.; Chan, C.; He, J. Enhanced Biobutanol Production from Starch Waste via Orange Peel Doping. Renew. Energy 2022, 193, 576–583. [Google Scholar] [CrossRef]
- Unyay, H.; Piersa, P.; Zabochnicka, M.; Romanowska-Duda, Z.; Kuryło, P.; Kuligowski, K.; Kazimierski, P.; Hutsol, T.; Dyjakon, A.; Wrzesińska-Jędrusiak, E.; et al. Torrefaction of Willow in Batch Reactor and Co-Firing of Torrefied Willow with Coal. Energies 2023, 16, 8083. [Google Scholar] [CrossRef]
- Landrat, M.; Abawalo, M.; Pikoń, K.; Fufa, P.A.; Seyid, S. Assessing the Potential of Teff Husk for Biochar Production through Slow Pyrolysis: Effect of Pyrolysis Temperature on Biochar Yield. Energies 2024, 17, 1988. [Google Scholar] [CrossRef]
- Başar, A.; Kökdemir Ünşar, E.; Ünyay, H.; Perendeci, N.A. Ethanol, Methane, or Both? Enzyme Dose Impact on Ethanol and Methane Production from Untreated Energy Crop Switchgrass Varieties. Renew. Energy 2020, 149, 287–297. [Google Scholar] [CrossRef]
- Regis, F.; Monteverde, A.H.A.; Fino, D. A Techno-Economic Assessment of Bioethanol Production from Switchgrass through Biomass Gasification and Syngas Fermentation. Energy 2023, 274, 127318. [Google Scholar] [CrossRef]
- Paluri, B.; Patel, D. Combustion and Performance Characteristics of SI Engine with Bioethanol Blended Fuels. Int. J. Energy Res. 2022, 46, 24454–24464. [Google Scholar] [CrossRef]
- EPA n.d. EPA Non-CO2 Greenhouse Gas (GHG) Technical Report. Available online: https://www.epa.gov/global-mitigation-non-co2-greenhouse-gases (accessed on 14 July 2024).
- Rostocki, A.; Unyay, H.; Ławińska, K.; Obraniak, A. Granulates Based on Bio and Industrial Waste and Biochar in a Sustainable Economy. Energies 2022, 16, 56. [Google Scholar] [CrossRef]
- Stelmach, J.; Kuncewicz, C.; Szufa, S.; Jirout, T.; Rieger, F. The Influence of Hydrodynamic Changes in a System with a Pitched Blade Turbine on Mixing Power. Processes 2020, 9, 68. [Google Scholar] [CrossRef]
- Wright, L.; Turhollow, A. Switchgrass Selection as a “Model” Bioenergy Crop: A History of the Process. Biomass Bioenergy 2010, 34, 851–868. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Yuan, X.; Wang, X.; Zhu, W.; Yang, F.; Cui, Z. Effect of Dairy Manure to Switchgrass Co-Digestion Ratio on Methane Production and the Bacterial Community in Batch Anaerobic Digestion. Appl. Energy 2015, 151, 249–257. [Google Scholar] [CrossRef]
- Głowacka, A.; Szostak, B.; Klebaniuk, R. Effect of Biogas Digestate and Mineral Fertilisation on the Soil Properties and Yield and Nutritional Value of Switchgrass Forage. Agronomy 2020, 10, 490. [Google Scholar] [CrossRef]
- Başar, İ.A.; Çoban, Ö.; Göksungur, M.Y.; Eskicioğlu, Ç.; Perendeci, N.A. Enhancement of Lignocellulosic Biomass Anaerobic Digestion by Optimized Mild Alkaline Hydrogen Peroxide Pretreatment for Biorefinery Applications. J. Environ. Manag. 2021, 298, 113539. [Google Scholar] [CrossRef]
- Ünyay, H.; Yılmaz, F.; Başar, İ.A.; Altınay Perendeci, N.; Çoban, I.; Şahinkaya, E. Effects of Organic Loading Rate on Methane Production from Switchgrass in Batch and Semi-Continuous Stirred Tank Reactor System. Biomass Bioenergy 2022, 156, 106306. [Google Scholar] [CrossRef]
- Akman, H.E.; Perendeci, N.A.; Ertekin, C.; Yaldiz, O. Energy Crops and Methane: Process Optimization of Ca(OH)2 Assisted Thermal Pretreatment and Modeling of Methane Production. Molecules 2022, 27, 6891. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.-S.; Lee, D.-J. Inhibitor Formation and Detoxification during Lignocellulose Biorefinery: A Review. Bioresour. Technol. 2022, 361, 127666. [Google Scholar] [CrossRef] [PubMed]
- Unyay, H.; Piersa, P.; Perendeci, N.A.; Wielgosinski, G.; Szufa, S. Valorization of Anaerobic Digestate: Innovative Approaches for Sustainable Resource Management and Energy Production—Case Studies from Turkey and Poland. Int. J. Green Energy 2023, 21, 1928–1943. [Google Scholar] [CrossRef]
- Jensen, J.R.; Morinelly, J.E.; Gossen, K.R.; Brodeur-Campbell, M.J.; Shonnard, D.R. Effects of Dilute Acid Pretreatment Conditions on Enzymatic Hydrolysis Monomer and Oligomer Sugar Yields for Aspen, Balsam, and Switchgrass. Bioresour. Technol. 2010, 101, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic Biomass: Acid and Alkaline Pretreatments and Their Effects on Biomass Recalcitrance—Conventional Processing and Recent Advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K.; Kumar, J.; Ahluwalia, V. A Critical Review on Current Strategies and Trends Employed for Removal of Inhibitors and Toxic Materials Generated during Biomass Pretreatment. Bioresour. Technol. 2020, 299, 122633. [Google Scholar] [CrossRef]
- Hu, M.; Cai, Z.; Zhang, J.; Yuan, L.; Fu, Q.; Ji, D. Ammonia Fiber Expansion (AFEX) Combined with White-Rot Fungi Pretreatment to Improve Enzymatic Hydrolysis of Lignocellulose. Biomass Convers. Biorefinery 2024, 14, 10085–10099. [Google Scholar] [CrossRef]
- Khan, M.U.; Usman, M.; Ashraf, M.A.; Dutta, N.; Luo, G.; Zhang, S. A Review of Recent Advancements in Pretreatment Techniques of Lignocellulosic Materials for Biogas Production: Opportunities and Limitations. Chem. Eng. J. Adv. 2022, 10, 100263. [Google Scholar] [CrossRef]
- Zabochnicka, M.; Krzywonos, M.; Romanowska-Duda, Z.; Szufa, S.; Darkalt, A.; Mubashar, M. Algal Biomass Utilization toward Circular Economy. Life 2022, 12, 1480. [Google Scholar] [CrossRef]
- MOSIER, N. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv Pretreatment of Lignocellulosic Biomass for Enzymatic Hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Leu, S.-Y.; Zhu, J.Y. Substrate-Related Factors Affecting Enzymatic Saccharification of Lignocelluloses: Our Recent Understanding. BioEnergy Res. 2013, 6, 405–415. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Cui, Y.; Yuan, W. Ultrasound for Microalgal Cell Disruption and Product Extraction: A Review. Ultrason. Sonochem. 2022, 87, 106054. [Google Scholar] [CrossRef] [PubMed]
- Amrutha, S.R.; Suja, N.R.; Menon, S. Morphological Analysis of Biomass. In Handbook of Biomass; Springer Nature: Singapore, 2023; pp. 1–31. [Google Scholar]
- Pereira, J.; de Melo, M.M.R.; Silva, C.M.; Lemos, P.C.; Serafim, L.S. Impact of a Pretreatment Step on the Acidogenic Fermentation of Spent Coffee Grounds. Bioengineering 2022, 9, 362. [Google Scholar] [CrossRef]
- Aktas, K.; Liu, H.; Basar, I.A.; Eskicioglu, C. Adsorption Enhanced Biological Treatment of Hydrothermal Liquefaction Aqueous Phase Derived from Municipal Sludge. Bioresour. Technol. 2024, 407, 131093. [Google Scholar] [CrossRef]
- Zhai, R.; Hu, J.; Jin, M. Towards Efficient Enzymatic Saccharification of Pretreated Lignocellulose: Enzyme Inhibition by Lignin-Derived Phenolics and Recent Trends in Mitigation Strategies. Biotechnol. Adv. 2022, 61, 108044. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Bokhary, A.; Leitch, M.; Liao, B.Q. Liquid–Liquid Extraction Technology for Resource Recovery: Applications, Potential, and Perspectives. J. Water Process Eng. 2021, 40, 101762. [Google Scholar] [CrossRef]
- Roque, L.R.; Morgado, G.P.; Nascimento, V.M.; Ienczak, J.L.; Rabelo, S.C. Liquid-Liquid Extraction: A Promising Alternative for Inhibitors Removing of Pentoses Fermentation. Fuel 2019, 242, 775–787. [Google Scholar] [CrossRef]
- Gyan, K.; Afedzi, A.E.K.; Tanypramphan, P.; Parakulsuksatid, P. A Review of the Advances in Detoxification Strategies of Lignocellulosic Hydrolysate for Bio-Based Succinic Acid Production. Biocatal. Agric. Biotechnol. 2024, 60, 103323. [Google Scholar] [CrossRef]
- Cantarella, M.; Cantarella, L.; Gallifuoco, A.; Spera, A.; Alfani, F. Comparison of Different Detoxification Methods for Steam-Exploded Poplar Wood as a Substrate for the Bioproduction of Ethanol in SHF and SSF. Process Biochem. 2004, 39, 1533–1542. [Google Scholar] [CrossRef]
- Arminda, M.; Josúe, C.; Cristina, D.; Fabiana, S.; Yolanda, M. Use of Activated Carbons for Detoxification of a Lignocellulosic Hydrolysate: Statistical Optimisation. J. Environ. Manag. 2021, 296, 113320. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.H.; Turanlı-Yıldız, B.; Liu, D.; Resch, M.G.; Fink, G.R.; Stephanopoulos, G. Engineered Yeast Tolerance Enables Efficient Production from Toxified Lignocellulosic Feedstocks. Sci. Adv. 2021, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Caspeta, L.; Castillo, T.; Nielsen, J. Modifying Yeast Tolerance to Inhibitory Conditions of Ethanol Production Processes. Front. Bioeng. Biotechnol. 2015, 3, 184. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic Conversion of Lignocellulosic Biomass into Chemicals and Fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Shahbazi, A.; Zhang, B. Dilute and Concentrated Acid Hydrolysis of Lignocellulosic Biomass. In Bioalcohol Production; Elsevier: Amsterdam, The Netherlands, 2010; pp. 143–158. [Google Scholar]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in Sustainable Bioethanol Production: A Review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic Biomass Fractionation by Mineral Acids and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Molecules 2019, 24, 4273. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Acid-Based Hydrolysis Processes for Ethanol from Lignocellulosic Materials: A Review. BioResources 2007, 2, 472–499. [Google Scholar] [CrossRef]
- Behera, S.S.; Saranraj, P.; Ray, R.C. Microbial Bioethanol Fermentation Technologies—Recent Trends and Future Prospects. In Biofuels and Biorefining; Elsevier: Amsterdam, The Netherlands, 2022; pp. 75–108. [Google Scholar]
- Kumar, D.; Singh, B.; Korstad, J. Utilization of Lignocellulosic Biomass by Oleaginous Yeast and Bacteria for Production of Biodiesel and Renewable Diesel. Renew. Sustain. Energy Rev. 2017, 73, 654–671. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent Advances in Understanding the Effects of Lignin Structural Characteristics on Enzymatic Hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and Biotechnological Applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Leung, K.T.; Qin, W. The Prospects of Cellulase-Producing Bacteria for the Bioconversion of Lignocellulosic Biomass. Int. J. Biol. Sci. 2009, 5, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Oh, S.E. Production and Characterization of Multiple Cellulolytic Enzymes by Isolated Streptomyces Sp. MDS. Biomass Bioenergy 2012, 47, 302–315. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Dey, P.; Pal, P.; Kevin, J.D.; Das, D.B. Lignocellulosic Bioethanol Production: Prospects of Emerging Membrane Technologies to Improve the Process—A Critical Review. Rev. Chem. Eng. 2020, 36, 333–367. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Dahnum, D.; Tasum, S.O.; Triwahyuni, E.; Nurdin, M.; Abimanyu, H. Comparison of SHF and SSF Processes Using Enzyme and Dry Yeast for Optimization of Bioethanol Production from Empty Fruit Bunch. Energy Procedia 2015, 68, 107–116. [Google Scholar] [CrossRef]
- Papa, G.; Rodriguez, S.; George, A.; Schievano, A.; Orzi, V.; Sale, K.L.; Singh, S.; Adani, F.; Simmons, B.A. Comparison of Different Pretreatments for the Production of Bioethanol and Biomethane from Corn Stover and Switchgrass. Bioresour. Technol. 2015, 183, 101–110. [Google Scholar] [CrossRef]
- Alizadeh, H.; Teymouri, F.; Gilbert, T.I.; Dale, B.E. Pretreatment of Switchgrass by Ammonia Fiber Explosion (AFEX). Appl. Biochem. Biotechnol. 2005, 124, 1133–1141. [Google Scholar] [CrossRef]
- Afedzi, A.E.K.; Parakulsuksatid, P. Recent Advances in Process Modifications of Simultaneous Saccharification and Fermentation (SSF) of Lignocellulosic Biomass for Bioethanol Production. Biocatal. Agric. Biotechnol. 2023, 54, 102961. [Google Scholar] [CrossRef]
- Permatasari, N.S.; Zainuri, M.; Kusumaningrum, H.P.; Mishbach, I.; Hastuti, E.D. Bioethanol Production Using the SSF Method (Simultaneous Saccharification and Fermentation) of Microalgae Anabaena Sp. J. Phys. Conf. Ser. 2020, 1524, 012071. [Google Scholar] [CrossRef]
- Oehlenschläger, K.; Schepp, E.; Stiefelmaier, J.; Holtmann, D.; Ulber, R. Simultaneous Fermentation and Enzymatic Biocatalysis—A Useful Process Option? Biotechnol. Biofuels Bioprod. 2024, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H.; Charpentier, C. Biochemical Aspects of Stuck and Sluggish Fermentation in Grape Must. J. Ind. Microbiol. Biotechnol. 1998, 20, 20–27. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cui, Q.; Feng, Y.; Xuan, J. Composition of Lignocellulose Hydrolysate in Different Biorefinery Strategies: Nutrients and Inhibitors. Molecules 2024, 29, 2275. [Google Scholar] [CrossRef]
- Capecchi, L.; Galbe, M.; Wallberg, O.; Mattarelli, P.; Barbanti, L. Combined Ethanol and Methane Production from Switchgrass (Panicum virgatum L.) Impregnated with Lime Prior to Steam Explosion. Biomass Bioenergy 2016, 90, 22–31. [Google Scholar] [CrossRef]
- Derman, E.; Abdulla, R.; Marbawi, H.; Sabullah, M.K. Oil Palm Empty Fruit Bunches as a Promising Feedstock for Bioethanol Production in Malaysia. Renew. Energy 2018, 129, 285–298. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Cannella, D.; Jørgensen, H.; Felby, C.; Thygesen, L.G. Cellulase Inhibition by High Concentrations of Monosaccharides. J. Agric. Food Chem. 2014, 62, 3800–3805. [Google Scholar] [CrossRef]
- Wang, X.; Feng, H.; Li, Z. Pretreatment of Switchgrass with Electrolyzed Water and a Two-Stage Method for Bioethanol Production. Biotechnol. Bioprocess Eng. 2012, 17, 624–633. [Google Scholar] [CrossRef]
- Bals, B.; Rogers, C.; Jin, M.; Balan, V.; Dale, B. Evaluation of Ammonia Fibre Expansion (AFEX) Pretreatment for Enzymatic Hydrolysis of Switchgrass Harvested in Different Seasons and Locations. Biotechnol. Biofuels 2010, 3, 1. [Google Scholar] [CrossRef]
- Tao, L.; Aden, A.; Elander, R.T.; Pallapolu, V.R.; Lee, Y.Y.; Garlock, R.J.; Balan, V.; Dale, B.E.; Kim, Y.; Mosier, N.S.; et al. Process and Technoeconomic Analysis of Leading Pretreatment Technologies for Lignocellulosic Ethanol Production Using Switchgrass. Bioresour. Technol. 2011, 102, 11105–11114. [Google Scholar] [CrossRef] [PubMed]
- Smullen, E.; Finnan, J.; Dowling, D.; Mulcahy, P. Bioconversion of Switchgrass: Identification of a Leading Pretreatment Option Based on Yield, Cost and Environmental Impact. Renew. Energy 2017, 111, 638–645. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Bakalinsky, A.; Penner, M.H. Enzymatic Saccharification and Fermentation of Xylose-Optimized Dilute Acid–Treated Lignocellulosics. Appl. Biochem. Biotechnol. 2005, 124, 0947–0962. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Liu, E.; Saeed, A.; Williams, D.W.; Hu, H.; Li, C.; Ray, A.E.; Shi, J. Industrial Hemp as a Potential Bioenergy Crop in Comparison with Kenaf, Switchgrass and Biomass Sorghum. Bioresour. Technol. 2017, 244, 641–649. [Google Scholar] [CrossRef]
- El-Mashad, H.M. Biomethane and Ethanol Production Potential of Spirulina Platensis Algae and Enzymatically Saccharified Switchgrass. Biochem. Eng. J. 2015, 93, 119–127. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Cheng, J.J.; Sharma-Shivappa, R.R.; Burns, J.C. Delignification of Switchgrass Cultivars for Bioethanol Production. BioResources 2011, 6, 707–720. [Google Scholar] [CrossRef]
- Garlock, R.J.; Balan, V.; Dale, B.E.; Ramesh Pallapolu, V.; Lee, Y.Y.; Kim, Y.; Mosier, N.S.; Ladisch, M.R.; Holtzapple, M.T.; Falls, M.; et al. Comparative Material Balances around Pretreatment Technologies for the Conversion of Switchgrass to Soluble Sugars. Bioresour. Technol. 2011, 102, 11063–11071. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Zhang, M.; Wang, D. Modified Simultaneous Saccharification and Fermentation to Enhance Bioethanol Titers and Yields. Fuel 2018, 215, 647–654. [Google Scholar] [CrossRef]
- Chang, V.S.; Kaar, W.E.; Burr, B.; Holtzapple, M.T. Simultaneous Saccharification and Fermentation of Lime-Treated Biomass. Biotechnol. Lett. 2001, 23, 1327–1333. [Google Scholar] [CrossRef]
- Keshwani, D.R.; Cheng, J.J. Microwave-Based Alkali Pretreatment of Switchgrass and Coastal Bermudagrass for Bioethanol Production. Biotechnol. Prog. 2010, 26, 644–652. [Google Scholar] [CrossRef]
- Ewanick, S.; Bura, R. The Effect of Biomass Moisture Content on Bioethanol Yields from Steam Pretreated Switchgrass and Sugarcane Bagasse. Bioresour. Technol. 2011, 102, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Chundawat, S.P.S.; Pal, R.K.; Zhao, C.; Campbell, T.; Teymouri, F.; Videto, J.; Nielson, C.; Wieferich, B.; Sousa, L.; Dale, B.E.; et al. Ammonia Fiber Expansion (AFEX) Pretreatment of Lignocellulosic Biomass. J. Vis. Exp. 2020, 158, e57488. [Google Scholar] [CrossRef]
- Teymouri, F.; Laureano-Perez, L.; Alizadeh, H.; Dale, B.E. Optimization of the Ammonia Fiber Explosion (AFEX) Treatment Parameters for Enzymatic Hydrolysis of Corn Stover. Bioresour. Technol. 2005, 96, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Ben Atitallah, I.; Antonopoulou, G.; Ntaikou, I.; Soto Beobide, A.; Dracopoulos, V.; Mechichi, T.; Lyberatos, G. A Comparative Study of Various Pretreatment Approaches for Bio-Ethanol Production from Willow Sawdust, Using Co-Cultures and Mono-Cultures of Different Yeast Strains. Molecules 2022, 27, 1344. [Google Scholar] [CrossRef] [PubMed]
- Larnaudie, V.; Ferrari, M.D.; Lareo, C. Switchgrass as an Alternative Biomass for Ethanol Production in a Biorefinery: Perspectives on Technology, Economics and Environmental Sustainability. Renew. Sustain. Energy Rev. 2022, 158, 112115. [Google Scholar] [CrossRef]
| Pretreatments | Pretreatment Conditions | Fermentation Process | Glucose Yield % | Bioethanol | |
|---|---|---|---|---|---|
| Raw switchgrass | SHF | 14.7 g/kg | [63] | ||
| Raw switchgrass | SSF | 80 g/kg | [64] | ||
| 2 stage (H2O2)+autoclave pretreatment | 5% solid loading (w/w) 3% H2O2 50 °C 150 rpm, 24 h + DI water, 12.5% solid loading 121 °C for 30 min | SHF | 89 | 59 g/kg | [73] |
| AEW | 20% solid loading (w/w) 200 °C for 25 min in tubular reactor | SHF | 66 | 26.5 g/kg | [73] |
| Ammonia fiber explosion (AFEX) | 1:1 kg of ammonia: kg of dry weight 100 °C, 5 min | SSF | 93 | 200 g/kg | [64] |
| Ammonia fiber explosion (AFEX) | 0.9 g/g ammonia 80 °C 20 min | SHF | 276 g/kg | 30 g/L | [74] |
| Ammonia fiber explosion (AFEX) | 0.81 g/g ammonia 150 °C 30 min | SSF | 76 | 196.75 g/kg | [75] |
| Ammonia fiber explosion (AFEX) | 0.5 g biomass 20 m3 of 2 mol/L ammonia solution (NH3aq), 40 °C for 48 h | SSF | 55 | 316 g/kg | [76] |
| Dilute acid | 1.2% sulfuric acid, 180 °C, and 0.5 min | SSF | 90.3 | [77] | |
| Dilute acid | 10 mg sulfuric acid/g enzyme 40 °C 40 min or 160 °C 10 min | SHF | 86 | 218 g/kg | [78] |
| Dilute acid | 0.2% w/w H2SO4 195 °C 10 min | SSF | 85 | 157.5 g/kg | [70] |
| Dilute acid | 0.5 g biomass 20 m3 of 1 mol/L sulfuric acid (H2SO4), 40 °C for 48 h | SSF | 47 | 285 g/kg | [76] |
| Dilute acid | 1% sulfuric acid (H2SO4) 140 °C, 40 min | SSF | 74.5 | 193 g/kg | [75] |
| Dilute alkali | 2% w/v NaOH at 140 ± 2 °C for 1 h | SHF | 79.9 | 244 g/kg | [78] |
| Dilute alkali | 1% NaOH 10:1 solid/liquid ratio 50 °C 12 h 100 rpm in water bath | SHF | 130.9 g/kg | [79] | |
| Dilute alkali | 1% (w/v) NaOH 30 min 121 °C | SHF | 60.8 | [80] | |
| Dilute alkali | 0.5 g biomass 20 m3 of 1 mol/L sodium hydroxide (NaOH), 40 °C for 48 h | SSF | 38 | 218 g/kg | [76] |
| Dilute sulfur dioxide SO2 | 10% solid (w/w) 5%SO2 180 °C 10 min | SHF | 83 | [81] | |
| Dilute sulfur dioxide SO2 | 30% solid loading, 180 °C, 10 min | SSF | 80.5 | 202.42 g/kg | [75] |
| Hydrothermal and ethanol | 20% solid loading at 170 °C for 60 min at an ethanol concentration of 80% (v/v) | mSSF | 87.9 | 83.50% | [82] |
| Hydrogen peroxide and acetic acid (HPAC) | 2% H2O2 + 0% HAc, 24 h, and 100 °C | SSF | 81.65 g/kg | [3] | |
| Ionic liquid (IL) | 3 h, 10% (w/w), (100 °C), 5% dry biomass with ionic liquid 1-ethyl-3-methylimidazolium acetate [C2C1Im][OAc] | SHF | 722 g/kg | 85.7 g/kg | [63] |
| Lime-pretreated | 120 °C, 2 h, 0.1 g Ca(OH)2 g−1 dry biomass, and 9 mL H2O g−1 dry weight | SSF | 72 | 14.1 g/kg | [83] |
| Lime-pretreated | 0.4% w/w Ca(OH)2 lime 195 °C, 10 min | SSF | 78 | 167.5 g/kg | [70] |
| Lime-pretreated | 2 g H2O/gDB 1.52 g/g anhydrous NH3/DB 150 °C 30 min | SHF | 87 | [81] | |
| Lime-pretreated | 0.1/1 lime/dry weight, 120 °C, 4 h | SSF | 80.9 | 180.90 g/kg | [75] |
| Methanol pretreatment | 0.5 g biomass 20 m3 of 1 mol/L methanol (CH3OH), 40 °C for 48 h | SSF | 69 | 268 g/kg | [76] |
| Microwave-based alkali pretreatment | 2% w/v alkali solution (NaOH), microwave radiation for 10 min | SHF | 82 | [84] | |
| Pressurized hot water (PHW) | 190 C, 15 min, 10% (w/w) | SHF | 334 g/kg | 52.5 g/kg | [63] |
| Soaking in aqueous ammonia (SAA) | 20% solid content, 1.35 g pure NH3/g dry biomass 160 °C 60 min | SSF | 65.5 | 132.75 g/kg | [75] |
| Steam explosion pretreatment | SO2 impregnated (3% w/dry weight), 195 °C, 7 min, 1.5 L batch steam gun | SSF | 113.22 g/kg | [85] | |
| Steam explosion pretreatment | 20:1 water/dry weight 195 °C 1 h | SSF | 53 | 149 g/kg | [70] |
| Washed liquid hot water (LHW) | 15% solid (w/w) 200 °C, 10 min | SHF | 85 | [81] | |
| Washed liquid hot water (LHW) | 20% solid content 200 °C, 10 min | SSF | 78.8 | 155.50 g/kg | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unyay, H.; Perendeci, N.A.; Piersa, P.; Szufa, S.; Skwarczynska-Wojsa, A. Harnessing Switchgrass for Sustainable Energy: Bioethanol Production Processes and Pretreatment Technologies. Energies 2024, 17, 4812. https://doi.org/10.3390/en17194812
Unyay H, Perendeci NA, Piersa P, Szufa S, Skwarczynska-Wojsa A. Harnessing Switchgrass for Sustainable Energy: Bioethanol Production Processes and Pretreatment Technologies. Energies. 2024; 17(19):4812. https://doi.org/10.3390/en17194812
Chicago/Turabian StyleUnyay, Hilal, Nuriye Altınay Perendeci, Piotr Piersa, Szymon Szufa, and Agata Skwarczynska-Wojsa. 2024. "Harnessing Switchgrass for Sustainable Energy: Bioethanol Production Processes and Pretreatment Technologies" Energies 17, no. 19: 4812. https://doi.org/10.3390/en17194812
APA StyleUnyay, H., Perendeci, N. A., Piersa, P., Szufa, S., & Skwarczynska-Wojsa, A. (2024). Harnessing Switchgrass for Sustainable Energy: Bioethanol Production Processes and Pretreatment Technologies. Energies, 17(19), 4812. https://doi.org/10.3390/en17194812









