Systematic Literature Review on Pipeline Transport Losses of Hydrogen, Methane, and Their Mixture, Hythane
Abstract
1. Introduction
- Synthesis of current research. We systematically compile and synthesize research findings related to the losses experienced during the transportation of hydrogen, methane, and hythane, highlighting key factors that influence these losses.
- Identification of knowledge gaps. Our review identifies critical gaps in the current body of knowledge, pointing out areas that require further investigation to optimize transport systems for these gases.
- Comparative analysis. We offer a comparative assessment of transportation losses for hydrogen, methane, and hythane, providing insights into how the transport characteristics differ between these gases and the implications for efficiency and safety.
- Recommendations for future research. Based on our findings, we propose recommendations for future research directions, focusing on enhancing transport efficiency and reducing losses, which can contribute to the development of more sustainable energy systems.
2. Materials and Methods
- RQ1:
- can a natural gas pipeline network be used for H2 transport?
- RQ2:
- what are the main pressure loss indicators in H2 transport?
- RQ3:
- are the risks of transporting H2 vs. CH4 higher?
- Pipes for H2 transport;
- H2 and CH4 blended transport;
- Replacement of CH4 with H2 in the actual pipe transport networks.
3. Results
3.1. RQ1: Can a Natural Gas Pipeline Network Be Used for H2 Transport?
3.1.1. Parametric Characteristics of the Two Gases
3.1.2. H2 Transportation
- In a gaseous state in specialized cylinders or tube trailers and pipeline transport (compressed hydrogen to high pressures—typically around 35–70 MPa—is one of the most common methods; this approach is suitable for short to medium distances and for smaller volumes);
- In a liquid state (H2 can be transported in liquid form, which is advantageous for long distances and large-scale transport due to its higher energy density compared with gaseous hydrogen; however, the liquefaction process is energy-intensive and requires cryogenic temperatures around −253 °C);
- In a bound form using solid or liquid carriers, including by land transport in cylindrical containers (H2 can also be transported using solid or liquid carriers, such as metal hydrides or chemical hydrogen storage systems [25]; these carriers allow for safer and more efficient storage and transportation, adding additional complexity to the hydrogen release process);
- Condensation of gaseous H2 on a steel surface (dispersive forces or Van der Waals forces) leads to surface coverage. The coverage of hydrogen atoms on the steel surface can be up to a monolayer under typical conditions. At room temperature and moderate pressures (0.1–1 MPa), surface coverage might range from 10⁻³ to 10⁻² molecules per square nanometer (molecules/nm²) [26,29].
- Dissociation of molecules into atoms—chemisorption. The energy of chemisorption is the energy required for hydrogen molecules to dissociate and chemisorb onto steel surfaces and typically ranges between 20 and 50 kJ/mol. The degree of dissociation depends on temperature and surface properties but could result in a significant proportion of surface hydrogen in an atomic form at temperatures above 200 °C [26,29].
- Transition of atoms through a steel surface—gas dissolution in steel. The surface permeation flux representing the flux of hydrogen atoms penetrating the steel surface (depending on pressure and temperature) could range between 10⁻⁶ and 10⁻⁴ mol/m²/s under typical industrial conditions (e.g., 200–400 °C and 1–10 MPa pressure). The actual flux depends significantly on the steel type and environmental conditions [26,29].
- Diffusion of H2 atoms from the surface into the interior of the steel wall, characterized by:
- ➢
- ➢
3.2. RQ2: What Are the Main Pressure Loss Indicators in H2 Transport?
- Mixture H2/CH4 [%];
- Gas or liquid state;
- Pipeline diameter [mm];
- Wall thickness [mm];
- Length of pipe [m];
- Pipe material;
- Strength class;
- Outside pipe pressure [Pa];
- Inside pipe pressure [Pa];
- Velocity [m/s];
- Temperature [°C];
- Diffusion coefficient of H2 in metal [m2/s];
- Solubility of H2 in Fe [m3/m3 solid*MPa0.5];
- H2 leaks [m3];
- Power usage for transport [MW];
- Load loss [MPa];
- Inner surface roughness of pipe [μm];
- Loss due to diffusion [%]
- Enthalpic jump [kJ/kg];
- Mass flow [kg/s];
- Pipe production technology.
3.3. RQ3: Are Risks of Transporting H2 vs. CH4 Higher?
- Correctly assessing the vulnerabilities of metal piping;
- Understanding the behavior of H2 released into the atmosphere, including its rapid dispersal and tendency not to accumulate near the ground, where ignition sources are more likely to be present;
- Accidental release of H2 means a much higher flammable mixture, necessitating a review of applicable safety measures;
- Compared with methane (CH4), hydrogen possesses a significantly higher flame propagation velocity, which can increase the risk of fire hazards;
- The flammability and radiation hazard should be reconsidered;
- Equipment built for use in gas group IIC (ATEX) is to be used [58];
- Gas measurement and leak detection equipment must be specifically suitable for hydrogen to effectively identify potential leaks and mitigate risks associated with its release.
4. Expanded Comparative Analysis and Safety Considerations in Hydrogen and Methane Transport Systems
- Pipe diameter and wall thickness. Smaller pipe diameters and thicker walls generally result in greater pressure losses due to increased friction and reduced flow area. For hydrogen, which is less dense and more prone to turbulent flow, optimizing pipe dimensions is crucial to minimize losses.
- Average flow velocity. Hydrogen has a higher flow velocity at the same pressure gradient compared with methane. This increased velocity can lead to higher frictional losses, especially in long-distance transport pipelines. Maintaining optimal flow velocity is essential to reducing pressure drop.
- Surface roughness. The internal surface roughness of the pipeline has a significant impact on pressure loss. Hydrogen’s low viscosity exacerbates the effects of surface roughness, increasing frictional resistance. Pipelines designed for hydrogen transport might require smoother internal surfaces to mitigate these losses [71].
- Operating pressure and temperature. Higher operating pressures tend to reduce pressure losses due to a higher density of hydrogen, which can compensate for its low molecular weight. However, operating at elevated temperatures can decrease hydrogen’s density, increasing pressure loss.
- Hydrogen embrittlement. Hydrogen can penetrate steel pipelines, leading to embrittlement—a phenomenon whereby the metal becomes brittle and more susceptible to cracking. This risk is particularly pronounced in high-strength steels and requires the use of special alloys or coatings to mitigate [28,29,42].
- Leakage. Due to its small molecular size, hydrogen is more likely to leak through pipeline joints, fittings, and even through the steel itself. This increases the risk of explosions, especially since hydrogen has a wide flammability range (4–75% in air) and a low ignition energy compared with methane [43,57,74].
- Explosion hazard. Hydrogen’s high diffusivity and wide flammability limits make it more prone to accidental ignition. When leaks occur, hydrogen can form explosive mixtures more readily than methane, especially in confined spaces. The energy released in hydrogen explosions is also typically higher, posing a significant safety risk [7].
- Joule–Thomson effect. When hydrogen is released from high pressure (as might happen during a leak), its temperature increases, unlike methane, which cools upon expansion. This temperature rise can lead to ignition risks if there are sparks or other ignition sources nearby [36].
- Accidental damage to a pipe in an excavation activity. Here, the failure frequencies are similar to those for natural gas “from 2016 to 2022 it resulted in 11 fatalities, 36 injuries, and over USD 144 M in damages” [74] in the US only.
5. Conclusions
- Incremental integration of hydrogen. The initial phase of integrating hydrogen into existing natural gas systems involves blending H2 with methane in concentrations ranging from 5% to 30%. This blending is contingent upon local regulations and, as such, the adjustment of risk assessment matrices and maintenance practices for metal piping and associated infrastructure becomes imperative. Effective management of this transition requires a thorough understanding of the materials properties and behavior of piping systems under mixed gas conditions.
- Adaptation of combustion systems. Converting large-scale CH4 combustion systems to utilize hythane (a mixture of hydrogen and natural gas) up to 30% H2 allows for a competitive alternative that aids in maintaining the necessary flow within transport pipelines. This conversion not only facilitates the transition to hydrogen but also leverages existing infrastructure and technologies for a more gradual shift.
- Innovative liquid hydrogen transport. Exploring the feasibility of transporting liquid hydrogen through cryogenic pipelines presents a revolutionary approach to reducing transport costs, with theoretical reductions in energy costs of up to 99.5% compared with traditional methane transport [41]. This method could potentially open new avenues for hydrogen distribution, although it requires innovative material solutions to handle the extreme conditions.
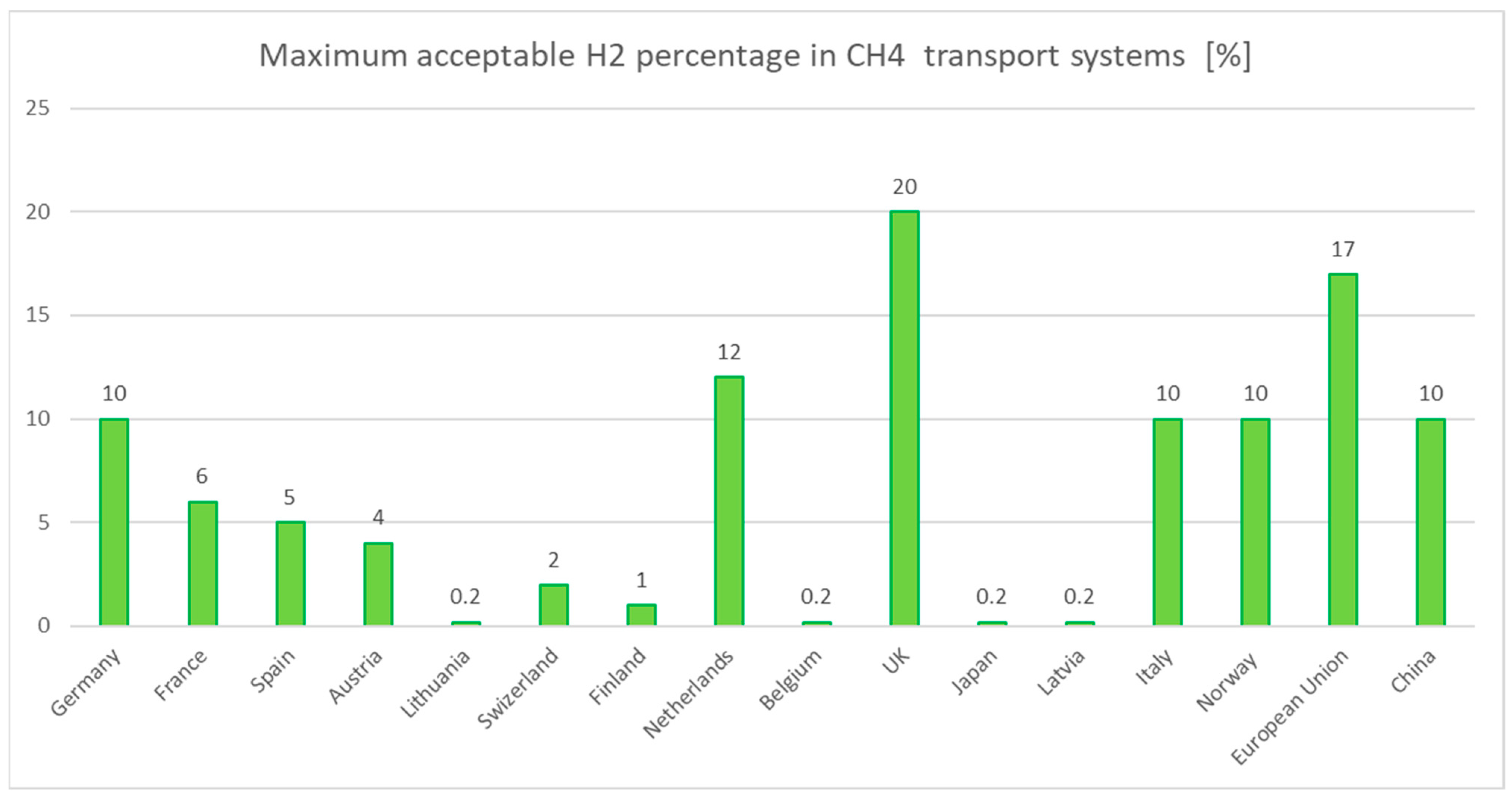
- Repurposing natural gas pipelines. While repurposing existing natural gas pipelines for hydrogen transport is achievable, it necessitates extensive upgrades to ensure safety and efficiency. This includes material adjustments to withstand hydrogen’s unique properties, the implementation of enhanced monitoring systems, and limitations on hydrogen concentration to mitigate mechanical stress risks. Additionally, integrating an inner lining designed to reduce friction could further optimize energy loss during transport [55].
- Advanced pipeline design. The introduction of pipes with a 3D-structured surface—such as triangular or knife-blade geometries—aimed at minimizing drag, represents a cutting-edge solution for enhancing hydrogen flow efficiency. These advanced designs can significantly reduce turbulence and pressure losses, thus optimizing the overall performance of hydrogen transport systems.
- Optimization of transport parameters. To ensure efficient hydrogen transportation, a comprehensive analysis of pipeline dimensions, flow velocity, surface roughness, and operating conditions is critical. Effective design and optimization of these factors are essential to minimize pressure losses and maximize throughput.
- Risk management. The inherent risks associated with hydrogen transport—such as embrittlement of materials, leakage, and explosion potential—are notably higher than those for methane. Addressing these risks demands substantial advancements in technology and materials science, along with rigorous safety protocols that may include continuous monitoring and rapid response mechanisms.
- Leak detection technologies. Development of advanced sensors and monitoring systems for real-time detection of hydrogen and hythane leaks in pipelines. This can include research on nanomaterials or IoT-enabled devices for enhanced sensitivity.
- Material innovation. Investigating new materials for piping and storage that reduce permeability to hydrogen and minimize transport losses. This could include coatings or composite materials that are more resistant to hydrogen embrittlement.
- Integration with renewable energy sources. Analyzing how integrating hydrogen transport with renewable energy systems (like solar or wind) can enhance energy efficiency and minimize loss during transport.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-Dimensional |
| BW | Box and Whiskers Chart |
| CAPEX | Capital Expenditures |
| Fe | Iron |
| H2 | Hydrogen |
| KPI | Key Performance Indicator |
| NAF | Not Accounted For |
| OPEX | Operating Expenses |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RQ | Research Questions |
| SLR | Systematic Literature Review |
| WW | World Wide |
Appendix A
- 1.
- Disciplines used for each criterion: Agriculture and Agribusiness, Applied Sciences, Architecture, Biotechnology, Chemistry, Computer science, Construction and Building, Earth and Atmospheric sciences, Engineering, Environmental Sciences, Life Sciences, Mining and natural resources, Power and Energy, Science, Technology
- 2.
- Search mode used for criterion 1 to 5:
- 3.
- Search mode used for criterion 6: online recommended research papers during skim and scan.
Appendix B
| Properties | Hydrogen (H2) | Methane (CH4) | Hythane 10% H2-90% CH4 | Hythane 25% H2-75% CH4 | Hythane 30% H2-70% CH4 | Mixture 40% H2-60% CH4 | Mixture 60% H2-40% CH4 | Unit |
| Molar mass | 2.02 | 16.04 | 14.64 | 11.48 | 15.06 | 10.43 | 7.63 | g/mole |
| Critical temperature | 33.20 | 190.65 | 174.91 | 139.48 | 179.63 | 127.67 | 96.18 | C |
| Critical pressure | 1.315 | 4.540 | 4.218 | - | 4.314 | - | - | MPa |
| Vapor density at normal boiling point | 1.34 | 1.82 | 1.77 | - | 1.79 | - | - | kg/m3 |
| Vapor density at 20 C and 0.1 MPa | 0.0838 | 0.6510 | 0.5943 | 0.4667 | 0.6113 | 0.4241 | 0.3107 | kg/m3 |
| Specific heat capacity at 20 C and constant pressure | 14.40 | 2.21 | 3.43 | 6.17 | 3.06 | 7.09 | 9.52 | kJ/kg/K |
| Specific heat ratio (Cp/Cv) | 1.40 | 1.31 | 1.32 | 1.34 | 1.32 | 1.35 | 1.36 | |
| Lower calorific value by mass (lower heating value, weight basis) | 120.00 | 48.00 | 55.20 | 71.40 | 53.04 | 76.80 | 91.20 | MJ/kg |
| Lower calorific value by volume at 1 atm | 11.00 | 35.00 | 32.60 | 27.20 | 33.32 | 25.40 | 20.60 | MJ/m3 |
| Higher calorific value by mass | 142.00 | 53.00 | 61.90 | 81.93 | 59.23 | 88.60 | 106.40 | MJ/kg |
| Higher calorific value by volume at 1 Atm | 13.00 | 39.00 | 36.00 | 30.00 | 27.00 | 28.00 | 20.00 | MJ/m3 |
| Maximum flame temperature | 1526.85 | 1221.85 | 1252.35 | 1320.98 | 1243.2 | 1343.85 | 1404.85 | °C |
| Explosive (deniability) limits | 18.20 | 5.70 | - | - | - | 10.70 | 13.20 | Vol % in air |
| Limiting oxygen for combustion | 5.00 | 12.00 | - | - | - | - | - | Vol % |
| Flammability limits | 4.10 | 5.30 | 4.40 | 4.40 | 4.50 | 4.60 | 4.60 | Vol % in air |
| Auto-ignition temperature | 560.00 | 600.00 | 590.00 | 590.00 | 580.00 | 580.00 | 570.00 | °C |
| Laminar burning velocity | 3.10 | 0.40 | 0.68 | 1.31 | 0.60 | 1.52 | 2.08 | m/s |
| Dilute gas viscosity at T ¼ 299 K | 0.000009 | 0.000011 | 0.000011 | 0.000010 | 0.000011 | 0.000010 | 0.000010 | Pa × s |
| Molecular diffusivity in air | 0.000061 | 0.000160 | 0.000150 | 0.000128 | 0.000153 | 0.000120 | 0.000101 | m2/s |
| Solubility in water | 0.0016 | 0.0250 | 0.0227 | 0.0174 | 0.0234 | 0.0156 | 0.0110 | kg/m3 |
References
- Grasso, N. Fire prevention technical rule for gaseous hydrogen transport in pipelines. Int. J. Hydrogen Energy 2008, 34, 4675–4683. [Google Scholar] [CrossRef]
- Paul, W. Pipeline Transportation of Hydrogen: Regulation, Research, and Policy. Congressional Research Service. Available online: https://www.everycrsreport.com/files/2021-03-02_R46700_294547743ff4516b1d562f7c4dae166186f1833e.pdf (accessed on 30 September 2022).
- Europe Could Operate 40,000 km of Hydrogen Pipelines by 2040. Reuters 2022. Available online: https://www.reuters.com/business/sustainable-business/europe-could-operate-40000-km-hydrogen-pipelines-by-2040-operators-2021-04-13/ (accessed on 12 August 2024).
- Messaoudani, Z.L.; Rigas, F.; Hamid, M.D.B.; Hassan, C.R.C. Hazards, safety and knowledge gaps on hydrogen transmission via natural gas grid: A critical review. Int. J. Hydrogen Energy 2016, 41, 17511–17525. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S. Economic Analysis on Hydrogen Pipeline Infrastructure Establishment Scenarios: Case Study of South Korea. Energies 2022, 15, 6824. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues. NREL. Available online: http://www.nrel.gov/docs/fy13osti/51995.pdf (accessed on 15 November 2022).
- Vidas, L.; Castro, R.; Pires, A. A Review of the Impact of Hydrogen Integration in Natural Gas Distribution Networks and Electric Smart Grids. Energies 2022, 15, 3160. [Google Scholar] [CrossRef]
- Pyza, D.; Gołda, P.; Sendek-Matysiak, E. Use of hydrogen in public transport systems. J. Clean. Prod. 2022, 335, 130247. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.-X.; Yao, R.; Wu, Y.-H.; Qiu, J.-S. Progress and prospects of hydrogen production: Opportunities and challenges. J. Electron. Sci. Technol. 2021, 19, 100080. [Google Scholar] [CrossRef]
- Hora, C.; Dan, F.C.; Rancov, N.; Badea, G.E.; Secui, C. Main Trends and Research Directions in Hydrogen Generation Using Low Temperature Electrolysis: A Systematic Literature Review. Energies 2022, 15, 6076. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Corporate Author, Kyoto Protocol. 2017. Available online: http://unfccc.int/kyoto_protocol/items/2830.php (accessed on 12 August 2024).
- Paris Agreement to the United Nations Framework Convention on Climate Change. 2015. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 12 August 2024).
- Pires, A.L.G.; Junior, P.R.; Morioka, S.N.; Rocha, L.C.S.; Bolis, I. Main Trends and Criteria Adopted in Economic Feasibility Studies of Offshore Wind Energy: A Systematic Literature Review. Energies 2021, 15, 12. [Google Scholar] [CrossRef]
- Kovács, K.E.; Dan, B.; Hrabéczy, A.; Bacskai, K.; Pusztai, G. Is Resilience a Trait or a Result of Parental Involvement? The Results of a Systematic Literature Review. Educ. Sci. 2022, 12, 372. [Google Scholar] [CrossRef]
- Ciccarelli, G.; Chaumeix, N.; Mendiburu, A.Z.; N’Guessan, K.; Comandini, A. Fast-flame limit for hydrogen/methane-air mixtures. Proc. Combust. Inst. 2019, 37, 3661–3668. [Google Scholar] [CrossRef]
- Scientific Data Curation Team. Thermodynamic and transport properties of hydrogen containing streams. Sci. Data 2020, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Ilbas, M.; Crayford, A.; Yilmaz, I.; Bowen, P.; Syred, N. Laminar-burning velocities of hydrogen–air and hydrogen–methane–air mixtures: An experimental study. Int. J. Hydrogen Energy 2006, 31, 1768–1779. [Google Scholar] [CrossRef]
- Cheng, R.K.; Oppenheim, A.K. Autoignition in methane-hydrogen mixtures. Combust. Flame 1984, 58, 125–139. [Google Scholar] [CrossRef]
- Conti, R.S.; Hertzberg, M. Thermal Autoignition Temperatures for Hydrogen-Air and Methane-Air Mixtures. J. Fire Sci. 1988, 6, 348–355. [Google Scholar] [CrossRef]
- Miao, H.; Lu, L.; Huang, Z. Flammability limits of hydrogen-enriched natural gas. Int. J. Hydrogen Energy 2011, 36, 6937–6947. [Google Scholar] [CrossRef]
- Vandenschoor, F.; Verplaetsen, F. The upper flammability limit of methane/hydrogen/air mixtures at elevated pressures and temperatures. Int. J. Hydrogen Energy 2007, 32, 2548–2552. [Google Scholar] [CrossRef]
- Pandey, A.; Mohan, S.V.; Chang, J.-S.; Hallenbeck, P.C.; Larroche, C. (Eds.) Biohydrogen. In Biomass, Biofuels, Biochemicals, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Cambridge, MA, USA, 2019. [Google Scholar]
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Veluswamy, H.P. Energy Storage in Hydrates: Status, Recent Trends, and Future Prospects. ACS Appl. Energy Mater. 2024. [Google Scholar] [CrossRef]
- Bolobov, V.I.; Latipov, I.U.; Popov, G.G.; Buslaev, G.V.; Martynenko, Y.V. Estimation of the Influence of Compressed Hydrogen on the Mechanical Properties of Pipeline Steels. Energies 2021, 14, 6085. [Google Scholar] [CrossRef]
- Schefer, R.; Houf, W.; Sanmarchi, C.; Chernicoff, W.; Englom, L. Characterization of leaks from compressed hydrogen dispensing systems and related components. Int. J. Hydrogen Energy 2006, 31, 1247–1260. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Zhang, J.; Akiyama, E.; Wang, Y.; Song, X. Review of Hydrogen Embrittlement in Metals: Hydrogen Diffusion, Hydrogen Characterization, Hydrogen Embrittlement Mechanism and Prevention. Acta Metall. Sin. (Engl. Lett.) 2020, 33, 759–773. [Google Scholar] [CrossRef]
- Li, H.; Niu, R.; Li, W.; Lu, H.; Cairney, J.; Chen, Y.-S. Hydrogen in pipeline steels: Recent advances in characterization and embrittlement mitigation. J. Nat. Gas Sci. Eng. 2022, 105, 104709. [Google Scholar] [CrossRef]
- Eames, I.; Austin, M.; Wojcik, A. Injection of gaseous hydrogen into a natural gas pipeline. Int. J. Hydrogen Energy 2022, 47, 25745–25754. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Thian, T.C.; Othman, M.R. Evaluation of hydrogen concentration effect on the natural gas properties and flow performance. Int. J. Hydrogen Energy 2021, 46, 974–983. [Google Scholar] [CrossRef]
- Gönczi, G. Unconventional methods for pressure loss reduction in standard pipe elements. Water Pract. Technol. 2018, 13, 355–361. [Google Scholar] [CrossRef]
- Di Lullo, G.; Oni, A.O.; Kumar, A. Blending blue hydrogen with natural gas for direct consumption: Examining the effect of hydrogen concentration on transportation and well-to-combustion greenhouse gas emissions. Int. J. Hydrogen Energy 2021, 46, 19202–19216. [Google Scholar] [CrossRef]
- Vaccariello, E.; Trinchero, R.; Stievano, I.S.; Leone, P. A Statistical Assessment of Blending Hydrogen into Gas Networks. Energies 2021, 14, 5055. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V.; Salgansky, E.A.; Arutyunov, A.V.; Arutyunov, V.S. A Comprehensive Review on the Prospects of Using Hydrogen–Methane Blends: Challenges and Opportunities. Energies 2022, 15, 2265. [Google Scholar] [CrossRef]
- Shaaban, S. Design and optimization of a novel flowmeter for liquid hydrogen. Int. J. Hydrogen Energy 2017, 42, 14621–14632. [Google Scholar] [CrossRef]
- Njoka, F.; Ookawara, S.; Ahmed, M. Influence of design and operating conditions on the performance of tandem photoelectrochemical reactors. Int. J. Hydrogen Energy 2018, 43, 1285–1302. [Google Scholar] [CrossRef]
- Pozzi, A.; Tognaccini, R. The effect of the Eckert number on impulsively started pipe flow. Eur. J. Mech. B Fluids 2012, 36, 120–127. [Google Scholar] [CrossRef]
- Urbanowicz, K. Fast and accurate modelling of frictional transient pipe flow. ZAMM Z. Für Angew. Math. Mech. J. Appl. Math. Mech. 2018, 98, 802–823. [Google Scholar] [CrossRef]
- Dyachenko, S.A.; Zlotnik, A.; Korotkevich, A.O.; Chertkov, M. Operator splitting method for simulation of dynamic flows in natural gas pipeline networks. Phys. D. Nonlinear Phenom. 2017, 361, 1–11. [Google Scholar] [CrossRef]
- Bulckaen, V. Energy losses in the transport in 4000 km pipelines of liquid hydrogen and oxygen derived from the splitting of water, and of liquid methane. Int. J. Hydrogen Energy 1992, 17, 613–622. [Google Scholar] [CrossRef]
- Kürten, D.; Khader, I.; Kailer, A. Determining the effective hydrogen diffusion coefficient in 100Cr6. Mater. Corros. 2020, 71, 918–923. [Google Scholar] [CrossRef]
- Johnson, D.; Covington, A.; Clark, N. Environmental and Economic Assessment of Leak and Loss Audits at Natural Gas Compressor and Storage Facilities. Energy Technol. 2014, 2, 1027–1032. [Google Scholar] [CrossRef]
- Peet, Y.; Sagaut, P.; Charron, Y. Pressure loss reduction in hydrogen pipelines by surface restructuring. Int. J. Hydrogen Energy 2009, 34, 8964–8973. [Google Scholar] [CrossRef]
- Li, J.; Han, G.; Zhao, M.; Qu, W.; Nie, M.; Song, W.; Xie, B.; Eller, F. Nitrogen input weakens the control of inundation frequency on soil organic carbon loss in a tidal salt marsh. Estuar. Coast. Shelf Sci. 2020, 243, 106878. [Google Scholar] [CrossRef]
- Mohanty, S.; Nayak, A.K.; Swain, C.K.; Dhal, B.R.; Kumar, A.; Kumar, U.; Tripathi, R.; Shahid, M.; Behera, K.K. Impact of integrated nutrient management options on GHG emission, N loss and N use efficiency of low land rice. Soil Tillage Res. 2020, 200, 104616. [Google Scholar] [CrossRef]
- Zhuang, C.; Shao, J.; Wang, Z.; Lu, Y.; Zhang, K.; Dou, Z. Explosion suppression of porous materials in a pipe-connected spherical vessel. J. Loss Prev. Process Ind. 2020, 65, 104106. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, A. The effects of aspect ratio on CH4/air flame stability in rectangular mesoscale combustors. J. Energy Inst. 2020, 93, 792–801. [Google Scholar] [CrossRef]
- RoyChowdhury, T.; Bramer, L.; Hoyt, D.W.; Kim, Y.M.; Metz, T.O.; McCue, L.A.; Diefenderfer, H.L.; Jansson, J.K.; Bailey, V. Temporal dynamics of CO2 and CH4 loss potentials in response to rapid hydrological shifts in tidal freshwater wetland soils. Ecol. Eng. 2018, 114, 104–114. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Wang, W.; Li, L.; Huo, Y. Numerical investigation on the evolution of forces and energy features in thermo-sensitive cavitating flow. Eur. J. Mech. B Fluids 2020, 84, 233–249. [Google Scholar] [CrossRef]
- Sánchez-Orgaz, E.M.; Denia, F.D.; Baeza, L.; Kirby, R. Numerical mode matching for sound propagation in silencers with granular material. J. Comput. Appl. Math. 2019, 350, 233–246. [Google Scholar] [CrossRef]
- Dhamala, T.N.; Pyakurel, U.; Dempe, S. A Critical Survey on the Network Optimization Algorithms for Evacuation Planning Problems; TU Bergakademie Freiberg, Fakultät für Mathematik und Informatik: Leipzig, Germany, 2018; Volume 15, p. 101. [Google Scholar]
- Yang, L.; Ji, Z.L.; Wu, T.W. Transmission loss prediction of silencers by using combined boundary element method and point collocation approach. Eng. Anal. Bound. Elem. 2015, 61, 265–273. [Google Scholar] [CrossRef]
- Hemme, C.; van Berk, W. Hydrogeochemical modeling to identify potential risks of underground hydrogen storage in depleted gas fields. Appl. Sci. 2018, 8, 2282. [Google Scholar] [CrossRef]
- Lei, Y.; Hosseini, E.; Liu, L.; Scholes, C.A.; Kentish, S.E. Internal polymeric coating materials for preventing pipeline hydrogen embrittlement and a theoretical model of hydrogen diffusion through coated steel. Int. J. Hydrogen Energy 2022, 47, 31409–31419. [Google Scholar] [CrossRef]
- Ekhtiari, A.; Flynn, D.; Syron, E. Investigation of the Multi-Point Injection of Green Hydrogen from Curtailed Renewable Power into a Gas Network. Energies 2020, 13, 6047. [Google Scholar] [CrossRef]
- Eileen, S.; Melissa, K.V.G. 1 Existing Natural Gas Pipeline Materials and Associated Operational Characteristics. DOE Hydrog. Program 2006. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/progress05/v_g_1_schmura.pdf?sfvrsn=79a23ec1_1.pdf (accessed on 12 August 2024).
- Huising, O.J.C.; Krom, A.H.M. H2 in an Existing Natural Gas Pipeline. In Volume 1: Pipeline and Facilities Integrity, 2020 13th International Pipeline Conference, Virtual, 28–30 September 2020; American Society of Mechanical Engineers: New York City, NY, USA, 2020. [Google Scholar] [CrossRef]
- Injecting Hydrogen in Natural Gas Grids Could Provide Steady Demand the Sector Needs to DeveloElectric Power. Natural Gas. SP Global, 19 May 2020. Available online: https://www.spglobal.com/platts/en/market-insights/blogs/natural-gas/051920-injecting-hydrogen-in-natural-gas-grids-could-provide-steady-demand-the-sector-needs-to-develop (accessed on 12 August 2024).
- Tong, S.; Li, X.; Sun, S.; Tu, C.; Xia, X. Interchangeability of Hydrogen Injection in Zhejiang Natural Gas Pipelines as a Means to Achieve Carbon Neutrality. Energies 2022, 15, 6394. [Google Scholar] [CrossRef]
- Tsui, L.; Garzon, F.; Agi, K. Solid-State Mixed-Potential Electrochemical Sensors for Natural Gas Leak Detection and Quality Control (Final Technical Report); University of New Mexico: Albuquerque, NM, USA, 2024; DOE-UNM--FE0031864; p. 2382681. [Google Scholar] [CrossRef]
- Mercuri, A.; Gianfelici, F.; Blasioli, G.; Luci, V.; Branduardi, L.; Arcangeletti, G.; Aloigi, E. Safe Delivery of H2 and CO2 in Offshore Pipeline Systems: Novel Methodology and Tools for Technological Risk Assessment. In Proceedings of the Offshore Technology Conference, OTC, Houston, TX, USA, 2–5 May 2022. [Google Scholar] [CrossRef]
- Isaac, T. HyDeploy: The UK’s First Hydrogen Blending Deployment Project. Clean Energy 2019, 3, 114–125. [Google Scholar] [CrossRef]
- Mark, F.R.; Paige, J.; Nicholas, G.; Elizabeth, C.; Richard, B.; Simon, A.J.; Amgad, E.; Jarett, Z. The Technical and Economic Potential of the H2@Scale Concept within the United States; National Renewable Energy Laborator: Golden, CO, USA, 2020; NREL/TP-6A20-77610. Available online: https://www.nrel.gov/docs/fy21osti/77610.pdf (accessed on 12 August 2024).
- Lamari, F.; Weinberger, B.; Langlois, P.; Fruchart, D. Instances of Safety-Related Advances in Hydrogen as Regards Its Gaseous Transport and Buffer Storage and Its Solid-State Storage. Hydrogen 2024, 5, 387–402. [Google Scholar] [CrossRef]
- Smith, J. The Role of Hydrogen Fuel in Hard-to-Decarbonise Modes of Transport: An Energy Systems Perspective. Ph.D. Thesis, Apollo—University of Cambridge Repository, Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Klopčič, N.; Stöhr, T.; Grimmer, I.; Sartory, M.; Trattner, A. Refurbishment of Natural Gas Pipelines towards 100% Hydrogen—A Thermodynamic-Based Analysis. Energies 2022, 15, 9370. [Google Scholar] [CrossRef]
- Zhang, J.X.; An, C.; Wei, D.F.; Chen, B.Q.; Soares, C.G. Structural Behaviour of Hydrogen Flexible Pipe under Internal Pressure. In Trends in Renewable Energies Offshore; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Victor FAGNON. Techno-Economic Assessment of Flexible Composite Pipelines for Decentralised Production of H2 with Electrolysers Integrated in Wind Turbine of Stavanger, 2022. Available online: https://uis.brage.unit.no/uis-xmlui/bitstream/handle/11250/3032550/no.uis:inspera:102983723:64696369.pdf?sequence=1 (accessed on 12 August 2024).
- Nemeth, A.; Czapski, D.; Alexander, C. Optimizing Operator Systems Through the Use of Flexible Composite Pipe. In Volume 2: Pipeline and Facilities Integrity; American Society of Mechanical Engineers: Calgary, AB, Canada, 2022. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, Y.; Shen, W.; Li, H.; Nan, Z.; Dong, M.; Bian, J.; Cao, X. Key Technologies of Pure Hydrogen and Hydrogen-Mixed Natural Gas Pipeline Transportation. ACS Omega 2023, 8, 19212–19222. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Mahajan, D.; Venkatesh, T.A. Computational fluid dynamic modeling of methane-hydrogen mixture transportation in pipelines: Understanding the effects of pipe roughness, pipe diameter and pipe bends. Int. J. Hydrogen Energy 2024, 49, 1028–1042. [Google Scholar] [CrossRef]
- Thawani, B.; Hazael, R.; Critchley, R. Assessing the pressure losses during hydrogen transport in the current natural gas infrastructure using numerical modelling. Int. J. Hydrogen Energy 2023, 48, 34463–34475. [Google Scholar] [CrossRef]
- Ruiz-Tagle, A.; Groth, K.M. Comparing the risk of third-party excavation damage between natural gas and hydrogen pipelines. Int. J. Hydrogen Energy 2024, 57, 107–120. [Google Scholar] [CrossRef]

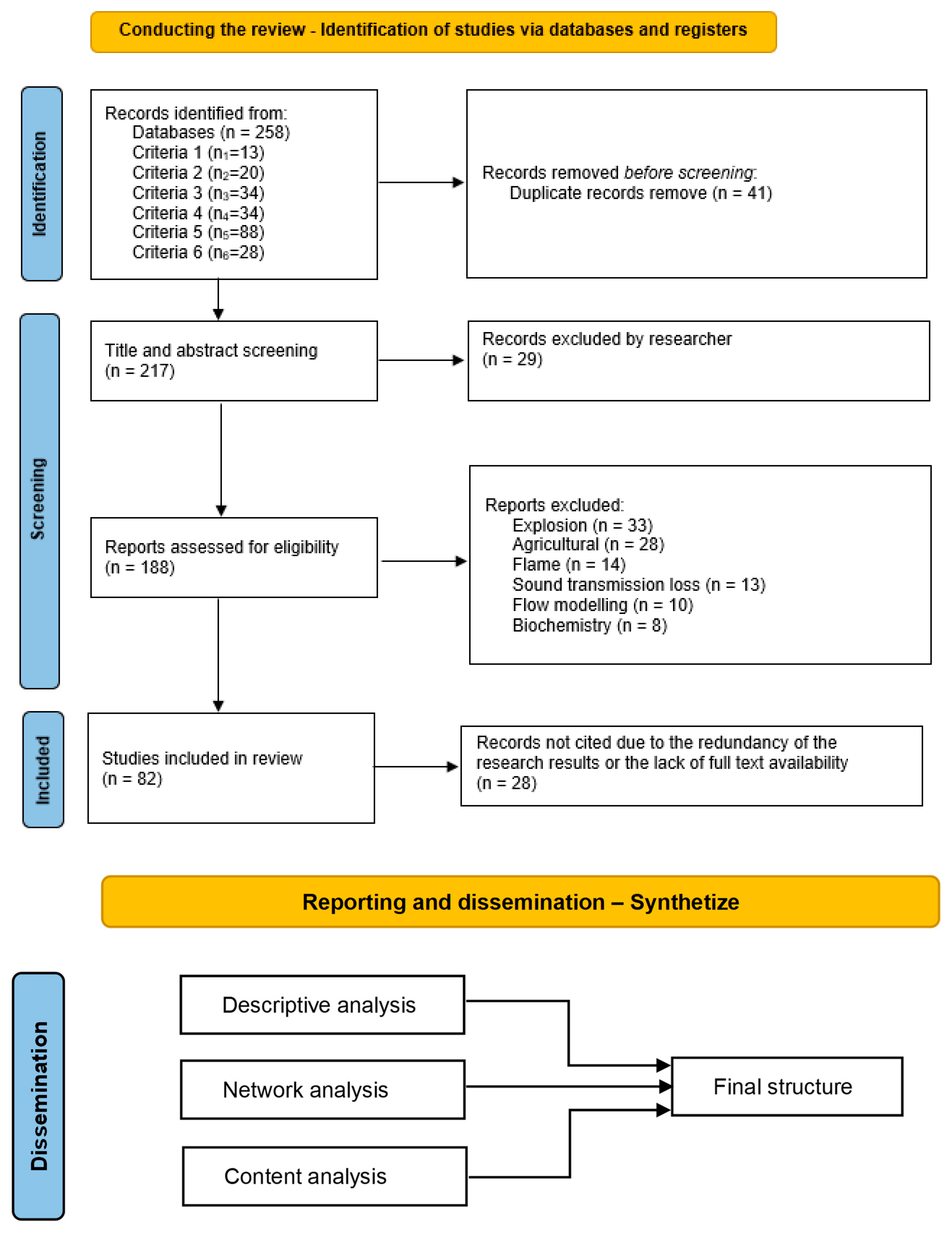



| Criterion 1 | Criterion 2 | Criterion 3 | Criterion 4 | Criterion 5 | Criterion 6 | |
|---|---|---|---|---|---|---|
| Key words | H2 losses in pipes | Losses in pipes | Fluid transmission losses | Hythane losses | CH4 losses | NA 1 |
| Disciplines | Appendix A | All | ||||
| Expanders | Also search within the full text of the articles/apply equivalent subjects | NA | ||||
| Search modes | Appendix A | Appendix A | ||||
| Results’ limits | Full text peer-reviewed | |||||
| Published date | 2012 ÷ 2024 | No time limit | ||||
| Language | English | |||||
| Result [papers] | 13 | 200 | 40 | 34 | 88 | 5 |
| Properties | Hydrogen (H2) | Methane (CH4) | Hythane 10% H2-90% CH4 | Hythane 25% H2-75% CH4 | Hythane 30% H2-70% CH4 | Mixture 40% H2-60% CH4 | Mixture 60% H2-40% CH4 | Unit |
|---|---|---|---|---|---|---|---|---|
| Lower calorific value by volume at 1 atm | 11.00 | 35.00 | 32.60 | 27.20 | 33.32 | 25.40 | 20.60 | MJ/m3 |
| Higher calorific value by volume at 1 atm | 13.00 | 39.00 | 36.00 | 30.00 | 27.00 | 28.00 | 20.00 | MJ/m3 |
| Maximum flame temperature | 1800.00 | 1495.00 | 1525.50 | 1594.13 | 1516.35 | 1617.00 | 1678.00 | K |
| Auto-ignition temperature | 560.00 | 600.00 | 590.00 | 590.00 | 580.00 | 580.00 | 570.00 | C |
| Laminar burning velocity | 3.10 | 0.40 | 0.68 | 1.31 | 0.60 | 1.52 | 2.08 | m/s |
| Consequences | Occurrence Probability | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–20% | 21–40% | 41–60% | 61–80% | 81–100% | ||||||
| A | B | C | D | E | ||||||
| Severity | Health/Safety | Environment | PR | Corporate Impact | Almost Certain | Rare | Unlikely | Possible | Likely | Certain |
| 5 Severe | Potential for multiple fatalities | Potential catastrophic damage | Governmental level | Lack of functionality >7 days | >3 days | 5A | 5B | 5C | 5D | 5E |
| 4 Major | Potential for single fatality | Potential long-term effects | Public disruption | Lack of functionality >1 day | <3 days | 4A | 4B | 4C | 4D | 4E |
| 3 Moderate | Potential for a single major injury | Potential medium-term effects | Small public disruption | Lack of functionality <1 day | >2 days | 3A | 3B | 3C | 3D | 3E |
| 2 Minor | Potential for lost time | Potential long-term effects | Local media coverage | Corrective maintenance | >2 days | 2A | 2B | 2C | 2D | 2E |
| 1 Marginal | Potential for first aid injury | Potential long-term effects | No media coverage | Malfunction | No imp. | 1A | 1B | 1C | 1D | 1E |
| CH4 Infrastructure | Pressure Regulation | Meters | CHG Storage Tanks | House Install | Seals /Valves | Transmission Pipelines | Co-Gen Plants | Home Gas Burners/Stove | Compression Station | Gas Turbines |
|---|---|---|---|---|---|---|---|---|---|---|
| H2 [%] | 67 | 30 | 30 | 30 | 30 | 30 | 20 | 10 | 2 | 1 |
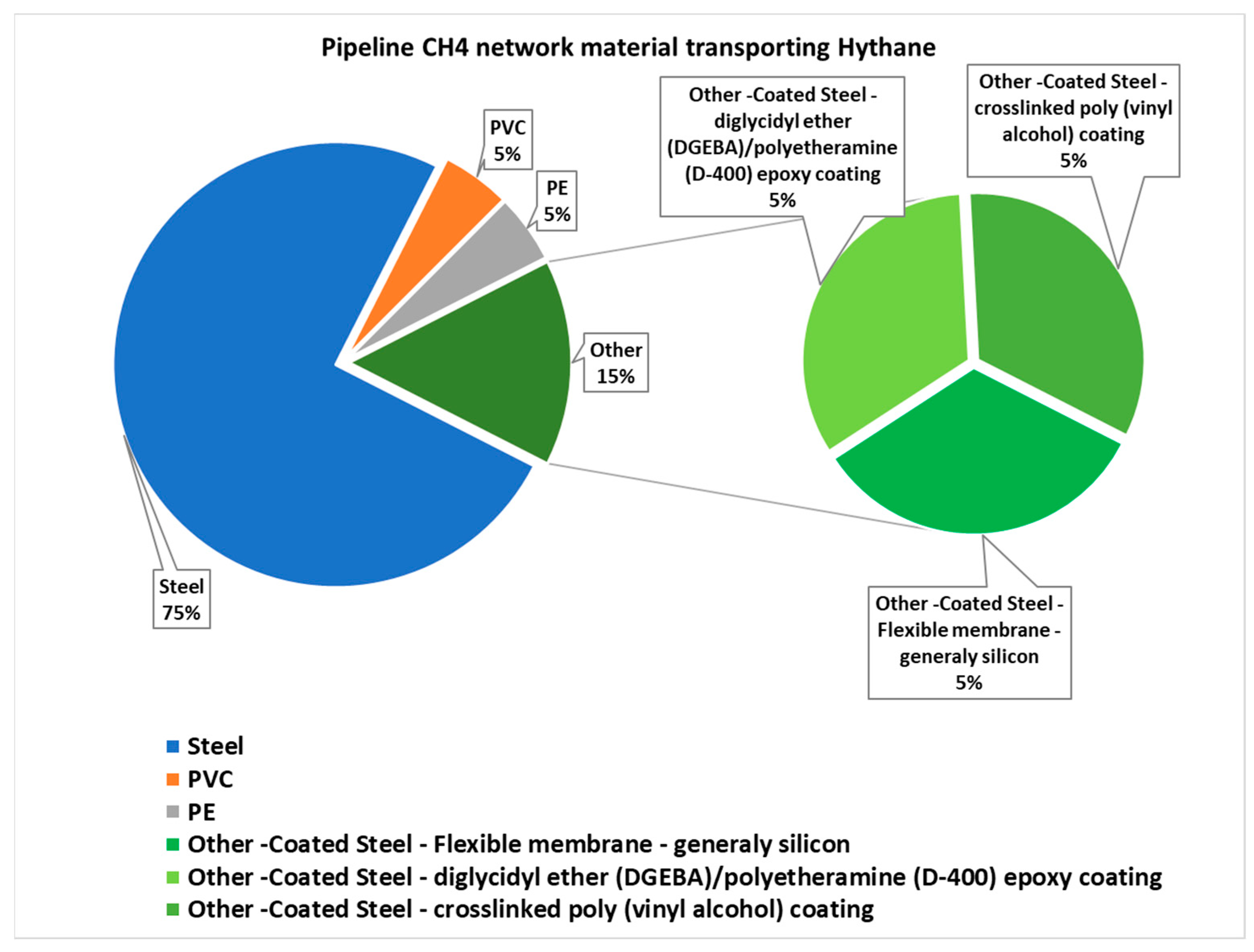
| Transport network | Steel pipes | PVC pipeline | Sealants | Connectors | Fittings | Flow valves | Domestic pipe | |||
| H2 [%] | 30 | 70 | 30 | 30 | 30 | 15 | 30 | |||
| Transport equipment | Pipeline | Turbine | Compressor | |||||||
| 30 | 1 | 5 |
| Methane | Hydrogen |
|---|---|
| greater density | higher heat capacity |
| greater viscosity | higher diffusivity |
| higher calorific value by volume | higher calorific value by mass |
| higher solubility in water | higher flame temperature |
| higher auto-ignition temperature | |
| wider explosive and fire danger |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hora, C.; Dan, F.C.; Secui, D.-C.; Hora, H.N. Systematic Literature Review on Pipeline Transport Losses of Hydrogen, Methane, and Their Mixture, Hythane. Energies 2024, 17, 4709. https://doi.org/10.3390/en17184709
Hora C, Dan FC, Secui D-C, Hora HN. Systematic Literature Review on Pipeline Transport Losses of Hydrogen, Methane, and Their Mixture, Hythane. Energies. 2024; 17(18):4709. https://doi.org/10.3390/en17184709
Chicago/Turabian StyleHora, Cristina, Florin Ciprian Dan, Dinu-Calin Secui, and Horea Nicolae Hora. 2024. "Systematic Literature Review on Pipeline Transport Losses of Hydrogen, Methane, and Their Mixture, Hythane" Energies 17, no. 18: 4709. https://doi.org/10.3390/en17184709
APA StyleHora, C., Dan, F. C., Secui, D.-C., & Hora, H. N. (2024). Systematic Literature Review on Pipeline Transport Losses of Hydrogen, Methane, and Their Mixture, Hythane. Energies, 17(18), 4709. https://doi.org/10.3390/en17184709







