Potential of Wheat Straw for Biogas Production by Anaerobic Digestion in South Africa: A Review
Abstract
1. Introduction
2. Availability of Wheat Straw in South Africa
3. Structural Composition of Wheat Straw and Limitations for Biogas Production
4. Wheat Straw Residue to Biogas
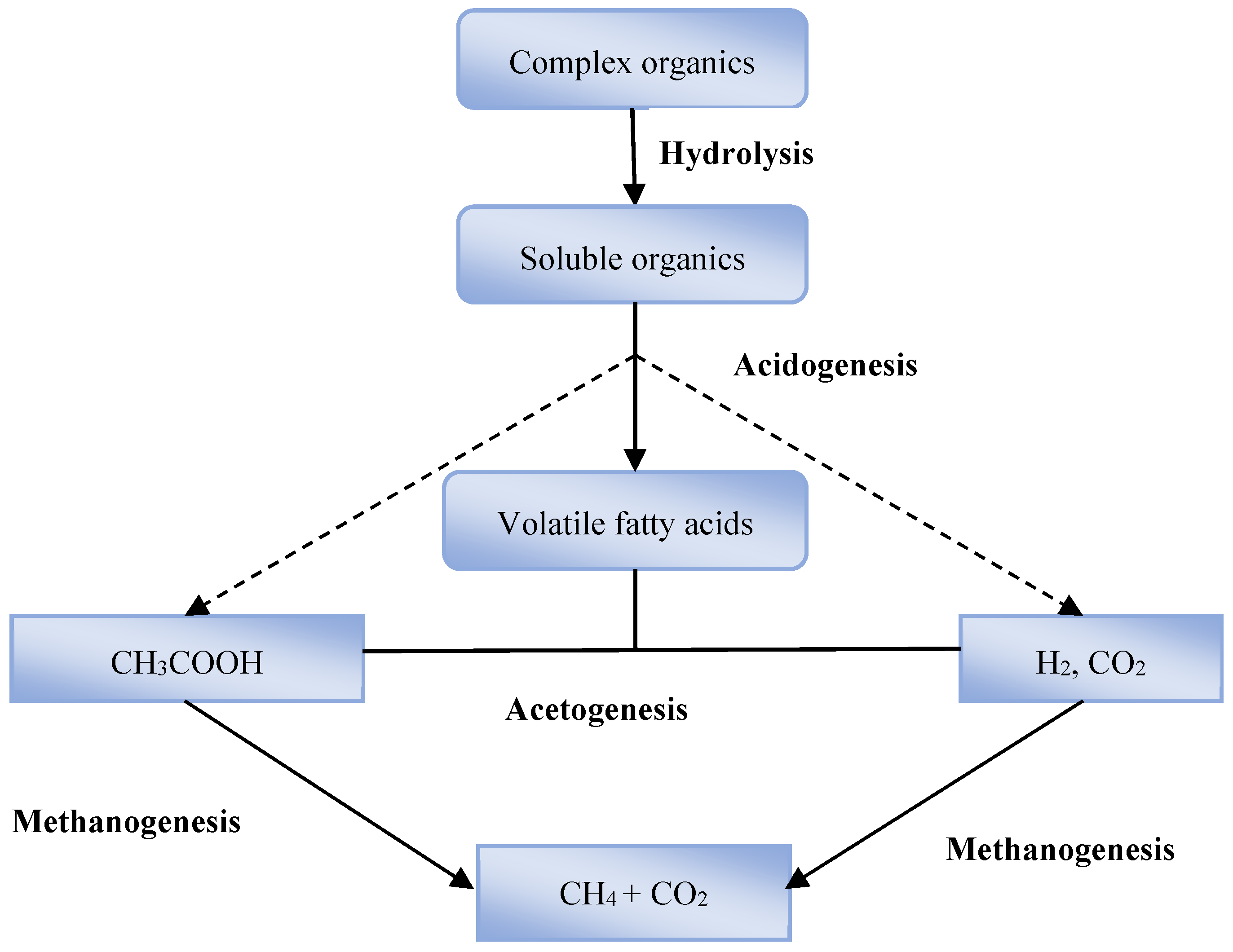
4.1. The Anaerobic Digestion Pathway
4.2. Biomethane Potential of Wheat Straw
5. Potential Strategies to Enhance Biogas Production from Wheat Straw
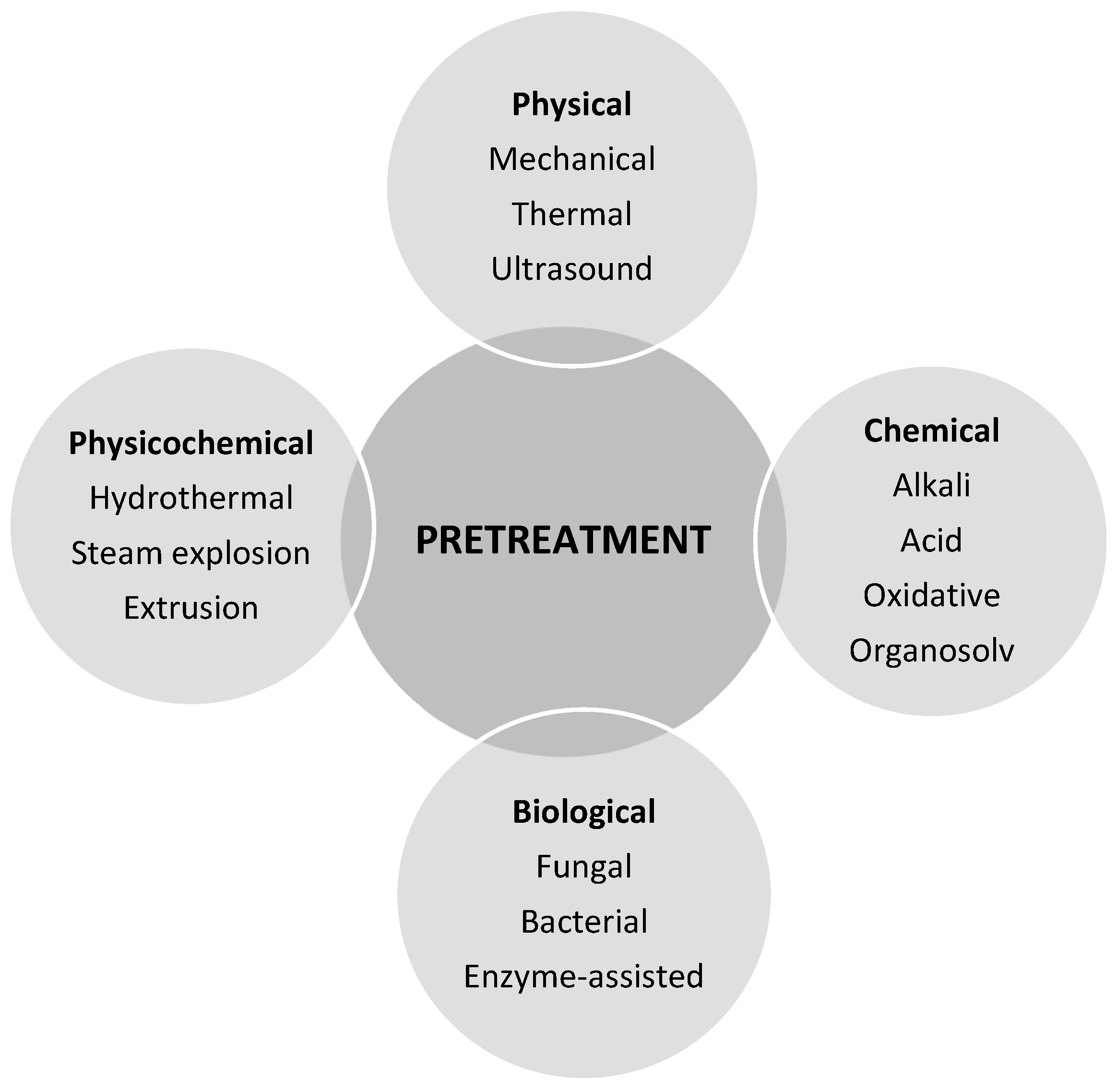
5.1. Pre-Treatment of Wheat Straw
5.1.1. Physical Pre-Treatment
| Physical Agent | Pre-Treatment Conditions | Findings | Reference |
|---|---|---|---|
| Mechanical | Knife milling, 0.3–1.2 mm particle size | Methane yield increased by 49.3% | [53] |
| Roll milling | 21% increase in methane yield | [14] | |
| Cutting (3–5 cm), milling (<1 mm) | 5–13% more methane for 3–5 cm particles with faster kinetics | [43] | |
| Chopping (2 cm), extruder-grinding (0.2 cm) | Size reduction improved methane yield by 26% | [60] | |
| Conventional thermal | 150–220 °C, 1–15 min | 20% increase in methane yield | [43] |
| 200 °C, 5 min | 27% more methane production | [4] | |
| 121 °C, 60 min | 20% increase in methane yield | [55] | |
| 150–220 °C, 1–15 min | Methane yield enhanced by 20% | [56] | |
| Microwave | Power of 400–1600 W, 150 °C | 28% increase in methane yield | [57] |
| 200–300 °C, 15 min | No increase in methane yield | [61] | |
| Ultrasound | 4% KOH, 20 kHz, ambient temperature, 36 h | 63% higher methane yield | [59] |
| Hydrodynamic cavitation, 2300–2700 rpm, 2–6 min | 145% increased methane yield | [62] | |
| 4% (w/w) H2O2, 36 °C, 10 min, 25 kHz | 64% enhanced methane yield | [63] |
5.1.2. Chemical Pre-Treatment
| Chemical Agent | Pre-Treatment Conditions | Findings | Reference |
|---|---|---|---|
| Acid | 1% H2SO4, 121 °C, 10–120 min | Increased methane yield by 16% | [65] |
| 0.5–5% H2SO4, 90–100 °C, 2 h | Biogas yield increased by 32% using 0.5% H2SO4 while 5% H2SO4 did not improve biogas yield | [69] | |
| 2% HCl, 90 °C, 2 h | 59% more biogas yield | [70] | |
| Alkaline | 1.6% NaOH, 30 °C, 24 h | 15% enhanced methane yield | [66] |
| NH3 (2, 4, 6%), 35 °C, 7 d | 52% increased methane yield | [71] | |
| 4% NaOH, 37 °C, 5 d | Biogas yield increased by 87.5% | [64] | |
| 7 g KOH, 25 °C, 24 h | 128% methane yield increment | [47] | |
| 75 mM NaOH, 16 h | Methane yield increased by 23% | [72] | |
| 0.05 M NaOH, 25 °C, 22 h | 22% increase in cumulative methane | [44] | |
| 0.08 M Ca(OH)2, 20 °C, 48 h | Methane yield increased by 315% | [73] | |
| Oxidative | TiO2-assisted photo-oxidation | Improved methane yield by 27% | [67] |
| NMMO, 120 °C, 3 h | 11% methane yield improvement | [66] | |
| Organosolv | NMMO, 90 °C, 7 h | 47% increase in methane production | [68] |
| 50% ethanol, 180 °C, 1 h | 15% improved methane yield | [66] | |
| NMMO, 120 °C, 3 h | 11% enhanced methane yield | [66] |
5.1.3. Physico-Chemical Pre-Treatment
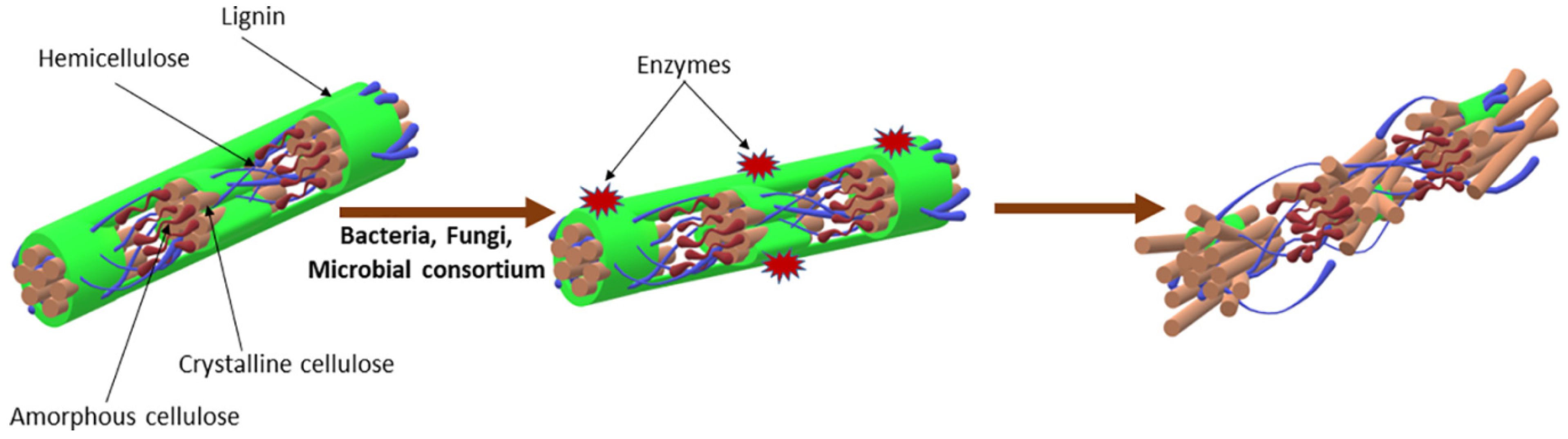
5.1.4. Biological Pre-Treatment
| Biological Agent | Microbes and Enzymes | Pre-Treatment Conditions | Findings | Reference |
|---|---|---|---|---|
| Fungi | Penicillium aurantiogriseum, Trichoderma reesei, Gilbertellapersicaria, Rhizomucormiehei | 100 mL batch reactors, 37 °C, 10 d | Highest methane yield increase of 48% from P. aurantiogriseum pre-treated wheat straw | [94] |
| Polyporusbrumalis | 40 L aerobic reactors, 31 °C, 90% moisture, 13 d | 18% increase in methane yield | [86] | |
| Chaetomium globosporum | Reagent bottles, 36 °C, 81% moisture, 14 d | 31% enhanced methane yield | [87] | |
| Ganoderma lobatum, Gloeophyllumtrabeum | 250 mL Erlenmeyer flasks, dark, 25 °C, 10–40 d | 43.6 and 26.1% increase in glucose yield by G. lobatum and G. trabeum, respectively | [95] | |
| Ligninolytic fungi | 250 mL Erlenmeyer flasks, 28 °C, 150 rpm, 7 d | Five-fold higher biogas yield | [96] | |
| Microbial consortium | Microbial consortium TC-5 | 1 L anaerobic bottles, 50 °C, 3 d | 22.2 and 36.6% increase in methane yield under mesophilic and thermophilic conditions, respectively | [92] |
| Microbial consortium | Batch, 37 °C, 20 d | 80.34% improved methane yield | [91] | |
| Cow rumen-derived microbial consortium | 35 °C, 15 d | 55.5% lignocellulose degradation | [1] | |
| Microbial consortium composed of fungi and bacteria | 39.24 and 80.34% increase in biogas and methane yield, respectively | [91] | ||
| Enzymes | Cellulase, xylanase, arabinase, pectinase, other carbohydrases, β-glucosidase | 100 mL glass reactors, 50 °C, 16 h | 14% enhanced methane yield | [72] |
| Laccase, peroxidase | 30 °C, 60 rpm, 6 and 24 h | 11% increased methane yield after 6 h pre-treatment and 15% decreased methane yield after 24 h pre-treatment | [97] |
5.2. Anaerobic Co-Digestion
| Co-Substrate | Inoculum | Experimental Conditions | Methane Potential (mL g VS−1) | Reference |
|---|---|---|---|---|
| Food waste, cattle manure | Sewage sludge from anaerobic digester | 610 mL glass bottles, 35 °C, 100 rpm, 45 d | 416 | [101] |
| Rapeseed meal | Effluent from mesophilic digester | 150 mL serum glass vials, 42 °C, 30 d | 375 | [106] |
| Herbal extraction process residues | Anaerobic sludge of pig manure | 250 mL batch digesters, 30 d | 178 | [107] |
| Swine manure | [108] | |||
| Cattle manure | Cattle manure | 1 L glass bottles, 35 °C, 50 d | 109 | [109] |
| Swine manure | [102] | |||
| Animal manure | Anaerobic sludge of dairy manure | 1 L ground flasks, 3 g magnetite, 35 °C | 228 | [42] |
| Animal manure | Cow dung | 1 L aspirator glass bottles, 25–30 °C, 20 d | 566 | [20] |
| Sunflower meal | Digested manure | 300 mL serum bottles, 35 °C, 60 d | 591 | [46] |
| Rice straw | Digested manure | 300 mL glass bottles, pH 7–7.5, 35 °C, 60 d | 339 | [110] |
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lazuka, A.; Auer, L.; Bozonnet, S.; Morgavi, D.P.; O’Donohue, M.; Hernandez-Raquet, G. Efficient anaerobic transformation of raw wheat straw by a robust cow rumen-derived microbial consortium. Bioresour. Technol. 2015, 196, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.M.; Gahlot, P.; Moustakas, K.; Kazmi, A.A.; Ojha, C.S.P.; Tyagi, V.K. Pre-treatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [Google Scholar] [CrossRef]
- Batidzirai, B.; Valk, M.; Wicke, B.; Junginger, M.; Daioglou, V.; Euler, W.; Faaij, A.P.C. Current and future technical, economic and environmental feasibility of maize and wheat residues supply for biomass energy application: Illustrated for South Africa. Biomass Bioenergy 2016, 92, 106–129. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Nilsen, P.J.; Fdz-Polanco, F.; Perez-Elvira, S.I. Biomethane potential of wheat straw: Influence of particle size, water impregnation and thermal hydrolysis. Chem. Eng. J. 2014, 242, 254–259. [Google Scholar] [CrossRef]
- Kamusoko, R.; Jingura, R.M.; Chikwambi, Z.; Parawira, W. Strategies for valorization of crop residues into biofuels and other value-added products. Biofuels Bioprod. Biorefining 2021, 15, 1950–1964. [Google Scholar] [CrossRef]
- Shinde, R.; Shahi, D.K.; Mahapatra, P.; Singh, C.S.; Naik, S.K.; Thombare, N.; Singh, A.K. Management of crop residues with special reference to the on-farm utilization methods: A review. Ind. Crops Prod. 2022, 181, 114772. [Google Scholar] [CrossRef]
- Ge, X.; Xu, F.; Li, Y. Solid-state anaerobic digestion of lignocellulosic biomass: Recentprogress and perspectives. Bioresour. Technol. 2016, 205, 239–249. [Google Scholar] [CrossRef]
- Majd, S.S.; Abdoli, M.A.; Karbassi, A.; Pourzamani, H.R.; Rezaee, M. Effect of physical and chemical operating parameters on anaerobic digestion of manure and biogas production: A review. J. Environ. Health Sustain. Dev. 2017, 2, 235–247. [Google Scholar]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pre-treatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef]
- Srivastava, N.; Shrivastav, A.K.; Srivastava, M.; Mishra, P.K. Recent Developments in Bioenergy Research; Gupta, V.K., Treichel, H.H., Kuhad, R.C., Rodriguez-Cout, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 22; p. 433. [Google Scholar]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B. Pre-treatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Rehman, M.L.U.; Iqbal, A.; Chang, C.; Li, W.; Ju, M. Anaerobic digestion. Water Environ. Res. 2019, 91, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wei, S. Efficient short time pre-treatment on lignocellulosic waste using an isolated fungus Trametes sp. W-4 for the enhancement of biogas production. Heliyon 2023, 9, e14573. [Google Scholar] [CrossRef]
- Victorin, M.; Davidsson, A.; Wallberg, O. Characterization of mechanically pre-treated wheat straw for biogas production. Bionergy Res. 2020, 13, 833–844. [Google Scholar] [CrossRef]
- Rangseesuriyachai, T.; Boonnorat, J.; Glanpracha, N.; Khetkorn, W.; Thiamngoen, P.; Pinpathanapong, K. Anaerobic co-digestion of elephant dung and biological pre-treated Napier grass: Synergistic effect and kinetics of methane production. Biomass Bioenergy 2023, 175, 106849. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Ye, J.; Wu, Y.; Fang, W.; Gou, X.; Zeng, G. Improvement of methane production from rice straw with rumen fluid pre-treatment: A feasibility study. Int. J. Biodeterior. Biodegrad. 2016, 113, 9–16. [Google Scholar] [CrossRef]
- Awogbemi, O.; Kallon, D.V.V. Pre-treatment techniques for agricultural waste. Case Stud. Chem. Environ. Eng. 2022, 6, 100229. [Google Scholar] [CrossRef]
- Ameen, F.; Ranjitha, J.; Ahsan, N.; Shankar, V. Co-digestion of microbial biomass with animal manure in three-stage anaerobic digestion. Fuel 2021, 306, 121746. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Khanal, S.K. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef]
- Rani, P.; Pathak, V.V.; Bansal, M. Co-digestion of wheat straw and animal manure pretreated with calcium hydroxide for biomethane production: Kinetic study. Curr. Res. Green Sustain. Chem. 2021, 4, 100145. [Google Scholar] [CrossRef]
- Department of Agriculture, Forestry & Fisheries. A Profile of the South African Wheat Market Value Chain; Department of Agriculture, Forestry & Fisheries: Pretoria, South Africa, 2021.
- Barahira, D.S.; Okudoh, V.I.; Eloka-Eboka, A.C. Suitability of crop residues as feedstock for biofuel production in South Africa: A sustainable win-win scenario. J. Oleo Sci. 2021, 70, 213–226. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Bergamo, T.F.; Kikas, T. Potential of cereal-based agricultural residues available for bioenergy production. Data Brief 2019, 23, 103829. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.P.; Poonia, A.K.; Chaudhari, P.K. Pre-treatment of lignocellulosic agricultural waste for delignification, rapid hydrolysis and enhanced biogas production: A review. J. Indian Chem. Soc. 2021, 98, 100147. [Google Scholar] [CrossRef]
- Tabussam, T.; Farhan, S.; Muhammad, I.; Muhammad, U.A.; Faqir, M.A.; Muhammad, A.; Huma, B.U.A.; Muhammad, S.; Tanweer, A.G.; Shahzad, H. Biochemical characterization of wheat straw cell wall with special reference to bioactive profile. Int. J. Food Prop. 2018, 21, 1303–1310. [Google Scholar]
- Tsapekos, P.; Kougias, P.G.; Vasileiou, S.A.; Treu, L.; Campanaro, S.; Lyberatos, G.; Angelidaki, I. Bioaugmentation with hydrolytic microbes to improve the anaerobic biodegradability of lignocellulosic agricultural residues. Bioresour. Technol. 2017, 234, 350–359. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Hong, Y.; La, D.D.; Nguyen, X.G.; Ngo, H.H.; et al. Review on pre-treatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Swain, M.R.; Singh, A.; Sharma, A.K.; Tulli, D.K. Bioethanol Production from Food Crops; Ray, R.C., Ramachandran, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 11; p. 213. [Google Scholar]
- Passoth, V.; Sandgren, M. Biofuel production from straw hydrolysates: Current achievements and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5105. [Google Scholar] [CrossRef]
- Kamusoko, R.; Jingura, R.M.; Parawira, W.; Chikwambi, Z. Characterization of lignocellulosic crop residues for potential biogas production in Zimbabwe. Biofuels Bioprod. Bioref. 2022, 16, 1165–1171. [Google Scholar] [CrossRef]
- Sambusiti, C. Physical, Chemical and Biological Pre-Treatment to Enhance Biogas Production from Lignocellulosic Substrates. Ph.D. Thesis, Politecnico Di Milano, Milan, Italy, 2013. [Google Scholar]
- Kumar, S.; Paritosh, K.; Pareek, N.; Chawade, A.; Vivekanand, V. Deconstruction of major Indian cereal crop residues through chemical pre-treatment for improved biogas production: An overview. Renew. Sustain. Energy Rev. 2018, 90, 160–170. [Google Scholar] [CrossRef]
- Dar, R.A.; Parmar, M.; Dar, E.A.; Sani, R.K.; Phutela, U.G. Biomethanation of agricultural residues: Potential, limitations and possible solutions. Renew. Sustain. Energy Rev. 2021, 135, 110217. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pre-treatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 3. [Google Scholar] [CrossRef]
- Scholes, C.A. Advances in Carbon Capture: Methods, Technologies and Applications; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; Chapter 16; p. 357. [Google Scholar]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pre-treatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Prasad, S.; Rathore, D.; Singh, A. Recent advances in biogas production. Chem. Eng. Process Techniq. 2017, 3, 1038. [Google Scholar]
- Raju, C.S.; Ward, A.J.; Nielsen, L.; Moller, H.B. Comparison of near infra-red spectroscopy, neutral detergent fiber assay and in-vitro organic matter digestibility assay for rapid determination of the biochemical methane potential of meadow grasses. Bioresour. Technol. 2011, 102, 7835–7839. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Davila, E.; Hernandez, J.J.; Gonzalez, L.M.L.; Cardoso, E.L.B.; Amarante, E.B.; Velazquez, L.M.C.; Romero-Romero, O. Biochemical methane potential of agro-wastes as a renewable source alternative for electrical energy production in Cuba. Cienc. Y Tecnol. Agropecu. 2022, 23, e1890. [Google Scholar] [CrossRef]
- Ozbayram, E.G.; Kleinsteuber, S.; Nikolausz, M.; Ince, B.; Ince, O. Effect of bioaugmentation by cellulolytic bacteria enriched from sheep rumen on methane production from wheat straw. Anaerobe 2017, 46, 122–130. [Google Scholar] [CrossRef]
- Sukhesh, M.J.; Rao, P.V. Anaerobic digestion of crop residues: Technological developments and environmental impact in the Indian context. Biocatal. Agric. Biotechnol. 2018, 16, 513–528. [Google Scholar] [CrossRef]
- Liu, X.; Zicari, S.M.; Liu, G.; Li, Y.; Zhang, R. Pre-treatment of wheat straw with potassium hydroxide for increasing enzymatic and microbial degradability. Bioresour. Technol. 2015, 185, 150–157. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Donoso-Bravo, A.; Nilsen, P.J.; Fdz-Polanco, F.; Perez-Elvira, S.I. Influence of thermal pre-treatment on the biochemical methane potential of wheat straw. Bioresour. Technol. 2013, 143, 251–257. [Google Scholar] [CrossRef]
- Zdeb, M. Anaerobic digestion of wheat straw pre-treated with soaking in water and alkali medium. J. Ecol. Eng. 2021, 22, 246–254. [Google Scholar] [CrossRef]
- Moset, V.; Xavier, C.; de Almeida, N.; Feng, L.; Wahid, R.; Møller, H.B. Combined low thermal alkali addition and mechanical pre-treatment to improve biogas yield from wheat straw. J. Clean. Prod. 2018, 172, 1391–1398. [Google Scholar] [CrossRef]
- Rajput, A.A.; Zeshan; Hassan, M. Enhancing biogas production through co-digestion and thermal pre-treatment of wheat straw and sunflower meal. Renew. Energy 2021, 168, 1–10. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Wahid, R.; Hernández, V.; Møller, H.; Fernandez, B. Improvement of wheat straw anaerobic digestion through alkali pre-treatment: Carbohydrates bioavailability evaluation and economic feasibility. Sci. Total Environ. 2017, 595, 651–695. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S. Handbook of Biofuels; Sahay, S., Ed.; Academic Press: Cambridge, MA, USA, 2022; Chapter 10; p. 207. [Google Scholar]
- Yan, L.; Gao, Y.; Wang, Y.; Liu, Q.; Sun, Z.; Fu, B.; Wen, X.; Cui, Z.; Wang, W. Diversity of a mesophilic lignocellulolytic microbial consortium which is useful for enhancement of biogas production. Bioresour. Technol. 2012, 111, 49–54. [Google Scholar] [CrossRef]
- Moodley, P.; Trois, C. Applied Biotechnology Reviews, Sustainable Biofuels: Opportunities and Challenges; Ray, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2021; Chapter 2; p. 21. [Google Scholar]
- Rodriguez, C.; Alaswad, A.; Benyounis, K.Y.; Olabi, A.G. Pre-treatment techniques used for biogas production from grass. Renew. Sustain. Energy Rev. 2016, 68, 1193–1204. [Google Scholar] [CrossRef]
- Dell’Omo, P.P.; Spena, V.A. Mechanical pre-treatment of lignocellulosic biomass to improve biogas production: Comparison of results for giant reed and wheat straw. Energy 2020, 203, 117798. [Google Scholar] [CrossRef]
- Dell’Omo, P.; Froscia, S.L. Enhancing anaerobic digestion of wheat straw through multistage milling. Model. Meas. Control C 2018, 79, 127–132. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, R.; Li, K.; Ma, R. A review of crop straw pre-treatment methods for biogas production by anaerobic digestion in China. Renew. Sustain. Energy Rev. 2019, 107, 51–58. [Google Scholar] [CrossRef]
- Bolado-Rodriguez, S.; Toquero, C.; Martin-Juarez, J.; Travaini, R.; Garcia-Encina, P.A. Effect of thermal, acid, alkaline and alkaline-peroxide pre-treatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour. Technol. 2016, 201, 182–190. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.A.; En, S.C.F.; Elkamel, A.; Ahmadi, L.; Yetilmezsoy, K. A review of standards and guidelines set by international bodies for the parameters of indoor air quality. Atmos. Pollut. Res. 2015, 6, 751–767. [Google Scholar] [CrossRef]
- Jackowiak, D.; Bassard, D.; Pauss, A.; Ribeiro, T. Optimization of a microwave pre-treatment of wheat straw for methane production. Bioresour. Technol. 2011, 102, 6750–6756. [Google Scholar] [CrossRef]
- Jain, S.; Jain, S.; Wolf, I.T.; Lee, J.; Tong, Y.W. A comprehensive review on operating parameters and different pre-treatment technologies for anaerobic digestion of municipal solid waste. Renew. Sustain. Energy Rev. 2015, 52, 142–154. [Google Scholar] [CrossRef]
- Korai, R.M.; Wachemo, A.C.; Yue, L.; Jaffar, M.; Li, Z.; Shahbaz, M.; Yuana, H.; Li, X. Effect of ultrasonic application during KOH pre-treatment and anaerobic process on digestion performance of wheat straw. RSC Adv. 2020, 10, 9290. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, D.; Wedwitschka, H.; Moeller, L.; Zehnsdorf, A.; Stinner, W. Effect of particle size reduction and ensiling fermentation on biogas formation and silage quality of wheat straw. Bioresour. Technol. 2017, 245, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Sapci, Z. The effect of microwave pre-treatment on biogas production from agricultural straws. Bioresour. Technol. 2013, 128, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.N.; Gogate, P.R.; Csoka, L.; Dregelyi-Kiss, A.; Horvath, M. Intensification of biogas production using pre-treatment based on hydrodynamic cavitation. Ultrason. Sonochem. 2016, 30, 79–86. [Google Scholar] [CrossRef]
- Ouahabi, Y.R.; Bensadok, K.; Ouahabi, A. Optimization of the biomethane production process by anaerobic digestion of wheat straw using chemical pre-treatments coupled with ultrasonic disintegration. Sustainability 2021, 13, 7202. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T.; Kumar, R. Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pre-treatments. Energy 2012, 3, 273–282. [Google Scholar] [CrossRef]
- Taherdanak, M.; Zilouei, H.; Karimi, K. The influence of dilute sulfuric acid pre-treatment on biogas production form wheat plant. Int. J. Green Energy 2016, 13, 1129–1134. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Increased biogas production from wheat straw by chemical pre-treatments. Renew. Energy 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Aiwas, M.; Alvarado-Morales, M.; Tsapekos, P.; Gulfraz, M.; Angelidaki, I. TiO2 assisted photo-oxidative pre-treatment of wheat straw for biogas production. In Biogas Science; Technical University of Denmark: Lyngby, Denmark, 2016. [Google Scholar]
- Akhand, M.; Blancas, A.M. Optimization of NMMO Pre-Treatment of Straw for Enhanced Biogas Production. Master’s Thesis, University of Boras, Boras, Sweden, 2012. [Google Scholar]
- Jankovicova, B.; Hutnan, M.; Czolderova, M.N.; Hencelova, K.; Imreova, Z. Comparison of acid and alkali pre-treatment of lignocellulosic materials for biogas production. Plant Soil Environ. 2022, 68, 195–204. [Google Scholar] [CrossRef]
- Simioni, T.; Agustini, C.B.; Dettmer, A.; Gutterres, M. Enhancement of biogas production by anaerobic co-digestion of leather waste with raw and pre-treated wheat straw. Energy 2022, 253, 124051. [Google Scholar] [CrossRef]
- Yang, D.; Pang, Y.; Yuan, H.; Chen, S.; Ma, J.; Yu, L.; Li, X. Enhancing biogas production from anaerobically digested wheat straw through ammonia pre-treatment. Chin. J. Chem. Eng. 2014, 22, 576–582. [Google Scholar] [CrossRef]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Galletti, S. Biogas production from wheat straw pre-treated with hydrolytic enzymes or sodium hydroxide. Environ. Eng. Manag. J. 2017, 16, 1827–1835. [Google Scholar] [CrossRef]
- Reilly, M.; Dinsdale, R.; Guwy, A. Enhanced biomethane potential from wheat straw by low temperature alkaline calcium hydroxide pre-treatment. Bioresour. Technol. 2015, 189, 258–265. [Google Scholar] [CrossRef]
- Hjorth, M.; Gränitz, K.; Adamsen, A.P.S.; Møller, H.B. Extrusion as a pre-treatment to increase biogas production. Bioresour. Technol. 2011, 102, 4989–4994. [Google Scholar] [CrossRef]
- Chen, G.; Cao, H.; Zhao, C.; Zhang, W.; Zheng, J.; Wang, E. A comparative study of the effects of extrusion on lignocellullose structure and biogas production from wheat straw and digested wheat straw. Bioenergy Res. 2022, 16, 1939–1949. [Google Scholar] [CrossRef]
- Wahid, R.; Hjorth, M.; Kristensen, S.; Møller, H.B. Extrusion as pre-treatment for boosting methane production: Effect of screw configurations. Energy Fuel 2015, 29, 4030–4037. [Google Scholar] [CrossRef]
- Ziegler-Devin, S.; Chrusciel, L.; Brosse, N. Steam explosion pre-treatment of lignocellulosic biomass: A mini-review of theoretical and experimental approaches. Front. Chem. 2021, 9, 705358. [Google Scholar] [CrossRef]
- Kaldis, F.; Cysneiros, D.; Day, J.; Karatzas, K.G.; Chatzzifragkou, A. Anaerobic digestion of steam-exploded wheat straw and co-digestion strategies for enhanced biogas production. Appl. Sci. 2020, 10, 8284. [Google Scholar] [CrossRef]
- Risberg, K.; Sun, L.; Leven, T.; Horn, S.J.; Schnurer, A. Biogas production from wheat straw and manure—Impact of pre-treatment and processing parameters. Bioresour. Technol. 2013, 149, 232–237. [Google Scholar] [CrossRef]
- Theuretzbacher, F.; Lizasoain, J.; Lefever, C.; Saylor, M.K.; Enguidanos, R.; Weran, N.; Gronauer, A.; Bauer, A. Steam explosion pre-treatment of wheat straw to improve methane yields: Investigation of degradation kinetics of structural compounds during anaerobic digestion. Bioresour. Technol. 2015, 179, 299–305. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Murto, M. Biogas production from wheat straw in batch and UASB reactors: The roles of pre-treatment and seaweed hydrolysate as a co-substrate. Bioresour. Technol. 2013, 128, 164–172. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Tyagi, V.K.; Gunjyal, N.; Kazmi, A.A.; Ojha, C.S.P.; Moustakas, K. Hydrothermal and thermal-alkali pre-treatments of wheat straw: Co-digestion, substrate solubilization, biogas yield and kinetic study. Environ. Res. 2023, 216, 114436. [Google Scholar] [CrossRef]
- He, C.; Hu, J.; Shen, F.; Huang, M.; Zhao, L.; Zou, J.; Tian, D.; Jiang, Q.; Zeng, Y. Turning hydrothermal pre-treatment severity of wheat straw to match energy application scenarios. Ind. Crops Prod. 2022, 176, 114326. [Google Scholar] [CrossRef]
- Rajput, A.A.; Visvanathan, C. Effect of thermal pre-treatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J. Environ. Manag. 2018, 221, 45–52. [Google Scholar] [CrossRef]
- Perez-Pimienta, J.A.; Toro, E.E.R.; Amezquita-Garcia, H.J.; Escamilla-Alvarado, C. Applied Biotechnology Reviews, Sustainable Biofuels; Ray, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2021; Chapter 5; pp. 101–130. [Google Scholar]
- Rouches, E.; Escudié, R.; Latrille, E.; Carrère, H. Solid-state anaerobic digestion of wheat straw: Impact of S/I ratio and pilot-scale fungal pre-treatment. Waste Manag. 2019, 85, 464–476. [Google Scholar] [CrossRef]
- Yadav, M.; Singh, A.; Balan, V.; Pareek, N.; Vivekanand, V. Biological treatment of lignocellulosic biomass by Chaetomium globosporum: Process derivation and improved biogas production. Int. J. Biol. Macromol. 2019, 128, 176–183. [Google Scholar] [CrossRef]
- Kainthola, J.; Podder, A.; Fechner, M.; Goel, R. An overview of fungal pre-treatment processes for anaerobic digestion: Applications, bottlenecks and future needs. Bioresour. Technol. 2021, 321, 124397. [Google Scholar] [CrossRef]
- Barati, B.; Zafar, F.F.; Rupani, P.F.; Wang, S. Bacterial pre-treatment of microalgae and the potential of novel nature hydrolytic sources. Environ Technol. Innov. 2021, 21, 101362. [Google Scholar] [CrossRef]
- Xu, W.; Fu, S.; Yang, Z.; Lu, J.; Guo, R. Improved methane production from maize straw by micro-aerobic pre-treatment with pure bacteria system. Bioresour. Technol. 2018, 259, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Wang, C.; Wang, F.; Jia, H.; Wei, P.; Zhao, Y. Enhanced biogas production from wheat straw with the application of synergistic microbium consortium pre-treatment. RSC Adv. 2016, 6, 60187. [Google Scholar] [CrossRef]
- Kong, X.P.; Du, J.; Ye, X.; Xi, Y.; Jin, H.; Zhang, M.; Guo, D. Enhanced methane production from wheat straw with the assistance from lignocellulolytic microbial consortium TC-5. Bioresour. Technol. 2018, 263, 33–39. [Google Scholar] [CrossRef]
- Gaudino, E.C.; Grillo, G.; Tabasso, S.; Stevanato, L.; Gravotto, G.; Marjamaa, K.; Pihlajaniemi, V.; Koivula, A.; Aro, N.; Uusitalo, J.; et al. Optimization of ultrasound pre-treatment and enzymatic hydrolysis of wheat straw: From lab to semi-industrial scale. J. Clean. Prod. 2022, 380, 134897. [Google Scholar] [CrossRef]
- Kovács, E.; Szűcs, C.; Farkas, A.; Szuhaj, M.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Pre-treatment of lignocellulosic biogas substrates by filamentous fungi. J. Biotechnol. 2022, 360, 160–170. [Google Scholar] [CrossRef]
- Carrasco, E.D.H. A Combined Biological Pre-Treatment of Wheat Straw Using Native Wood-Rotting Fungi for Improving Its Biodegradability. Ph.D. Thesis, Universidad De La Frontera, Temmuco, Chile, 2017. [Google Scholar]
- Shah, T.A.; Ulla, R. Pre-treatment of wheat straw with ligninolytic fungi for increased biogas productivity. Int. J. Environ. Sci. Technol. 2019, 16, 7497–7508. [Google Scholar] [CrossRef]
- Schroyen, M.; Vervaeren, H.; Vandepitte, H.; Van Hulle, S.W.H.; Raes, K. Effect of enzymatic pre-treatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour. Technol. 2015, 192, 696–702. [Google Scholar] [CrossRef]
- Rowan, M.; Umenweke, G.C.; Epelle, E.I.; Afolabi, I.C.; Okoye, P.U.; Gunes, B.; Okolie, J.A. Anaerobic co-digestion of food waste and agricultural residues: An overview of feedstock properties and the impact of biochar addition. Digit. Chem. Eng. 2022, 4, 100046. [Google Scholar] [CrossRef]
- Induchoodan, T.G.; Haq, I.; Kalamdhad, A.S. Advanced Organic Waste Management; Hussain, C., Hait, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 14; p. 233. [Google Scholar]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Tyagi, V.K.; Ahmed, B.; Kazmi, A.A.; Ojha, C.S.P.; Singh, R. Critical insights into anaerobic co-digestion of wheat straw with food waste and cattle manure: Synergistic effects on biogas yield and kinetic modeling. Environ. Res. 2022, 212, 113382. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gavala, H.N.; Skiadas, I.V.; Ahring, B.K. Wet explosion of wheat straw and co-digestion with swine manure: Effect on methane productivity. Waste Manag. 2009, 29, 2830–2835. [Google Scholar] [CrossRef]
- Chu, L.; Li, Y.; Feng, Y.; Yang, G. Characteristics of co-digestion of pig dung and wheat straw in various ratios. Trans. Chin. Soc. Agric. Mach. 2011, 42, 100–104. [Google Scholar]
- Esposito, G.; Liotta, F.; Frunzo, L.; Panico, A.; Giordano, A.; Pirozzi, F. Anaerobic co-digestion of organic wastes. Rev. Environ. Sci. Biotechnol. 2012, 11, 325–341. [Google Scholar] [CrossRef]
- Pan, S.; Tsai, C.; Liu, C.; Wang, S.; Kim, H.; Fan, C. Anaerobic co-digestion of agricultural wastes toward circular bioeconomy. iScience 2021, 24, 102704. [Google Scholar] [CrossRef]
- Kaldis, F. Steam Explosion as Pre-Treatment Method to Improve Biogas Production from Wheat Straw. Ph.D. Thesis, The University of Reading, Reading, UK, 2020. [Google Scholar]
- Xi, Y.; Liu, Y.; Ye, X.; Du, J.; Kong, X.; Guo, D.; Xiao, Q. Enhanced anaerobic biogas production from wheat straw by herbal-extraction process residues supplementation. Front. Bioengin. Biotechnol. 2021, 9, 623594. [Google Scholar] [CrossRef]
- Wu, X.; Yao, W.; Zhu, J.; Miller, C. Biogas and CH4 productivity by co-digesting swine manure with three crop residues as an external carbon source. Bioresour. Technol. 2010, 101, 4042–4047. [Google Scholar] [CrossRef]
- Harsha, G.; Maurya, N.S. Liquid state anaerobic co-digestion of cattle manure and wheat straw at various mix ratios for optimal biogas production. Orient. J. Chem. 2022, 38, 777–784. [Google Scholar] [CrossRef]
- Meraj, S.; Liaquat, R.; Naqvi, S.R.; Sheikh, Z.; Zainab, A.; Khoja, A.H.; Juchelkova, D.; Atabani, A. Enhanced methane production from anaerobic co-digestion of wheat straw rice straw and sugarcane bagasse: A kinetic analysis. Appl. Sci. 2021, 11, 6069. [Google Scholar] [CrossRef]
- GreenCape. Large-Scale Renewable Energy Market Intelligence Report; GreenCape: Cape Town, South Africa, 2022. [Google Scholar]
- Msibi, S.S.; Kornelius, G. Potential for domestic biogas as household energy supply in South Africa. J. Energy S. Afr. 2017, 28, 1–13. [Google Scholar] [CrossRef]

| Province | Quantity (Million t yr−1) |
|---|---|
| Western Cape | 0.002 |
| Northern Cape | 0.385 |
| Free State | 0.009 |
| Eastern Cape | 0.003 |
| KwaZulu Natal | 0.025 |
| Mpumalanga | 0.019 |
| Limpopo | 0.099 |
| Gauteng | 0.005 |
| North West | 0.055 |
| Cellulose (%) | Hemicelluloses (%) | Lignin (%) | Reference |
|---|---|---|---|
| 30–55 | 18–37 | 10–30 | [1] |
| 35–45 | 20–30 | 8–15 | [25] |
| 33–40 | 20–25 | 15–20 | [28] |
| 30–40 | 20–30 | 15–20 | [4] |
| 35–39 | 23–30 | 12–16 | [29] |
| 35–38 | 20–28 | 16–24 | [30] |
| 27–42 | 11–27 | 14–21 | [31] |
| 30–49 | 22–34 | 7–22 | [32] |
| 30–40 | 20–25 | 20–25 | [20] |
| 35–50 | 15–25 | 10–15 | [33] |
| Country | Inoculum | Reactor Conditions | BMP (mL g VS−1) | Reference |
|---|---|---|---|---|
| Spain | Activated sludge | 2 L borosilicate glass, 35 °C, 45 d | 233 | [4] |
| USA | Inoculum from food waste thermophilic digester | 1 L anaerobic reactors, 50 °C, 25 d | 179 | [42] |
| Spain | Mixed sludge from municipal wastewater treatment plant | 2 L borosilicate glass, 35 °C, 40 d | 226 | [43] |
| Denmark | Co-digested mixture of animal manure and ethanol wastes | 337 mL glass bottles, thermophilic conditions | 221 | [26] |
| Germany | Inoculum from pilot plant treating cow manure and maize silage | Automated Methane Potential Testing System II, 37 °C, 30 d | 154 | [40] |
| Poland | Digested sewage sludge from wastewater treatment plant | 2 L glass bioreactors, 37 °C, 40 d | 339 | [44] |
| Denmark | Sludge from wastewater treatment plant digester | 500 mL bottles, 37 °C, 35 d, stirring at 150 rpm | 237 | [14] |
| Denmark | Inoculum from mesophilic anaerobic digester | 500 mL bottles, 35 °C, 96 d | 217 | [45] |
| Pakistan | Digested manure | 300 mL serum bottles, 35 °C, 45 d | 365 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamusoko, R.; Mukumba, P. Potential of Wheat Straw for Biogas Production by Anaerobic Digestion in South Africa: A Review. Energies 2024, 17, 4662. https://doi.org/10.3390/en17184662
Kamusoko R, Mukumba P. Potential of Wheat Straw for Biogas Production by Anaerobic Digestion in South Africa: A Review. Energies. 2024; 17(18):4662. https://doi.org/10.3390/en17184662
Chicago/Turabian StyleKamusoko, Reckson, and Patrick Mukumba. 2024. "Potential of Wheat Straw for Biogas Production by Anaerobic Digestion in South Africa: A Review" Energies 17, no. 18: 4662. https://doi.org/10.3390/en17184662
APA StyleKamusoko, R., & Mukumba, P. (2024). Potential of Wheat Straw for Biogas Production by Anaerobic Digestion in South Africa: A Review. Energies, 17(18), 4662. https://doi.org/10.3390/en17184662








