Abstract
Although in general ions are not able to migrate in the solid-state position due to rigid skeletal structure, in some solid electrolytes with a low energy barrier and high ionic conductivities, these ion transition can occur. In this work, we considered several solid electrolytes including lithium phosphorus oxy-nitride (LIPON), a lithium super-ionic conductor (SILICON), and thio-LISICON. For the fabrication and characterization of the solid electrolyte’s fabrication, we used a single-step ball milling (SSBM) procedure. Through this research on all-solid-state rechargeable lithium-ion batteries, our target is to discuss solving several problems in solid LIBs that have recently escalated due to raised concerns relating to safety hazards such as solvent leakage and the flammability of the liquid electrolytes used for commercial LIBs. Through this research, we tested the conductivity amounts of various substrates containing amorphous glass, SSBM, and glass-ceramic samples. Obviously, the SSBM glass-ceramics increased the conductivity, and we also found that the values for conductivity attained by SSBM were higher than those values for glass-ceramics. Using an SSBM technique, silicon nanoparticles were used as an anode material and it was found that the charge and discharge curves in the battery cell cycled between 0.009 and 1.45 V versus Li+/Li at a current density of 210 mA g−1 at room temperature. Since high resistance causes degradation between the cathode material (LiCoO2) and the solid electrolyte, we added GeS2 and SiS2 to the Li2S-P2S5 system to obtain higher conductivities and better stability of the electrode–electrolyte interface.
1. Introduction
Although with advanced technologies liquid electrolytes are the most frequently used electrolyte application for LIB fabrication, the use of these types of electrolytes is hazardous and flammable in batteries. Therefore, in order to fabricate LIBs with an extreme degree of safety, liquid electrolytes should be exchanged for solid electrolytes. Solid electrolytes have several advantages compared to liquid electrolytes, including high stability, long life and durability, being non-flammable, and insulation to prevent thermal increase inside internal circuits. Moreover, these batteries do not need extra safety layers, which can cause the overall energy density to remain at high voltages and amperages [1,2,3,4,5]. In addition, based on particular phenomena in solid electrolytes (such as the fact that only Li+ ions are mobile in solid electrolytes), several unfavorable parallel chemical reactions can be removed from the electrolytes, which causes the safety condition to be increased [6,7,8,9]. It is notable that, besides these advantages, there are some disadvantages; for instance, solid-state electrolytes (SSE) are not comparable with liquid electrolytes due to a lower achievable conductivity. Our target in this study is a discussion on functional solid electrolyte LIBs to understand the action of the electrochemistry phenomenon. This work consists of a developmental concept of solid electrolytes for manufacturing methods and a characterization of these types of electrodes. The initial step in this research was accomplished to synthesize and characterize suitable conducting solid electrolytes. Interpreting the electrochemical properties for quick ion conduction via solid compounds is the key to the fabrication of solid-electrolyte LIBs [10,11,12,13,14,15]. A proper understanding of chemical reactions inside the LIBs can be yielded through using cathode mixed materials, as well as from the selection of proper composites for anodes during electrolyte–electrode interaction. Finally, our third target was to find the condition of a high-energy-density solid electrolyte rechargeable battery due to the efficiency of the kinetic reactions of cathode and anode compounds resulting from a decrease in nanoparticle sizes through electrochemical testing [15,16,17,18,19,20]. Through this research on all-solid-state rechargeable lithium-ion batteries, our target is to discuss solutions to several problems in solid LIBs that have recently escalated due to raised concerns relating to safety hazards such as solvent leakage and the flammability of the liquid electrolytes used for commercial LIBs. Since solid-state electrolytes do not inherently carry the safety burdens of liquid electrolytes, a large worldwide attempt is underway to provide a viable solid electrolyte instead of liquid electrolytes. Beyond the obvious safety advantages of solid electrolytes, all-solid-state batteries can be constructed with a wide variety of solid electrolyte products, varying in form and design and having high reliability.
2. Theoretical Backgrounds
Rechargeable LIBs work by charging and discharging, alternatively, through the exchange of Li+ inside electrolytes via cathode and anode electrodes. The lithium diffusion process begins due to a current of Li+ via the electrolyte, through a redox reaction and also through an external circuit (Scheme 1).
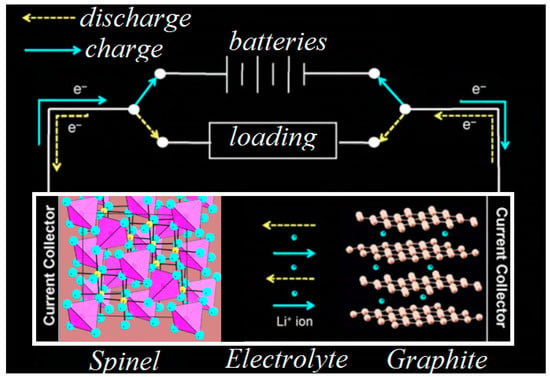
Scheme 1.
Operating LIBs.
2.1. Ionic Position in Cathode Materials
The insertion of Li+ inside cathode materials is largely related to the potential interaction among all ions, particularly the Li+ and the host compounds. Obviously, the insertion of Li+ in a crystal should be calculated, and it is ascertained that ion transport was accomplished through an optimal potential. Based on the shape of the insertion in the crystal, several structure items can be presented and be categorized, for example, LiFePO4, similar structures to LiCoO2, and 3D arrays of LiMn2O4 tunnels. The cathode materials directly affect insertion amounts of the Li ion as can be seen in Table 1. LiCoO2 with two-dimensional layered structures was found to have the largest Li ion diffusivity amongst the classes, while LiFePO4 with one-dimensional channels was found to have the lowest. Recently, several quantum chemical calculations of LixCoO2 diffusivity have been accomplished at various amounts of Li (0 ≤ x ≤ 1), which indicate the reason for the width of the range of lithium’s presence in electrolytes (Table 1 and Table 2).

Table 1.
Resistivity of cathode and anode electrodes.

Table 2.
Ionic conductivities of various solid-state electrolytes (SSEs).
In addition, these calculations found that the (010) surface in LiFePO4 has the optimum structure for Li+ insertion according to the approximation of numerical data. Obviously, ab initio methods through artificial modeling might be inapplicable for multiple phases and disordered amorphous structure calculations.
2.2. Electrical Position in Anode Materials
The electronic details of graphite were completely evaluated in the past decades. Graphite is an important electrode due to having a zero-band gap and exhibiting special phenomena during the influence of electrical fields [23,24]. Interlayer interactions in graphite are too low and, due to the large distance between these layers, easily allow Li ions to move between these sheets. In addition, these intercalations cause unique crystal and electronic situations. In amorphous carbons, as disorder appears, electrical conductivity considerably reduces. Since quantum calculations and ab initio methods are not able to analyze the electronic situation of amorphous structure in graphite, several models have been developed to predict electrical conductivity in graphite [43,44].
2.3. Conduction Evaluating in Electrolytes
A perfect electrolyte for LIBs has not yet been found. Obviously, solvent electrolytes are extremely common because of higher ionic conductivities. Although various electrolyte compounds have been analyzed as replacements, some of them do not exhibit acceptable ionic conductivity, at least 10−3 Scm−1, in LIBs [45]. The importance of electrolytes is to create an ionic conduction among the electrolytes and anodic and cathodic electrodes. Therefore, the main concern in electrolyte investigation is understanding how to increase ionic conductivity [46,47,48]. During the dissolving of cathode material salts in LiPF6 as an important electrolyte, cathode ions (Li+) and anions such as are produced. Since the dissociation of the salt is relevant to dialectical conditions such as the constant of the solvent, when a high dielectric constant is present, strong solvating occurs [49]. Consequently, large anions of any salts, via high conductivity and solubility, can be properly distributed due to their negative charges.
2.4. Solid-State Electrolytes (SSEs)
SSEs have several advantages compared to liquid electrolytes, such as a simple design, suitable sealing, resistance to pressure and any vibrations or explosions under high temperatures, wide electrochemical stability, and better safety [50,51,52]. The important disadvantage of these types of electrolytes is lower ionic conductivity. The ionic conductivities of several solid-state electrolytes are listed in Table 3 [53,54,55,56,57,58,59,60,61,62,63,64,65,66]. The ion movement of gelled macromolecules and polymers containing high solubility is considerably different from that in crystalline materials [67,68,69,70,71,72,73,74]. Solid electrolytes fabricated in semiconductors have also been used widely as an important ingredient of solid-state micro batteries. Moreover, inorganic elements used in solid-state electrolytes often consist of rare metals such as Ge, Ti, Sc, In, Lu, La and Y that are too expensive [75]. Because of the mentioned problems for using solid-state electrolytes, only gel polymer electrolytes have been applied as commercial fabrications in markets [76].

Table 3.
Ionic conductivity of various non-solid and SSEs.
Since the electrolyte material has a good Li+ conductivity and is also electrochemically properly mobile, it must tolerate volume changes in both electrodes. In other words, Li+ should tolerate the flux along cracks that causes glassy materials such as polycrystalline films to form leakage paths. Electrical measurements by Yu have demonstrated potentials up to 5.6 V in stable LiPON films in contact with lithium metal and the cathode [81] (Table 3). Since the ionic conductivity of LiPON is too low, this electrolyte is usually useful only in a ‘thin film’ configuration with solid electrolyte layer thicknesses around ~1000 nm. The thin film construction of batteries employing LiPON electrolyte limits overall capacity and therefore limits commercial application to small medical and MEMS devices. Fabrication of the LiSICON structure has been performed to create a 3D rigid framework for moving alkali ions through internal spaces. LiSICON electrolytes are made based on the formula LixM2M’3O12, where M and M’ are generally metals and represent metal ions containing octahedral and tetrahedral coordination to the O2− ions such as Li8Mg2Si3O12 [82].
3. Materials and Methods
3.1. Ball Milling
Recently, several manufacturing techniques have been developed to prepare solid electrolytes, the most well-known being SSE, which is obtained by a high conductivity melt quenching method. During the preparation of SSE, an ionic conductivity of approximately 10−2 cm−1 was obtained and found to have suitable electrochemical stability [83]. Although there are different methods for fusion systems, the cooling method is one of the most important cases in that can be applied as an example of the usual method. In this way, the powders are first pressed through a suspension in a carbon-coated quartz tube and then vacuum sealed to obtain an ampoule of these materials. To prepare the glass substrate, the bulb is heated to 750 °C for half a day to obtain a single phase and uniform shape. Later, the temperature of the quenched material must be reduced with cold water to produce a solid solution. It is notable that for the crystallization of solutes, the bulb should be heated to 750 °C for half a day, and then the product can be kept for about two days at a certain temperature to promote nucleation and crystal growth. [84]. Figure 1 shows a general diagram of different phases containing 75% Li2S with 25% P2S5 [84].
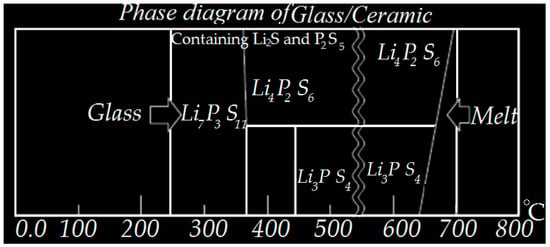
Figure 1.
XRD of crystals glass showing a general phase diagram of the 75% Li2S with %25P2S5.
Recently, ball milling methods have investigated the production of ultrafine amorphous at 298 K. The ideal powders are suitable for achieving large ionic conductivities and are also used to achieve the best contact between electrolytes and electrodes in SSE [85,86]. Ball milling probably also causes increased textural properties due to amorphous compounds, which are extracted using melt quenching techniques [87,88]. Ball-milled amorphous powders (BMAPs) are sometimes heated strongly to obtain crystalline units capable of higher conductivity compared to previous powders [89,90]. HEBM, which stands for high-energy ball milling, is an ideal technology for creating amorphous materials, and both contain the same concepts of pre-peel alloys using crushing technology. HEBM is generally carried out using shaker mills where the rotation takes place on a fixed axis. Although a high energy essentially causes a high-attrition grinding motion with the consideration of certain elements and proper control, the chambers rotating on a primary disk can be set at a selective speed. Since during grinding the compounds are alternately flattened, crushed, and welded, the steel balls trap certain particles between their surfaces. These impacts with enormous energies can severely destroy the molecules, reshape them atomically, and eliminate unusual surfaces and other structural defects [91,92]. Furthermore, the deforming and cracking of composites results in a progressive decrease in particle size and leads to significantly lower diffusion distances and temperatures [93,94,95]. BMAP is generally very stable and has stronger chemical interactions, which allows novel chemical reactions to be carried out while forming different nanostructures in our experiments. The behavior of hot milled electrolytes (BMEs) is confirmed by their excellent conductivity and solid electrolyte forming effects. For example, scientists have shown that heating ball-milled materials beyond their glass transition temperature can lead to highly conductive crystalline solid electrolytes [96].
3.1.1. Cryogenic Milling/Grinding and Jet Milling
Cryogenic grinding is another technology that has many advantages, including temperature control and mechanical control of grinding by cooling or quenching the compound at low temperatures to reduce the particle size of ‘soft’ materials. Jet milling is also another method without any grinding system, but it works with near-sonic-speed jets. This technique is usually applied to reduce size reduction. Volume reduction and material mixing are the results of high-speed collisions among particles of the same processing material.
3.1.2. Single-Step Ball Milling (SSBM)
Although SSBM has advantages such as saving time and being efficient, its performance improvement compared to ball milling followed by heat treatment is not significant.
In addition, although ball milling followed by heat treatment has been shown to be effective, several steps of this process are time consuming and inefficient and should be modified. It is important to note that heat treatment typically produces glass-ceramic materials with larger particle size whose morphology may not be desirable for use in all-solid-state Li-ion batteries. A single-step ball milling (SSBM) procedure is proposed in this work in which ball milling and heat treatment are carried out simultaneously. Through this work, we will report the glass-ceramic binary system xLi2S (100-x) P2S5 produced by the SSBM process in terms of structural and electrochemical properties.
3.2. Experimental
3.2.1. Preparation of P2S5, TiS2, and Li2S
Necessary and sufficient experiments were performed, as Li2S (Sigma -Aldrich, 99%, Hamburg, Germany) and P2S5 (Sigma-Aldrich, 99%, Hamburg, Germany) were applied as initial compounds for mechanical ball milling through using stoichiometry concentrations with the proper fractions of zirconia: exactly one gram with two zirconia balls (1 × 10 mm, 1 × 13 mm in diameter) for grinding. Energy ball milling took place for 24 h in a glove box containing Argon gas at a temperature controller around 60 °C (Spex2000, Jiangsu, China). Ball-milled vials were alternatingly turned on and turned off to drive the temperature towards reaching the vial temperature. A perfect correlation was observed between the ambient vial temperature and that of the vials due to milling without pausing. In addition to maintaining room temperature, the vials were frequently milled in 40 min intervals. All prepared materials from ball milling at room temperature were amorphous and their conductivity values were confirmed through previous experimental values [85,86,90]. In this work, we changed the amorphous materials into pellets 15 mm in diameter and 0.8 mm thick in a titanium die through cold pressing (5 metric tons). The product samples were characterized through X-ray diffraction using Cu-Kα radiation and were sealed in an air-isolated aluminum box with beryllium vents, then fixed to the X-ray diffraction meter (PanAlytical PW3830 X-Ray, London, UK). The diffraction values were evaluated for all morphological structures as well as unique materials between 15 and 45 degrees. The ionic conductivities were calculated by AC impedance spectroscopy (Solartron 1280 C, München, Germany) for all SSBM samples. The weighed composites were then cold pressed using 10 tons/metric pressures to prevent the lithium electrode plates from being pressed to both sides of the pellet at 1 metric ton. The impedances of selected cells were calculated from 25 MHz to 110 mHz at 298 K and the conductivities were measured using complex impedance analysis. For providing TiS2, several steps were accomplished as follows: 1. TiS2 (Sigma-Aldrich, 99%, Hamburg, Germany) was ball milled in a 120 mL agate vial at a net weight of 600 mg. 2. Large-ball-milled (LBM) samples were produced via 8 agate balls (8 mm diameter) and small-ball-milled (SBM) composites with 45 agate balls (5 mm diameter) used for grinding. Ball milling took place for 3 h for large-ball-milled material and 6 h for small-ball-milled products in an argon gas before the composites were used without further modification.
3.2.2. Preparation of Anode Materials
The anode electrode was made through a combination of crystalline nano-silicon (n-Si) powder (50–100 nm, Sigma-Aldrich, Hamburg, Germany) and bulk silicon (B-Si) powder (1–45 μm, Alfa Aesar brand of chemicals), solid-state electrolyte (SE) 76.0 Li2S% -24.0P 2S 5% provided from ball milling methods, and also multi-walled-carbon-nanotubes (NC7000, Namur, Belgium) at a weight ratio of 1:6:1, respectively. We applied a conductive additive due to the increased contact surface (400 m2g−1) compared to acetylene black (65 m2g−1) (Figure 2A,B) and thus observed an acceptable increase in conductivity in our system. Double-layer pellets were produced by compressing 1 milligram of electrode material on top of 200 milligrams of SSE powder via five metric tons. Finally, the lithium sheet was pressed with SSE using one metric ton. A schematic diagram of the SSE-LIB cell can be seen in Figure 2C. All experiments and evaluations were performed in a poly-aryl-ether-ketone (PEEK) chip (I = 1.4 cm) and were also performed in an Ar-filled glove box with Ti metal rods as current collectors. Galvano/static measurement of charge-discharge cycling was performed for several items at a fixed density of 110 mAg−1 at 298 K using an Arbin BT2000 galvanostat. The product samples were characterized through X-ray diffraction using Cu-. Kα radiation and were sealed in an air-isolated aluminum box with beryllium vents and fixed on an X-ray diffraction meter (PanAlytical PW3830 X-Ray, London, UK).
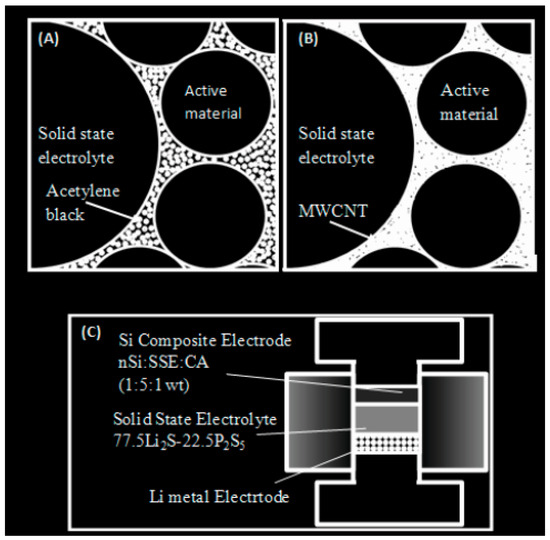
Figure 2.
(A) Anode electrode containing acetylene black. (B) Multi-wall carbon nanotubes in anode. (C) Solid-state lithium battery including titanium.
3.2.3. Preparation of Cathode Materials
An important problem of SSE-LIBs is the difficulty of creating a suitable electrode–electrolyte interaction. In sulfide-based SSE-LIBs, high interfacial resistances among the cathode composite materials and the solid electrolyte have been seen during the initial charging process of LiCoO2 as an effective cathode material. It is obvious that the high resistance is related to the destroying of the interface between the LiCoO2 and the Li2S-P2S5 solid electrolyte [97]. There are two ways of overcoming this problem: 1, using electrolyte additives for enhancing ionic conductivities and fixing the electrolyte in contact with the electrode, 2, creating a favorable electrode–electrolyte interface by interfacial modification. It has been confirmed that by using compounds such as GeS2 and SiS2 to create the network of the Li2S-P2S5 system, not only can higher conductivities for SSE be gained, but a better stability of the electrode–electrolyte system can also be formed [98]. Due to the second crystal structure, the elements with large ionic radius and high polarizability can be used, which consequently cause an increasing situation of mobility and conducting ions towards improving the solid crystal of the electrolytes [99]. These types of additive materials can decrease the large chemical potential difference among the components of a sulfide electrolyte as well as an oxide electrode. Creating a suitable electrode–electrolyte system by the optimization of cathode material surfaces has also confirmed an effective reduction in the resistance through coatings of Li4Ti5O12 on LiCoO2 particles [100]. In addition, a novel molecule was discovered, Li4Ti5O12, that can prevent dissolution [101]; coating by this molecule prevents the diffusion of the LiCoO2 inside the electrolyte. In this study, we investigated the mentioned additives in solid electrolytes to increase the stability of electrode materials with electrolytes. GeSe2 was used as a second network to improve the electrical conductivity and stabilize the SSE in our work, because sulfur is used as an additive to help stabilize the electrolyte for the high voltage in cathode electrodes.
Li2S-P2S5 as an SSE provided through ball milling as well as milling outputs in amorphous compounds elevated temperature conductivity values to 8.99 × 10−3 S cm−1 and 9.93 × 10−4 S cm−1, respectively. Cathode composite materials were mixed using both ball-milled and non-ball-milled titanium sulfide powders and SSE at a ratio of 2:3, respectively. For composites containing acetylene black (Alfa-Aesar, 60% compressed) as a conductive additive, a ratio of 15:20:2 was applied for three compounds consisting of TiS2, solid electrolyte, and acetylene black, respectively. Battery pellets were made by cold pressing (6 metric tons) and 12 mg of the composite cathode electrode above 150 mg of SE for 5 min. Li foil (Alfa-Aesar, 0.70 mm thick) was then attached to the SSE surface at 3 metric tons. All pressing and testing products were accomplished in a polyaryletheretherketone (PEEK) mold (I = 1.5 cm) with titanium rods as current collectors for both electrodes in an Ar-filled glove box.
3.2.4. Electrolyte Fabrication
Li2S-GeS2-P2S5 SSE was created using the SSBM method [102,103]. Reagent-grade powders of Li2S (Aldrich, 99.0%), P2S5 (Aldrich, 99%), and GeS2 (City Chemical, 99.0%) were consumed as initial products. Suitable amounts of composites were mixed into a zirconia vial (Spex) at a net weight of 1.5 g with two zirconia balls for grinding. HEBM (Spex8000) tool was selected and kept for 15 h in an Ar-filled dry box. The heat action of SSBM:SSE powders was accomplished through the initial mixing of sulfur and SSE powders and pelletizing the powders at 6 metric tons in a stainless steel die (I = 1.5 cm) with 500 mg of initial compounds. The provided pellets were fixed in a sealed glass container and were heated to 250 °C for 15 h. Both Li2S-GeSe2-P2S5 and Li4xGe1−xPxS2(1+x)Se2(1−x) electrolytes were prepared similarly with GeSe2 (Stream, 99.9%) and instead used temperatures of 240 °C, 350 °C, and 450 °C.
3.2.5. Electrode Fabrication with Bilayer Electrolyte
Cathode materials were provided through mixing LiCoO2 powder (Sigma-Aldrich) as the active material, SSE Li2S-GeS2-P2S5 powder for quick conduction of lithium, and acetylene black (Alfa, 55% compressed) for electron conduction at a weight ratio of 25:50:2, respectively. Double-layered pellets were made through a 120 mg hand pressing of Li2S-GeSe2-P2S5 or Li2S-GeS2-P2S5 SSE over an 80 mg hand-pressed layer of 69.5 Li2S -19.5 P2S5 SSE created using the SSBM method. Then, a 12 mg layer of the cathode compounds was carefully diffused over the Li2S-GeSe2-P2S5 or Li2S-GeS2-P2S5 solid electrolyte and the cell was pelletized through cold pressing for 6 min. All experiments were performed in an argon-gas-filled glove box. Galvanostatic charge-discharge cycling occurred at initial cycle cut off voltages of 4.5 and 2.0 V at a 45 μA cm−2 current at 298K using an Arbin BT2000. Schematic diagrams for the Li/SSE/LiCoO2 and AC impedance cells can be seen in Figure 3.
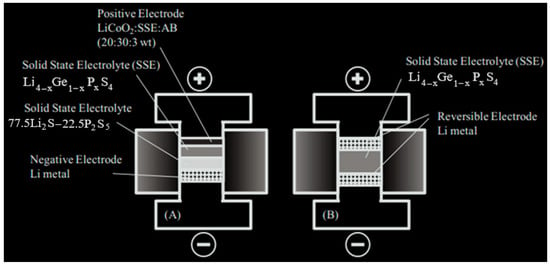
Figure 3.
Titanium test with Li2S–GeS2–P2S5 SSE, (A) solid electrolyte battery, and (B) conductivity tester.
4. Results and Discussion
4.1. Characterization
Figure 4 exhibits the XRD spectrum of several glass-ceramic morphologies in the combination of 65 < x < 85 for [xLi2S(100−x)P2S5]. A sharp peak can be seen among all items denoting the crystalline composition prepared under the SSBM method. For x = 76.5 and x = 79, electrolytes with sharp diffraction peaks are observed near 24.9 and 30.5 due to excess Li2S content in their composition [104].
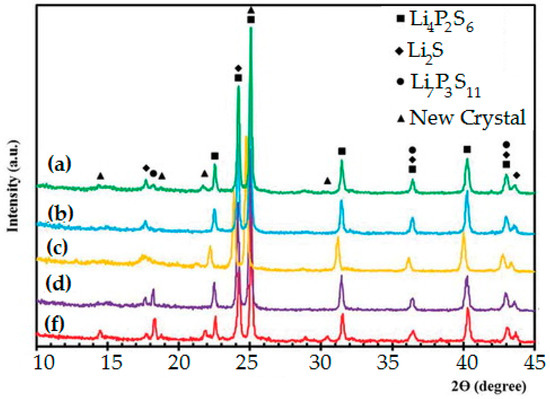
Figure 4.
XRD patterns for all tested electrolytes, (a) Li2S, (b) P2S5, (c) x = GeS2, (d) x = 64, (f) x = 76.
The diffraction patterns for SSBM composites denote a linearly increasing intensity of crystalline peaks up to x = 76.5, at which point only the Li2S peaks intensify for x = 79. A new crystalline pattern, identical to that of the thio-LISICON II analog, can be seen through positioned peaks among the materials ranges [104]. The specific behavior of composites such as the low melting temperature of P2S5 might be a reason for the crystallization of these compounds during SSBM. Measurements were accomplished from the cathodic and then the anodic situations up to 4.5 V vs. Li+/Li and down to 0.5 V vs. Li+/Li. No reactions were observed in these voltammograms except for the lithium deposition (Li+ + e and dissolution ( Li+ + e) reactions between 0.5 and 0.6 V up to 4.5 V vs. Li+/Li. This suggests that the SSBM composites have a wider electrochemical window than 4.5 V [105].
4.2. Conductivities
Figure 5 exhibits the details of a conductive situation according to values that were measured from amorphous and SSBM ceramic glasses. Through two-step testing, increasing conductivities over glass electrolytes appeared due to the formation of unique crystalline structures [26]. In other words, the enhancement in conductivity by the crystallization of sulfide-based glass solid electrolytes appeared due to the crystalline phase, which is produced during the ball milling process. In addition, the LISICON phase of Li(4−x)Ge(1−x)PxS4 (0.5 < x < 0.9) with high conductivities was created through monoclinic superstructure [41]. We predicted that enhancements of conductivities are caused mostly by SSBM when they are around one or two orders of magnitude compared to other glass electrolytes as well as higher than these values for glass-ceramics, which were created using two heating steps [26].
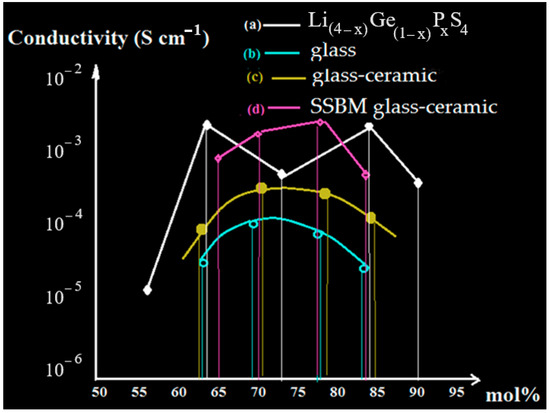
Figure 5.
(a) Conductivity map for Li(4–x)Ge(1–x)PxS4; (b) glass, (c) glass-ceramic, (d) SSBM glass-ceramic.
Conductive Additive Modification by MWCNTs
Multiwall carbon nanotubes (MWCNTs), due to their unique one-dimensional structure and tubular structure, high electrical and thermal conductivities, and extremely large surface area, have been considered as ideal additive materials to improve the electrochemical characteristics of the anodes and cathodes of Li-ion batteries. A recent development of electrode materials for LIBs has been driven mainly by hybrid nanostructures consisting of Li storage compounds and MWCNTs. The electrochemical performance of LIBs affected by the presence of MWCNTs in terms of energy and power densities, rate capacity, cyclic life and safety are highlighted in comparison with those without or containing other types of carbonaceous materials. Figure 6 exhibits the extra efficiency of the conductive additive modification including n-Si cells using MWCNT at three temperatures (298, 300, 302 K) under galvanic-static charge/discharge cycling between 1.5 V and 5 V (vs. Li/Li+). It is usual to show the specific capacities based on the mass of the active materials such as nano-Si in the anode. In addition, the lithium interaction capacity of MWCNTs can considerably help the total capacities of the anode materials. Consequently, the capacities of the conductive additives were gained in the cell using several amounts of the MWCNTs as the active material. The specific capacities of these amounts in the anode were then estimated from the data for the corresponding capacity of nano-Si according to the use of MWCNT amounts. We found a considerable enhancing of the specific surface area of the MWCNTs for flexion for maintaining the electrical contact with the nano-Si particles upon cycling [7]. This test confirms that solid-state lithium batteries using nano-Si and MWCNTs as a conductive additive maintain a considerable capacity and amazingly exhibit higher efficiency compared with those batteries that do not use nano-Si. MWCNTs were found to improve cycling capacity in all-solid-state lithium batteries over acetylene black as a conductive additive.
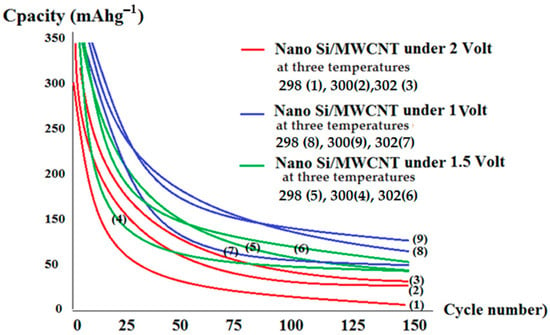
Figure 6.
Comparison showing superior performance of MWCNT as a conductive additive for all solid–state lithium batteries over acetylene black in different voltages and three temperatures.
4.3. Liquid vs. Solid
The battery cell was cycled between 0 and 150 and voltages between 1.5 V to 5 V versus Li+/Li at a current density of 500 mAg−1 at room temperature. The first discharge and charge values of nano-Si were found to be various values based on Figure 7 from A to H, respectively. During the first cycle (Figure 7A), the potential quickly drops to 3.2 V, then gradually reaches 2.4 V. Figure 7B compares the cycling performance of solid-state and liquid electrolyte systems using nano-Si; the charge capacity of the solid glass-ceramic electrolyte system reduces from 250 to 50 mAhg-1 within 0–50 cycles (Figure 7C). The nano-Si in solid-state electrolyte demonstrated a capacity retention of 71.5% after 50 charge/discharge cycles in these ranges of voltages (Figure 7D). In contrast, the nano-Si in the conventional liquid electrolyte cell displayed a maximum of 20% capacity retention (Figure 7E). This difference is related to the amount of expansion during Li accumulation, because the amount changes of Li-Si permit Si absorption in lithium through a liquid electrolyte compared with solid electrolytes (Figure 7F).
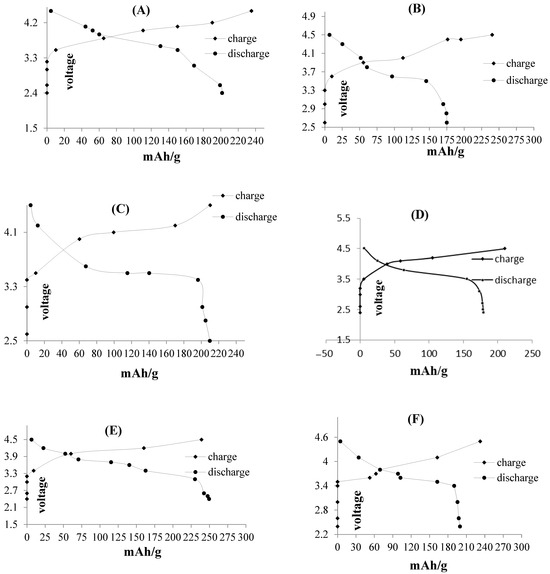
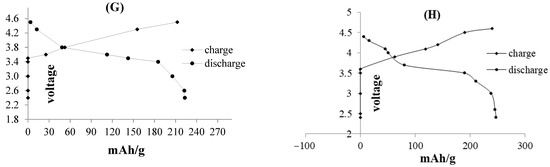
Figure 7.
Charge–discharge curves of all-solid-state cell fabricated with n-Si as anode material by different voltage ranges. (A–F) compare the cycling performance of solid state and liquid electrolyte systems using nano-Si. (G,H) exhibit the charge-discharge performance of ion cells, tested with lithium phosphorus oxy-nitride (LIPON) and lithium super-ionic conductor (SILICON) solid electrolyte, respectively.
5. Conclusions
Here, we found that electrodes composed of silicon rods control the electrolytes of LIBs because they can provide more stability with optimized crushing, as well as creating an appropriate lifespan and sufficient Coulomb efficiency. The simplicity of the SSBM process and high ionic conductivity of SSBM samples make the SSBM method superior for the development of SSE for all-solid-state battery production. Through this work, we confirmed, using the glass-ceramic electrolytes produced by the SSBM technique, that silicon nanoparticles can be used as the anode material in all-solid-state Li-ion batteries. This work also confirms that solid-state lithium batteries using nano-Si and MWCNTs as a conductive additive maintain a considerable capacity and, amazingly, exhibit higher efficiency compared to those batteries without using nano-Si. As a result, MWCNTs can improve cycling capacity in all-solid-state lithium batteries over acetylene black as a conductive additive for any further research.
Author Contributions
Conceptualization, M.M. and F.M.; methodology, M.M.; software, F.M.; validation, M.M.; formal analysis, M.M. and F.M.; investigation, M.M. and F.M.; resources, M.M.; data curation, M.M.; writing—original draft preparation, F.M.; writing—review and editing, F.M.; visualization, M.M. and F.M.; supervision, M.M.; project administration, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data available on request due to restrictions (e.g., privacy, legal or ethical reasons).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, W.; Yi, J.; He, P.; Zhou, H. Solid-State Electrolytes for Lithium-Ion Batteries: Fundamentals, Challenges and Perspectives. Electrochem. Energ. Rev. 2019, 2, 574–605. [Google Scholar] [CrossRef]
- Flamme, B.; Rodriguez Garcia, G.; Weil, M.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidala, V.; Chagnes, A. Guidelines to design organic electrolytes for lithium-ion batteries: Environmental impact, physicochemical and electrochemical properties. Green Chem. 2017, 19, 1828. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2017, 8, 1702184. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, H.Q. Strategic Structural Design of a Gel Polymer Electrolyte toward a High Efficiency Lithium-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 3937–3971. [Google Scholar] [CrossRef]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Cai, H.; Chen, Z.; Guo, S.; Ma, D.; Wang, J. Polyacrylamide gel electrolyte for high-performance quasi-solid-state electrochromic devices. Sol. Energy Mater. Sol. Cells 2023, 256, 112310. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, D.; Yue, J.; Zhang, X.; Wu, Z.; Zhang, T.; Chen, C.; Fei, T. Optical Waveguide Sensors for Measuring Human Temperature and Humidity with Gel Polymer Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 60384–60392. [Google Scholar] [CrossRef]
- Kim, K.J.; Hyeon, J.S.; Kim, H.; Mun, T.J.; Haines, C.S.; Li, N.; Baughman, R.H.; Kim, S.J. Enhancing the Work Capacity of Electrochemical Artificial Muscles by Coiling Plies of Twist-Released Carbon Nanotube Yarns. ACS Appl. Mater. Interfaces 2019, 11, 13533–13537. [Google Scholar] [CrossRef]
- Yang, H.X.; Liu, Z.K.; Wang, Y.; Li, N.W.; Yu, L. Multiscale Structural Gel Polymer Electrolytes with Fast Li+ Transport for Long-Life Li Metal Batteries. Adv. Funct. Mater. 2023, 33, 2209837. [Google Scholar] [CrossRef]
- Sohn, J.-Y.; Choi, J.H.; Kim, P.-W.; Hwang, I.T.; Shin, J.; Jung, C.-H.; Lee, Y.-M. In-situ preparation of chemically-crosslinked polyvinylpyrrolidone gel polymer electrolyte for lithium ion battery via room-temperature electron beam-induced gelation. Radiat. Phys. Chem. 2023, 211, 111047. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Xia, Y.; Liang, Y.; Xia, X.; Tu, J. A polyacrylonitrile (PAN)-based double-layer multifunctional gel polymerelectrolyte for lithium-sulfur batteries. J. Membr. Sci. 2019, 582, 37–47. [Google Scholar] [CrossRef]
- Zhou, Z.; Pei, X.; Zhang, T.; Wang, L.; Hong, J.; Lu, Y.; He, G. A Gel Polymer Electrolyte with 2D Filler-reinforced for Dendrite Suppression Li-Ion Batteries. Electroanalysis 2023, 35, 2200306. [Google Scholar] [CrossRef]
- Dennis, J.O.; Shukur, M.F.; Aldaghri, O.A.; Ibnaouf, K.H.; Adam, A.A.; Usman, F.; Hassan, Y.M.; Alsadig, A.; Danbature, W.L.; Abdulkadir, B.A. A Review of Current Trends on Polyvinyl Alcohol (PVA)-Based Solid Polymer Electrolytes. Molecules 2023, 28, 1781. [Google Scholar] [CrossRef]
- Marchiori, C.F.N.; Carvalho, R.P.; Ebadi, M.; Brandell, D.; Moyses Araujo, C. Understanding the Electrochemical Stability Window of Polymer Electrolytes in Solid-State Batteries from Atomic-Scale Modeling: The Role of Li-Ion Salts. Chem. Mater. 2020, 32, 7237–7246. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent Advances in Innovative Polymer Electrolytes based on Poly (ionic liquid)s. Electrochim. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Cho, Y.G.; Hwang, C.; Cheong, D.S.; Kim, Y.-S.; Son, H.-K. Gel/Solid Polymer Electrolytes Characterized by In Situ Gelation or Polymerization for Electrochemical Energy Systems. Adv. Mater. 2019, 31, 1804909. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, S.; Sun, Y.; Xiang, N.; Jiang, X.; Hou, L. Facile preparation and characterization of poly(vinyl alcohol)-NaCl-glycerol. Eur. Polym. J. 2018, 106, 206–213. [Google Scholar] [CrossRef]
- Shi, S.; Peng, X.; Liu, T.; Chen, Y.-N.; He, C.; Wang, H. Facile preparation of hydrogen-bonded supramolecular polyvinylalcoholglycerol gels with excellent thermoplasticity and mechanical properties. Polymer 2017, 111, 168–176. [Google Scholar] [CrossRef]
- Sun, Y.; Xiang, N.; Jiang, X.; Hou, L. Preparation of high tough poly(vinyl alcohol) hydrogel by soaking in NaCl aqueous solution. Mater. Lett. 2017, 194, 34–37. [Google Scholar] [CrossRef]
- Di, X.; Ma, Q.; Xu, Y.; Yang, M.; Wu, G.; Sun, P. High-performance ionic conductive poly(vinyl alcohol) hydrogels for flexible strain sensors based on a universal soaking strategy. Mater. Chem. Front. 2021, 5, 315–323. [Google Scholar] [CrossRef]
- Callister, W.D., Jr. Materials Science and Engineering: An Introduction, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1997; pp. 796–801. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Partoens, B.; Peeters, F.M. From graphene to graphite: Electronic structure around the K point. Phys. Rev. B 2006, 74, 075404. [Google Scholar] [CrossRef]
- Hess, M.; Lebraud, E.; Levasseur, A. Graphite multilayer thin films: A new anode material for Li-ion microbatteries synthesis and characterization. Power Sources 1997, 68, 204–207. [Google Scholar] [CrossRef]
- Segall, B. Energy bands of aluminum. Phys. Rev. 1961, 124, 1797–1806. [Google Scholar] [CrossRef]
- Kemp, J.P.; Cox, P.A. Electronic structure of LiCoO2 and related materials; photoemission studies. Phys. Condens. Matter 1990, 2, 9653–9667. [Google Scholar] [CrossRef]
- Shi, S.; Ouyang, C.; Lei, M.; Tang, W. Effect of Mg-doping on the structural and electronic properties of LiCoO2: A first-principles investigation. Power Sources 2007, 171, 908–912. [Google Scholar] [CrossRef]
- van Elp, J.; Wieland, J.L.; Eskes, H.; Kuiper, P.; Sawatzky, G.A. Electronic structure of CoO, Li-doped CoO, and LiCOO2. Phys. Rev. B 1991, 44, 6090–6103. [Google Scholar] [CrossRef]
- Ragavendran, K.; Nakkiran, A.; Kalyani, P.; Veluchamy, A.; Jagannathan, R. Nickel doped spinel lithium manganate–some insights using opto-impedance. Chem. Phys. Lett. 2008, 456, 110–115. [Google Scholar] [CrossRef]
- Marzec, J.; Swierczek, K.; Przewoznik, J.; Molenda, J.; Simon, D.R.; Kelder, E.M.; Schoonman, J. Conduction mechanism in operating a LiMn2O4 cathode. Solid State Ion. 2002, 146, 225–237. [Google Scholar] [CrossRef]
- Molenda, J.; Kucza, W. Transport properties of LiMn2O4. Solid State Ion. 1999, 117, 41–46. [Google Scholar] [CrossRef]
- Shi, S.; Liu, L.; Ouyang, C.; Wang, D.S.; Wang, Z.; Chen, L.; Huang, X. Enhancement of electronic conductivity of LiFePO4 by Cr doping and its identification by first-principles calculations. Phys. Rev. B 2003, 68, 195108-1–195108-5. [Google Scholar] [CrossRef]
- Xu, Y.N.; Chung, S.Y.; Bloking, J.T.; Chiang, Y.M.; Ching, W.Y. Electronic structure and electrical conductivity of undoped LiFePO4. Electrochem. Solid State Lett. 2004, 7, A131–A134. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kijima, N.; Tokiwa, K.; Watanabe, T.; Akimoto, J. Single-crystal synthesis, structure refinement and electrical properties of Li0.5CoO2. Phys. Condens. Matter 2007, 19, 436202–1–436202-12. [Google Scholar] [CrossRef]
- Levasseur, S.; Ménétrier, M.; Delmas, C. On the Dual Effect of Mg Doping in LiCoO2 and Li1+δCoO2: Structural, Electronic Properties, and 7Li MAS NMR Studies. Chem. Mater. 2002, 14, 3584–3590. [Google Scholar] [CrossRef]
- Carlier, D.; Ménétrier, M.; Delmas, C.J. 7Li MAS NMR study of electrochemically deintercalated LixNi0.30Co0.70O2 phases: Evidence of electronic and ionic mobility, and redox processes. J. Mater. Chem. 2001, 11, 594–603. [Google Scholar] [CrossRef]
- Lala, S.M.; Montoro, L.A.; Abbate, M.; Rosolen, J.M. The negative and positive structural effects of Ga doping in the electrochemical performance of LiCoO2. Electrochim. Acta 2005, 51, 7–13. [Google Scholar] [CrossRef]
- Ramos Ferrer, P.; Mace, A.; Thomas, S.N.; Jeon, J.W. Nanostructured porous graphene and its composites for energy storage applications. Nano Converg. 2017, 4, 29. [Google Scholar] [CrossRef]
- Molenda, J.; Swierczek, K.; Marzec, J.; Liu, R.S. Charge transport mechanism in LiCoyMn2−yO4 cathode material. Solid State Ion. 2003, 157, 101–108. [Google Scholar] [CrossRef]
- Lazarraga, M.G.; Pascual, L.; Gadjov, H.; Kovacheva, D.; Petrov, K.; Amarilla, J.M.; Rojas, R.M.; Martin-Luengo, M.A.; Rojo, J.M. Nanosize LiNiyMn2 − yO4 (0 < y ≤ 0.5) spinels synthesized by a sucrose-aided combustion method. Characterization and electrochemical performance. Mater. Chem. 2004, 14, 1640–1647. [Google Scholar]
- Kim, D.K.; Park, H.M.; Jung, S.J.; Jeong, Y.U.; Lee, J.H.; Kim, J.J. LiMnPO4: Review on Synthesis and Electrochemical Properties. Power Sources 2006, 159, 237–240. [Google Scholar] [CrossRef]
- Zhou, F.; Cococcioni, M.; Marianetti, C.A.; Morgan, D.; Ceder, G. First-principles prediction of redox potentials in transition-metal compounds with LDA+U. Phys. Rev. B 2004, 70, 235121–1–235121–8. [Google Scholar] [CrossRef]
- Ravagnan, L.; Piseri, P.; Bruzzi, M.; Miglio, S.; Bongiorno, G.; Baserga, A.; Casari, C.S.; Bassi, A.L.; Lenardi, C.; Yamaguchi, Y.; et al. Influence of Cumulenic Chains on the Vibrational and Electronic Properties of sp-sp2 Amorphous Carbon. Phys. Rev. Lett. 2007, 98, 216103-1–216103-4. [Google Scholar] [CrossRef]
- Vishwakarma, P.N.; Subramanyam, S.V. Hopping conduction in boron doped amorphous carbon films. J. Appl. Phys. 2006, 100, 113702–1–113702-5. [Google Scholar] [CrossRef]
- Brodd, R.J.; Huang, W.; Akridge, J.R. Advances in Lithium-Ion Batteries. Macromol. Symp. 2000, 159, 229–245. [Google Scholar] [CrossRef]
- Chagnes, A.; Carré, B.; Willmann, P.; Lemordant, D. Guidelines to Design Electrolytes for Lithium-ion Batteries: Environmental Impact, Physicochemical and Electrochemical Properties. Power Sources 2002, 109, 203–213. [Google Scholar] [CrossRef]
- Geoffroy, I.; Willmann, P.; Mesfar, K.; Carré, B.; Lemordant, D. Electrolytic characteristics of ethylene carbonate–diglyme-based electrolytes for lithium batteries. Electrochim. Acta 2000, 45, 2019–2027. [Google Scholar] [CrossRef]
- Ding, M.S.; Jow, T.R. Evaluation of Fluorinated Alkyl Phosphates as Flame Retardants in Electrolytes for Li-Ion Batteries I. Physical and Electrochemical Properties. J. Electrochem. Soc. 2003, 150, A620–A628. [Google Scholar] [CrossRef]
- Blint, R.J. Chemistry-Informed Machine Learning for Polymer Electrolyte Discovery. Electrochem. Soc. 1995, 142, 696–702. [Google Scholar] [CrossRef]
- Ribes, M. Thin Film Technology and Characterization: Their Use in Microionic Devices. Solid State Microbatteries; James, R., Akridge, M.B., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 41–58. [Google Scholar]
- Wakihara, M. Recent developments in lithium ion batteries. Mater. Sci. Eng. R 2001, 33, 109–134. [Google Scholar] [CrossRef]
- Zaghib, K.; Charest, P.; Guerfi, A.; Shim, J.; Perrier, M.; Striebel, K. Safe Li-ion polymer batteries for HEV applications. Power Sources 2004, 134, 124–129. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Shen, Y.J.; Reddy, M.J.; Chu, P.P. Complexation of poly(vinylidene fluoride): LiPF6 solid polymer electrolyte with enhanced ion conduction in ‘wet’form. Power Sources 2003, 123, 222–229. [Google Scholar] [CrossRef]
- Kaneko, M.; Nakayama, M.; Wakihara, M. Lithium-ion conduction in elastomeric binder in Li-ion batteries. Solid State Electrochem. 2007, 11, 1071–1076. [Google Scholar] [CrossRef]
- Egashira, M.; Todo, H.; Yosimoto, N.; Morita, M. Lithium ion conduction in ionic liquid-based gel polymer electrolyte. Power Sources 2008, 178, 729–735. [Google Scholar] [CrossRef]
- Zhang, S.; Lee, J.Y.; Hong, L. Li+ conducting ‘fuzzy’poly (ethylene oxide)–SiO2 polymer composite electrolytes. Power Sources 2004, 134, 95–102. [Google Scholar] [CrossRef]
- Abouimrane, A.; Whitfield, P.S.; Niketic, S.; Davidson, I.J. Investigation of Li salt doped succinonitrile as potential solid electrolytes for lithium batteries. Power Sources 2007, 174, 883–888. [Google Scholar] [CrossRef]
- Yang, K.Y.; Fung, K.Z.; Leu, I.C. Study on the structural change and lithium ion conductivity for the perovskite-type LaAlO3–La0.50Li0.50TiO3 solid solution. Alloys Compd. 2007, 438, 207–216. [Google Scholar] [CrossRef]
- Savitha, T.; Selvasekarapandian, S.; Ramya, C.S.; Bhuvaneswari, M.S.; Hirankumar, G.; Baskaran, R.; Angelo, P.C. Structural and ionic transport properties of Li2AlZr[PO4]3. Power Sources 2006, 157, 533–536. [Google Scholar] [CrossRef]
- Kanno, R.; Hata, T.; Kawamoto, Y.; Irie, M. Synthesis of a new lithium ionic conductor, thio-LISICON–lithium germanium sulfide system. Solid State Ion. 2000, 130, 97–104. [Google Scholar] [CrossRef]
- Tomita, Y.; Matsushita, H.; Kobayashi, K.; Maeda, Y.; Yamada, K. Substitution effect of ionic conductivity in lithium ion conductor, Li3InBr6−xClx. Solid State Ion. 2008, 179, 867–870. [Google Scholar] [CrossRef]
- Thangadurai, V.; Schwenzel, J.; Weppner, W. Tailoring ceramics for specific applications: A case study of the development of all-solid-state lithium batteries. Ionics 2005, 11, 11–23. [Google Scholar] [CrossRef]
- Hamon, Y.; Douard, A.; Sabary, F.; Marcel, C.; Vinatier, P.; Pecquenard, B.; Levasseur, A. Influence of sputtering conditions on ionic conductivity of LiPON thin films. Solid State Ion. 2006, 177, 257–261. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Zhang, Z. Further Characterization of Sis2-Li2s Glasses Doped with Lithium Halide. Electrochem. Soc. 1988, 135, 859–862. [Google Scholar] [CrossRef]
- Inada, T.; Takada, K.I.; Kajiyama, A.; Kouguchi, M.; Sasaki, H.; Kondo, S.; Watanabe, M.; Murayama, M.; Kanno, R. Fabrications and properties of composite solid-state electrolytes. Solid State Ion. 2003, 158, 275–280. [Google Scholar] [CrossRef]
- Chowdari, B.V.R.; Rao, G.V.S.; Lee, G.Y.H. XPS and ionic conductivity studies on Li2O–Al2O3–(TiO2 or GeO2)–P2O5 glass–ceramics. Solid State Ion. 2000, 136–137, 1067–1075. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Sakaguchi, I.; Zhang, L.; Ma, R.; Fukuda, K.; Osada, M.; Sasaki, T. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 2007, 9, 1486–1490. [Google Scholar] [CrossRef]
- Ratner, M.A.; Shriver, D.F. Ion transport in solvent-free polymers. Chem. Rev. 1988, 88, 109–124. [Google Scholar] [CrossRef]
- Papke, B.L.; Ratner, M.A.; Shriver, D.F. Conformation and ion-transport models for the structure and ionic conductivity in complexes of polyethers with alkali Metal Salts. Electrochem. Soc. 1982, 129, 1694–1701. [Google Scholar] [CrossRef]
- Brandup, J.; Immergut, E.H.; Grulke, E.A.; Abe, A.; Bloch, D.R. (Eds.) Solution Properties, Polymer Handbook, 4th ed.; John Wiley and Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Le Nest, J.F.; Callens, S.; Gandini, A.; Armand, M. Additives for Solid Polymer Electrolytes: The Layered Nanoparticles. Electrochim. Acta 1992, 37, 1585–1588. [Google Scholar] [CrossRef]
- Nishimoto, A.; Watanabe, M.; Ikeda, Y.; Kohjiya, S. High ionic conductivity of new polymer electrolytes based on high molecular weight polyether comb polymers. Electrochim. Acta 1998, 43, 1177–1184. [Google Scholar] [CrossRef]
- Scrosati, B. Advances in Lithium–Ion Batteries; van Schalkwijk Walter, A., Scrosati, B., Eds.; Kluwer Academic/Plenum Publishers: Norwell, MA, USA, 2002; pp. 252–266. [Google Scholar]
- Svanberg, C.; Adebahr, J.; Bergman, R.; Börjesson, L.; Scrosati, B.; Jacobsson, P. Polymer concentration dependence of the dynamics in gel electrolytes. Solid State Ion. 2000, 136–137, 1147–1152. [Google Scholar] [CrossRef]
- Jak, M.J.G.; Ooms, F.G.B.; Kelder, E.M.; Legerstee, W.J.; Schoonman, J.; Weisenburger, A. Design of Organoboron Solid Electrolytes/Solid Electrolyte Interface for Enhanced Performance of Lithium Ion Secondary Batteries. Power Sources 1999, 80, 83–89. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Gozdz, A.S.; Schmutz, C.; Shokoohi, F.; Warren, P.C. Performance of Bellcore`s plastic rechargeable Li-ion batteries. Solid State Ion. 1996, 86–88, 49–54. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Ue, M.; Mori, S. Mobility and ionic association of lithium salts in a propylene carbonate-ethyl methyl carbonate mixed solvent. Electrochem. Soc. 1995, 142, 2577–2581. [Google Scholar] [CrossRef]
- Schmidta, M.; Heidera, U.; Kuehnera, A.; Oestena, R.; Jungnitza, M.; Ignat’evb, N.; Sartorib, P. Lithium fluoroalkylphosphates: A new class of conducting salts for electrolytes for high energy lithium-ion batteries. Power Sources 2001, 97/98, 557–560. [Google Scholar]
- Walker, C.W., Jr.; Cox, J.D.; Salomon, M. Conductivity and electrochemical stability of electrolytes containing organic solvent mixtures with lithium tris(trifluoromethanesulfonyl) methide. Electrochem. Soc. 1996, 143, L80–L82. [Google Scholar] [CrossRef]
- Bates, J.; Dudney, N.; Neudecker, B.; Ueda, A.; Evans, C. Thin-film lithium and lithiumion batteries. Solid State Ion. 2000, 135, 33–45. [Google Scholar]
- Hong, H. Crystal-structure and ionic-conductivity of li14zn(geo4)4 and other new Li+ superionic conductors. Mater. Res. Bull. 1978, 13, 117–124. [Google Scholar]
- Seino, Y.; Takada, K.; Kim, B.; Zhang, L.; Ohta, N.; Wada, H.; Osada, M.; Sasaki, T. Synthesis of phosphorous sulfide solid electrolyte and all-solid-state lithium batteries with graphite electrode. Solid State Ion. 2005, 176, 2389–2393. [Google Scholar] [CrossRef]
- Adachi, G.; Imanaka, N.; Aono, H. Fast Li-circle plus conducting ceramic electrolytes. Adv. Mater. 1996, 8, 127. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Mizuno, F.; Tadanaga, K.; Minami, T.; Tatsumisago, M. Characterization of Li2S-P2S5 glass-ceramics as a solid electrolyte for lithium secondary batteries. Solid State Ion. 2004, 175, 683. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Minami, T.; Tatsumisago, M. Formation of superionic crystals from mechanically milled Li2S-P2S5 glasses. Electrochem. Commun. 2003, 5, 111. [Google Scholar] [CrossRef]
- Machida, N.; Yamamoto, H.; Asano, S.; Shigematsu, T. Preparation of amorphous 75L(2)S center dot chi P2S3 center dot(25-chi)P2S5 (mol%) solid electrolytes by a highenergy ball-milling process and their application for an all-solid-state lithium battery. Solid State Ion. 2005, 176, 473. [Google Scholar] [CrossRef]
- Yamamoto, H.; Machida, N.; Shigematsu, T. A mixed-former effect on lithium-ion conductivities of the Li2S-GeS2-P2S5 amorphous materials prepared by a high-energy ball-milling process. Solid State Ion. 2004, 175, 707. [Google Scholar] [CrossRef]
- Tatsumisago, M. Glassy materials based on Li2S for all-solid-state lithium secondary batteries. Solid State Ion. 2004, 175, 13. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Mizuno, F.; Hayashi, A. All-solid-state lithium secondary batteries using sulfide-based glass-ceramic electrolytes. J. Power Sources 2006, 159, 193. [Google Scholar] [CrossRef]
- Koch, C.C. Amorphization by mechanical alloying. J. Non Cryst. Solids 1990, 117, 670. [Google Scholar] [CrossRef]
- Zhou, E.H.; Suryanarayana, C.; Fores, F.H. Effect of premilling elemental powders on solid solubility extension of magnesium in titanium by mechanical alloying. Mater. Lett. 1995, 23, 27. [Google Scholar] [CrossRef]
- Lecaer, G.; Matteazzi, P. Mechanosynthesis of nanocrystalline materials. Hyperfine Interact. 1994, 90, 229. [Google Scholar] [CrossRef]
- Schaffer, G.B.; McCormick, P.G. Reduction of metal-oxides by mechanical alloying. Appl. Phys. Lett. 1989, 55, 45. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Wuhrer, R.; Liu, Z.W.; Ringer, S.P. In situ formation of BN nanotubes during nitriding reactions. Chem. Mater. 2005, 17, 5172. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.P.; Chen, H.; Chen, Y.J. One-dimensional nanomaterials synthesized using high-energy ball milling and annealing process. Sci. Technol. Adv. Mater. 2006, 7, 839. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Wan, C.; Du, K.; Xie, J.; Xu, N. Sulfur composite cathode materials for rechargeable lithium batteries. Adv. Funct. Mater. 2003, 13, 487–492. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hu, J.; Wan, L. Nanostructured Materials for Electrochemical Energy Conversion and Storage Devices. Adv. Mater. 2008, 22, 4384. [Google Scholar]
- Sakuda, A.; Hayashi, A.; Ohtomo, T.; Hama, S.; Tatsumisago, M. All-Solid-State Lithium Secondary Batteries Using LiCoO2 Particles with PLD Coatings of Li2S-P2S5. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2010; p. 570. [Google Scholar]
- Sata, N.; Eberman, K.; Eberl, K.; Maier, J. Mesoscopic fast ion conduction in nanometre-scale planar heterostructures. Nature 2000, 408, 946–949. [Google Scholar] [CrossRef]
- Trevey, J.E.; Jung, Y.S.; Lee, S.H. Preparation of Li2S-GeSe2-P2S5 electrolytes by a single step ball milling for all-solid-state lithium secondary batteries. J. Power Sources 2010, 195, 4984–4989. [Google Scholar] [CrossRef]
- Trevey, J.; Jang, J.; Jung, Y.; Stoldt, C.; Lee, S. Glass-ceramic Li2S-P2S5 electrolytes prepared by a single step ball billing process and their application for all-solid-state lithium-ion batteries. Electrochem. Commun. 2009, 11, 1830–1833. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Morimoto, H.; Tatsumisago, M.; Minami, T. Preparation of Li2S-P2S5 amorphous solid electrolytes by mechanical milling. J. Am. Ceram. Soc. 2001, 84, 477–479. [Google Scholar] [CrossRef]
- Kim, Y.; Martin, S. Ionic conductivities of various GeS2-based oxy-sulfide amorphous materials prepared by melt-quenching and mechanical milling methods. Solid State Ion. 2006, 177, 2881–2887. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).