Development of Solid-State Lithium-Ion Batteries (LIBs) to Increase Ionic Conductivity through Interactions between Solid Electrolytes and Anode and Cathode Electrodes
Abstract
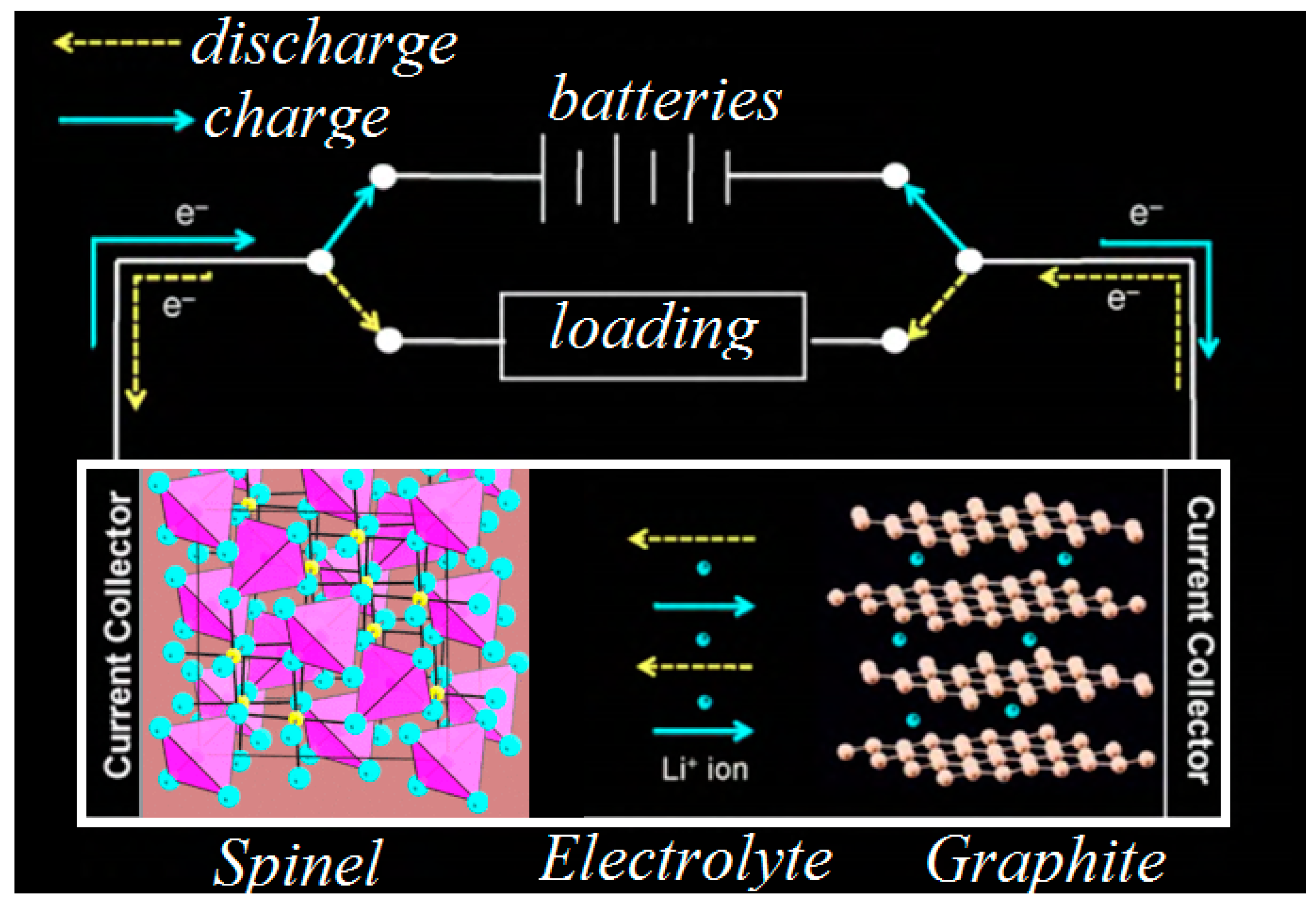
1. Introduction
2. Theoretical Backgrounds
2.1. Ionic Position in Cathode Materials
2.2. Electrical Position in Anode Materials
2.3. Conduction Evaluating in Electrolytes
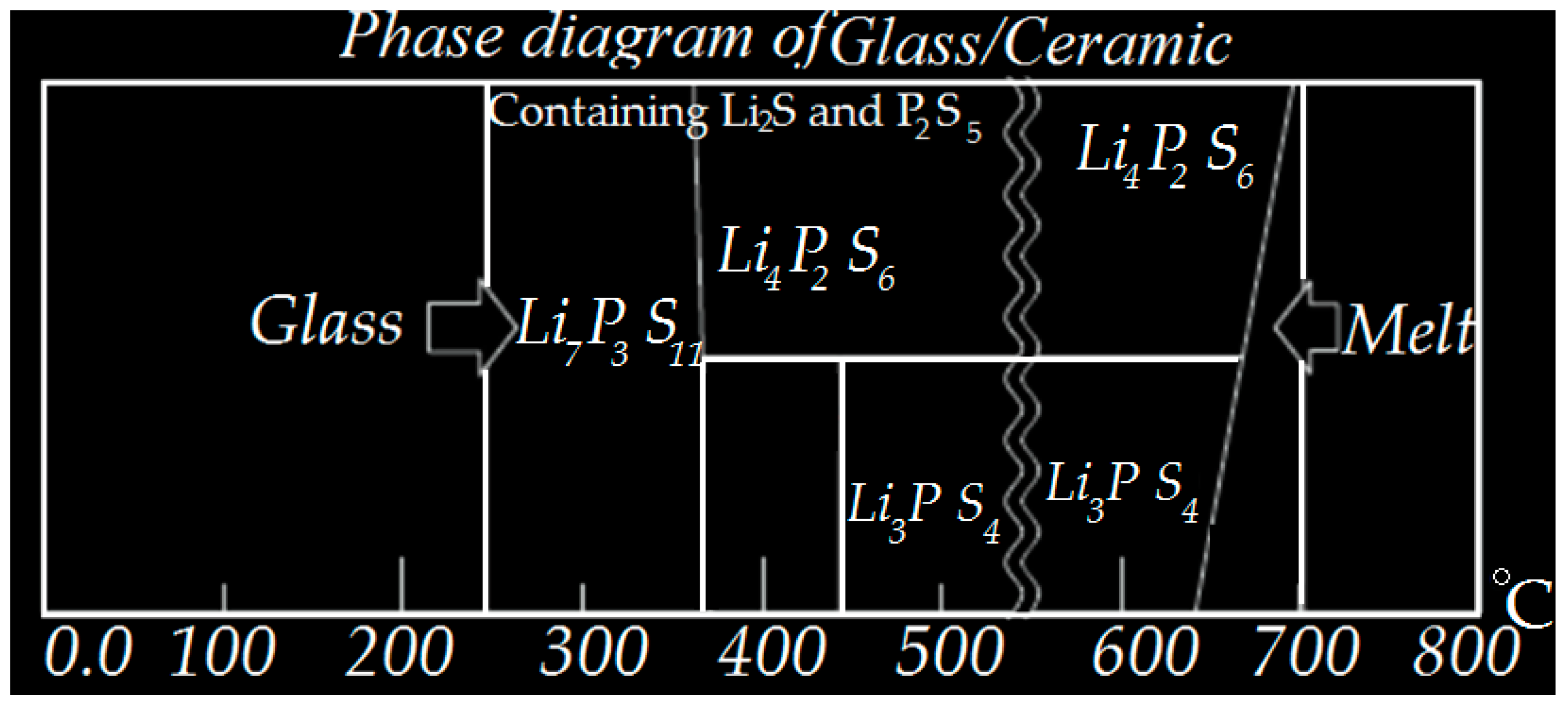
2.4. Solid-State Electrolytes (SSEs)
3. Materials and Methods
3.1. Ball Milling
3.1.1. Cryogenic Milling/Grinding and Jet Milling
3.1.2. Single-Step Ball Milling (SSBM)
3.2. Experimental
3.2.1. Preparation of P2S5, TiS2, and Li2S
3.2.2. Preparation of Anode Materials
3.2.3. Preparation of Cathode Materials
3.2.4. Electrolyte Fabrication
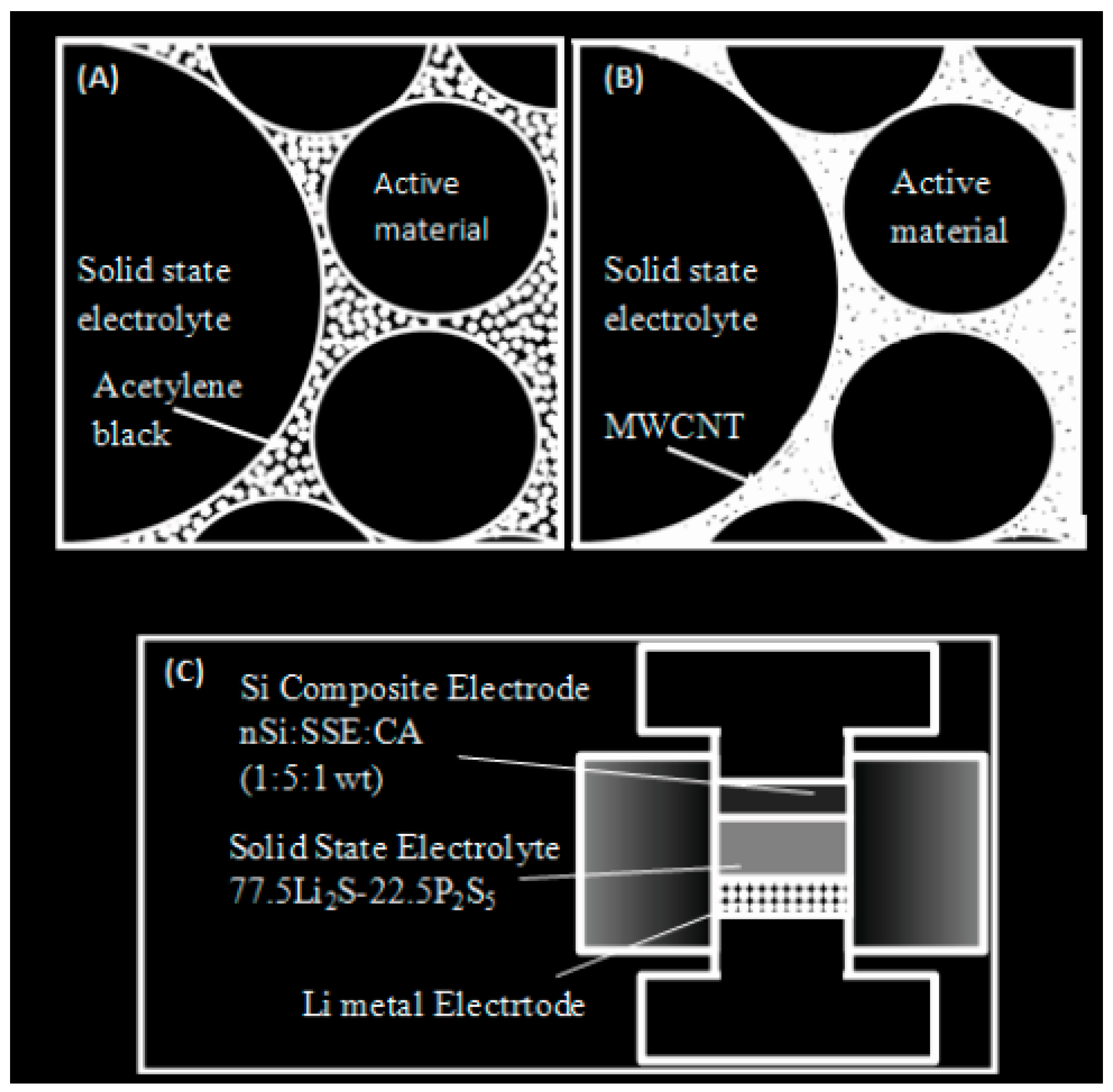
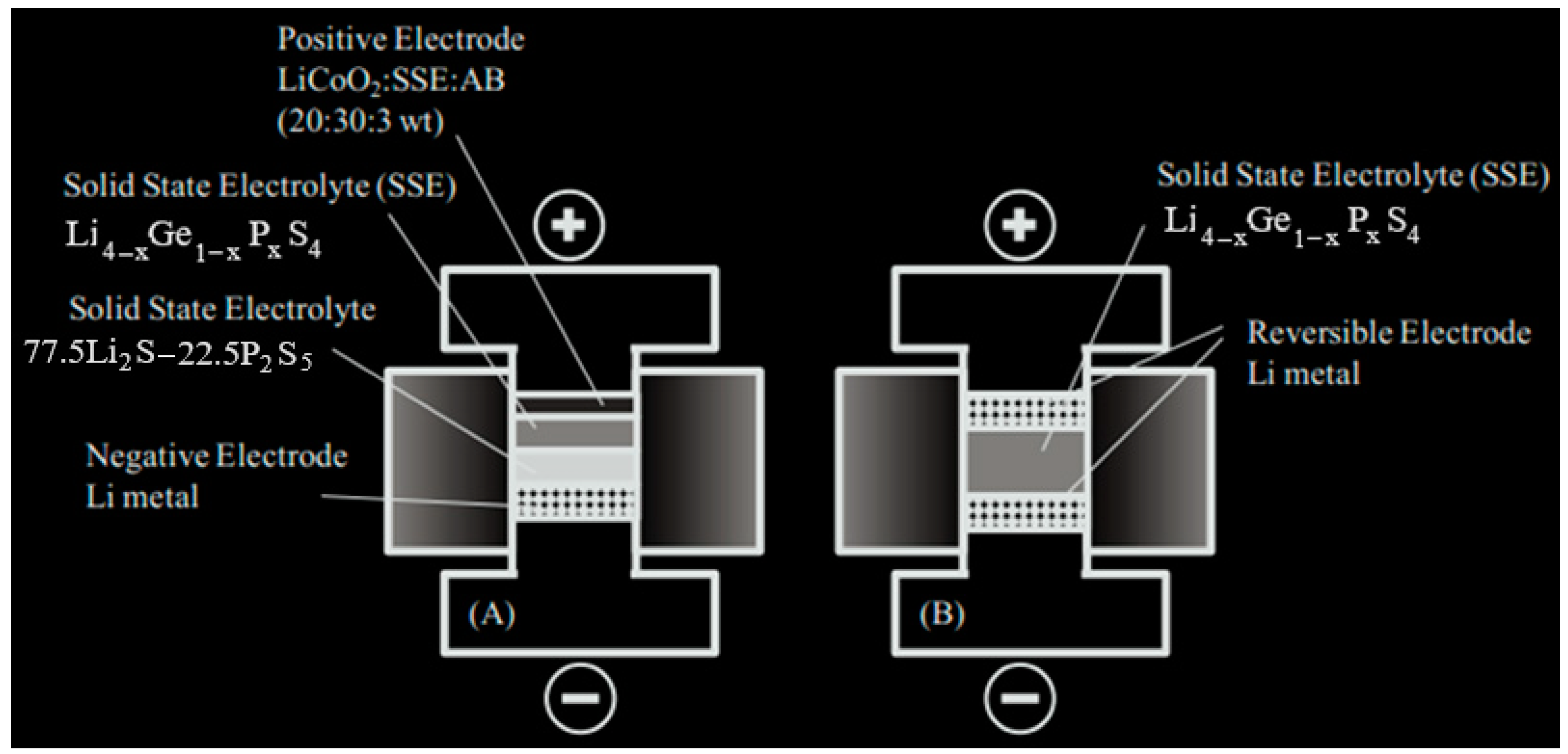
3.2.5. Electrode Fabrication with Bilayer Electrolyte
4. Results and Discussion
4.1. Characterization
4.2. Conductivities
Conductive Additive Modification by MWCNTs
4.3. Liquid vs. Solid
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Yi, J.; He, P.; Zhou, H. Solid-State Electrolytes for Lithium-Ion Batteries: Fundamentals, Challenges and Perspectives. Electrochem. Energ. Rev. 2019, 2, 574–605. [Google Scholar] [CrossRef]
- Flamme, B.; Rodriguez Garcia, G.; Weil, M.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidala, V.; Chagnes, A. Guidelines to design organic electrolytes for lithium-ion batteries: Environmental impact, physicochemical and electrochemical properties. Green Chem. 2017, 19, 1828. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2017, 8, 1702184. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, H.Q. Strategic Structural Design of a Gel Polymer Electrolyte toward a High Efficiency Lithium-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 3937–3971. [Google Scholar] [CrossRef]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Cai, H.; Chen, Z.; Guo, S.; Ma, D.; Wang, J. Polyacrylamide gel electrolyte for high-performance quasi-solid-state electrochromic devices. Sol. Energy Mater. Sol. Cells 2023, 256, 112310. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, D.; Yue, J.; Zhang, X.; Wu, Z.; Zhang, T.; Chen, C.; Fei, T. Optical Waveguide Sensors for Measuring Human Temperature and Humidity with Gel Polymer Electrolytes. ACS Appl. Mater. Interfaces 2021, 13, 60384–60392. [Google Scholar] [CrossRef]
- Kim, K.J.; Hyeon, J.S.; Kim, H.; Mun, T.J.; Haines, C.S.; Li, N.; Baughman, R.H.; Kim, S.J. Enhancing the Work Capacity of Electrochemical Artificial Muscles by Coiling Plies of Twist-Released Carbon Nanotube Yarns. ACS Appl. Mater. Interfaces 2019, 11, 13533–13537. [Google Scholar] [CrossRef]
- Yang, H.X.; Liu, Z.K.; Wang, Y.; Li, N.W.; Yu, L. Multiscale Structural Gel Polymer Electrolytes with Fast Li+ Transport for Long-Life Li Metal Batteries. Adv. Funct. Mater. 2023, 33, 2209837. [Google Scholar] [CrossRef]
- Sohn, J.-Y.; Choi, J.H.; Kim, P.-W.; Hwang, I.T.; Shin, J.; Jung, C.-H.; Lee, Y.-M. In-situ preparation of chemically-crosslinked polyvinylpyrrolidone gel polymer electrolyte for lithium ion battery via room-temperature electron beam-induced gelation. Radiat. Phys. Chem. 2023, 211, 111047. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Xia, Y.; Liang, Y.; Xia, X.; Tu, J. A polyacrylonitrile (PAN)-based double-layer multifunctional gel polymerelectrolyte for lithium-sulfur batteries. J. Membr. Sci. 2019, 582, 37–47. [Google Scholar] [CrossRef]
- Zhou, Z.; Pei, X.; Zhang, T.; Wang, L.; Hong, J.; Lu, Y.; He, G. A Gel Polymer Electrolyte with 2D Filler-reinforced for Dendrite Suppression Li-Ion Batteries. Electroanalysis 2023, 35, 2200306. [Google Scholar] [CrossRef]
- Dennis, J.O.; Shukur, M.F.; Aldaghri, O.A.; Ibnaouf, K.H.; Adam, A.A.; Usman, F.; Hassan, Y.M.; Alsadig, A.; Danbature, W.L.; Abdulkadir, B.A. A Review of Current Trends on Polyvinyl Alcohol (PVA)-Based Solid Polymer Electrolytes. Molecules 2023, 28, 1781. [Google Scholar] [CrossRef]
- Marchiori, C.F.N.; Carvalho, R.P.; Ebadi, M.; Brandell, D.; Moyses Araujo, C. Understanding the Electrochemical Stability Window of Polymer Electrolytes in Solid-State Batteries from Atomic-Scale Modeling: The Role of Li-Ion Salts. Chem. Mater. 2020, 32, 7237–7246. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent Advances in Innovative Polymer Electrolytes based on Poly (ionic liquid)s. Electrochim. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Cho, Y.G.; Hwang, C.; Cheong, D.S.; Kim, Y.-S.; Son, H.-K. Gel/Solid Polymer Electrolytes Characterized by In Situ Gelation or Polymerization for Electrochemical Energy Systems. Adv. Mater. 2019, 31, 1804909. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Liu, S.; Sun, Y.; Xiang, N.; Jiang, X.; Hou, L. Facile preparation and characterization of poly(vinyl alcohol)-NaCl-glycerol. Eur. Polym. J. 2018, 106, 206–213. [Google Scholar] [CrossRef]
- Shi, S.; Peng, X.; Liu, T.; Chen, Y.-N.; He, C.; Wang, H. Facile preparation of hydrogen-bonded supramolecular polyvinylalcoholglycerol gels with excellent thermoplasticity and mechanical properties. Polymer 2017, 111, 168–176. [Google Scholar] [CrossRef]
- Sun, Y.; Xiang, N.; Jiang, X.; Hou, L. Preparation of high tough poly(vinyl alcohol) hydrogel by soaking in NaCl aqueous solution. Mater. Lett. 2017, 194, 34–37. [Google Scholar] [CrossRef]
- Di, X.; Ma, Q.; Xu, Y.; Yang, M.; Wu, G.; Sun, P. High-performance ionic conductive poly(vinyl alcohol) hydrogels for flexible strain sensors based on a universal soaking strategy. Mater. Chem. Front. 2021, 5, 315–323. [Google Scholar] [CrossRef]
- Callister, W.D., Jr. Materials Science and Engineering: An Introduction, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1997; pp. 796–801. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Partoens, B.; Peeters, F.M. From graphene to graphite: Electronic structure around the K point. Phys. Rev. B 2006, 74, 075404. [Google Scholar] [CrossRef]
- Hess, M.; Lebraud, E.; Levasseur, A. Graphite multilayer thin films: A new anode material for Li-ion microbatteries synthesis and characterization. Power Sources 1997, 68, 204–207. [Google Scholar] [CrossRef]
- Segall, B. Energy bands of aluminum. Phys. Rev. 1961, 124, 1797–1806. [Google Scholar] [CrossRef]
- Kemp, J.P.; Cox, P.A. Electronic structure of LiCoO2 and related materials; photoemission studies. Phys. Condens. Matter 1990, 2, 9653–9667. [Google Scholar] [CrossRef]
- Shi, S.; Ouyang, C.; Lei, M.; Tang, W. Effect of Mg-doping on the structural and electronic properties of LiCoO2: A first-principles investigation. Power Sources 2007, 171, 908–912. [Google Scholar] [CrossRef]
- van Elp, J.; Wieland, J.L.; Eskes, H.; Kuiper, P.; Sawatzky, G.A. Electronic structure of CoO, Li-doped CoO, and LiCOO2. Phys. Rev. B 1991, 44, 6090–6103. [Google Scholar] [CrossRef]
- Ragavendran, K.; Nakkiran, A.; Kalyani, P.; Veluchamy, A.; Jagannathan, R. Nickel doped spinel lithium manganate–some insights using opto-impedance. Chem. Phys. Lett. 2008, 456, 110–115. [Google Scholar] [CrossRef]
- Marzec, J.; Swierczek, K.; Przewoznik, J.; Molenda, J.; Simon, D.R.; Kelder, E.M.; Schoonman, J. Conduction mechanism in operating a LiMn2O4 cathode. Solid State Ion. 2002, 146, 225–237. [Google Scholar] [CrossRef]
- Molenda, J.; Kucza, W. Transport properties of LiMn2O4. Solid State Ion. 1999, 117, 41–46. [Google Scholar] [CrossRef]
- Shi, S.; Liu, L.; Ouyang, C.; Wang, D.S.; Wang, Z.; Chen, L.; Huang, X. Enhancement of electronic conductivity of LiFePO4 by Cr doping and its identification by first-principles calculations. Phys. Rev. B 2003, 68, 195108-1–195108-5. [Google Scholar] [CrossRef]
- Xu, Y.N.; Chung, S.Y.; Bloking, J.T.; Chiang, Y.M.; Ching, W.Y. Electronic structure and electrical conductivity of undoped LiFePO4. Electrochem. Solid State Lett. 2004, 7, A131–A134. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kijima, N.; Tokiwa, K.; Watanabe, T.; Akimoto, J. Single-crystal synthesis, structure refinement and electrical properties of Li0.5CoO2. Phys. Condens. Matter 2007, 19, 436202–1–436202-12. [Google Scholar] [CrossRef]
- Levasseur, S.; Ménétrier, M.; Delmas, C. On the Dual Effect of Mg Doping in LiCoO2 and Li1+δCoO2: Structural, Electronic Properties, and 7Li MAS NMR Studies. Chem. Mater. 2002, 14, 3584–3590. [Google Scholar] [CrossRef]
- Carlier, D.; Ménétrier, M.; Delmas, C.J. 7Li MAS NMR study of electrochemically deintercalated LixNi0.30Co0.70O2 phases: Evidence of electronic and ionic mobility, and redox processes. J. Mater. Chem. 2001, 11, 594–603. [Google Scholar] [CrossRef]
- Lala, S.M.; Montoro, L.A.; Abbate, M.; Rosolen, J.M. The negative and positive structural effects of Ga doping in the electrochemical performance of LiCoO2. Electrochim. Acta 2005, 51, 7–13. [Google Scholar] [CrossRef]
- Ramos Ferrer, P.; Mace, A.; Thomas, S.N.; Jeon, J.W. Nanostructured porous graphene and its composites for energy storage applications. Nano Converg. 2017, 4, 29. [Google Scholar] [CrossRef]
- Molenda, J.; Swierczek, K.; Marzec, J.; Liu, R.S. Charge transport mechanism in LiCoyMn2−yO4 cathode material. Solid State Ion. 2003, 157, 101–108. [Google Scholar] [CrossRef]
- Lazarraga, M.G.; Pascual, L.; Gadjov, H.; Kovacheva, D.; Petrov, K.; Amarilla, J.M.; Rojas, R.M.; Martin-Luengo, M.A.; Rojo, J.M. Nanosize LiNiyMn2 − yO4 (0 < y ≤ 0.5) spinels synthesized by a sucrose-aided combustion method. Characterization and electrochemical performance. Mater. Chem. 2004, 14, 1640–1647. [Google Scholar]
- Kim, D.K.; Park, H.M.; Jung, S.J.; Jeong, Y.U.; Lee, J.H.; Kim, J.J. LiMnPO4: Review on Synthesis and Electrochemical Properties. Power Sources 2006, 159, 237–240. [Google Scholar] [CrossRef]
- Zhou, F.; Cococcioni, M.; Marianetti, C.A.; Morgan, D.; Ceder, G. First-principles prediction of redox potentials in transition-metal compounds with LDA+U. Phys. Rev. B 2004, 70, 235121–1–235121–8. [Google Scholar] [CrossRef]
- Ravagnan, L.; Piseri, P.; Bruzzi, M.; Miglio, S.; Bongiorno, G.; Baserga, A.; Casari, C.S.; Bassi, A.L.; Lenardi, C.; Yamaguchi, Y.; et al. Influence of Cumulenic Chains on the Vibrational and Electronic Properties of sp-sp2 Amorphous Carbon. Phys. Rev. Lett. 2007, 98, 216103-1–216103-4. [Google Scholar] [CrossRef]
- Vishwakarma, P.N.; Subramanyam, S.V. Hopping conduction in boron doped amorphous carbon films. J. Appl. Phys. 2006, 100, 113702–1–113702-5. [Google Scholar] [CrossRef]
- Brodd, R.J.; Huang, W.; Akridge, J.R. Advances in Lithium-Ion Batteries. Macromol. Symp. 2000, 159, 229–245. [Google Scholar] [CrossRef]
- Chagnes, A.; Carré, B.; Willmann, P.; Lemordant, D. Guidelines to Design Electrolytes for Lithium-ion Batteries: Environmental Impact, Physicochemical and Electrochemical Properties. Power Sources 2002, 109, 203–213. [Google Scholar] [CrossRef]
- Geoffroy, I.; Willmann, P.; Mesfar, K.; Carré, B.; Lemordant, D. Electrolytic characteristics of ethylene carbonate–diglyme-based electrolytes for lithium batteries. Electrochim. Acta 2000, 45, 2019–2027. [Google Scholar] [CrossRef]
- Ding, M.S.; Jow, T.R. Evaluation of Fluorinated Alkyl Phosphates as Flame Retardants in Electrolytes for Li-Ion Batteries I. Physical and Electrochemical Properties. J. Electrochem. Soc. 2003, 150, A620–A628. [Google Scholar] [CrossRef]
- Blint, R.J. Chemistry-Informed Machine Learning for Polymer Electrolyte Discovery. Electrochem. Soc. 1995, 142, 696–702. [Google Scholar] [CrossRef]
- Ribes, M. Thin Film Technology and Characterization: Their Use in Microionic Devices. Solid State Microbatteries; James, R., Akridge, M.B., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 41–58. [Google Scholar]
- Wakihara, M. Recent developments in lithium ion batteries. Mater. Sci. Eng. R 2001, 33, 109–134. [Google Scholar] [CrossRef]
- Zaghib, K.; Charest, P.; Guerfi, A.; Shim, J.; Perrier, M.; Striebel, K. Safe Li-ion polymer batteries for HEV applications. Power Sources 2004, 134, 124–129. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Shen, Y.J.; Reddy, M.J.; Chu, P.P. Complexation of poly(vinylidene fluoride): LiPF6 solid polymer electrolyte with enhanced ion conduction in ‘wet’form. Power Sources 2003, 123, 222–229. [Google Scholar] [CrossRef]
- Kaneko, M.; Nakayama, M.; Wakihara, M. Lithium-ion conduction in elastomeric binder in Li-ion batteries. Solid State Electrochem. 2007, 11, 1071–1076. [Google Scholar] [CrossRef]
- Egashira, M.; Todo, H.; Yosimoto, N.; Morita, M. Lithium ion conduction in ionic liquid-based gel polymer electrolyte. Power Sources 2008, 178, 729–735. [Google Scholar] [CrossRef]
- Zhang, S.; Lee, J.Y.; Hong, L. Li+ conducting ‘fuzzy’poly (ethylene oxide)–SiO2 polymer composite electrolytes. Power Sources 2004, 134, 95–102. [Google Scholar] [CrossRef]
- Abouimrane, A.; Whitfield, P.S.; Niketic, S.; Davidson, I.J. Investigation of Li salt doped succinonitrile as potential solid electrolytes for lithium batteries. Power Sources 2007, 174, 883–888. [Google Scholar] [CrossRef]
- Yang, K.Y.; Fung, K.Z.; Leu, I.C. Study on the structural change and lithium ion conductivity for the perovskite-type LaAlO3–La0.50Li0.50TiO3 solid solution. Alloys Compd. 2007, 438, 207–216. [Google Scholar] [CrossRef]
- Savitha, T.; Selvasekarapandian, S.; Ramya, C.S.; Bhuvaneswari, M.S.; Hirankumar, G.; Baskaran, R.; Angelo, P.C. Structural and ionic transport properties of Li2AlZr[PO4]3. Power Sources 2006, 157, 533–536. [Google Scholar] [CrossRef]
- Kanno, R.; Hata, T.; Kawamoto, Y.; Irie, M. Synthesis of a new lithium ionic conductor, thio-LISICON–lithium germanium sulfide system. Solid State Ion. 2000, 130, 97–104. [Google Scholar] [CrossRef]
- Tomita, Y.; Matsushita, H.; Kobayashi, K.; Maeda, Y.; Yamada, K. Substitution effect of ionic conductivity in lithium ion conductor, Li3InBr6−xClx. Solid State Ion. 2008, 179, 867–870. [Google Scholar] [CrossRef]
- Thangadurai, V.; Schwenzel, J.; Weppner, W. Tailoring ceramics for specific applications: A case study of the development of all-solid-state lithium batteries. Ionics 2005, 11, 11–23. [Google Scholar] [CrossRef]
- Hamon, Y.; Douard, A.; Sabary, F.; Marcel, C.; Vinatier, P.; Pecquenard, B.; Levasseur, A. Influence of sputtering conditions on ionic conductivity of LiPON thin films. Solid State Ion. 2006, 177, 257–261. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Zhang, Z. Further Characterization of Sis2-Li2s Glasses Doped with Lithium Halide. Electrochem. Soc. 1988, 135, 859–862. [Google Scholar] [CrossRef]
- Inada, T.; Takada, K.I.; Kajiyama, A.; Kouguchi, M.; Sasaki, H.; Kondo, S.; Watanabe, M.; Murayama, M.; Kanno, R. Fabrications and properties of composite solid-state electrolytes. Solid State Ion. 2003, 158, 275–280. [Google Scholar] [CrossRef]
- Chowdari, B.V.R.; Rao, G.V.S.; Lee, G.Y.H. XPS and ionic conductivity studies on Li2O–Al2O3–(TiO2 or GeO2)–P2O5 glass–ceramics. Solid State Ion. 2000, 136–137, 1067–1075. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Sakaguchi, I.; Zhang, L.; Ma, R.; Fukuda, K.; Osada, M.; Sasaki, T. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 2007, 9, 1486–1490. [Google Scholar] [CrossRef]
- Ratner, M.A.; Shriver, D.F. Ion transport in solvent-free polymers. Chem. Rev. 1988, 88, 109–124. [Google Scholar] [CrossRef]
- Papke, B.L.; Ratner, M.A.; Shriver, D.F. Conformation and ion-transport models for the structure and ionic conductivity in complexes of polyethers with alkali Metal Salts. Electrochem. Soc. 1982, 129, 1694–1701. [Google Scholar] [CrossRef]
- Brandup, J.; Immergut, E.H.; Grulke, E.A.; Abe, A.; Bloch, D.R. (Eds.) Solution Properties, Polymer Handbook, 4th ed.; John Wiley and Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Le Nest, J.F.; Callens, S.; Gandini, A.; Armand, M. Additives for Solid Polymer Electrolytes: The Layered Nanoparticles. Electrochim. Acta 1992, 37, 1585–1588. [Google Scholar] [CrossRef]
- Nishimoto, A.; Watanabe, M.; Ikeda, Y.; Kohjiya, S. High ionic conductivity of new polymer electrolytes based on high molecular weight polyether comb polymers. Electrochim. Acta 1998, 43, 1177–1184. [Google Scholar] [CrossRef]
- Scrosati, B. Advances in Lithium–Ion Batteries; van Schalkwijk Walter, A., Scrosati, B., Eds.; Kluwer Academic/Plenum Publishers: Norwell, MA, USA, 2002; pp. 252–266. [Google Scholar]
- Svanberg, C.; Adebahr, J.; Bergman, R.; Börjesson, L.; Scrosati, B.; Jacobsson, P. Polymer concentration dependence of the dynamics in gel electrolytes. Solid State Ion. 2000, 136–137, 1147–1152. [Google Scholar] [CrossRef]
- Jak, M.J.G.; Ooms, F.G.B.; Kelder, E.M.; Legerstee, W.J.; Schoonman, J.; Weisenburger, A. Design of Organoboron Solid Electrolytes/Solid Electrolyte Interface for Enhanced Performance of Lithium Ion Secondary Batteries. Power Sources 1999, 80, 83–89. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Gozdz, A.S.; Schmutz, C.; Shokoohi, F.; Warren, P.C. Performance of Bellcore`s plastic rechargeable Li-ion batteries. Solid State Ion. 1996, 86–88, 49–54. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Ue, M.; Mori, S. Mobility and ionic association of lithium salts in a propylene carbonate-ethyl methyl carbonate mixed solvent. Electrochem. Soc. 1995, 142, 2577–2581. [Google Scholar] [CrossRef]
- Schmidta, M.; Heidera, U.; Kuehnera, A.; Oestena, R.; Jungnitza, M.; Ignat’evb, N.; Sartorib, P. Lithium fluoroalkylphosphates: A new class of conducting salts for electrolytes for high energy lithium-ion batteries. Power Sources 2001, 97/98, 557–560. [Google Scholar]
- Walker, C.W., Jr.; Cox, J.D.; Salomon, M. Conductivity and electrochemical stability of electrolytes containing organic solvent mixtures with lithium tris(trifluoromethanesulfonyl) methide. Electrochem. Soc. 1996, 143, L80–L82. [Google Scholar] [CrossRef]
- Bates, J.; Dudney, N.; Neudecker, B.; Ueda, A.; Evans, C. Thin-film lithium and lithiumion batteries. Solid State Ion. 2000, 135, 33–45. [Google Scholar]
- Hong, H. Crystal-structure and ionic-conductivity of li14zn(geo4)4 and other new Li+ superionic conductors. Mater. Res. Bull. 1978, 13, 117–124. [Google Scholar]
- Seino, Y.; Takada, K.; Kim, B.; Zhang, L.; Ohta, N.; Wada, H.; Osada, M.; Sasaki, T. Synthesis of phosphorous sulfide solid electrolyte and all-solid-state lithium batteries with graphite electrode. Solid State Ion. 2005, 176, 2389–2393. [Google Scholar] [CrossRef]
- Adachi, G.; Imanaka, N.; Aono, H. Fast Li-circle plus conducting ceramic electrolytes. Adv. Mater. 1996, 8, 127. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Mizuno, F.; Tadanaga, K.; Minami, T.; Tatsumisago, M. Characterization of Li2S-P2S5 glass-ceramics as a solid electrolyte for lithium secondary batteries. Solid State Ion. 2004, 175, 683. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Minami, T.; Tatsumisago, M. Formation of superionic crystals from mechanically milled Li2S-P2S5 glasses. Electrochem. Commun. 2003, 5, 111. [Google Scholar] [CrossRef]
- Machida, N.; Yamamoto, H.; Asano, S.; Shigematsu, T. Preparation of amorphous 75L(2)S center dot chi P2S3 center dot(25-chi)P2S5 (mol%) solid electrolytes by a highenergy ball-milling process and their application for an all-solid-state lithium battery. Solid State Ion. 2005, 176, 473. [Google Scholar] [CrossRef]
- Yamamoto, H.; Machida, N.; Shigematsu, T. A mixed-former effect on lithium-ion conductivities of the Li2S-GeS2-P2S5 amorphous materials prepared by a high-energy ball-milling process. Solid State Ion. 2004, 175, 707. [Google Scholar] [CrossRef]
- Tatsumisago, M. Glassy materials based on Li2S for all-solid-state lithium secondary batteries. Solid State Ion. 2004, 175, 13. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Mizuno, F.; Hayashi, A. All-solid-state lithium secondary batteries using sulfide-based glass-ceramic electrolytes. J. Power Sources 2006, 159, 193. [Google Scholar] [CrossRef]
- Koch, C.C. Amorphization by mechanical alloying. J. Non Cryst. Solids 1990, 117, 670. [Google Scholar] [CrossRef]
- Zhou, E.H.; Suryanarayana, C.; Fores, F.H. Effect of premilling elemental powders on solid solubility extension of magnesium in titanium by mechanical alloying. Mater. Lett. 1995, 23, 27. [Google Scholar] [CrossRef]
- Lecaer, G.; Matteazzi, P. Mechanosynthesis of nanocrystalline materials. Hyperfine Interact. 1994, 90, 229. [Google Scholar] [CrossRef]
- Schaffer, G.B.; McCormick, P.G. Reduction of metal-oxides by mechanical alloying. Appl. Phys. Lett. 1989, 55, 45. [Google Scholar] [CrossRef]
- Yu, J.; Chen, Y.; Wuhrer, R.; Liu, Z.W.; Ringer, S.P. In situ formation of BN nanotubes during nitriding reactions. Chem. Mater. 2005, 17, 5172. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.P.; Chen, H.; Chen, Y.J. One-dimensional nanomaterials synthesized using high-energy ball milling and annealing process. Sci. Technol. Adv. Mater. 2006, 7, 839. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Wan, C.; Du, K.; Xie, J.; Xu, N. Sulfur composite cathode materials for rechargeable lithium batteries. Adv. Funct. Mater. 2003, 13, 487–492. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hu, J.; Wan, L. Nanostructured Materials for Electrochemical Energy Conversion and Storage Devices. Adv. Mater. 2008, 22, 4384. [Google Scholar]
- Sakuda, A.; Hayashi, A.; Ohtomo, T.; Hama, S.; Tatsumisago, M. All-Solid-State Lithium Secondary Batteries Using LiCoO2 Particles with PLD Coatings of Li2S-P2S5. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2010; p. 570. [Google Scholar]
- Sata, N.; Eberman, K.; Eberl, K.; Maier, J. Mesoscopic fast ion conduction in nanometre-scale planar heterostructures. Nature 2000, 408, 946–949. [Google Scholar] [CrossRef]
- Trevey, J.E.; Jung, Y.S.; Lee, S.H. Preparation of Li2S-GeSe2-P2S5 electrolytes by a single step ball milling for all-solid-state lithium secondary batteries. J. Power Sources 2010, 195, 4984–4989. [Google Scholar] [CrossRef]
- Trevey, J.; Jang, J.; Jung, Y.; Stoldt, C.; Lee, S. Glass-ceramic Li2S-P2S5 electrolytes prepared by a single step ball billing process and their application for all-solid-state lithium-ion batteries. Electrochem. Commun. 2009, 11, 1830–1833. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Morimoto, H.; Tatsumisago, M.; Minami, T. Preparation of Li2S-P2S5 amorphous solid electrolytes by mechanical milling. J. Am. Ceram. Soc. 2001, 84, 477–479. [Google Scholar] [CrossRef]
- Kim, Y.; Martin, S. Ionic conductivities of various GeS2-based oxy-sulfide amorphous materials prepared by melt-quenching and mechanical milling methods. Solid State Ion. 2006, 177, 2881–2887. [Google Scholar] [CrossRef]

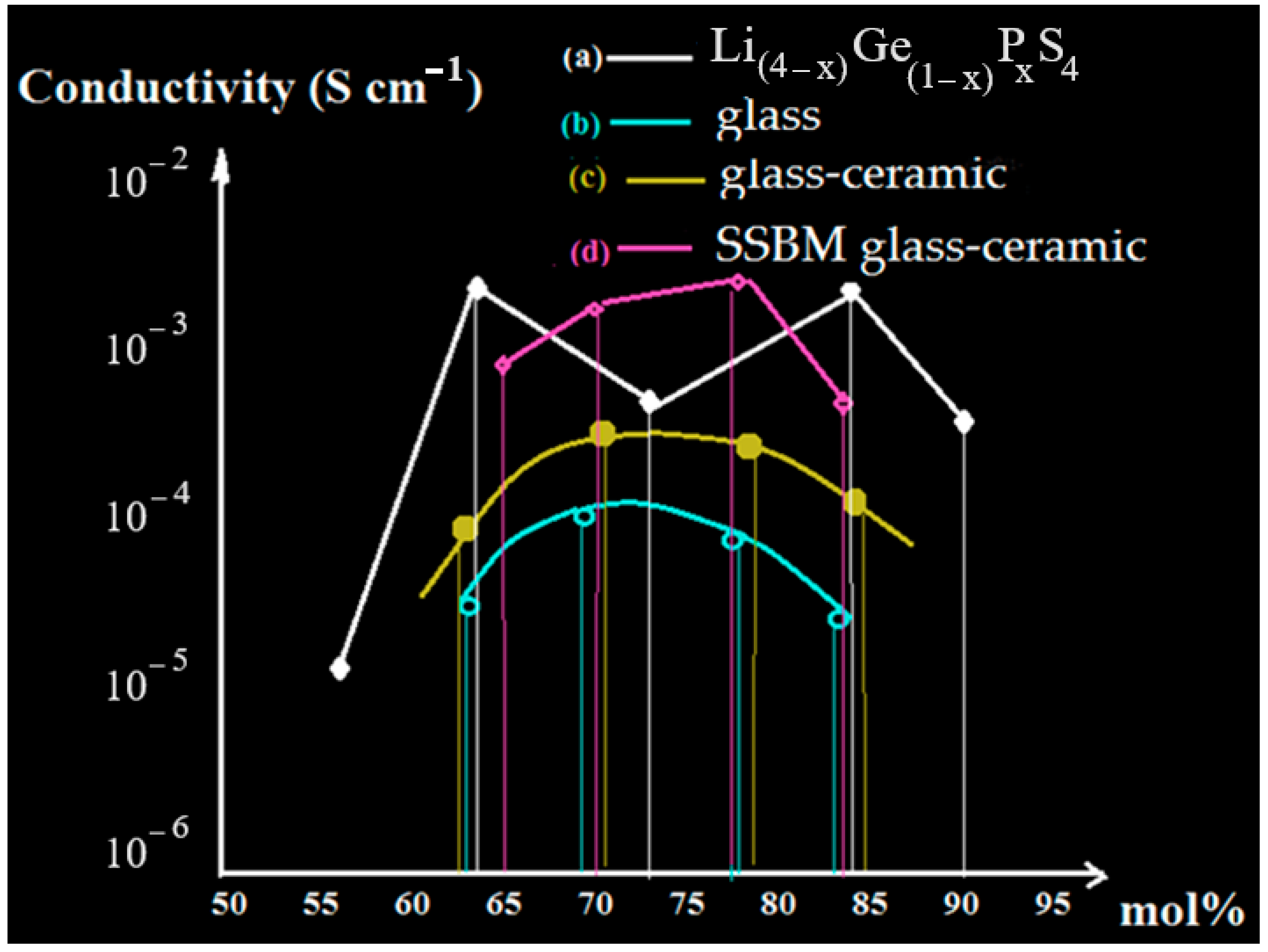
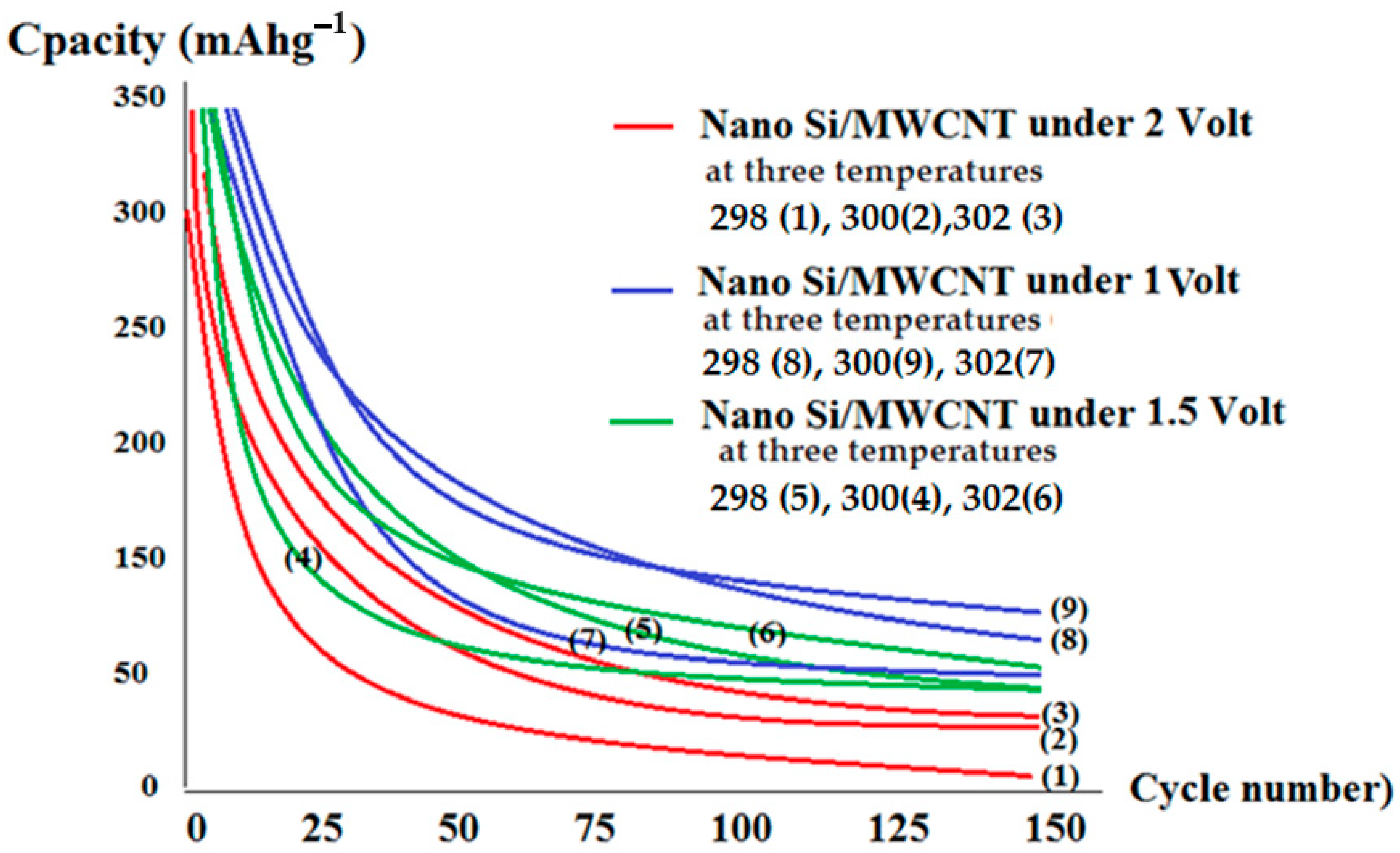

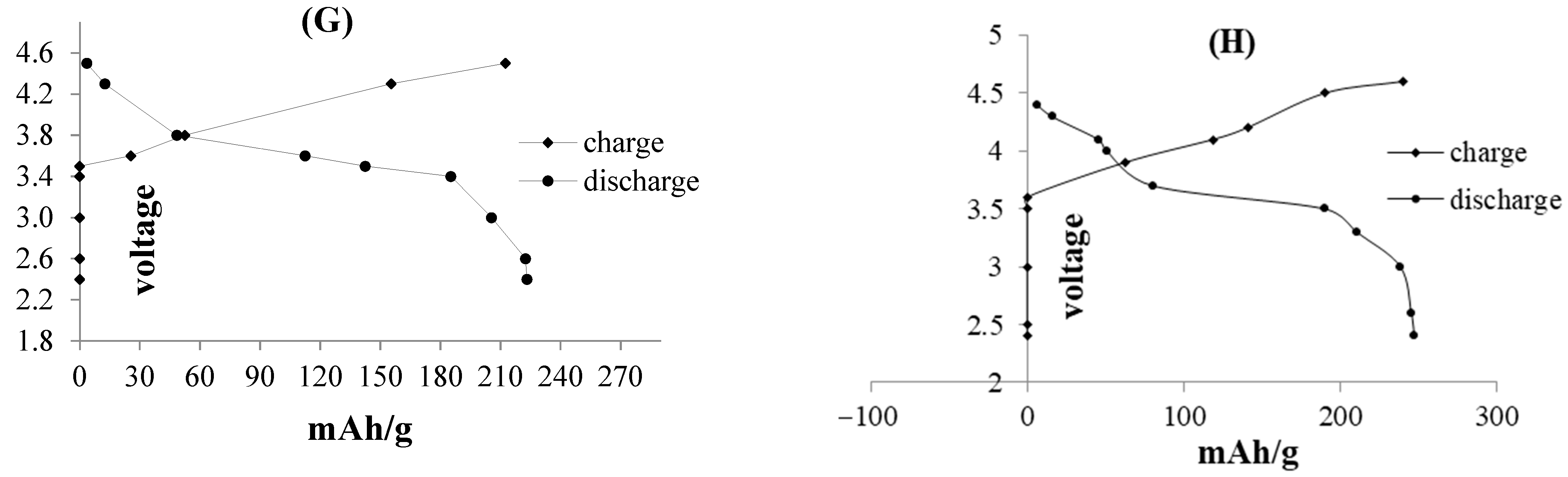
| Electrode | Compound | Band Gap (eV) | Conductivity Scm−1 | Reference |
|---|---|---|---|---|
| anode | Copper | 0 | 6 × 105 | [21,22] |
| anode | Graphite | 0 | 2 × 103 | [23,24] |
| anode | Aluminum | 0 | 3.5 × 105 | [25] |
| cathode | LiCoO2 | 0.6–2.5 | 10−5 | [26,27,28] |
| cathode | LiMn2O4 | 0.25–2.1 | 10−4 | [29,30,31] |
| cathode | LiFePO4 | 0.2–0.9 | 10−6 | [32,33] |
| Cathode Composite | Electrical Conductivity (Scm−1) | Technique | Reference |
|---|---|---|---|
| LixCoO2 | 2.1 × 10−2 | 4 Point Probe DC | [34] |
| Li1.0Mgy Co1−yO2 | (10−3.77: y = 0); (10−1.2: y = 0.04); (10−0.70: y = 0.05) | 4 Point Probe DC | [35] |
| LixNi0.30Co0.70O2 | 10−4 to 10−3: 0.70 < x < 1 | 4 Point Probe DC | [36] |
| LiGay Co1−yO2 | 6.65 × 10−4: un-doped system | EIS | [37] |
| LixMn2O4 | 10−6.5 (x = 1.00) | 2 Point Probe AC | [29,30,31] |
| LixMn2O4 | 10−4.5 (x = 0.90) | 2 Point Probe AC | [38] |
| LiCoyMn2−yO4 | (2.3 × 10−4 (y = 0.1); (2.5 × 10−2 (y = 1):at RT | AC impedance | [39] |
| LiNiyMn2−yO4 | 0−4.5 (y = 0, to 0.6): at RT | 4 Point Probe DC | [40] |
| LixFePO4 | 10−2:x = 0.8(impurity:Fe2P2O7) | 2 Point Probe AC | [41] |
| Li1−xMxFePO4(M = Zr, Nb, Mg) | 10−3 (size: 30 nm, C content: 5 wt%) | 2 Point Probe AC | [42] |
| Salt | Solvent | Ionic Conductivities (Scm−1) | Reference |
|---|---|---|---|
| Solid-state electrolytes (SSEs) | |||
| Wet polymer | - | 8.5 × 10−7 (LiPF6 6 wt% in PVdF) | [53] |
| Gel polymer | - | 7.8 × 10−2 (at 25 °C) | [54] |
| Plastic crystal | - | 1 × 10−4 (6%), 5 × 10−3 (12%):LiBF4 | [57] |
| Crystalline (perovskite) | - | 1.0 × 10−3 (x = 0, 0.2) | [58] |
| Crystalline (NASICON) | - | 6.5 × 10−5(at 200 °C) | [59] |
| Crystalline (thio-LISICON) | - | 4.3 × 10−5 (x = 0) | [60] |
| Crystalline (Garnet) | - | 5.2 × 10−4 | [62] |
| Glass (LiPON) | - | 1.8 × 10−6 to 7.7 × 10−7 | [63] |
| Composite (glass + polymer) | - | 1.2 × 10−3 (dry process) | [65] |
| Glass-ceramics | - | 1. 3 × 10−8 | [66] |
| Solvent electrolytes | |||
| LiClO4 | PC | 5.5 | [77] |
| LiAsF6 | EC/DMC | 8.5 | [78] |
| LiBF4 | EC/DMC | 11.3 | [79] |
| LiPF6 | EC/DMC | 10.5 | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monajjemi, M.; Mollaamin, F. Development of Solid-State Lithium-Ion Batteries (LIBs) to Increase Ionic Conductivity through Interactions between Solid Electrolytes and Anode and Cathode Electrodes. Energies 2024, 17, 4530. https://doi.org/10.3390/en17184530
Monajjemi M, Mollaamin F. Development of Solid-State Lithium-Ion Batteries (LIBs) to Increase Ionic Conductivity through Interactions between Solid Electrolytes and Anode and Cathode Electrodes. Energies. 2024; 17(18):4530. https://doi.org/10.3390/en17184530
Chicago/Turabian StyleMonajjemi, Majid, and Fatemeh Mollaamin. 2024. "Development of Solid-State Lithium-Ion Batteries (LIBs) to Increase Ionic Conductivity through Interactions between Solid Electrolytes and Anode and Cathode Electrodes" Energies 17, no. 18: 4530. https://doi.org/10.3390/en17184530
APA StyleMonajjemi, M., & Mollaamin, F. (2024). Development of Solid-State Lithium-Ion Batteries (LIBs) to Increase Ionic Conductivity through Interactions between Solid Electrolytes and Anode and Cathode Electrodes. Energies, 17(18), 4530. https://doi.org/10.3390/en17184530






