Development of an Effective Microalgae Cultivation System Utilizing CO2 in the Air by Injecting CaCO3
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Strains and Culture Conditions
2.2. Analytical Methods
2.2.1. Analysis of Microalgal Biomass from Mixed Samples
2.2.2. Analysis of pH and Dissolved Inorganic Carbon (DIC) Concentration
2.2.3. Analysis of CA Activity
2.2.4. Lipid Extraction and Analysis of Total Lipid Content from Microalgal Cells
2.2.5. Analysis of 13C Isotope Ratios in Culture Media and Cell Biomass
3. Results and Discussion
3.1. Introduction of CaCO3 from Biomineralization to Microalgae Cultivation System for Preventing Cell Death from Ambient Air CO2 Supply
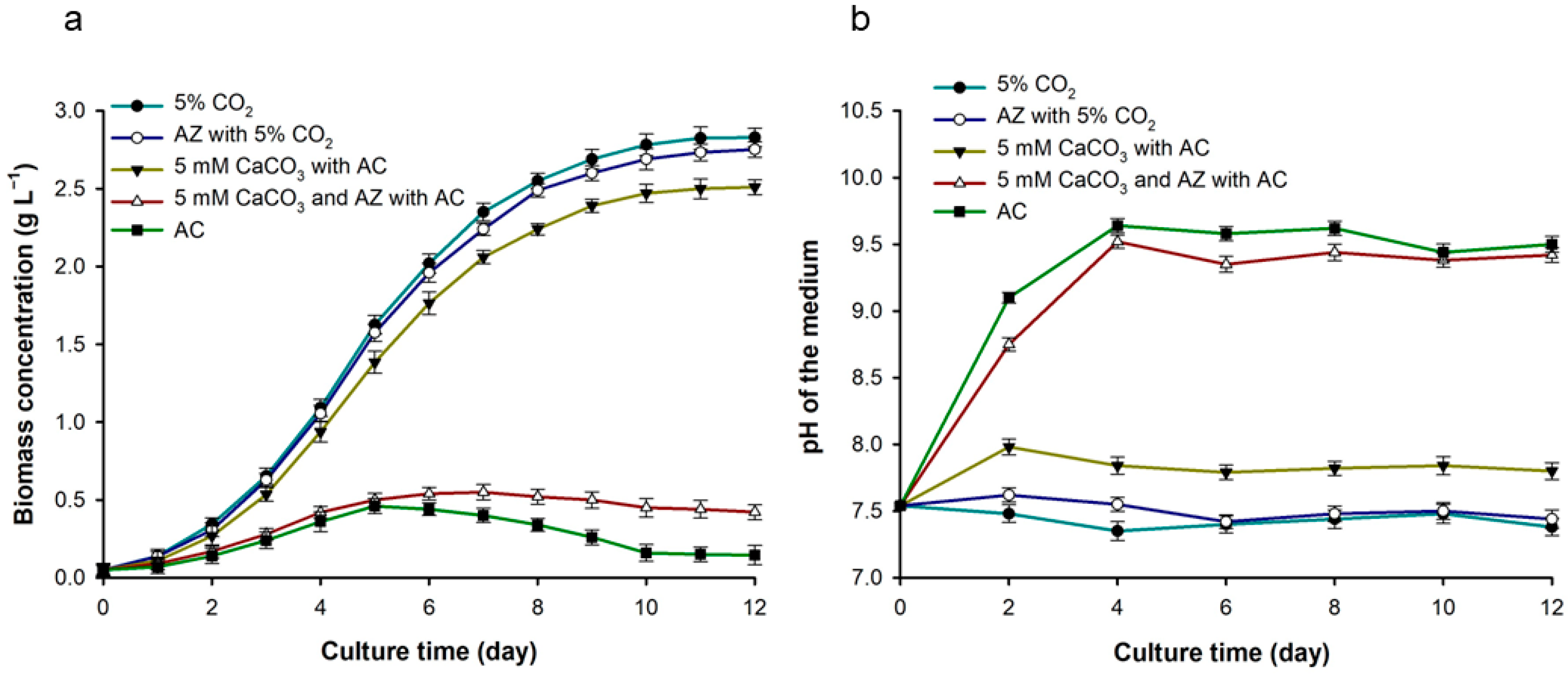
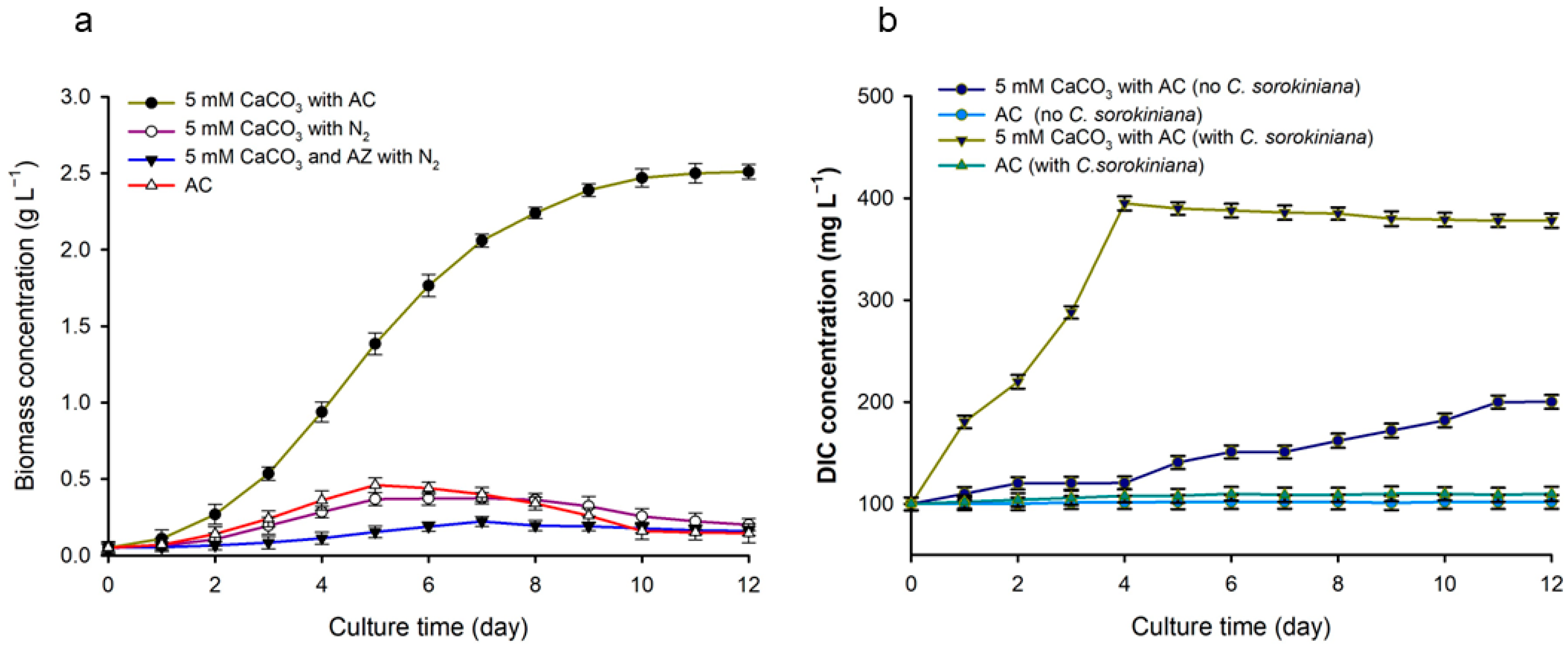
3.2. Effect of Cost-Effective Materials CaCO3 on Microalgal Biomass Production
3.2.1. Effect of CaCO3 Concentration on Biomass Production
3.2.2. Effect of Carbonic Anhydrase Enzyme on Biomass Production and Resulting pH Changes
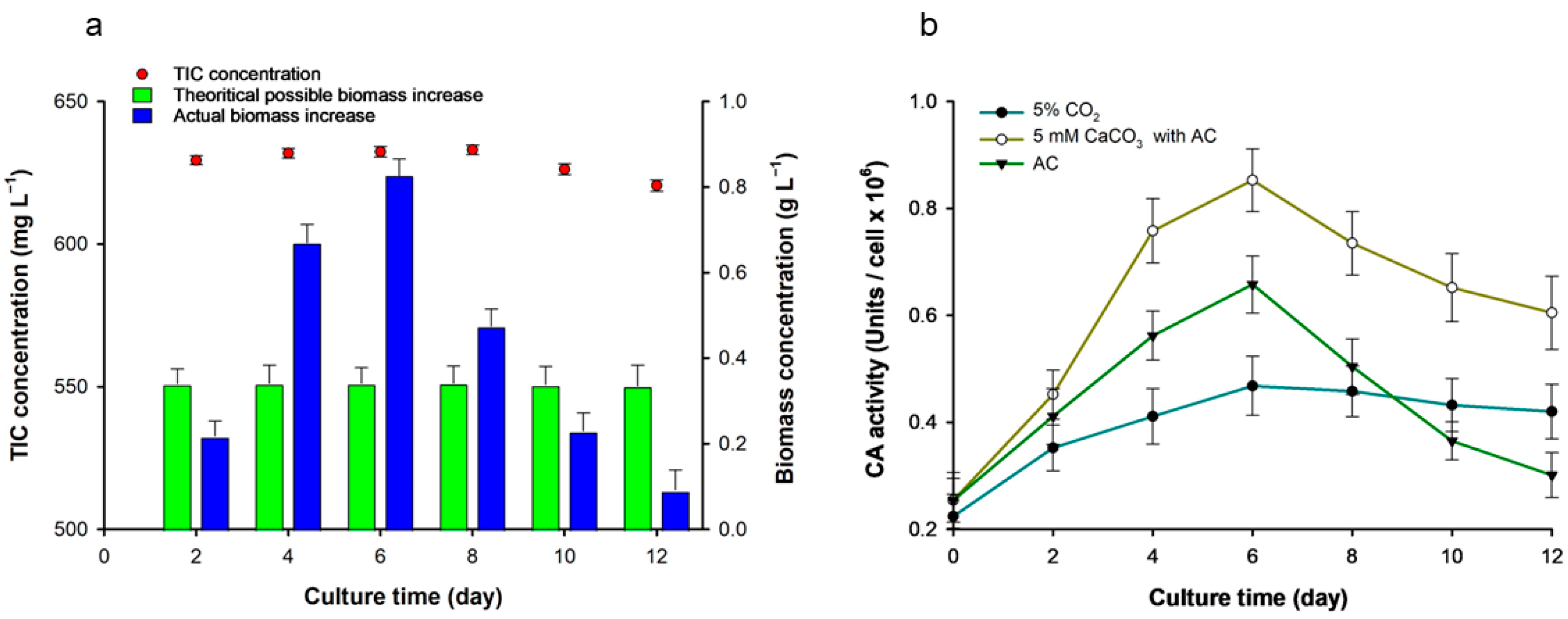
3.3. Analysis of the Cause of Biomass Increase When 5 mM CaCO3 Was Injected with AC
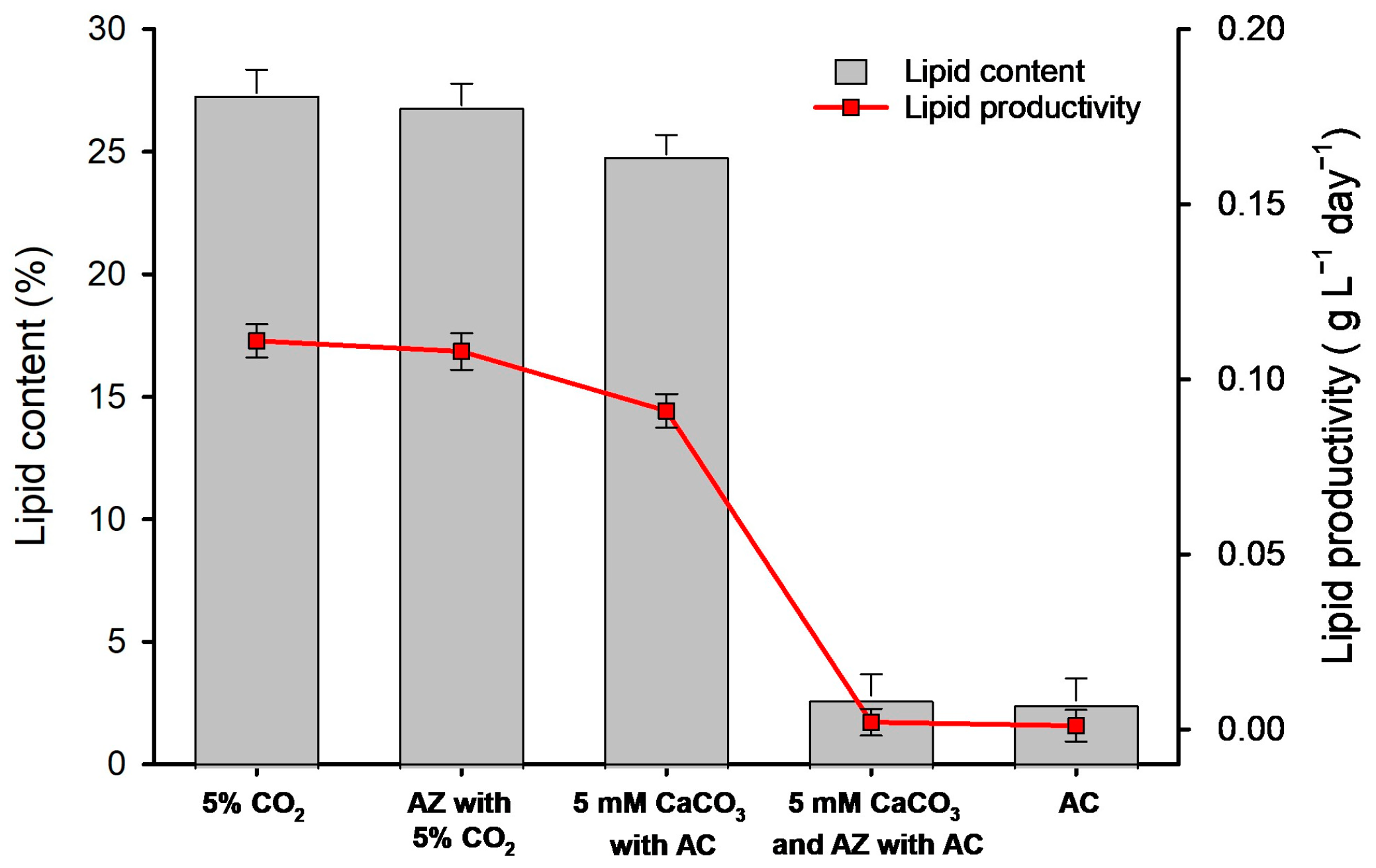
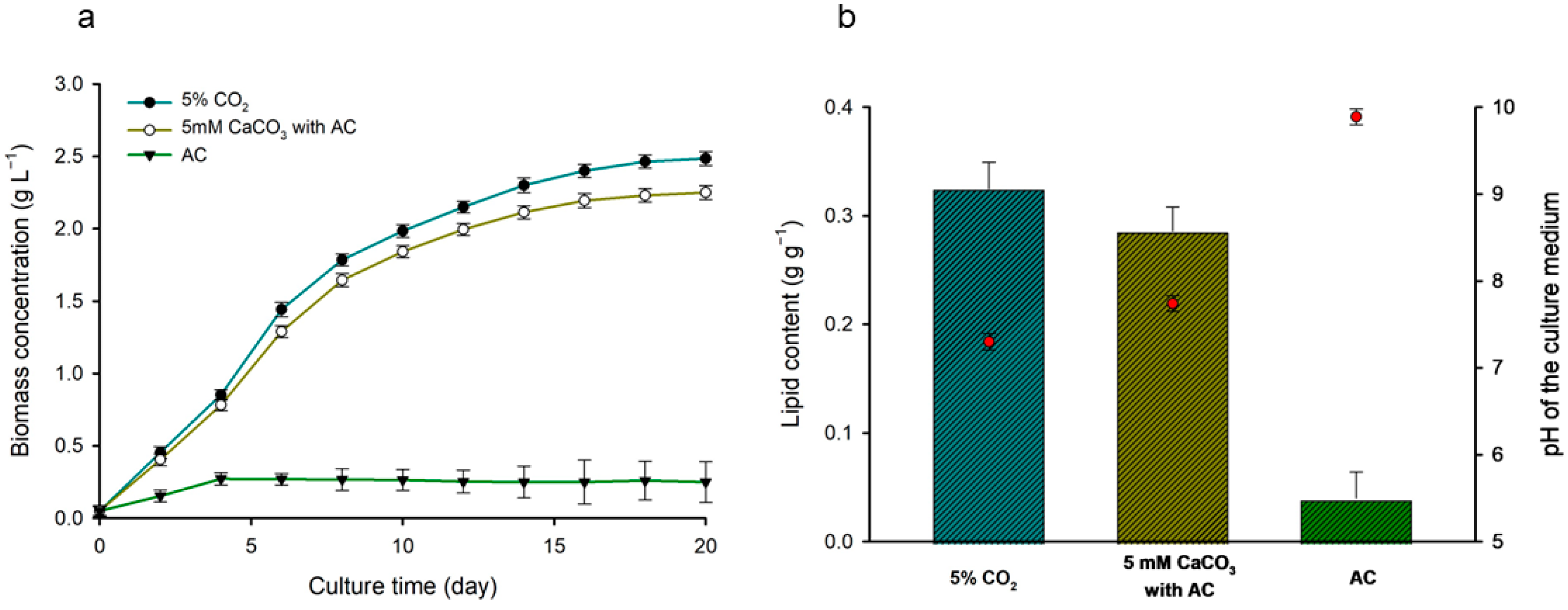
3.4. Comparison of Microalgal Lipid Content and Productivity According to Different Culture Systems
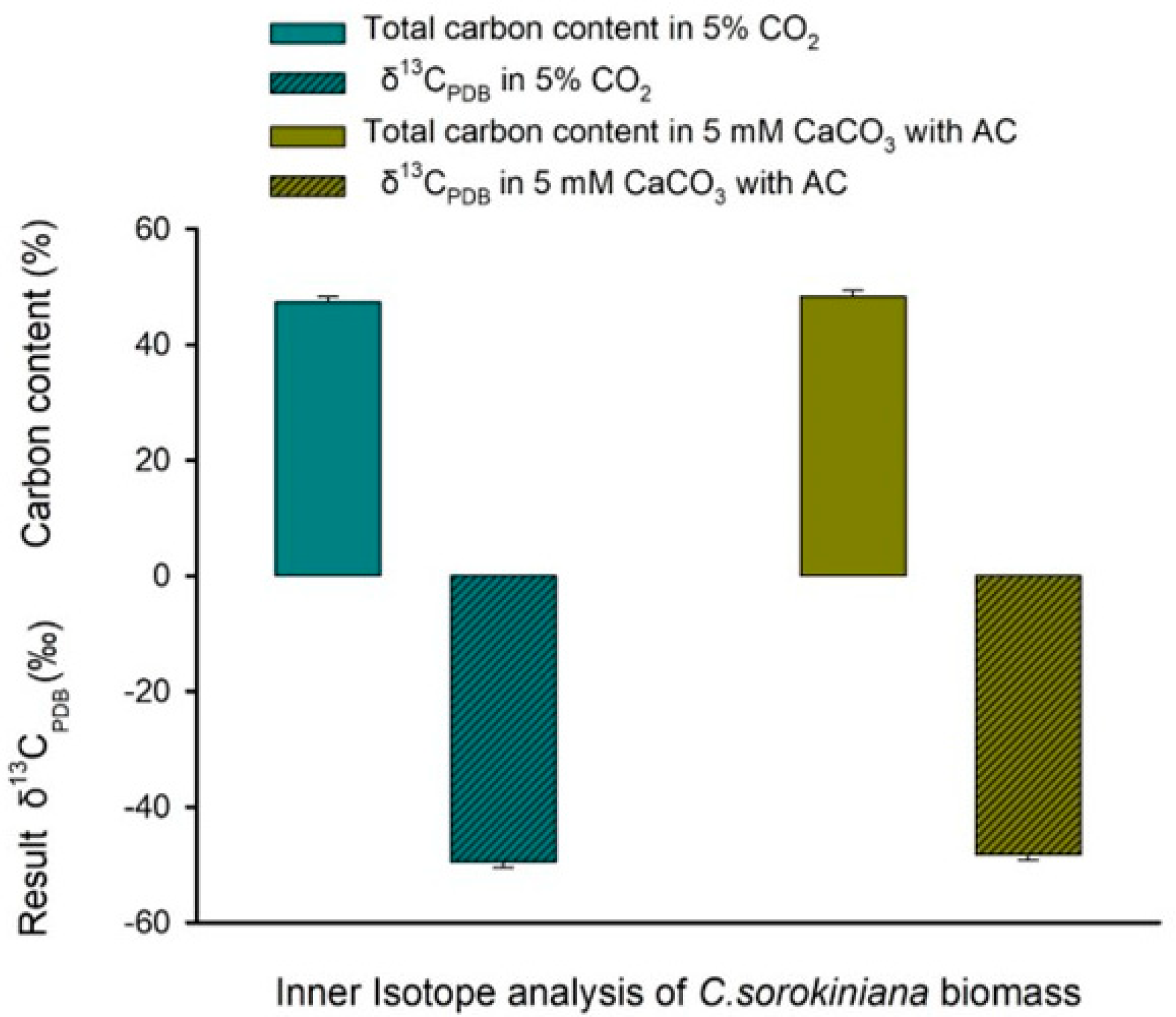
3.5. Isotopic Analysis to Determine Whether the Carbon Source Utilized by C. sorokiniana Cells for Biomass Accumulation Was from Air or CaCO3
3.6. Analyzing the Effect of CaCO3 on Microalgal Cultures and DIC Concentration Changes and the Role of CA Enzymes
3.7. Analyzing the Effect of CaCO3 on Biomass Production and Resulting pH Changes in Microalgal Cells with AC under Bench-Scale Culture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dvork, M.T.; Armour, K.C.; Friersin, D.M.W.; Proistosescu, C.; Baker, M.B.; Smith, C.J. Estimating the timing of geophysical commitment to 1.5 and 2.0 °C of global warming. Nat. Clim. Chang. 2022, 12, 547–552. [Google Scholar] [CrossRef]
- Gupta, A.K.; Nair, S.S. Environmental Extremes Disaster Risk Management—Addressing Climate Change; National Institute of Disaster Management: New Delhi, India, 2012; p. 40.
- Muruganandam, M.; Rajamanickam, S.; Sivarethinamohan, S.; Reddy, M.K.; Velusamy, P.; Gomathi, R.; Ravindiran, G.; Gurugubelli, T.R.; Munisamy, S.K. Impact of Climate Change and Anthropogenic Activities on Aquatic Ecosystem—A Review. Environ. Res. 2023, 238, 117233. [Google Scholar]
- Rogelj, J.; Den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Tapia, J.F.D.; Lee, J.Y.; Ooi, R.E.; Foo, D.C.; Tan, R.R. A review of optimization and decision-making models for the planning of CO2 capture, utilization and storage (CCUS) systems. Sustain. Prod. Consum. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Bruhn, T.; Naims, H.; Olfe-Kräutlein, B. Separating the debate on CO2 utilization from carbon capture and storage. Environ. Sci. Policy 2016, 60, 38–43. [Google Scholar] [CrossRef]
- Zoback, M.D.; Gorelick, S.M. Earthquake triggering and large-scale geologic storage of carbon dioxide. Proc. Natl. Acad. Sci. USA 2012, 109, 10164–10168. [Google Scholar] [CrossRef] [PubMed]
- Brandl, P.; Bui, M.; Hallett, J.P.; Mac Dowell, N. Beyond 90% capture: Possible, but at what cost? Int. J. Greenh. Gas Control. 2021, 105, 103239. [Google Scholar] [CrossRef]
- Daneshvar, E.; Wicker, R.J.; Show, P.L.; Bhatnagar, A. Biologically-Mediated Carbon Capture and Utilization by Microalgae Towards Sustainable CO2 Biofixation and Biomass Valorization—A Review. J. Chem. Eng. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.H.; Kang, S.G.; Park, Y.G. Development of Environmental Impact Monitoring Protocol for Offshore Carbon Capture and Storage (CCS): A Biological Perspective. Environ. Impact Assess. Rev. 2016, 57, 139–150. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Nirmala, N.; Dawn, S.S.; Baskaran, A.; SundarRajan, P.; Gopinath, K.P.; Arun, J. Genetic Improvement of MIcroalgae for Enhanced Carbon Dioxide Sequestration and Enriched Biomass Productivity: Review on CO2 Bio-Fixation Pathways Modifications. Algal Res. 2022, 66, 102810. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z. Advances in the biological fixation of carbon dioxide by microalgae. J. Chem. Technol. Biotechnol. 2021, 96, 1475–1495. [Google Scholar] [CrossRef]
- Alami, A.H.; Alasad, S.; Ali, M.; Alshamsi, M. Investigating algae for CO2 capture and accumulation and simultaneous production of biomass for biodiesel production. Sci. Total Environ. 2021, 759, 143529. [Google Scholar] [CrossRef]
- Xu, P.; Shao, S.; Qian, J.; Li, J.; Xu, R.; Liu, J.; Zhou, W. Scale-up of microalgal systems for decarbonization and bioproducts: Challenges and opportunities. Bioresour. Technol. 2024, 398, 130528. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chang, H.; Zhang, S.; Ho, S.H. Production of sustainable biofuels from microalgae with CO2 bio-sequestration and life cycle assessment. Environ. Res. 2023, 227, 115730. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zuo, L.; Li, X.; Liu, X. Enhancing Shale Gas Recovery by Carbon Dioxide Injection: A Method of Carbon Capture, Utilization and Storage (CCUS). Process Saf. Environ. Prot. 2023, 179, 484–492. [Google Scholar] [CrossRef]
- Yu, B.S.; Sung, Y.J.; Choi, H.I.; Sirohi, R.; Sim, S.J. Concurrent enhancement of CO2 fixation and productivities of omega-3 fatty acids and astaxanthin in Haematococcus pluvialis culture via calcium-mediated homeoviscous adaptation and biomineralization. Bioresour. Technol. 2021, 340, 125720. [Google Scholar] [CrossRef]
- Ali, S.; Peter, A.P.; Chew, K.W.; Munawaroh, H.S.H.; Show, P.L. Resource Recovery from Industrial Effluents through the Cultivation of Microalgae: A Review. Bioresour. Technol. 2021, 337, 125461. [Google Scholar] [CrossRef]
- Xie, M.; Qiu, Y.; Song, C.; Qi, Y.; Li, Y.; Kitamura, Y. Optimization of Chlorella sorokiniana cultivation condition for simultaneous enhanced biomass and lipid production via CO2 fixation. Bioresour. Technol. 2018, 2, 15–20. [Google Scholar]
- Montoya-Vallejo, C.; Guzmán Duque, F.L.; Quintero Díaz, J.C. Biomass and Lipid Production by the Native Green Microalgae Chlorella Sorokiniana in Response to Nutrients, Light Intensity, and Carbon Dioxide: Experimental and Modeling Approach. Front. Bioeng. Biotechnol. 2023, 11, 1149762. [Google Scholar] [CrossRef]
- Klinthong, W.; Yang, Y.H.; Huang, C.H.; Tan, C.S. A Review: Microalgae and Their Applications in CO2 Capture and Renewable Energy. Aerosol Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Singh, U.B.; Ahluwalia, A.S. Microalgae: A Promising Tool for Carbon Sequestration. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 73–95. [Google Scholar] [CrossRef]
- Dahai, H.; Zhihong, Y.; Lin, Q.; Yuhong, L.; Lei, T.; Jiang, L.; Liandong, Z. The Application of Magical Microalgae in Carbon Sequestration and Emission Reduction: Removal Mechanisms and Potential Analysis. Renew. Sustain. Energy Rev. 2024, 197, 114417. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Dulta, K.; Kurniawan, S.B.; Omoarukhe, F.O.; Ewuzie, U.; Eshiemogie, S.O.; Ojo, A.U.; Abdullah, S.R.S. Progress in microalgae application for CO2 sequestration. Clean. Chem. Eng. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Bhola, V.; Swalaha, F.; Ranjith Kumar, R.; Singh, M.; Bux, F. Overview of the potential of microalgae for CO2 sequestration. Int. J. Environ. Sci. Technol 2014, 11, 2103–2118. [Google Scholar] [CrossRef]
- Maghzian, A.; Aslani, A.; Zahedi, R. Review on the direct air CO2 capture by microalgae: Bibliographic mapping. Energy Rep. 2022, 8, 3337–3349. [Google Scholar] [CrossRef]
- Zaidi, S.; Srivastava, N.; Khare, S.K. Microbial Carbonic Anhydrase Mediated Carbon Capture, Sequestration & Utilization: A Sustainable Approach to Delivering Bio-Renewables. Bioresour. Technol. 2022, 365, 128174. [Google Scholar]
- Mondal, M.; Khanra, S.; Tiwari, O.N.; Gayen, K.; Halder, G.N. Role of Carbonic Anhydrase on the Way to Biological Carbon Capture Through Microalgae–A Mini Review. Environ. Prog. Sustain. Energy. 2016, 35, 1605–1615. [Google Scholar] [CrossRef]
- Ores, J.D.C.; Amarante, M.C.A.D.; Fernandes, S.S.; Kalil, S.J. Production of carbonic anhydrase by marine and freshwater microalgae. Biocatal. Biotransformation 2016, 34, 57–65. [Google Scholar] [CrossRef]
- Mishra, S.; Joshi, B.; Dey, P.; Pathak, H.; Pandey, N.; Kohra, A. CCM in photosynthetic bacteria and marine alga. J. Pharmacogn. Phytochem. 2018, 7, 928–937. [Google Scholar]
- Vikramathithan, J.; Hwangbo, K.; Lim, J.M.; Lim, K.M.; Park, Y.I.; Jeong, W.J. Overexpression of Chlamydomonas reinhardtii LCIA (CrLCIA) gene increases growth of Nannochloropsis salina CCMP1776. Algal Res. 2020, 46, 101807. [Google Scholar] [CrossRef]
- Yu, B.S.; Sung, Y.J.; Hong, M.E.; Sim, S.J. Improvement of photoautotrophic algal biomass production after interrupted CO2 supply by urea and KH2PO4 injection. Energies 2021, 14, 778. [Google Scholar] [CrossRef]
- Xie, T.; Wu, Y. The role of microalgae and their carbonic anhydrase on the biological dissolution of limestone. Environ. Earth Sci. 2014, 71, 5231–5239. [Google Scholar] [CrossRef]
- Moroney, J.V.; Husic, H.D.; Tolbert, N.E. Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol. 1985, 79, 177–183. [Google Scholar] [CrossRef]
- Yu, B.S.; Yang, H.E.; Sirohi, R.; Sim, S.J. Novel effective bioprocess for optimal CO2 fixation via microalgae-based biomineralization under semi-continuous culture. Bioresour. Technol. 2022, 364, 128063. [Google Scholar] [CrossRef]
- Rigobello-Masini, M.; Aidar, E.; Masini, J.C. Extra and intracellular activities of carbonic anhydrase of the marine microalga Tetraselmis gracilis (Chlorophyta). Braz. J. Microbiol. 2003, 34, 267–272. [Google Scholar] [CrossRef]
- Willbur, K.M.; Anderson, N.G. Electrometric and colorimetric determination of carbonic anhydrase. J. Biol. Chem. 1948, 176, 147–154. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Iverson, S.J.; Lang, S.L.; Cooper, M.H. Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 2021, 36, 1283–1287. [Google Scholar] [CrossRef]
- Shim, S.J.; Hong, M.E.; Chang, W.S.; Sim, S.J. Repeated-batch production of omega-3 enriched biomass of Chlorella sorokiniana via calcium-induced homeoviscous adaptation. Bioresour. Technol. 2020, 303, 122944. [Google Scholar] [CrossRef]
- Hutchinson, T.F.; Kessler, A.J.; Wong, W.W.; Hall, P.; Leung, P.M.; Jirapanjawat, T.; Greening, C.; Glud, R.N.; Cook, P.L. Microorganisms oxidize glucose through distinct pathways in permeable and cohesive sediments. ISME J. 2024, 18, wrae001. [Google Scholar] [CrossRef] [PubMed]
- Kitadai, N.; Nakamura, R.; Yamamoto, M.; Okada, S.; Takahagi, W.; Nakano, Y.; Takahashi, Y.; Takai, K.; Oono, Y. Thioester synthesis through geoelectrochemical CO2 fixation on Ni sulfides. Commun. Chem 2021, 4, 37. [Google Scholar] [CrossRef]
- Nisar, A.; Khan, S.; Hameed, M.; Nisar, A.; Ahmad, H.; Mehmood, S.A. Bio-conversion of CO2 into biofuels and other value-added chemicals via metabolic engineering. Microbiol. Res. 2021, 251, 126813. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Bermudez, S.P.; Garcia-Perez, J.S.; Rittmann, B.E.; Parra-Saldivar, R. Photosynthetic bioenergy utilizing CO2: An approach on flue gases utilization for third generation biofuels. J. Clean. Prod. 2015, 98, 53–65. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Alvim-Ferraz, M.C.; Martins, F.G.; Simões, M.; Pires, J.C. Integration of Microalgae-Based Bioenergy Production into a Petrochemical Complex: Techno-Economic Assessment. Energies 2016, 9, 224. [Google Scholar] [CrossRef]
- Seth, J.R.; Wangikar, P.P. Challenges and opportunities for microalgae-mediated CO2 capture and biorefinery. Biotechnol. Bioeng. 2015, 112, 1281–1296. [Google Scholar] [CrossRef]
- Hoang, A.T.; Sirohi, R.; Pandey, A.; Nižetić, S.; Lam, S.S.; Chen, W.H.; Luque, R.; Thomas, S.; Arici, M.; Pham, V.V. Biofuel production from microalgae: Challenges and chances. Phytochem. Rev. 2023, 22, 1089–1126. [Google Scholar] [CrossRef]
- Hong, M.E.; Yu, B.S.; Patel, A.K.; Choi, H.I.; Song, S.; Sung, Y.J.; Chang, W.S.; Shim, S.J. Enhanced biomass and lipid production of Neochloris oleoabundans under high light conditions by anisotropic nature of light-splitting CaCO3 crystal. Bioresour. Technol. 2019, 287, 121483. [Google Scholar] [CrossRef] [PubMed]
- Kupriyanova, E.V.; Pronina, N.A.; Los, D.A. Adapting from low to high: An update to CO2-concentrating mechanisms of cyanobacteria and microalgae. Plants 2023, 12, 1569. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wu, L.; Tan, D.; Yu, Y.; Jiang, Q.; Wu, Y.; Wang, H.; Liu, Y. Enhancing CO2 fixation by microalgae in a Photobioreactor: Molecular mechanisms with exogenous carbonic anhydrase. Bioresour. Technol. 2024, 408, 131176. [Google Scholar] [CrossRef]
- Gao, P.; Guo, L.; Zhao, Y.; Jin, C.; She, Z.; Gao, M. Enhancing microalgae growth and product accumulation with carbon source regulation: New perspective for the coordination between photosynthesis and aerobic respiration. Chemosphere 2021, 278, 130435. [Google Scholar]
- Elfadil, D.; Palmieri, S.; Della Pelle, F.; Sergi, M.; Amine, A.; Compagnone, D. Enzyme inhibition coupled to molecularly imprinted polymers for acetazolamide determination in biological samples. Talanta 2022, 240, 123195. [Google Scholar] [CrossRef]
- Zhao, X.C.; Tan, X.B.; Yang, L.B.; Liao, J.Y.; Li, X.Y. Cultivation of Chlorella pyrenoidosa in anaerobic wastewater: The coupled effects of ammonium, temperature and pH conditions on lipids compositions. Bioresour. Technol. 2019, 284, 90–97. [Google Scholar] [CrossRef]

| Parameters (during Autotrophic Induction) | Chlorella sorokiniana (UTEX 2714) | ||||
|---|---|---|---|---|---|
| 5% CO2 | AZ with 5% CO2 | 5 mM CaCO3 with AC | 5 mM CaCO3 and AZ with AC | AC | |
| CaCO3 consumption (g) | - | - | 0.272 | 0.056 | - |
| Biomass conversion rate (g) from dissolved calcite | - | - | 0.066 | 0.014 | - |
| Biomass conversion rate (g) from dissolved CO2 | 2.829 | 2.814 | 2.497 | 0.288 | 0.145 |
| Biomass concentration (g L–1) | 2.829 | 2.814 | 2.563 | 0.302 | 0.145 |
| Biomass productivity (g L–1 day–1) | 0.404 | 0.402 | 0.366 | 0.043 | 0.021 |
| Lipid content (g g–1) | 0.274 | 0.269 | 0.249 | 0.028 | 0.026 |
| Lipid productivity (g L–1 day–1) | 0.111 | 0.108 | 0.091 | 0.001 | 0.001 |
| CO2 removal rate (g L–1 day–1) * | 1.029 | 1.024 | 0.932 | 0.11 | 0.053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyo, S.; Yu, B.-S.; Han, K. Development of an Effective Microalgae Cultivation System Utilizing CO2 in the Air by Injecting CaCO3. Energies 2024, 17, 4475. https://doi.org/10.3390/en17174475
Pyo S, Yu B-S, Han K. Development of an Effective Microalgae Cultivation System Utilizing CO2 in the Air by Injecting CaCO3. Energies. 2024; 17(17):4475. https://doi.org/10.3390/en17174475
Chicago/Turabian StylePyo, Seonju, Byung-Sun Yu, and Kyudong Han. 2024. "Development of an Effective Microalgae Cultivation System Utilizing CO2 in the Air by Injecting CaCO3" Energies 17, no. 17: 4475. https://doi.org/10.3390/en17174475
APA StylePyo, S., Yu, B.-S., & Han, K. (2024). Development of an Effective Microalgae Cultivation System Utilizing CO2 in the Air by Injecting CaCO3. Energies, 17(17), 4475. https://doi.org/10.3390/en17174475







