Possibilities of Liquefied Spruce (Picea abies) and Oak (Quercus robur) Biomass as an Environmentally Friendly Additive in Conventional Phenol–Formaldehyde Resin Wood Adhesives
Abstract
1. Introduction
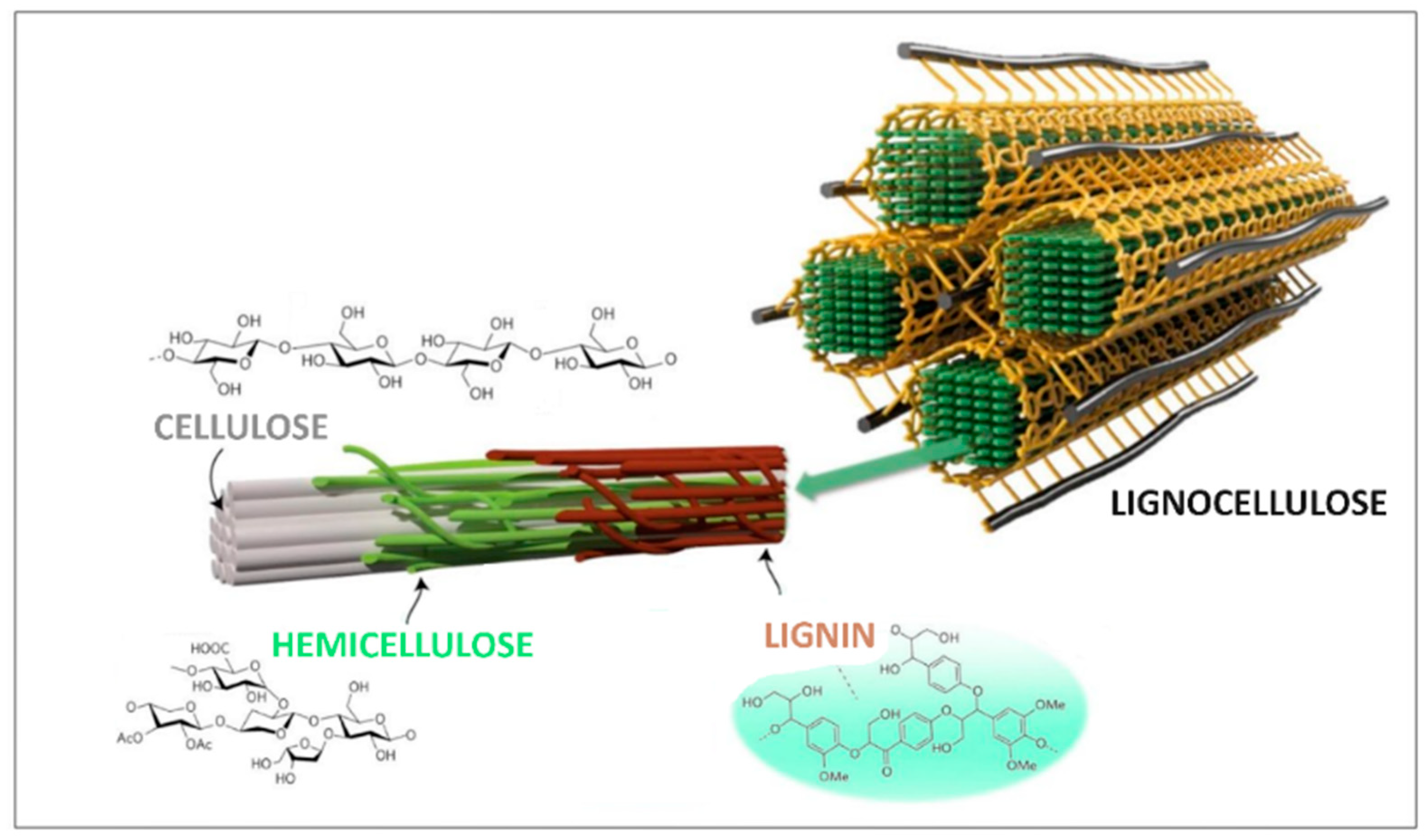
2. Materials and Methods
2.1. Wood Samples
2.2. Sample Preparation
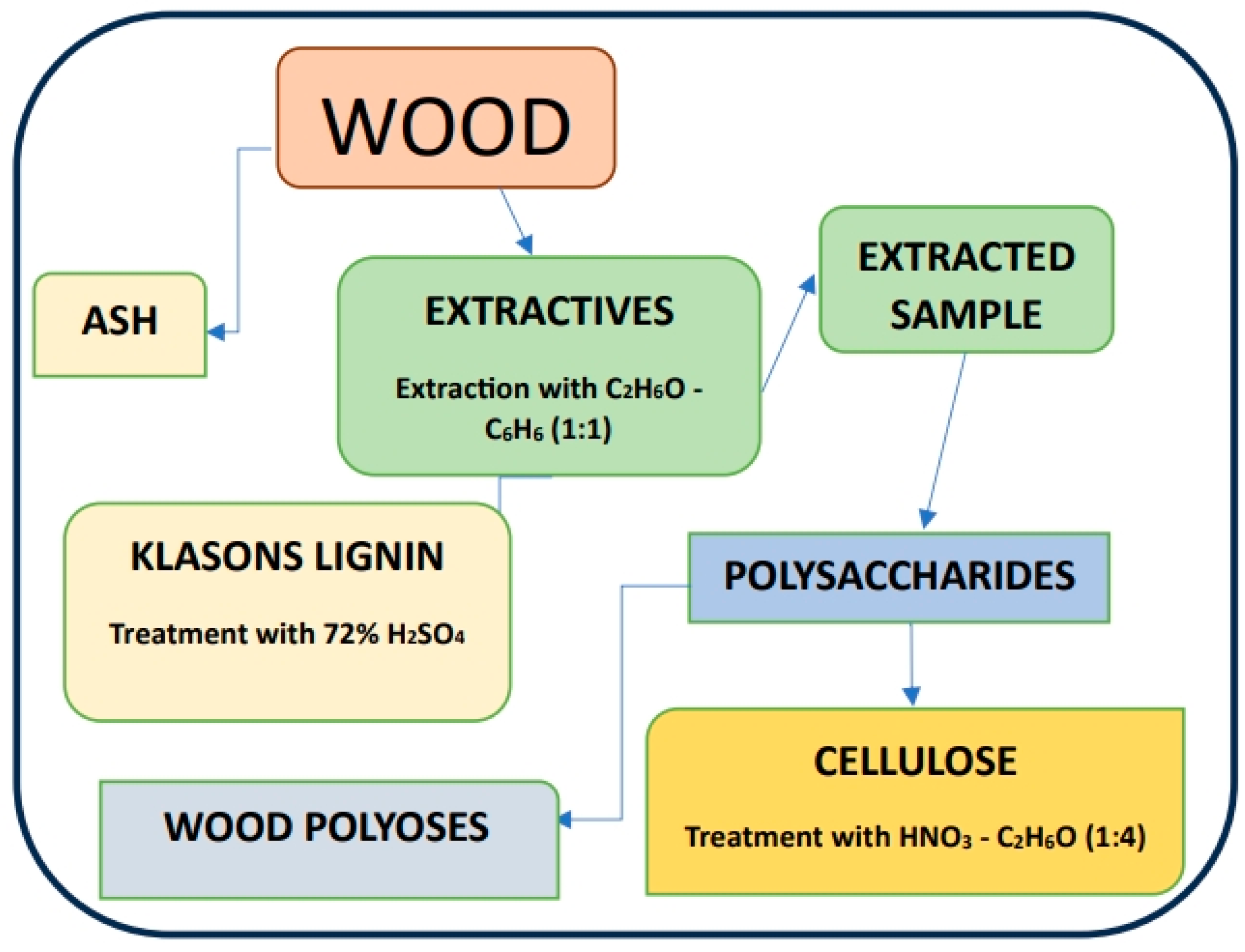
2.3. Analysis of the Physicochemical Composition
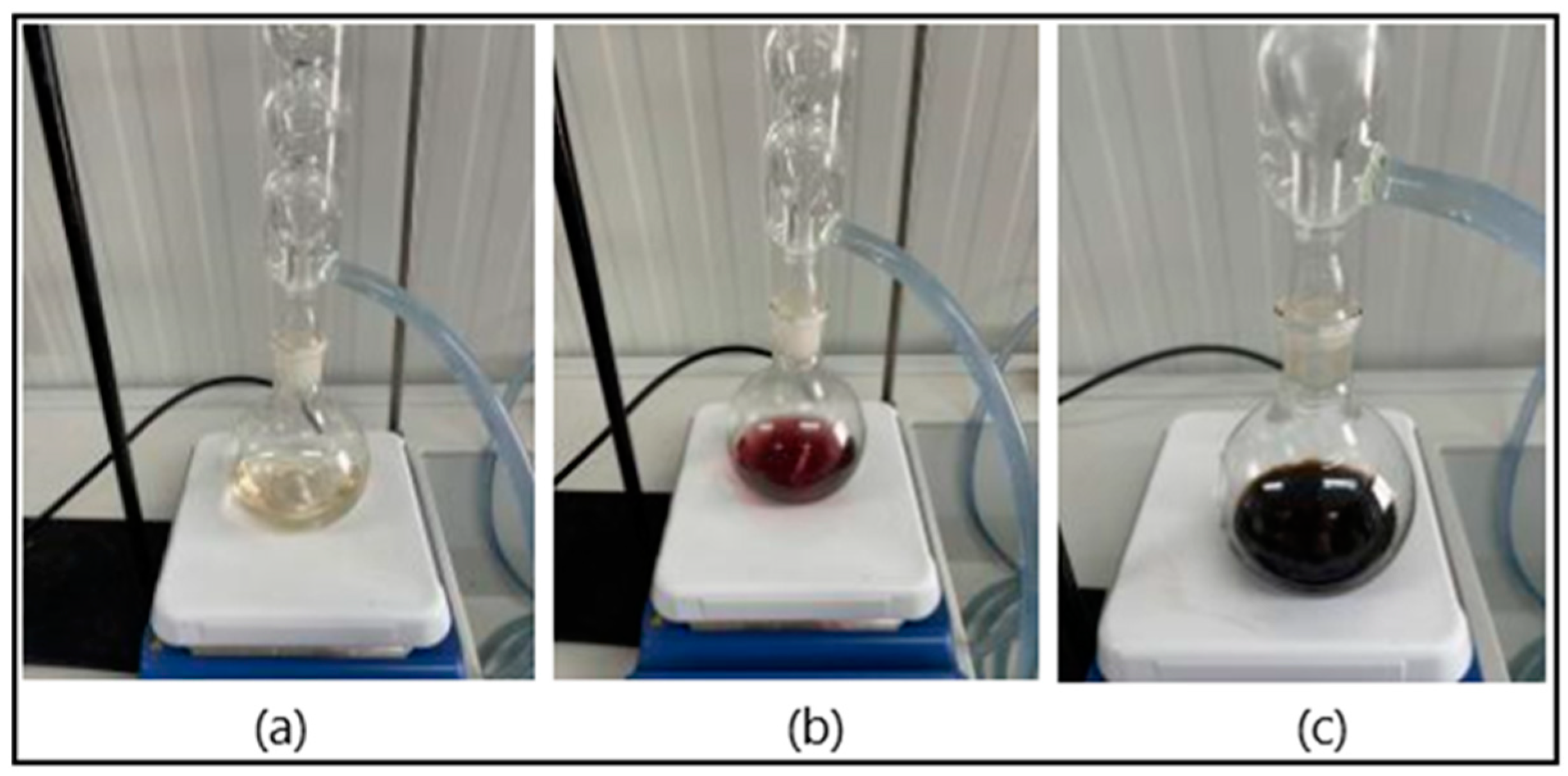
2.4. Liquefaction of the Wood Biomass Samples
2.5. Analysis of the Liquefied Samples
2.5.1. Insoluble Residue
2.5.2. Liquefaction Percentage
2.5.3. Dry Matter
2.5.4. Hydroxyl (OH) Number
2.6. Statistics
3. Results
Chemical Composition of the Biomass of Spruce and Oak
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kalak, T. Potential use of industrial biomass waste as a sustainable energy source in the future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Ikubanni, P.P.; Orhadahwe, T.A.; Christopher, C.T.; Akano, J.M.; Agboola, O.O.; Adegoke, S.O.; Balogun, A.O.; Ibikunle, R.A. Sustainability of multifaceted usage of biomass: A review. Heliyon 2021, 7, e08025. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, Y.; Van Le, Q.; Yang, H.; Hosseinzadeh-Bandbafha, H.; Yang, Y.; Sonne, C.; Tabatabaei, M.; Lam, S.S.; Peng, W. An Overview on the Conversion of Forest Biomass into Bioenergy. Front. Energy Res. 2021, 9, 684234. [Google Scholar] [CrossRef]
- Omer, S.; Kopljar, A.; Hodžić, A. Biomasa kao Gorivo. In Univerzitetski udžbenik; Univerzitet u Bihaću, Tehnički fakultet: Bihać, Bosnia and Herzegovina, 2020. [Google Scholar]
- Zhang, X.; Li, L.; Xu, F. Chemical characteristics of wood cell wall with an emphasis on ultrastructure: A mini-review. Forests 2022, 13, 439. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Adewuyi, A. Underutilized lignocellulosic waste as sources of feedstock for biofuel production in developing countries. Front. Energy Res. 2022, 10, 741570. [Google Scholar] [CrossRef]
- Khan, R.; Jolly, R.; Fatima, T.; Shakir, M. Extraction processes for deriving cellulose: A comprehensive review on green approaches. Polym. Adv. Technol. 2022, 33, 2069–2090. [Google Scholar] [CrossRef]
- Beluhan, S.; Mihajlovski, K.; Šantek, B.; Ivančić Šantek, M. The production of bioethanol from lignocellulosic biomass: Pretreatment methods, fermentation, and downstream processing. Energies 2023, 16, 7003. [Google Scholar] [CrossRef]
- Castro Garcia, A.; Cheng, S.; Cross, J.S. Lignin gasification: Current and future viability. Energies 2022, 15, 9062. [Google Scholar] [CrossRef]
- Nechita, P.; Mirela, R.; Ciolacu, F. Xylan Hemicellulose: A renewable material with potential properties for food packaging applications. Sustainability 2021, 13, 13504. [Google Scholar] [CrossRef]
- Zhang, L.; Larsson, A.; Moldin, A.; Edlund, U. Comparison of Lignin Distribution, Structure, and Morphology in Wheat Straw and Wood. Ind. Crops Prod. 2022, 187, 115432. [Google Scholar] [CrossRef]
- Soltanian, S.; Aghbashlo, M.; Almasi, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.S.; Ok, Y.S.; Lam, S.S.; Tabatabaei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Shah, A.A.; Ahmed, J.; Rehman, S.; Sultan, S.H.; Shah, A.K.; Raza, A.; Mubarak, N.M.; Hashmi, Z.; Usto, M.A.; et al. Hydrothermal Liquefaction of Lignocellulosic and Protein-Containing Biomass: A Comprehensive Review. Catalysts 2022, 12, 1621. [Google Scholar] [CrossRef]
- Brethauer, S.; Shahab, R.L.; Studer, M.H. Impacts of biofilms on the conversion of cellulose. Appl. Microbiol. Biotechnol. 2020, 104, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Sar, T.; Gowd, S.C.; Rajendran, K.; Kumar, V.; Sarsaiya, S.; Li, Y.; Sindhu, R.; Binod, P.; Zhang, Z.; et al. A Comprehensive Review on Thermochemical, and Biochemical Conversion Methods of Lignocellulosic Biomass into Valuable End Product. Fuel 2023, 342, 127790. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, L.; Zhang, H.; Zhai, H.; Ren, H. Research status, industrial application demand and prospects of phenolic resin. RSC Adv. 2019, 9, 28924–28935. [Google Scholar] [CrossRef]
- Kariuki, S.W.; Wachira, J.; Kawira, M.; Murithi, G. Formaldehyde use and alternative biobased binders for particleboard formulation: A review. J. Chem. 2019, 2019, 5256897. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Bio-based alternatives to phenol and formaldehyde for the production of resins. Polymers 2020, 12, 2237. [Google Scholar] [CrossRef]
- Antonović, A.; Ištvanić, J.; Medved, S.; Antolović, S.; Stanešić, J.; Kukuruzović, J.; Đurović, A.; Španić, N. Influence of Different Wood Specie Chemical Composition on the Liquefaction Properties. In Proceedings of the 30th International Conference on Wood Science and Technology, Zagreb, Croatia, 12–13 December 2019; Volume 25. [Google Scholar]
- Kosmela, P.; Kazimierski, P. Comparison of the Efficiency of Hetero-and Homogeneous Catalysts in Cellulose Liquefaction. Materials 2023, 16, 6135. [Google Scholar] [CrossRef]
- Yue, D.; Oribayo, O.; Rempel, G.L.; Pan, Q. Liquefaction of waste pine wood and its application in the synthesis of a flame retardant polyurethane foam. RSC Adv. 2017, 7, 30334–30344. [Google Scholar] [CrossRef]
- Jovičić, N.; Antonović, A.; Matin, A.; Antolović, S.; Kalambura, S.; Krička, T. Biomass valorization of walnut shell for liquefaction efficiency. Energies 2022, 15, 495. [Google Scholar] [CrossRef]
- Jiang, W. Acid-Catalyzed Liquefaction of Industrial Side-Streams for Producing Wood Adhesives and Particleboard. Ph.D. Thesis, Linnaeus University Press, Växjö, Sweden, 2022. [Google Scholar]
- Tshikovhi, A.; Motaung, T.E. Technologies and Innovations for Biomass Energy Production. Sustainability 2023, 15, 12121. [Google Scholar] [CrossRef]
- Bontaș, M.G.; Diacon, A.; Călinescu, I.; Rusen, E. Lignocellulose Biomass Liquefaction: Process and Applications Development as Polyurethane Foams. Polymers 2023, 15, 563. [Google Scholar] [CrossRef]
- Janiszewska-Latterini, D.; Pizzi, A. Application of Liquefied Wood Products for Particleboard Manufacturing: A Meta-Analysis Review. Curr. For. Rep. 2023, 9, 291–300. [Google Scholar] [CrossRef]
- Jiang, W.; Kumar, A.; Adamopoulos, S. Liquefaction of Lignocellulosic Materials and Its Applications in Wood Adhesives—A Review. Ind. Crops Prod. 2018, 124, 325–342. [Google Scholar] [CrossRef]
- Hill, C.; Altgen, M.; Rautkari, L. Thermal modification of wood—A review: Chemical changes and hygroscopicity. J. Mater. Sci. 2021, 56, 6581–6614. [Google Scholar] [CrossRef]
- Sut, S.; Maccari, E.; Zengin, G.; Ferrarese, I.; Loschi, F.; Faggian, M.; Paolo, B.; De Zordi, N.; Dall’Acqua, S. “Smart Extraction Chain” with Green Solvents: Extraction of Bioactive Compounds from Picea abies Bark Waste for Pharmaceutical, Nutraceutical and Cosmetic Uses. Molecules 2022, 27, 6719. [Google Scholar] [CrossRef]
- Schoss, K.; Kočevar Glavač, N.; Kreft, S. Volatile Compounds in Norway Spruce (Picea abies) Significantly Vary with Season. Plants 2023, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Obratov-Petković, D.; Beloica, J.; Čavlović, D.; Djurdjević, V.; Belanović Simić, S.; Bjedov, I. Modelling Response of Norway Spruce Forest Vegetation to Projected Climate and Environmental Changes in Central Balkans Using Different Sets of Species. Forests 2022, 13, 666. [Google Scholar] [CrossRef]
- Backs, J.R.; Ashley, M.V. Quercus genetics: Insights into the past, present, and future of oaks. Forests 2021, 12, 1628. [Google Scholar] [CrossRef]
- Dvořák, O.; Kvietková, M.S.; Šimůnková, K.; Machanec, O.; Pánek, M.; Pastierovič, F.; Lin, C.F.; Jones, D. The Influence of the Initial Treatment of Oak Wood on Increasing the Durability of Exterior Transparent Coating Systems. Polymers 2023, 15, 3251. [Google Scholar] [CrossRef]
- Popović, M.; Katičić Bogdan, I.; Varga, F.; Šatović, Z.; Bogdan, S.; Ivanković, M. Genetic Diversity in Peripheral Pedunculate Oak (Quercus robur L.) Provenances—Potential Climate Change Mitigators in the Center of Distribution despite Challenges in Natural Populations. Forests 2023, 14, 2290. [Google Scholar] [CrossRef]
- TAPPI T 257; 2014 ed.; 2021–Sampling and Preparing Wood for Analysis. Technical Association of the Pulp and Paper Industry (TAPPI): Peachtree Corners, GA, USA, 2021.
- HRN EN ISO 14780:2017; Solid Biofuels–Sample Preparation (ISO 14780:2017; EN ISO 14780:2017). Croatian Standard Institute: Zagreb, Croatia, 2017.
- HRN EN ISO 17827-2:2024; Solid Biofuels–Determination of Particle Size Distribution for Uncompressed Fuels–Part 2: Vibrating Screen Method Using Sieves with Aperture of 3,15 mm and Below (ISO 17827-2:2024; EN ISO 17827-2:2024). Croatian Standard Institute: Zagreb, Croatia, 2024.
- ISO 3310-1:2016; Test Sieves—Technical Requirements and Testing—Part 1: Test Sieves of Metal Wire Cloth. International Organization for Standardization: Geneva, Switzerland, 2016.
- TAPPI T 204; 2007 ed.; 2017–Solvent Extractives of Wood and Pulp. Technical Association of the Pulp and Paper Industry (TAPPI): Peachtree Corners, GA, USA, 2017.
- TAPPI T 222; 2002 ed.; 2021–Acid-Insoluble Lignin in Wood and Pulp. Technical Association of the Pulp and Paper Industry (TAPPI): Peachtree Corners, GA, USA, 2021.
- HRN EN 18134-2:2024; Determination of Moisture Content–-Part 2: Total Moisture–Simplified Method (ISO 18134-2:2024; EN ISO 18134-2:2024). Croatian Standard Institute: Zagreb, Croatia, 2024.
- HRN EN ISO 18122:2022; Solid Biofuels–Determination of Ash Content (ISO 18122:2022; EN ISO 18122:2022). Croatian Standard Institute: Zagreb, Croatia, 2022.
- HRN EN ISO 18123:2023; Solid Biofuels–Determination of Volatile Matter (ISO 18123:2023; EN ISO 18123:2023). Croatian Standard Institute: Zagreb, Croatia, 2023.
- HRN EN ISO 18125:2017; Solid Biofuels–Determination of Calorific Value (ISO 18125:2017; EN ISO 18125:2017). Croatian Standard Institute: Zagreb, Croatia, 2017.
- Shahbazi, S.; Goodpaster, J.V.; Smith, G.; Becker, T.; Lewis, S.W. Studies into exfoliation and coating of Egyptian blue for application to the detection of latent fingermarks. Sci. Justice 2022, 62, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Dyjakon, A.; Noszczyk, T. Alternative Fuels from Forestry Biomass Residue: Torrefaction Process of Horse Chestnuts, Oak Acorns, and Spruce Cones. Energies 2020, 13, 2468. [Google Scholar] [CrossRef]
- Pikoń, K.; Ścierski, W.; Klejnowska, K.; Myćka, Ł.; Janoszka, A.; Sinek, A. Determination of fuel properties of char obtained during the pyrolysis of waste pharmaceutical blisters. Energies 2021, 14, 1782. [Google Scholar] [CrossRef]
- Shadangi, K.P.; Sarangi, P.K.; Behera, A.K. Characterization Techniques of Biomass: Physico-Chemical, Elemental, and Biological. In Bioenergy Engineering; Woodhead Publishing: Sawston, UK, 2023; pp. 51–66. [Google Scholar] [CrossRef]
- Haverly, M.R.; Ghosh, A.; Brown, R.C. The effect of moisture on hydrocarbon-based solvent liquefaction of pine, cellulose and lignin. J. Anal. Appl. Pyrolysis 2020, 146, 104758. [Google Scholar] [CrossRef]
- Ulusal, A.; Apaydın Varol, E.; Bruckman, V.J.; Uzun, B.B. Opportunity for sustainable biomass valorization to produce biochar for improving soil characteristics. Biomass Convers. Biorefinery 2021, 11, 1041–1051. [Google Scholar] [CrossRef]
- Čajová Kantová, N.; Holubčík, M.; Čaja, A.; Trnka, J.; Jandačka, J. Analyses of pellets produced from spruce sawdust, spruce bark, and pine cones in different proportions. Energies 2022, 15, 2725. [Google Scholar] [CrossRef]
- Öcal, B.; Yüksel, A. Liquefaction of Oak Wood Using Various Solvents for Bio-oil Production. ACS Omega 2023, 8, 40944–40959. [Google Scholar] [CrossRef]
- Kwiatkowski, J.; Graban, Ł.; Stolarski, M.J. The Quality of Virginia Fanpetals Biomass as an Energy Source, Depending on the Type of Propagating Material and Plantation Age. Energies 2023, 17, 218. [Google Scholar] [CrossRef]
- Vilas-Boas, A.C.M.; Tarelho, L.A.C.; Oliveira, H.S.M.; Silva, F.G.C.S.; Pio, D.T.; Matos, M.A.A. Valorisation of Residual Biomass by Pyrolysis: Influence of Process Conditions on Products. Sustain. Energy Fuels 2024, 8, 379–396. [Google Scholar] [CrossRef]
- Wang, L.; Olsen, M.N.; Moni, C.; Dieguez-Alonso, A.; de la Rosa, J.M.; Stenrød, M.; Liu, X.; Mao, L. Comparison of Properties of Biochar Produced from Different Types of Lignocellulosic Biomass by Slow Pyrolysis at 600 °C. Appl. Energy Combustion Sci. 2022, 12, 100090. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, N.; Ma, Y.; Wang, W.; Quan, C.; Tu, X.; Miskolczi, N. Biomass Volatiles Reforming by Integrated Pyrolysis and Plasma-Catalysis System for H2 Production: Understanding Roles of Temperature and Catalyst. Energy Convers. Manag. 2023, 288, 117159. [Google Scholar] [CrossRef]
- Abdollahi, S.A.; Ranjbar, S.F.; Razeghi Jahromi, D. Applying Feature Selection and Machine Learning Techniques to Estimate the Biomass Higher Heating Value. Sci. Rep. 2023, 13, 16093. [Google Scholar] [CrossRef]
- Yamamoto, K.; Fukuda, M.; Hanatani, A. Ultrasupercritical and Advanced Ultrasupercritical Power Plants. In Advances in Power Boilers; Elsevier: Amsterdam, The Netherlands, 2021; pp. 345–390. [Google Scholar]
- Siddique, M.; Soomro, S.A.; Aziz, S.; Suri, S.U.K.; Akhter, F.; Naeem Qaisrani, Z. Potential Techniques for Conversion of Lignocellulosic Biomass into Biofuels. Pak. J. Anal. Environ. Chem. 2022, 23, 21–31. [Google Scholar] [CrossRef]
- Romero, M.J.; Duca, D.; Maceratesi, V.; Di Stefano, S.; De Francesco, C.; Toscano, G. Preliminary Study on the Thermal Behavior and Chemical-Physical Characteristics of Woody Biomass as Solid Biofuels. Processes 2023, 11, 154. [Google Scholar] [CrossRef]
- Gendek, A.; Piętka, J.; Aniszewska, M.; Malaťák, J.; Velebil, J.; Tamelová, B.; Krilek, J.; Moskalik, T. Energy Value of Silver Fir (Abies alba) and Norway Spruce (Picea abies) Wood Depending on the Degree of Its Decomposition by Selected Fungal Species. Renew. Energy 2023, 2023, 118948. [Google Scholar] [CrossRef]
- Jasinskas, A.; Mieldažys, R.; Jotautienė, E.; Domeika, R.; Vaiciukevičius, E.; Marks, M. Technical, Environmental, and Qualitative Assessment of the Oak Waste Processing and Its Usage for Energy Conversion. Sustainability 2020, 12, 8113. [Google Scholar] [CrossRef]
- Tumuluru, J.S. Pelleting of pine and switchgrass blends: Effect of process variables and blend ratio on the pellet quality and energy consumption. Energies 2019, 12, 1198. [Google Scholar] [CrossRef]
- Park, S.Y.; Oh, K.C.; Kim, S.J.; Cho, L.H.; Jeon, Y.K.; Kim, D. Development of a Biomass Component Prediction Model Based on Elemental and Proximate Analyses. Energies 2023, 16, 5341. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Franco, J.M. Lignocellulosic Materials for the Production of Biofuels, Biochemicals and Biomaterials and Applications of Lignocellulose-Based Polyurethanes: A Review. Polymers 2022, 14, 881. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The Critical Role of Lignin in Lignocellulosic Biomass Conversion and Recent Pretreatment Strategies: A Comprehensive Review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Esteves, B.; Sen, U.; Pereira, H. Influence of Chemical Composition on Heating Value of Biomass: A Review and Bibliometric Analysis. Energies 2023, 16, 4226. [Google Scholar] [CrossRef]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification. Molecules 2022, 27, 266. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Ghavidel, A.; Hofmann, T.; Bak, M.; Sandu, I.; Vasilache, V. Comparative Archaeometric Characterization of Recent and Historical Oak (Quercus spp.) Wood. Wood Sci. Technol. 2020, 54, 1121–1137. [Google Scholar] [CrossRef]
- Antonović, A.; Krička, T.; Voća, N.; Jurišić, V.; Matin, A.; Grubor, M.; Bilandžija, N.; Stanešić, J.; Ištvanić, J. Kemijska karakterizacija utekućene trave Miscanthus × giganteusa. In Proceedings of the 54. Hrvatski i 14. Međunarodni Simpozij Agronoma, Vodice, Hrvatska, 17–22 February 2019; pp. 573–577. [Google Scholar]
- Gosz, K.; Tercjak, A.; Olszewski, A.; Haponiuk, J.; Piszczyk, Ł. Bio-based polyurethane networks derived from liquefied sawdust. Materials 2021, 14, 3138. [Google Scholar] [CrossRef]
- Mathanker, A.; Pudasainee, D.; Kumar, A.; Gupta, R. Hydrothermal liquefaction of lignocellulosic biomass feedstock to produce biofuels: Parametric study and products characterization. Fuel 2020, 271, 117534. [Google Scholar] [CrossRef]
- Kumar, A.; Sethy, A.; Chauhan, S. Liquefaction behaviour of twelve tropical hardwood species in phenol. Maderas. Cienc. Y Tecnol. 2018, 20, 211–220. [Google Scholar] [CrossRef]
- Đurović, A.; Matin, B.; Brandić, I.; Tomić, I.; Matin, A.; Antonović, A. Influence of soybean (Glycine max) and hemp (Cannabis sativa) stalks biomass to glycerol solvent ratio on liquefied properties. In Proceedings of the 59. Hrvatski i 19. Međunarodni Simpozij Agronoma, Dubrovnik, Hrvatska, 11–16 February 2024; pp. 507–513. [Google Scholar]
- HRN EN 13986:2015; Wood-Based Panels for Use in Construction—Characteristics, Evaluation of Conformity and Marking (EN 13986:2004+A1:2015). Croatian Standard Institute: Zagreb, Croatia, 2015.







| Wood Species | Chemical Composition | |||
|---|---|---|---|---|
| MC | Ash | FC | VM | |
| Spruce | 8.60 ± 0.20 b | 0.39 ± 0.07 b | 17.63 ± 0.45 b | 80.2 ± 1.11 a |
| Oak | 8.18 ± 0.07 a | 0.25 ± 0.04 a | 12.99 ± 0.09 a | 79.87 ± 0.19 a |
| Wood Species | Chemical Composition | |
|---|---|---|
| HHV | LHV | |
| Spruce | 19.20 ± 0.36 b | 18.20 ± 0.36 b |
| Oak | 18.43 ± 0.47 a | 17.47 ± 0.42 a |
| Wood Species | Lignocellulosic Components | |||
|---|---|---|---|---|
| C | L | HC | E | |
| Spruce | 49.77 ± 0.29 a | 26.38 ± 0.54 a | 21.72 ± 0.2 a | 2.44 ± 0.21 b |
| Oak | 49.81 ± 0.58 a | 25.97 ± 0.15 a | 21.43 ± 0.4 a | 1.70 ± 0.02 a |
| Liquefaction Ratio | Liquefaction Properties | |||
|---|---|---|---|---|
| LP (%) | IR (%) | DM (%) | OH (mg KOH/g) | |
| 1:1 | 71.34 ± 1.21 a | 28.66 ± 1.22 d | 69.74 ± 1.60 d | 338.36 ± 0.48 a |
| 1:2 | 73.94 ± 0.23 a | 26.06 ± 0.44 c | 63.63 ± 1.05 c | 352.21 ± 0.15 a |
| 1:3 | 85.09 ± 0.42 b | 14.91 ± 0.11 b | 60.08 ± 2.97 b | 415.88 ± 0.51 b |
| 1:4 | 87.74 ± 0.13 c | 12.26 ± 0.23 a | 55.64 ± 1.83 a | 499.01 ± 0.22 c |
| 1:5 | 89.54 ± 0.10 c | 10.46 ± 0.47 a | 54.70 ± 1.17 a | 569.74 ± 0.33 d |
| Liquefaction Ratio | Liquefaction Properties | |||
|---|---|---|---|---|
| LP (%) | IR (%) | DM (%) | OH (mg KOH/g) | |
| 1:1 | 77.85 ± 0.40 a | 22.15 ± 0.50 c | 62.37 ± 0.48 c | 492.12 ± 0.05 a |
| 1:2 | 79.93 ± 3.910 a | 20.07 ± 1.24 b | 55.53 ± 2.54 b | 513.42 ± 0.11 a |
| 1:3 | 87.24 ± 0.38 b | 12.76 ± 1.01 a | 57.68 ± 0.81 b | 645.08 ± 0.29 b |
| 1:4 | 89.69 ± 0.38 c | 10.31 ± 0.19 a | 51.35 ± 2.13 a | 751.23 ± 0.20 c |
| 1:5 | 87.84 ± 0.44 b | 12.16 ± 0.59 a | 52.25 ± 1.14 a | 839.01 ± 0.18 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matin, B.; Brandić, I.; Matin, A.; Ištvanić, J.; Antonović, A. Possibilities of Liquefied Spruce (Picea abies) and Oak (Quercus robur) Biomass as an Environmentally Friendly Additive in Conventional Phenol–Formaldehyde Resin Wood Adhesives. Energies 2024, 17, 4456. https://doi.org/10.3390/en17174456
Matin B, Brandić I, Matin A, Ištvanić J, Antonović A. Possibilities of Liquefied Spruce (Picea abies) and Oak (Quercus robur) Biomass as an Environmentally Friendly Additive in Conventional Phenol–Formaldehyde Resin Wood Adhesives. Energies. 2024; 17(17):4456. https://doi.org/10.3390/en17174456
Chicago/Turabian StyleMatin, Božidar, Ivan Brandić, Ana Matin, Josip Ištvanić, and Alan Antonović. 2024. "Possibilities of Liquefied Spruce (Picea abies) and Oak (Quercus robur) Biomass as an Environmentally Friendly Additive in Conventional Phenol–Formaldehyde Resin Wood Adhesives" Energies 17, no. 17: 4456. https://doi.org/10.3390/en17174456
APA StyleMatin, B., Brandić, I., Matin, A., Ištvanić, J., & Antonović, A. (2024). Possibilities of Liquefied Spruce (Picea abies) and Oak (Quercus robur) Biomass as an Environmentally Friendly Additive in Conventional Phenol–Formaldehyde Resin Wood Adhesives. Energies, 17(17), 4456. https://doi.org/10.3390/en17174456







