Comparative and Descriptive Study of Biomass Gasification Simulations Using Aspen Plus
Abstract
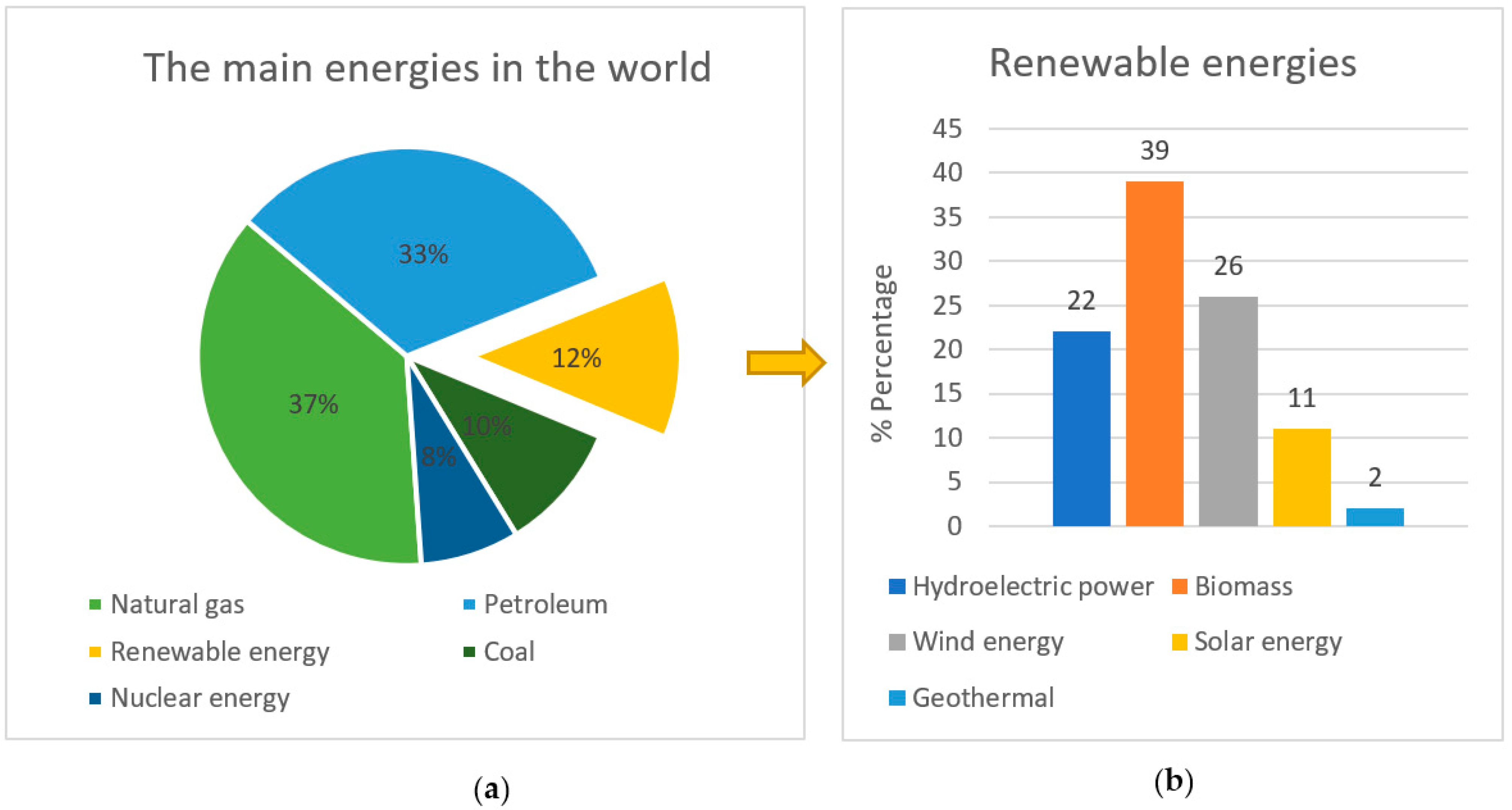
1. Introduction
Objectives
2. Different Approaches to Biomass Gasification Simulation
2.1. Thermodynamic Equilibrium Modeling
2.2. Kinetic Modeling
3. Parameters Evaluated in the Different Simulations of Biomass Gasification
3.1. Parameters That Affect the Biomass Gasification
3.1.1. Type of Biomass
3.1.2. Characteristics of Biomass
- Moisture;
- Calorific value of biomass;
- Volatiles content;
- Biomass particle size;
3.1.3. Reactor Diameter
4. Definition of Properties
4.1. Inlet Streams
4.2. Thermodynamic Methods
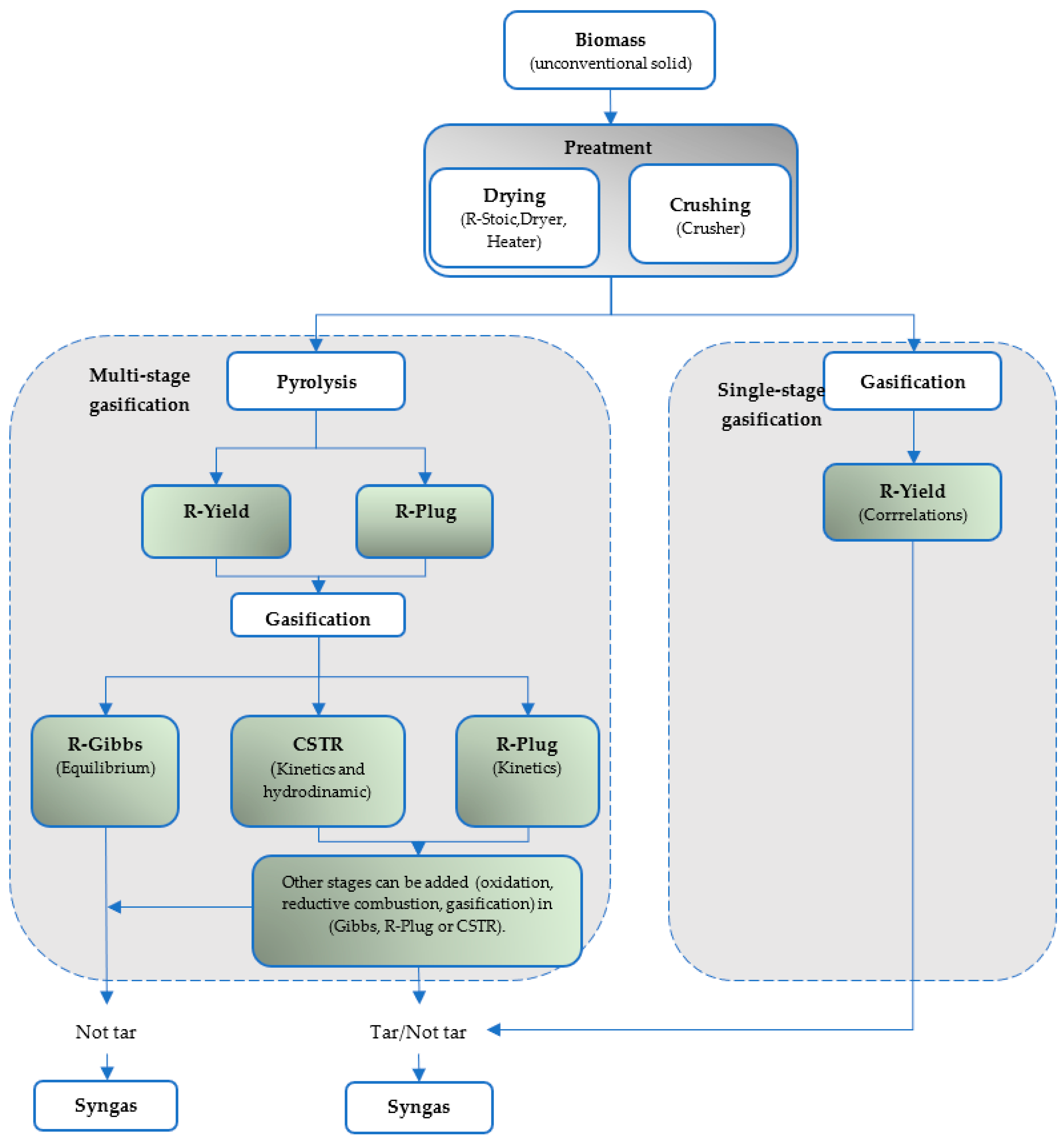
5. Stages of a Simulation in Aspen Plus
5.1. Pretreatment
5.2. Crusher
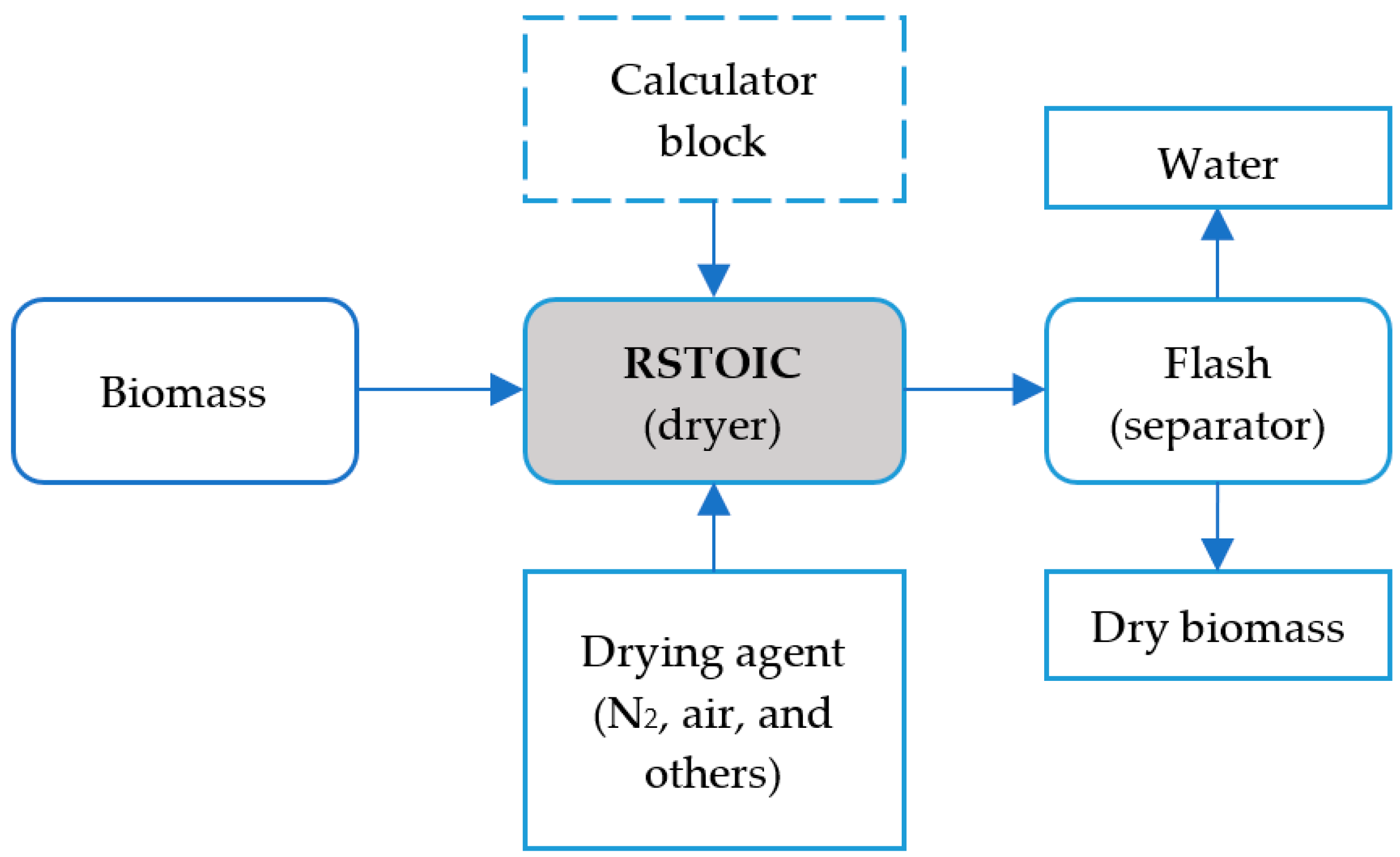
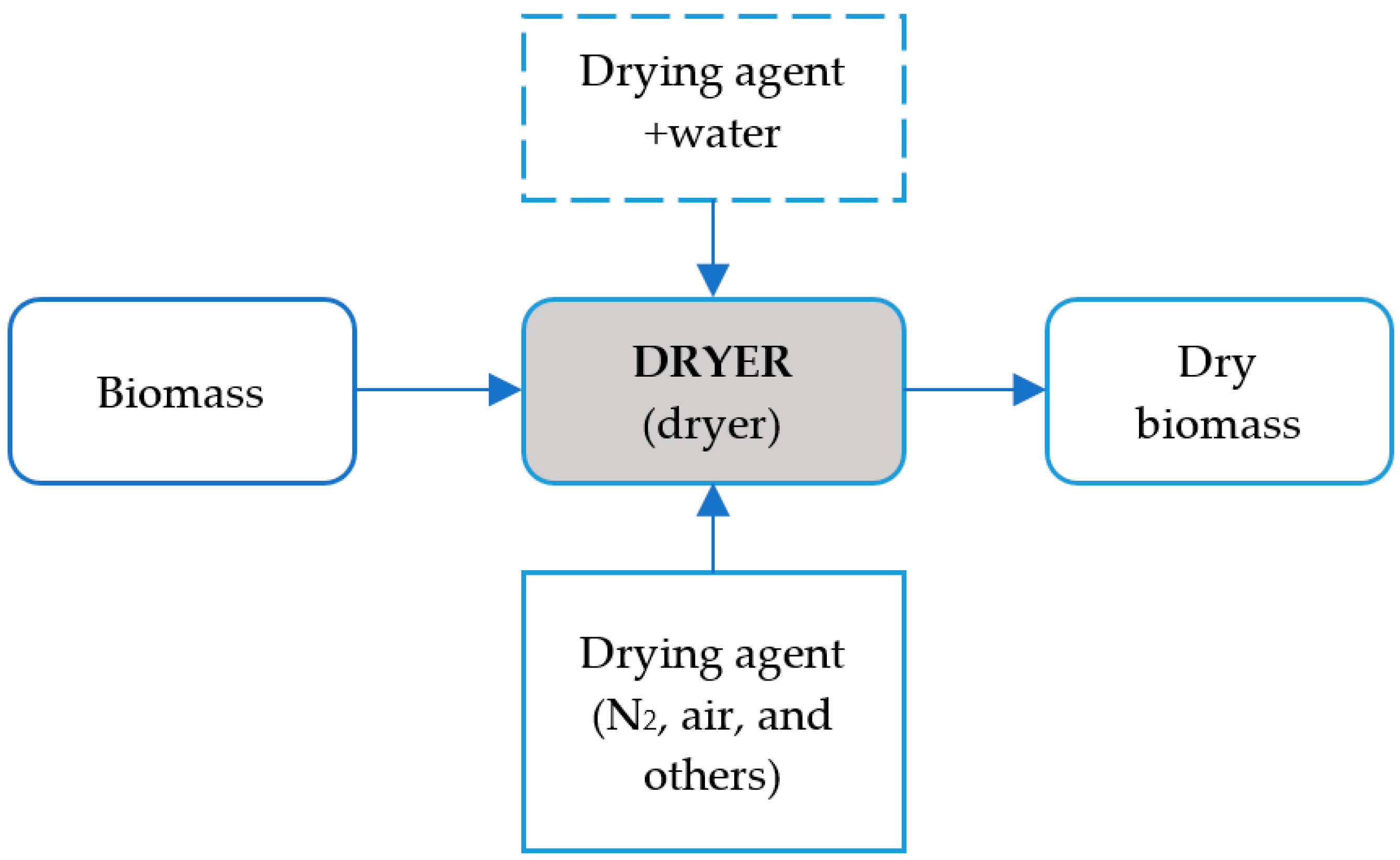
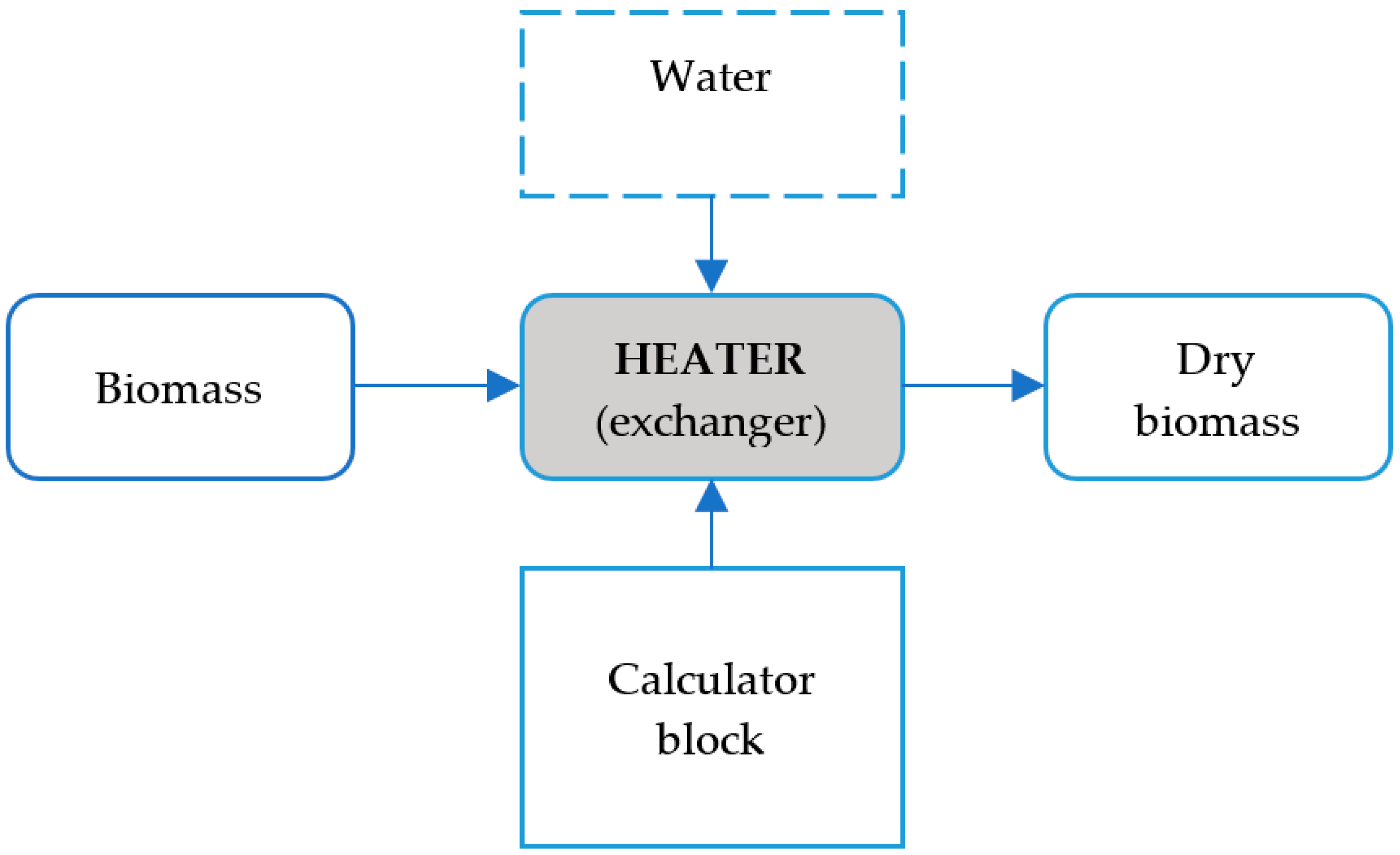
5.3. Dryer
- First approach:
- Second approach:
- Third approach:
5.4. Simulation of Biomass Gasification
5.5. Pyrolysis Stage
5.5.1. Approach with Correlations Based on Elemental Analysis
5.5.2. Correlations Approach for Biomass Pyrolysis from Experimental Data
5.5.3. Kinetic Approach for Biomass Pyrolysis
5.6. Gasification Stage
5.6.1. Thermodynamic Equilibrium (GIBBS)
5.6.2. Kinetic Approach for Biomass Gasification
5.6.3. Correlations Approach for Biomass Gasification from Experimental Data
6. Synthesis Gas Cleaning
6.1. Tar Treatment
6.1.1. Primary Methods
6.1.2. Secondary Methods
- Secondary physical methods:
- 2.
- Secondary chemical methods:
- Thermal cracking:
- Catalytic cracking of tar:
- Tar reforming:
6.2. CO2 Capture
Use CO2 as a Gasification Agent
7. Recaps
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| 2D | Two-dimensional simulation |
| ANN | Artificial neural network |
| ATR | Autothermal reforming |
| BG-FT | Fischer–Tropsch |
| BIGCC | Biomass Integrated Gasification Combined Cycle |
| CaS | Unstable solid waste |
| CFD | Computational fluid dynamics |
| CHP | Combined heat and power |
| CIPSD | Conventional solids with particle size distribution |
| CISOLID | Conventional solids |
| CISOLID | Conventional solids |
| DES | Deep eutectic solvent |
| DFB | Dual fluidized bed gasification |
| DGOC | Dry gasification oxy-combustion power cycle |
| ER | Equivalence ratio |
| EPA | United States Environmental Protection Agency |
| HHV | Higher heating value |
| IL | Ionic liquid |
| IEA | International Energy Agency |
| LHHW | Langmuir–Hinshelwood–Hougen–Watson |
| LHV | Lower heating value |
| MEA | Monoethanolamine |
| MIXCINC | Aqueous mixtures of conventional components and conventional solids |
| MIXCISLD | Conventional solid mixtures |
| MIXED | Aqueous conventional component mixtures |
| MIXNCPSD | Aqueous mixtures of conventional components, conventional solids, and unconventional solids with particle size distribution. |
| mm | Millimeter |
| NC | Unconventional solid |
| NFM | N-Formylmorpholine |
| NCPSD | Unconventional solids with particle distribution |
| POX | Partial oxidation |
| PR-BM | Peng–Robinson with Boston–Mathias function |
| RADFRAC | Fractional distillation column |
| RCSTR | Stoichiometrically balanced reactor |
| RGIBBS | Thermodynamic equilibrium reactor |
| R-PLUG | Stoichiometrically balanced reactor |
| R-Yield | Yield reactor |
| RMSE | Root-mean-square error |
| S/B | Steam biomass ratio |
| SCWG | Supercritical water gasification |
| SRK | Soave–Redlich–Kwong |
| WGS | Water–Gas shift reactions |
References
- Mariyam, S.; Shahbaz, M.; Al-Ansari, T.; Mackey, H.R.; McKay, G. A Critical Review on Co-Gasification and Co-Pyrolysis for Gas Production. Renew. Sustain. Energy Rev. 2022, 161, 112349. [Google Scholar] [CrossRef]
- EIA. Renewable Energy Explained. In What Renewable Energy 2023; EIA: Washington, DC, USA, 2023; U.S. Primary Energy Consumption by Energy Source, 2022. Available online: https://www.eia.gov/energyexplained/renewable-sources/ (accessed on 28 August 2024).
- IEA. Renewables 2021. In Analysis and Forecast to 2026; International Energy Agency (IEA): Paris, France, 2021; p. 175. Available online: https://iea.blob.core.windows.net/assets/5ae32253-7409-4f9a-a91d-1493ffb9777a/Renewables2021-Analysisandforecastto2026.pdf (accessed on 28 August 2024).
- EIA. Monthly Energy Review May 2022; U.S. Energy Information Administration: Washington, DC, USA, 2022. Available online: https://www.eia.gov/totalenergy/data/monthly/archive/00352205.pdf (accessed on 28 August 2024).
- Mohtasham, J. Review Article-Renewable Energies. Energy Procedia 2015, 74, 1289–1297. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Biomass Explained. In National Energy Educcation Projection; U.S. Energy Information Administration: Washington, DC, USA, 2020. Available online: https://www.eia.gov/energyexplained/biomass/ (accessed on 28 August 2024).
- Garba, A. Biomass Conversion Technologies for Bioenergy Generation: An Introduction. In Biomass [Working Title]; IntechOpen: Rijeka, Croatia, 2020; Available online: https://www.intechopen.com/online-first/biomass-conversion-technologies-for-bioenergy-generation-an-introduction (accessed on 28 August 2024).
- Gómez, A.; Klose, W.; Rincón, S.L.; Wiest1, W. Thermochemical Transformation of the Residual Biomass from the Palm Oil Extraction Process: Technologies and Prospects. Rev. Palmas 2004, 25, 388–397. Available online: https://publicaciones.fedepalma.org/index.php/palmas/article/download/1104/1104 (accessed on 28 August 2024).
- Sreejith, C.C.; Muraleedharan, C.; Arun, P. Performance Prediction of Steam Gasification of Wood Using an ASPEN PLUS Thermodynamic Equilibrium Model. Int. J. Sustain. Energy 2014, 33, 416–434. [Google Scholar] [CrossRef]
- Tasaka, K.; Furusawa, T.; Ujimine, K.; Tsutsumi, A. Surface Analyses of Cobalt Catalysts for the Steam Reforming of Tar Derived from Biomass Gasification. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2006; Volume 159, pp. 517–520. ISBN 978-0-444-51733-3. [Google Scholar] [CrossRef]
- Niu, M.; Xie, J.; Liang, S.; Liu, L.; Wang, L.; Peng, Y. Simulation of a New Biomass Integrated Gasification Combined Cycle (BIGCC) Power Generation System Using Aspen Plus: Performance Analysis and Energetic Assessment. Int. J. Hydrogen Energy 2021, 46, 22356–22367. [Google Scholar] [CrossRef]
- Bolhar-Nordenkampf, M.; Hofbauer, H.; Rauch, R.; Aichernig, C. Biomass CHP Plant Güssing—Using Gasification for Power Generation. In Proceedings of the 2nd Regional Conference on Energy Technology Towards a Clean Environment, Phuket, Thailand, 12–14 February 2003; Volume 7. Available online: https://www.researchgate.net/publication/270160090_Biomass_CHP_Plant_Gussing_-_Using_Gasification_for_Power_Generation (accessed on 28 August 2024).
- Jayanarasimhan, A.; Pathak, R.M.; Shivapuji, A.M.; Rao, L. Tar Formation in Gasification Systems: A Holistic Review of Remediation Approaches and Removal Methods. ACS Omega 2024, 9, 2060–2079. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Tanksale, A. Review of Recent Developments in Ni-Based Catalysts for Biomass Gasification. Renew. Sustain. Energy Rev. 2014, 38, 428–438. [Google Scholar] [CrossRef]
- Dattatray, A.D.; Shilapuram, V. Detailed Parametric Investigation of Dry Gasification Oxy-Combustion Power Cycle Using ASPEN Plus Simulations. Fuel 2019, 236, 501–515. [Google Scholar] [CrossRef]
- Shamsi, M.; Obaid, A.A.; Farokhi, S.; Bayat, A. A Novel Process Simulation Model for Hydrogen Production via Reforming of Biomass Gasification Tar. Int. J. Hydrogen Energy 2022, 47, 772–781. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Bhattacharya, S. Process Modelling for the Production of Hydrogen-Rich Gas from Gasification of Coal Using Oxygen, CO2 and Steam Reactants. Int. J. Hydrogen Energy 2021, 46, 24051–24059. [Google Scholar] [CrossRef]
- Rabea, K.; Michailos, S.; Akram, M.; Hughes, K.J.; Ingham, D.; Pourkashanian, M. An Improved Kinetic Modelling of Woody Biomass Gasification in a Downdraft Reactor Based on the Pyrolysis Gas Evolution. Energy Convers. Manag. 2022, 258, 115495. [Google Scholar] [CrossRef]
- Dincer, I.; Bicer, Y. 4.22 Electrochemical Energy Conversion. In Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 856–894. ISBN 978-0-12-814925-6. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128095973004399?via%3Dihub (accessed on 28 August 2024).
- Abdelouahed, L.; Authier, O.; Mauviel, G.; Corriou, J.P.; Verdier, G.; Dufour, A. Detailed Modeling of Biomass Gasification in Dual Fluidized Bed Reactors under Aspen Plus. Energy Fuels 2012, 26, 3840–3855. [Google Scholar] [CrossRef]
- Beheshti, S.M.; Ghassemi, H.; Shahsavan-Markadeh, R. Process Simulation of Biomass Gasification in a Bubbling Fluidized Bed Reactor. Energy Convers. Manag. 2015, 94, 345–352. [Google Scholar] [CrossRef]
- Donaldson, D.J. Laboratory Kinetics. In Encyclopedia of Atmospheric Sciences; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1090–1097. ISBN 978-0-12-227090-1. Available online: https://www.sciencedirect.com/sdfe/pdf/download/eid/3-s2.0-B0122270908004759/first-page-pdf (accessed on 28 August 2024).
- Faraji, M.; Saidi, M. Hydrogen-Rich Syngas Production via Integrated Configuration of Pyrolysis and Air Gasification Processes of Various Algal Biomass: Process Simulation and Evaluation Using Aspen Plus Software. Int. J. Hydrogen Energy 2021, 46, 18844–18856. [Google Scholar] [CrossRef]
- Singh, D.K.; Tirkey, J.V. Process Modelling and Thermodynamic Performance Optimization of Biomass Air Gasification Fuelled with Waste Poultry Litter Pellet by Integrating Aspen plus with RSM. Biomass Bioenergy 2022, 158, 106370. [Google Scholar] [CrossRef]
- Erdem, K.; Gündüz Han, D.; Midilli, A. A Parametric Study on Hydrogen Production by Fluidized Bed Co-Gasification of Biomass and Waste Plastics. Int. J. Hydrogen Energy 2024, 52, 1434–1444. [Google Scholar] [CrossRef]
- Okati, A.; Reza Khani, M.; Shokri, B.; Monteiro, E.; Rouboa, A. Parametric Studies over a Plasma Co-Gasification Process of Biomass and Coal through a Restricted Model in Aspen Plus. Fuel 2023, 331, 125952. [Google Scholar] [CrossRef]
- Yu, M.; Kim, M.; Byun, J.; Lee, S. Energy, Techno-Economic Analysis, and Life Cycle Assessment for Co-Gasification of Polyethylene Terephthalate and Olive Husk by Chemical Process Simulation. Chem. Eng. Sci. 2024, 295, 120164. [Google Scholar] [CrossRef]
- Rosha, P.; Ibrahim, H. Technical Feasibility of Biomass and Paper-Mill Sludge Co-Gasification for Renewable Fuel Production Using Aspen Plus. Energy 2022, 258, 124883. [Google Scholar] [CrossRef]
- Atikah, M.S.N.; Harun, R. Simulation and Optimization of Chlorella Vulgaris Gasification Using Aspen Plus. Process Integr. Optim. Sustain. 2019, 3, 349–357. [Google Scholar] [CrossRef]
- Shehzad, A.; Bashir, M.J.K.; Horttanainen, M.; Manttari, M.; Havukainen, J.; Abbas, G. Modeling and Comparative Assessment of Bubbling Fluidized Bed Gasification System for Syngas Production—A Gateway for a Cleaner Future in Pakistan. Environ. Technol. 2018, 39, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Tauqir, W.; Zubair, M.; Nazir, H. Parametric Analysis of a Steady State Equilibrium-Based Biomass Gasification Model for Syngas and Biochar Production and Heat Generation. Energy Convers. Manag. 2019, 199, 111954. [Google Scholar] [CrossRef]
- Ugwuodo, C.B.; Ugwuoke, E.C.; Owabor, C.N.; Ogbeide, S.E. A Thermodynamic Equilibrium Model of Fluidized Bed Gasifier Using ASPEN HYSYS. Int. J. Eng. Bus. Manag. 2020, 4, 1–11. [Google Scholar] [CrossRef]
- Bassyouni, M.; ul Hasan, S.W.; Abdel-Aziz, M.H.; Abdel-hamid, S.M.-S.; Naveed, S.; Hussain, A.; Ani, F.N. Date Palm Waste Gasification in Downdraft Gasifier and Simulation Using ASPEN HYSYS. Energy Convers. Manag. 2014, 88, 693–699. [Google Scholar] [CrossRef]
- Kartal, F.; Özveren, U. A Comparative Study for Biomass Gasification in Bubbling Bed Gasifier Using Aspen HYSYS. Bioresour. Technol. Rep. 2021, 13, 100615. [Google Scholar] [CrossRef]
- Wilk, V.; Hofbauer, H. Analysis of Optimization Potential in Commercial Biomass Gasification Plants Using Process Simulation. Fuel Process. Technol. 2016, 141, 138–147. [Google Scholar] [CrossRef]
- Gröbl, T.; Walter, H.; Haider, M. Biomass Steam Gasification for Production of SNG—Process Design and Sensitivity Analysis. Appl. Energy 2012, 97, 451–461. [Google Scholar] [CrossRef]
- Sharma, P.; Sen, S.; Sheth, P.N.; Mohapatra, B.N. Multizone Model of a Refused Derived Fuel Gasification: A Thermodynamic Semi-Empirical Approach. Energy Convers. Manag. 2022, 260, 115621. [Google Scholar] [CrossRef]
- Hoo, K.K.; Md Said, M.S. Simulation of Air Gasification of Napier Grass Using Aspen Plus. Sustain. Energy Technol. Assess. 2022, 50, 101837. [Google Scholar] [CrossRef]
- Vikram, S.; Rosha, P.; Kumar, S.; Mahajani, S. Thermodynamic Analysis and Parametric Optimization of Steam-CO2 Based Biomass Gasification System Using Aspen PLUS. Energy 2022, 241, 122854. [Google Scholar] [CrossRef]
- Faraji, M.; Saidi, M. Process Simulation and Optimization of Groundnut Shell Biomass Air Gasification for Hydrogen-Enriched Syngas Production. Int. J. Hydrogen Energy 2022, 47, 13579–13591. [Google Scholar] [CrossRef]
- Singh, M.; Salaudeen, S.A.; Gilroyed, B.H.; Dutta, A. Simulation of Biomass-Plastic Co-Gasification in a Fluidized Bed Reactor Using Aspen Plus. Fuel 2022, 319, 123708. [Google Scholar] [CrossRef]
- Kumar, G.S.; Gupta, A.; Viswanadham, M. Sensitivity Analysis and Optimization of Parameters for the Gasification of High Ash Indian Coal. Int. J. Appl. Eng. Res. 2017, 12, 7184–7193. [Google Scholar]
- Fajimi, L.I.; Oboirien, B.O.; Adams, T.A. Simulation Studies on the Co-Production of Syngas and Activated Carbon from Waste Tyre Gasification Using Different Reactor Configurations. Energy Convers. Manag. X 2021, 11, 100105. [Google Scholar] [CrossRef]
- Almpantis, D.; Zabaniotou, A. Technological Solutions and Tools for Circular Bioeconomy in Low-Carbon Transition: Simulation Modeling of Rice Husks Gasification for CHP by Aspen PLUS V9 and Feasibility Study by Aspen Process Economic Analyzer. Energies 2021, 14, 2006. [Google Scholar] [CrossRef]
- Hashemisohi, A.; Wang, L.; Shahbazi, A. Numerical Analysis of Tar and Syngas Formation during the Steam Gasification of Biomass in a Fluidized Bed. Energies 2023, 16, 5283. [Google Scholar] [CrossRef]
- Hamaidi, B.E.; Boumeddane, B.; Benarous, A.; Vera, D. Unlocking the Potential of Date Palm Waste for Syngas and Methanol Production: Process Simulation and Optimization. Process Saf. Environ. Prot. 2024, 182, 98–108. [Google Scholar] [CrossRef]
- Rabea, K.; Michailos, S.; Hughes, K.J.; Ingham, D.; Pourkashanian, M. Comprehensive Process Simulation of a Biomass-Based Hydrogen Production System through Gasification within the BECCS Concept in a Commercial Two-Stage Fixed Bed Gasifier. Energy Convers. Manag. 2023, 298, 117812. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Mahamood, R.M.; Jen, T.-C.; Loha, C.; Akinlabi, E.T. An Overview of Biomass Solid Fuels: Biomass Sources, Processing Methods, and Morphological and Microstructural Properties. J. Bioresour. Bioprod. 2023, 8, 333–360. [Google Scholar] [CrossRef]
- Sharma, A. Assessing the Suitability of Various Feedstocks for Biomass Gasification; Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 2011; Available online: https://repository.lsu.edu/cgi/viewcontent.cgi?article=4757&context=gradschool_theses (accessed on 28 August 2024).
- González-Vázquez, M.P.; García, R.; Gil, M.V.; Pevida, C.; Rubiera, F. Comparison of the Gasification Performance of Multiple Biomass Types in a Bubbling Fluidized Bed. Energy Convers. Manag. 2018, 176, 309–323. [Google Scholar] [CrossRef]
- Migliaccio, R.; Brachi, P.; Montagnaro, F.; Papa, S.; Tavano, A.; Montesarchio, P.; Ruoppolo, G.; Urciuolo, M. Sewage Sludge Gasification in a Fluidized Bed: Experimental Investigation and Modeling. Ind. Eng. Chem. Res. 2021, 60, 5034–5047. [Google Scholar] [CrossRef]
- Rabah, A.A. Syngas Production from Agriculture Residues: Sudan. J. Energy 2022, 2022, 2944552. [Google Scholar] [CrossRef]
- Puri, L.; Hu, Y.; Naterer, G. Critical Review of the Role of Ash Content and Composition in Biomass Pyrolysis. Front. Fuels 2024, 2, 1378361. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Raheem, A.; Wang, F.; Wei, J.; Xu, D.; Song, X.; Bao, W.; Huang, A.; Zhang, S.; et al. Syngas Production from Biomass Gasification: Influences of Feedstock Properties, Reactor Type, and Reaction Parameters. ACS Omega 2023, 8, 31620–31631. [Google Scholar] [CrossRef]
- Louw, J.; Schwarz, C.E.; Knoetze, J.H.; Burger, A.J. Thermodynamic Modelling of Supercritical Water Gasification: Investigating the Effect of Biomass Composition to Aid in the Selection of Appropriate Feedstock Material. Bioresour. Technol. 2014, 174, 11–23. [Google Scholar] [CrossRef]
- Vaezi, M.; Passandideh-Fard, M.; Moghiman, M. On a Numerical Model for Gasification of Biomass Materials. In Proceedings of the 1st WSEAS International Conference on Computational Chemistry, Cairo, Egypt, 29–31 December 2007; Available online: https://www.researchgate.net/publication/237227503_On_a_Numerical_Model_for_Gasification_of_Biomass_Materials (accessed on 28 August 2024).
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An Overview of Advances in Biomass Gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Shahbaz, M.; Al-Ansari, T.; Inayat, M.; Sulaiman, S.A.; Parthasarathy, P.; McKay, G. A Critical Review on the Influence of Process Parameters in Catalytic Co-Gasification: Current Performance and Challenges for a Future Prospectus. Renew. Sustain. Energy Rev. 2020, 134, 110382. [Google Scholar] [CrossRef]
- Contractor, E.D.; Mehta, K.P.; Thattil, A.; Gautam, A.; Gautam, S. Detailed Analysis of Downdraft Gasifier Using Different Parameters for Biomass Gasification: A Review. EMERG Energy Environ. Effic. Resour. Glob. 2024, 10, 94. Available online: https://emerg.ro/wp-content/uploads/2024/04/7-DETAILED-ANALYSIS-OF-DOWNDRAFT-GASIFIER.pdf (accessed on 28 August 2024). [CrossRef]
- Pérez, J.F.; Melgar, A.; Benjumea, P.N. Effect of Operating and Design Parameters on the Gasification/Combustion Process of Waste Biomass in Fixed Bed Downdraft Reactors: An Experimental Study. Fuel 2012, 96, 487–496. [Google Scholar] [CrossRef]
- AlNouss, A.; Parthasarathy, P.; Shahbaz, M.; Al-Ansari, T.; Mackey, H.; McKay, G. Techno-Economic and Sensitivity Analysis of Coconut Coir Pith-Biomass Gasification Using ASPEN PLUS. Appl. Energy 2020, 261, 114350. [Google Scholar] [CrossRef]
- Salisu, J.; Gao, N.; Quan, C. Techno-Economic Assessment of Co-Gasification of Rice Husk and Plastic Waste as an Off-Grid Power Source for Small Scale Rice Milling—An Aspen Plus Model. J. Anal. Appl. Pyrolysis 2021, 158, 105157. [Google Scholar] [CrossRef]
- Kombe, E.Y.; Lang’at, N.; Njogu, P.; Malessa, R.; Weber, C.-T.; Njoka, F.; Krause, U. Numerical Investigation of Sugarcane Bagasse Gasification Using Aspen Plus and Response Surface Methodology. Energy Convers. Manag. 2022, 254, 115198. [Google Scholar] [CrossRef]
- Piazzi, S.; Patuzzi, F.; Baratieri, M. Energy and Exergy Analysis of Different Biomass Gasification Coupled to Fischer-Tropsch Synthesis Configurations. Energy 2022, 249, 123642. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Khalili, M.; Sharifzadeh, M.M.M. Model Development and Energy and Exergy Analysis of the Biomass Gasification Process (Based on the Various Biomass Sources). Renew. Sustain. Energy Rev. 2018, 91, 869–887. [Google Scholar] [CrossRef]
- Montiel-Bohórquez, N.D.; Pérez, J.F. Energy Valorization Strategies of Fallen Leaves and Woody Biomass in a Based Downdraft Gasification-Engine Power Plant. Sustain. Energy Technol. Assess. 2022, 49, 101749. [Google Scholar] [CrossRef]
- Yong, Y.S.; Abdul Rasid, R. Process Simulation of Hydrogen Production through Biomass Gasification: Introduction of Torrefaction Pre-Treatment. Int. J. Hydrogen Energy 2022, 47, 42040–42050. [Google Scholar] [CrossRef]
- Han, D.; Yang, X.; Li, R.; Wu, Y. Environmental Impact Comparison of Typical and Resource-Efficient Biomass Fast Pyrolysis Systems Based on LCA and Aspen Plus Simulation. J. Clean. Prod. 2019, 231, 254–267. [Google Scholar] [CrossRef]
- Li, B.; Wei, L.; Yang, H.; Wang, X.; Chen, H. The Enhancing Mechanism of Calcium Oxide on Water Gas Shift Reaction for Hydrogen Production. Energy 2014, 68, 248–254. [Google Scholar] [CrossRef]
- Akhator, P.; Asibor, J. Simulation of Air-Gasification of Wood Wastes Using Aspen Plus. Int. J. Eng. Sci. Appl. 2021, 5, 86–97. Available online: https://www.researchgate.net/publication/355908878_Simulation_of_Air-Gasification_of_Wood_Wastes_Using_Aspen_Plus (accessed on 28 August 2024).
- Safarian, S.; Saryazdi, S.M.E.; Unnthorsson, R.; Richter, C. Dataset of Biomass Characteristics and Net Output Power from Downdraft Biomass Gasifier Integrated Power Production Unit. Data Brief 2020, 33, 106390. [Google Scholar] [CrossRef]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam Gasification of Biomass with Subsequent Syngas Adjustment Using Shift Reaction for Syngas Production: An Aspen Plus Model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Gye, H.-R.; Lee, C.-J. Process Modeling for Steam Biomass Gasification in a Dual Fluidized Bed Gasifier. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; Volume 44, pp. 343–348. ISBN 978-0-444-64241-7. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780444642417500525?via%3Dihub (accessed on 28 August 2024).
- Salisu, J.; Gao, N.; Quan, C.; Yanik, J.; Artioli, N. Co-Gasification of Rice Husk and Plastic in the Presence of CaO Using a Novel ANN Model-Incorporated Aspen plus Simulation. J. Energy Inst. 2023, 108, 101239. [Google Scholar] [CrossRef]
- Rosha, P.; Kumar, S.; Vikram, S.; Ibrahim, H.; Al-Muhtaseb, A.H. H2-Enriched Gaseous Fuel Production via Co-Gasification of an Algae-Plastic Waste Mixture Using Aspen PLUS. Int. J. Hydrogen Energy 2021, 47, 26294–26302. [Google Scholar] [CrossRef]
- Xiang, Y.; Cai, L.; Guan, Y.; Liu, W.; Cheng, Z.; Liu, Z. Study on the Effect of Gasification Agents on the Integrated System of Biomass Gasification Combined Cycle and Oxy-Fuel Combustion. Energy 2020, 206, 118131. [Google Scholar] [CrossRef]
- Gao, N.; Chen, C.; Magdziarz, A.; Zhang, L.; Quan, C. Modeling and Simulation of Pine Sawdust Gasification Considering Gas Mixture Reflux. J. Anal. Appl. Pyrolysis 2021, 155, 105094. [Google Scholar] [CrossRef]
- Aspen Technology. Getting Started Modeling Processes with Solids. In Aspentech Plus; Aspen Technology: Burlington, MA, USA, 2013; Available online: https://profsite.um.ac.ir/~fanaei/_private/Solids%208_4.pdf (accessed on 28 August 2024).
- Puig-Gamero, M.; Argudo-Santamaria, J.; Valverde, J.L.; Sánchez, P.; Sanchez-Silva, L. Three Integrated Process Simulation Using Aspen Plus®: Pine Gasification, Syngas Cleaning and Methanol Synthesis. Energy Convers. Manag. 2018, 177, 416–427. [Google Scholar] [CrossRef]
- Nikoo, M.B.; Mahinpey, N. Simulation of Biomass Gasification in Fluidized Bed Reactor Using ASPEN PLUS. Biomass Bioenergy 2008, 32, 1245–1254. [Google Scholar] [CrossRef]
- Lan, W.; Chen, G.; Zhu, X.; Wang, X.; Liu, C.; Xu, B. Biomass Gasification-Gas Turbine Combustion for Power Generation System Model Based on ASPEN PLUS. Sci. Total Environ. 2018, 628–629, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, V.; Bocci, E.; Ouweltjes, J.P.; Del Zotto, L.; Monarca, D. Evaluation of Sorbents for High Temperature Removal of Tars, Hydrogen Sulphide, Hydrogen Chloride and Ammonia from Biomass-Derived Syngas by Using Aspen Plus. Int. J. Hydrogen Energy 2020, 45, 6651–6662. [Google Scholar] [CrossRef]
- Hannula, I.; Kurkela, E. A Semi-Empirical Model for Pressurised Air-Blown Fluidised-Bed Gasification of Biomass. Bioresour. Technol. 2010, 101, 4608–4615. [Google Scholar] [CrossRef]
- Kakati, U.; Sakhiya, A.K.; Baghel, P.; Trada, A.; Mahapatra, S.; Upadhyay, D.; Kaushal, P. Sustainable Utilization of Bamboo through Air-Steam Gasification in Downdraft Gasifier: Experimental and Simulation Approach. Energy 2022, 252, 124055. [Google Scholar] [CrossRef]
- Alcazar-Ruiz, A.; Ortiz, M.L.; Dorado, F.; Sanchez-Silva, L. Gasification versus Fast Pyrolysis Bio-Oil Production: A Life Cycle Assessment. J. Clean. Prod. 2022, 336, 130373. [Google Scholar] [CrossRef]
- Detchusananard, T.; Wuttipisan, N.; Limleamthong, P.; Prasertcharoensuk, P.; Maréchal, F.; Arpornwichanop, A. Pyrolysis and Gasification Integrated Process of Empty Fruit Bunch for Multi-Biofuels Production: Technical and Economic Analyses. Energy Convers. Manag. 2022, 258, 115465. [Google Scholar] [CrossRef]
- Ismail, H.Y.; Abbas, A.; Azizi, F.; Zeaiter, J. Pyrolysis of Waste Tires: A Modeling and Parameter Estimation Study Using Aspen Plus®. Waste Manag. 2017, 60, 482–493. [Google Scholar] [CrossRef]
- Kushwah, A.; Reina, T.R.; Short, M. Modelling Approaches for Biomass Gasifiers: A Comprehensive Overview. Sci. Total Environ. 2022, 834, 155243. [Google Scholar] [CrossRef]
- Kaushal, P.; Tyagi, R. Advanced Simulation of Biomass Gasification in a Fluidized Bed Reactor Using ASPEN PLUS. Renew. Energy 2017, 101, 629–636. [Google Scholar] [CrossRef]
- Cohce, M.K.; Dincer, I.; Rosen, M.A. Thermodynamic Analysis of Hydrogen Production from Biomass Gasification. Int. J. Hydrogen Energy 2010, 35, 4970–4980. [Google Scholar] [CrossRef]
- Pei, X.; He, B.; Yan, L.; Wang, C.; Song, W.; Song, J. Process Simulation of Oxy-Fuel Combustion for a 300MW Pulverized Coal-Fired Power Plant Using Aspen Plus. Energy Convers. Manag. 2013, 76, 581–587. [Google Scholar] [CrossRef]
- Singh, D.K.; Tirkey, J.V. Modeling and Multi-Objective Optimization of Variable Air Gasification Performance Parameters Using Syzygium Cumini Biomass by Integrating ASPEN Plus with Response Surface Methodology (RSM). Int. J. Hydrogen Energy 2021, 46, 18816–18831. [Google Scholar] [CrossRef]
- Aghaalikhani, A.; Schmid, J.C.; Borello, D.; Fuchs, J.; Benedikt, F.; Hofbauer, H.; Rispoli, F.; Henriksen, U.B.; Sárossy, Z.; Cedola, L. Detailed Modelling of Biomass Steam Gasification in a Dual Fluidized Bed Gasifier with Temperature Variation. Renew. Energy 2019, 143, 703–718. [Google Scholar] [CrossRef]
- Tavares, R.; Monteiro, E.; Tabet, F.; Rouboa, A. Numerical Investigation of Optimum Operating Conditions for Syngas and Hydrogen Production from Biomass Gasification Using Aspen Plus. Renew. Energy 2020, 146, 1309–1314. [Google Scholar] [CrossRef]
- Cruz, P.L.; Iribarren, D.; Dufour, J. Exergy Analysis of Alternative Configurations of a System Coproducing Synthetic Fuels and Electricity via Biomass Gasification, Fischer-Tropsch Synthesis and a Combined-Cycle Scheme. Fuel 2017, 194, 375–394. [Google Scholar] [CrossRef]
- Rupesh, S.; Muraleedharan, C.; Arun, P. ASPEN plus Modelling of Air–Steam Gasification of Biomass with Sorbent Enabled CO2 Capture. Resour.-Effic. Technol. 2016, 2, 94–103. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Pio, D.T.; Tarelho, L.A.C.; Sánchez, P.; Sanchez-Silva, L. Simulation of Biomass Gasification in Bubbling Fluidized Bed Reactor Using Aspen Plus®. Energy Convers. Manag. 2021, 235, 113981. [Google Scholar] [CrossRef]
- Zeng, X.; Ueki, Y.; Yoshiie, R.; Naruse, I.; Wang, F.; Han, Z.; Xu, G. Recent Progress in Tar Removal by Char and the Applications: A Comprehensive Analysis. Carbon Resour. Convers. 2020, 3, 1–18. [Google Scholar] [CrossRef]
- Gündüz Han, D.; Erdem, K.; Midilli, A. Investigation of Hydrogen Production via Waste Plastic Gasification in a Fluidized Bed Reactor Using Aspen Plus. Int. J. Hydrogen Energy 2023, 48, 39315–39329. [Google Scholar] [CrossRef]
- Hosseingholilou, B.; Tavakoli, N.; Saidi, M. Cost-Effectiveness and Economic Growth Potential Evaluation of Olive Pomace Gasification Process to Sustainable Fuel: Comparison Study of Different Gasifying Agent. Process Saf. Environ. Prot. 2024, 187, 533–548. [Google Scholar] [CrossRef]
- Ali, A.M.; Shahbaz, M.; Shahzad, K.; Inayat, M.; Naqvi, S.; Al-Zahrani, A.A.; Rashid, M.I.; Rehan, M.; Mahpudz, A.B. Polygeneration Syngas and Power from Date Palm Waste Steam Gasification through an Aspen Plus Process Modeling. Fuel 2023, 332, 126120. [Google Scholar] [CrossRef]
- Zaman, S.A.; Roy, D.; Ghosh, S. Process Modeling and Optimization for Biomass Steam-Gasification Employing Response Surface Methodology. Biomass Bioenergy 2020, 143, 105847. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, J.; Chen, G.; Liu, J.; Cheng, Z.; Wang, L.; Yi, W.; Xu, S. Energy, Efficiency, and Environmental Analysis of Hydrogen Generation via Plasma Co-Gasification of Biomass and Plastics Based on Parameter Simulation Using Aspen Plus. Energy Convers. Manag. 2023, 295, 117623. [Google Scholar] [CrossRef]
- Ranjan, N.; Yadav, N.; Singh, H.; Kumar, S.; Mahajani, S.M. Modelling and Simulation of Autothermal Downdraft Co-Gasification of Biomass and Plastic Wastes Using Aspen Plus. Energy Convers. Manag. 2023, 297, 117714. [Google Scholar] [CrossRef]
- Gagliano, A.; Nocera, F.; Bruno, M.; Cardillo, G. Development of an Equilibrium-Based Model of Gasification of Biomass by Aspen Plus. Energy Procedia 2017, 111, 1010–1019. [Google Scholar] [CrossRef]
- HajiHashemi, M.; Mazhkoo, S.; Dadfar, H.; Livani, E.; Naseri Varnosefaderani, A.; Pourali, O.; Najafi Nobar, S.; Dutta, A. Combined Heat and Power Production in a Pilot-Scale Biomass Gasification System: Experimental Study and Kinetic Simulation Using ASPEN Plus. Energy 2023, 276, 127506. [Google Scholar] [CrossRef]
- NREL(National Renewable Energy Laboratory); Spath, P.; Aden, A.; Eggeman, T.; Ringe, M.; Wallace, B.; Jechura, J. Biomass to Hydrogen and Economics Utilizing the Battelle Columbus Laboratory Indirectly-Heated Gasifier; National Renewable Energy Laboratory: Golden, CO, USA, 2005; p. 161. Available online: https://www.nrel.gov/docs/fy05osti/37408.pdf (accessed on 28 August 2024).
- Hernández, L.M.; Feliz, M.L.; Alonzo, L.R.; Abdelouahed, L.; Taouk, B. Aspen Plus® Modeling Approach of Beechwood Gasification in a Fluidized Bed Reactor Using Biochar as Bed Material. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2022; Volume 51, pp. 571–576. ISBN 978-0-323-95879-0. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780323958790500965?via%3Dihub (accessed on 28 August 2024).
- Harb, R.; Rivera-Tinoco, R.; Zeghondy, B.; Bouallou, C. Evaluating the Impact of Several Scrubbing Systems on the Tar Removal Efficiency from Producer Gas. Clean Technol. Environ. Policy 2022, 24, 379–392. [Google Scholar] [CrossRef]
- Thapa, S.; Indrawan, N.; Bhoi, P.R.; Kumar, A.; Huhnke, R.L. Tar Reduction in Biomass Syngas Using Heat Exchanger and Vegetable Oil Bubbler. Energy 2019, 175, 402–409. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A Review of the Primary Measures for Tar Elimination in Biomass Gasification Processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Cortazar, M.; Santamaria, L.; Lopez, G.; Alvarez, J.; Zhang, L.; Wang, R.; Bi, X.; Olazar, M. A Comprehensive Review of Primary Strategies for Tar Removal in Biomass Gasification. Energy Convers. Manag. 2023, 276, 116496. [Google Scholar] [CrossRef]
- Panayiotis, N. Removal, Utilization and Separation of Tars from Syngas 2016.
- Lotfi, S. Technologies for Tar Removal from Biomass-Derived Syngas. Pet. Petrochem. Eng. J. 2021, 5, 1–35. [Google Scholar] [CrossRef]
- Lateh, H.; Taweekun, J.; Maliwan, K.; Ishak, A. The Removal of Mixed Tar in Biomass Fuel Gas through the Thermal and Catalytic Treatment Methods: Review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 1003, 012141. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar Reduction in Biomass Producer Gas via Mechanical, Catalytic and Thermal Methods: A Review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Hsiau, S.-S.; Liao, C.-E.; Chou, S.-H. Development of New Technology for Tar Removal in IGCC. J. Clean. Prod. 2023, 384, 135575. [Google Scholar] [CrossRef]
- Font Palma, C. Modelling of Tar Formation and Evolution for Biomass Gasification: A Review. Appl. Energy 2013, 111, 129–141. [Google Scholar] [CrossRef]
- Singh, S.; Kumar Bhaumik, S.; Dong, L.; Vuthaluru, H. Enhanced Tar Removal in Syngas Cleaning through Integrated Steam Catalytic Tar Reforming and Adsorption Using Biochar. Fuel 2023, 331, 125912. [Google Scholar] [CrossRef]
- Nunes, L.J.R. Biomass Gasification as an Industrial Process with Effective Proof-of-Concept: A Comprehensive Review on Technologies, Processes and Future Developments. Results Eng. 2022, 14, 100408. [Google Scholar] [CrossRef]
- Vreugdenhil, B.J.; Zwart, R.W.R. Tar Formation in Pyrolysis and Gasification; TNO Publications: Amsterdam, The Netherlands, 2009; Available online: https://www.researchgate.net/publication/264090579_Tar_formation_in_pyrolysis_and_gasification (accessed on 28 August 2024).
- Monir, M.U.; Khatun, F.; Ramzilah, U.R.; Aziz, A.A. Thermal Effect on Co-Product Tar Produced with Syngas Through Co-Gasification of Coconut Shell and Charcoal. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022007. [Google Scholar] [CrossRef]
- Basu, P. Tar Production and Destruction. In Biomass Gasification, Pyrolysis and Torrefaction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 189–210. ISBN 978-0-12-812992-0. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128129920000066?via%3Dihub (accessed on 28 August 2024).
- Tang, W.; Cao, J.; He, Z.; Jiang, W.; Wang, Z.; Zhao, X. Recent Progress of Catalysts for Reforming of Biomass Tar/Tar Models at Low Temperatures—A Short Review. ChemCatChem 2023, 15, e202300581. [Google Scholar] [CrossRef]
- Deng, C.; Song, W.; Chai, Z.; Guo, S.; Zhu, Z. Characteristics of Tar Thermal Cracking and Catalytic Conversion during Circulating Fluidized Bed Char Gasification. Energy Fuels 2020, 34, 142–149. [Google Scholar] [CrossRef]
- Kaisalo, N. Tar Reforming in Biomass Gasification Gas Cleaning. Ph.D. Thesis, Aalto University, Aalto, Finland, 2017. Available online: https://aaltodoc.aalto.fi/server/api/core/bitstreams/8b234e2f-b59c-4f86-b110-effd5c8f4c76/content (accessed on 28 August 2024).
- Xu, Y.; Zuo, Y.; Yang, W.; Shu, X.; Chen, W.; Zheng, A. Targeted Catalytic Cracking to Olefins (TCO): Reaction Mechanism, Production Scheme, and Process Perspectives. Engineering 2023, 30, 100–109. [Google Scholar] [CrossRef]
- Fjellerup, J.; Ahrenfeldt, J.; Henriksen, U.; Gøbel, B. Formation, Decomposition and Cracking of Biomass Tars in Gasification; Technical University of Denmark, Department of Mechanical Engineering: Lyngby, Denmark, 2005; Available online: https://www.researchgate.net/publication/265115976_Formation_Decomposition_and_Cracking_of_Biomass_Tars_in_Gasification (accessed on 28 August 2024).
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic Steam Reforming of Biomass Tar: Prospects and Challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461. [Google Scholar] [CrossRef]
- Binte Mohamed, D.K.; Veksha, A.; Ha, Q.L.M.; Chan, W.P.; Lim, T.-T.; Lisak, G. Advanced Ni Tar Reforming Catalysts Resistant to Syngas Impurities: Current Knowledge, Research Gaps and Future Prospects. Fuel 2022, 318, 123602. [Google Scholar] [CrossRef]
- Hoo, K.K.; Md Said, M.S. Air Gasification of Empty Fruit Bunch: An Aspen Plus Model. Bioresour. Technol. Rep. 2021, 16, 100848. [Google Scholar] [CrossRef]
- Ghiat, I.; AlNouss, A.; McKay, G.; Al-Ansari, T. Biomass-Based Integrated Gasification Combined Cycle with Post-Combustion CO2 Recovery by Potassium Carbonate: Techno-Economic and Environmental Analysis. Comput. Chem. Eng. 2020, 135, 106758. [Google Scholar] [CrossRef]
- Haider, M.B.; Maheshwari, P.; Kumar, R. CO2 Capture from Flue Gas Using Phosphonium Based Deep Eutectic Solvents: Modeling and Simulation Approach. J. Environ. Chem. Eng. 2021, 9, 106727. [Google Scholar] [CrossRef]
- Jana, K.; De, S. Biomass Integrated Combined Power Plant with Post Combustion CO2 Capture—Performance Study by ASPEN Plus®. Energy Procedia 2014, 54, 166–176. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Paramio, C.; Palomar, J. Aspen plus Supported Design of Pre-Combustion CO2 Capture Processes Based on Ionic Liquids. Sep. Purif. Technol. 2022, 290, 120841. [Google Scholar] [CrossRef]
- Li, S.; Zhu, L.; He, Y.; Fan, J.; Lv, L. Thermodynamic Evaluation of CCHP System Based on Biomass Gasification by Exploring the Feasibility of Using CO2 as Gasification Agent. Sustain. Energy Technol. Assess. 2020, 42, 100867. [Google Scholar] [CrossRef]
- Sadhwani, N.; Adhikari, S.; Eden, M.R. Biomass Gasification Using Carbon Dioxide: Effect of Temperature, CO2/C Ratio, and the Study of Reactions Influencing the Process. Ind. Eng. Chem. Res. 2016, 55, 2883–2891. [Google Scholar] [CrossRef]
- Reyes, L.; Abdelouahed, L.; Buvat, J.-C.; Campusano, B.; Boyer, C.D.; Taouk, B. ☆Presented during the French Chemical Engineering Congress SFGP 2019, Nantes 15–17 October 2019. Energetic Study of Beech Wood Gasification in Fluidized Bed Reactor under Different Gasification Conditions. Chem. Eng. Res. Des. 2020, 164, 23–34. [Google Scholar] [CrossRef]
- Hasnain, M.F.U.; Ali, H.O.; Aziz, S.; Kouotou, P.M.; Waqas, M.; Naqvi, S.M.A.; Athar, M.H.; Ammar, M.; Shah, I.; Jung, D. Optimization and Techno-Economic Analysis of Catalytic Gasification of Wheat Straw Biomass Using ASPEN PLUS Model. Arab. J. Chem. 2024, 17, 105821. [Google Scholar] [CrossRef]
- De Oliveira, D.C.; De Rezende, T.T.G.; Lora, E.E.S.; Venturini, O.J.; Maya, D.M.Y. Tar Removal from Biomass-Derived Syngas for Hydrogen Production: Oil Absorption Process Considering Brazilian Sources. Int. J. Hydrogen Energy 2024, in press. [CrossRef]
- Njuguna, F.; Ndiritu, H.; Gathitu, B.; Hawi, M.; Munyalo, J. Kinetic Modeling and Optimization of Process Parameters for Gasification of Macadamia Nutshells with Air Preheating: A Combined Use of Aspen Plus and Response Surface Methodology (RSM). Bioresour. Technol. Rep. 2023, 22, 101477. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, C.; Cai, L.; Long, X. Steady State Modelling of Steam-Gasification of Biomass for H2-Rich Syngas Production. Energy 2022, 238, 121616. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, Y.; Du, J. Air-Steam Gasification of Biomass Based on a Multi-Composition Multi-Step Kinetic Model: A Clean Strategy for Hydrogen-Enriched Syngas Production. Sci. Total Environ. 2021, 753, 141690. [Google Scholar] [CrossRef] [PubMed]
- Dhrioua, M.; Ghachem, K.; Hassen, W.; Ghazy, A.; Kolsi, L.; Borjini, M.N. Simulation of Biomass Air Gasification in a Bubbling Fluidized Bed Using Aspen Plus: A Comprehensive Model Including Tar Production. ACS Omega 2022, 7, 33518–33529. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.; Wang, L.; Qian, T.; Zhu, Y.; Sun, H.; Gao, J.; Chen, H.; Gao, Y.; Liu, C. COSMO-Based Solvent Selection and Aspen Plus Process Simulation for Tar Absorptive Removal. Appl. Energy 2019, 251, 113314. [Google Scholar] [CrossRef]
- Francois, J.; Abdelouahed, L.; Mauviel, G.; Feidt, M.; Rogaume, C.; Mirgaux, O.; Patisson, F.; Dufour, A. Estimation of the Energy Efficiency of a Wood Gasification Chp Plant Using Aspen Plus. Chem. Eng. Trans. 2012, 29, 769–774. [Google Scholar] [CrossRef]
- Susmozas, A.; Iribarren, D.; Dufour, J. Life-Cycle Performance of Indirect Biomass Gasification as a Green Alternative to Steam Methane Reforming for Hydrogen Production. Int. J. Hydrogen Energy 2013, 38, 9961–9972. [Google Scholar] [CrossRef]
- Hejazi, B.; Grace, J.R. Simulation of Tar-Free Biomass Syngas Enhancement in a Calcium Looping Operation Using Aspen Plus Built-in Fluidized Bed Model. Int. J. Greenh. Gas Control 2020, 99, 103096. [Google Scholar] [CrossRef]
- Kum, J.; Cho, S.; Ko, Y.; Lee, C.-H. Blended-Amine CO2 Capture Process without Stripper for High-Pressure Syngas. Chem. Eng. J. 2024, 486, 150226. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, N.; Li, X.; Li, Z.; Li, K.; Jin, H.; Qu, S.; Xia, H.; Cheng, H.; Tan, X.; et al. Process Optimization, Energy Consumption Analysis, and Environmental Assessment of Total CO2 Capture from Syngas Based on [NEMH][Ac] Protic Ionic Liquid. Int. J. Greenh. Gas Control 2024, 131, 104037. [Google Scholar] [CrossRef]
- Shahbaz, M.; Yusup, S.; Inayat, A.; Ammar, M.; Patrick, D.O.; Pratama, A.; Naqvi, S.R. Syngas Production from Steam Gasification of Palm Kernel Shell with Subsequent CO2 Capture Using CaO Sorbent: An Aspen Plus Modeling. Energy Fuels 2017, 31, 12350–12357. [Google Scholar] [CrossRef]
- Canepa, R.; Wang, M.; Biliyok, C.; Satta, A. Thermodynamic Analysis of Combined Cycle Gas Turbine Power Plant with Post-Combustion CO2 Capture and Exhaust Gas Recirculation. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2013, 227, 89–105. [Google Scholar] [CrossRef]
- Pellegrini, L.A.; De Guido, G.; Moioli, S. Design of the CO2 Removal Section for PSA Tail Gas Treatment in a Hydrogen Production Plant. Front. Energy Res. 2020, 8, 77. [Google Scholar] [CrossRef]
- Leonzio, G.; Zondervan, E. Surface-Response Analysis for the Optimization of a Carbon Dioxide Absorption Process Using [Hmim][Tf2N]. Processes 2020, 8, 1063. [Google Scholar] [CrossRef]
- Frigo, S.; Flori, G.; Barontini, F.; Gabbrielli, R.; Sica, P. Experimental and Numerical Performance Assessment of Green-Hydrogen Production from Biomass Oxy-Steam Gasification. Int. J. Hydrogen Energy 2024, 71, 785–796. [Google Scholar] [CrossRef]
- Pilar González-Vázquez, M.; Rubiera, F.; Pevida, C.; Pio, D.T.; Tarelho, L.A.C. Thermodynamic Analysis of Biomass Gasification Using Aspen Plus: Comparison of Stoichiometric and Non-Stoichiometric Models. Energies 2021, 14, 189. [Google Scholar] [CrossRef]
- Antonopoulos, I.-S.; Karagiannidis, A.; Gkouletsos, A.; Perkoulidis, G. Modelling of a Downdraft Gasifier Fed by Agricultural Residues. Waste Manag. 2012, 32, 710–718. [Google Scholar] [CrossRef]


| Parameter | Software * | References | Conclusions |
|---|---|---|---|
| Temperature | Aspen Plus | [9,23,24,25,26,27,28,29,30,31] | With the increase in temperature, a decrease in the composition of CH4 and CO2 was observed, while the production of H2 and CO increased until reaching a specific maximum temperature, after which some of these authors noticed a slight decrease. |
| Aspen HYSYS | [32,33,34] | ||
| IPSEpro | [35,36] | ||
| MATLAB | [37] | ||
| Aspen Plus | [9] | When the temperature increases, the LHV decreases (427–627 °C). Afterwards, an increase is observed (727–1227 °C). | |
| IPSEpro | [36] | When the temperature increases, the LHV decreases (700–900 °C). | |
| Aspen Plus | [38,39] | When the temperature increases, the LHV and HHV increase (650–750 °C). After this temperature, a decrease is observed (750–950 °C). | |
| Pressure (not as influential as temperature) | Aspen Plus | [9,40,41,42] | Increasing pressure enhances the generation of CH4 and CO2, while the concentrations of CO and H2 decrease (0–30 atm). |
| Aspen Plus | [41] | As pressure increases, calorific value increases HHV (1–20 atm). | |
| Aspen Plus | [9] | The energy efficiency and exergy efficiency decrease marginally with pressure. | |
| Equivalence ratio (ER) | Aspen Plus | [25,26,30,31,42,43,44] | As ER increases, CO2 production increases, while H2 and CO production decreases (0.1–1). |
| Aspen Plus | [39,43] | An increase in ER leads to a decrease in the LHV of the synthetic gas (0.18–0.38). | |
| Steam/biomass ratio (S/B) | Aspen HYSYS | [32,34] | When S/B is increased, the concentrations of H2 and CO2 increase, while the concentration of CO decreases (0.1–2.5). |
| IPSEpro | [35,36] | ||
| Numerical simulation/Cantera | [45] | ||
| Aspen Plus | ([9,25,26,27,46] used Aspen and MATLAB) | ||
| Aspen Plus | [9,25,26,27,46] ([47] used Aspen and MATLAB) | The LHV value decreases with the increase in the steam-to-biomass ratio (0.2–5). | |
| IPSEpro | [36] | ||
| Percentage of oxygen (OP) | Aspen Plus | [11] | An increase in the percentage of oxygen enhances the quality of the synthetic gas and the efficiency of gasification. |
| Biomass moisture | IPSEpro | [36] | An increase in the moisture content of the biomass leads to a decrease in the cold gas efficiency for both gasification and the entire process. |
| Treatment | Equipment | Technologies | Description | Tars | Reference |
|---|---|---|---|---|---|
| Tar treatment | R-CSTR and R-GIBBS | Steam reforming | The tar is produced during the pyrolysis stage. It is then separated and sent to a gas cleaning unit, where nickel reactions occur. It is then sent to a CSTR reactor, where steam reforming reactions occur. | Biomass: wheat straw, Gasification agent: steam, Catalyst of TSR: Ni-Co-Al2O3 (15, 10, 5)%, Tars: phenol, toluene, naphthalene. | [139] |
| RADFRAC | Absorption of tar with oils (canola, soybean, palm, used cooking oils, tallow biodiesel, and resin oil) | A gas mixture containing H2, CO, CO2, CH4, H2O, N2, and tars was introduced. Then, oil was introduced at the top of the absorber. The tar was absorbed by the oils. | Feed: syngas, Tars: benzene, toluene, xylene, styrene, naphthalene, 2-methylnaphtalene, 1 methylnaphthalene, diphenanthrene, fluorene, phenanthrene and anthracene. | [140] | |
| R-PLUG | Thermal cracking (oxidation kinetics and reduction stage) | In this simulation, reactions with their kinetics are used to simulate the thermal cracking. | Biomass: macadamia nutshells, Tars: phenol, naphthalene, benzene toluene. | [141] | |
| R-CSTR | Tar thermal cracking | The tars produced in the pyrolysis stage were cracked using kinetics. | Biomass: pomegranate wood, Tars: phenol, naphthalene, acetol (C3H6O2). | [106] | |
| R-GIBBS/R-CSTR | Combustion and cracking | In this simulation, the tar is separated from the mixture after the pyrolysis process. Subsequently, it is directed to a cracking reactor using a Gibbs reactor. Then, these products are also subjected to cracking through reaction kinetics using a CSTR reactor. | Biomass: white pine, Gasification agent: steam, Tars: benzene, toluene, phenol y naphthalene. | [142] | |
| R-GIBBS | Thermal cracking combustion of tar | The tar is separated from the volatiles and the char, and it is burned in a combustion reactor. The final product does not contain tars. | Biomass: pine sawdust, Gasification agent: air–steam, T: 700–800 °C. | [143] | |
| RADFRAC | Scrubbing | It consists of two scrubbers followed by a stripper. The producer gas is cooled (and washed in the first scrubber with water) to condense the heavy tar fraction. Then, in the second scrubber, the light tar fraction is absorbed. Finally, the stripper allows for the regeneration of the washing liquid. | Feed: syngas, Tars: toluene, benzene phenol, naphthalene, indene and fluoranthene. | [109] | |
| R-STOIC | Thermal cracking combustion of tar | The tar produced in the pyrolysis stage is taken to a reactor that models oxidation reactions, incorporating tar cracking reactions. | Biomass: Prosopis juliflora, Gasification agent: air gasification, T:800–1000 °C, Tars: phenol, toluene, naphthalene, and benzene. | [144] | |
| Steam reforming (Aspen HYSYS). | The kinetics is introduced into a stoichiometric reactor. | Feed: syngas, Tars: benzene, toluene, naphthalene, pyrene, p-xylene, indene, ethylbenzene, anthracene, acenaphthylene. | [16] | ||
| R-GIBBS | Tar cracking | The tar produced in the devolatilization stage, which is a primary tar, is cracked in the gasification stage through a general tar cracking kinetics; CO, CO2, CH4, H2, and inert tar are involved. | The tar was considered as a substance. | [89] | |
| R-STOIC | Cracking | The tar produced in the pyrolysis stage is modeled using correlations. | Biomass: sawdust; tar is considered as a substance. | [96] | |
| RADFRAC | Absorption and desorption | The synthesis gas was introduced in a counter-current with oils in the absorption tower; the tar components were absorbed, heated, and separated from the solvent in a desorption tower for recirculation. | Tars: benzene, toluene, phenol, and naphthalene. | [145] | |
| R-CSTR | Thermal cracking | The tar produced in the pyrolysis stage is directed along with the other gases and cracked using reaction kinetics. | Tars: toluene, hydrogen, naphthalene, phenol, CnHm). | [21] | |
| R-PLUG/ R-EQUIL | Catalytic cracking, steam reforming, and thermal cracking reactions | The conversion degrees are derived from empirical results, and olivine is employed as a catalyst. | Tars: phenol, benzene, toluene, naphthalene. | [146] | |
| R-PLUG | Thermal and catalytic cracking with biochar | Conversions were used. | Tars: phenol, benzene, toluene, naphthalene. | [20], | |
| R-GIBBS | Tar reformer | The tar reforming involves a bubbling fluidized bed that also uses olivine as the catalytic bed material. | Biomass: poplar, Tar: naphthalene. | [147] | |
| R-GIBBS | Tar reformer/water scrubbing | The tar reformer consists of a fluidized bed reactor operating at 766 °C and 1.5 bar, with olivine as the bed material. | [95] | ||
| R-STOIC | Tar reformer (dolomite) | Reactions were used to simulate the reforming stage. | Biomass: pine, Tar: benzene. | [79] | |
| CO2 capture | Absorption tower | Chemical absorption with MDEA-PZ (10 wt.% methyl diethanolamine and 30 wt.% piperazine). | Solvent: MDEA (Aspen HYSYS). | [16] | |
| RADFRAC | Chemical absorption | An amine-based absorption column and a stripper are used to simulate the solvent recovery. | Solvent: MEA. | [134] | |
| RADFRAC/FLASH2 | Chemical absorption | In this simulation, the synthesis gas was counter-current fed through an absorption column coupled with a regeneration section consisting of a flash liquid–gas separation column available in the Aspen Plus library to regenerate the solvent. This regeneration section consists of a series of expansion drums operating at lower pressures. The DESs have negligible volatility, so at low pressures they only results in the release of carbon dioxide from the top and the bottom of the DESs. | Solvent: deep eutectic solvents (DESs). | [133], | |
| RADFRAC | Chemical absorption | The synthesis gas is introduced in a counter-current with the ionic liquid that absorbs CO2. RADFRAC columns were used for both the absorption simulation and the regeneration part of the ionic liquids. | Solvent: ionic liquids. | [135], | |
| FluidBed (unit solid in Aspen Plus) | In situ lime-based CO2 capture in a bubbling fluidized bed (BFB); calcium looping | Limestone was used assuming complete calcination in this simulation. CaO and syngas were introduced into a carbonation reactor, using an LHHW-type kinetics. | Feed: syngas. | [148] | |
| Absorption/flash column | CO2 | In this simulation, an absorption column is used, followed by a depressurization in the flash column to simulate solvent regeneration. Pre-combustion capture. | Feed: syngas, Solvent: (MDEA/PZ), Capture percentage: 90%. | [149] | |
| RADFRAC | Physical absorption | The synthesis gas is dehydrated with triethylene glycol (TEG) and then mixed with recycled gas, and the ionic liquid absorbs the acidic gas. The purified gas is extracted and the CO and H2 are recycled. The ionic liquid is regenerated in a flash tank and the acidic gases are separated in a distillation tower. | Feed: coal, syngas, Impurities: CO2 and H2S, Capture percentage: (98%CO2), Solvent: ionic liquid/N-ethylmorpholine acetate ([NEMH][Ac]). | [150] | |
| R-GIBBS | Carbonation | Carbon dioxide is captured through carbonation with calcium oxide (CaO), and then the formed CaCO3 is separated using a cyclone. | Biomass: palm kernels, Efficiency: the CO2 content decreased from 20 to 5.32%, Sorbent: CaO. | [151] | |
| RADFRAC | Chemical absorption | The absorber and stripper, designed as packed-bed columns, allow a counter-current flow between the exhaust gases and the lean potassium carbonate. Using the RADFRAC model, the kinetic reactions in both columns are considered. | Solvent: piperazine is added to the potassium carbonate solvent, Capture percentage: 80%. | [132] | |
| RADFRAC | Absorption | A post-combustion capture was performed to clean the synthesis gas through absorption. | Solvent: 30 wt.% MEA, Capture percentage: 90%. | [152] | |
| Absorption | A post-combustion capture was performed to clean the synthesis gas through absorption. | Solvent: MDEA, Capture percentage: 96.49%. | [153] | ||
| RADFRAC | Physic absorption | The overall process includes a packed-bed absorber and a flash distiller for IL regeneration. Additionally, it features an intermediate-pressure cooling system for separating carbon dioxide from water, followed by compressors with intermediate cooling to increase the pressure of the captured carbon dioxide. | Solvent: ionic liquid using [hmim][Tf2N], Capture percentage: 93.7%. | [154] | |
| Absorption | A syngas cleaning process was simulated. The syngas was cooled to 300 °C, preventing tar condensation. Then, the syngas was introduced into an absorption tower that operates through chemical absorption, using a solvent. The CO2 was absorbed by the solvent and subsequently separated in a desorption tower. | Feed: syngas from woody biomass, Solvent: hot potassium carbonate solution, Capture percentage: 94.9%. | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loweski Feliz, M.; Abdelouahed, L.; Taouk, B. Comparative and Descriptive Study of Biomass Gasification Simulations Using Aspen Plus. Energies 2024, 17, 4443. https://doi.org/10.3390/en17174443
Loweski Feliz M, Abdelouahed L, Taouk B. Comparative and Descriptive Study of Biomass Gasification Simulations Using Aspen Plus. Energies. 2024; 17(17):4443. https://doi.org/10.3390/en17174443
Chicago/Turabian StyleLoweski Feliz, Minda, Lokmane Abdelouahed, and Bechara Taouk. 2024. "Comparative and Descriptive Study of Biomass Gasification Simulations Using Aspen Plus" Energies 17, no. 17: 4443. https://doi.org/10.3390/en17174443
APA StyleLoweski Feliz, M., Abdelouahed, L., & Taouk, B. (2024). Comparative and Descriptive Study of Biomass Gasification Simulations Using Aspen Plus. Energies, 17(17), 4443. https://doi.org/10.3390/en17174443







