CCUS Perspectives: Assessing Historical Contexts, Current Realities, and Future Prospects
Abstract
1. Background and Significance of CCUS
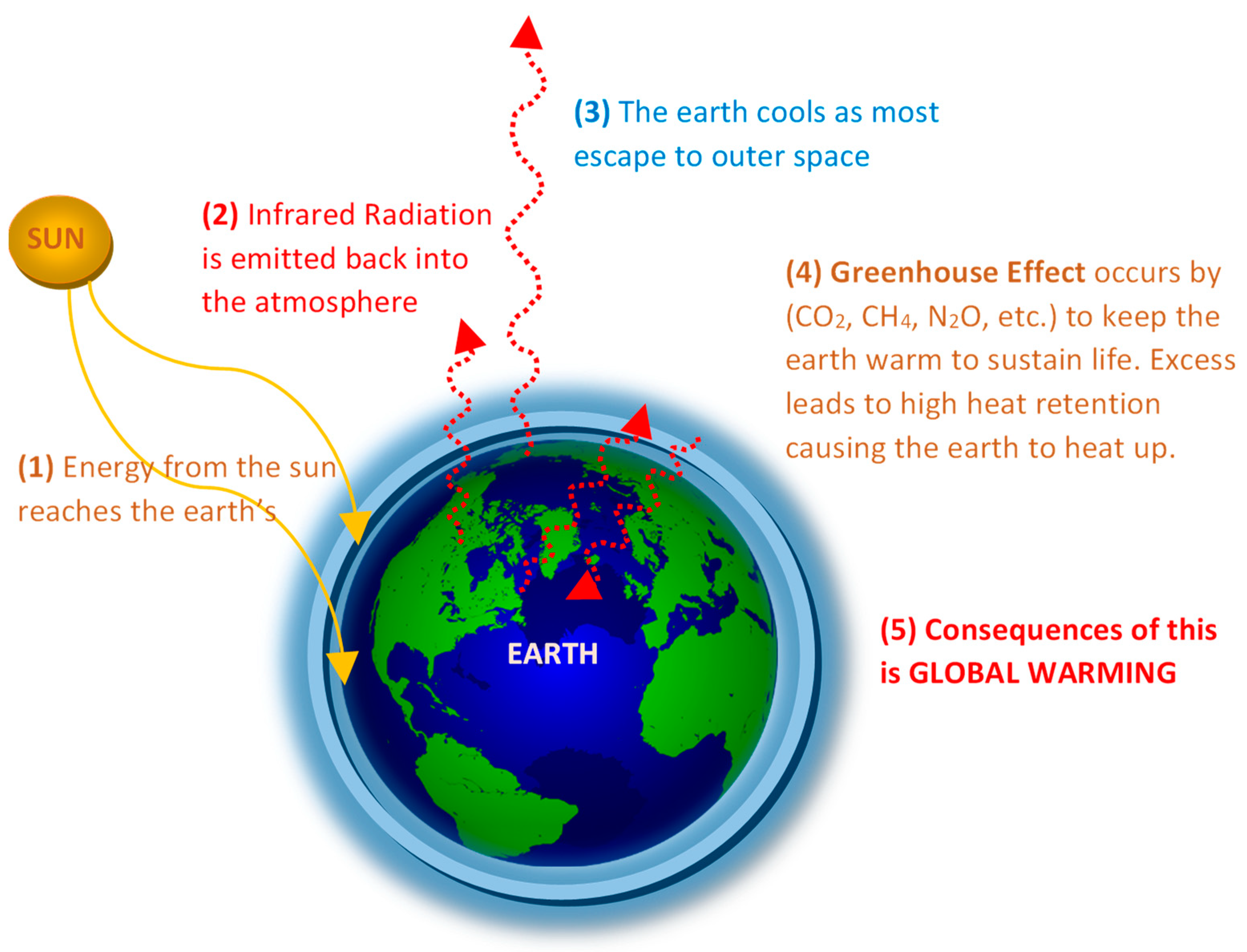
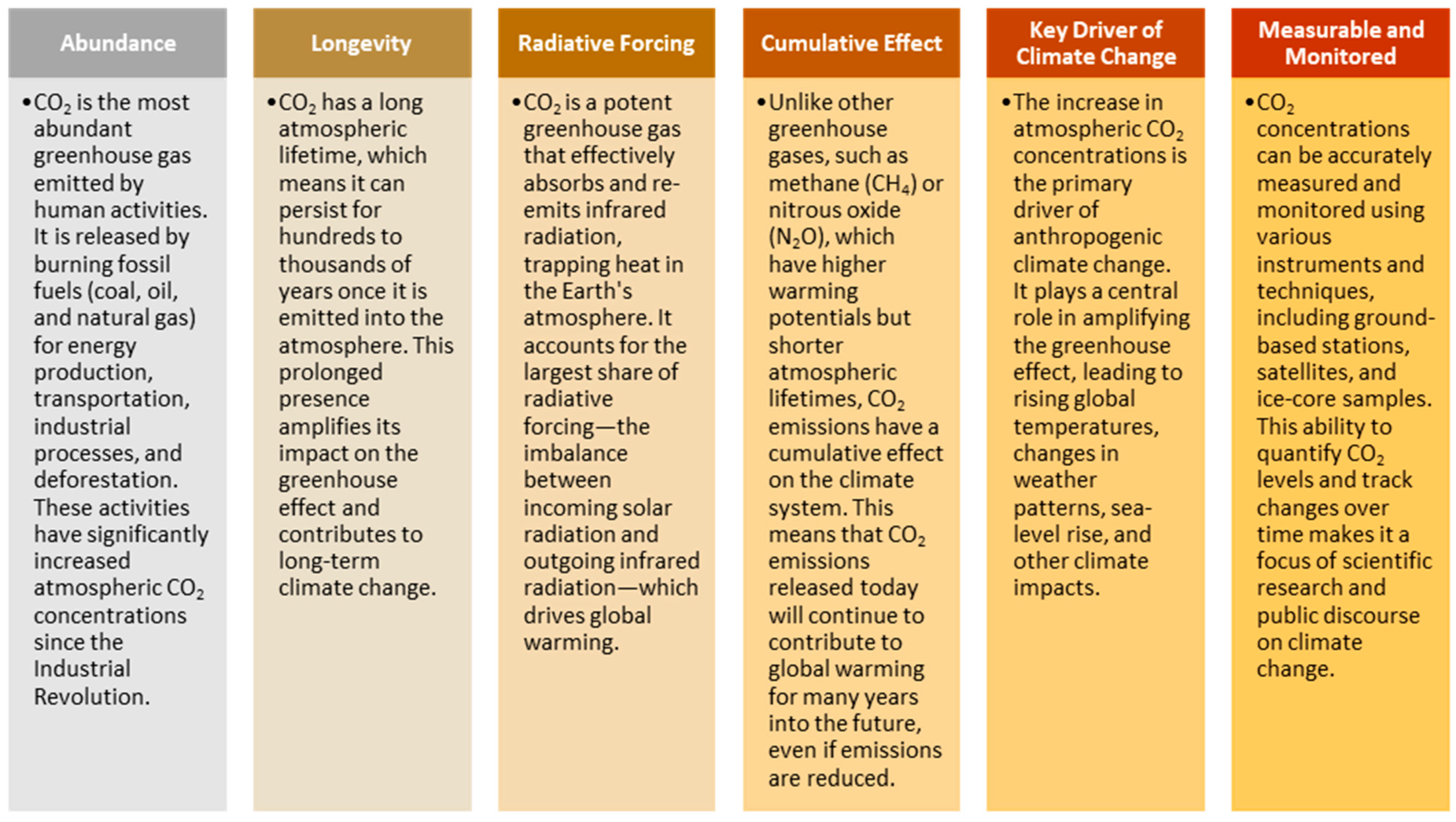
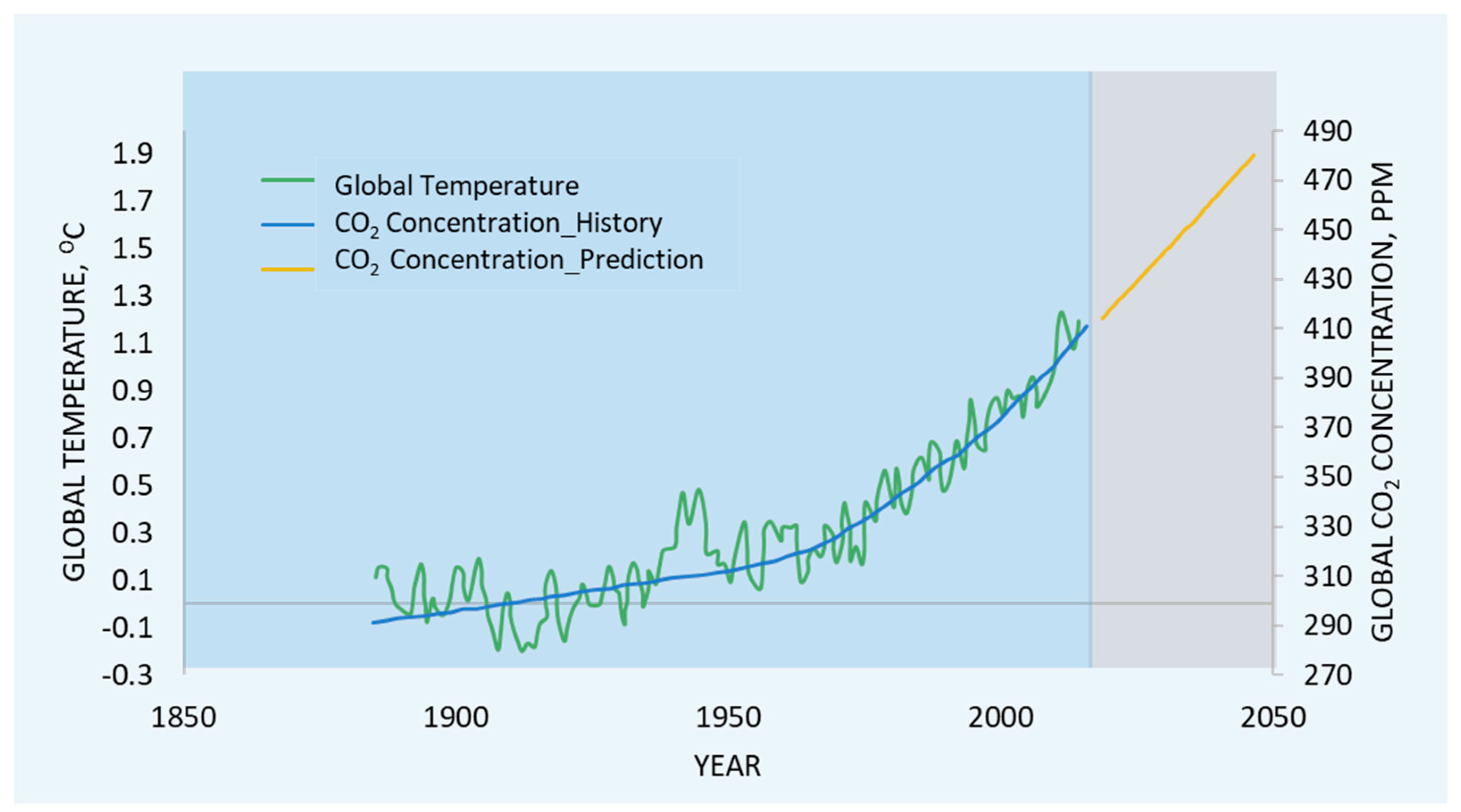
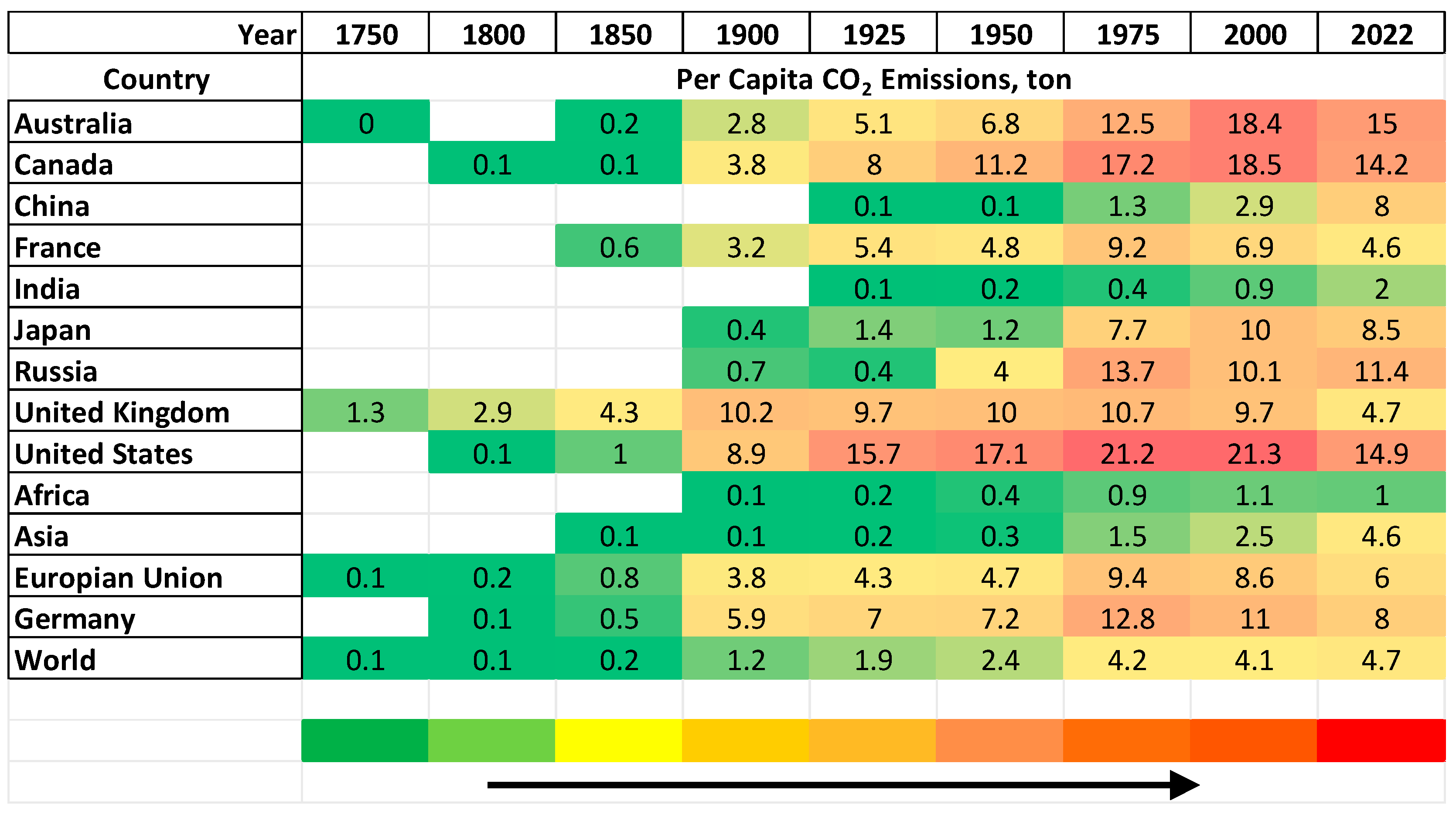
1.1. CO2 as a Key Target in Global Warming Menace
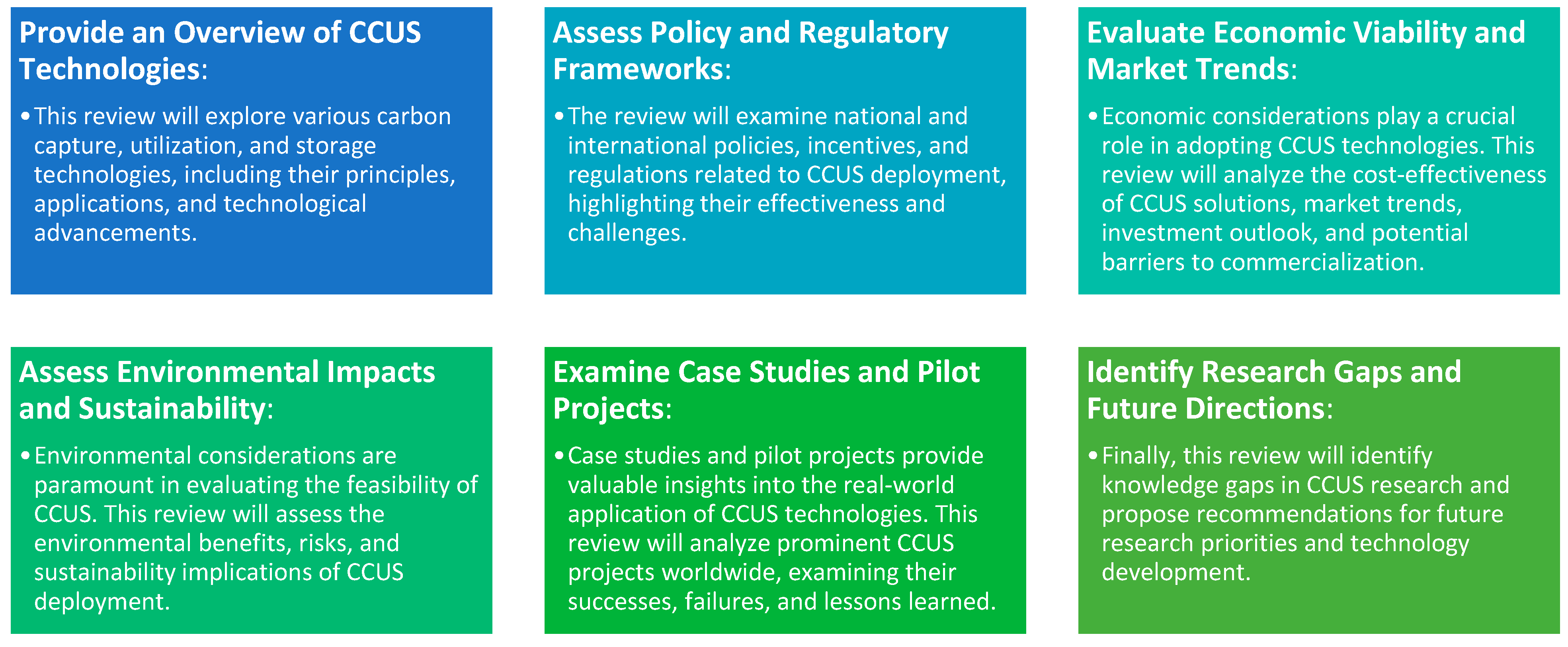
1.2. Scope and Objectives
2. Overview of Carbon Capture, Utilization, and Storage (CCUS)
2.1. Future Prospects and Strategic Directions in CCUS
2.2. Harnessing CCUS Potential in Emerging Markets
Current Underground Injection Control (UIC) Class VI Permit Applications
3. CCUS Pathways and Technological Advancement
3.1. Carbon Capture and Separation Technologies
3.1.1. Industrial Process
3.1.2. Post Combustion
3.1.3. Pre-Combustion
3.1.4. Oxy-Fuel Combustion
3.1.5. Chemical Looping Combustion (CLC)
3.1.6. Direct Air Capture
3.2. Separation Technologies
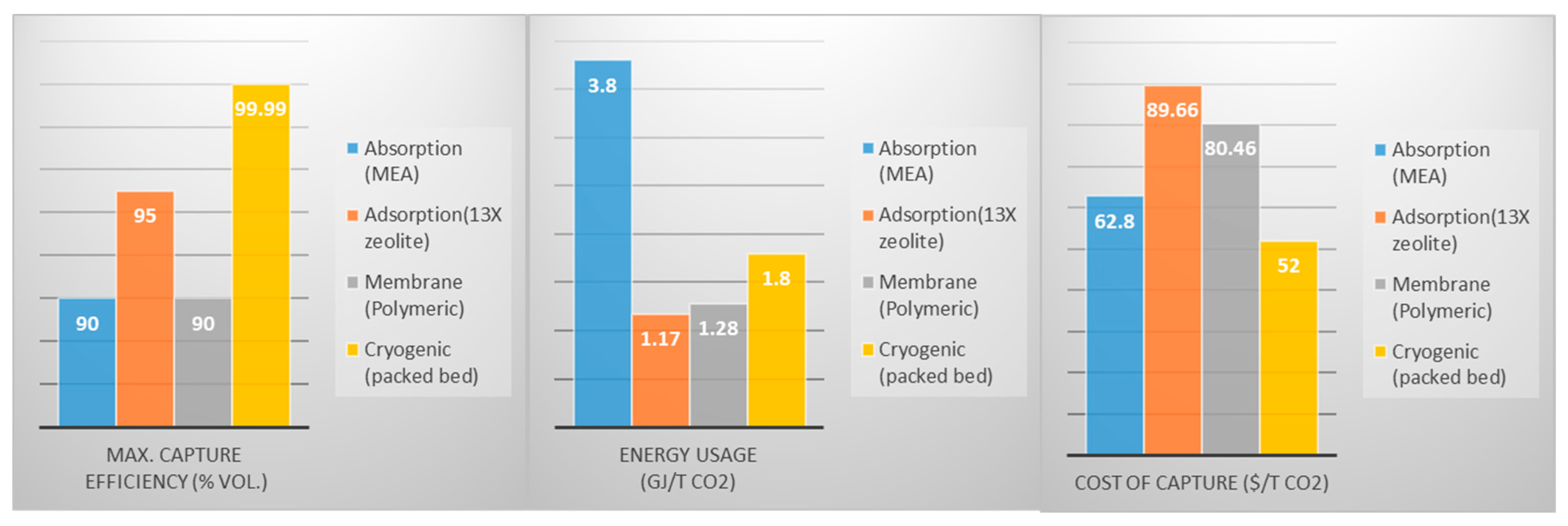
3.2.1. Absorption
3.2.2. Adsorption
3.2.3. Membrane Separation
3.2.4. Cryogenic Separation
3.2.5. Biological Separation
3.3. Transportation of Captured CO2

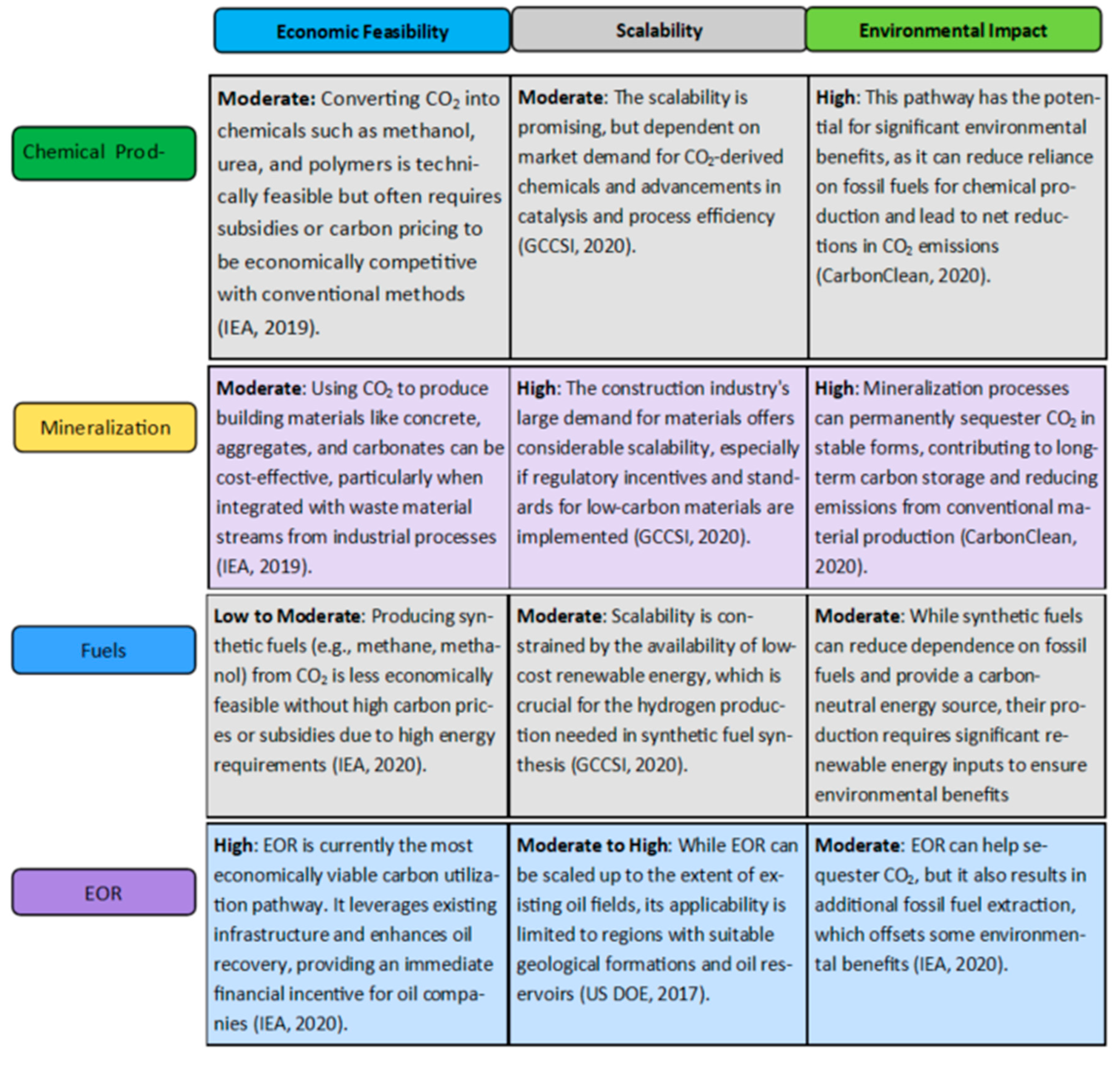
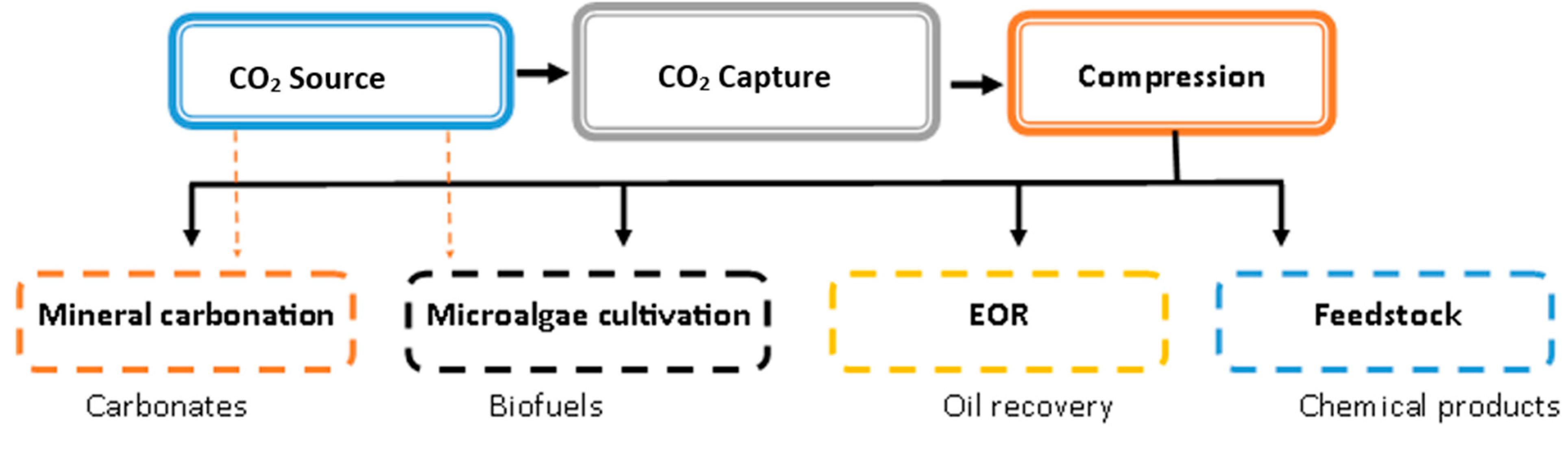
3.4. Carbon Capture and Utilization (CCU) Pathways
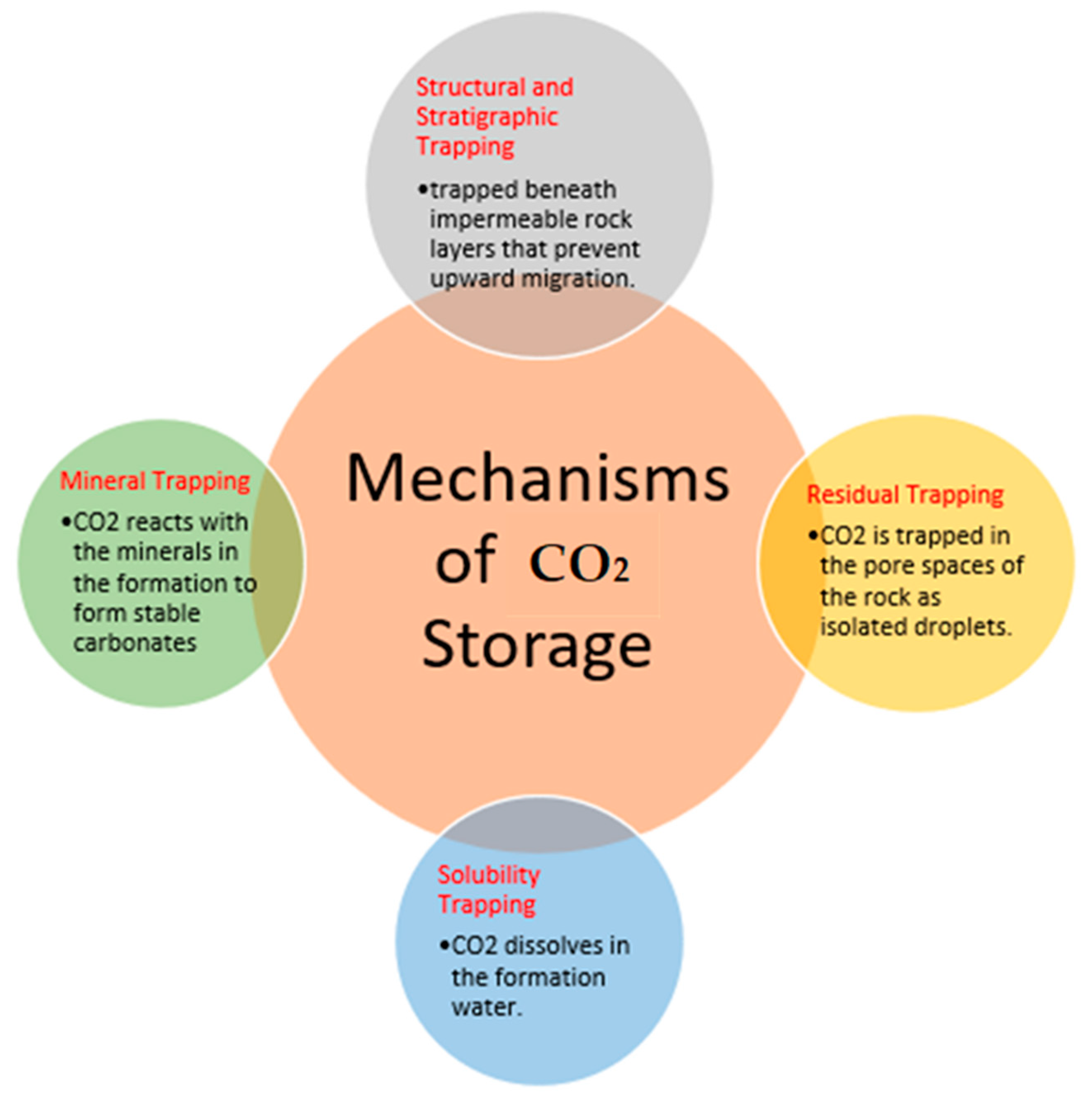
4. Carbon Storage Technologies (CCS Pathways)
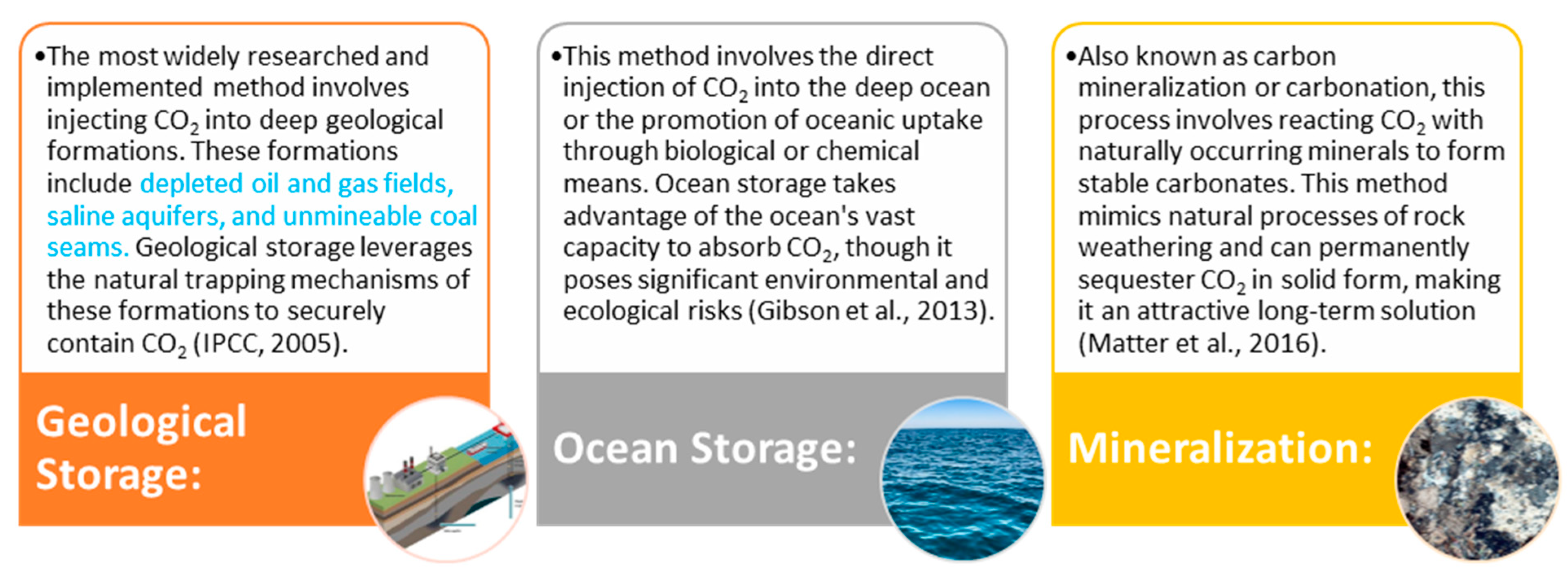
4.1. Geological Storage
4.1.1. CO2—EOR with Storage
4.1.2. CCS in Depleted Oil and Gas Reservoirs (DOGR)
4.1.3. Saline Aquifers
4.1.4. CO2-Enhanced Coalbed Methane (ECBM)
4.2. CO2 Mineralization
4.3. Oceanic Storage
| Parameter | Storage Medium | ||
|---|---|---|---|
| Geological | Oceanic | Mineralization | |
| Storage Capacity | High capacity, particularly in deep saline aquifers, but site-specific. | Very high capacity due to the vast volume of the ocean. | Limited by the availability of reactive minerals but offers permanent storage. |
| Stability and Performance | Generally stable, but risks of leakage and induced seismicity. | Long-term stability is uncertain, and there is potential for acidification and ecological impacts. | Permanent and stable, forming solid carbonates. |
| Monitoring and Verification | Requires extensive and continuous monitoring. | Difficult to monitor, especially for deep-sea injections. | Minimal monitoring is needed post-reaction. |
| Environmental Impact | Potential for groundwater contamination and induced seismicity. | Ocean acidification, ecological disruptions. | Mining and processing impacts but stable final products. |
| Economic Consideration | High initial costs, potential revenue from EOR. | High costs for infrastructure and monitoring | High energy and material costs, the potential for utilization of industrial waste. |
| Types | Mechanism | Advantage | Challenge | |
|---|---|---|---|---|
| Geological | Depleted Oil/gas | Utilizes existing reservoirs that have held hydrocarbons for millions of years, providing a proven trap for CO2. | Well-understood geology, existing infrastructure, and potential for enhanced oil recovery (EOR). | Limited capacity, and potential for CO2 leakage through old wells, require detailed site characterization. |
| Deep Saline | Injects CO2 into porous rock formations saturated with saline water. | Vast storage potential, and widespread availability. | Requires extensive monitoring, potential for induced seismicity, less characterized compared to oil and gas reservoirs. | |
| Unmineable Coal | CO2 adsorbs onto the surface of coal, displacing methane. | Potential for enhanced coalbed methane recovery (ECBM). | Limited storage capacity, complex adsorption dynamics, potential for CO2 leakage. | |
| Oceanic | Direct Injection | CO2 is injected into the deep ocean where it forms a dense liquid or hydrates. | High potential storage capacity, and long-term sequestration potential. | Ocean acidification, ecological disruptions, and uncertain long-term stability |
| Enhance Weathering | Adding alkaline minerals to the ocean to increase CO2 uptake. | Natural process acceleration, potential co-benefits for ocean chemistry. | Large-scale feasibility, and environmental impact of mineral extraction and distribution | |
| Ocean fertilization | Adding nutrients to stimulate phytoplankton growth, enhancing biological carbon pump. | Can sequester CO2 in organic matter, relatively low-cost. | Ecological risks, limited and variable efficacy, and potential for negative feedback | |
| Mineralization | In Situ | CO2 is injected into subsurface rock formations, such as basalt, where it reacts with minerals to form carbonates. | Permanent storage, natural process, minimal monitoring post-injection. | Slow reaction rates, limited suitable sites, and energy-intensive |
| Ex Situ | Reactive minerals are mined, crushed, and reacted with CO2 in an industrial setting. | Controlled conditions, and use of industrial by-products. | High energy and resource requirements, environmental impact of mining and processing |
5. Policy and Regulatory Framework
5.1. National and International Policies
5.2. Incentive and Funding Mechanisms
5.3. Regulatory Challenges and Opportunities
6. Economic Viability and Market Trends
6.1. Cost Analysis of CCUS Technologies
6.1.1. Cost Components of CCUS Technologies
6.1.2. Economic and Policy Implications
6.2. Market Trends and Investment Outlook
6.2.1. Market Trends in CCUS
6.2.2. Investment Patterns in CCUS
6.2.3. Future Outlook for CCUS
6.3. Economic Barriers and Potential Solutions
6.3.1. Economic Barriers to CCUS
6.3.2. Potential Solutions to Economic Barriers
7. Environmental Impacts and Sustainability
7.1. Assessment of Environmental Benefits and Risks
7.1.1. Environmental Benefits of CCUS
7.1.2. Environmental Risks of CCUS
7.2. How Green Is CCUS: Life Cycle Analysis of CCUS Technologies
7.2.1. Life Cycle Assessment of CCS
| Capture Technology | |||||||
|---|---|---|---|---|---|---|---|
| Plant Type | Pre-Comb | Post Comb | Oxy-Fuel Comb | Functional Unit | LCA Boundary | Sequestration | References |
| 1 | Y | Y | Y | 0.001 MWh | C2Gv | GF | [184] |
| 1 | Y | 0.001 MWh | C2Gv | O | [185] | ||
| 1 | Y | 1 MWh | C2Gv | O | [186] | ||
| 1 | Y | 1 tCO2 | G2Gt | [187] | |||
| 1 | Y | Y | 0.001 MWh | C2Gv | O | [188] | |
| 2 | Y | Y | Y | 0.001 MWh | G2Gv | GF | [189] |
| 1 | Y | 1 MWh | C2Gv | GF | [190] | ||
| 1 | Y | 1 MWh | C2Gv | [191] | |||
| 2 | Y | C2Gv | GF | [192] | |||
| 1 | Y | 1 tCO2 | G2Gt | [193] | |||
| 3 | Y | Y | 1 MWh | C2Gv | [194] | ||
| 4 | Y | 1 MWh | C2Gv | GF | [195] | ||
| 1 | Y | 1 MWh | C2Gt | [196] | |||
| 4 | Y | 1 MWh | C2Gt | [197] | |||

7.2.2. Environmental Impact Assessment of Carbon Capture and Utilization (CCU)
7.3. Socioeconomic and Sustainability Considerations
7.3.1. Job Creation
7.3.2. Energy Security
7.3.3. Public Acceptance
8. Case Studies and Pilot Projects
8.1. Overview of Prominent CCUS Projects Worldwide
Summary of U.S. Department of Energy (DOE) Sponsored Projects
8.2. Lessons Learned from Successful and Unsuccessful Projects
8.2.1. Success Factors
8.2.2. Challenges and Failures
8.3. Implications for Future Deployment
9. Research Gaps and Future Directions of CCUS
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Summary of CCUS Projects Worldwide
| No. | Project Name | Location | Start | Capacity | Description |
|---|---|---|---|---|---|
| 1 | Boundary Dam CCS | Saskatchewan, Canada | 2014 | 1 Mt/yr. | Captures CO2 emissions from a coal-fired power plant and stores them underground. |
| 2 | Petra Nova Carbon Capture | Texas, USA | 2017 | 1.6 Mt/yr. | Captures CO2 emissions from a coal-fired power plant and utilizes them for enhanced oil recovery. |
| 3 | Sleipner CCS | North Sea, Norway | 1996 | 20 M to date | Captures CO2 emissions from natural gas production and stores them underground. World’s first commercial CCS project. |
| 4 | Quest CCS | Alberta, Canada | 2015 | 1.1 Mt/yr. | Captures CO2 emissions from an oil sands upgrader and stores them underground. |
| 5 | Gorgon CCS | Western Australia | 2019 | 4 Mt/yr. | Captures CO2 emissions from a natural gas processing plant and stores them underground. |
| 6 | Weyburn-Midale CO2 | Saskatchewan, Canada | 2000 | 1.8 Mt/yr. | Involves the injection of captured CO2 into oil fields for secondary oil recovery. |
| 7 | In Salah Gas CCS | Algeria | 2004 | 17 Mt inj. | Captures and stores CO2 emissions from natural gas production. |
| 8 | Troll Gas CCS | North Sea, Norway | 1996 | 2 Mt | Captures CO2 emissions from a natural gas processing facility and stores them underground. |
| 9 | Decatur Carbon Capture | Illinois, USA | 2017 | 1 MT/yr. | Captures CO2 emissions from an ethanol production facility and stores them underground. |
| 10 | Mountaineer CCS Project | West Virginia, USA | 2009 | 0.1 Mt | A pilot project aimed to capture CO2 emissions from a coal-fired power plant for storage underground. |
| 11 | Saline Aquifer Storage Site Project | Otway Basin, Australia | 2008 | Research | Involves the injection of captured CO2 into a saline aquifer for storage and monitoring. |
| 12 | Southwest Regional Carbon Sequestration Partnership (SWP) Projects | USA | 2000 | variable capacities | A collaborative effort among industry, government, and research institutions to study and demonstrate carbon capture and storage in the southwestern United States. |
| 13 | Interstate Oil and Gas Compact Commission (IOGCC) CCS Projects | USA | A collaborative effort among states to promote and facilitate the development of CCS projects in the oil and gas industry. | ||
| 14 | Midwest Geological Sequestration Consortium (MGSC) Projects | USA | 2000 | variable capacities | A consortium focused on studying geological CO2 storage in the Midwest region of the United States. |
| 15 | Carbon Sequestration Leadership Forum (CSLF) Projects | International | 2003 | variable capacities | An international collaboration to advance CCS technologies and practices through knowledge sharing and research. |
| 16 | Alberta Carbon Trunk Line (ACTL) CCS Project | Alberta, Canada | 2020 | A project aimed at capturing CO2 emissions from industrial sources and transporting them via pipeline for enhanced oil recovery. | |
| 17 | Tomakomai CCS Demonstration Project | Hokkaido, Japan | 2016 | Involves capturing CO2 emissions from a hydrogen plant and storing them underground. | |
| 18 | CO2CRC Otway Project | Otway Basin, Australia | Involves the injection of captured CO2 into a saline aquifer for storage and monitoring. | ||
| 19 | SaskPower Boundary Dam CCS Project | Saskatchewan, Canada | 2014 | 1 Mt/yr. | Captures CO2 emissions from a coal-fired power plant and stores them underground. |
| 20 | Saline Aquifer Storage Site Project | Ketzin, Germany | 2008 | A research project aimed at studying the feasibility of storing CO2 in a saline aquifer formation. | |
| 21 | North West Redwater Sturgeon Refinery CCS Project | Alberta, Canada | The Sturgeon Refinery is one of the first refineries in the world designed from the ground up to incorporate carbon capture and storage (CCS) technology. | ||
| 22 | Hellisheidi CCS Project | Iceland | 2014 | 12,000 t/yr. | This project captures CO2 emissions from a geothermal power plant and stores them underground by mineralizing the CO2 into basalt rock. |
| 23 | Petrobras CO2 Injection Project | Brazil | 2010 | This project involves the injection of captured CO2 for enhanced oil recovery in offshore oil fields. First CCUS project in ultra-deep waters. Currently the largest CO2 injection project in the world (annual reinjection). | |
| 24 | Questerre Project | Alberta, Canada | Aimed at capturing and storing CO2 from shale gas production operations. | ||
| 25 | LaBarge CCS Project | Wyoming, USA | The project captures CO2 emissions from a natural gas processing plant and stores them underground in a saline aquifer. |
| No | Project Name | Location | Status | Capacity | Description |
|---|---|---|---|---|---|
| 1 | Northern Lights CCS Project | Norway | Currently in development | A full-chain CCS project aiming to capture CO2 emissions from industrial sources and store them offshore. | |
| 2 | Acorn CCS Project | Scotland, UK | A project aiming to develop a full-chain CCS system, including capture, transportation, and storage in depleted oil and gas fields. | ||
| 3 | Carson Hydrogen Power Plant CCS Project | Under development | Under development | Planned to capture CO2 emissions from a hydrogen production plant and store them underground. | |
| 4 | Carbon Capture Project | Utah, USA | Project ongoing | Aimed at capturing CO2 emissions from industrial sources for storage underground in deep saline formations. | |
| 5 | Carson CCS Project | California, USA | A project aimed at capturing CO2 emissions from a cement plant and storing them underground. | ||
| 6 | Val Verde CCS Project | Texas, USA | Aimed at capturing CO2 emissions from industrial sources for storage underground. | ||
| 7 | Huntly Power Station CCS Project | under consideration | Proposed project aiming to capture CO2 emissions from a power plant for storage underground. | ||
| 8 | Carlsbad CCS Project | New Mexico, USA | under development | Aimed at capturing CO2 emissions from industrial sources for storage underground. | |
| 9 | CarbonNet Project | Victoria, Australia | A project aiming to capture and store CO2 emissions from industrial sources in the Gippsland Basin. | ||
| 10 | Wabash Valley Resources CCS Project | Indiana, USA | Aimed at capturing CO2 emissions from a fertilizer plant for storage underground. | ||
| 11 | Netherlands—ROAD Project | Rotterdam, Netherlands | Aimed at establishing a CO2 transport and storage infrastructure to support emissions reduction in the Rotterdam area. | ||
| 12 | Porthos CCS Project | Rotterdam, Netherlands | A project aiming to develop a shared CO2 transport and storage infrastructure to reduce emissions in the region. | ||
| 13 | H21 North of England CCS Project | United Kingdom | A proposed project aiming to decarbonize industrial clusters in the north of England, utilizing CCS technology. | ||
| 14 | Amager Bakke CCS Project | Copenhagen, Denmark | 0.5 Mt/yr. | A project aiming to capture CO2 emissions from a waste-to-energy plant for storage underground. | |
| 15 | Tianjin CCS Project | Tianjin, China | Aimed at capturing CO2 emissions from a coal-fired power plant and storing them underground. | ||
| 16 | Project Tundra CCS Project | North Dakota, USA | final project development phase | Up to 4 million metric tons annually | A proposed project aiming to capture CO2 emissions from a coal-fired power plant for storage underground. |
| 17 | Tulsa Regional Carbon Capture & Sequestration (CCS) Project | Oklahoma, USA | A project aimed at studying the feasibility of CCS in the Tulsa region, focusing on industrial emissions. | ||
| 18 | Port Arthur CCS Project | Texas, USA | Planned as part of a refinery expansion project, aiming to capture and store around 1.5 million tonnes of CO2 per year underground. |
| No | Project Name | Location | Status | Capacity | Description |
|---|---|---|---|---|---|
| 1 | Natchez CCS Project | Mississippi, USA | canceled in 2017 | 1.5 Mt/yr. | Planned to capture CO2 emissions from a coal-fired power plant for storage underground. |
| 2 | Hydrogen Energy California (HECA) CCS Project | California, USA | canceled in 2017 | 2.5 Mt/yr. | Planned as an integrated gasification combined cycle (IGCC) coal-fired power plant with CCS for enhanced oil recovery. |
| 3 | Texas Clean Energy Project | Texas, USA | discontinued | 2.7 Mt/yr. | Originally planned as an IGCC coal-fired power plant with CCS for enhanced oil recovery. |
| 4 | Peterhead CCS Project | Scotland, UK | canceled in 2015 | Planned to capture CO2 emissions from a power plant and store them in depleted gas fields beneath the North Sea. | |
| 5 | Kemper County Energy Facility CCS Project | Mississippi, USA | Project transitioned to natural gas without CCS | 3.5 Mt/yr. | Originally intended as a coal gasification plant with integrated CCS for enhanced oil recovery. |
| Hazelwood CCS Project | Victoria, Australia | Proposed as a retrofit to a coal-fired power plant, aiming to capture CO2 emissions for storage underground. However, the project did not proceed beyond the planning stage. | |||
| Lake Charles CCS Project | Cancelled in October 2014 | 4.5 Mt/yr. |
References
- IEA Fact Sheet and Analysis. 2015. Available online: https://www.iea-shc.org/fact-sheets (accessed on 20 November 2023).
- DOE/NETL Advanced Carbon Dioxide R&D Program: Technology Update. 2011. (p. 118). National Energy Technology Laboratory. Available online: http://www.netl.doe.gov/technologies/coalpower/ewr/pubs/CO2Handbook (accessed on 26 November 2023).
- Morgan, A.; Grigg, R.; Ampomah, W. A Gate-to-Gate Life Cycle Assessment for the CO2-EOR Operations at Farnsworth Unit (FWU). Energies 2021, 14, 2499. [Google Scholar] [CrossRef]
- CO2CRC. Report, CO2CRC Annual, 2022/2023 20th Anniversary. 2023. Available online: https://co2crc.com.au/wp-content/uploads/2023/11/CO2CRC-Annual-Report-22-23.pdf (accessed on 17 January 2024).
- Brown, K.; Whittaker, S.; Wilson, M.; Srisang, W.; Smithson, H.; Tontiwachwuthikul, P. The history and development of the IEA GHG Weyburn-Midale CO2 Monitoring and Storage Project in Saskatchewan, Canada (the world largest CO2 for EOR and CCS program). Petroleum 2017, 3, 3–9. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-Industrial Levels and related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; IPPC: Geneva, Switzerland, 2018. [Google Scholar]
- GML. Carbon Cycle Greenhouse Gases. Available online: https://gml.noaa.gov/ccgg/ (accessed on 12 February 2024).
- National Oceanic and Atmospheric Administration (NOAA). Despite pandemic shutdowns, carbon dioxide and methane surged in 2020. Available online: https://research.noaa.gov/2021/04/07/despite-pandemic-shutdowns-carbon-dioxide-and-methane-surged-in-2020/ (accessed on 19 March 2024).
- IPCC Special Report on Carbon Dioxide Capture and Storage. Prepared by Working Group III of the Intergovernmental Panel on Climate Change. 2005. Available online: https://www.ipcc.ch/report/carbon-dioxide-capture-and-storage/ (accessed on 5 January 2024).
- NOAA, Global Monitoring Laboratory; Trends in atmospheric carbon dioxide (CO2). Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 20 February 2024).
- Nath, F.; Mahmood, M.N.; Yousuf, N. Recent advances in CCUS: A critical review on technologies, regulatory aspects and economics. Geoenergy Sci. Eng. 2024, 238, 212726. [Google Scholar] [CrossRef]
- Singh, J.D.; Wattal, D. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Front. Mar. Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Kelemen, P.; Benson, S.; Pilorgé, H.; Psarras, P.C.; Wilcox, J. An Overview of the Status and Challenges of CO2 Storage in Minerals and Geological Formations. Front. Clim. 2019, 1, 9. [Google Scholar] [CrossRef]
- IEA. Net zero by 2050, IEA, Paris. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 10 January 2024).
- Martin-Roberts, E.; Scott, V.; Flude, S.; Johnson, G.; Haszeldine, R.S.; Gilfillan, S. Carbon capture and storage at the end of a lost decade. One Earth 2021, 4, 1569–1584. [Google Scholar] [CrossRef]
- GCB. Country Chart Figures Contain Data on Land Use Emissions and Fossil Fuel Emissions. Global Carbon Budget. 2023. Available online: https://globalcarbonbudget.org/carbonbudget2023/ (accessed on 6 April 2024).
- Gale, J.; Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. Carbon Dioxide Capture and Storage. Intergovernmental Panel on Climate Change; IPPC: Geneva, Switzerland, 2005. [Google Scholar]
- Scott, V.; Gilfillan, S.; Markusson, N.; Chalmers, H.; Haszeldine, R.S. Last chance for carbon capture and storage. Nat. Clim. Chang. 2012, 3, 105–111. [Google Scholar] [CrossRef]
- Lu, J.; Wilkinson, M.; Haszeldine, R.S.; Fallick, A.E. Long-term performance of a mudrock seal in natural CO2 storage. Geology 2009, 37, 35–38. [Google Scholar] [CrossRef]
- Juanes, R.; Hager, B.H.; Herzog, H.J. No geologic evidence that seismicity causes fault leakage that would render large-scale carbon capture and storage unsuccessful. Proc. Natl. Acad. Sci. USA 2012, 109, 201215026. [Google Scholar] [CrossRef]
- Ampomah, W.; Acheampong, S.A.; McMillan, M.; Bratton, T.; El-Kaseeh, G. Development of a Machine Learning Assisted Framework in Calibrating In-situ Stress Changes Through Time-lapse Vertical Seismic Profiling. In Proceedings of the 16th International Conference on Greenhouse Gas Control Technologies GHGT-16, Lyon, France, 23–24 October 2022. [Google Scholar] [CrossRef]
- Global CCSInstitute. Global Status of CCS Report: 2020. Available online: https://www.globalccsinstitute.com/wp-content/uploads/2021/03/Global-Status-of-CCS-Report-English.pdf (accessed on 5 December 2023).
- Bachu, S. Screening and ranking of sedimentary basins for sequestration of CO2 in geological media in response to climate change. Environ. Geol. 2003, 44, 277–289. [Google Scholar] [CrossRef]
- Ma, J.; Li, L.; Wang, H.; Du, Y.; Ma, J.; Zhang, X.; Wang, Z. Carbon Capture and Storage: History and the Road Ahead. Engineering 2022, 14, 33–43. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.S.; Cather, M.; Will, R.; Gunda, D.; Dai, Z.; Soltanian, M.R. Optimum design of CO2 storage and oil recovery under geological uncertainty. Appl. Energy 2017, 195, 80–92. [Google Scholar] [CrossRef]
- IEA. CCUS in Clean Energy Transitions. Available online: https://www.iea.org/reports/ccus-in-clean-energy-transitions (accessed on 28 January 2024).
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Hong, W.Y. A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Sci. Technol. 2021, 3, 100044. [Google Scholar] [CrossRef]
- You, J.; Ampomah, W.; Morgan, A.; Sun, Q.; Huang, X. A comprehensive techno-eco-assessment of CO2 enhanced oil recovery projects using a machine-learning assisted workflow. Int. J. Greenh. Gas Control. 2021, 111, 103480. [Google Scholar] [CrossRef]
- Morgan, A.; Ampomah, W.; Grigg, R.; Dai, Z.; You, J.; Wang, S. Echno-economic life cycle assessment of CO2-EOR operations towards net negative emissions at farnsworth field unit. Fuel 2023, 342, 127897. [Google Scholar] [CrossRef]
- EPA. Current Class VI Projects under Review at EPA. U.S. Environmental Protection Agency. Available online: https://www.epa.gov/uic/current-class-vi-projects-under-review-epa (accessed on 28 June 2024).
- EPA. Table of EPA’s Draft and Final Class VI Well Permits. U.S. Environmental Protection Agency. Available online: https://www.epa.gov/uic/table-epas-draft-and-final-class-vi-well-permits (accessed on 30 April 2024).
- GCCSI. U.S. EPA Releases Class VI Well Permit Dashboard and Data Repository. Global CCS institute. Available online: https://www.globalccsinstitute.com/news-media/latest-news/u-s-epa-releases-class-vi-well-permit-dashboard-and-data-repository/ (accessed on 30 April 2024).
- CRC. Carbon TerraVault Provides 2023 Update. California Resources Corporation. Available online: https://crc.com/news/news-details/2024/Carbon-TerraVault-Provides-2023-Update/default.aspx (accessed on 30 April 2024).
- NETL. NETL-Supported Petra Nova Project Celebrates Three Years of Sustainable Operation. Available online: https://www.netl.doe.gov/node/9405 (accessed on 5 March 2024).
- Ampomah, W.; Balch, R.S.; Grigg, R.B.; Will, R.; Dai, Z.; White, M.D. Farnsworth Field CO2-EOR Project: Performance Case History. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016; Society of Petroleum Engineers: Tulsa, OK, USA, 2016; Volume 18. [Google Scholar] [CrossRef]
- Kheirinik, M.; Ahmed, S.; Rahmanian, N. Comparative Techno-Economic Analysis of Carbon Capture Processes: Pre-Combustion, Post-Combustion, and Oxy-Fuel Combustion Operations. Sustainability 2021, 13, 13567. [Google Scholar] [CrossRef]
- Kearns, D.; Liu, H.; Consoli, C. Technology readiness and costs of CCS. Glob. CCS Inst. 2021, 3, 6–43. [Google Scholar]
- Beck, L. Carbon capture and storage in the USA: The role of US innovation leadership in climate-technology commercialization. Clean Energy 2019, 4, 2–11. [Google Scholar] [CrossRef]
- C. Beumelburg. Heidelberg Cement’ Commitment to Carbon Abatement. Available online: https://www.heidelbergmaterials.com/en/sustainability/we-decarbonize-the-construction-industry (accessed on 12 April 2024).
- GCCSI. Global Status of CCS 2021. Available online: https://www.globalccsinstitute.com/wp-content/uploads/2021/10/2021-Global-Status-of-CCS-Report_Global_CCS_Institute.pdf (accessed on 7 March 2024).
- Gomilšek, R.; Čuček, L.; Homšak, M.; Tan, R.R.; Kravanja, Z. Carbon Emissions Constrained Energy Planning for Aluminum Products. Energies 2020, 13, 2753. [Google Scholar] [CrossRef]
- Worth, K.; White, D.; Chalaturnyk, R.; Sorensen, J.; Hawkes, C.; Rostron, B.; Johnson, J.; Young, A. Aquistore Project Measurement, Monitoring, and Verification: From Concept to CO2 Injection. Energy Procedia 2014, 63, 3202–3208. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Carbon Capture From Flue Gas and the Atmosphere: A Perspective. Front. Energy Res. 2020, 8, 560849. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Eide, L.I.; Bailey, D.W. Precombustion Decarbonisation Processes. Oil Gas Sci. Technol. 2005, 60, 475–484. [Google Scholar] [CrossRef]
- Zhai, H.; Rubin, E.S. Systems Analysis of Ionic Liquids for Post-combustion CO2 Capture at Coal-fired Power Plants. Energy Procedia 2014, 63, 1321–1328. [Google Scholar] [CrossRef]
- Rubin, E.S.; Mantripragada, H.; Marks, A.; Versteeg, P.; Kitchin, J. The outlook for improved carbon capture technology. Prog. Energy Combust. Sci. 2012, 38, 630–671. [Google Scholar] [CrossRef]
- Wienchol, P.; Szlęk, A.; Ditaranto, M. Waste-to-energy technology integrated with carbon capture—Challenges and opportunities. Energy 2020, 198, 117352. [Google Scholar] [CrossRef]
- Herzog, H.; Golomb, D. Carbon Capture and Storage from Fossil Fuel Use. Encycl. Energy 2004, 1, 277–287. [Google Scholar]
- López, R.; Fernández, C.; Martínez, O.; Sánchez, M.E. Techno-economic analysis of a 15 MW corn-rape oxy-combustion power plant. Fuel Process. Technol. 2016, 142, 296–304. [Google Scholar] [CrossRef]
- Portillo, E.; Alonso-Fariñas, B.; Vega, F.; Cano, M.; Navarrete, B. Alternatives for oxygen-selective membrane systems and their integration into the oxy-fuel combustion process: A review. SePurif. Technol. 2019, 229, 115708. [Google Scholar] [CrossRef]
- Ma, Y.; Liao, Y.; Su, Y.; Wang, B.; Yang, Y.; Ji, D.; Li, H.; Zhou, H.; Wang, D. Comparative Investigation of Different CO2 Capture Technologies for Coal to Ethylene Glycol Process. Processes 2021, 9, 207. [Google Scholar] [CrossRef]
- Rosa, L.; Sanchez, D.L.; Mazzotti, M. Assessment of carbon dioxide removal potential via BECCS in a carbon-neutral Europe. Energy Environ. Sci. 2021, 14, 3086–3097. [Google Scholar] [CrossRef]
- Ashkanani, H.E.; Wang, R.; Shi, W.; Siefert, N.S.; Thompson, R.L.; Smith, K.; Steckel, J.A.; Gamwo, I.K.; Hopkinson, D.; Resnik, K.; et al. Levelized Cost of CO2 Captured Using Five Physical Solvents in Pre-combustion Applications. Int. J. Greenh. Gas Control 2020, 101, 103135. [Google Scholar] [CrossRef]
- Abuelgasim, S.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2020, 764, 142892. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Linderholm, C.; Rydén, M.; Lyngfelt, A. Avoiding CO2 capture effort and cost for negative CO2 emissions using industrial waste in chemical-looping combustion/gasification of biomass. Mitig. Adapt. Strateg. Glob. Chang. 2019, 25, 1–24. [Google Scholar] [CrossRef]
- Rubin, E.S.; Davison, J.E.; Herzog, H.J. The cost of CO2 capture and storage. Int. J. Greenh. Gas Control 2015, 40, 378–400. [Google Scholar] [CrossRef]
- Joshi, A.; Shah, V.; Mohapatra, P.; Kumar, S.; Joshi, R.K.; Kathe, M.; Qin, L.; Tong, A.; Fan, L.-S. Chemical looping-A perspective on the next-gen technology for efficient fossil fuel utilization. Adv. Appl. Energy 2021, 3, 100044. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Yang, Q.; Yao, M.; Wu, Y.-N.; Gu, Y. Direct air capture of CO2 in designed metal-organic frameworks at lab and pilot scale. Carbon Capture Sci. Technol. 2023, 9, 100145. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef]
- Li, B.; Duan, Y.; Luebke, D.; Morreale, B. Advances in CO2 capture technology: A patent review. Appl. Energy 2013, 102, 1439–1447. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Dubey, A.; Arora, A. Advancements in carbon capture technologies: A review. J. Clean. Prod. 2022, 373, 133932. [Google Scholar] [CrossRef]
- Chamwudhiprecha, N.; Blunt, M.J. CO2 Storage Potential in the North Sea. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 15 November 2011. [Google Scholar] [CrossRef]
- Otitoju, O.; Oko, E.; Wang, M. Technical and economic performance assessment of post-combustion carbon capture using piperazine for large scale natural gas combined cycle power plants through process simulation. Appl. Energy 2021, 292, 116893. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Xu, G.; Liang, F.; Yang, Y.; Hu, Y.; Zhang, K.; Liu, W. An Improved CO2 Separation and Purification System Based on Cryogenic Separation and Distillation Theory. Energies 2014, 7, 3484–3502. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Kuo, C.-C.; Yang, M.-W.; Zhuang, Z.-Y.; Lin, P.-W.; Chen, Y.-F.; Yang, H.-S.; Chou, C.-T. CO2 Capture from Flue Gas of a Coal-Fired Power Plant Using Three-Bed PSA Process. Energies 2021, 14, 3582. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Seoane, B.; Coronas, J.; Gascon, I.; Benavides, M.E.; Karvan, O.; Caro, J.; Kapteijn, F.; Gascon, J. Metal–organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture? Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar] [CrossRef]
- Plaza, M.G.; García, S.; Rubiera, F.; Pis, J.J.; Pevida, C. Post-combustion CO2 capture with a commercial activated carbon: Comparison of different regeneration strategies. Chem. Eng. J. 2010, 163, 41. [Google Scholar] [CrossRef]
- Jiang, N.; Shen, Y.; Liu, B.; Zhang, D.; Tang, Z.; Li, G.; Fu, B. CO2 capture from dry flue gas by means of VPSA, TSA and TVSA. J. CO2 Util. 2020, 35, 153–168. [Google Scholar] [CrossRef]
- Yuan, Z.; Eden, M.R.; Gani, R. Toward the Development and Deployment of Large-Scale Carbon Dioxide Capture and Conversion Processes. Ind. Eng. Chem. Res. 2015, 55, 3383–3419. [Google Scholar] [CrossRef]
- Zhao, L.; Riensche, E.; Blum, L.; Stolten, D. Multi-stage gas separation membrane processes used in post-combustion capture: Energetic and economic analyses. J. Memb. Sci. 2010, 359, 160–172. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- He, X.; Fu, C.; Hägg, M.-B. Membrane system design and process feasibility analysis for CO2 capture from flue gas with a fixed-site-carrier membrane. Chem. Eng. J. 2015, 268, 1–9. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Safdarnejad, S.M.; Hedengren, J.D.; Baxter, L.L. Dynamic optimization of a hybrid system of energy-storing cryogenic carbon capture and a baseline power generation unit. Appl. Energy 2016, 172, 66–79. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Lacerda, L.M.C.F.; Franco, T.T. Biomass production and carbon dioxide fixation by Aphanothece microscopica Nägeli in a bubble column photobioreactor. Biochem. Eng. J. 2008, 40, 27–34. [Google Scholar] [CrossRef]
- Maurice, N. Bioenergy Production: Opportunities for Microorganisms (Part I). Bioenergy Res. Commer. Oppor. Chall. 2021, 43–89. [Google Scholar] [CrossRef]
- Hosseini, N.S.; Shang, H.; Scott, J.A. Biosequestration of industrial off-gas CO2 for enhanced lipid productivity in open microalgae cultivation systems. Renew. Sustain. Energy Rev. 2018, 92, 458–469. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- WRI. Guidelines for Carbon Dioxide Capture, Transport, and Storage. World Resource Institue. Available online: https://www.wri.org/research/guidelines-carbon-dioxide-capture-transport-and-storage (accessed on 25 May 2024).
- NPC. Meeting the Dual Challenge A Roadmap to At-Scale Deployment of Carbon Capture, Use, and Storage. National Petroleum Council Report. Available online: https://dualchallenge.npc.org/ (accessed on 9 March 2024).
- GCCSI. The Costs of CO2 Transport: Post-Demonstration CCS in the EU. Global CCS Institute. Available online: https://www.globalccsinstitute.com/resources/publications-reports-research/the-costs-of-co2-transport-post-demonstration-ccs-in-the-eu/ (accessed on 15 May 2024).
- N. P. Council. Enhanced_Oil_Recovery. Library of Congress No. 84-061296, Washington, DC. Available online: https://www.academia.edu/117000967/Enhanced_Oil_Recovery_Field_Case_Studies (accessed on 20 March 2024).
- IEA. The Future of Petrochemicals. Available online: https://www.iea.org/reports/the-future-of-petrochemicals (accessed on 7 December 2023).
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Johansson, T.; Williams, R.; Ishitani, H.; A Edmonds, J. Options for reducing CO2 emissions from the energy supply sector. Energy Policy 1996, 24, 985–1003. [Google Scholar] [CrossRef]
- Yun, X.; Soo, D.; Joo, J.; Lee, C.; Wu, W.-Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. Advancements in CO2 capture by absorption and adsorption: A comprehensive review. J. CO2 Util. 2024, 81, 102727. [Google Scholar] [CrossRef]
- Liu, E.; Lu, X.; Wang, D. A Systematic Review of Carbon Capture, Utilization and Storage: Status, Progress and Challenges. Energies 2023, 16, 2865. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef]
- IEA. Putting CO2 to Use. IEA. Available online: https://www.iea.org/reports/putting-co2-to-use (accessed on 3 June 2024).
- Patricio, J.; Angelis-Dimakis, A.; Castillo-Castillo, A.; Kalmykova, Y.; Rosado, L. Method to identify opportunities for CCU at regional level—Matching sources and receivers. J. CO2 Util. 2017, 22, 330–345. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. The changing paradigm in CO2 utilization. J. CO2 Util. 2013, 3, 65–73. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Becker, C.; Kläring, H.-P. CO2 enrichment can produce high red leaf lettuce yield while increasing most flavonoid glycoside and some caffeic acid derivative concentrations. Food Chem. 2015, 199, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Pappijn, C.A.R.; Ruitenbeek, M.; Reyniers, M.-F.; Van Geem, K.M. Challenges and Opportunities of Carbon Capture and Utilization: Electrochemical Conversion of CO2 to Ethylene. Front. Energy Res. 2020, 8, 557466. [Google Scholar] [CrossRef]
- Azarabadi, H.L.; Klaus, S. A sorbent-focused techno-economic analysis of direct air capture. Appl. Energy 2019, 250, 959–975. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Comans, R.N.J.; Witkamp, G.-J. Cost evaluation of CO2 sequestration by aqueous mineral carbonation. Energy Convers. Manag. 2007, 48, 1923–1935. [Google Scholar] [CrossRef]
- Adu-Gyamfi, B.; Ampomah, W.; Tu, J.; Sun, Q.; Erzuah, S.; Acheampong, S. Assessment of chemo-mechanical impacts of CO2 sequestration on the caprock formation in Farnsworth oil field, Texas. Sci. Rep. 2022, 12, 13023. [Google Scholar] [CrossRef]
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1644. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Kiwalabye, J.; Junin, R.; Augustine, A. A review of gas enhanced oil recovery schemes used in the North Sea. J. Pet. Explor. Prod. Technol. 2018, 8, 1373–1387. [Google Scholar] [CrossRef]
- Belhaj, H.; Abukhalifeh, H.; Javid, K. Miscible oil recovery utilizing N2 and/or HC gases in CO2 injection. J. Pet. Sci. Eng. 2013, 111, 144–152. [Google Scholar] [CrossRef]
- Pourhadi, S.; Fath, A.H. Performance of the injection of different gases for enhanced oil recovery in a compositionally grading oil reservoir. J. Pet. Explor. Prod. Technol. 2020, 10, 641–661. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wang, F.; Wu, J.; Tahir, M.U.; Li, Q.; Yuan, L.; Liu, Z. Effect of thickener and reservoir parameters on the filtration property of CO2 fracturing fluid. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 42, 1705–1715. [Google Scholar] [CrossRef]
- Choubineh, A.; Helalizadeh, A.; Wood, D.A. The impacts of gas impurities on the minimum miscibility pressure of injected CO2-rich gas–crude oil systems and enhanced oil recovery potential. Pet. Sci. 2019, 16, 117–126. [Google Scholar] [CrossRef]
- Khabibullin, R.; Ltd, A.E.; Abu Grin, Z.; Ltd, R.O.; Alkan, H.; Grivet, M.; Elgridi, K. Investigation of CO2 Application for Enhanced Oil Recovery in a North African Field—A New Approach to EOS Development. In Proceedings of the IOR 2017—19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017. [Google Scholar] [CrossRef]
- Wang, X.; Luo, P.; Er, V.; Huang, S.-S.S. Assessment of CO2 Flooding Potential for Bakken Formation, Saskatchewan. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, SPE-137728-MS, Calgary, AB, Canada, 19 October 2010. [Google Scholar] [CrossRef]
- Vicencio, O.; Sepehrnoori, K.; Miller, M. Simulation of Nitrogen Injection into Naturally Fractured Reservoirs. In Proceedings of the SPE International Petroleum Conference in Mexico, Puebla Pue, Mexico, 7 November 2004. SPE-92110-MS. [Google Scholar] [CrossRef]
- IEA. Storing CO2 through Enhanced Oil Recovery. IEA. Available online: https://www.iea.org/reports/storing-co2-through-enhanced-oil-recovery (accessed on 3 March 2024).
- Li, Q.; Li, Y.; Cheng, Y.; Li, Q.; Wang, F.; Wei, J.; Liu, Y.; Zhang, C.; Song, B.; Yan, C.; et al. Numerical simulation of fracture reorientation during hydraulic fracturing in perforated horizontal well in shale reservoirs. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 1807–1813. [Google Scholar] [CrossRef]
- Ross, D.J.; Bustin, R.M. Shale gas potential of the Lower Jurassic Gordondale Member, northeastern British Columbia, Canada. Bull. Can. Pet. Geol. 2007, 55, 51–75. [Google Scholar] [CrossRef]
- Sun, Q.; Ampomah, W.; Kutsienyo, E.J.; Appold, M.; Adu-Gyamfi, B.; Dai, Z.; Soltanian, M.R. Assessment of CO2 trapping mechanisms in partially depleted oil-bearing sands. Fuel 2020, 278, 118356. [Google Scholar] [CrossRef]
- Bachu, S. Carbon dioxide storage capacity in uneconomic coal beds in Alberta, Canada: Methodology, potential and site identification. Int. J. Greenh. Gas Control 2007, 1, 374–385. [Google Scholar] [CrossRef]
- Hovorka, S.D.; Benson, S.M.; Doughty, C.; Freifeld, B.M.; Sakurai, S.; Daley, T.M.; Kharaka, Y.K.; Holtz, M.H.; Trautz, R.C.; Nance, H.S.; et al. Measuring permanence of CO2 storage in saline formations: The Frio experiment. Environ. Geosci. 2006, 13, 105–121. [Google Scholar] [CrossRef]
- Gislason, S.R.; Wolff-Boenisch, D.; Stefansson, A.; Oelkers, E.H.; Gunnlaugsson, E.; Sigurdardottir, H.; Sigfusson, B.; Broecker, W.S.; Matter, J.M.; Stute, M.; et al. Mineral sequestration of carbon dioxide in basalt: A pre-injection overview of the CarbFix project. Int. J. Greenh. Gas Control 2010, 4, 537–545. [Google Scholar] [CrossRef]
- de Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; de Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Bock, B.; Rhudy, R.; Herzog, H.; Klett, M.; Davison, J.; Simbeck, D. Economic Evaluation of CO2 Storage and Sink Enhancement Options. Available online: https://www.osti.gov/servlets/purl/826435 (accessed on 9 May 2024).
- Bachu, S. Review of CO2 storage efficiency in deep saline aquifers. Int. J. Greenh. Gas Control 2015, 40, 188–202. [Google Scholar] [CrossRef]
- de Coninck, H.; Benson, S.M. Carbon Dioxide Capture and Storage: Issues and Prospects. Annu. Rev. Environ. Resour. 2014, 39, 243–270. [Google Scholar] [CrossRef]
- Hepple, R.P.; Benson, S.M. Geologic storage of carbon dioxide as a climate change mitigation strategy: Performance requirements and the implications of surface seepage. Env. Geol. 2005, 47, 576–585. [Google Scholar] [CrossRef]
- Barry, J.P.; Buck, K.R.; Lovera, C.F.; Kuhnz, L.; Whaling, P.J.; Peltzer, E.T.; Walz, P.; Brewer, P.G. Effects of Direct Ocean CO2 Injection on Deep-Sea Meiofauna. J. Oceanogr. 2004, 60, 759–766. [Google Scholar] [CrossRef]
- Fan, C.; Elsworth, D.; Li, S.; Chen, Z.; Luo, M.; Song, Y.; Zhang, H. Modelling and optimization of enhanced coalbed methane recovery using CO2/N2 mixtures. Fuel 2019, 253, 1114–1129. [Google Scholar] [CrossRef]
- White, C.M.; Smith, D.H.; Jones, K.L.; Goodman, A.L.; Jikich, S.A.; LaCount, R.B.; DuBose, S.B.; Ozdemir, E.; Morsi, B.I.; Schroeder, K.T. Sequestration of Carbon Dioxide in Coal with Enhanced Coalbed Methane RecoveryA Review. Energy Fuels 2005, 19, 659–724. [Google Scholar] [CrossRef]
- Koperna, G.J.; Riestenberg, D.E. Carbon Dioxide Enhanced Coalbed Methane and Storage: Is There Promise? In Proceedings of the SPE International Conference on CO2 Capture, Storage, and Utilization, San Diego, California, USA, November 2009. [CrossRef]
- Godec, M.; Koperna, G.; Gale, J. CO2-ECBM: A Review of its Status and Global Potential. Energy Procedia 2014, 63, 5858–5869. [Google Scholar] [CrossRef]
- Reeves, S.R. An overview of CO2-ECBM and sequestration in coal seams. In Carbon Dioxide Sequestration in Geological Media—State of the Science: AAPG Studies in Geology; Grobe, M., Pashin, J.C., Dodge, R.L., Eds.; AAPG: Tulsa, OK, USA, 2009; Volume 59, pp. 17–32. [Google Scholar] [CrossRef]
- Mukherjee, M.; Misra, S. A review of experimental research on Enhanced Coal Bed Methane (ECBM) recovery via CO2 sequestration. Earth-Sci. Rev. 2018, 179, 392–410. [Google Scholar] [CrossRef]
- Taillefert, A.; Reeves, S. Screening Model for ECBM Recovery and CO2 Sequestration in Coal. 2003. Available online: https://adv-res.com/pdf/Screening%20Model%20for%20ECBM%20Recovery%20and%20CO2%20Sequestration%20-%20Topical%20Report%20-%20Coal-Seq%20V1.0%20Manual.pdf (accessed on 20 June 2024).
- Zhang, X.; Ranjith, P.G. Experimental investigation of effects of CO2 injection on enhanced methane recovery in coal seam reservoirs. J. CO2 Util. 2019, 33, 394–404. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.H. Carbon Storage in Basalt. Science 2014, 344, 373–374. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Park, A.-H.A.; Fan, L.-S. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Rau, G.H.; Knauss, K.G.; Langer, W.H.; Caldeira, K. Reducing energy-related CO2 emissions using accelerated weathering of limestone. Energy 2007, 32, 1471–1477. [Google Scholar] [CrossRef]
- Williamson, P.; Wallace, D.W.; Law, C.S.; Boyd, P.W.; Collos, Y.; Croot, P.; Denman, K.; Riebesell, U.; Takeda, S.; Vivian, C. Ocean fertilization for geoengineering: A review of effectiveness, environmental impacts and emerging governance. Process Saf. Environ. Prot. 2012, 90, 475–488. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F.; Lackner, K.S. Permanent carbon dioxide storage in deep-sea sediments. Proc. Natl. Acad. Sci. USA. 2006, 103, 12291–12295. [Google Scholar] [CrossRef]
- Matter, J.M.; Stute, M.; Hall, J.; Mesfin, K.; Snæbjörnsdóttir, S.; Gislason, S.; Oelkers, E.; Sigfusson, B.; Gunnarsson, I.; Aradottir, E.; et al. Monitoring permanent CO2 storage by in situ mineral carbonation using a reactive tracer technique. Energy Procedia 2014, 63, 4180–4185. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; De Coninck, H.C.; Loos, M.; Meyer, L. Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Godec, M.; Kuuskraa, V.; Van Leeuwen, T.; Melzer, S. The Role of CCUS in Low-Carbon Power Systems; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2020. [Google Scholar] [CrossRef]
- B. C. A. Resource. Low Carbon Fuel Standard. CA.Gov. Available online: https://ww2.arb.ca.gov/our-work/programs/low-carbon-fuel-standard (accessed on 14 May 2024).
- E. Commission. EU Emissions Trading System (EU ETS). Energy, Climate change, Environment. Available online: https://climate.ec.europa.eu/eu-action/eu-emissions-trading-system-eu-ets_en (accessed on 3 April 2024).
- Davis, S.J.; Lewis, N.S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo, I.L.; Benson, S.M.; Bradley, T.; Brouwer, J.; Chiang, Y.-M.; et al. Net-zero emissions energy systems. Science 2018, 360, eaas9793. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Yao, J.; Han, H.; Yang, Y.; Song, Y.; Li, G. A Review of Recent Progress of Carbon Capture, Utilization, and Storage (CCUS) in China. Applied Sciences 2023, 13, 1169. [Google Scholar] [CrossRef]
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef]
- UNFCCC. The Paris Agreement. United Nations Climate Change. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement (accessed on 2 March 2024).
- Simonis, U. Caching the carbon. The politics and policy of carbon capture and storage. Environ. Polit. Env. POLIT. 2011, 20, 602–604. [Google Scholar] [CrossRef]
- ZeThe Zero Emissions Platform. Zero Emission Platform. Available online: https://zeroemissionsplatform.eu/ (accessed on 1 May 2024).
- UNFCCC. UN Climate Change Conference—United Arab Emirates. United Nations Climate Change. Available online: https://unfccc.int/cop28 (accessed on 25 March 2024).
- C. UAE. COP28 UAE. Available online: https://www.cop28.com/en/ (accessed on 20 April 2024).
- Gov.Uk. The Carbon Capture and Storage Infrastructure Fund: An Update on Its Design. Gov.UK. Available online: https://www.gov.uk/government/publications/design-of-the-carbon-capture-and-storage-ccs-infrastructure-fund/the-carbon-capture-and-storage-infrastructure-fund-an-update-on-its-design-accessible-webpage (accessed on 12 May 2024).
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Government. Safeguard Mechanism. Clean Energy Regulator. Available online: https://cer.gov.au/schemes/safeguard-mechanism (accessed on 16 April 2024).
- BloombergNEF. Corporate Clean Energy Buying Grew 18% in 2020, Despite Mountain of Adversity. BloombergNEF. Available online: https://about.bnef.com/blog/corporate-clean-energy-buying-grew-18-in-2020-despite-mountain-of-adversity/ (accessed on 7 January 2024).
- Herzog, H.; Meldon, J.; Hatton, A. Advanced Post-Combustion CO2 Capture. Clean Air Task Force. 2009. Available online: https://www.researchgate.net/publication/265454631_Advanced_Post-Combustion_CO_2_Capture (accessed on 26 February 2024).
- Middleton, R.S.; Eccles, J.K. The complex future of CO2 capture and storage: Variable electricity generation and fossil fuel power. Energy Procedia 2013, 108, 66–73. [Google Scholar] [CrossRef]
- Benson, S. Carbon Dioxide Sequestration in Deep Sedimentary Formations. 2012. Available online: https://www.geo.arizona.edu/~reiners/geos195K/CO2Sequestration_Benson_ELEMENTS.pdf (accessed on 12 May 2024).
- N. P. Council. Meeting the Dual Challenge. National Petroleum Council Report. Available online: https://dualchallenge.npc.org/ (accessed on 17 March 2024).
- WorldBank. State and Trends of Carbon Pricing 2022. Available online: https://openknowledge.worldbank.org/entities/publication/a1abead2-de91-5992-bb7a-73d8aaaf767f (accessed on 19 June 2024).
- DOE. FY 2022 Budget Justification. Available online: https://www.energy.gov/cfo/articles/fy-2022-budget-justification (accessed on 2 February 2024).
- GCCSI. Global Status of CCS 2023—Report & Executive Summary. Global CCS Institute. Available online: https://www.globalccsinstitute.com/resources/publications-reports-research/global-status-of-ccs-2023-executive-summary/ (accessed on 13 April 2024).
- BP. BP Sustainability. BP. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/sustainability/group-reports/bp-esg-datasheet-2023.pdf (accessed on 12 May 2024).
- Chevron. chevron Invests in Carbon Capture and Removal Technology Company, Svante. Chevron. Available online: https://www.chevron.com/newsroom/2022/q4/chevron-invests-in-carbon-capture-and-removal-technology-company-svante (accessed on 9 January 2024).
- ExxonMobil. ExxonMobil Low Carbon Solutions to Commercialize Emission-Reduction Technology. ExxonMobil. Available online: https://corporate.exxonmobil.com/news/news-releases/2021/0201_exxonmobil-low-carbon-solutions-to-commercialize-emission-reduction-technology (accessed on 19 May 2024).
- PitchBook. CarbonCapture Overview. PitchBook. Available online: https://pitchbook.com/profiles/company/468036-91 (accessed on 4 February 2024).
- Equinor. The Northern Lights Project. Equinor. Available online: https://www.equinor.com/energy/northern-lights (accessed on 12 December 2023).
- CEM. Carbon Capture Utilization And Storage. Clean Energy Ministries. Available online: https://www.cleanenergyministerial.org/initiatives-campaigns/carbon-capture-utilization-and-storage/ (accessed on 8 December 2023).
- BlackRock. Investment Implication of U.S. Transition Policy. BlackRock. Available online: https://www.blackrock.com/corporate/insights/blackrock-investment-institute/publications/us-transition-policy-implications#:~:text=Investment%20implication%20of%20U.S.%20transition,other%20drivers%2C%20like%20monetary%20policy. (accessed on 14 March 2024).
- Oxyburgh. Lowest Cost Decarbonisation for the UK: The Critical Role of CCS. British Institute of Energy Economics. Available online: https://stemm-ccs.eu/sites/stemm-ccs/files/documents/Parliamentary_Advisory_Group_on_CCS_-_Final_report.PDF (accessed on 10 April 2024).
- Aresta, M.; Caroppo, A.; Dibenedetto, A.; Narracci, M. Life Cycle Assessment (LCA) Applied to the Synthesis of Methanol. Comparison of the Use of Syngas with the Use of CO2 and Dihydrogen Produced from Renewables BT—Environmental Challenges and Greenhouse Gas Control for Fossil Fuel Utilization in the 21st; Maroto-Valer, M.M., Song, C., Soong, Y., Eds.; Springer: Boston, MA, USA, 2002; pp. 331–347. [Google Scholar] [CrossRef]
- Porthos. Porthos Project Overview. Porthos CO2 Transport and Storage. Available online: https://www.porthosco2.nl/en/project/ (accessed on 18 November 2023).
- Spigarelli, B.P.; Kawatra, S.K. Opportunities and challenges in carbon dioxide capture. J. CO2 Util. 2013, 1, 69–87. [Google Scholar] [CrossRef]
- Marx, J.; Schreiber, A.; Zapp, P.; Haines, M.; Hake, J.-F.; Gale, J. Environmental evaluation of CCS using Life Cycle Assessment–A synthesis report. Energy Procedia 2011, 4, 2448–2456. [Google Scholar] [CrossRef]
- Zoback, M.D.; Gorelick, S.M. Earthquake triggering and large-scale geologic storage of carbon dioxide. Proc. Natl. Acad. Sci. USA 2012, 109, 10164–10168. [Google Scholar] [CrossRef]
- Manuilova, A.; Koiwanit, J.; Piewkhaow, L.; Wilson, M.; Chan, C.; Tontiwachwuthikul, P. Life Cycle Assessment of Post-Combustion CO2 Capture and CO2-Enhanced Oil Recovery based on the Boundary Dam Integrated Carbon Capture and Storage Demonstration Project in Saskatchewan. Energy Procedia 2014, 63, 7398. [Google Scholar] [CrossRef]
- Klüppel, H.J. ISO 14041: Environmental management—Life cycle assessment—Goal and scope definition—Inventory analysis. Int. J. Life Cycle Assess. 1998, 3, 301. [Google Scholar] [CrossRef]
- Bras-Klapwijk, R. Procedures and tools for generating and selecting alternatives in LCA. Int. J. Life Cycle Assess. 2003, 8, 266–272. [Google Scholar] [CrossRef]
- Volkart, K.; Bauer, C.; Boulet, C. Life cycle assessment of carbon capture and storage in power generation and industry in Europe. Int. J. Greenh. Gas Control 2013, 16, 91–106. [Google Scholar] [CrossRef]
- Tang, L.; Yokoyama, T.; Kubota, H.; Shimota, A. Life Cycle Assessment of a Pulverized Coal-fired Power Plant with CCS Technology in Japan. Energy Procedia 2014, 63, 7437–7443. [Google Scholar] [CrossRef]
- Zhang, X.; Singh, B.; He, X.; Gundersen, T.; Deng, L.; Zhang, S. Post-combustion carbon capture technologies: Energetic analysis and life cycle assessment. Int. J. Greenh. Gas Control 2014, 27, 289–298. [Google Scholar] [CrossRef]
- Grant, T.; Anderson, C.; Hooper, B. Comparative life cycle assessment of potassium carbonate and monoethanolamine solvents for CO2 capture from post combustion flue gases. Int. J. Greenh. Gas Control 2014, 28, 35–44. [Google Scholar] [CrossRef]
- Schakel, W.; Meerman, H.; Talaei, A.; Ramírez, A.; Faaij, A. Comparative life cycle assessment of biomass co-firing plants with carbon capture and storage. Appl. Energy 2014, 131, 441–467. [Google Scholar] [CrossRef]
- Troy, S.; Schreiber, A.; Zapp, P. Life cycle assessment of membrane-based carbon capture and storage. Clean Technol. Environ. Policy 2016, 18, 1641–1654. [Google Scholar] [CrossRef]
- Asante-Okyere, S.; Daqing, T.; Enemuoh, E.; Kwofie, S. Life Cycle Assessment of Supercritical Coal Power Plant With Carbon Capture and Sequestration in Chin. Asian J. Environ. Ecol. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Petrescu, L.; Bonalumi, D.; Valenti, G.; Cormos, A.-M.; Cormos, C.-C. Life Cycle Assessment for supercritical pulverized coal power plants with post-combustion carbon capture and storage. J. Clean. Prod. 2017, 157, 10–21. [Google Scholar] [CrossRef]
- Petrescu, L.; Cormos, C.-C. Environmental assessment of IGCC power plants with pre-combustion CO2 capture by chemical & calcium looping methods. J. Clean. Prod. 2017, 158, 233–244. [Google Scholar] [CrossRef]
- Giordano, L.; Roizard, D.; Favre, E. Life cycle assessment of post-combustion CO2 capture: A comparison between membrane separation and chemical absorption processes. Int. J. Greenh. Gas Control 2018, 68, 146–163. [Google Scholar] [CrossRef]
- Navajas, A.; Mendiara, T.; Goñi, V.; Jiménez, A.; Gandía, L.M.; Abad, A.; García-Labiano, F.; de Diego, L.F. Life cycle assessment of natural gas fuelled power plants based on chemical looping combustion technology. Energy Convers. Manag. 2019, 198, 111856. [Google Scholar] [CrossRef]
- Yang, B.; Wei, Y.-M.; Hou, Y.; Li, H.; Wang, P. Life cycle environmental impact assessment of fuel mix-based biomass co-firing plants with CO2 capture and storage. Appl. Energy 2019, 252, 113483. [Google Scholar] [CrossRef]
- Young, B.; Krynock, M.; Carlson, D.; Hawkins, T.R.; Marriott, J.; Morelli, B.; Jamieson, M.; Cooney, G.; Skone, T.J. Comparative environmental life cycle assessment of carbon capture for petroleum refining, ammonia production, and thermoelectric power generation in the United States. Int. J. Greenh. Gas Control 2019, 91, 102821. [Google Scholar] [CrossRef]
- Zang, G.; Zhang, J.; Jia, J.; Lora, E.S.; Ratner, A. Life cycle assessment of power-generation systems based on biomass integrated gasification combined cycles. Renew. Energy 2020, 149, 336–346. [Google Scholar] [CrossRef]
- Wildboiz, C. Life Cycle Assessment of Selected Technologies for CO2 Transport and Sequestration. Institute of Environmental Engineering (IfU). 2007. Available online: https://doka.ch/CCSDiplomaWildbolz07.pdf (accessed on 14 May 2024).
- Nie, Z.; Korre, A.; Durucan, S. Life cycle modelling and comparative assessment of the environmental impacts of oxy-fuel and post-combustion CO2 capture, transport and injection processes. Energy Procedia 2011, 4, 2510–2517. [Google Scholar] [CrossRef]
- Koornneef, J.; van Keulen, T.; Faaij, A.; Turkenburg, W. Life cycle assessment of a pulverized coal power plant with post-combustion capture, transport and storage of CO2. Int. J. Greenh. Gas Control 2008, 2, 448–467. [Google Scholar] [CrossRef]
- Odeh, N.A.; Cockerill, T.T. Life cycle GHG assessment of fossil fuel power plants with carbon capture and storage. Energy Policy 2008, 36, 367–380. [Google Scholar] [CrossRef]
- You, J.; Ampomah, W.; Sun, Q.; Kutsienyo, E.J.; Balch, R.S.; Dai, Z.; Cather, M.; Zhang, X. Machine learning based co-optimization of carbon dioxide sequestration and oil recovery in CO2-EOR project. J. Clean. Prod. 2020, 260, 120866. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.S.; Grigg, R.B.; McPherson, B.; Will, R.A.; Lee, S.; Dai, Z.; Pan, F. Co-optimization of CO2-EOR and storage processes in mature oil reservoirs. Greenh. Gases Sci. Technol. 2017, 7, 128–142. [Google Scholar] [CrossRef]
- Khoo, H.H.; Bu, J.; Wong, R.L.; Kuan, S.Y.; Sharratt, P.N. Carbon capture and utilization: Preliminary life cycle CO2, energy, and cost results of potential mineral carbonation. Energy Procedia 2011, 4, 2494–2501. [Google Scholar] [CrossRef]
- Azzolina, N.A.; Hamling, J.A.; Peck, W.D.; Gorecki, C.D.; Nakles, D.V.; Melzer, L.S. A Life Cycle Analysis of Incremental Oil Produced via CO2 EOR. Energy Procedia 2017, 114, 6588–6596. [Google Scholar] [CrossRef]
- Hertwich, E.G.; Aaberg, M.; Singh, B.; Strømman, A.H. Life-cycle Assessment of Carbon Dioxide Capture for Enhanced Oil Recovery. Chinese J. Chem. Eng. 2008, 16, 343–353. [Google Scholar] [CrossRef]
- Morgan, A.; Akanji, L.; Udoh, T.; Mohammed, S.; Anumah, P.; Kyeremeh, S.J. Fractional Flow Behaviour and Transport Mechanisms During Low salinity and Protein Enzyme Bio-surfactant EOR Flooding. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Virtual, 11–13 August 2020; p. D013S009R002. [Google Scholar] [CrossRef]
- Lardon, L.; Hélias, A.; Sialve, B.; Steyer, J.-P.; Bernard, O. Life-Cycle Assessment of Biodiesel Production from Microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [Google Scholar] [CrossRef]
- Stephenson, A.L.; Kazamia, E.; Dennis, J.S.; Howe, C.J.; Scott, S.A.; Smith, A.G. Life-Cycle Assessment of Potential Algal Biodiesel Production in the United Kingdom: A Comparison of Raceways and Air-Lift Tubular Bioreactors. Energy Fuels 2010, 24, 4062–4077. [Google Scholar] [CrossRef]
- Borkowski, M.G.; Zaimes, G.G.; Khanna, V. Integrating LCA and thermodynamic analysis for sustainability assessment of algal biofuels: Comparison of renewable diesel vs. biodiesel. In Proceedings of the 2012 IEEE International Symposium on Sustainable Systems and Technology (ISSST), Boston, MA, USA, 16–18 May 2012; pp. 1–6. [Google Scholar] [CrossRef]
- Azzolina, N.A.; Peck, W.D.; Hamling, J.A.; Gorecki, C.D.; Ayash, S.C.; Doll, T.E.; Nakles, D.V.; Melzer, L.S. How green is my oil? A detailed look at greenhouse gas accounting for CO2-enhanced oil recovery (CO2-EOR) sites. Int. J. Greenh. Gas Control 2016, 51, 369. [Google Scholar] [CrossRef]
- Sminchak, J.R.; Mawalkar, S.; Gupta, N. Large CO2 Storage Volumes Result in Net Negative Emissions for Greenhouse Gas Life Cycle Analysis Based on Records from 22 Years of CO2-Enhanced Oil Recovery Operations. Energy Fuels 2020, 34, 3566–3577. [Google Scholar] [CrossRef]
- Nduagu, E.; Romão, I.; Fagerlund, J.; Zevenhoven, R. Performance assessment of producing Mg(OH)2 for CO2 mineral sequestration. Appl. Energy 2013, 106, 116–126. [Google Scholar] [CrossRef]
- Mora, M.A.M.; Vergara, C.P.; Leiva, M.A.; Delgadillo, S.A.M.; Rosa-Domínguez, E.R. Life cycle assessment of carbon capture and utilization from ammonia process in Mexico. J. Environ. Manag. 2016, 183, 998–1008. [Google Scholar] [CrossRef]
- GCCSI. The Global Status of CCS: 2016 Summary Report. Global CCS Institute. Available online: https://www.globalccsinstitute.com/resources/publications-reports-research/the-global-status-of-ccs-2016-summary-report/ (accessed on 6 January 2024).
- Healy, N.; Stephens, J.C.; Malin, S.A. Embodied energy injustices: Unveiling and politicizing the transboundary harms of fossil fuel extractivism and fossil fuel supply chains. Energy Res. Soc. Sci. 2019, 48, 219–234. [Google Scholar] [CrossRef]
- Kuuskraa, V. The role of enhanced oil recovery for carbon capture, use, and storage. Greenh. Gases Sci. Technol. 2013, 3, 3–4. [Google Scholar] [CrossRef]
- Wallquist, L.; Visschers, V.; Siegrist, M. Impact of Knowledge and Misconceptions on Benefit and Risk Perception of CCS. Environ. Sci. Technol. 2010, 44, 6557–6562. [Google Scholar] [CrossRef] [PubMed]
- Torp, T.A.; Gale, J. Demonstrating storage of CO2 in geological reservoirs: The Sleipner and SACS projects. Energy 2004, 29, 1361–1369. [Google Scholar] [CrossRef]
- ter Mors, E.; van Leeuwen, E. It matters to be heard: Increasing the citizen acceptance of low-carbon technologies in the Netherlands and United Kingdom. Energy Res. Soc. Sci. 2023, 100, 103103. [Google Scholar] [CrossRef]
- Betiku, A.; Bassey, B. Exploring the Barriers to Implementation of Carbon Capture, Utilisation and Storage in Nigeria. In Proceedings of the International Petroleum Technology Conference, Riyadh, Saudi Arabia, 21–23 February 2022. [Google Scholar] [CrossRef]
- Gollakota, S.; McDonald, S. CO2 capture from ethanol production and storage into the Mt Simon Sandstone. Greenh. Gases Sci. Technol. 2012, 2, 346–351. [Google Scholar] [CrossRef]
- Finley, R. An overview of the Illinois Basin—Decatur Project. Greenh. Gases Sci. Technol. 2014, 4, 571–579. [Google Scholar] [CrossRef]
- Rock, L.; O’brien, S.; Tessarolo, S.; Duer, J.; Bacci, V.O.; Hirst, B.; Randell, D.; Helmy, M.; Blackmore, J.; Duong, C.; et al. The Quest CCS Project: 1 st Year Review Post Start of Injection. Energy Procedia 2017, 114, 5320–5328. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sawada, Y.; Tanase, D.; Tanaka, J.; Shiomi, S.; Kasukawa, T. Tomakomai CCS Demonstration Project of Japan, CO2 Injection in Process. Energy Procedia 2017, 114, 5836–5846. [Google Scholar] [CrossRef]
- Preston, C. 2018-05 The CCS Project at Air Products’ Port Arthur Hydrogen Production Facility (Compressed Secured). 2018. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3365795 (accessed on 18 December 2023).
- CRS. DOE’s Carbon Capture and Storage (CCS) and Carbon Removal Programs. Congressional Research Services. Available online: https://crsreports.congress.gov/AppropriationsStatusTable (accessed on 7 April 2024).
- IEA. Regional Carbon Sequestration Partnerships (RCSP). IEA50. Available online: https://www.iea.org/policies/14137-regional-carbon-sequestration-partnerships-rcsp (accessed on 12 January 2024).
- DOE NETL. Regional Carbon Sequestration Partnership (RCSP). National Energy Technology Laboratory. Available online: https://netl.doe.gov/carbon-management/carbon-storage/RCSP (accessed on 5 December 2023).
- Ross-Coss, D.; Ampomah, W.; Cather, M.; Balch, R.S.; Mozley, P.; Rasmussen, L. An Improved Approach for Sandstone Reservoir Characterization. In Proceedings of the SPE Western Regional Meeting, Anchorage, AK, USA, 23–26 May 2016. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.S.; Grigg, R.B.; Dai, Z.; Pan, F. Compositional Simulation of CO2 Storage Capacity in Depleted Oil Reservoirs. In Proceedings of the Carbon Management Technology Conference, Sugar Land, TX, USA, 17–19 November 2015. [Google Scholar] [CrossRef]
- Cather, M.; Rose-Coss, D.; Gallagher, S.; Trujillo, N.; Cather, S.; Hollingworth, R.S.; Mozley, P.; Leary, R.J. Deposition, Diagenesis, and Sequence Stratigraphy of the Pennsylvanian Morrowan and Atokan Intervals at Farnsworth Unit. Energies 2021, 14, 1057. [Google Scholar] [CrossRef]
- Acheampong, S.A.; Ampomah, W.; Khaniani, H.; Will, R.; Sarkodie-Kyeremeh, J. Quantitative interpretation of time-lapse seismic data at Farnsworth field unit: Rock physics modeling, and calibration of simulated time-lapse velocity responses. Greenh. Gases Sci. Technol. 2022, 12, 671–697. [Google Scholar] [CrossRef]
- DOS. Carbon Sequestration Leadership Forum. US Department of State. Available online: https://2001-2009.state.gov/g/oes/rls/fs/46392.htm (accessed on 17 December 2023).
- MIT. FutureGen Fact Sheet: Carbon Dioxide Capture and Storage Project. Carbon capture and sequestration technologies @MIT. Available online: https://sequestration.mit.edu/tools/projects/futuregen.html (accessed on 16 May 2024).
- EPA. Archer Daniels Midland Company Illinois Industrial Carbon Capture and Sequestration Project. U.S. Environmental Protection Agency. Available online: https://www.epa.gov/ghgreporting/archer-daniels-midland-company-illinois-industrial-carbon-capture-and-sequestration (accessed on 23 February 2024).
- DOE. APCI PORT ARTHUR ICCS PROJECT. Available online: https://www.energy.gov/fecm/air-products-chemicals-inc (accessed on 26 February 2024).
- Sullivan, M.; Rodosta, T.; Mahajan, K.; Damiani, D. An overview of the Department of Energy’s CarbonSAFE Initiative: Moving CCUS toward commercialization. AIChE J. 2019, 66, e16855. [Google Scholar] [CrossRef]
- UK. Unique Public-Private Research Consortium Established CAER as CO2 Capture Pioneers. University of Kentucky. Available online: https://caer.uky.edu/co2capture/ (accessed on 4 May 2024).







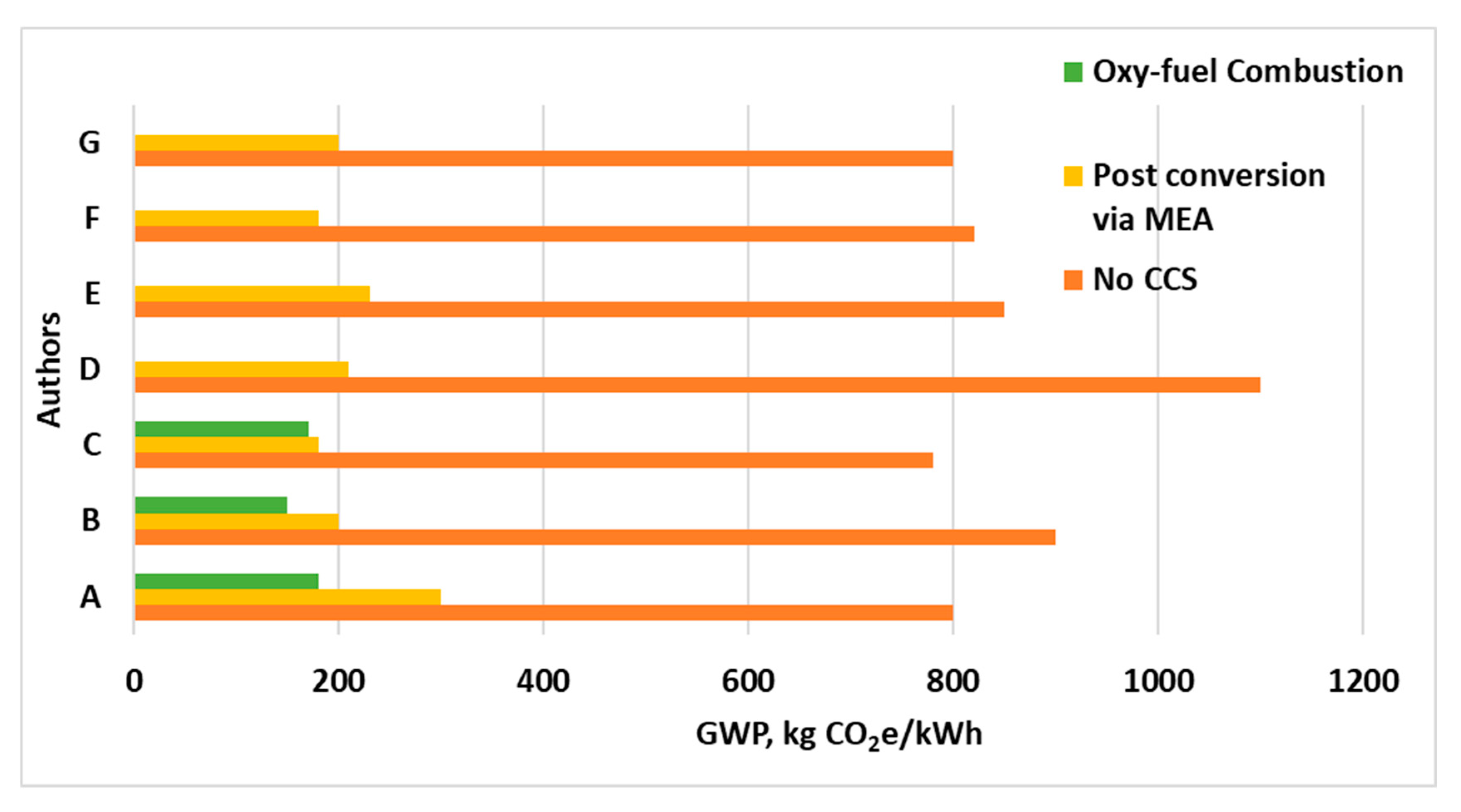

| Plant Type | Capture Type | Functional Unit | LCA Impact | Utilization | References |
|---|---|---|---|---|---|
| CCGT | Post Combustion | 1 MWh | GWP | Mineral Carbonation | [204] |
| synthesis of DMC with CO2 as feedstock | MEA | 1 kg of DMC | GWP, AP, ODP | Production of chemicals | [176] |
| IGCC | Pre combustion | MWh | GWP | Enhanced oil Recovery | [205] |
| CCGT | Pre combustion | 1 MWh, 1 m3 oil | GWP, AP | Enhanced oil Recovery | [206] |
| FWU oil field | from industrial sources | kgCO2e/bbl. oil | GWP | Enhanced oil Recovery | [3,30,207] |
| Biodiesel from microalgae | from industrial sources | 1 MJ of fuel | GWP, AP, EP, | biodiesel production | [208] |
| biodiesel production | CCGT power plant | 1 tonne of biodiesel | GWP | biodiesel production | [209] |
| biodiesel production | Power plants via MEA | 1 MJ of fuel | GWP | biodiesel production | [210] |
| Oil Field | Coal power plant | CO2e/bbl. | GWP | Enhanced oil Recovery | [211] |
| Oil Field | natural gas power plant | CO2e/bbl. | GWP | Enhanced oil Recovery | [212] |
| Coal power plant | post-combustion via MEA | 1 tonne of CO2 in silicate | GWP | Mineral Carbonation | [213] |
| Ammonia plant | post-combustion capture | kgCO2e/bbl. oil | GWP | Urea, carbonated drinks, EOR | [214] |
| Research Scope | Gaps | Details |
|---|---|---|
| Carbon Capture Efficiency and Cost | While advancing, current carbon capture technologies still face challenges related to efficiency and cost-effectiveness [14]. | Research is needed to improve the capture efficiency of various technologies (e.g., amine scrubbing and solid sorbents) and reduce the associated costs [67]. |
| Long-Term Storage Security | Uncertainties remain about the long-term stability and security of stored CO2 in geological formations [126]. | Understanding the potential for leakage, monitoring technologies, and the integrity of storage sites over extended periods is crucial [9]. |
| Integration with Renewable Energy | Limited research on integrating CCUS with renewable energy sources [92]. | Further investigation is required to explore how CCUS can work synergistically with renewable energy systems to provide low-carbon solutions. |
| Environmental Impact and Risk Assessment | Comprehensive environmental impact assessments of CCUS operations are lacking [125]. | Evaluating potential impacts on ecosystems, groundwater, and soil and developing robust risk assessment frameworks is essential [229]. |
| Public Perception and Policy | Insufficient understanding of public perception and the socio-political dimensions of CCUS deployment. | Research must address public concerns, policy frameworks, and regulatory environments to facilitate broader acceptance and implementation. |
| Opportunity | Examples | |
|---|---|---|
| Advanced Materials and Sorbents | Development of novel materials with higher CO2 capture efficiency and lower energy requirements. | Metal–organic frameworks (MOFs), advanced solid sorbents, and hybrid materials [72]. |
| Enhanced Oil Recovery (EOR) and Beyond | Optimizing CCUS for enhanced oil recovery and exploring other industrial uses for captured CO2 [145]. | Utilization of CO2 in chemical synthesis, carbonates, and polymers production [100]. |
| Digital and Smart Monitoring Systems | Leveraging digital technologies for real-time monitoring and management of CCUS systems [53]. | IoT sensors, machine learning algorithms for predictive maintenance, and blockchain for transparency and security. |
| Hybrid Systems | Combining CCUS with other carbon mitigation strategies, such as bioenergy with carbon capture and storage (BECCS) [83]. | Integration with algae cultivation for biofuel production and simultaneous CO2 capture. |
| Pilot Projects and Demonstrations | Establishing more pilot projects to demonstrate the viability of new CCUS technologies [41] | Large-scale field trials in diverse geological settings and industrial applications. |
| Recommendation | Action | |
|---|---|---|
| Focus on Cost Reduction | Prioritize research on reducing the capital and operational costs of CCUS technologies [14]. | Funding for projects aimed at material innovations, process optimization, and scale-up studies [228]. |
| Long-Term Monitoring and Risk Assessment | Develop and implement long-term monitoring protocols for storage sites. | Collaborative research programs to study storage integrity, potential leakage pathways, and environmental impacts [9]. |
| Interdisciplinary Approaches | Encourage interdisciplinary research combining engineering, environmental, and social sciences. | Grants and funding opportunities for projects that address technical, environmental, and socio-political aspects of CCUS. |
| Policy and Regulatory Frameworks | Support research on developing robust policy and regulatory frameworks. | Collaboration with policymakers, industry stakeholders, and academic institutions to create guidelines and incentives for CCUS deployment. |
| Public Engagement and Education | Enhance efforts to educate the public and stakeholders about the benefits and safety of CCUS. | Public outreach programs, transparent communication strategies, and educational campaigns are used to build trust and acceptance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ampomah, W.; Morgan, A.; Koranteng, D.O.; Nyamekye, W.I. CCUS Perspectives: Assessing Historical Contexts, Current Realities, and Future Prospects. Energies 2024, 17, 4248. https://doi.org/10.3390/en17174248
Ampomah W, Morgan A, Koranteng DO, Nyamekye WI. CCUS Perspectives: Assessing Historical Contexts, Current Realities, and Future Prospects. Energies. 2024; 17(17):4248. https://doi.org/10.3390/en17174248
Chicago/Turabian StyleAmpomah, William, Anthony Morgan, Desmond Ofori Koranteng, and Warden Ivan Nyamekye. 2024. "CCUS Perspectives: Assessing Historical Contexts, Current Realities, and Future Prospects" Energies 17, no. 17: 4248. https://doi.org/10.3390/en17174248
APA StyleAmpomah, W., Morgan, A., Koranteng, D. O., & Nyamekye, W. I. (2024). CCUS Perspectives: Assessing Historical Contexts, Current Realities, and Future Prospects. Energies, 17(17), 4248. https://doi.org/10.3390/en17174248









