Abstract
In this paper, we introduce an environmentally friendly approach to recycle used batteries and recover highly valuable manganese-based cathode materials. This study demonstrates the feasibility of fast plasma pyrolysis to recover LiMn2O4 electrode materials (e.g., lithium manganese oxide, LMO) and demonstrate their reuse in newly assembled Li-ion cells. The electrochemical performance of as-recycled cathodes shows an initial discharge capacity of 72 mAh/g and is stable for 100 cycles at 0.1 C. After adding 20 mole % of excess LiOH, the recycled LMO after relithiation at 660 °C can deliver an initial discharge capacity of 96 mAh/g and retain a decent discharge capacity of 88 mAh/g after 50 cycles at a 0.2 C rate. Without relithiation, the as-recycled LMO cathode after heating at 1000 °C delivers the best electrochemical cycling performance, including an initial discharge capacity of 94 mAh/g and 50th cycle capacity of 91 mAh/g at a 0.2 C rate. This study highlights a feasible approach for recycling electrode materials in spent LIBs. Recycling of lithium-ion batteries and especially electrode materials is crucial for the sustained growth of the lithium-ion battery industry and reduced environmental issues.
1. Introduction
The global energy consumption has been rising rapidly due to an increase in population, economic development, and technological advancement. Climate change and environmental challenges are mostly caused by the vast use of fossil fuels and production of greenhouse gases and pollution [1,2]. In the last twenty years, a lot of research has been conducted on alternative energy sources like wind energy, solar energy, hydro energy, and tidal energy. However, most renewable energy sources are irregular in nature, and it is necessary to use storage devises to provide a constant flow of energy from these sources [3]. Lithium-ion batteries (LIBs) are known to be the best candidate for energy storage devices due to their high energy density, high specific energy, good rechargeable capability, and stability [4,5]. LIBs are already the first choice for energy storage in portable electronic devices like laptops, smart phones, and tablets. Furthermore, they are the most attractive battery technology for electric vehicles (EVs) and hybrid electric vehicles (HEVs), as well as a strong candidate for stationary storage solutions, and for a widespread use of lithium-ion batteries in private and industrial applications [6,7,8].
The global market for lithium-ion batteries is undergoing an enormous growth from 259 to 2500 GWh within the years of 2020–2030, representing an average increase of 25.4% per year [9]. This dramatically increasing trend indicates a drastically rising demand for raw materials for LIBs and a drastically rising number of spent Li-ion batteries due to limited service life [10,11]. It is becoming imperative that a major portion of raw materials to fabricate LIBs can be extracted from the spent LIBs instead of mineral mining. According to a report, by the year of 2030, the number of spent LIBs globally will reach 11 million tons, and the recycling market could exceed US 23 billion [12]. By 2030, the demand for these batteries is expected to soar significantly [13]. Most lithium-ion batteries that are now in use have a shelf life of 5–6 years, after which they are either recycled or transferred to the municipal waste stream. The majority of lithium batteries contain lithium (Li), nickel (Ni), cobalt (Co), and manganese (Mn), which are essential for producing new batteries. We cannot throw away used lithium-ion battery after the end of their life cycle, because it poses a risk to environmental safety. Many countries do not have resources for lithium, nickel, cobalt, and manganese, and the recycling of lithium batteries would enhance the supply chain, reduce the reliance on new materials, control the price of materials, as well as have a smaller impact on the environment [14,15]. The United States, Argentina, and China strongly dominate the global lithium market, presenting themselves as important geopolitical players for the electric battery vehicle global chain [16,17].
Therefore, LIB recycling is becoming urgent and vital. The popular cathode materials in LIBs include LiCoO2 (LCO), LiNiMnCoO2 (NMC), LiNiCoAlO2 (NCA), and LiPFeO4 (LFP). Among them, LCO (1:1)-, NCA (0.8:0.15:0.05)-, and NMC (1:1:1)-based batteries have higher recycling values than LFP considering the price of elements (Ni, Co) [18]. Also, depending on the ratio of Ni to Co, the NMC also includes NMC90505, NMC811, NMC721, NMC622, NMC532, NMC442, NMC111, etc. In addition, LIBs also contain liquid electrolytes with Li-salts that are dissolved in various solvents, such as lithium hexafluorophosphate in propylene carbonate (PC), ethylene carbonate (EC), diethyl carbonate (DEC), dimethyl carbonate (DMC), and ethyl methyl carbonate (EMC) solvents, etc., with a small amount of 1–2 wt% of additives on it like vinylene carbonate (VC), 1,3-propane sultone (PS), and 4-Fluoro-1,3-dioxolan-2-one (FEC). All these organic liquid electrolytes are flammable and will cause safety concerns for large quantities of long-distance transportation [19,20,21].
Battery recycling technology plays a crucial role to realize the sustainable management of battery waste and the recovery of valuable materials. To date, there are three dominant recycling methods, pyrometallurgy, hydrometallurgy, and direct recycling. In the pyrometallurgy process, LIB recycling is carried out by heating the furnace at a low temperature of 150–500 °C to remove organic solvents and electrolytes [22,23]. After that, a high-temperature treatment of 1400–1600 °C is used to form slag (lithium oxide and lithium carbonate) and alloys of cobalt products [15]. In the hydrometallurgical process, the pre-treatment process includes the discharging, physical dismantling, and separation of active materials. The hydrometallurgy process could recover cathode materials like lithium and transition metal oxides [24]. This method does not consume too much energy due to a relative low temperature for operation [15,25], but it requires a large amount of water. In the direct recycling approach, the batteries are first discharged, and then, discharged batteries are put into a controlled environment to remove electrolytes. In this process, relithiation processes are often carried out to reuse the cathode material in the batteries [26,27]. The direct recycling process plays a crucial role in recovering the active materials of the battery directly by retaining its compound structure [28,29]. Compared to the pyrometallurgy and hydrometallurgy recycling processes, the direct recycling method has advantages in terms of safety, cost, energy consumption, and economic aspects [30,31].
Atmospheric plasmas are finding applications in various processing applications. In our earlier work, we employed this technique for the synthesis of nanowire materials such as SnO2, Cu2O, TiO2, LiNi02Mn0.6Co0.2O2, and Li6WO6 and their applications usage in solar cells, CO2 reduction, semiconductors, batteries, and water splitting reactions [32,33,34,35,36,37]. In this work, we report the use of an atmospheric microwave plasma flame to pyrolyze the plastics and volatile electrolytes in the spent LIBs to obtain the separated anode and cathode materials, respectively. After the plasma pyrolysis, the recycled electrode materials can be stored and transported as nonhazardous. The as-recycled LiMn2O4 cathode was directly used to assemble new LIBs to test their electrochemical performance. Moreover, 20 mole % of excess LiOH relithiation was performed to improve the cycling performance of the recycled cathode. Additionally, the post-heating treatment at 800–1000 °C without relithiation was also demonstrated to enhance the electrochemical cycling performance. The main reason is that the cathode’s active material needs to be separated from metallic foils. Otherwise, the metallic foils will negatively influence the cathode’s active material composition. Secondly, it is important to ensure that the majority of the flammable and polymeric components do not leave any residue in the product. It is impossible to directly achieve a high temperature post-heat treatment for the batteries if the batteries have pouch, cylinder, and prismatic cells. The plasma pyrolysis process mentioned in our study allows for a fast way to produce nonhazardous materials from unsafe lithium-ion battery components for shipping and recycling purposes. Our suggested process also reduces the vast amount of water waste used in pyrometallurgy and hydrometallurgy in the current battery recycling process. The plasma exposure treatment process allows us to safely separate and recover the cathode’s active materials, and the subsequent thermal treatment enables the complete rejuvenation of the structure and composition and retaining >90% of the original capacity.
This study highlights a scalable plasma method for recycling and reusing electrode materials in spent LIBs. The reported plasma-based pyrolysis process could be used for the pre-treatment of recycled lithium-ion batteries at a large scale to supply recyclable materials for cell manufacturing.
2. Experimental Procedure
2.1. Plasma Treatment of Used LIBs
A LiMn2O4 waste cathode was obtained from dismantling of retired pouch cell inside an argon-filled glove box. The disassembled LIB cell was then used for the plasma treatment, as shown in Figure S1. The atmospheric plasma pyrolysis experiments were performed using a custom-made upstream atmospheric plasma system. In this setup, an electrode-less atmospheric microwave plasma torch system was assembled with a commercially available magnetron (MKS ASteX FI20162-2, 2.45 GHz, MA, USA) with a maximum stationary power of 2 kW. At the far end of the waveguide, a quartz tube of about 1 inch in diameter and 20 inch in length was vertically attached and a cooling water line with a maximum flow of 3 slpm (standard liters per minute), which supplies cooling to the magnetron, and the waveguide. The microwave plasma was ignited by inserting a copper wire into the resonant cavity from the top of the reactor, gently moving it around the walls of the resonant cavity until a plasma jet was generated. The flow rates of gases were controlled with mass flow controllers that were connected to the top and side of the quartz tube. During the plasma treatment process, the disassembled cell electrode was directly exposed to the plasma flame to completely burn to remove the organic solvents to form the exhaust gas (nonhazardous). The resulting gases were analyzed using gas chromatography (Thermo Scientific Model: Trace 1310, MA, USA). After the plasma treatment, the cathode and anode electrode were separated and collected by scrapping off from the current collectors, respectively. The collected electrode powder was subjected to ball milling for 2 h to obtain a fine powder, followed by washing with ethanol and drying at 60 °C for overnight. The collection of the as-recycled cathode and anode carbon powder after washing and sieving is shown in Figure 1. The as-recycled materials were divided into three parts and subjected to different post-treatments: (1) One portion of as-recycled cathodes was directly mixed at different weight ratios with the commercial spinel LiMn2O4 cathode for a comparison of their electrochemical performance. (2) The second portion of as-recycled cathode material was mixed with 0.2 M LiOH and calcined at 660 °C for 10 h with a slow heating rate of 2 °C/min to compensate for the loss of lithium in the LiMn2O4 cathode structure. (3) The third portion of as-recycled cathode was heated at 800–1000 °C for 10 h with a ramp speed of 5 °C/min to remove the impurities such as conductive carbon and binder.
Figure 1.
Images of the as-recycled (a) LiMn2O4 cathode and (b) anode (carbon) powders (after washing with ethanol and sieving).
2.2. Characterizations
The recovered LiMn2O4 powders were analyzed using field emission scanning electron microscopy (FE-SEM; FEI Nova600, OR, USA) and energy-dispersive X-ray analysis (EDAX) and X-ray diffraction (XRD; Bruker D8 Discover, MA, USA) with CuKα (λ = 1.541 Å) at a scan speed of 0.02° s−1 at the range of 10–80° (2θ). Bruker EVA software (version 6) and powder diffraction file (PDF) were used for phase identification. The gas analysis was performed using gas chromatography using a thermal conductivity detector (Thermofischer Scientific, MA, USA).
2.3. Electrode Preparation and Electrochemical Characterizations
The as-recycled LiMn2O4 electrode was directly used to assemble new LIBs in 2032-Coin-type cell for the electrochemical characterizations. The as-recycled LiMn2O4 electrode was also mixed with different concentrations of commercial spinel material (LiMn2O4, MTI Corporation) as an active cathode for comparison, namely, pure as-recycled LiMn2O4 (100 wt%), 80 wt% as-recycled +20 wt% commercial LiMn2O4, 40 wt% as-recycled +60 wt% commercial LiMn2O4, and pure commercial LiMn2O4. In addition, the LiOH—added as a recycled LiMn2O4 (LiOH:LMO molar ratio 0.2:1) cathode sintered at 660 °C-10 h, and as-recycled LiMn2O4 without relithiation after high-temperature treatment (800–1000 °C) were also compared for the electrochemical cycling. For the as-recycled LiMn2O4, a mixture of as-recycled LiMn2O4 (40 wt%) and commercial LiMn2O4 (60 wt%), a mixture of as-recycled LiMn2O4 (80 wt%) and commercial LiMn2O4 (20 wt%), a commercial LiMn2O4, and LiOH-added as-recycled LiMn2O4 cathode was prepared using a mixture of 10 mg of active material and 6 mg of Teflonized acetylene black binder using an agate mortar. Then, the mixture was pressed onto a stainless steel mesh (area of 2 cm2) with a hydraulic press, followed by drying at 160 °C for 5 h in a vacuum oven. Coin cell assembly was carried out in an argon-filled dry glove box. The as-recycled LiMn2O4 was used as a working electrode and Li foil as a counter-electrode. They were separated by two piece of glass fiber filter (ADVANTEC, GB-100R, Toyo Roshi Company Ltd., Japan) using 2032 coin-type cells. The electrolyte used was 1M LiPF6-EC (ethylene carbonate): DMC (dimethyl carbonate) (1:2 by weight) and 1M LiPF6-EC:DMC (1:2)-2 wt% FEC (fluoroethylene carbonate). The galvanostatic charge–discharge measurements were carried out using an Arbin instrument (TX, USA, 16 channel). The charge–discharge measurements were carried out in the voltage range between 3.0 and 4.3 V with a current density of 11 (0.1 C) and 22 (0.2 C) mA g−1 at room temperature, respectively. For the regenerated LiMn2O4 cathode, charge–discharge measurements were carried out at a high voltage range between 4.7 and 2.5 V with a current of 22 mA/g (0.2 C), respectively. The cyclic voltammetry of as-recycled cathode and commercial and LiOH-added LiMn2O4 electrodes were measured at the voltage range of 4.3–3.0 V with a scan speed of 0.1 mV/s. For the heat-treated (800–1000 °C) as-recycled LiMn2O4 cathodes, cyclic voltammetry measurements were carried out at a high voltage range of 4.7–2.5 V with a scan speed of 0.1 mV/s, respectively.
3. Results and Discussion
After the plasma treatment process of the pouch cell, the separated cathode and anode electrodes coated with Al and Cu foil are shown in Figure S2. The generated gases were analyzed as shown in Figure S3. The GC curve shows the presence of only CO and CO2 gases and N2 gas coming from air. Figure 2 shows the XRD patterns of the as-recycled LiMn2O4, with 20 mole % excess LiOH added to as-recycled LiMn2O4 (LiOH:LMO molar ratio of 0.2:1) compared with the commercial material. All three samples show the characteristic diffraction peaks of the spinel phase with a strong diffraction plane of (111), (311), (511), and (400). The spinel of LiMn2O4 has an Fd3m group in which Li+ ion occupies the (8a) tetrahedral sites. The Mn3+ and Mn4+ ion in the LiMn2O4 structure occupy (16d) octahedral sites, and O2− occupies octahedral (32e) sites. The spinel phase in the as-recycled sample clearly indicates that the LiMn2O4 cathode materials have been successfully reconstructed to their original structure after our plasma pyrolysis treatment. It is evident from the analysis that the LiMn2O4 phase is intact after the recycling process, as well as the heat treatment at different temperatures after the addition of LiOH. Later, the as-recycled LiMn2O4 was further heat-treated at higher temperatures (800–1000 °C) to fully remove the binders. The XRD patterns of the recycled LiMn2O4 after post-heating treatment are shown in Figure S4. However, the heat-treated sample at 1000 °C shows impurity peaks at 2θ of 33.8°, 35.02°, and 44.81°. The peaks at 33.8° and 35.02° are indicative of Li2O2 (100) and (101) planes [38]. The magnified scan from 2θ of 18° to 19° in Figure S4 shows the shift in the (111) plane for the as-recycled and heat-treated materials. The shift in the peak indicates the change in the crystal structure of the material. The shift to a higher 2θ angle suggests a contraction in the lattice [39]. Figure 3 displays scanning electron microscopy (SEM) images for the as-recycled LiMn2O4 sample, which shows the irregular particle size of about 1–6 μm in diameter with a great crystalline surface.
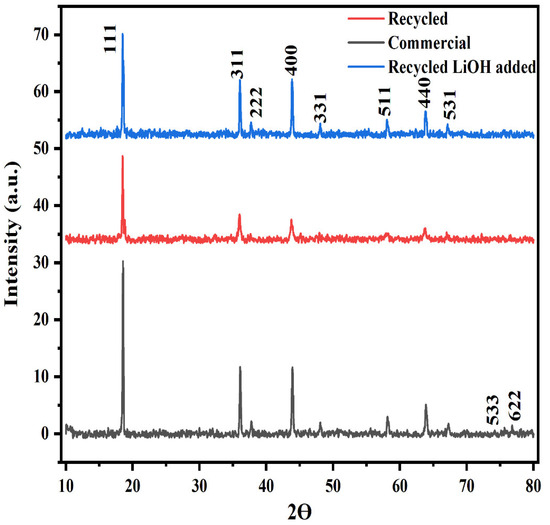
Figure 2.
X-ray diffraction patterns of commercial LiMn2O4 (bottom), as-recycled LiMn2O4 (middle), and LiOH-added LiMn2O4 (top).
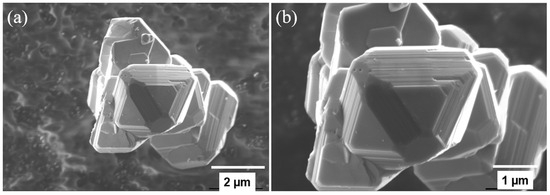
Figure 3.
(a,b) SEM images of as-recycled LiMn2O4 powder under different magnifications.
Figure 4a show the first two charge–discharge curves of the as-recycled LiMn2O4 sample without any further treatment at a rate of 0.1C (11 mAh/g) within the voltage range of 3.0–4.3 V (electrolyte of 1M LiPF6- EC:DMC with 1:2 by volume), and the initial discharge capacity is 72 mAh/g. Figure 4b displays the charge–discharge capacities and columbic efficiency of the cell with the as-recycled LiMn2O4 for up to 100 cycles. After 100 cycles, the discharge capacity decreases to 50 mAh/g, corresponding to a capacity retention rate of 70%. The capacity decrease of the cell with the as-recycled spinel cathode during cycling is similar to the aging mechanism due to Jahn–Teller distortion structural deformation and manganese dissolution [40,41,42]. The manganese ion moving through the separator is reduced on the anode surface, which accelerates the reaction of solid electrolyte interface (SEI) thickening with an increasing resistance and loss of lithium-ion [43,44,45,46]. In addition, since the as-recycled cathode is directly used after the plasma treatment without further purification, the impurities such as Li2O and binder may also cause a decrease in capacity and structural degradation from crystal to amorphous [47,48,49]. The initial coulombic efficiency (CE) of the as-recycled LiMn2O4 sample was 83.7%; nevertheless, the CE value slightly increased to beyond 100% after the fourth cycle and continued until 100 cycles. The continuous increase in coulombic efficiency of the as-recycled LiMn2O4 cathode was due to the dissolution of manganese in the electrolyte, which leads to a loss of cathode active material and electrolytes. The reason for the high coulombic efficiencies is that if the Li-ion is less intercalated due to some structural interference during the discharge process and the maximum amount of Li-ion is released during the charge process, the coulombic efficiency may exceed over 100%. The structural failure of active materials and side reactions during a charge process leads to the generation of higher capacity than that are released from the recycled cathode’s active electrode. The cathode material used in this study was directly used as recycled without further purification.
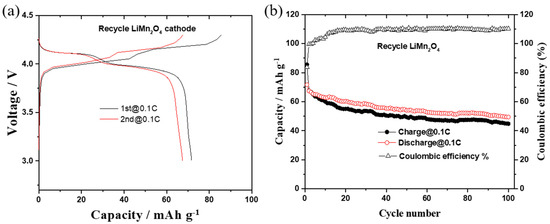
Figure 4.
(a) Charge–discharge curves and (b) cycle performance of as-recycled LiMn2O4 cathode from plasma treatment. The cells were cycled at 0.1 C rate with liquid electrolyte 1M LiPF6-EC:DMC (1:2) within voltage window of 3.0–4.3 V.
It is very important to do relithiation as well as optimization of the capacity of cathode materials to convince the manufacturers to reuse the recycled cathode [50]. Using a 1M LiPF6-EC:DMC liquid electrolyte with 2 wt% FEC (fluoroethylene carbonate) as an additive, Figure 5 shows the first charge–discharge curves and cycling performance for different cathode samples, specifically, the as-recycled LiMn2O4 (100%), mixture of 80 wt% as-recycled + 20 wt% commercial LiMn2O4, mixture of 40 wt% as-recycled + 60 wt% commercial LiMn2O4, commercial LiMn2O4, as well as the 20 mole % excess LiOH-added recycled LiMn2O4. With 80 wt% of as-recycled and 20 wt% commercial LiMn2O4, the cell shows an initial discharge capacity of 79.4 mAh/g and a stable capacity of 65.5 mAh/g after 50 cycles at a 0.2 C rate. When increasing the commercial material content to 60 wt%, the cell delivers an initial discharge capacity of 97.8 mAh/g and remains at a stable capacity of 91mAh/g after 50 cycles at a 0.2 C rate. The controlled cell with a commercial LiMn2O4 electrode displays an initial discharge capacity of 100 mAh/g, but this value increases to 110 mAh/g and is stable for 50 cycles at 0.2 C. In comparison, the recycled LiMn2O4 after relithiation (adding LiOH) shows an initial discharge capacity of 96 mAh/g during the first cycle and still retains a capacity of 82.5 mAh/g after 50 cycles at a 0.2 C rate. For the as-recycled LiMn2O4, it is possible that there is an impurity layer (Li2O, or Li2O2) on the cathode surface that comes from the plasma pyrolysis of the electrolyte. Due to the poor conductivity of Li2O, this layer may reduce the active material’s weight and cause some cathode material to be inaccessible. This is evident from the fact that we obtain a discharge capacity of 70 mAh/g for 100% as-recycled LiMn2O4 and 110 mAh/g for the commercial LiMn2O4 material. The mixture of as-recycled 40 wt% and commercial 60 wt% LiMn2O4 with a 40:60 ratio delivers a discharge capacity of 95 mAh/g (0.4 × 70 + 0.6 × 110 = 94 mAh/g). The mixture of as-recycled 80 wt% and commercial 20 wt% LiMn2O4 with a 80:20 ratio delivers a discharge capacity of 79.8 mAh/g (0.8 × 70 + 0.2 × 110 = 78 mAh/g).
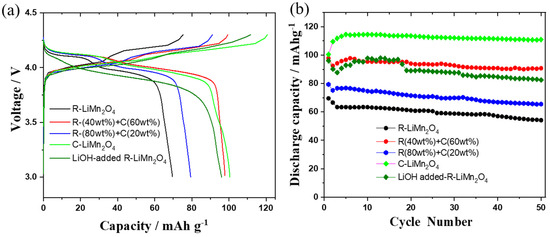
Figure 5.
(a) The charge–discharge curves and (b) cycle performance of new LIBs with five different cathode materials: as-recycled LiMn2O4 cathode; a mixture of 80 wt% as-recycled + 20 wt% commercial LiMn2O4 cathode; a mixture of 40 wt% as-recycled + 60 wt% commercial LiMn2O4 cathode; commercial LiMn2O4, and recycled LiMn2O4 after adding 0.2M LiOH for relithiation. The cells were cycled at a 0.2 C rate with the liquid electrolyte 1M LiPF6-EC:DMC (1:2)-2 wt% FEC within a voltage window of 3.0–4.3 V.
The SEM images of the heat-treated materials are shown in Figure S5. In contrast to the as-recycled particles with defects, the heat-treated samples show well-defined particles with smooth surfaces without any defects. Figure 6 shows the cyclic voltammetry of the as-recycled LiMn2O4, commercial LiMn2O4, and 20 mole % excess LiOH-added LiMn2O4 (LiOH:LMO molar ratio 0.2:1) cathodes at the voltage range of 4.3–3.0 V with a scan speed of 0.1 mV/s. The CV measurement of the as-recycled LiMn2O4 clearly shows two pairs of oxidation and reduction peaks at 4.05/3.95 V and 4.17/4.08 eV, respectively. The oxidation peak at 4.17 V is slightly lower than the other peak at 4.05 V. The redox peak splitting is associated with the two-step reversible extraction and insertion of Li+ ion into the LiMn2O4 framework [51,52,53], corresponding to a two-step process corresponding to LiMn2O4/Li0.5Mn2O4 and Li0.5Mn2O4/λ-MnO2 during charging and discharging [54]. This observation further confirms that the plasma pyrolysis treatment successfully reconstructs the LiMn2O4 electrode from the spent LIBs. On the other hand, for the relithiated LiMn2O4 electrode, after adding LiOH, its CV curve displays two strong pairs of peaks with narrower positions. The oxidation/reduction voltages are located at 4.08/3.96 V and 4.19/4.1 V, respectively, which is closer to the peaks of the commercial LiMn2O4 electrode. Adding LiOH for relithiation is beneficial for the second step of the process at a higher voltage, specifically the redox of Li0.5Mn2O4/λ-MnO2.
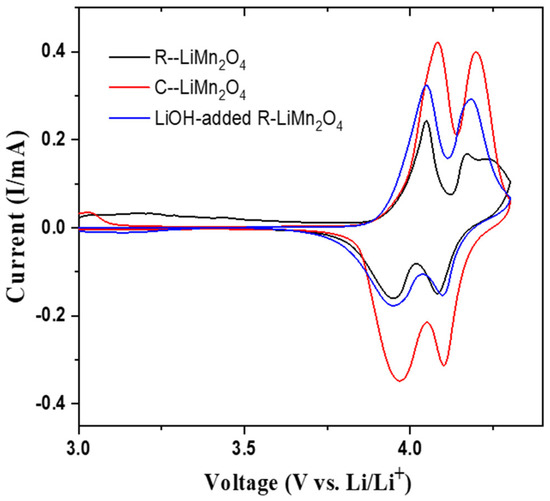
Figure 6.
Cyclic voltammetry curves of as-recycled LiMn2O4, recycled LiMn2O4 after adding LiOH, and commercial LiMn2O4 cathode electrodes scan at speed of 0.1 mV/s.
After the plasma pyrolysis treatment, the as-recycled LiMn2O4 cathode still contains some impurities, and thus, a higher calcination temperature (800–1000 °C) was used to fully remove the possible impurities such as carbon, binder, and manganese dissolution with electrolytes. The electrochemical performance of the high temp-heated LiMn2O4 cathode (regenerated) was examined at a current of 22 mA/g (0.2 C) within a voltage of 2.5–4.7 V. Figure 7a,b display the first charge–discharge curves and cycling performance of the regenerated LiMn2O4 cathode after different temperatures (800–900 °C) without the addition of LiOH. As shown in Figure 7a, the recycled 800 °C-LiMn2O4 cathode exhibits the highest initial discharge capacity of 97.6 mAh/g, followed by the 1000 °C recycled sample (93.5 mAh/g) and the 900 °C recycled sample (87 mAh/g). After 50 cycles at 0.2 C, the remaining capacity for the recycled 800 °C-LiMn2O4 cathode is 87.6 mAh/g (90% of capacity retention), and that of the recycled 900 °C-LiMn2O4 cathode is 87.6 mAh/g. In contrast, the discharge capacity of the 1000 °C-LiMn2O4 cathode remains at 92.1 mAh/g after 50 cycles, suggesting a best capacity retention of 98.2%. The reason for the high capacity retention rate of the regenerated LiMn2O4 at 1000 °C is due to the highly crystalline structure of the sample.
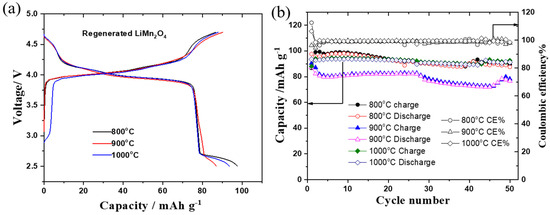
Figure 7.
(a) The charge–discharge curves and (b) cycle performance of the regenerated LiMn2O4 cathode at 800, 900, and 1000 °C-10 h. The cells were cycled at the current of a 0.2 C rate within a voltage of 2.5–4.7 V.
In this study, we found out that the regenerated LiMn2O4 cathode after calcination at 800–1000 °C reconstructed its original crystal structure, as confirmed by the X-ray diffraction patterns with high-intensity peaks. Moreover, it delivers a discharge capacity of 97 mAh/g, which is almost 90% of its practical capacity. The reason for the high discharge capacities and 100% coulombic efficiency of the regenerated cathode materials is that the remaining Li2O or Li2O2 after the plasma exposure of the cathode help fulfill the lithium loss in the LiMn2O4 electrode. In our future study, we will also try to apply a direct recycling process for the NMC cathode too.
The CV curves of the regenerated LiMn2O4 cathode calcined at 800, 900, and 1000 °C are shown in Figure 8. The recycled 800 °C-LiMn2O4 and 1000 °C-LiMn2O4 show similar pairs of oxidation/reduction peaks at 4.1/3.91 V and 4.2/4.08 V, corresponding to the two-step lithium extraction/insertion process into the tetrahedral sites [55,56,57]. Moreover, they also display an additional oxidation peak at 3.1 V and two reduction peaks at 2.61 and 4.59 V. A pair of well-defined oxidation/reduction peaks at 3.1/2.6 V are attributed to the typical cubic to tetragonal phase transition of the spinel from LiMn2O4 to Li2Mn2O4 [58]. In contrast, the CV of the recycled 900 °C-LiMn2O4 shows a sharp oxidation/reduction peak at 4.08/3.91 V with two additional reduction peaks at 4.59 and 4.09 V, respectively.
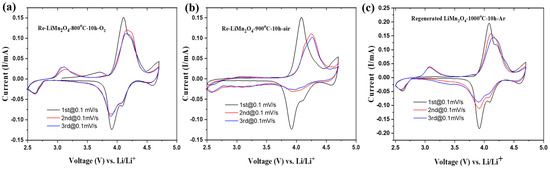
Figure 8.
Cyclic voltammetry results of regenerated LiMn2O4 cathode electrodes at (a) 800 °C, (b) 900 °C, and (c) 1000 °C with scanning speed of 0.1 mV/s.
4. Conclusions
In this study, we successfully demonstrated the atmosphere plasma pyrolysis technique as an efficient method to recycle and reuse the spinel LiMn2O4 cathode from spent LIBs. The as-recycled LiMn2O4 cathode delivers an initial discharge capacity of 72 mAh/g and retains a discharge capacity of 54 mAh/g after 100 cycles at 0.1 C. The mixture of as-recycled and commercial LiMn2O4 cathodes shows enhanced charge/discharge capacities and retains a capacity at a 0.2 C rate; specifically, the 40 wt% as-recycled + 60 wt% commercial sample delivers a discharge capacity of 97.8 mAh/g at 0.2 C and still retains a discharge capacity of 91 mAh/g after 50 cycles at a 0.2 C rate. The recycled LiMn2O4 after relithiation with added LiOH exhibits improved performance in both its initial capacity (96 mAh/g) and 50th capacity (82 mAh/g) compared with the as-recycled LiMn2O4. A high-temperature treatment of the as-recycled LiMn2O4 cathode also benefits the electrochemical performance. The 1000 °C-LiMn2O4 cathode delivers an initial discharge capacity of 93.5 mAh/g at 0.2 C and remains at 98.2% of capacity after 50 cycles due to the highly crystalline structure. Our future work may focus on further improving the cycling performance of the recycled cathode from plasma technology.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/en17163996/s1, Figure S1: Images of disassembled cell (a) at inert Ar glove box and (b) plasma flame exposure of pouch cells; Figure S2: Image after plasma flame exposure of pouch cell and separated cathode, and anode electrode coated with Al and Cu foils; Figure S3: Gas chromatography analysis of plasma-exposed Li-ion pouch cell; Figure S4: Left: X-ray diffraction patterns of as-recycled LiMn2O4 heat-treated at 800 °C, 900 °C, and 1000 °C. Right: X-ray diffraction of 111 peak for commercial LiMn2O4, as-recycled LiMn2O4, and as-recycled LiMn2O4 heat-treated at 800 °C, 900 °C, and 1000 °C; Figure S5: SEM images of as-recycled LiMn2O4 regenerated at (a) 800 °C, (b) 900 °C, and (c) 1000 °C.
Author Contributions
Conceptualization, A.K.T., H.W. and M.K.S.; Methodology, A.K.T., A.C.N., M.M., H.P.R.K., L.B., H.W. and M.K.S.; Formal analysis, A.K.T. and A.C.N.; Investigation, M.M., H.P.R.K., L.B. and H.W.; Writing—original draft, A.K.T., A.C.N., H.P.R.K., L.B., H.W. and M.K.S.; Writing—review and editing, A.K.T., A.C.N., M.M., H.P.R.K., L.B., H.W. and M.K.S.; Supervision, L.B. and M.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
Hari Prasad Reddy Kannapu is a research scientist working at Advanced Energy Materials, LLC. Mahendra Sunkara has financial interests in Advanced Energy Materials, LLC. No external funding was there for Advanced energy Materials, LLC for this project.
References
- Innocenzi, V.; Prisciandaro, M. Technical feasibility of biodiesel production from virgin oil and waste cooking oil: Comparison between traditional and innovative process based on hydrodynamic cavitation. Waste Manag. 2021, 122, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, H.A.; Pecht, M.G. Circular economy of Li Batteries: Technologies and trends. J. Energy Storage 2021, 40, 102690. [Google Scholar] [CrossRef]
- Yang, J.; Gu, F.; Guo, J. Environmental feasibility of secondary use of electric vehicle lithium-ion batteries in communication base stations. Resour. Conserv. Recycl. 2020, 156, 104713. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P. Lithium-ion batteries–Current state of the art and anticipated developments. J. Power Sources 2020, 479, 228708. [Google Scholar] [CrossRef]
- Melin, H.E.; Rajaeifar, M.A.; Ku, A.Y.; Kendall, A.; Harper, G.; Heidrich, O. Global implications of the EU battery regulation. Science 2021, 373, 384–387. [Google Scholar] [CrossRef]
- Habib, K.; Hansdóttir, S.T.; Habib, H. Critical metals for electromobility: Global demand scenarios for passenger vehicles, 2015–2050. Resour. Conserv. Recycl. 2020, 154, 104603. [Google Scholar] [CrossRef]
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Del Campo, F.; Lanceros-Méndez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. Energy Storage Mater. 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Shaqsi, A.Z.A.; Sopian, K.; Al-Hinai, A. Review of energy storage services, applications, limitations, and benefits. Energy Rep. 2020, 6, 288–306. [Google Scholar] [CrossRef]
- Altiparmak, S.O. China and lithium geopolitics in a changing global market. Chin. Political Sci. Rev. 2023, 8, 487–506. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. Burgeoning prospects of spent lithium-ion batteries in multifarious applications. Adv. Energy Mater. 2018, 8, 1802303. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Wanger, T.C. The Lithium future—Resources, recycling, and the environment. Conserv. Lett. 2011, 4, 202–206. [Google Scholar] [CrossRef]
- Sun, X.; Hao, H.; Zhao, F.; Liu, Z. Global lithium flow 1994–2015: Implications for improving resource efficiency and security. Environ. Sci. Technol. 2018, 52, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.L.; Lee, K.T.; Nazar, L.F. Positive electrode materials for Li-ion and Li-batteries. Chem. Mater. 2010, 22, 691–714. [Google Scholar] [CrossRef]
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwade, A. Ecological recycling of lithium-ion batteries from electric vehicles with focus on mechanical processes. J. Electrochem. Soc. 2016, 164, A6184. [Google Scholar] [CrossRef]
- Kawamura, T.; Okada, S.; Yamaki, J.-I. Decomposition reaction of LiPF6-based electrolytes for lithium ion cells. J. Power Sources 2006, 156, 547–554. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of recycling on cradle-to-gate energy consumption and greenhouse gas emissions of automotive lithium-ion batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Jinglong, L.; Hui, L.; Dongbin, W. Status and prospects of treatment methods for valuable metals in spent lithium-ion battery. Hot Work. Technol. 2018, 22, 12–15. [Google Scholar]
- Lee, C.K.; Rhee, K.-I. Preparation of LiCoO2 from spent lithium-ion batteries. J. Power Sources 2002, 109, 17–21. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Li, J.; Qu, L.; Yang, Y.; Guo, X.; Xie, W. Improved hydrometallurgical extraction of valuable metals from spent lithium-ion batteries via a closed-loop process. J. Alloys Compd. 2020, 847, 156489. [Google Scholar] [CrossRef]
- Sloop, S.E.; Parker, R. System and Method for Processing an End-of-Life or Reduced Performance Energy Storage and/or Conversion Device Using a Supercritical Fluid. U.S. Patent 8067107B2, 29 November 2011. [Google Scholar]
- Li, X.; Zhang, J.; Song, D.; Song, J.; Zhang, L. Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J. Power Sources 2017, 345, 78–84. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, G.; Liu, F.; Yue, X.; Chen, Z. Resolving the compositional and structural defects of degraded LiNixCoyMnzO2 particles to directly regenerate high-performance lithium-ion battery cathodes. ACS Energy Lett. 2018, 3, 1683–1692. [Google Scholar] [CrossRef]
- Gaines, L. The future of automotive lithium-ion battery recycling: Charting a sustainable course. Sustain. Mater. Technol. 2014, 1, 2–7. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Y.; Jiao, B.; Chang, N.; Wu, M.; Liu, G.; Lin, X.; Weng, X.; Chen, J.; Zhang, L. Direct Recycling of Spent Li-ion Batteries: Challenges and Opportunities towards Practical Applications. iScience 2023, 26, 107676. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Meduri, P.; Pendyala, C.; Kumar, V.; Sumanasekera, G.U.; Sunkara, M.K. Hybrid tin oxide nanowires as stable and high capacity anodes for Li-ion batteries. Nano Lett. 2009, 9, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, S.; Vendra, V.K.; Kim, J.H.; Druffel, T.; Sunkara, M.K. Scalable synthesis and photoelectrochemical properties of copper oxide nanowire arrays and films. Catal. Today 2013, 199, 27–35. [Google Scholar] [CrossRef]
- Kumar, V.; Kim, J.H.; Jasinski, J.B.; Clark, E.L.; Sunkara, M.K. Alkali-assisted, atmospheric plasma production of titania nanowire powders and arrays. Cryst. Growth Des. 2011, 11, 2913–2919. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Atla, V.; Vendra, V.K.; Thapa, A.K.; Jasinski, J.B.; Druffel, T.L.; Sunkara, M.K. Scalable solvo-plasma production of porous tin oxide nanowires. Chem. Eng. Sci. 2016, 154, 20–26. [Google Scholar] [CrossRef]
- Ajayi, B.P.; Thapa, A.K.; Cvelbar, U.; Jasinski, J.B.; Sunkara, M.K. Atmospheric plasma spray pyrolysis of lithiated nickel-manganese-cobalt oxides for cathodes in lithium ion batteries. Chem. Eng. Sci. 2017, 174, 302–310. [Google Scholar] [CrossRef]
- Akram, M.Z.; Atla, V.; Nambo, A.; Ajayi, B.P.; Jasinski, J.B.; He, J.; Gong, J.R.; Sunkara, M. Low-temperature and fast kinetics for CO2 sorption using Li6WO6 nanowires. Nano Lett. 2018, 18, 4891–4899. [Google Scholar] [CrossRef]
- Lau, K.C.; Qiu, D.; Luo, X.; Greeley, J.; Curtiss, L.A.; Lu, J.; Amine, K. Theoretical exploration of various lithium peroxide crystal structures in a Li-air battery. Energies 2015, 8, 529–548. [Google Scholar] [CrossRef]
- Nam, K.-W.; Yoon, W.-S.; Shin, H.; Chung, K.Y.; Choi, S.; Yang, X.-Q. In situ X-ray diffraction studies of mixed LiMn2O4−LiNi1/3Co1/3Mn1/3O2 composite cathode in Li-ion cells during charge–discharge cycling. J. Power Sources 2009, 192, 652–659. [Google Scholar] [CrossRef]
- Jang, D.H.; Shin, Y.J.; Oh, S.M. Dissolution of spinel oxides and capacity losses in 4 V Li/LixMn2O4 cells. J. Electrochem. Soc. 1996, 143, 2204. [Google Scholar] [CrossRef]
- Nishimura, K.; Douzono, T.; Kasai, M.; Andou, H.; Muranaka, Y.; Kozono, Y. Spinel-type lithium–manganese oxide cathodes for rechargeable lithium batteries. J. Power Sources 1999, 81, 420–424. [Google Scholar] [CrossRef]
- Terada, Y.; Nishiwaki, Y.; Nakai, I.; Nishikawa, F. Study of Mn dissolution from LiMn2O4 spinel electrodes using in situ total reflection X-ray fluorescence analysis and fluorescence XAFS technique. J. Power Sources 2001, 97, 420–422. [Google Scholar] [CrossRef]
- Lu, C.-H.; Lin, S.-W. Dissolution kinetics of spinel lithium manganate and its relation to capacity fading in lithium ion batteries. J. Mater. Res. 2002, 17, 1476–1481. [Google Scholar] [CrossRef]
- Leung, K. First-principles modeling of Mn (II) migration above and dissolution from LixMn2O4 (001) surfaces. Chem. Mater. 2017, 29, 2550–2562. [Google Scholar] [CrossRef]
- Shilina, Y.; Ziv, B.; Meir, A.; Banerjee, A.; Ruthstein, S.; Luski, S.; Aurbach, D.; Halalay, I.C. Combined electron paramagnetic resonance and atomic absorption spectroscopy/inductively coupled plasma analysis as diagnostics for soluble manganese species from Mn-based positive electrode materials in Li-ion cells. Anal. Chem. 2016, 88, 4440–4447. [Google Scholar] [CrossRef]
- Banerjee, A.; Shilina, Y.; Ziv, B.; Ziegelbauer, J.M.; Luski, S.; Aurbach, D.; Halalay, I.C. On the oxidation state of manganese ions in Li-ion battery electrolyte solutions. J. Am. Chem. Soc. 2017, 139, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.; Li, Z. A comparative study of commercial lithium ion battery cycle life in electrical vehicle: Aging mechanism identification. J. Power Sources 2014, 251, 38–54. [Google Scholar] [CrossRef]
- Zhan, C.; Lu, J.; Jeremy Kropf, A.; Wu, T.; Jansen, A.N.; Sun, Y.-K.; Qiu, X.; Amine, K. Mn (II) deposition on anodes and its effects on capacity fade in spinel lithium manganate–carbon systems. Nat. Commun. 2013, 4, 2437. [Google Scholar] [CrossRef] [PubMed]
- Hawley, W.B.; Parejiya, A.; Bai, Y.; Meyer, H.M., III; Wood, D.L., III; Li, J. Lithium and transition metal dissolution due to aqueous processing in lithium-ion battery cathode active materials. J. Power Sources 2020, 466, 228315. [Google Scholar] [CrossRef]
- Gao, H.; Yan, Q.; Xu, P.; Liu, H.; Li, M.; Liu, P.; Luo, J.; Chen, Z. Efficient direct recycling of degraded LiMn2O4 cathodes by one-step hydrothermal relithiation. ACS Appl. Mater. Interfaces 2020, 12, 51546–51554. [Google Scholar] [CrossRef]
- Bang, H.J.; Donepudi, V.; Prakash, J. Preparation and characterization of partially substituted LiMyMn2−yO4 (M=Ni, Co, Fe) spinel cathodes for Li-ion batteries. Electrochim. Acta 2002, 48, 443–451. [Google Scholar] [CrossRef]
- Liu, W.; Farrington, G.; Chaput, F.; Dunn, B. Synthesis and electrochemical studies of spinel phase LiMn2O4 cathode materials prepared by the Pechini process. J. Electrochem. Soc. 1996, 143, 879. [Google Scholar] [CrossRef]
- Hashem, A.; Abdel-Ghany, A.; Abuzeid, H.; El-Tawil, R.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C. EDTA as chelating agent for sol-gel synthesis of spinel LiMn2O4 cathode material for lithium batteries. J. Alloys Compd. 2018, 737, 758–766. [Google Scholar] [CrossRef]
- Xia, Y.; Yoshio, M. An investigation of lithium ion insertion into spinel structure li-mn-o compounds. J. Electrochem. Soc. 1996, 143, 825. [Google Scholar] [CrossRef]
- Huang, W.; Wang, G.; Luo, C.; Xu, Y.; Xu, Y.; Eckstein, B.J.; Chen, Y.; Wang, B.; Huang, J.; Kang, Y. Controllable growth of LiMn2O4 by carbohydrate-assisted combustion synthesis for high performance Li-ion batteries. Nano Energy 2019, 64, 103936. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable recovery of cathode materials from spent lithium-ion batteries using lactic acid leaching system. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Palaniyandy, N.; Rambau, K.; Musyoka, N.; Ren, J. A facile segregation process and restoration of LiMn2O4 cathode material from spent lithium-ion batteries. J. Electrochem. Soc. 2020, 167, 090510. [Google Scholar] [CrossRef]
- Lin, J.; Fan, E.; Zhang, X.; Li, Z.; Dai, Y.; Chen, R.; Wu, F.; Li, L. Sustainable upcycling of spent lithium-ion batteries cathode materials: Stabilization by in situ Li/Mn disorder. Adv. Energy Mater. 2022, 12, 2201174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).