Abstract
In recent years, algae have emerged as a promising feedstock for biofuel production, due to their eco-friendly, sustainable, and renewable nature. Various methods, including chemical, biochemical, and thermochemical processes, are used to convert algal biomass into biofuels. Pyrolysis, a widely recognized thermochemical technique, involves high temperature and pressure to generate biochar and bio-oil from diverse algal sources. Various pyrolytic processes transform algal biomass into biochar and bio-oil, including low pyrolysis, fast pyrolysis, catalytic pyrolysis, microwave-assisted pyrolysis, and hydropyrolysis. These methods are utilized to convert a range of microalgae and cyanobacteria into biochar and bio-oil. In this publication, we will discuss catalytic pyrolysis using mesoporous materials, such as SBA-15. Mesoporous catalysts have earned significant attention for catalytic reactions, due to their high surface area, facilitating the better distribution of impregnated metal. Pyrolysis conducted in the presence of a mesoporous catalyst is viewed more as efficient, compared to reactions occurring within the smaller microporous cavities of traditional zeolites. SBA-15 supports with incorporated Zr and/or Ce were synthesized using the direct hydrothermal synthesis method. The catalyst was characterized using structural and morphological technical analysis and utilized for the pyrolysis reaction of the algal biomass.
1. Introduction
Green microalgae have previously been tested for their yield of bio-oil, and the results are promising. Green macroalgae and microalgae are potential fuel sources for many reasons. Microalgal bio-oils have a high concentration of aromatic compounds, sugars, and more high-value chemicals. These microalgal oils could be used to produce biodiesel through various chemical conversion techniques [1]. Biofuels from microalgae are considered third-generation biofuels, meaning they are used to improve the environmental performance of biofuels produced from the existing feedstocks. The integration of microalgae in biorefineries is a priority, and studies are ongoing [2]. Green microalgae are frequently regarded as an important source for the production of bio-oil.
The pyrolysis process seems to be a great option for converting energy. The advantage is that it can utilize various organic material sources without being restricted by the lipid content, unlike biodiesel production. The pyrolysis of biomass is considered a renewable process because during the process, the biomass is converted into different gasses, such as carbon dioxide, which is absorbed by the algae for their growth, establishing a self-sustaining process. The relative yield of each phase generated in the process depends on several factors. These include operating parameters, such as temperature, heating rate, residence time, and flow rate of inert gas, as well as properties of the biomass, including particle size and moisture content. Additionally, the type of pyrolysis used, whether slow, fast, or flash pyrolysis, also influences the outcome [3].
The process of pyrolysis involves decomposing organic compounds in the total biomass in a controlled environment without oxygen and atmospheric pressure, producing distinct phases: liquid (bio-oil), gas, and solid (char). An endothermic reaction takes place at a temperature of 300–700 °C. The pyrolysis temperature depends on the characteristics of the material to be pyrolyzed [4]. The petrochemical, automotive, and aviation industries have a growing interest and investment in biomass catalytic hydroprocessing technology and demonstrate that this technology will have a significant impact on the biofuel industry in the near future.
Compared to their fossil counterparts, pyrolysis technology products have improved characteristics; they have a high heating value, increased oxidation stability, reduced acidity, and an elevated saturation level.
The outcomes of pyrolysis technology exhibit enhanced attributes relative to both their fossil-derived counterparts and traditional biofuels. These advancements encompass a heightened heating value and cetane number, augmented oxidation stability, minimal acidity, and an elevated saturation level.
In addition to its application in producing high-quality paraffinic fuels, catalytic pyrolysis serves as an effective method for converting liquid, gaseous, and solid biofuels. It facilitates the upgrading of intermediate products generated by solid biomass conversion technologies, including pyrolysis oils and Fischer–Tropsch wax. Generally, this catalytic process enables the transformation of triglycerides and lipids into paraffins and iso-paraffins. Zeolites are commonly used as catalysts in pyrolysis processes because of their crystallinity, well-defined pore structures, large surface area, strong acidity, and good thermal stability [5].
In the present study, several mesoporous SBA-15-based catalysts with ceria, zirconia, and ceria–zirconia were synthesized using the direct synthesis method (hydrothermal method) [6] to use them for algal (C. homosphaera 424 CCAP 211/121 strain) biomass pyrolysis.
There are several methods for the synthesis of mesoporous materials. The mesostructured hybrid network could be previously formed, or a cooperative process could be used. Alternatively, such mesostructured hybrid networks could be prepared by using preformed nano building blocks [7].
In the various synthesis methods used for crafting mesoporous materials, it is crucial to meticulously adjust the texturing agent’s chemical, spatial, and structural characteristics, often referred to as “reaction pockets.” This adjustment involves the careful manipulation of chemical reaction rates, interface properties, and the encapsulation process of the developing inorganic phase. Precisely tailoring the organomineral interface stands out as the pivotal stage in achieving clearly defined textured phases [8]. Achieving control over the chemical, spatial, and temporal aspects of this “hybrid interface” presents a significant challenge in the endeavor to develop cooperatively assembled inorganic–organic integrated systems [9].
The objective of this research was to produce catalytic materials with open mesoporosity, which would help in the transfer of reagents and products through the pores of the catalytic supports. Using the large surface area of the mesoporous SBA-15 material, adding metal oxides was an effective way to improve catalytic performance. The combination of cerium’s acidity and zirconium’s acidity may improve the catalytic material’s performance and selectivity by introducing chemical alterations into the support’s composition.
2. Materials and Methods
2.1. Materials
All reagents used to prepare the zeolite catalysts were purchased from Sigma-Aldrich (Merck Group, Darmstadt, Germany). The algae biomass was produced, using as the strain C. homosphaera 424 CCAP 211/121, from our institute collection.
2.2. Preparation of Zeolite Catalysts
Ce/SBA-15, Zr/SBA-15, and Zr-Ce/SBA-15 catalysts were synthesized using the direct hydrothermal method [10].
We followed the standard procedure for the synthesis of the catalyst without adding any modifications or optimizations.
The direct hydrothermal method started usually with the preparation of a precursor solution including metal salts, organic ligands, and a solvent. The pH and temperature were adjusted to encourage nucleation and crystal formation. The characteristics and quality of the final product were determined by the choice of metal salts and organic ligands (template).
Amphiphilic P123 triblock copolymer (Pluronic P123, EO20PO70EO20, molecular weight = 5800, Sigma-Aldrich) was used as the template, and tetraethyl orthosilicate (TEOS, Sigma-Aldrich) was used as the silica source. Initially, 1 g of Pluronic P123 was dissolved in 40.0 g of 2 M HCl solution while stirring until the polymer was completely dissolved. Afterward, 2.1 g of TEOS was gradually added to the solution drop by drop. Following this, Ce(NO3)3·H2O) (Sigma-Aldrich) and/or ZrOCl2·8H2O (Sigma-Aldrich) were added into the solution under continuous stirring. The mixed solution was continuously stirred for 24 h at 40 °C and subsequently crystallized in a Teflon-lined stainless steel autoclave at 100 °C for another 24 h. The resulting precipitate was filtered, washed with DI water, and dried overnight at 80 °C. The organic template was then removed from the porous structure by calcination at 550 °C for 6 h, with a heating rate of 1.5 °C/min. The obtained products were designated as Ce/SBA15-DSM (molar ratio of Ce/Si = 0.1), Zr/SBA15-DSM (molar ratio of Zr/Si = 0.1), and Zr-Ce-SBA15-DSM (molar ratio of Zr/Si = 0.05 and Ce/Si = 0.05), according to Scheme 1.
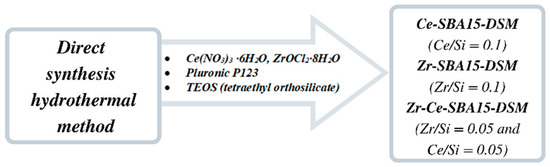
Scheme 1.
Synthesis steps of mesoporous Ce, Zr/SBA15.
2.3. Physico-Chemical Characterization of the Prepared Catalysts
The prepared catalysts were characterized by TGA, XRD, N2 adsorption–desorption analysis, and Raman analysis.
Thermogravimetric analyses were conducted on a TA Q5000 (TA Instruments, New Castle, DE, USA). Samples were heated at a rate of 10 °C/min from room temperature and reaching 1000 °C, within a nitrogen atmosphere, with a gas flow of 50 mL/min gas.
X-ray diffraction (XRD) patterns were recorded on a Dron UN-1 diffractometer (Bourevestnik, Saint-Petersburg, Russia) using a setup equipped with an anti-cathode CuKα (λ = 1.54178 Å). For the full-range XRD analysis, patterns were recorded across 2θ values ranging from 10° to 80°, with a scanning rate of 5 s/step and with 0.05 step sizes. Additionally, low-angle XRD patterns were recorded using a Drom UN-1 diffractometer, with a CuKα (λ = 1.54178 Å) radiation within the 2θ range of 0.3–6°, with 0.02° steps.
Raman analysis was carried out using an inVia Confocal Raman microscope system (Renishaw, Wotton-under-edge, UK). The excitation laser wavelength was 473 nm.
The Raman spectra were acquired in the extended spectral region from 100 to 3200 cm−1. Raman studies were carried out under ambient conditions.
Textural characteristics of the catalysts (surface area, pore volume, average pore diameter, and pore size distribution) were determined on an Autosorb 1 Nova 2200 Analyzer (Quantacrome Instruments, Boynton Beach, FL, USA). Texture data were obtained by the automatic recording and processing of adsorption–desorption isotherms of nitrogen. The specific surface area was calculated using the equation in the linear part of the BET adsorption isotherm. To assess the distribution of pores and the pore size, the desorption branch of isotherms with hysteresis was used by applying the BJH method [11].
The samples in fine powder form were contacted with formvar/carbon nickel grids (Emdiasum, Hatfield, PA, USA) and analyzed by transmission electron microscopy (TEM). The obtained images were taken in bright-field mode (BF-TEM) with a TECNAI F20 G2 TWIN Cryo-TEM (FEI, Hillsboro, OR, USA) at an accelerating voltage of 200 kV.
2.4. Microalgae Cultivation and Biomass Harvesting
The C. homosphaera 424 CCAP 211/121 strain was cultivated in BBM media. Cultivation was performed in a tubular photobioreactor at 25 °C and with a photosynthetic photon flux density of 100 µmoli·m−2·s−1. Harvesting was performed by electroflocculation.
2.5. The Pyrolysis Reaction
The pyrolysis reactions were performed in a laboratory-scale fixed-bed reactor with a descending flow of liquid and gas, Series 5400, coupled with a 487-process controller (Parr Instrument Company, Moline, IL, USA), as shown in Figure 1. The fixed-bed reactor possessed a long, cylindrical shape and was equipped with a gas-cooling system. The reaction was carefully controlled, with heat provided by an external furnace. To maintain inert atmospheric conditions, a gas cylinder was externally attached, with nitrogen serving as the preferred inert carrier gas. Additionally, a cooling system was integrated into the reactor to facilitate the condensation of vapors into pyrolysis oil. These reactors are well-suited for low-scale production. It is important to note that fixed-bed reactors are designed to accommodate a specific pressure drop, and the mass of the catalyst filling the reactor must not exceed the specification for the adiabatic fixed-bed reactor.
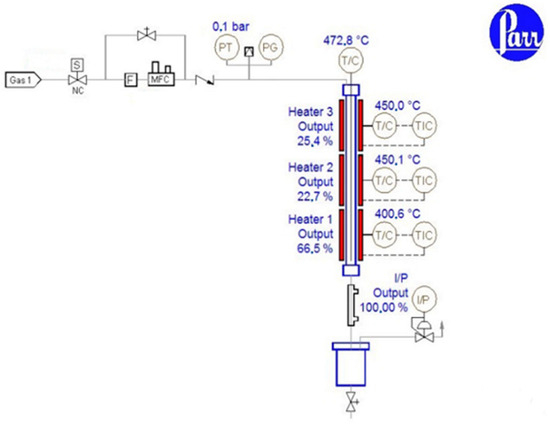
Figure 1.
Schematic diagram of the reaction system.
The suspension obtained from the mixture of wet biomass and catalyst was fed into the Parr reactor. (Wet biomass/catalyst ratios were 35 g:0.35 g, corresponding to a ratio of 1/100 catalyst/wet biomass.) The reaction parameters for the pyrolysis reaction were: temperature 450 °C, atmospheric pressure, catalyst concentration 1–100 wt.%, and residence time of 1–10 s.
2.6. Analysis of the Pyrolysis Products
The liquid fraction was continuously analyzed using a GC–MS/MS TRIPLE QUAD chromatograph (Agilent 7890 A from Agilent Technologies, Santa Clara, CA, USA) equipped with a DB-WAX capillary column. For the determination of the hydrocarbon C1-C4 fraction in the gaseous fraction, a Varian 3800 gas chromatograpf from Conquer Scientific (Poway, CA, USA) coupled with a mass spectrometer (Varian 4000 from Conquer Scientific, Poway, CA, USA) was operated, utilizing an Agilent VF-5 from Agilent Technologies, Santa Clara, CA, USA, MS capillary column. Helium served as the carrier gas for GC/MS analysis, with the injector temperature maintained at 250 °C. Initially, the oven temperature was set to 30 °C, followed by a gradual increase of 3 °C/min up to 100 °C. The structural characterization of the resulting biochar was obtained by textural analysis and FTIR analysis.
Thermogravimetric analysis (TGA) of the biochar materials (in powder form) was conducted using a TA TGA Q500 IR instrument from TA Instruments, New Castle, DE, USA. Samples between 6–9 mg were loaded into aluminum pans and heated from 40 to 700 °C under a nitrogen atmosphere, with a heating rate of 10 °C/min.
Fourier transform infrared (FTIR) spectra of the biochars were obtained using a Jasco FT-IR 6300 instrument, manufactured by JASCO International Co., Ltd., Tokyo, Japan, equipped with an attenuated total reflectance (ATR) accessory of a diamond crystal. Data collection was conducted at room temperature, covering a wavenumber range of 400–4000 cm−1, with 30 scans performed at a resolution of 4 cm−1.
3. Results and Discussions
3.1. Preparation and Characterization of the Catalysts
TGA curves vs. temperatures performed on the solid Ce-SBA15-DSM (a), Zr-SBA15-DSM (b), and Zr-Ce-SBA15-DSM (c), prepared via the direct synthesis method, are reported in Figure 2 and Figure 3. The thermograms highlight the presence of two critical steps of decomposition of the mixed oxides: for the first decomposition, a weight loss in the range of 70–200 °C was reported, with 2 peaks. The first peak was centered at 175 °C, due to dehydration, and the second peak was centered at 200 °C, associated with the decomposition of nitrates.
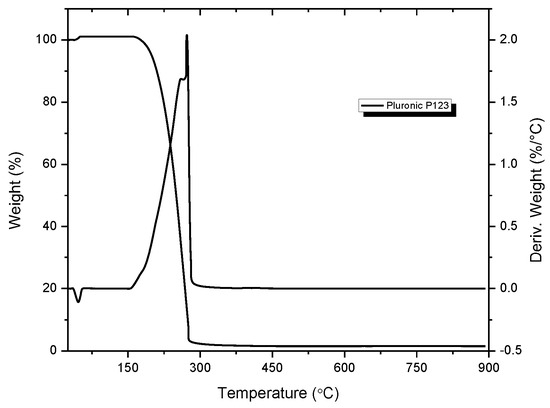
Figure 2.
Thermal decomposition of the surfactant P123.
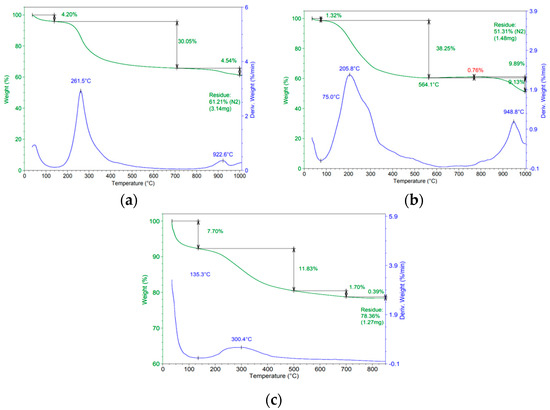
Figure 3.
Thermal decomposition of the Ce-SBA15-DSM (a), Zr-SBA15-DSM (b), and Zr-Ce-SBA15-DSM (c), dried at 100 °C.
The second decomposition interval was in the range of 200–400 °C, with 2 peaks: the first peak, centered at 300 °C, can be attributed to the decomposition of polymer P123 (and hybrid with P123); as we can also see in the thermogram of decomposition of P123, the second peak, centered at 400 °C, was associated with the interaction between the template and precursors and the crystallization of Ce-SBA15.
For temperatures higher than 400 °C, there were no additional peaks in the breakdown curves and no weight loss in the TG curve. Such behavior indicates that the sample’s total crystallization occurred under these conditions, and all the organic materials were removed [12].
The X-ray diffraction (XRD) analysis, as depicted in Figure 4 and Figure 5, provided significant insights into the crystalline phases formed during synthesis.
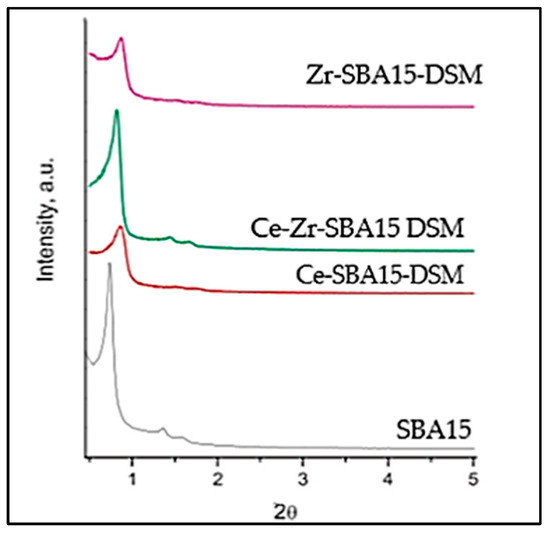
Figure 4.
Small-angle XRD for Ce-SBA15-DSM, Zr-SBA15-DSM, and Ce-Zr-SBA15 DSM.
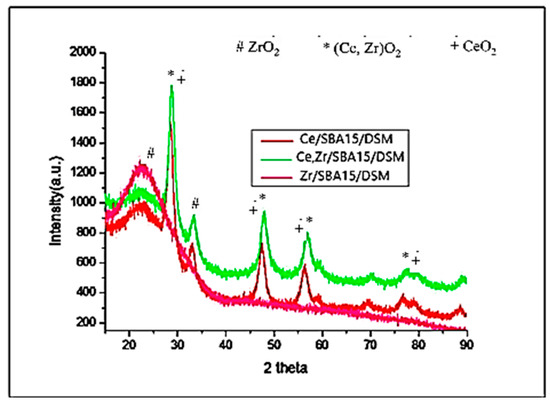
Figure 5.
Wide angle XRD for Ce-SBA15-DSM, Zr-SBA15-DSM, and Ce-Zr-SBA15 DSM.
In the small-angle XRD patterns, a well-defined reflection centered at 0.55° was observed for the SBA-15 catalytic support, with a broadening and shifting of the X-ray line toward a higher 2θ angle (1°) for the Ce-SBA15-DSM, Zr-SBA15-DSM, and Ce-Zr-SBA15 DSM. This result evidenced the genesis of an ordered mesophase (P6mm hexagonal symmetry). During the surfactant’s decomposition in the presence of the inorganic species, there was little chance that the mesostructure partially decreased.
Wide-angle XRD of the catalysts indicated that for the Zr-SBA15-DSM pattern, there was a broad peak typical of materials that are amorphous.
The diffraction angles corresponding to reference cerium oxide phases were evident in these catalysts. The Ce-SBA15-DSM exhibited a face-centered, cubic, fluorite-type structure.
The XRD patterns of Ce-Zr-SBA15 DSM were indicative of the well-crystallized inorganic frameworks in a crystalline tetragonal phase [13,14].
The nitrogen adsorption–desorption isotherms exhibited a characteristic type IV isotherm with a type H1 hysteresis cycle, characteristic of the hexagonal array typical of the SBA-15 mesoporous structure. Adding promoters like Ce and Zr via the direct-synthesis method kept the mesoporous structure almost unchanged, as can be seen in Figure 6.
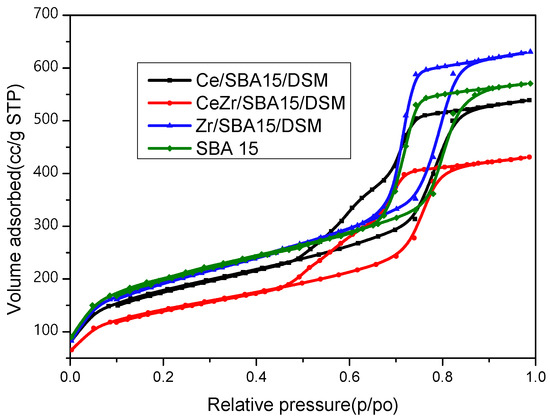
Figure 6.
Nitrogen adsorption–desorption isotherms for the catalysts and for the support.
The specific surface was almost the same for all the catalysts, according to the SBA 15 surface, as seen in Table 1.

Table 1.
N2-BET surface area of catalysts.
Figure 7 shows the Raman spectra for different ZrO2, CeO2, and Ce-Zr/SBA-15 catalysts. It can be seen that a strong peak at 1200 cm−1 and a weak peak at 600 cm−1 can be observed in all the samples. Furthermore, a distinct peak at 455 cm−1 can be observed on all the samples, due to the CeO2 (460 cm−1) presence, and it is attributed to the presence of oxygen vacancies in the CeO2 lattice. The presence of oxygen vacancies in all catalysts is attributed to the incorporation of CeO2 or ZrO2 into the SBA-15 support [15].
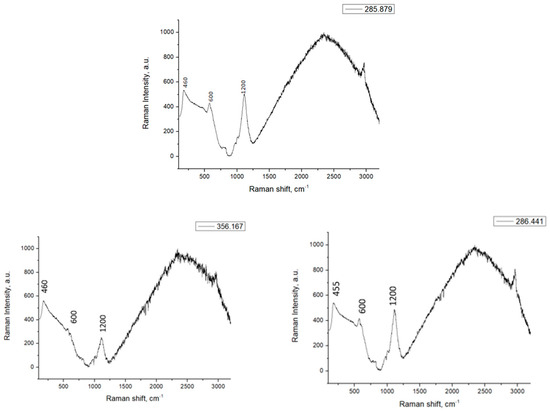
Figure 7.
Raman spectra of ZrO2, CeO2, and Ce-Zr/SBA-15 catalysts.
Transmission electron microscopy was used to evaluate the morphologies of the cerium–zirconia grains and the locations of the metal particles. TEM investigations were performed on different sections of the 500 °C-calcined Ce/SBA-15, Zr/SBA-15, and Ce-Zr/SBA-15 samples (refer to Figure 8). First, the Ce and Zr elements that composed the Ce–Zr grains were distributed uniformly.
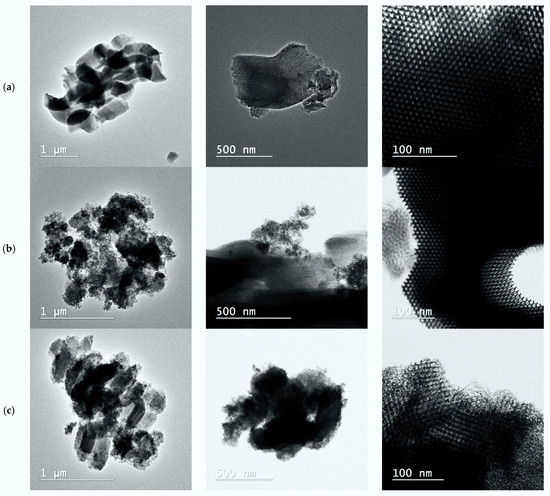
Figure 8.
TEM images recorded on Ce/SBA-15 (a), Zr/SBA-15 (b), and Ce-Zr/SBA-15 (c) catalysts calcined at 550 °C.
As a general trend, relatively good incorporations of Ce and Zr were discernible on all samples. However, greater homogeneity in the composition appeared on the series Ce/SBA-15, Zr/SBA-15, and Ce-Zr/SBA-15, with the detection of Ce-, Zr-, or Ce/Zr-enriched microdomains likely ascribed to the agglomeration process. In contrast, Ce or Zr seemed to be orderly distributed on SBA15. The Zr- and Ce-enriched areas are highlighted on all samples, suggesting a homogeneous distribution to a slight extent.
3.2. Characterization of Catalytic Pyrolysis Products
For the best bio-oil yield, a moderate temperature range of 400–550 °C was ideal. Beyond this temperature range, the bio-oil and char were transformed into gaseous products as the secondary cracking occurrence increased in speed. Furthermore, at temperatures higher than 700 °C, there was a rise in the decarboxylation and dehydration processes, which resulted in an increase in the amount of polycyclic aromatic hydrocarbons (PAHs), like phenanthrene and pyrene, in the bio-oil. The yields were similar, and the maximum yield of 59% for the bio-oil was achieved for the Ce-Zr/SBA-15, with 55% and 50% for Ce/SBA-15 and Zr/SBA-15, respectively.
The composition of the liquid phase separated from the pyrolysis of the algal biomass, as determined from gas chromatograms, is presented in the next tables for each catalyst.
The identification and quantification of the pyrolysis products, such as palmitic, stearic, and linoleic acids, phenol, p-cresol, and indole, were performed by using GC-MS quantitative analysis of the extracted samples. A total of twelve fatty acids were identified by the GC-MS analysis of the methyl transesterified algal oil, which was extracted using ethanol. By comparing retention times and mass spectra of recognized standards, the saturated fatty acids (SFA) and unsaturated fatty acids (PUFAs and MUFA) in the analyzed microalgae oil samples were identified at their peaks (Table 2, Table 3, Table 4 and Table 5).

Table 2.
Composition of the hydrocarbon fractions in the bio-oil for the pyrolysis reaction without a catalyst.

Table 3.
The composition of the hydrocarbon fractions in the bio-oil, as determined from gas chromatograms for the Ce/SBA 15 DSM.

Table 4.
The composition of the hydrocarbon fractions in the bio-oil, as determined from gas chromatograms for the Zr/SBA 15 DSM.

Table 5.
The composition of the hydrocarbon fractions in the bio-oil, as determined from gas chromatograms for the Ce-Zr/SBA 15 DSM.
The experimental test results for the pyrolysis reaction using the Ce-Zr-SBA15DSM catalyst revealed a complex mixture comprising numerous different hydrocarbons and heteroatoms (primarily O and N) that were present in the algal biocrude. Various groups of compounds were identified in the bio-oil, including aromatic hydrocarbons, heterocyclic compounds, phenols, amines, amides, indoles, alkanes, and nitriles. The carbon chain length in the bio-oil ranged from C7 to C17. The oxygen-containing compounds primarily consisted of saturated fatty acids (such as hexadecanoic acid) and phenol derivatives, like p- and m-cresol. Additionally, fatty alcohols, such as dodecanol, were observed in small quantities. The compositions of the bio-oils for the pyrolysis reactions without and with a catalyst are presented in Table 2, Table 3, Table 4 and Table 5. In the absence of a catalyst, the bio-oil mainly comprised oxygenated compounds, such as acetic acid, ketones, aldehydes, and furans, likely resulting from the decomposition of carbohydrate constituents. It also contained N-compounds, such as pyrroles, indole, pyrazoles, oleamides, and fatty nitriles, which are products of protein decomposition. Furthermore, glycerides, fatty esters, and aliphatic and fatty acids (predominantly octadecanoic, hexadecanoic, heptanoic, and octanoic acid) were presented, resulting from the decomposition and/or volatilization of lipid constituents in microalgae. As described recently [16], each of the microalgae components undergoes various pathways to olefins—(a) oxygenates from carbohydrate degradation undergo deoxygenation, followed by cracking; (b) proteins undergo deamination, followed by cracking to aromatics; and (c) lipids undergo decarboxylation and decarbonylation to form long-chain hydrocarbons, which are then cracked to shorter olefins. Following these processes, dienes and olefins undergo cyclization and dehydrogenation to form aromatics [17]. The amide compounds are produced from the long-chain fatty acids by replacing their hydroxyl groups with ammonia (derived from the deamination of amino acids). Esters are formed from the reaction between the fatty acids and alcohols (resulting from the reduction of an organic acid). Additionally, N&O-heterocyclic compounds, such as pyrrole, pyridine, pyrrolidinone, pyrrolidinone, imidazole, thiazole, and their derivatives, are formed through the Maillard reaction between amino acids and reducing sugars. Further, specific nitrogen and oxygen heterocyclic compounds might react with long-chain fatty acids to form pyrrolidinone derivatives of fatty acids.
The main compositions in bio-oil were indoles and phenols, with similar concentrations of indoles and phenols, with a maximum phenol percentage of 60% for the Ce-Zr/SBA 15 catalyst. Concerning the biochar obtained after the pyrolysis of the algal biomass, in the composition, there were some C compounds, suggesting that algal biochar can act directly as a fertilizer without the addition of other constituents. There were no significant differences between the three catalysts used for the pyrolysis.
FT-IR analysis was performed for the biochars, and the results are described in the next section.
As seen in Figure 9, the FTIR spectra shared the corresponding bands as follows: SBA-15.
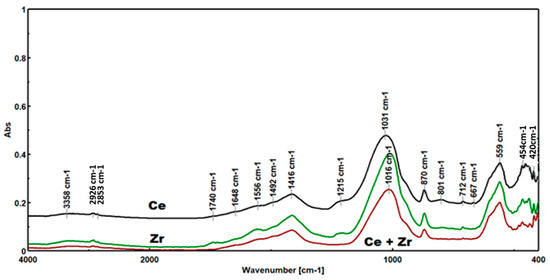
Figure 9.
The FTIR spectra of the biochars obtained after pyrolysis.
- 3358 cm−1: Stretching vibration of the associated O-H.
- 1648 cm−1: Binding vibration of water molecules.
- 1016–1031 cm−1: Asymmetrical tension vibration of the Si-O-Si link.
Very wide: Si-O-C; C-O-C (carbohydrates); Si-OH (shoulder); and C-OH (carbohydrates).
- 870 cm−1: Symmetrical tension vibration of the SI-O-Si link.
- 559 cm−1: Deformation vibration of the SI-O-Si connection.
Organic residues were present in the char, as well as in SBA-15.
- 2968 cm−1: Link tension vibration C-H (of groups CH3).
- 2926 cm−1: Asymmetrical tension vibration of the C-H link (of groups CH2).
- 2853 cm−1: Symmetrical tension vibration of the C-H link (of groups CH2).
- 945 cm−1: Si-O-Zr linkage tension vibration (shoulder).
- 667 cm−1: Zr-O H linkage tension vibration
- 454 cm−1: Tension vibration of the connection.
- 420 cm−1: Zr-O linkage tension vibration.
The characteristics bands for biochar:
- 1740 cm−1: Bonding of tension vibration C=O;
- 1556 cm−1: Bonding of tension vibration C=C aromatic;
- 870 cm−1: Out-of-plane deflection vibration of the C-H aromatic connection [18,19,20].
The TGA analysis of the biochar was performed in order to establish if there were residual components after pyrolysis. The thermogram is shown in Figure 10.
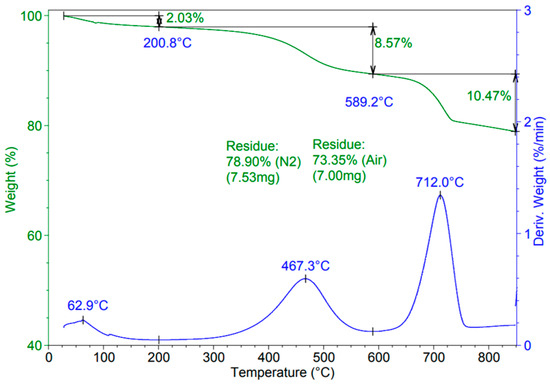
Figure 10.
TGA of biochar that resulted after pyrolysis.
As is evident in Figure 10, all residues were burned and activated at 700 °C and had lower ash contents. After 750 °C and 800 °C, no thermal activities were identified. The thermogram was characteristic of inactivate biochar. There were also residues of proteins, lipides, and carbohydrates at 467 °C (the residue was 78.9 % (N2) and 73.35% (Air), which can lead us to the conclusion that the pyrolysis temperature could be increased to 500 °C for the valorification of all the biomass components [21].
For a variety of the catalytic effects, metal oxides and zeolites are typically combined. Wang et al. [22] studied the pyrolysis of Enteromorpha clathrata using the catalysis of Mg-Ce oxides supported on ZSM-5. They discovered that the modified ZSM-5 catalysts showed a promising performance in increasing the relative content of C5–C7 and decreasing that of acids. Gao et al. [23] presented a type of Mg-Al layered double oxide/ZSM-5 composite and studied its catalytic performance on the pyrolysis of Cyanobacteria. They noticed that the MgAl-LDO/ZSM-5 catalyst with the best structure might achieve bio-oils that have higher yields and quality, in contrast with the non-catalytic process and the catalytic reaction with zeolite or only oxides.
The pyrolysis reaction of Rhizoclonium sp. algae to produce bio-oil was investigated by Casoni et al. [24]. It was discovered that the SBA-15 or SBA-15-based catalysts decreased the quantities of aliphatic alcohols and several high-molecular-weight products in the bio-oil. As a result, the use of catalysts enhanced the amounts of visible products collected. Three different zeolite types (H-Y, H-β, and H-ZSM-5) were utilized by Du et al. [25] to catalyze the pyrolysis of Chlorella vulgaris. The outcomes demonstrated that catalytic pyrolysis produced significantly more aromatic hydrocarbons than the non-catalytic method.
4. Conclusions
In this study, ceria-, zirconia-, and ceria-zirconia-based mesoporous catalysts were combined to improve the performance and physicochemical properties of the catalysts. The characterization results of the catalysts showed the presence of mesopores in the catalysts and confirmed the formation of a composite structure of mesoporous zeolites, as evidenced by characterization results. Sustained by the results of the characterization, all catalysts presented a high surface area and a well-ordered hexagonal array of channels. The prepared catalysts exhibited significant activity for the pyrolysis reaction of the algal biomass, yielding specific products, such as palmitic, stearic, and linoleic acids. Additionally, phenol, p-cresol, and indole were obtained as products in the pyrolysis reaction.
The pyrolysis of algae yielded a higher content of bio-oil, which is rich in heteroatoms, such as N, O, and S.
We obtained bio-oil rich in heteroatoms, such as N, O, and S, and the next research will focus on the challenge of reducing the heteroatom content in bio-oil in order to ensure the improvement of upgraded bio-oil, with respect to the specifications for drop-in fuels. However, reducing the heteroatom content in bio-oil poses a current challenge and necessitates further research and development efforts.
In a recent publication, Dirgarini et al. [26] concluded that when Ni/SBA-15 was used as a catalyst in the pyrolysis of algal biomass, products were chemically composed of furans, furfurals, nitrogen aromatic compounds, and alkyl aromatics, and the efficiency of molecular collisions increased. Usually, the pyrolysis and heat decomposition of proteins and carbohydrates produce these aromatic chemicals. Compared with our results, the conclusions were comparable, with the exception that in our case, we decreased the reaction temperature, which can be economically advantageous.
Author Contributions
Conceptualization, S.-B.G. and G.V.; methodology, S.-B.G., G.V. and F.O.; validation, G.V. and F.O.; formal analysis and investigation, S.-B.G., G.V., M.-F.R., M.C.-U., B.T. and A.-L.M.; resources, G.V. and F.O.; writing—original draft preparation, S.-B.G. and G.V.; writing—review and editing, S.-B.G. and F.O.; visualization, S.-B.G.; supervision, G.V. and F.O.; project administration, F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out through the PN 23.06 Core Program, ChemNewDeal, within the National Plan for Research, Development, and Innovation 2022–2027, and developed with the support of the Ministry of Research, Innovation, and Digitization, project no. PN 23.06.02.01 InteGral, including Program 1, Development of the national research and development system, and Subprogram 1.2, Institutional Performance—Projects to finance excellence in RDI, Grant no. 15PFE/2021.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
Fierascu Radu is acknowledged for the XRD analysis. Verziu Marian is acknowledged for the Raman analysis, and Nicolae Cristian is acknowledged for the thermogravimetric analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Budarin, V.L.; Shuttleworth, P.S.; Dodson, J.R.; Hunt, A.J.; Lanigan, B.; Marriott, R.; Milkowski, K.J.; Wilson, A.J.; Breeden, S.W.; Fan, J.; et al. Use of green chemical technologies in an integrated biorefinery. Energy Environ. Sci. 2010, 4, 471–479. [Google Scholar] [CrossRef]
- Golberg, A.; Liberzon, A. Modeling of smart mixing regimes to improve marine biorefinery productivity and energy efficiency. Algal Res. 2015, 11, 28–32. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Martini, P.R.R. Conversão Pirolítica de Bagaço Residual da Indústria de Suco de Laranja e Caracterização Química dos Produtos. Master’s Dissertation, PPGQ, Universidade Federal de Santa Maria, Santa Maria, Brazil, 2009. [Google Scholar]
- Zhang, M.; Hu, Y.; Wang, H.; Li, H.; Han, X.; Zeng, Y.; Xu, C.C. A review of bio-oil upgrading by catalytic hydrotreatment: Advances, challenges, and prospects. Mol. Catal. 2021, 504, 111438. [Google Scholar]
- Beims, R.F.; Hu, Y.; Shui, H.; Xu, C.C. Hydrothermal liquefaction of biomass to fuels and value-added chemicals: Products applications and challenges to develop large-scale operations. Biomass Bioenergy 2020, 135, 105510. [Google Scholar] [CrossRef]
- Issa, G.; Kormunda, M.; Tumurbaatar, O.; Szegedi, Á.; Kovacheva, D.; Karashanova, D.; Popova, M. Impact of Ce/Zr Ratio in the Nanostructured Ceria and Zirconia Composites on the Selective CO2 Adsorption. Nanomaterials 2023, 13, 2428. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Amin, N.S. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energ. Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Yanik, J.; Stahl, R.; Troeger, N.; Sinag, A. Pyrolysis of algal biomass. J. Anal. Appl. Pyrolysis 2013, 103, 134–141. [Google Scholar] [CrossRef]
- Hognon, C.; Delrue, F.; Texier, J.; Grateau, M.; Thiery, S.; Miller, H.; Roubaud, A. Comparison of pyrolysis and hydrothermal liquefaction of Chlamydomonas reinhardti. Growth studies on the recovered hydrothermal aqueous phase. Biomass Bioenergy 2015, 73, 23–31. [Google Scholar]
- Huber, G.W.; Corma, A. Synergies between bio-and oil refineries for the production of fuels from biomass. Angew. Chem. Intern. Edn. 2007, 46, 7184–7201. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Jardim, E.O.; Ramos-Fernandez, E.V.; Villora-Picó, J.J.; Pastor-Blas, M.M.; Silvestre-Albero, J.; Órfão, J.J.M.; Pereira, M.F.R.; Sepúlveda-Escribano, A. Highly N2-Selective Activated CarbonSupported Pt-In Catalysts for the Reduction of Nitrites in Water. Front. Chem. 2021, 9, 733881. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fan, L.; Jiang, X.; Guo, J.; Liu, H.; Tian, M. Preparation of CexZr1–xO2 by Different Methods and Its Catalytic Oxidation Activity for Diesel Soot. ACS Omega 2022, 7, 16352–16360. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.M.; Bharali, P.; Saikia, P.; Park, S.E.; van den Berg, M.W.E.; Muhler, M.; Grünert, W. Structural Characterization and Catalytic Activity of Nanosized CexM1-xO2 (M = Zr and Hf) Mixed Oxides. J. Phys. Chem. C 2008, 112, 11729–11737. [Google Scholar] [CrossRef]
- Weber, W.H.; Hass, K.C.; McBride, J.R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 178. [Google Scholar]
- Zhang, Y.; Xiong, Q.; Chen, Y.; Liu, M.; Jin, P.; Yan, Y.; Pan, J. Synthesis of Ceria and Sulfated Zirconia Catalysts Supported on Mesoporous SBA-15 toward Glucose Conversion to 5-Hydroxymethylfurfural in a Green Isopropanol-Mediated System. Ind. Eng. Chem. Res. 2018, 57, 1968–1979. [Google Scholar] [CrossRef]
- Soler-Illia, G.J.A.A.; Sanchez, C.; Lebeau, B. Patarin, Chemical Strategies To Design Textured Materials: from Microporous and Mesoporous Oxides to Nanonetworks and Hierarchical Structures. J. Chem. Rev. 2002, 102, 4093. [Google Scholar]
- Kumar, G.; Shobana, S.; Chen, W.-H.; Bach, Q.-V.; Kim, S.H.; Atabani, A.E.; Chang, J.-S. A review of thermochemical conversion of microalgal biomass for biofuels: Chemistry and processes. Green Chem. 2017, 19, 44–67. [Google Scholar] [CrossRef]
- Liu, C.; Pan, R.; Hong, C.; Zhang, X.; Han, W.; Han, J.; Du, S. Effects of Zr on the precursor architecture and high-temperature nanostructure evolution of SiOCpolymer-derived ceramics. J. Eur. Ceram. Soc. 2016, 36, 395–402. [Google Scholar] [CrossRef]
- Das, I.; Chattopadhyay, S.; Mahato, A.; Kundu, B.; De, G. Fabrication of a cubic zirconia nanocoating on a titanium dental implant with excellent adhesion, hardness and biocompatibility. RSC Adv. 2016, 6, 59030–59038. [Google Scholar] [CrossRef]
- Behazin, E.; Ogunsona, E.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M.; Anyia, A.O. Biochars for composites. BioResources 2016, 11, 1334–1348. [Google Scholar]
- Wang, S.; Cao, B.; Liu, X.; Xu, L.; Hu, Y.; Afonaa-Mensah, S.; Abomohra, A.E.-F.; He, Z.; Wang, Q.; Xu, S. A comparative study on the quality of bio-oil derived from green macroalga Enteromorpha clathrata over metal modified ZSM-5 catalysts. Bioresour. Technol. 2018, 256, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, J.; Xu, W.; Xiao, G. Catalytic pyrolysis of natural algae over Mg-Al layered double oxides/ZSM-5 (MgAl-LDO/ZSM-5) for producing bio-oil with low nitrogen content. Bioresour. Technol. 2017, 225, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Lasoni, A.I.; Zunino, J.; Piccolo, M.C.; Volpe, M.A. Valorization of Rhizoclonium sp. algae via pyrolysis and catalytic pyrolysis. Bioresour. Technol. 2016, 216, 302–307. [Google Scholar]
- Du, Z.; Ma, X.; Li, Y.; Chen, P.; Liu, Y.; Lin, X.; Lei, H.; Ruan, R. Production of aromatic hydrocarbons by catalytic pyrolysis of microalgae with zeolites: Catalyst screening in a pyroprobe. Bioresour. Technol. 2013, 139, 397–401. [Google Scholar] [CrossRef]
- Subagyono RD, J.; Putri, S.A.; Manawan, M.; Mollah, M.; Nugroho, R.A.; Gunawan, R. Catalytic Pyrolysis of the Green Microalgae Botryococcus braunii over Ni/SBA-15 Prepared by the Ultrasonic-Assisted Sol-Gel Method. ACS Omega 2023, 8, 8582–8595. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).