1. Introduction
Energy is a fundamental component in sustaining socio-economic growth. The concerns regarding resource depletion and air emissions due to the rising dependence on fossil fuels lead to the necessity of finding alternative energy sources [
1]. Considering the climate change issue and other problems related to it (i.e., extreme weather—such as heat waves, hurricanes—more frequent and more intensity), it is crucial to make the switch to cleaner, more sustainable energy sources to defend the planet and future generations [
2]. In the above-presented context, the development of renewable energy sources is one of the urgent tasks of the scientific community [
3]. By transitioning to renewable energy sources, climate change can be combated, air pollution can be reduced, and a sustainable energy future can be ensured, increasing energy independence and stimulating economic growth. The shift towards renewable energy is gaining momentum, and many countries are setting ambitious targets to decrease their habit on fossil fuels and the shift to an economy with low emissions [
2].
The European Union (EU) has approved its latest Renewable Energy Directive (RED III), which aims to double renewable energy shares by 2030. The EU member states are required to align their domestic policies by 2025 with either a 29% renewable energy target or a 14.5% reduction in greenhouse gas (GHG) emissions [
4]. Biodiesel is an alternative fuel source that can substitute diesel [
5]. Biodiesel, referred to as a fatty acid methyl ester (FAME), is a significant global biofuel valued for its status as a sustainable and renewable energy source [
6]. Sustainable biodiesel production has two main goals: (i) minimize negative impact both over the environment and society, and (ii) maximize its potential as a low-carbon alternative to conventional fossil fuels [
7].
Biodiesel’s environmental advantages come from its production process using biomass as a raw material, which requires carbon dioxide for its growth. When burned, biodiesel releases the stored carbon, effectively maintaining a carbon cycle balance [
8]. Consequently, biodiesel is widely regarded as a carbon-neutral fuel. The notable and clear advantages of biodiesel, such as low GHG emissions, increased lubricity, and a high cetane ignition rating compared to petroleum-derived diesel, although it does result in higher NO
x emissions [
9]. Biodiesel demonstrates a significant decrease in emissions of particulate matter (PM), hydrocarbon (HC), and CO in diesel engines [
8]. The main challenges in biodiesel production stem from the costly process, leading to a higher price compared to conventional fuel. A high percentage of the production cost is attributed to first-generation edible feedstocks such as palm, sunflower, and soybean. Efforts are made to obtain economically viable biodiesel with a good profit [
10].
The global demand for biofuels is increasing rapidly. There are countries in the world that invest their resources in the biodiesel production process to meet the criteria for production and development in order to address the desire to facilitate energy sustainability and mitigate the impacts of weather change [
11]. Biodiesel is obtained globally, as presented in
Figure 1. The data summarized in the above-mentioned figure represent percentages for the year 2022 [
12].
According to an International Energy Agency (IEA) report, biofuel demand is set to expand 38 billion liters over 2023–2028, a near 30% increase from the last five-year period. Total biofuel demand rises 23% to 200 billion liters by 2028, with renewable diesel and ethanol accounting for two-thirds of this growth and biodiesel and bio-jet fuel making up the remainder [
4].
Biodiesel is widely obtained through the reaction of triglycerides, from various sources, with a compound with a hydroxyl group using an acidic, basic, or enzymatic catalyst, homogeneous or heterogeneous. The traditional method for producing biodiesel involves transesterification using homogeneous alkaline catalysis. The reaction of the traditional method takes place at different temperatures up to 60 °C, with a small amount of methanol in excess, being in a molar ratio 6 times higher for methanol. Sodium or potassium hydroxide are commonly used as catalysts due to their cost-effectiveness, usually in the ratio of up to 1% mass/mass. Mass/mass is used to express the concentration of a substance in a mixture as a ratio of the mass of the solute to the total mass of the solution [
13]. Biodiesel production through homogeneous alkaline transesterification necessitates pre-treatment procedures, leading to higher process expenses [
14]. This also poses challenges in utilizing residual oils as feedstock, which could potentially offer a cost-effective alternative, given that raw material expenses make up a substantial portion of the overall production costs [
15,
16]. The existence of free fatty acids (FFA) and moisture can lead to saponification, resulting in the production of soap, which decreases the yield of the reaction by using catalyst and fat and complicates glycerol separation downstream. Therefore, it is recommended that the oil used should not have FFA levels exceeding 0.5% and water levels above 0.06% [
17,
18]. A key issue with transesterification is the production of glycerol, which needs to be separated, and its surplus demand impacts profitability. The global demand for biodiesel continues to rise, and the production of glycerol is also increasing significantly. This surplus has led to a decrease in market price, making the purification process less economically viable. In 2011, there was an excess of 3 million tons of glycerol produced, while the production for 2020 was about 7.66 million tons [
19]. Two potentials solutions to the issue of the higher amount of glycerol have been proposed. These solutions also increase the competitivenes of obtaining biodiesel. One option involves utilizing glycerol to produce a diverse range of chemicals, a concept referred to as glycerol chemistry [
20]. Another option is to produce biodiesel using alternative methods that do not result in the production of glycerol as a by-product. Although the price of this new by-product decreases due to increased supply and its production may not offer immediate benefits, this by-product would not pose the same technical challenges as glycerol, which in itself is an advantage. Hence, one potential solution is to investigate industrial processes that yield by-products other than glycerol, adding value to the overall process.
To find a solution to these problems, various different processes have been studied over time, including methods like homogeneous and heterogeneous acid catalysis [
21], enzymatic catalysis [
22], transesterification [
23], or interesterification [
24]. For instance, instead of producing glycerol as a by-product, interesterification generates triacins, such as triacetin, tripropionin, or tributyrin, depending on the type of carboxylate esters used in the reaction [
25].
First introduced by Kusdiana and Saka [
26], the biodiesel production under critical temperature and critical pressure eliminates the need for a catalyst. Since the primary reactions take place in a critical state, catalysts are unnecessary, and the purification process is simpler compared to traditional methods. This route has been shown to be technically viable [
22], offering the benefits of minimal or no treatment of the feedstocks and simplified product purification stages, as issues related to water and FFA presence are no longer a concern [
9]. With these two compounds, three reaction processes can take place: the conversion of triglycerides into FAME through the transesterification, the breakdown of triglycerides into free fatty acids through hydrolysis (especially with high water proportions), and the formation of FAME through esterification reaction of fatty acids. Surprisingly, water and free fatty acids, instead of hindering the reaction, exhibit acid catalytic properties that enhance conversion rates. Furthermore, the supercritical process allows for achieving yields comparable to those obtained from virgin oils even with residual oils that have higher FFA levels, which is a significant advantage [
16]. Goembira and co-authors investigated various esters (i.e., methyl carboxylates, ethyl carboxylates, propyl carboxylates, butyl carboxylates). Among the various esters, methyl acetate is the one that showed the highest yield in biodiesel production by the supercritical process. The same authors concluded also that the mixtures of fatty acid alkyl esters and triacins have no detrimental effects on biodiesel properties [
27]. According to Prestigiacomo and collaborators [
28], triacetin has a higher market value compared to glycerol since it can be added to the biodiesel mixture up to 10 wt.% without changing the fuel quality. Beside the above-mentioned advantages, triacetin can also be used as a green plasticizer for polymers and as an additive in the cosmetic and pharmaceutical industries [
29]. Triacetin has recently attracted attention as a renewable oxygenated fuel for diesel engines, and the effects of mixing triacetin with diesel or biodiesel fuel have been thoroughly examined [
30]. One benefit of adding it to the diesel-biodiesel blend is that it can lower emissions of NO
x, CO, HC, and CO
2. Yet, it appears that using triacetin in diesel engines is not cost-effective [
31].
The interesterification process emerges as a feasible alternative to classical transesterification for biodiesel production, providing the benefit of glycerol-free biodiesel while also producing triacetin (C
9H
14O
6) as a desirable by-product [
32]. During the interesterification reaction, one ester exchanges its alcohol group with another ester [
24]. This inter-esterification technology has several advantages over conventional catalytic processes, such as improving the biodiesel quality, minimizing the cost of processing biodiesel additives, increasing the yield of the product, and simplifying downstream separation and purification [
33].
The goal of this research is to evaluate, from a technical perspective, the production of biodiesel by interesterification through the reaction in supercritical conditions of triglycerides with methyl acetate. The novelty of the present research consists of the proposed approach. To the authors best knowledge, such a route was not proposed up to this moment in the scientific literature. The proposed non-conventional method may offer innovative solutions that could potentially improve the overall production process, leading to more environmentally friendly and cost-effective biodiesel production.
2. Materials and Methods
Figure 2 presents a simplified block flow diagram of the concept investigated in the present study.
The current research focuses on biodiesel as its target product. Therefore, a number of chemical processes were proposed and implemented by means of CHEMCAD process simulation software, version 7.1.2, for its manufacturing [
34]. Details regarding the process description, as well as the modeling and simulation aspects, are provided in the following section.
2.1. Biodiesel Production by Interesterification with Methyl Acetate
This process involves one reaction step where the interesterification reaction occurs between methyl acetate and triglycerides, resulting in the production of FAME and triacetin as a secondary product. The primary advantage of this method lies in the generation of triacetin as a by-product. The combination of the by-product volume and the primary product stream might prove to be an advantageous strategy since triacetin has a higher economic value than glycerol. This would yield more biofuel, which would be a triacetin and FAME mixture, while also removing the need to purify triacetin [
35]. Triacetin does not impact the key characteristics of biodiesel as a fuel—in fact, it might improve certain features. These improvements comply with the requirements established by European and North American legislation [
36].
According to literature studies [
22], as opposed to other non-conventional methods and supercritical transesterification, this approach demands higher temperatures or longer reaction times. The possible influence of heat degradation on triacetin and FAME makes this a major disadvantage. Yet, since triacetin may be blended into biodiesel, the process of separating the products is simple and consists of removing the unreacted oil and excess methyl acetate.
Since soybean oil is among the most commonly used, the oil was modeled using its fatty acid composition, which is mostly made up of oleic and linoleic triglycerides. The reaction mechanism consists of three reversible steps, along with transesterification: initially, a triglyceride molecule reacts with methyl acetate to yield FAME and mono-acetyl diglyceride. Subsequently, the mono-acetyl diglyceride reacts with another molecule of methyl acetate to produce additional FAME and diacetyl monoglyceride. Finally, the diacetyl monoglyceride reacts with methyl acetate to form more FAME and triacetin. If free fatty acids are present in the oil, they react with methyl acetate to produce acetic acid as a concomitant by-product [
35]. The reverse reaction and partial consumption of free fatty acids are facilitated by the continued presence of acetic acid in the reaction media, based on Le Chatelier’s principle [
37]. The overall reaction scheme involving methyl acetate is further presented in
Figure 3.
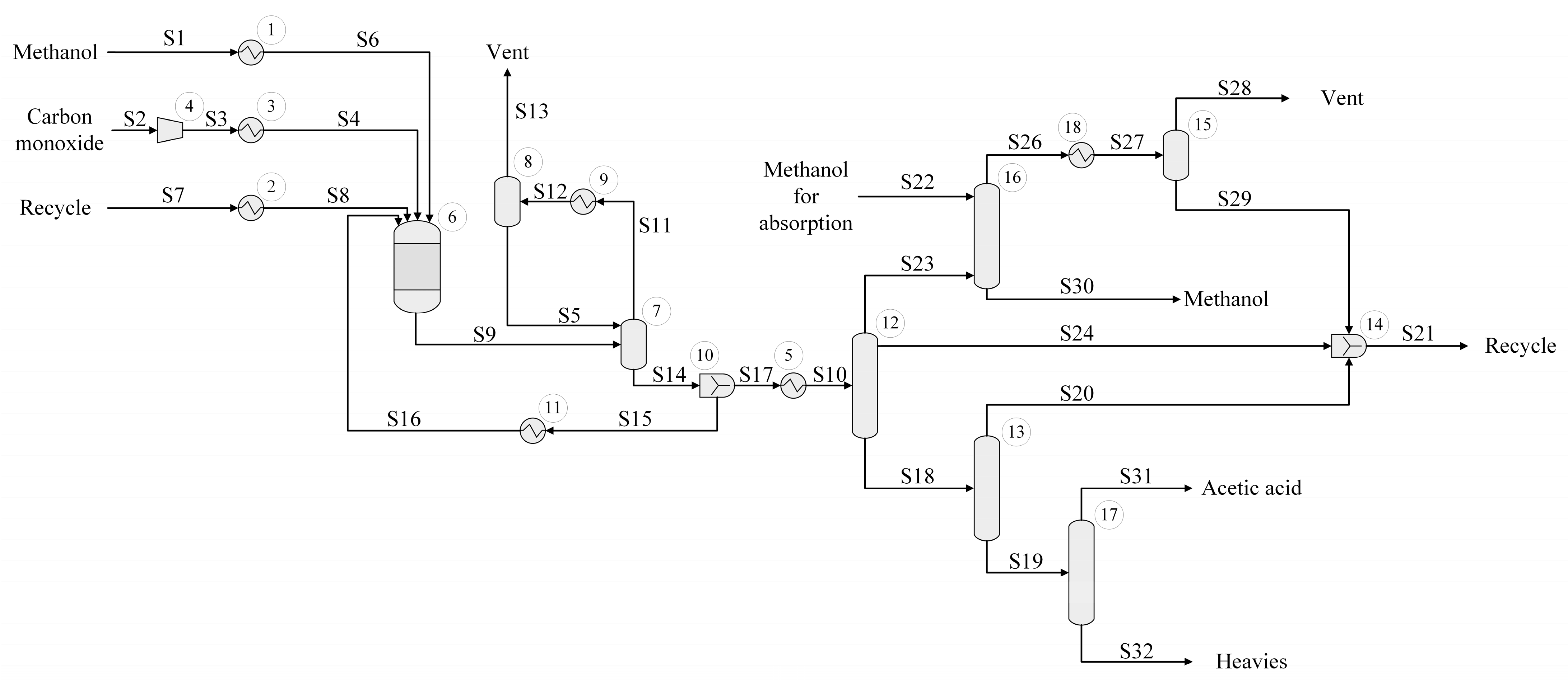
The process flow diagram for the biodiesel production using CHEMCAD process simulation software is illustrated in
Figure 4. The Ideal Vapor Pressure thermodynamic package was used to perform the study. At first, methyl acetate (S1) and oil (S2) feed streams are mixed and transported by a pump in a series of three heat exchangers, aiming to preheat the reaction mixture before entering the reaction unit. The output pressure of the pump is set at 200 bar. As for the reaction section, an isothermal Gibbs reactor is considered appropriate. The reactor outlet consisting of FAME, residual oil, triacetin, and excess methyl acetate is depressurized to 2 bars using an expander and cooled via two heat exchanger units. Afterwards, the excess of methyl acetate is removed in two steps. At first, a flash separator working at 100 °C and 2 bars is employed to perform a gas–liquid separation. The resulting gaseous stream solely contains methyl acetate; thus, it is recycled and mixed with fresh raw materials. The liquid phase is sent to a sequence of three distillation columns. The first distillation column (Unit 11) removes the excess of methyl acetate as distillate, while the bottom containing FAME, unreacted oil, and triacetin is further fed into the second separation unit. This unit operates on 5 stages (feed stream enters at stage 2) and a top pressure of 1.3 bar. In addition, a reflux ratio of 1.5 is set.
The second distillation column (Unit 14) aims to remove triacetin from the remaining mixture. This unit operates on 7 stages, the feed stream enters at the middle, and it has a top pressure of 0.1 bar. In addition, a reflux ratio of 6 and a bottom pump pressure of 0.5 bar were set. Although biodiesel and triacetin could be mixed together, some of the resulting by-product has to be eliminated from the fuel since its complete presence increased the density, reaching a level that was not allowed according to the National Agency of Petroleum, Natural Gas and Biofuels (ANP) specification [
38].
As triacetin is obtained at the top, the bottom stream is fed into a third separation unit to separate biodiesel from residual oil. The third unit (Unit 17) consists of 20 stages and operates at a top pressure of 0.1 bar, bottom pump pressure of 0.3 bar, and a reflux ratio of 0.5. Biodiesel is obtained at the top of the column, while residual oil is removed at the bottom.
2.2. Methyl Acetate Production by Esterification of Acetic Acid with Methanol
The most important process involves the esterification reaction of acetic acid with methanol to obtain methyl acetate, using a reactive divided wall distillation column (RDWC). In reactive distillation, high conversion is achieved by constantly removing products from the reaction section. The products are extracted through the bottom of a reactive distillation column (RDC) and separated within the same unit.
In an RDWC, a reactor and a separator are combined within a single unit. This setup allows for the isolation of excess reagents commonly found in reactive systems. By combining the RDC and separation column results in RDWC with methanol as a by-product stream, thus reducing the residence time of methanol with acetic acid and water at a minimum level. The intensified column provides enhanced reaction rate and selectivity while also removing azeotrope formation, reducing energy consumption, and minimizing solvent handling [
39].
The RDC and dividing wall column (DWC) exemplify process heat integration. The DWC features a highly integrated design with a condenser, reboiler, certain reactive zones, pre-fractionator and principal column all combined in a single distillation set [
39].
Figure 5 presents the process flow diagram for the methyl acetate production process.
The UNIFAC model was selected as a thermodynamic package to carry out this investigation. As can be observed, three separate streams enter the first distillation column (RDWC): acetic acid (S2), methanol (S4), and a recycled stream (S7). The operating parameters of the RDWC column are further presented (see
Table 1):
In the RDWC (Unit 1), methyl acetate is obtained as a result of the reaction between acetic acid and methanol. The reaction involving methanol and acetic acid for the production of methyl acetate is further shown (1):
In the present modeling approach, an adiabatic operation with the liquid phase was assumed. Methyl acetate (S10) is obtained at the top of the column, while water is removed as residue (S8). An intermediary stream (S5) is fed to the second distillation column (Unit 3) aiming to separate and recycle the unreacted methanol. This unit consists of 11 stages; the feed stream enters on stage 5; and a reflux ratio of 1.
Furthermore, as methanol and acetic acid are necessary to produce methyl acetate, which is afterwards used in the biodiesel synthesis process, their manufacture was also taken into account.
2.3. Methanol Production from Synthesis Gas
In addition to being a key raw material for the chemical industry, methanol is also regarded as a valuable alternative fuel. Although steam reforming of natural gas continues to be the main method used for the methanol manufacture, it produces a significant amount of GHG emissions. Therefore, to lessen the environmental impact, the process may switch to alternative feedstocks such as biomass or steel-mill gases for the syngas synthesis [
40]. It is thought that biomass use for syngas generation will limit waste while simultaneously providing socio-economic benefits, including generating income and creating jobs in rural regions [
41]. Utilizing biomass as a raw material offers a new strategy at the industrial level to address issues with pollution, energy consumption, as well as the storage and disposal of waste products [
42].
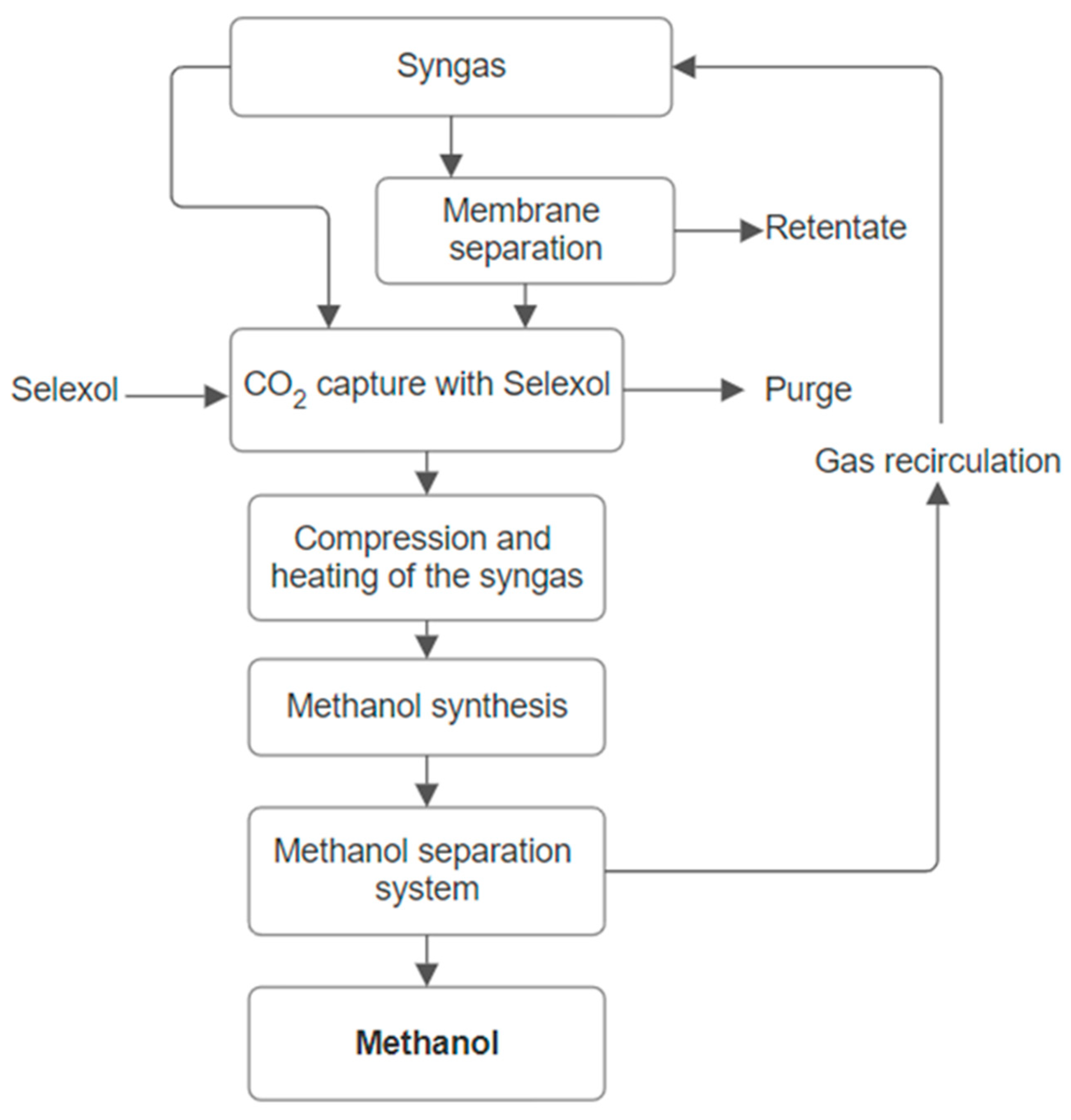
Synthesis gas, produced by gasification of biomass, is the initial raw material used in the manufacturing of methanol. There are similarities between the methanol production from biomass and the natural gas-based production method. Both include three stages: (i) generating synthesis gas; (ii) methanol manufacturing; and (iii) methanol purification.
Figure 6 schematically depicts the block flow diagram for the manufacturing of methanol, while
Figure 7 shows the process flowsheet.
The syngas input stream enters the process at 30 °C and 32 bar. A certain amount of the synthesis gas is directed toward a membrane, where carbon dioxide and methane are separated. Membranes are an important component of many industrial chemical processes since they are often employed in processes such as membrane filtration, reverse osmosis, or gas separation [
42]. The rate of permeability is independent of temperature, composition, or pressure and is based on the diffusion process.
The input flow is directed into a 474.4 m
2 gas separation membrane, where the chemical components’ permeability characteristics (H
2: 500 GPU, CO: 9 GPU, CO
2: 120 GPU, N
2: 6 GPU, CH
4: 4 GPU, and H
2O: 1800 GPU) are set. Following syngas separation through the membrane system, carbon dioxide is removed by a series of operations. A physical absorption method employing Selexol
® is used to absorb CO
2. Selexol
® serves as a solvent that is used in the gas processing industry to remove acid gases from natural gas streams, such as carbon dioxide and hydrogen sulfide [
42]. Carbon dioxide is captured using a ten-stage absorption column. During the capture process, a purge is used to dispose of the inert components in order to avoid them from accumulating up inside the system. The carbon capture rate is approximately 87%.
In the following stage, after being heated and compressed, the stream is fed into the reaction unit. Three parallel reactions were considered:
The reaction unit is modeled as an equilibrium reactor, working at about 250 °C. The output of the reactor is cooled prior to being fed into a flash unit, which serves to separate the gaseous from the liquid phase. The distillation column used for methanol separation operates at 50 °C and 60 bar. The PSRK thermodynamic model was utilized to perform the above-presented model.
2.4. Acetic Acid Production by Methanol Carbonylation
The carbonylation of methanol to acetic acid is acknowledged as an important homogeneous catalysis process in industry. Since Monsanto introduced a rhodium-catalyzed technique forty years ago, efforts have been made to improve both the process and knowledge of the fundamental chemical mechanisms. Different strategies were developed to improve the economic feasibility and efficiency of catalysts, resulting in a series of systems promoted by rhodium and iridium that demonstrate strong activity at lower concentrations of water, thus lowering the cost of product purification [
43].
Figure 8 presents the process flow diagram for acetic acid synthesis via methanol carbonylation. As can be noticed, three separate streams, namely methanol (S1), CO (S2), and a recycle stream (S7), enter the reaction unit (Unit 6). The reaction unit consists of an isothermal reactor, working at 220 °C. The following reactions are considered:
The reactor outlet contains approximately 80 wt.% acetic acid. The separation and purification section primarily consists of two distillation columns. The first one aims at removing water and light species, and it is modeled with 35 stages (feed on stage 18) and a top pressure of 1.3 bar. In addition, a reflux ratio of 1 is also set. The second separation unit targets acetic acid purification, and it is modeled with 30 stages (feed on stage 15). About 97 wt.% of acetic acid is recovered at the top of the column. The PSRK thermodynamic model was used to perform the current investigation.
Additional information regarding the input and output streams of the above-described processes is provided in the
Supplementary Material (see Tables S1–S4). Model validation was performed for each of the investigated processes based upon relevant literature studies (see
Tables S5–S7).
3. Results and Discussions
The current study aimed to investigate the biodiesel manufacturing process by means of interesterification of methyl acetate and triglyceride. The biodiesel productivity was set to 25 tons/h, with a required purity higher than 99 wt.%.
Table 2 summarizes the main processing units utilized within each section/process of the proposed biodiesel production pathway.
As can be noticed, biodiesel and methanol production processes have the highest number of unit operations, 17, making them the most complex of all the involved steps. Acetic acid production requires 16-unit operations, whereas methyl acetate only needs 4. Two distillation columns are required in the production of methyl acetate, with the reactive distillation column serving as the primary means of producing the desired product. Apart from the process complexity, the large number of processing units also entails a higher production cost.
In terms of product purity, over 99 wt.% purities were obtained for methanol, acetic acid, and biodiesel, while over 90 wt.% purity was achieved for methyl acetate.
The electricity and thermal energy consumptions when considering the proposed chain, from synthesis gas up to biodiesel generation, are summarized in
Table 3.
The total amount of power consumed is approximately 28 MWh, as indicated by the results shown in
Table 3. Of the total electricity consumption, about 91% is utilized in the biodiesel manufacturing process (i.e., 25,278.66 kWh of the total of 28,004.77 kWh), and this is mainly due to the transport and recycling of raw materials. About 2,420.50 kWh are used in the methanol manufacturing process. At this stage of the process, the compressors that transport the syngas to the methanol synthesis reactor are the main energy consumers. With 305.61 kWh used, the acetic acid synthesis process ranks third when it comes to power usage. Also worth mentioning is the expander installed in the biodiesel manufacturing facility, which generates around 226,159 kWh of electricity, enough to cover the chain’s use plus export any extra.
In regard to thermal energy consumption, biodiesel production represents the major consumer. The distillation column (Unit 11, see
Figure 4) that aims to separate and recycle unreacted methyl acetate uses approximately the same amount of thermal power as the three heat exchangers that come prior to the reactor. As previously mentioned, the heat exchangers are meant to preheat the temperature of the reaction mixture up to 345 °C. Methanol production ranks second in terms of thermal power consumption, while methyl acetate synthesis comes third. In the case of methyl acetate production, more than 90% of the total thermal power consumption is required in the first distillation column that separates the desired product. When compared to the thermal energy required in the biodiesel manufacturing process, the acetic acid manufacturing process consumes significantly lower amounts of thermal energy.
A thermal integration study (following the Pinch methodology [
44]) was additionally performed due to the manufacturing process of biodiesel being highly energy intensive. According to the Pinch methodology, the hot and cold process streams were coupled together in a heat exchanger network (HEN) in order to reduce the external utility consumptions. Achieving the process-to-process heat recovery, the overall ancillary energy consumption decreases and the energy efficiency increases, with benefic consequences on the overall techno-economic and environmental performances [
45]. The following paragraph presents the results of the detailed thermal integration analysis.
Table 4 presents the data gathered to perform the thermal integration analysis related to the biodiesel manufacturing process.
The composite heat curves and the grand composite curve are further illustrated in
Figure 9 and
Figure 10, respectively. The composite heat curves are graphical representations to analyze and optimize the heat-exchanger networks (HENs). These curves show the cumulative heat transfer requirements of hot and cold streams in this system for biodiesel production, allowing us to identify potential opportunities for heat recovery and energy savings. By plotting these curves, the heat exchange possibilities can be visualized and more efficient heat exchanger networks can be designed.
Figure 9 also integrates the hot and cold utility consumptions (resulting in balanced composite curves). There are sufficient hot streams throughout the process; thus, no additional external hot utility use is required. On the cold side, the external cold utility consumption is about 116 MW
th.
The grand composite curve (see
Figure 10) method is a technique used to optimize heat exchanger networks, and it involves plotting the composite curves of hot and cold streams to identify opportunities for heat recovery and minimize energy consumption, in this case. As can be noticed from
Figure 10, for both sub-systems (i.e., above and below the pinch point), the heat recovery potentials were identified (as the heat pockets above and below the pinch point, which correspond to a temperature of 115 °C). Based on
Figure 10, it can be observed that the hot pocket placed above the pinch point is considerably larger than the cold pocket found below the pinch point. This indicates that solely cold utilities are required throughout the biodiesel manufacturing process, with no extra hot utilities necessary.
Table 5 presents a comparison of the thermal energy consumption between the thermally integrated simulation and the baseline simulation.
By comparing the two simulations, it is shown that the thermally integrated simulation requires less thermal energy than the initial one, which required the use of external hot and cold utility consumptions, increasing the operational costs. In addition, the availability of hot process sources (without needing an external hot utility) significantly improves the overall operational flexibility of the process and increases the energy efficiency [
46]. As a key result of its reduced energy consumption and lower operational costs, the proposed model with a detailed thermal integration analysis has proved much more reliable, as well as simpler to implement.