Abstract
Approximately 25% of global carbon emissions come from food production. Renewable fuels are crucial for curbing greenhouse gas (GHG) emissions from vehicles, non-road machines, and agricultural machinery. Tractors, key to modern farming, are central to these efforts. As agriculture strives for sustainability, alternative fuels like methanol and hydrotreated vegetable oil (HVO) are arousing interest because they are renewable and offer potential for blending for use in diesel engines. Methanol and HVO have limited solubility in direct mixing, so the addition of a co-solvent is essential. This study addresses the research gap regarding the properties of HVO and methanol blends with co-solvents. It investigated the impact of three co-solvents, 1-dodecanol, 1-octanol, and methyl butyrate, on the miscibility of HVO and methanol. The experimental measurements cross-varied the co-solvent type with different blending ratios (MeOH5 and MeOH10). Investigated parameters include fuel density, kinematic viscosity, distillation properties, and surface tension. The co-solvents enabled the formation of a singular, clear, and homogeneous phase in methanol-HVO blends. The co-solvent 1-dodecanol demonstrated the highest solubilizing capacity for MeOH5 and MeOH10 blends, followed by 1-octanol. Adding co-solvents led to increased fuel density, decreased kinematic viscosity, and small changes in surface tension. These findings contribute to the optimization of methanol–HVO fuel blends for efficient and environmentally friendly use in vehicles, non-road machinery, and agricultural machinery.
1. Introduction
Diesel or compression-ignition (CI) engines are used in many applications such as road vehicles, ships, construction machinery, agricultural equipment, and generator sets because they are highly efficient and reliable. However, the prevalence of diesel engines poses challenges related to their emissions and the need to replace fossil fuel energy sources [1,2,3,4]. Constrained availability of petroleum resources and increasing awareness of environmental issues, particularly concerning greenhouse gas (GHG) emissions, have accelerated research and development of alternative fuels. Renewable alternative fuels play a pivotal role in mitigating environmental consequences across diverse sectors, including vehicles, non-road machinery, and agricultural equipment, with specific attention directed toward tractors. Tractors, key to modern farming, are central to these efforts [5,6,7].
Approximately 25% of global carbon emissions come from food production. Potential alternative power sources for agricultural machinery include alcohols (methanol and ethanol), pure plant oil, biodiesel, bio-methane, hydrotreated vegetable oil (HVO), hydrogen for fuel cells, and plug-in electrical power systems [7,8]. Methanol is considered a future alternative carbon-neutral fuel and energy carrier because it can be produced entirely from renewable sources, either through the thermochemical pathway or the biochemical pathway. The first method entails gasifying biomass into synthesis gas under favorable thermodynamic conditions. The biochemical pathway uses microbes’ anaerobic digestion of methane biogas [9]. In the future, methanol can be produced using renewable hydrogen and captured CO2. Methanol’s use in diesel engines has been limited due to its poor auto-ignition properties and low heating value [6,10]. Blending methanol with HVO addresses all these engineering and environmental challenges. HVO is renewable diesel derived from raw materials such as used cooking oil, animal fat from food industry waste, or crude tall oil, which is a residue of wood pulp production. HVO has lower life-cycle GHG emissions than conventional fossil diesel. High-quality HVO is compatible with all CI engines [11,12,13]. Crucially, in the pre-blending of methanol and HVO, emulsification can address methanol´s lubricity issues.
Research has been conducted on the use of HVO fuels in agricultural machinery. Sondors [14] investigated HVO fuel in a Claas Ares 557ATX tractor, revealing an average nitrogen oxides (NOx) reduction of 11.8%; total unburned hydrocarbons (THC) down by 26.4%; CO cut by 14.5%; and (tailpipe) CO2 down by 5.2% compared to conventional fossil diesel. Kumar et al. [15] observed a reduction in HC, CO, and smoke emissions of up to 30% in their study of blends of hydrotreated waste cooking oil with fossil diesel in agricultural engines. However, it was noted that emissions start increasing when the percentage of the hydrotreated oil is further increased. Simultaneously, NOx emissions were consistently lower across all test samples than those observed with diesel. Smigins et al. [16] assessed the impact of commercially available HVO and its blend with diesel fuel on performance parameters of an atmospheric internal combustion diesel engine for off-road applications. The HVO5 blend consisted of 5% (v/v) HVO and 95% (v/v) diesel. Their investigation evaluated the effects on power output, torque, fuel consumption, and the composition of exhaust gas. They found that diesel fuel yielded the lowest power and torque, whereas the HVO blend demonstrated slightly improved results, and a noteworthy reduction in the HC emissions, averaging 60% [16].
Methanol as an alternative fuel for diesel engines is considered to improve their performance and reduce emissions. Methanol’s high stoichiometric fuel/air ratio, high oxygen content, and high H/C ratio may be beneficial for improving combustion and reducing soot and smoke [1]. Methanol’s combustion emissions are lower than those from fossil fuels. Additionally, it possesses elevated heat of evaporation [17].
However, the direct mixing of methanol and HVO is challenging due to their limited solubility [18]. Separation of the fuel blends can cause damage to the engine. Adding co-solvents can improve the solubility and stability of the mixed fuel [6]. Despite the potential benefits of co-solvents, there is currently a lack of knowledge on the solubility and miscibility of methanol and HVO blends. Moreover, physical and chemical properties of the ternary MeOH–HVO–co-solvent blend need to be investigated. Co-solvents typically are used to improve the solubility and miscibility of alcohol and biodiesel/diesel blends, and to reduce phase separation. They enhance the intermolecular interactions between polar molecules acting as a bridging agent and thus producing a homogenous blend. There has been growing recent research into co-solvents such as furans, alcohols, ethers, and esters [1,19,20,21].
Gao et al. [19] studied the addition of alcohols, ethers, and esters as oxygen-containing compounds to prepare methanol/diesel blends. The inter-solubility of methanol and diesel was explored with the addition of various volume ratios of several alcohols, ethers, and esters as co-solvents. The differential effectiveness of these oxygen-containing compounds in facilitating the blending of methanol with diesel is attributed to their distinct molecular structures, including alkyl carbon chain length and oxygen-containing functional groups. These structural variances correspond to the compounds’ lipophilic and hydrophilic properties, respectively. The study concluded that ester methyl butyrate demonstrated the ability to enhance the dissolution of methanol in diesel. The co-solvent 1-octanol exhibited outstanding performance as a co-solvent, promoting the formulation of stable methanol/diesel blends [19].
Bayraktar [1] investigated the influence of a composite blend of diesel, methanol, and dodecanol on the operation of a CI engine. Murayama et al. [22] examined ten distinct solvents and determined that dodecanol exhibited optimal solvent properties for the diesel–methanol mixture. Building upon Murayama et al.’s findings, Bayraktar incorporated 1% 1-dodecanol into each mixture in their study and addressed challenges related to phase separation. Agarwal et al. [23] conducted an inquiry into the combustion characteristics, engine performance, emissions, and particulate characteristics of two blends of methanol and diesel (10% and 15% v/v methanol, with 1% v/v 1-dodecanol), while the remaining volume was composed of mineral diesel. Their study demonstrated a suitable combination of diesel–methanol blend with 1-dodecanol additive would improve blend stability as well as the BTE (brake thermal efficiency) or BSEC (brake-specific energy consumption), and EGT (exhaust gas temperature) in combustion.
Adding co-solvents to MeOH–HVO blends may change the fuel properties significantly. The properties have to be investigated carefully before engine experiments are conducted. The behavior of methanol blended with the larger molecules found in diesel and biodiesel fuels shows highly nonlinear physical properties with increasing alcohol content. One noteworthy property influenced by blending is surface tension. Surface tension emerges as a critical liquid fuel property in high-pressure injection because it significantly impacts spray atomization, the quality of spray formation (including penetration and angle) within the engine combustion chamber, and subsequently, combustion performance and emissions. Surface tension, density, and viscosity of the fuel are important in shaping injection, atomization, heat transfer, ignition, and combustion. The strategy of blending with a fuel characterized by low surface tension, low density, and low viscosity has garnered substantial attention in recent years, due to the associated advantages such as the absence of additional energy consumption, the absence of a need for engine modifications, and simplified operational procedures [6,24,25].
There are scarce investigations into the use of methanol–HVO blends in diesel engines for agricultural vehicles. This may be due to the issue of phase separation, caused by the inherent inability of methanol–HVO blends to form a homogeneous mixture. However, all the fuel and fuel blend options to enable carbon-neutral agriculture need to be investigated. Agricultural engine applications in particular would benefit from single-fuel solutions. This study investigated the addition of co-solvents to MeOH–HVO blends and studied these blends’ feasibility for non-road engines. The primary objective was to determine the fundamental characteristics of the novel blends consisting of MeOH–HVO and co-solvent additives in a single phase. The study evaluated the impact of co-solvents 1-dodecanol, 1-octanol, and methyl butyrate on the solubility and miscibility of methanol and HVO blends, and it also determined a suitable amount of each co-solvent for forming stable MeOH5 and MeOH10 samples. Based on the results, stable MeOH5 and MeOH10 samples were prepared, and their density, kinematic viscosity, and surface tension were measured to evaluate their feasibility in non-road engines.
2. Materials and Methods
2.1. Materials
Methanol and HVO were used as fuels for blends. The blend shares were calculated on the energy basis. The lower heating values used for calculations were 44 MJ kg−1 for HVO [26] and 20 MJ kg−1 for methanol [27]. Methanol was analysis grade by Merck KGaA, purchased from VWR, Finland. HVO was Neste MY renewable diesel without additives, supplied by Neste, Finland.
The co-solvents used for fuel blend stabilization were 1-dodecanol, 1-octanol, and methyl butyrate (Table 1). The co-solvents 1-dodecanol and 1-octanol are the products of Merck KGaA, and methyl butyrate is a product of Thermo Fisher Scientific (Waltham, MA, USA). All were purchased from VWR, Finland.

Table 1.
Physical and chemical properties of test fuels and the additives.
2.2. Titration Method to Measure the Needed Amount of Co-Solvents
A titration method was used to assess how much of each co-solvent was needed to form a stable mixture of methanol and HVO. The two fuel blends chosen for investigations were 5% of methanol and 95% of HVO (MeOH5), and 10% of methanol and 90% of HVO (MeOH10). The co-solvent 1-dodecanol is in a solid state at room temperature, with its melting or freezing points within the temperature range of 24 to 27 °C. In order to add the needed amount of co-solvent volume-based, the co-solvent was melted through immersion in a warm water bath maintained at approximately 30 °C.
The mixtures of methanol and HVO were prepared in plastic tubes with an initial volume of 5 mL. The required quantities of co-solvents for 5 mL of MeOH5 are 0.7 mL of 1-octanol, 0.72 mL of 1-dodecanol, and 1.57 mL of methyl butyrate. For 5 mL of MeOH10, the necessary amounts are 0.75 mL of 1-octanol, 0.83 mL of 1-dodecanol, and 2 mL of methyl butyrate. Titration was performed at room temperature, with the co-solvent being added gradually to the mixture using a high-precision pipette with an accuracy of 0.1 μL. The determination of the titration endpoint hinged upon the appearance of a clear, stable, one-phase liquid. At this point, the volume of co-solvent added was recorded. Test tubes with the MeOH–HVO blends were vortexed at 2400 rpm to prepare the blend solutions. Each solution was prepared in duplicates, and the final data presented here represent the average of two independent measurements. The final blends of MeOH–HVO and co-solvent were kept overnight and checked the next day to verify that the blends had remained stable.
2.3. Measurement of Density, Kinematic Viscosity, Distillation Properties, and Surface Tension
The density and the kinematic viscosity were measured by an SVM 3000 viscometer, and the measurements were produced according to Standard ASTM D7042 [28]. The viscometer’s measurement is based on torque and speed measurements. The device calculates the dynamic viscosity from the rotor speed. The device also has a density-measuring cell that employs an oscillating U-tube principle. The kinematic viscosity is calculated automatically based on these measurements. The reproducibility of the SVM 3000 viscometer is −0.04% for density and 0.06% for viscosity [29].
The distillation properties were measured by OptiPMD automatic distillation equipment, and the measurement was produced according to Standard ASTM D7345 [30]. A distillation curve shows the transition from liquid to vapor with respect to temperature. The reproducibility is not known for OptiPMD automatic distillation equipment [31].
Surface tension was measured with a Lauda Tensiometer TD 2 (Lauda Dr. R. Wobser GmbH & CO.KG). Measurements were made according to the manufacturer’s instructions (Tensiometer TD 2 operating instructions).
The results are arithmetic means of at least two replicant measurements. The relative standard deviations measured for methods are <1% for kinematic viscosity, <1% for density, and <1.1% for distillation. The relative standard deviation was not measured for surface tension.
3. Results and Discussion
3.1. Stabilizing Effect of Co-Solvents
The co-solvents used in this experiment were 1-dodecanol, 1-octanol, and methyl butyrate. The addition of co-solvent changed the layered/milky-white MeOH–HVO fuel into a single clear and homogeneous phase. Figure 1 shows the addition of the co-solvents 1-octanol, 1-dodecanol, and methyl butyrate to the MeOH10 blends and the formation of a single phase. The phase stability of all the mixtures was observed by storing them at room temperature (approximately 21 °C) in laboratory condition for two weeks. No phase separation or transition phase occurred in any of the blends.

Figure 1.
Addition of the co-solvents 1-octanol, 1-dodecanol, and methyl butyrate to MeOH10 blend to form a single phase.
The total volumes of MeOH5 and MeOH10 samples were 150 mL. Figure 2 presents the quantities of the three co-solvents. The MeOH5 samples needed 21.0 mL of 1-dodecanol, or 21.5 mL of 1-octanol, or 47.2 mL of methyl butyrate to achieve stable blends. The MeOH10 samples needed 22.5 mL of 1-dodecanol, or 25 mL of 1-octanol, or 60 mL of methyl butyrate. The co-solvents were added in quantities just sufficient to make MeOH5 and MeOH10 blends form a transparent, single-phase solution. The co-solvent 1-dodecanol emerged as the most effective co-solvent, followed by 1-octanol.
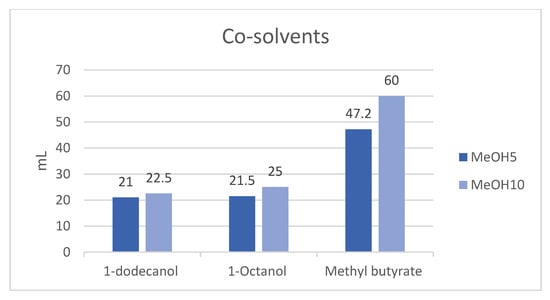
Figure 2.
Amounts of co-solvents added in 150 mL MeOH5 and MeOH10 samples.
Notably, the highest dosage of co-solvent was needed with methyl butyrate. What has to be kept in mind is that the addition of co-solvents changes the energy-based blend share of the samples. For example, after the co-solvent addition to MeOH5, this blend includes less methanol than 5% of the total sample.
Although 1-dodecanol had better solubilizing ability than 1-octanol, the amounts of either needed in MeOH5 were almost the same, and the amounts needed in MeOH10 were not very different. The amounts of co-solvents increased with the volume of methanol in MeOH–HVO.
Investigations into adding oxygenated fuels to diesel fuel to promote cleaner combustion have been carried out for half a century. Smoke emissions decrease with the increase in oxygen content in the blends, irrespective of the types of oxygenate additives. the. The oxygenated fuels contribute to more complete combustion in diesel engines, especially reducing HC, CO, toluene, and xylene emissions, while inhibiting soot formation and PM emissions. Similarly, different oxygenic additives also reduce engine emissions [32,33]. Table 1 shows that the co-solvents 1-dodecanol, 1-octanol, and methyl butyrate are all oxygen-containing reagents. Figure 2 shows that 1-dodecanol and 1-octanol, which are high alcohols, have a better solubilizing ability than methyl butyrate (an ester) as a co-solvent to prepare miscible MeOH–HVO blends. Methanol and the co-solvents studied are all oxygenated fuels, and the addition of co-solvents to the MeOH–HVO mixture increases the oxygen content and so is even more helpful in reducing engine emissions.
3.2. Distillation Curve Analysis
Figure 3 illustrates the distillation curves of HVO, MeOH10, MeOH5 methyl butyrate, and MeOH10 methyl butyrate blends. Data for the boiling properties of the study´s remaining blends were not obtained due to their inherently unstable boiling conditions. The initial boiling points (IBPs) for MeOH10, MeOH5 methyl butyrate, and MeOH10 methyl butyrate blends were recorded as 68 °C, 66 °C, and 67 °C, respectively. Their final boiling points (FBPs) were 283 °C, 265 °C, and 264 °C, respectively. Analysis of the test results presented in Figure 3 indicates that the addition of the co-solvent methyl butyrate to MeOH–HVO blends affects the distillation curve. Notably, the distillation temperature within the 10% to 90% range is observed to be higher than that of MeOH10. Additionally, the distillation temperature of MeOH5 methyl butyrate surpasses that of MeOH10 methyl butyrate in this part of the curve.
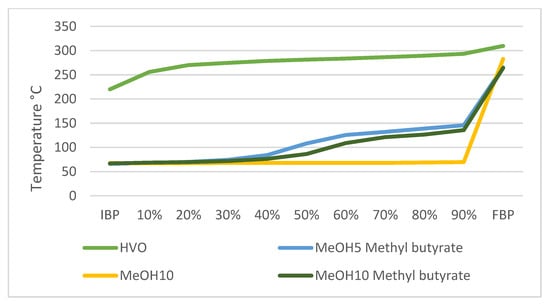
Figure 3.
Distillation curves of the tested samples: MeOH5 methyl butyrate, MeOH10, and MeOH10 methyl butyrate. No findings were obtained for other blends due to unstable boiling conditions.
3.3. Effects of Co-Solvents on Density and Kinematic Viscosity
Table 2 summarizes the key physical and chemical parameters of the fuels and blends. It is evident that the fuel density of MeOH5 and MeOH10 increased when co-solvents were added. Moreover, the density of MeOH–HVO (MeOH5 and MeOH10), MeOH–HVO–1-octanol (MeOH5 1-octanol and MeOH10 1-octanol), and MeOH–HVO–methyl butyrate (MeOH5 methyl butyrate and MeOH10 methyl butyrate) demonstrated an upward trend with the increasing methanol content in the fuel mixture. However, the densities of MeOH5 1-dodecanol and MeOH10 1-dodecanol were both the same. The densities of all blended fuels were within the density range of light fossil fuels (750–860 kg m−3) which have been identified by Krishnamoorthi et al. as suitable for low-temperature combustion [34].

Table 2.
Fuel properties of HVO, methanol, MeOH–HVO blends (MeOH5 and MeOH10), and MeOH–HVO–co-solvents blends: IBP—initial boiling point.
Fuel density directly impacts engine performance characteristics. Several fuel properties, including cetane number and heating value, are related to density [35]. Fuel injection systems meter fuel by volume, so alterations in fuel density can affect engine power output due to the injection of a different mass of fuel [36]. The addition of co-solvents to MeOH–HVO blends resulted in an increased density of the blends.
It was observed that the fuel kinematic viscosity of MeOH5 and MeOH10 decreased with the addition of co-solvents (Table 2). Furthermore, the viscosity of MeOH–HVO–1-octanol (MeOH5 1-octanol and MeOH10 1-octanol), MeOH–HVO–1-dodecanol (MeOH5 1-dodecanol and MeOH10 1-dodecanol), and MeOH–HVO–methyl butyrate (MeOH5 methyl butyrate and MeOH10 methyl butyrate) followed a decreasing trend with the increasing methanol content in the fuel mixture.
Viscosity is a pivotal property influencing spray formation and quality within the combustion chamber, particularly with regard to factors such as droplet size and the penetration of the fuel jet. The smaller the fuel droplet size, the greater is the heated and evaporated total area, and the faster is the evaporation rate [37,38]. Only MeOH5 methyl butyrate (1.38 mm2 s−1) and MeOH10 methyl butyrate (1.15 mm2 s−1) exhibited viscosities lower than 1.9 mm2 s−1. The viscosity values for all remaining blends in this study fell within the range of 2.5–3.08 mm2 s−1. The viscosities were on a suitable level for low temperature combustion fuels [34]. There are practical limitations for low-viscosity fuels in high-pressure injection systems. Kinematic viscosity lower than 1.9 mm2 s−1 poses the risk of fuel leakage from both the injector nozzle sealing and the high-pressure pump system. Addressing this issue necessitates the use of advanced hydraulic sealing systems [39,40]. Low-viscosity fuels usually have poor lubrication for injection systems and may cause pump wear.
The increase in fuel density and the reduction in kinematic viscosity upon the addition of co-solvents are pivotal findings for optimizing methanol–HVO blends. Higher fuel density can enhance the energy content per unit volume, potentially improving the overall fuel efficiency of engines. This is particularly advantageous in applications where volumetric fuel efficiency is critical [35,36]. Conversely, the reduction in kinematic viscosity improves the flow characteristics of the fuel, facilitating better atomization and more complete combustion [37,38,39,40]. This leads to smoother engine operation and reduced wear on fuel injection systems. These changes in physical properties suggest that the co-solvents not only stabilize the methanol–HVO blends but also enhance their performance characteristics, making them more suitable for use in various types of machinery. By optimizing these properties, it is possible to develop fuel blends that maximize efficiency and performance while maintaining stability, thereby bridging the gap between renewable fuels and practical engine applications.
3.4. Effects of Co-Solvents on Surface Tension
Figure 4 presents the test results of the surface tension. Following the addition of co-solvents to the MeOH5 blend, the surface tension values of MeOH5 1-octanol and MeOH5 1-dodecanol closely approximated that of MeOH5, both exceeding 26 mN m−1 and reaching the surface tension of HVO. Only the surface tension of MeOH5 methyl butyrate was lower, noted at 24.9 mN m−1. A comparison of the surface tension of MeOH10 revealed a slight increase after the addition of co-solvents, though by no more than 1 mN m−1.
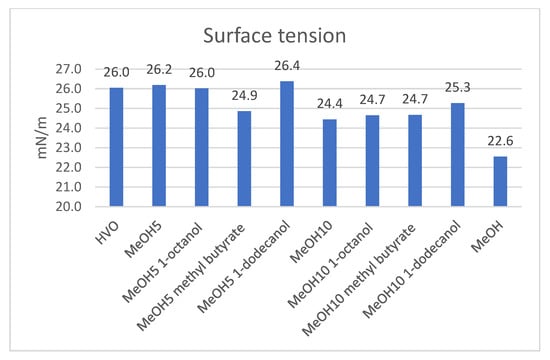
Figure 4.
Surface tension of all tested samples.
Figure 4 shows that the surface tensions for MeOH10 and its co-solvent blends were lower than those of MeOH5 and its co-solvent blends. Jin et al. [6] studied potential alcohol-based co-solvents for stabilizing ethanol and palm oil. The additives in their study caused a 2.00 to 4.00 mN m−1 variation in surface tension results of the samples [6]. The variation between all the samples in the current study was even smaller than in Jin et al.’s study, not exceeding 3.8 mN m−1. The effect of different co-solvents on the surface tension of the mixed fuels was relatively small.
Surface tension is a crucial property related to fuel ignition quality, injection, and atomization. Higher surface tension leads to poor fuel atomization, resulting in increased penetration and larger fuel droplet diameter. Conversely, lower surface tension promotes better atomization, leading to more efficient combustion, reduced soot formation, and cleaner combustion. Finely atomized fuel droplets can lead to more efficient combustion by enhancing the mixing of fuel with air. Smaller and more evenly distributed fuel droplets can result in cleaner and more complete combustion [32].
The minor changes in surface tension observed with the addition of co-solvents are significant in understanding the atomization and combustion behavior of methanol–HVO blends. Surface tension affects how the fuel breaks up into droplets during injection, which in turn influences the combustion process [32]. The fact that the co-solvents cause only minor changes in surface tension suggests that they do not adversely affect the atomization process. This is critical for ensuring a consistent fuel–air mixture and efficient combustion. Proper atomization is essential for achieving complete combustion, reducing unburned hydrocarbons, and minimizing soot formation [38,41]. The ability of the co-solvents to maintain appropriate surface tension while stabilizing the blend indicates that they can help optimize the combustion process, leading to lower emissions and better engine performance. These findings provide a robust foundation for future research into the ignition and emission characteristics of methanol–HVO blends with co-solvents.
3.5. Comparing the Physicochemical Properties of Blends with Those of HVO
Table 2’s comparison of physicochemical properties shows that MeOH5 methyl butyrate and MeOH10 methyl butyrate had very low kinematic viscosity (less than 1.9 mm2 s−1). The densities of these two fuels were higher than HVO’s, but within the acceptable diesel fuel density range. At the same time, their surface tensions (24.86 mN m−1 for the sample containing 5% of methanol and 24.68 mN m−1 for the sample containing 10% of methanol) were slightly lower than that of HVO (26.05 mN m−1). The lower surface tension values are more conducive to fuel atomization. However, the insufficient viscosities of MeOH5 methyl butyrate and MeOH10 methyl butyrate mean these fuels will cause poor lubrication of the injection system, resulting in injector leakage and pump wear [39,40].
The addition of 1-octanol and 1-dodecanol co-solvents to MeOH5 and MeOH10 had the best effect on forming a stable blend. The amount needed was smaller than when using methyl butyrate as the co-solvent. The densities of the four mixed fuels containing 1-octanol or 1-dodecanol additives were slightly higher (787 kg m−3) than that of HVO (780 kg m−3). The higher the density, the more mass burned per unit volume of fuel (Heywood, 2018). Comparison of the kinematic viscosity and surface tension values of these four mixed fuels shows the surface tension of only MeOH5 1-dodecanol was slightly higher than that of HVO, and the rest were slightly lower than that of HVO. This means that MeOH5 1-octanol, MeOH10 1-octanol, and MeOH10 1-dodecanol could have a slightly better atomization than HVO fuel. Consequently, this would lead to enhanced combustion efficiency, diminished soot formation, and a cleaner burning process [32]. A study by Agarwal et al. found the addition of 1-dodecanol to the methanol–diesel blend showed a lowering trend in density and kinematic viscosity [23]. However, the added amounts of 1-dodecanol differed from this study, and HVO has a lower density than fossil diesel.
In terms of compatibility with standard engine settings, the paramount criteria for fuel evaluation are cetane number (CN), lower heating value, density, kinematic viscosity, boiling point, and oxygen content. The longer-chain alcohols 1-octanol and 1-dodecanol exhibit densities and boiling points reminiscent of diesel, falling within the established range [41]. In contrast to shorter-chain alcohols such as methanol, longer-chain alcohols offer the advantage of a higher cetane number, coupled with a higher lower heating value (LHV) when compared to methanol’s LHV of 20 MJ kg−1. The energy output per kilogram of fuel rises with an increase in the alcohol chain length.
As the carbon chain length of alcohols increases, their compatibility with diesel and biodiesel becomes easier. This increase in the number of carbon atoms within alcohols is accompanied by a reduction in the mass percentage of oxygen, with a simultaneous increase in cetane number, density, and calorific value. In contrast to alcohols with fewer carbon atoms, those with higher carbon content exhibit heightened fuel properties, including cetane number, heating value, viscosity, auto-ignition temperature, and flash point. Importantly, these fuel properties of higher-carbon alcohols are closer to those of diesel, and also have enhanced safety characteristics during transportation and handling when compared to short-chain alcohols [42,43]. Although CN, LHV or oxygen content were not measured for the studied samples, the above explanation confirms 1-octanol’s and 1-dodecanol’s potential as an additive for HVO–methanol blends.
The interaction between the standard properties of fuel and fuel atomization is complex and important. First, the viscosity of the fuel affects atomization. Higher-viscosity fuels are more difficult to refine into small particles, reducing atomization efficiency [32]. Second, the surface tension of the fuel impacts atomization. Fuels with high surface tension disperse poorly in the airflow, preventing formation of fine droplets [44]. Finally, fuel density and particle size also affect atomization, since these properties determine the flow behavior and dispersion of the fuel in the atomizer [38]. Therefore, understanding the standard properties of fuel is crucial to optimize fuel atomization, leading to improved combustion efficiency and reduced emission generation.
Fuels that have lower density, viscosity, and surface tension than diesel exhibit smaller average droplet size, promoting better atomization. It is noteworthy that both kinematic viscosity and surface tension are dependent on temperature. But at already high fuel temperatures, additional changes in temperature show smaller effects on viscosity and surface tension than at low fuel temperatures [44,45]. HVO shows similarities to fossil diesel in terms of spray characteristics, air–fuel mixing, and combustion. These make it a favorable alternative, with positive impacts on emissions compared to fossil diesel [16]. High-quality HVO is compatible with all CI engines [11,12,13]. HVO was the major part of the MeOH–HVO–co-solvent blends in this study. Methanol, 1-octanol, and 1-dodecanol are all oxygenated alcohol fuels that are considered as alternative fuels for diesel engines to improve their performance and reduce emissions. The co-solvents 1-octanol and 1-dodecanol solve the separation problem of MeOH–HVO blends while forming a stable, single-phase fuel. Comparing the combination of their density, viscosity, and surface tension with that of HVO, it can be concluded that MeOH5 1-octanol, MeOH5 1-dodecanol, MeOH10 1-octanol, and MeOH10 1-dodecanol are all compatible with diesel engines in agricultural applications.
4. Conclusions
This research investigated the influence of co-solvents, namely 1-dodecanol, 1-octanol, and methyl butyrate, on the solubility and miscibility of HVO blends containing 5% and 10% methanol (MeOH5 and MeOH10). These blends were prepared with co-solvents to achieve single-phase compositions, and the fundamental characteristics of these novel blends were measured.
The following conclusions could be drawn from the results:
- MeOH5 and MeOH10 blends exhibit a singular, clear, and homogeneous phase upon the addition of co-solvents 1-dodecanol, 1-octanol, and methyl butyrate. They remain stable without separation for a duration of at least two weeks;
- Among the investigated co-solvents, 1-dodecanol demonstrated the highest solubilizing capacity for MeOH5 and MeOH10 blends, followed by 1-octanol;
- The addition of each co-solvent led to an increase in the density of MeOH5 and MeOH10. All the blends’ values aligned with the density range of conventional fossil fuels (750–860 kg m−3) suitable for CI engines. Additionally, the density of MeOH–HVO, MeOH–HVO–1-octanol, and MeOH–HVO–methyl butyrate blends increased with greater methanol content in the fuel mixture. However, it is noteworthy that the densities of MeOH5 1-dodecanol and MeOH10 1-dodecanol were the same;
- The kinematic viscosity of MeOH5 and MeOH10 fuels decreased upon the addition of co-solvents 1-dodecanol, 1-octanol, and methyl butyrate. Notably, only the viscosities of MeOH5 methyl butyrate (1.38 mm2 s−1) and MeOH10 methyl butyrate (1.15 mm2 s−1) fell below the specified range for proper operation of standard common-rail direct injection systems (1.90–6.00 mm2 s−1). The viscosities of all other blends remained within this range;
- The introduction of methyl butyrate to MeOH5 and MeOH10 raised the distillation temperature above MeOH10’s within the 10% to 90% range;
- Adding co-solvents to MeOH5 and MeOH10 either increased or decreased surface tension by around 1 mN m−1. Furthermore, the surface tensions of both MeOH10 and MeOH10–co-solvent blends were lower than those of MeOH5 and MeOH5–co-solvents blends;
- MeOH5 1-octanol, MeOH5 1-dodecanol, MeOH10 1-octanol, and MeOH10 1-dodecanol had higher density and lower kinematic viscosity than HVO fuel. Only MeOH5 1-dodecanol’s surface tension was higher than that of HVO: all the other co-solvent blends have lower surface tension than HVO.
Author Contributions
Conceptualization, H.W.-A. and K.S.; methodology, H.W.-A. and K.S.; investigation, H.W.-A. and K.S.; resources, K.S.; data curation, H.W.-A.; writing—original draft preparation, H.W.-A. and K.S.; writing—review and editing, H.W.-A., K.S., F.B., J.K. and C.N.; visualization, H.W.-A.; supervision, K.S., M.M. and S.N.; project administration: M.M.; funding acquisition: K.S., M.M. and S.N. All authors have read and agreed to the published version of the manuscript.
Funding
The work was conducted in the framework of the Flexible Clean Propulsion Technologies project with financial support from Business Finland (ref. 1310/31/2023).
Data Availability Statement
No new data were generated beyond what was documented in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bayraktar, H. An Experimental Study on the Performance Parameters of an Experimental CI Engine Fueled with Diesel-Methanol-Dodecanol Blends. Fuel 2008, 87, 158–164. [Google Scholar] [CrossRef]
- Buzikov, S.V.; Buzikova, O.M.; Motovilova, M.V. Reducing the Technosphere Impact of Agricultural Machines by Expanding the Low-Temperature Applicability of Alternative Fuels. IOP Conf. Series Earth Environ. Sci. 2022, 1076, 012012. [Google Scholar] [CrossRef]
- da Silveira, F.; Machado, F.M.; de Farias, M.S.; Schlosser, J.F. Fuel Consumption by Agricultural Machinery: A Review of Pollutant Emission Control Technologies. Ciência Rural. 2023, 53, e20220029. [Google Scholar] [CrossRef]
- Wang, X.; Geng, C.; Dong, J.; Li, X.; Xu, T.; Jin, C.; Liu, H.; Mao, B. Effect of Diesel/PODE/Ethanol Blends Coupled Pilot Injection Strategy on Combustion and Emissions of a Heavy Duty Diesel Engine. Fuel 2023, 335, 127024. [Google Scholar] [CrossRef]
- Mantoam, E.J.; Romanelli, T.L.; Gimenez, L.M. Energy Demand and Greenhouse Gases Emissions in the Life Cycle of Tractors. Biosyst. Eng. 2016, 151, 158–170. [Google Scholar] [CrossRef]
- Jin, C.; Liu, X.; Sun, T.; Dankwa Ampah, J.; Geng, Z.; Ikram, M.; Ji, J.; Wang, G.; Liu, H. Preparation of Ethanol and Palm Oil/Palm Kernel Oil Alternative Biofuels Based on Property Improvement and Particle Size Analysis. Fuel 2021, 305, 121569. [Google Scholar] [CrossRef]
- Bagagiolo, G.; Vigoroso, L.; De Paolis, G.; Caffaro, F.; Cavallo, E.; Pampuro, N. Barriers to Adoption of Alternative Fuels for Agricultural Machinery: A Study on a Group of Italian Farmers. In Proceedings of the SAE Technical Papers, 16 September 2022; SAE International: Warrendale PA, USA, 2022. [Google Scholar]
- Akande, F.B.; Oniya, O.O.; Adgidzi, D. Alternative Fuels and Their Potentials for Tractor Engines. Agric. Eng. Int. CIGR J. 2013, 15, 39–51. [Google Scholar]
- Domínguez, V.M.; Hernández, J.J.; Ramos, Á.; Giménez, B.; Rodríguez-Fernández, J. Exploring the Effect of Methanol and Ethanol on the Overall Performance and Substitution Window of a Dual-Fuel Compression-Ignition Engine Fueled with HVO. Fuel 2024, 359, 130529. [Google Scholar] [CrossRef]
- Liu, M. Methanol as a Marine Fuel-Availability and Pre-Trial; Nanyang Technological University: Singapore, 2020; Available online: https://www.ntu.edu.sg/docs/librariesprovider79/publication/mesd-webinar-2020-methanol-as-a-marine-fuel.pdf (accessed on 12 January 2024).
- Hunicz, J.; Mikulski, M.; Shukla, P.C.; Gęca, M.S. Partially Premixed Combustion of Hydrotreated Vegetable Oil in a Diesel Engine: Sensitivity to Boost and Exhaust Gas Recirculation. Fuel 2022, 307, 121910. [Google Scholar] [CrossRef]
- Niemi, S.; Vauhkonen, V.; Mannonen, S.; Ovaska, T.; Nilsson, O.; Sirviö, K.; Heikkilä, S.; Kiijärvi, J. Effects of Wood-Based Renewable Diesel Fuel Blends on the Performance and Emissions of a Non-Road Diesel Engine. Fuel 2016, 186, 1–10. [Google Scholar] [CrossRef]
- Spoof-Tuomi, K.; Vauhkonen, V.; Niemi, S.; Ovaska, T.; Lehtonen, V.; Heikkilä, S.; Nilsson, O. Effects of Crude Tall Oil Based Renewable Diesel on the Performance and Emissions of a Non-Road Diesel Engine. Proc. SAE Tech. Papers 2021. [Google Scholar] [CrossRef]
- Sondors, K.; Birkavs, A.; Dukulis, I.; Pirs, V.; Jesko, Z. Investigation in tractor claas ares 557atx operating parameters using hydrotreated vegetable oil fuel. Eng. Rural. Dev. 2014, 13, 63–68. [Google Scholar]
- Kumar, N.; Sonthalia, A.; Tomar, M.; Koul, R. An Experimental Investigation on Spray, Performance and Emission of Hydrotreated Waste Cooking Oil Blends in an Agricultural Engine. Int. J. Engine Res. 2021, 22, 2305–2317. [Google Scholar] [CrossRef]
- Smigins, R.; Sondors, K.; Pirs, V.; Dukulis, I.; Birzietis, G. Studies of Engine Performance and Emissions at Full-Load Mode Using HVO, Diesel Fuel, and HVO5. Energies 2023, 16, 4785. [Google Scholar] [CrossRef]
- Hassan, Q.H.; Shaker Abdul Ridha, G.; Hafedh, K.A.H.; Alalwan, H.A. The Impact of Methanol-Diesel Compound on the Performance of a Four-Stroke CI Engine. Mater. Today Proc. 2021, 42, 1993–1999. [Google Scholar] [CrossRef]
- Wang-Alho, H.; Sirviö, K.; Hissa, M.; Mikulski, M.; Niemi, S. Methanol-HVO Blends for Efficient Low-Temperature Combustion: Analytical Research on Fuel Properties. Agron. Res. 2023, 21, 994–1005. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, S.; Luo, J.; Zhang, B.; Zhang, H.; Xiao, R. Optimize the Co-Solvent for Methanol in Diesel with Group of Oxygen-Containing Reagents: Molecular Structure and Intermolecular Forces Analysis. Fuel Process. Technol. 2021, 222, 106980. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Geng, Z.; Pang, X.; Wang, X.; Ji, J.; Wang, G.; Liu, H. Effects of Various Co-Solvents on the Solubility between Blends of Soybean Oil with Either Methanol or Ethanol. Fuel 2019, 244, 461–471. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Han, W.; Geng, Z.; Tessa Margaret Thomas, M.; Dankwa Jeffrey, A.; Wang, G.; Ji, J.; Liu, H. Macro and Micro Solubility between Low-Carbon Alcohols and Rapeseed Oil Using Different Co-Solvents. Fuel 2020, 270, 117511. [Google Scholar] [CrossRef]
- Murayama, T.; Miyamoto, N.; Yamada, T.; Kawashima, J.-I.; Itow, K. A Method to Improve the Solubility and Combustion Characteristics of Alcohol-Diesel Fuel Blends; SAE International: Warrendale, PA, USA, 1982; Volume 91. [Google Scholar]
- Agarwal, A.K.; Sharma, N.; Singh, A.P.; Kumar, V.; Satsangi, D.P.; Patel, C. Adaptation of Methanol-Dodecanol-Diesel Blend in Diesel Genset Engine. J. Energy Resour. Technol. Trans. ASME 2019, 141, 102203. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, Y.; Zhang, X.; Jin, C.; Zheng, Z. Effect of Diesel/PODE/Ethanol Blends on Combustion and Emissions of a Heavy Duty Diesel Engine. Fuel 2019, 257, 116064. [Google Scholar] [CrossRef]
- Patiño-Camino, R.; Cova-Bonillo, A.; Lapuerta, M.; Rodríguez-Fernández, J.; Segade, L. Surface Tension of Diesel-Alcohol Blends: Selection among Fundamental and Empirical Models. Fluid. Phase Equilib. 2022, 555, 113363. [Google Scholar] [CrossRef]
- Neste Renewable Diesel Handbook, Neste Corporation 2020 Oct. Available online: https://www.sustainable-ships.org/stories/2023/neste-renewable-diesel-handbook (accessed on 12 January 2024).
- Lampinen, A. Uusiutuvan Liikenne-Energian Tiekartta; Karelia University of Applied Sciences: Joensuu, Finland, 2009; ISBN 9789516041004. Available online: https://www.theseus.fi/handle/10024/127014 (accessed on 2 January 2024). (In Finnish)
- ASTM D7042-21a; Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity). ASTM International: West Conshohocken, PA, USA, 2021.
- Novotny-Farkas, F.; Böhme, W.; Stabinger, H.; Belitsch, W. Customer Portrait. In The Stabinger Viscometer: A New and Unique Instrument for Oil Service Laboratories; Anton Paar: Vienna, Austria, 2010; p. 4. [Google Scholar]
- ASTM D7345-17; Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure (Micro Distillation Method). ASTM International: West Conshohocken, PA, USA, 2024.
- Kalghatgi, G.; Kalghatgi, G. FuelEngine Interactions; SAE International: Warrendale, PA, USA, 2014; p. 255. [Google Scholar]
- Esteban, B.; Riba, J.R.; Baquero, G.; Puig, R.; Rius, A. Characterization of the Surface Tension of Vegetable Oils to Be Used as Fuel in Diesel Engines. Fuel 2012, 102, 231–238. [Google Scholar] [CrossRef]
- Liu, J.; Feng, L.; Wang, H.; Zheng, Z.; Chen, B.; Zhang, D.; Yao, M. Spray Characteristics of Gasoline/PODE and Diesel/PODE Blends in a Constant Volume Chamber. Appl. Therm. Eng. 2019, 159, 113850. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R.; He, Z.; Kandasamy, S. A Review on Low Temperature Combustion Engines: Performance, Combustion and Emission Characteristics. Renew. Sustain. Energy Rev. 2019, 116, 109404. [Google Scholar] [CrossRef]
- Gülüm, M.; Bilgin, A. Density, Flash Point and Heating Value Variations of Corn Oil Biodiesel-Diesel Fuel Blends. Fuel Process. Technol. 2015, 134, 456–464. [Google Scholar] [CrossRef]
- Barabás, I.; Todoru, A.; Bldean, D. Performance and Emission Characteristics of an CI Engine Fueled with Diesel-Biodiesel-Bioethanol Blends. Fuel 2010, 89, 3827–3832. [Google Scholar] [CrossRef]
- Bietresato, M.; Bolla, A.; Caligiuri, C.; Renzi, M.; Mazzetto, F. The Kinematic Viscosity of Conventional and Bio-Based Fuel Blends as a Key Parameter to Indirectly Estimate the Performance of Compression-Ignition Engines for Agricultural Purposes. Fuel 2021, 298, 120817. [Google Scholar] [CrossRef]
- Hoang, A.T. Prediction of the Density and Viscosity of Biodiesel and the Influence of Biodiesel Properties on a Diesel Engine Fuel Supply System. J. Mar. Eng. Technol. 2021, 20, 299–311. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.M.; Roberts, W.L.; Dibble, R.W. Feasibility of Using Less Viscous and Lower Cetane (LVLC) Fuels in a Diesel Engine: A Review. Renew. Sustain. Energy Rev. 2015, 51, 1166–1190. [Google Scholar] [CrossRef]
- Yahya, S.I.; Aghel, B. Estimation of Kinematic Viscosity of Biodiesel-Diesel Blends: Comparison among Accuracy of Intelligent and Empirical Paradigms. Renew. Energy 2021, 177, 318–326. [Google Scholar] [CrossRef]
- Mousavi, N.S.; Romero-Martínez, A.; Ramírez-Verduzco, L.F. Predicting the Surface Tension of Mixtures of Fatty Acid Ethyl Esters and Biodiesel Fuels Using UNIFAC Activity Coefficients. Fluid. Phase Equilib. 2020, 507, 112430. [Google Scholar] [CrossRef]
- Preuß, J.; Munch, K.; Denbratt, I. Performance and Emissions of Long-Chain Alcohols as Drop-in Fuels for Heavy Duty Compression Ignition Engines. Fuel 2018, 216, 890–897. [Google Scholar] [CrossRef]
- Atmanli, A. Comparative Analyses of Diesel-Waste Oil Biodiesel and Propanol, n-Butanol or 1-Pentanol Blends in a Diesel Engine. Fuel 2016, 176, 209–215. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Zhang, Z.; Wang, X.; Geng, Z.; Jin, C.; Liu, H.; Yao, M. Effects of Diesel-Ethanol-THF Blend Fuel on the Performance and Exhaust Emissions on a Heavy-Duty Diesel Engine. Fuel 2020, 271, 117633. [Google Scholar] [CrossRef]
- Sirviö, K.; Niemi, S.; Help, R.; Heikkilä, S.; Hiltunen, E. Kinematic Viscosity Studies for Medium-Speed CI Engine Fuel Blends. Agron. Res. 2018, 16, 1247–1256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).