The Fate of Fluorine Post Per- and Polyfluoroalkyl Substances Destruction during the Thermal Treatment of Biosolids: A Thermodynamic Study
Abstract
1. Introduction
2. Materials and Methods
- The reaction would reach equilibrium; that is, the reaction time would be indefinite.
- Biosolids were introduced in the software platform in their elemental form.
- PFAS present in biosolids were introduced into the model as elemental fluorine with the assumption that PFAS would be fully destroyed.
- Pyrolysis and gasification temperatures were considered at 600 and 900 °C, respectively.
3. Results and Discussions
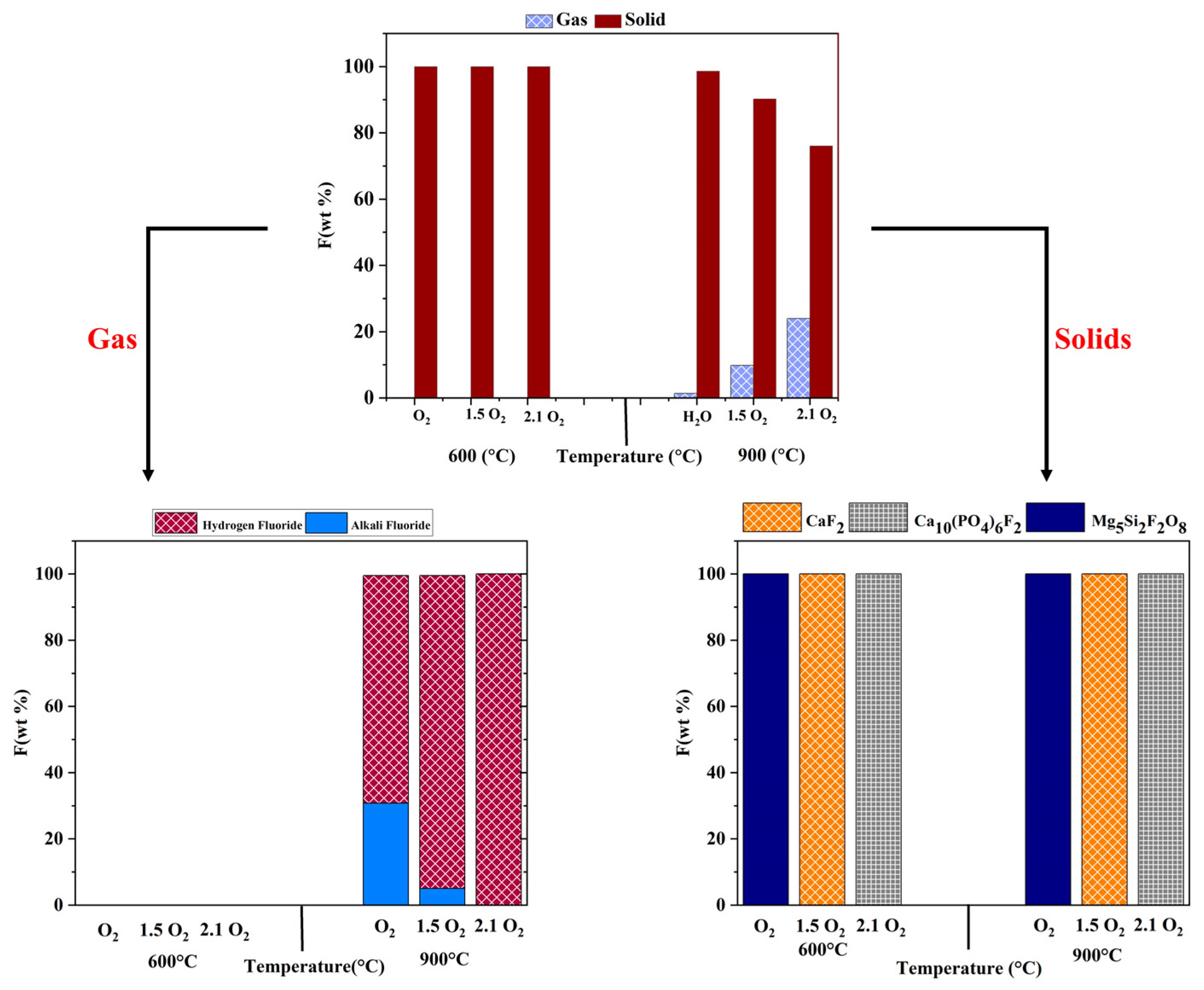
3.1. Effect of Oxygen Concentration
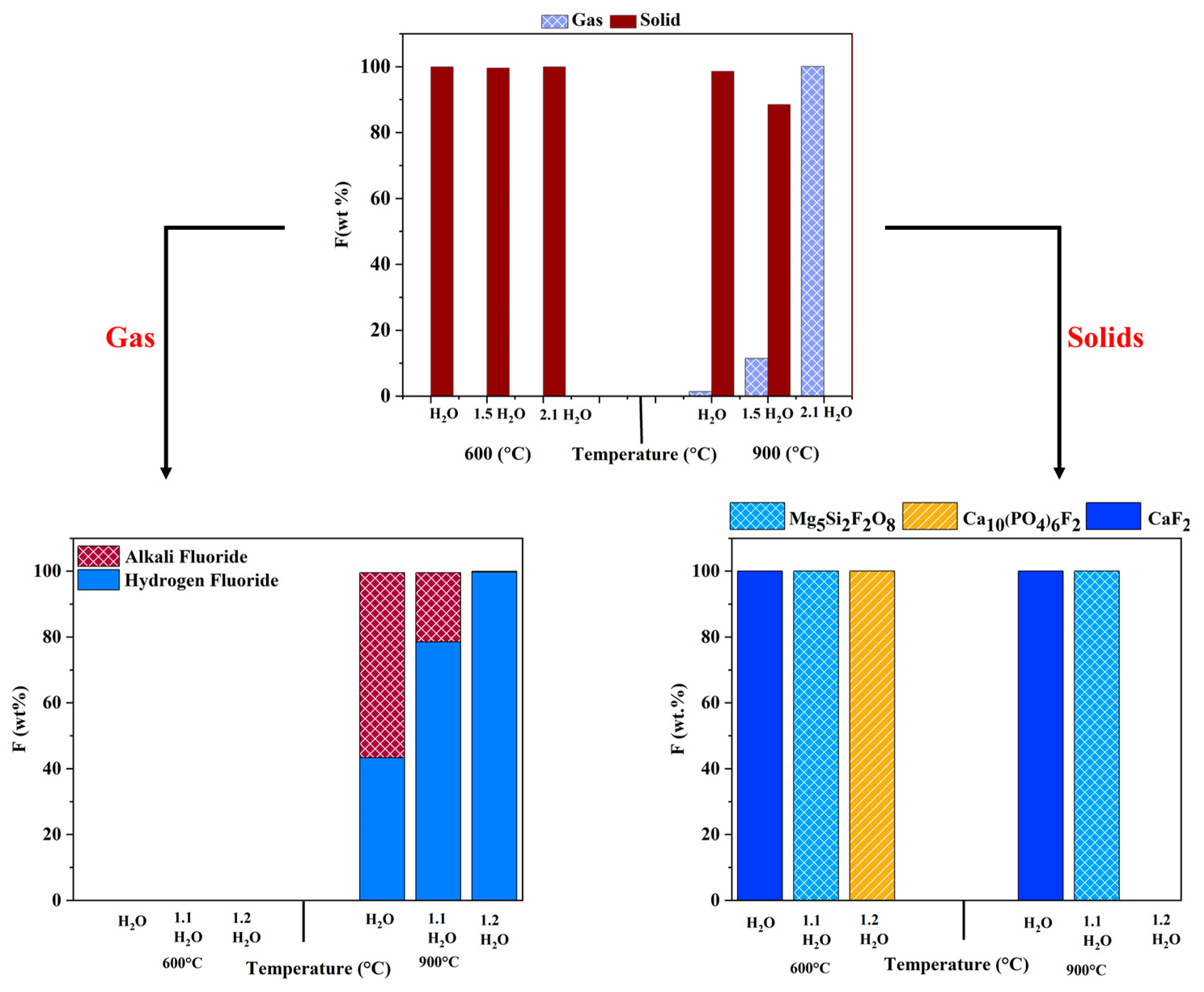
3.2. Effects of Moisture Content
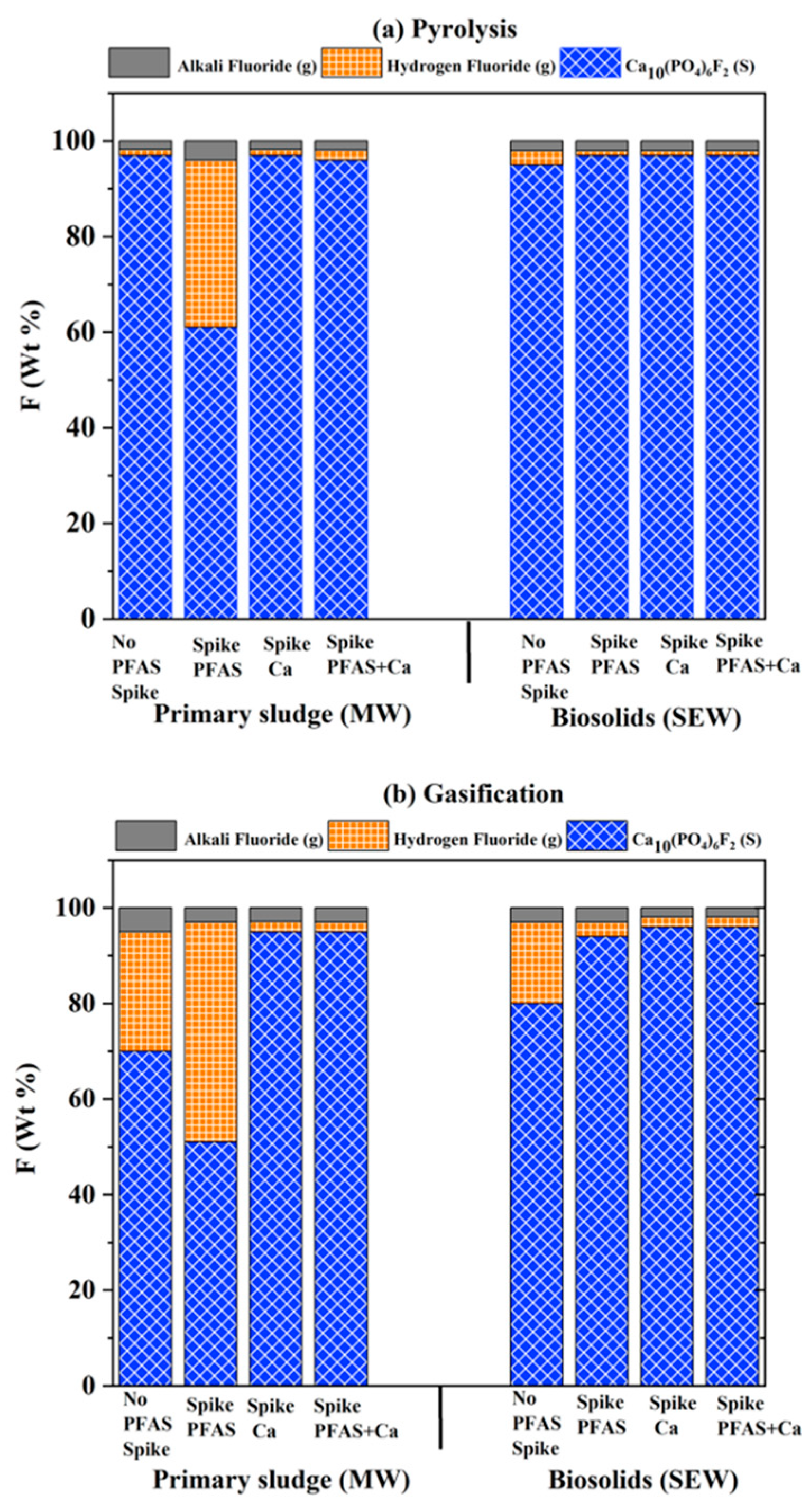
3.3. Effect of Minerals Addition
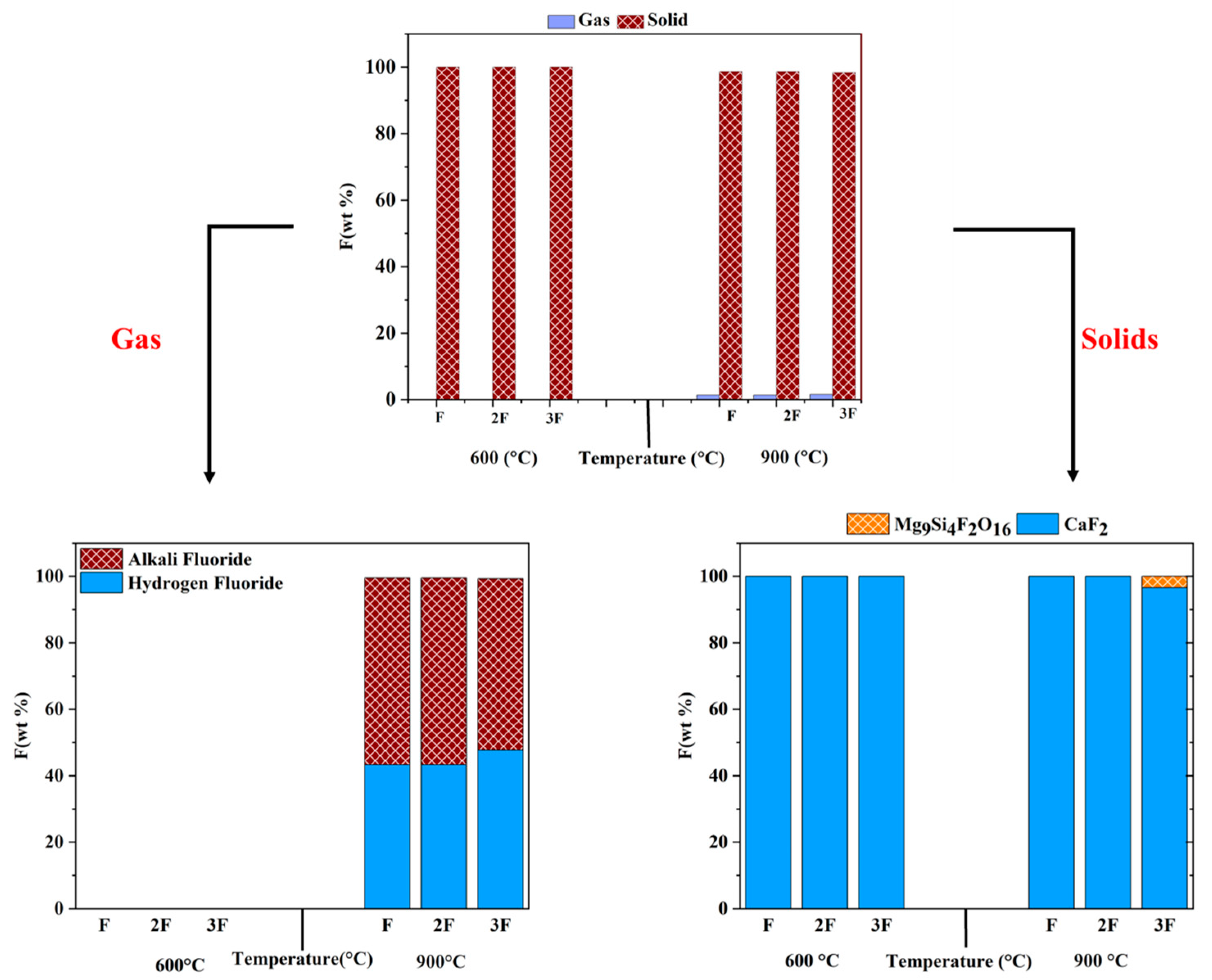
3.4. Effect of Fluorine Concentration
3.5. Effect of Variation in Biosolids’ Composition
4. Limitations and Future Directions
5. Conclusions
- Operating temperatures played an important role in determining the capture of fluorine post-PFAS destruction. Most fluorine was locked as calcium fluoride at low-temperature treatment (600 °C). In comparison, at higher temperature treatment (900 °C), fluorine was primarily available as hydrogen fluoride in the gas phase, and some were locked as CaF2 and their spinels, depending on the thermodynamic minimum Gibbs free energy.
- High oxygen and moisture concentrations at a higher temperature, such as 900 °C, significantly affected the product gas composition, with hydrogen fluoride as the major component. Increasing oxygen and moisture concentration affected the thermodynamic equilibrium and captured fluorine as magnesium and calcium fluoride spinels in the solid phase, irrespective of the operating temperature.
- Alkaline earth metals reduced hydrogen fluoride emissions. A significant decrease in hydrogen fluoride formation was observed at a higher concentration of calcium and magnesium. On the other hand, sodium and potassium had no major impact on gas-phase mass fraction distribution, with sodium being the least influencing element.
- Fluorine concentration had minimal effect on the volatilisation of fluorine even at a higher temperature and did not significantly affect the thermodynamic equilibrium during solid-phase reactions.
- Feedstock compositions directly influenced the fluorine pathway. Feedstock with less organic volatile matter and a high ash content decreased hydrogen fluoride emissions and locked most fluorine as solid-phase calcium fluoride compounds.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per-and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Corsolini, S.; Falandysz, J.; Fillmann, G.; Kumar, K.S.; Loganathan, B.G.; Mohd, M.A.; Olivero, J.; Van Wouwe, N.; Yang, J.H.; et al. Perfluorooctanesulfonate and Related Fluorochemicals in Human Blood from Several Countries. Environ. Sci. Technol. 2004, 38, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Scharf, S.; Weiss, S.; Gans, O.; Scheffknecht, C. Emissions of Perfluorinated Alkylated Substances (PFAS) from Point Sources—Identification of Relevant Branches. Water Sci. Technol. 2008, 58, 59–66. [Google Scholar] [CrossRef] [PubMed]
- CRC CARE. A Human Health Review of PFOS and PFOA; CRC CARE Technical Report No. 42, CRC for Contamination Assessment and Remediation of the Environment; CRC CARE: Newcastle, Australia, 2016. [Google Scholar]
- CSWAB. Perfluorohexane Sulfonic Acid (PFHxS), Its Salts and PFHxS-Related Compounds DRAFT RISK PROFILE Persistent Organic Pollutants Review Committee; CSWAB: Merrimac, WI, USA, 2018. [Google Scholar]
- Boulanger, B.; Vargo, J.D.; Schnoor, J.L.; Hornbuckle, K.C. Evaluation of Perfluorooctane Surfactants in a Wastewater Treatment System and in a Commercial Surface Protection Product. Environ. Sci. Technol. 2005, 39, 5524–5530. [Google Scholar] [CrossRef] [PubMed]
- Huset, C.A.; Chiaia, A.C.; Barofsky, D.F.; Jonkers, N.; Kohler, H.-P.E.; Ort, C.; Giger, W.; Field, J.A. Occurrence and Mass Flows of Fluorochemicals in the Glatt Valley Watershed, Switzerland. Environ. Sci. Technol. 2008, 42, 6369–6377. [Google Scholar] [CrossRef]
- Dauchy, X.; Boiteux, V.; Bach, C.; Colin, A.; Hemard, J.; Rosin, C.; Munoz, J.F. Mass Flows and Fate of per- and Polyfluoroalkyl Substances (PFASs) in the Wastewater Treatment Plant of a Fluorochemical Manufacturing Facility. Sci. Total Environ. 2017, 576, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Phong Vo, H.N.; Ngo, H.H.; Guo, W.; Hong Nguyen, T.M.; Li, J.; Liang, H.; Deng, L.; Chen, Z.; Hang Nguyen, T.A. Poly-and Perfluoroalkyl Substances in Water and Wastewater: A Comprehensive Review from Sources to Remediation. J. Water Process Eng. 2020, 36, 101393. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, H.; Li, F.; Hu, X.; Zhou, Q. Sorption of Short- and Long-Chain Perfluoroalkyl Surfactants on Sewage Sludges. J. Hazard. Mater. 2013, 260, 689–699. [Google Scholar] [CrossRef]
- Kor-Bicakci, G.; Eskicioglu, C. Recent Developments on Thermal Municipal Sludge Pretreatment Technologies for Enhanced Anaerobic Digestion. Renew. Sustain. Energy Rev. 2019, 110, 423–443. [Google Scholar] [CrossRef]
- Houtz, E.F.; Sutton, R.; Park, J.S.; Sedlak, M. Poly- and Perfluoroalkyl Substances in Wastewater: Significance of Unknown Precursors, Manufacturing Shifts, and Likely AFFF Impacts. Water Res. 2016, 95, 142–149. [Google Scholar] [CrossRef]
- Eriksson, U.; Haglund, P.; Kärrman, A. Contribution of Precursor Compounds to the Release of per- and Polyfluoroalkyl Substances (PFASs) from Waste Water Treatment Plants (WWTPs). J. Environ. Sci. 2017, 61, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Halbach, T.R.; Simcik, M.F.; Gulliver, J.S. Input Characterization of Perfluoroalkyl Substances in Wastewater Treatment Plants: Source Discrimination by Exploratory Data Analysis. Water Res. 2012, 46, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Lewis, A.J.; Sales, C.M.; Suri, R.; McKenzie, E.R. Linking PFAS Partitioning Behavior in Sewage Solids to the Solid Characteristics, Solution Chemistry, and Treatment Processes. Chemosphere 2021, 271, 129530. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, I.G.; Halder, P.; Patel, S.; Selezneva, E.; Rathnayake, N.; Marzbali, M.H.; Veluswamy, G.; Sharma, A.; Kundu, S.; Surapaneni, A.; et al. Current Understanding on the Transformation and Fate of per- and Polyfluoroalkyl Substances Before, During, and after Thermal Treatment of Biosolids. Chem. Eng. J. 2024, 493, 152537. [Google Scholar] [CrossRef]
- ANZBP. Australian Biosolids Statistics. Available online: https://www.biosolids.com.au/guidelines/australian-biosolids-statistics/ (accessed on 30 March 2024).
- Moodie, D.; Coggan, T.; Berry, K.; Kolobaric, A.; Fernandes, M.; Lee, E.; Reichman, S.; Nugegoda, D.; Clarke, B.O. Legacy and Emerging per- and Polyfluoroalkyl Substances (PFASs) in Australian Biosolids. Chemosphere 2021, 270, 129143. [Google Scholar] [CrossRef]
- Hamid, H.; Li, L. Role of Wastewater Treatment Plant in Environmental Cycling of Poly- and Perfluoroalkyl Substances. Ecocycles 2016, 2, 43–53. [Google Scholar] [CrossRef]
- EPA Victoria PFAS NEMP 2.0. Available online: https://www.epa.vic.gov.au/for-community/environmental-information/pfas/pfas-national-environmental-management-plan (accessed on 15 June 2024).
- Naidu, R.; Nadebaum, P.; Fang, C.; Cousins, I.; Pennell, K.; Conder, J.; Newell, C.J.; Longpré, D.; Warner, S.; Crosbie, N.D.; et al. Per- and Poly-Fluoroalkyl Substances (PFAS): Current Status and Research Needs. Environ. Technol. Innov. 2020, 19, 100915. [Google Scholar] [CrossRef]
- Kundu, S.; Patel, S.; Halder, P.; Patel, T.; Hedayati Marzbali, M.; Pramanik, B.K.; Paz-Ferreiro, J.; De Figueiredo, C.C.; Bergmann, D.; Surapaneni, A.; et al. Removal of PFASs from Biosolids Using a Semi-Pilot Scale Pyrolysis Reactor and the Application of Biosolids Derived Biochar for the Removal of PFASs from Contaminated Water. Environ. Sci. Water Res. Technol. 2021, 7, 638–649. [Google Scholar] [CrossRef]
- Chen, R.; Sheng, Q.; Dai, X.; Dong, B. Upgrading of Sewage Sludge by Low Temperature Pyrolysis: Biochar Fuel Properties and Combustion Behavior. Fuel 2021, 300, 121007. [Google Scholar] [CrossRef]
- Chanaka Udayanga, W.D.; Veksha, A.; Giannis, A.; Lisak, G.; Chang, V.W.C.; Lim, T.T. Fate and Distribution of Heavy Metals during Thermal Processing of Sewage Sludge. Fuel 2018, 226, 721–744. [Google Scholar] [CrossRef]
- Weber, N.H.; Stockenhuber, S.P.; Delva, C.S.; Fara, A.A.; Grimison, C.C.; Lucas, J.A.; Mackie, J.C.; Stockenhuber, M.; Kennedy, E.M. Kinetics of Decomposition of PFOS Relevant to Thermal Desorption Remediation of Soils. Ind. Eng. Chem. Res. 2021, 60, 9080–9087. [Google Scholar] [CrossRef]
- Ventia PFAS Remediation. Available online: https://www.ventia.com/what-we-do/capabilities/pfas-remediation (accessed on 15 June 2024).
- Winchell, L.J.; Ross, J.J.; Wells, M.J.M.; Fonoll, X.; Norton, J.W.; Bell, K.Y. Per- and Polyfluoroalkyl Substances Thermal Destruction at Water Resource Recovery Facilities: A State of the Science Review. Water Environ. Res. 2021, 93, 826–843. [Google Scholar] [CrossRef]
- Longendyke, G.K.; Katel, S.; Wang, Y. PFAS Fate and Destruction Mechanisms during Thermal Treatment: A Comprehensive Review. Environ. Sci. Process. Impacts 2022, 24, 196–208. [Google Scholar] [CrossRef]
- Wang, F.; Shih, K.; Lu, X.; Liu, C. Mineralization Behavior of Fluorine in Perfluorooctanesulfonate (PFOS) during Thermal Treatment of Lime-Conditioned Sludge. Environ. Sci. Technol. 2013, 47, 2621–2627. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Shih, K.; Liu, C. Influence of Calcium Hydroxide on the Fate of Perfluorooctanesulfonate under Thermal Conditions. J. Hazard. Mater. 2011, 192, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- García, A.N.; Viciano, N.; Font, R. Products Obtained in the Fuel-Rich Combustion of PTFE at High Temperature. J. Anal. Appl. Pyrolysis 2007, 80, 85–91. [Google Scholar] [CrossRef]
- Altarawneh, M.; Almatarneh, M.H.; Dlugogorski, B.Z. Thermal Decomposition of Perfluorinated Carboxylic Acids: Kinetic Model and Theoretical Requirements for PFAS Incineration. Chemosphere 2022, 286, 131685. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, X.; Lv, G.; Liu, B.; Jin, Y.; Yan, J. SO2, NOx, HF, HCl and PCDD/Fs Emissions during Co-Combustion of Bituminous Coal and Pickling Sludge in a Drop Tube Furnace. Fuel 2016, 186, 91–99. [Google Scholar] [CrossRef]
- WHO. WHO Fluorine and Fluorides; WHO: Geneva, Switzerland, 1984.
- Lee, Y.J.; Jeong, I.B. Chemical Pneumonitis by Prolonged Hydrogen Fluoride Inhalation. Respir. Med. Case Rep. 2021, 32, 101338. [Google Scholar] [CrossRef]
- Riedel, T.P.; Wallace, M.A.G.; Shields, E.P.; Ryan, J.V.; Lee, C.W.; Linak, W.P. Low Temperature Thermal Treatment of Gas-Phase Fluorotelomer Alcohols by Calcium Oxide. Chemosphere 2021, 272, 129859. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Li, X.; Shih, K. Effectiveness and Mechanisms of Defluorination of Perfluorinated Alkyl Substances by Calcium Compounds during Waste Thermal Treatment. Environ. Sci. Technol. 2015, 49, 5672–5680. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, R.; Feliciano, T.; Mimna, R.A.; Redding, A.M.; Matthis, J. Thermal Destruction of PFAS during Full-Scale Reactivation of PFAS-Laden Granular Activated Carbon. Remediat. J. 2022, 32, 231–238. [Google Scholar] [CrossRef]
- Kim, J.H.; Ok, Y.S.; Choi, G.H.; Park, B.J. Residual Perfluorochemicals in the Biochar from Sewage Sludge. Chemosphere 2015, 134, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Bioforcetech. Eliminating PFAS from Biosolids Is No Longer a Mystery; Bioforcetech Corporation: South San Francisco, CA, USA, 2019. [Google Scholar]
- Bamdad, H.; Papari, S.; Moreside, E.; Berruti, F. High-Temperature Pyrolysis for Elimination of Per- and Polyfluoroalkyl Substances (PFAS) from Biosolids. Processes 2022, 10, 2187. [Google Scholar] [CrossRef]
- Thoma, E.D.; Wright, R.S.; George, I.; Krause, M.; Presezzi, D.; Villa, V.; Preston, W.; Deshmukh, P.; Kauppi, P.; Zemek, P.G. Pyrolysis Processing of PFAS-Impacted Biosolids, a Pilot Study. J. Air Waste Manag. Assoc. 2022, 72, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Hušek, M.; Semerád, J.; Skoblia, S.; Moško, J.; Kukla, J.; Beňo, Z.; Jeremiáš, M.; Cajthaml, T.; Komárek, M.; Pohořelý, M. Removal of per- and Polyfluoroalkyl Substances and Organic Fluorine from Sewage Sludge and Sea Sand by Pyrolysis. Biochar 2024, 6, 31. [Google Scholar] [CrossRef]
- McNamara, P.; Samuel, M.S.; Sathyamoorthy, S.; Moss, L.; Valtierra, D.; Cortes Lopez, H.; Nigro, N.; Somerville, S.; Liu, Z. Pyrolysis Transports, and Transforms, PFAS from Biosolids to Py-Liquid. Environ. Sci. Water Res. Technol. 2023, 9, 386–395. [Google Scholar] [CrossRef]
- Logan City Council. Technical Report for Loganholme Wastewater Treatment Plant: Biosolids Gasification Demonstration Plant (PBE-075); Loganholme Council: Queensland, Australia, 2021. [Google Scholar]
- Sørmo, E.; Castro, G.; Hubert, M.; Licul-Kucera, V.; Quintanilla, M.; Asimakopoulos, A.G.; Cornelissen, G.; Arp, H.P.H. The Decomposition and Emission Factors of a Wide Range of PFAS in Diverse, Contaminated Organic Waste Fractions Undergoing Dry Pyrolysis. J. Hazard. Mater. 2023, 454, 131447. [Google Scholar] [CrossRef]
- Khan, M.Y.; So, S.; da Silva, G. Decomposition Kinetics of Perfluorinated Sulfonic Acids. Chemosphere 2020, 238, 124615. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, X.; Yao, Z.; Yu, Q.; Wang, Z.; Lin, Y. Study of the Pyrolysis of Municipal Sludge in N2/CO2 Atmosphere. Appl. Therm. Eng. 2018, 128, 662–671. [Google Scholar] [CrossRef]
- RMRC Sewage Sludge Ash. Available online: https://rmrc.wisc.edu/ug-mat-sewage-sludge-ash/ (accessed on 10 June 2024).
- Oguz, E. Adsorption of Fluoride on Gas Concrete Materials. J. Hazard. Mater. 2005, 117, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Appelman, E.H.; Thompson, R.C. Studies of the Aqueous Chemistry of Fluorine and Hypofluorous Acid. J. Am. Chem. Soc. 2002, 106, 4167–4172. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding Organofluorine Chemistry. An Introduction to the C–F Bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.C.Y.T.-H.I.Y.-S. Treatment of Hydrogen Fluoride Generated from the F-Gases Decomposition Processes. Asian J. Atmos. Environ. 2016, 10, 190–196. [Google Scholar] [CrossRef]
- Rampersadh, P. Removal of Hydrogen Fluoride from Gas Streams; University of Witwatersrand: Johannesburg, South Africa, 2005. [Google Scholar]
- Conesa, J.A.; Font, R. Polytetrafluoroethylene Decomposition in Air and Nitrogen. Polym. Eng. Sci. 2001, 41, 2137–2147. [Google Scholar] [CrossRef]
- Simon, C.M.; Kaminsky, W. Chemical Recycling of Polytetrafluoroethylene by Pyrolysis. Polym. Degrad. Stab. 1998, 62, 1–7. [Google Scholar] [CrossRef]
- Weber, N.H.; Delva, C.S.; Stockenhuber, S.P.; Grimison, C.C.; Lucas, J.A.; Mackie, J.C.; Stockenhuber, M.; Kennedy, E.M. Thermal Decomposition of Perfluorooctanesulfonic Acid (PFOS) in the Presence of Water Vapor. Ind. Eng. Chem. Res. 2022, 61, 15146–15155. [Google Scholar] [CrossRef]
- Uhle, J.M. ¨; Ganesan, A.L.; Miller, B.R.; Salameh, P.K.; Harth, C.M.; Greally, B.R.; Rigby, M.; Porter, L.W.; Steele, L.P.; Trudinger, C.M.; et al. Perfluorocarbons in the Global Atmosphere: Tetrafluoromethane, Hexafluoroethane, and Octafluoropropane. Atmos. Chem. Phys 2010, 10, 5145–5164. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.; He, X.; Song, M.; Westerhoff, P.; Doudrick, K.; Hanigan, D. Critical Review of Thermal Decomposition of Per- and Polyfluoroalkyl Substances: Mechanisms and Implications for Thermal Treatment Processes. Environ. Sci. Technol. 2022, 56, 5355–5370. [Google Scholar] [CrossRef]
- Hakeem, I.G.; Halder, P.; Patel, S.; Sharma, A.; Gupta, R.; Surapaneni, A.; Paz-Ferreiro, J.; Shah, K. Enhancing the Pyrolytic Conversion of Biosolids to Value-Added Products via Mild Acid Pre-Treatment. J. Anal. Appl. Pyrolysis 2023, 173, 106087. [Google Scholar] [CrossRef]
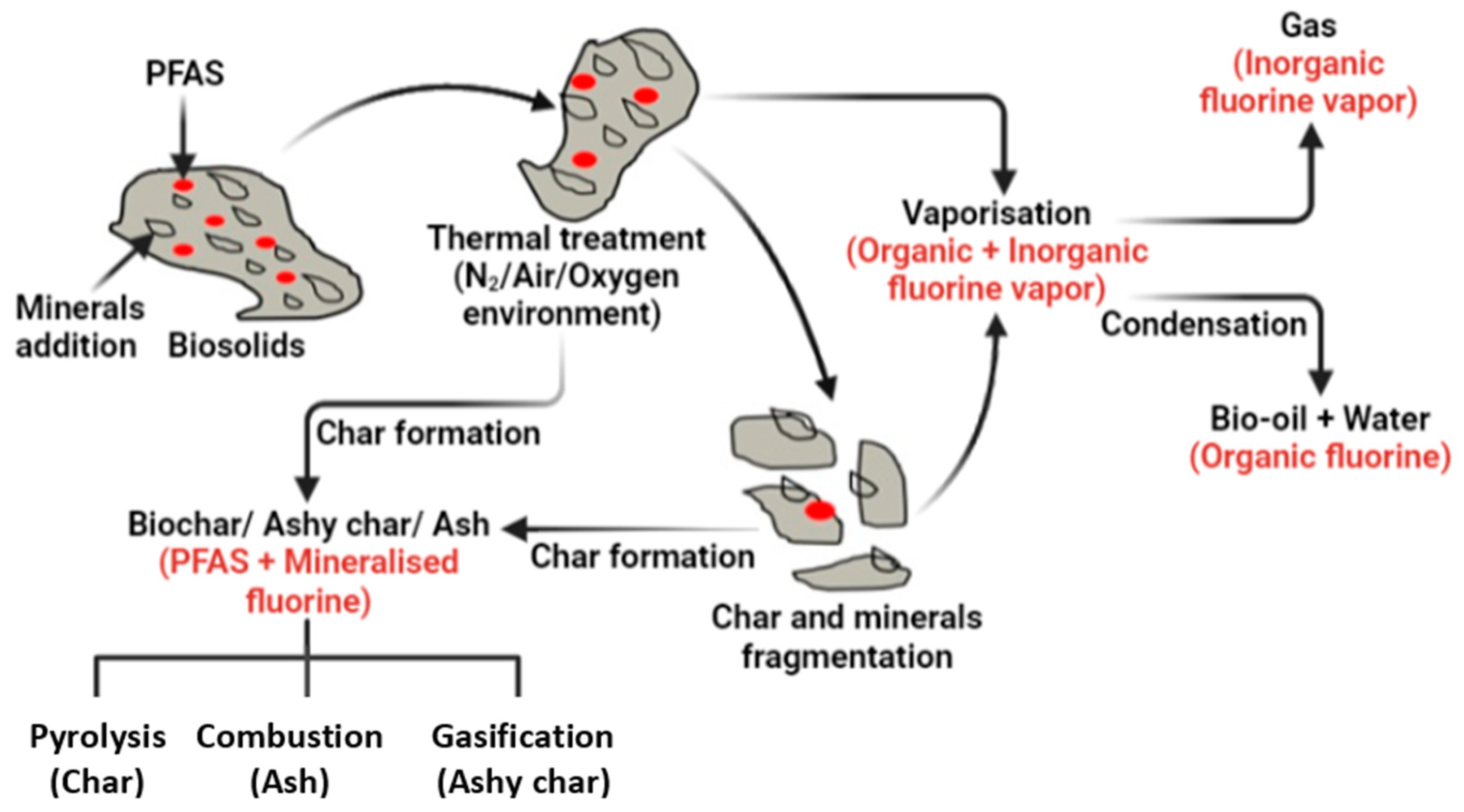

| Component | Mass (g/100 g Dry Mass) | ||
|---|---|---|---|
| Municipal Sludge [48,49] | Biosolids (SEW) a | Primary Sludge (MW) b | |
| H2O | 0 | 0 | 0 |
| C | 26.5 | 38.14 | 45.38 |
| H | 4.6 | 4.68 | 6.9 |
| O | 19.4 | 20.92 | 32.50 |
| N | 4.3 | 5.99 | 3.75 |
| S | 0.35 | 0.96 | 0.43 |
| Cl | 0.4 | 0.31 | |
| Ash | 44.6 | 28.89 | 10.7 |
| Volatile matter | 48.8 | 60.6 | 78.3 |
| Fixed carbon | 6.6 | 10.4 | 11.0 |
| Si | 19 | 11.69 | 4.33 |
| Ca | 8 | 4.68 | 1.73 |
| Fe | 4 | 2.34 | 0.87 |
| Al | 7 | 4.09 | 1.52 |
| Mg | 2 | 1.17 | 0.43 |
| Na | 0.3 | 0.18 | 0.06 |
| K | 0.3 | 0.29 | 0.11 |
| P | 6 | 3.51 | 1.30 |
| Cr | 0.01 | 0.01 | |
| Cu | 0.39 | 0.14 | |
| Pb | 0.01 | 0.00 | |
| Zn | 0.51 | 0.19 | |
| F | 0.05 c | 0.04 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.; Halder, P.; Hakeem, I.G.; Selezneva, E.; Jena, M.K.; Veluswamy, G.; Rathnayake, N.; Sharma, A.; Sivaram, A.K.; Surapaneni, A.; et al. The Fate of Fluorine Post Per- and Polyfluoroalkyl Substances Destruction during the Thermal Treatment of Biosolids: A Thermodynamic Study. Energies 2024, 17, 3476. https://doi.org/10.3390/en17143476
Patel S, Halder P, Hakeem IG, Selezneva E, Jena MK, Veluswamy G, Rathnayake N, Sharma A, Sivaram AK, Surapaneni A, et al. The Fate of Fluorine Post Per- and Polyfluoroalkyl Substances Destruction during the Thermal Treatment of Biosolids: A Thermodynamic Study. Energies. 2024; 17(14):3476. https://doi.org/10.3390/en17143476
Chicago/Turabian StylePatel, Savankumar, Pobitra Halder, Ibrahim Gbolahan Hakeem, Ekaterina Selezneva, Manoj Kumar Jena, Ganesh Veluswamy, Nimesha Rathnayake, Abhishek Sharma, Anithadevi Kenday Sivaram, Aravind Surapaneni, and et al. 2024. "The Fate of Fluorine Post Per- and Polyfluoroalkyl Substances Destruction during the Thermal Treatment of Biosolids: A Thermodynamic Study" Energies 17, no. 14: 3476. https://doi.org/10.3390/en17143476
APA StylePatel, S., Halder, P., Hakeem, I. G., Selezneva, E., Jena, M. K., Veluswamy, G., Rathnayake, N., Sharma, A., Sivaram, A. K., Surapaneni, A., Naidu, R., Megharaj, M., Vuppaladadiyam, A. K., & Shah, K. (2024). The Fate of Fluorine Post Per- and Polyfluoroalkyl Substances Destruction during the Thermal Treatment of Biosolids: A Thermodynamic Study. Energies, 17(14), 3476. https://doi.org/10.3390/en17143476










