Highly Conductive Single-Ion Polymeric Electrolyte for Long-Cycle-Life Lithium Metal Batteries
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization and Measurements
2.3. Electrochemical Performance Testing
3. Results and Discussion
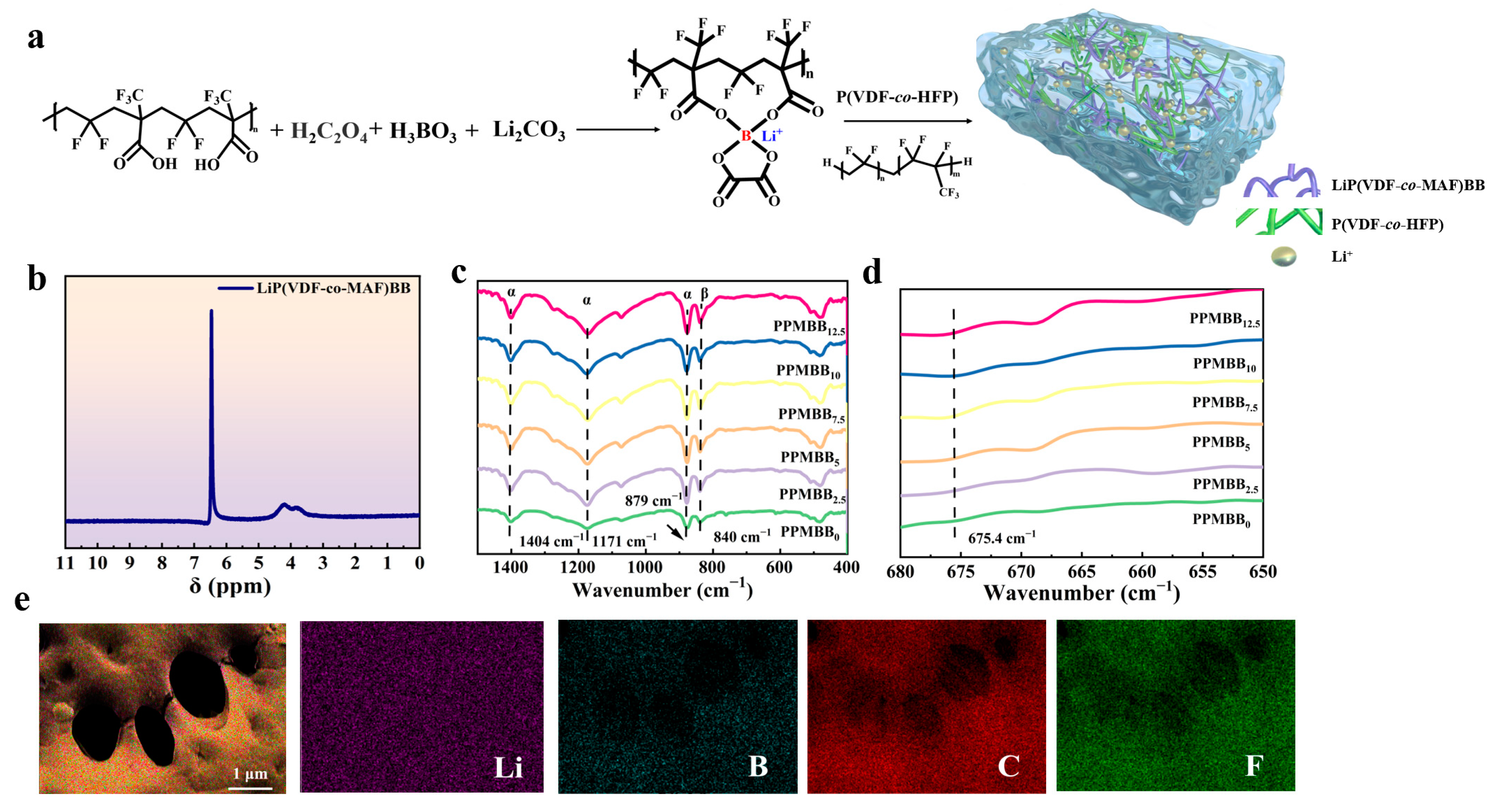
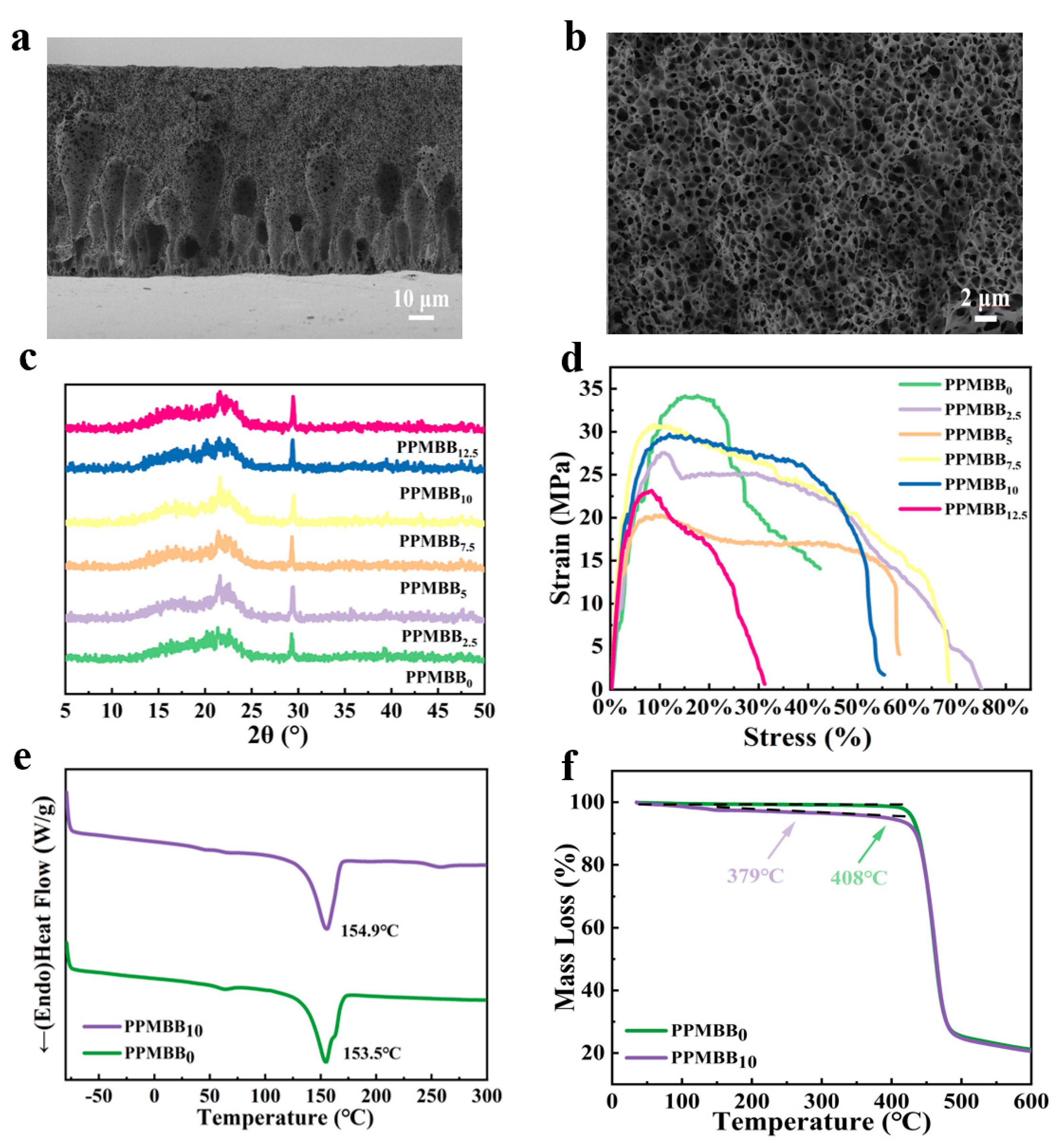
3.1. Structural Characteristics of LiP(VDF-co-MAF)BB and PPMBBn
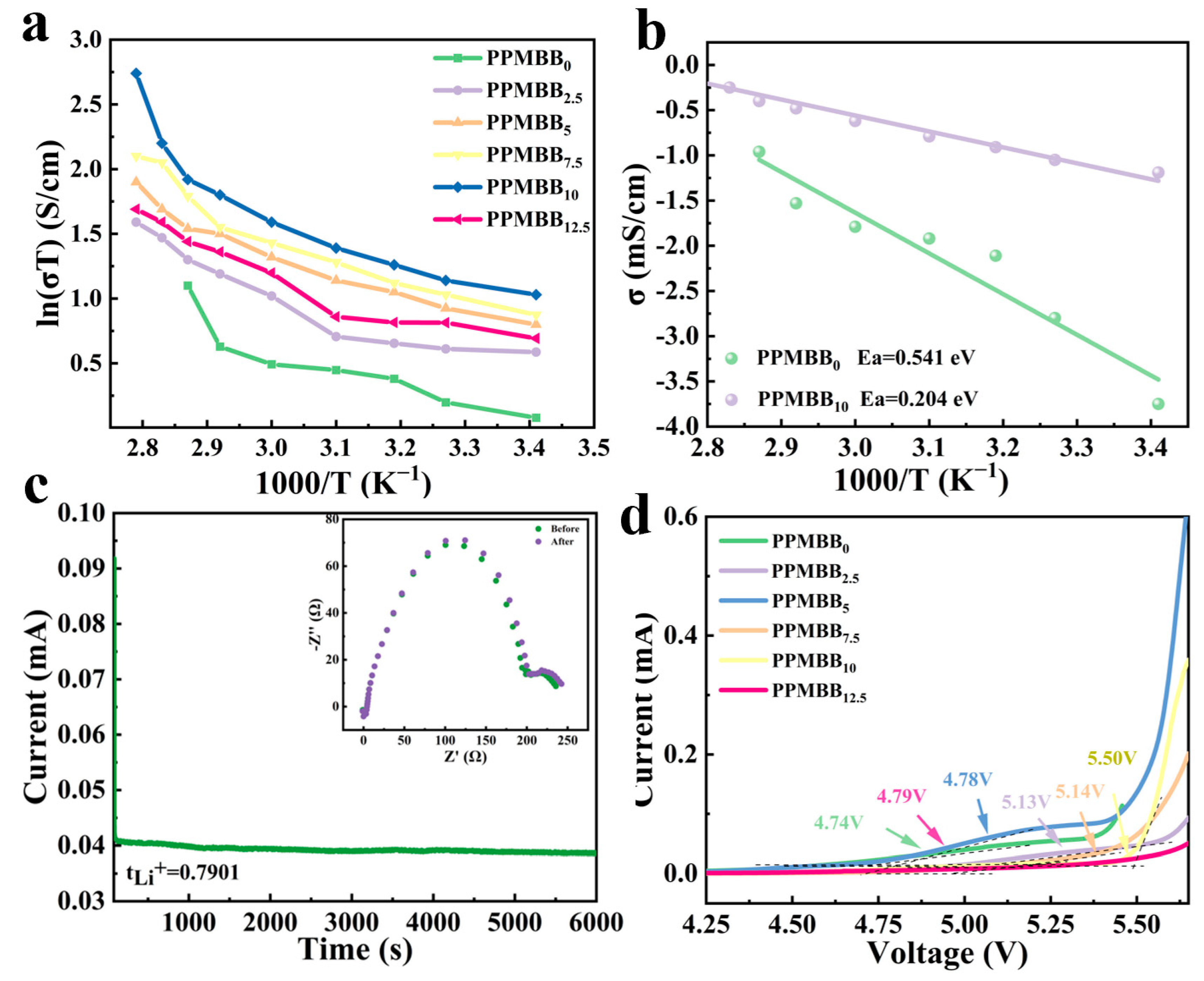
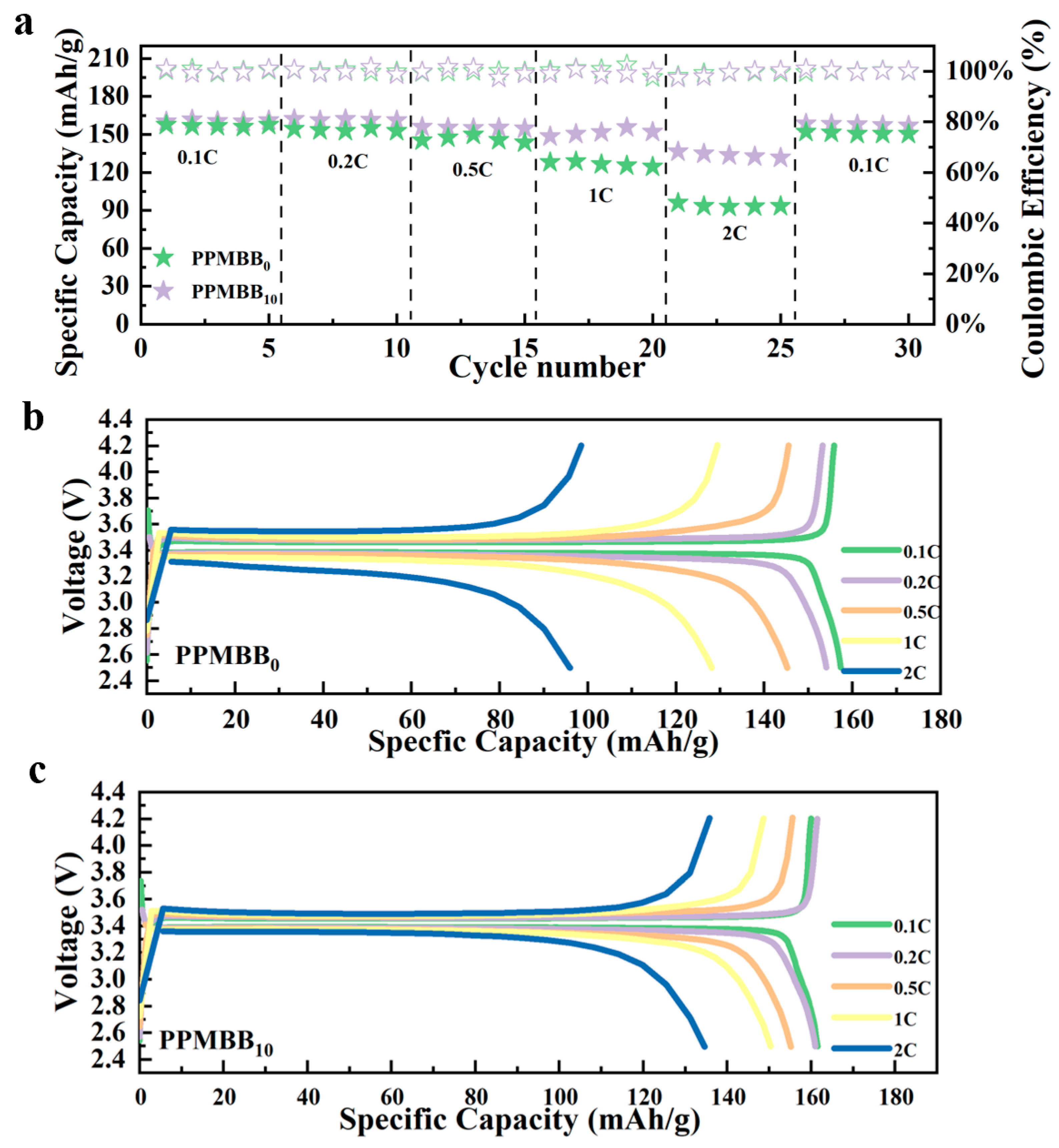
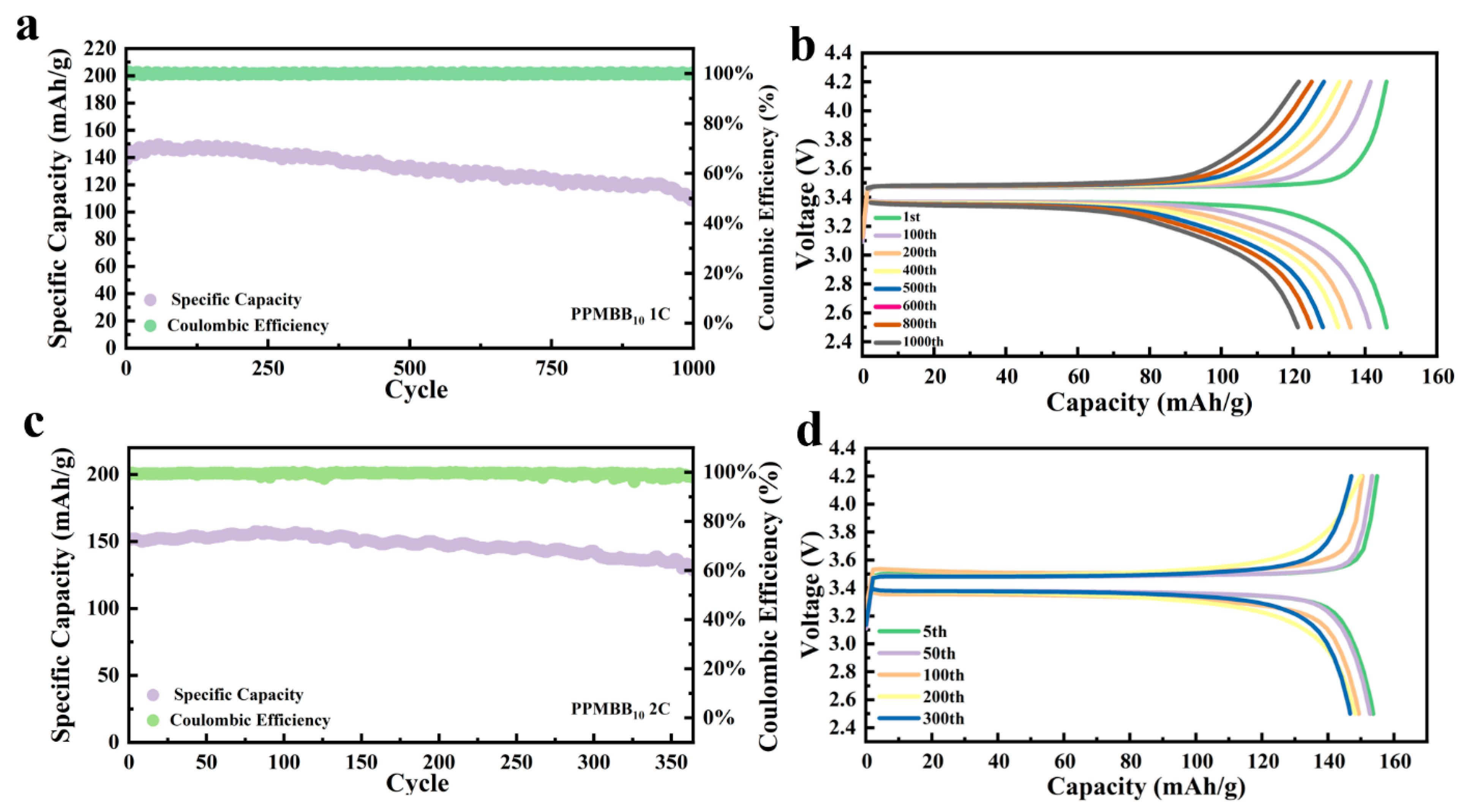
3.2. Electrochemical Performance
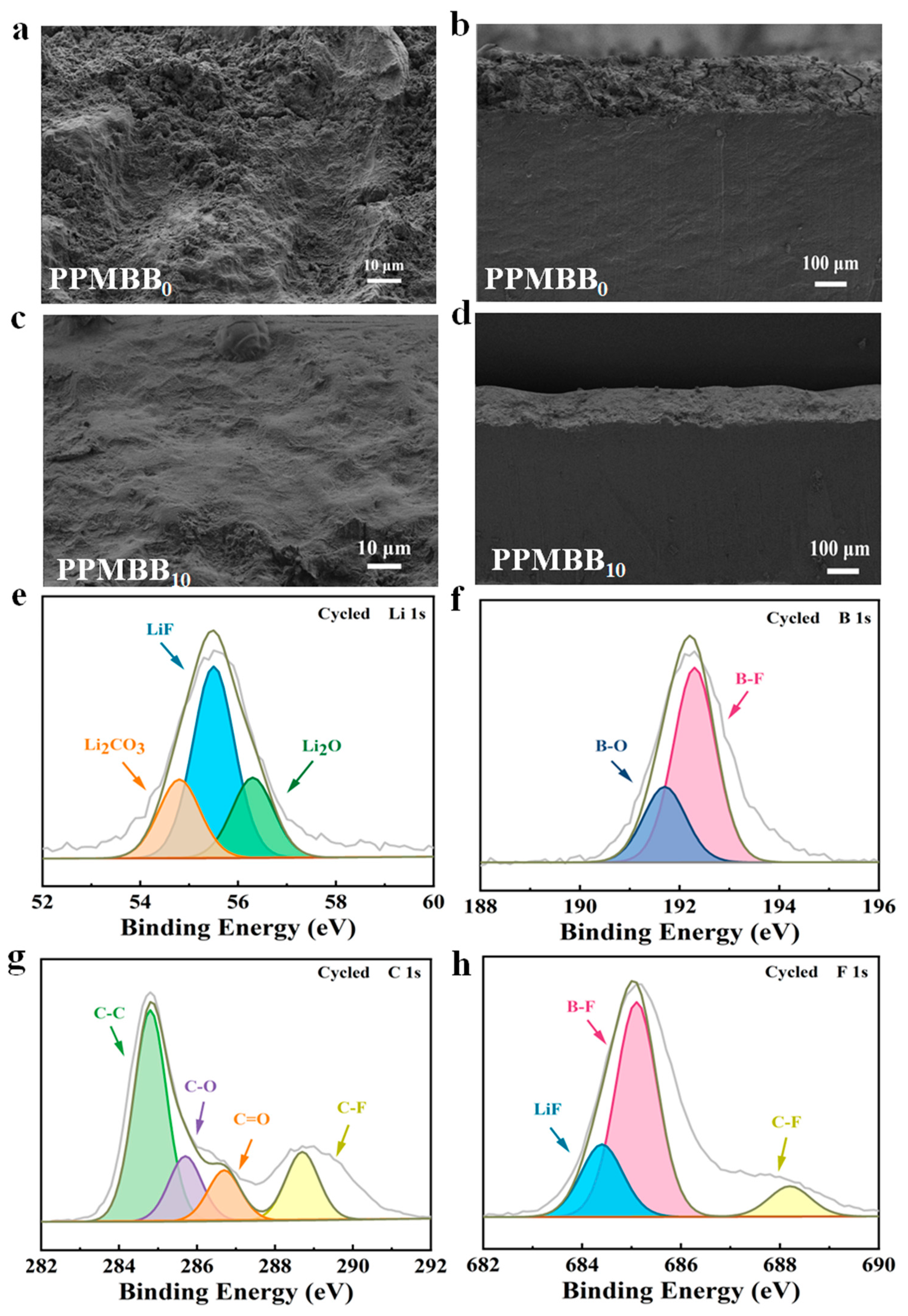
3.3. Anode Interface Stability
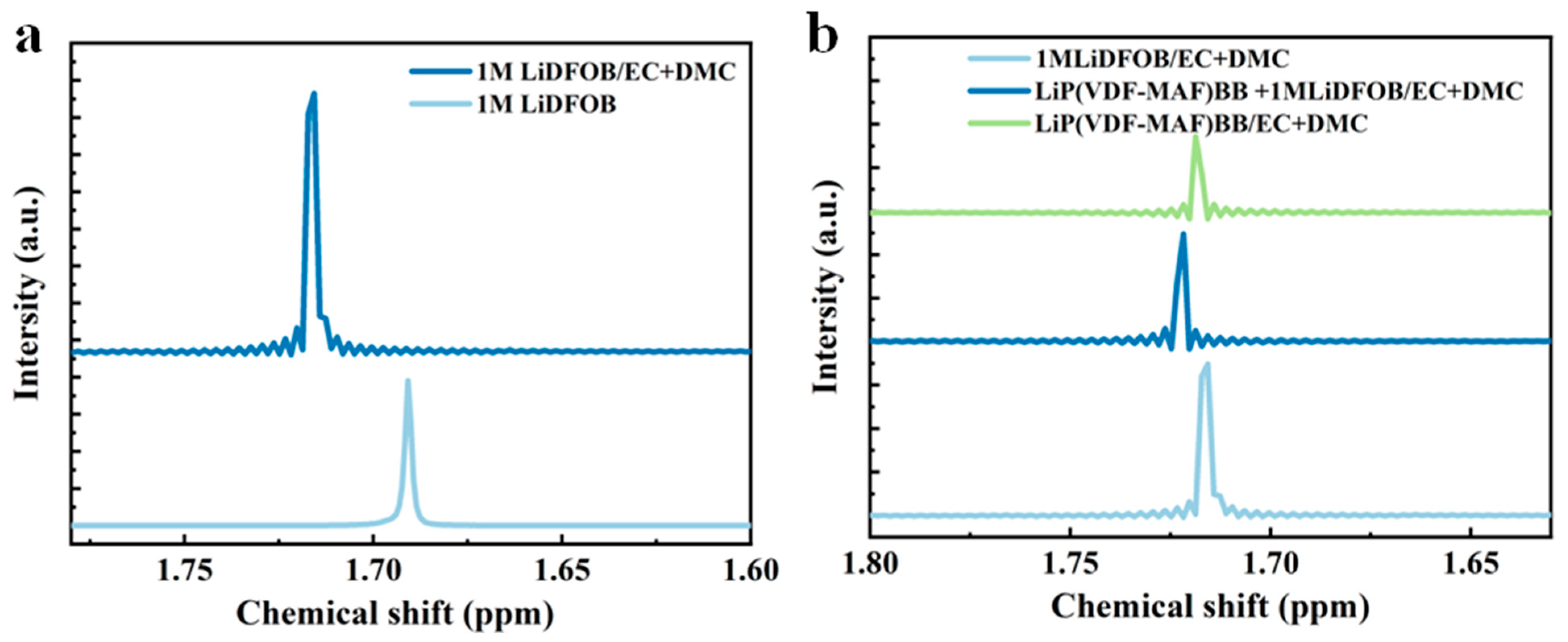
3.4. 7Li NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Zhu, T.; Zhu, M.; Kang, P.; Yuan, S.; Li, Y.; Lan, J.; Yang, X.; Sui, G. In Situ Constructing Ultrathin, Robust-Flexible Polymeric Electrolytes with Rapid Interfacial Ion Transport in Lithium Metal Batteries. Small Methods 2022, 6, 2201114. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, Y.; Wu, H. Polymers in Lithium-Ion and Lithium Metal Batteries. Adv. Energy Mater. 2021, 11, 2003239. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Ba, D.; Zhao, Y.; Ye, Y.; Li, Y.; Liu, J. Filler-Integrated Composite Polymer Electrolyte for Solid-State Lithium Batteries. Adv. Mater. 2022, 35, 2110423. [Google Scholar] [CrossRef] [PubMed]
- Long, M.-C.; Wu, G.; Wang, X.-L.; Wang, Y.-Z. Self-adaptable gel polymer electrolytes enable high-performance and all-round safety lithium ion batteries. Energy Storage Mater. 2022, 53, 62–71. [Google Scholar] [CrossRef]
- Choo, Y.; Halat, D.M.; Villaluenga, I.; Timachova, K.; Balsara, N.P. Diffusion and migration in polymer electrolytes. Prog. Polym. Sci. 2020, 103, 101220. [Google Scholar] [CrossRef]
- Doyle, M.; Newman, J.; Gozdz, A.S.; Schmutz, C.N.; Tarascon, J.M. Comparison of Modeling Predictions with Experimental Data from Plastic Lithium Ion Cells. J. Electrochem. Soc. 1996, 143, 1890. [Google Scholar] [CrossRef]
- Guan, S.; Wen, K.; Liang, Y.; Xue, C.; Liu, S.; Yu, J.; Zhang, Z.; Wu, X.; Yuan, H.; Lin, Z.; et al. An organic additive assisting with high ionic conduction and dendrite resistance of polymer electrolytes. J. Mater. Chem. A 2022, 10, 24269–24279. [Google Scholar] [CrossRef]
- Guan, T.; Yang, H.; Ou, M.; Zhang, J. Storage period affecting dynamic succession of microbiota and quality changes of strong-flavor Baijiu Daqu. LWT 2021, 139, 110544. [Google Scholar] [CrossRef]
- Guzmán-González, G.; Alvarez-Tirado, M.; Olmedo-Martínez, J.L.; Picchio, M.L.; Casado, N.; Forsyth, M.; Mecerreyes, D. Lithium Borate Ionic Liquids as Single-Component Electrolytes for Batteries. Adv. Energy Mater. 2023, 13, 2202974. [Google Scholar] [CrossRef]
- Hou, T.; Qian, Y.; Li, D.; Xu, B.; Huang, Z.; Liu, X.; Wang, H.; Jiang, B.; Xu, H.; Huang, Y. Electronegativity-Induced Single-Ion Conducting Polymer Electrolyte for Solid-State Lithium Batteries. Energy Environ. Mater. 2023, 6, e12428. [Google Scholar] [CrossRef]
- Huang, M.; Feng, S.; Zhang, W.; Giordano, L.; Chen, M.; Amanchukwu, C.V.; Anandakathir, R.; Shao-Horn, Y.; Johnson, J.A. Fluorinated Aryl Sulfonimide Tagged (FAST) salts: Modular synthesis and structure–property relationships for battery applications. Energy Environ. Sci. 2018, 11, 1326–1334. [Google Scholar] [CrossRef]
- Jurng, S.; Brown, Z.L.; Kim, J.; Lucht, B.L. Effect of electrolyte on the nanostructure of the solid electrolyte interphase (SEI) and performance of lithium metal anodes. Energy Environ. Sci. 2018, 11, 2600–2608. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, J.; Zhou, J.; Chen, S.; Jia, Y.; Han, X.; Meng, X.; Bielawski, C.W.; Geng, J. A Space-Confined Polymerization Templated by Ice Enables Large-Scale Synthesis of Two-Dimensional Polymer Sheets. Angew. Chem. Int. Ed. 2023, 62, e202301940. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.W.; Trapa, P.E.; Olugebefola, S.C.; Gonzalez-Leon, J.A.; Sadoway, D.R.; Mayes, A.M. Effect of Counter Ion Placement on Conductivity in Single-Ion Conducting Block Copolymer Electrolytes. J. Electrochem. Soc. 2005, 152, A158. [Google Scholar] [CrossRef]
- Sha, Z.; Boyer, C.; Li, G.; Yu, Y.; Allioux, F.-M.; Kalantar-Zadeh, K.; Wang, C.-H.; Zhang, J. Electrospun liquid metal/PVDF-HFP nanofiber membranes with exceptional triboelectric performance. Nano Energy 2022, 92, 106713. [Google Scholar] [CrossRef]
- Shan, X.; Morey, M.; Li, Z.; Zhao, S.; Song, S.; Xiao, Z.; Feng, H.; Gao, S.; Li, G.; Sokolov, A.P.; et al. A Polymer Electrolyte with High Cationic Transport Number for Safe and Stable Solid Li-Metal Batteries. ACS Energy Lett. 2022, 7, 4342–4351. [Google Scholar] [CrossRef]
- Sun, X.-G.; Angell, C.A. New single ion conductors (“polyBOP” and analogs) for rechargeable lithium batteries. Solid State Ion. 2004, 175, 743–746. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Zhang, W.; Wang, D.; Shen, Z.; Zheng, J.; Zhuang, H.L.; He, Y.; Lu, Y. Dual-salt-additive electrolyte enables high-voltage lithium metal full batteries capable of fast-charging ability. Nano Energy 2021, 89, 106353. [Google Scholar] [CrossRef]
- Weber, R.L.; Mahanthappa, M.K. Thiol–ene synthesis and characterization of lithium bis(malonato)borate single-ion conducting gel polymer electrolytes. Soft Matter 2017, 13, 7633–7643. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Q.; Li, Q.; An, Q.; Zhao, Y.; Peng, Z.; Jiang, Y.; Tan, S.; Yan, M.; Mai, L. Pseudocapacitive layered iron vanadate nanosheets cathode for ultrahigh-rate lithium ion storage. Nano Energy 2018, 47, 294–300. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, F.; Yang, Z.; Ka, O.; Wen, L.; Wang, X.; Liu, S.; Lu, W.; Dai, L. Multifunctionalizing electrolytes in situ for lithium metal batteries. Nano Energy 2023, 116, 108825. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Zeng, X.; Dong, L.; Fu, J.; Chen, L.; Zhou, J.; Zong, P.; Liu, G.; Shi, L. Enhanced interfacial stability with a novel boron-centered crosslinked hybrid polymer gel electrolytes for lithium metal batteries. Chem. Eng. J. 2022, 428, 131100. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, J.; Shen, S.; Zhu, Z.; Mao, S.; Xiao, X.; Zhu, C.; Tang, J.; Lu, X.; Chen, J. Recent Advances on Dual-Band Electrochromic Materials and Devices. Adv. Funct. Mater. 2022, 32, 2109848. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, L.; Hayakawa, T.; Ueda, M.; Higashihara, T. Polymer electrolyte membranes based on poly(phenylene ether)s with sulfonic acid via long alkyl side chains. J. Mater. Chem. A 2013, 1, 11389–11396. [Google Scholar] [CrossRef]
- Zhang, Y.; Irfan, M.; Yang, Z.; Liu, K.; Su, J.; Zhang, W. Lithium hydroxyphenyl propanesulfonate imparts composite solid polymer electrolytes with ultrahigh ionic conductivity for dendrite free lithium batteries. Chem. Eng. J. 2022, 435, 134775. [Google Scholar] [CrossRef]
- Zhang, Y.; Rohan, R.; Sun, Y.; Cai, W.; Xu, G.; Lin, A.; Cheng, H. A gel single ion polymer electrolyte membrane for lithium-ion batteries with wide-temperature range operability. RSC Adv. 2014, 4, 21163–21170. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Wang, X.J.; Hou, Y.Y.; Gao, X.W.; Liu, L.L.; Wu, Y.P.; Shimizu, M. A new single-ion polymer electrolyte based on polyvinyl alcohol for lithium ion batteries. Electrochim. Acta 2013, 87, 113–118. [Google Scholar] [CrossRef]
- Lin, Z.; Guo, X.; Zhang, R.; Tang, M.; Ding, P.; Zhang, Z.; Wu, L.; Wang, Y.; Zhao, S.; Zhang, Q.; et al. Molecular structure adjustment enhanced anti-oxidation ability of polymer electrolyte for solid-state lithium metal battery. Nano Energy 2022, 98, 107330. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, Y.; Li, Y.; Liu, Y.; Zhong, S.; Xie, M.; Yan, F.; Zhang, Z.; Peng, J.; Li, J.; et al. Regulating solvation structure in gel polymer electrolytes with covalent organic frameworks for lithium metal batteries. Energy Storage Mater. 2022, 53, 917–926. [Google Scholar] [CrossRef]
- He, F.; Hu, Z.; Tang, W.; Wang, A.; Wen, B.; Zhang, L.; Luo, J. Vertically Heterostructured Solid Electrolytes for Lithium Metal Batteries. Adv. Funct. Mater. 2022, 32, 2201465. [Google Scholar] [CrossRef]
- Jabbari, V.; Yurkiv, V.; Rasul, M.G.; Saray, M.T.; Rojaee, R.; Mashayek, F.; Shahbazian-Yassar, R. An efficient gel polymer electrolyte for dendrite-free and long cycle life lithium metal batteries. Energy Storage Mater. 2022, 46, 352–365. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, L.; Lin, Z.; Tang, M.; Ding, P.; Guo, X.; Zhang, Z.; Liu, S.; Wang, B.; Yin, X.; et al. Hydrogen bonds enhanced composite polymer electrolyte for high-voltage cathode of solid-state lithium battery. Nano Energy 2022, 96, 107105. [Google Scholar] [CrossRef]
- Lu, R.; Shokrieh, A.; Li, C.; Zhang, B.; Amin, K.; Mao, L.; Wei, Z. PVDF-HFP layer with high porosity and polarity for high-performance lithium metal anodes in both ether and carbonate electrolytes. Nano Energy 2022, 95, 107009. [Google Scholar] [CrossRef]
- Yu, S.; Wu, Z.; Holoubek, J.; Liu, H.; Hopkins, E.; Xiao, Y.; Xing, X.; Lee, M.H.; Liu, P. A Fiber-Based 3D Lithium Host for Lean Electrolyte Lithium Metal Batteries. Adv. Sci. 2022, 9, 2104829. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Sun, Z.; Hou, Q.; Duan, J.; Li, C.; Dou, W.; Fan, J.; Zheng, M.; Dong, Q. Regulating Lithium Plating/Stripping Behavior by a Composite Polymer Electrolyte Endowed with Designated Ion Channels. Small 2022, 18, 2205571. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; He, W.; Zeng, C.; Li, L.; Yang, W. Biomimetic plant-cell composite gel polymer electrolyte for boosting rate performance of lithium metal batteries. Chem. Eng. J. 2023, 451, 138414. [Google Scholar] [CrossRef]
- Park, S.; Sohn, J.-Y.; Hwang, I.-T.; Shin, J.; Yun, J.-M.; Eom, K.; Shin, K.; Lee, Y.-M.; Jung, C.-H. In-situ preparation of gel polymer electrolytes in a fully-assembled lithium ion battery through deeply-penetrating high-energy electron beam irradiation. Chem. Eng. J. 2023, 452, 139339. [Google Scholar] [CrossRef]
- Sun, P.; Chen, J.; Li, Y.; Tang, X.; Sun, H.; Song, G.; Mu, X.; Zhang, T.; Zha, X.; Li, F.; et al. Deep eutectic solvent-based gel electrolytes for flexible electrochromic devices with excellent high/low temperature durability. InfoMat 2022, 5, e12363. [Google Scholar] [CrossRef]
- Xia, J.; Gao, R.; Yang, Y.; Tao, Z.; Han, Z.; Zhang, S.; Xing, Y.; Yang, P.; Lu, X.; Zhou, G. TinO2n–1/MXene Hierarchical Bifunctional Catalyst Anchored on Graphene Aerogel toward Flexible and High-Energy Li–S Batteries. ACS Nano 2022, 16, 19133–19144. [Google Scholar] [CrossRef]
- Qiao, L.; Rodriguez Peña, S.; Martínez-Ibañez, M.; Santiago, A.; Aldalur, I.; Lobato, E.; Sanchez-Diez, E.; Zhang, Y.; Manzano, H.; Zhu, H.; et al. Anion π–π Stacking for Improved Lithium Transport in Polymer Electrolytes. J. Am. Chem. Soc. 2022, 144, 9806–9816. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, Y.; Guan, M.; Qiu, J.; Wang, Z. A High-Energy and Safe Lithium Battery Enabled by Solid-State Redox Chemistry in a Fireproof Gel Electrolyte. Adv. Mater. 2022, 34, 2201981. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Jin, L.; Lu, Y.; Wu, Q.; Zheng, J.; Zhang, C.; Chen, Z.; Zheng, J.P. Over-Potential Tailored Thin and Dense Lithium Carbonate Growth in Solid Electrolyte Interphase for Advanced Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103402. [Google Scholar] [CrossRef]
- Mi, J.; Ma, J.; Chen, L. Topology Crafting of Polyvinylidene Difluoride Electrolyte Creates Ultra-Long Cycling High-Voltage Lithium Metal Solid-State Batteries. Energy Storage Mater. 2022, 48, 375. [Google Scholar] [CrossRef]
- Badatya, S.; Kumar, A.; Sharma, C. Transparent flexible graphene quantum dot-(PVDF-HFP) piezoelectric nanogenerator. Mater. Lett. 2021, 290, 129493. [Google Scholar] [CrossRef]
- Luo, J.; Fang, C.C.; Wu, N.L. Anodes: High Polarity Poly(vinylidene difluoride) Thin Coating for Dendrite-Free and High-Performance Lithium Metal Anodes. Adv. Energy Mater. 2018, 8, 1701482. [Google Scholar] [CrossRef]
- Sim, L.N.; Majid, S.R.; Arof, A.K. FTIR studies of PEMA/PVdF-HFP blend polymer electrolyte system incorporated with LiCF3SO3 salt. Vib. Spectrosc. 2012, 58, 57–66. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Effect of polyaniline (PANI) on Poly(vinylidene fluoride-co-hexaflouro propylene) (PVDF-co-HFP) polymer electrolyte membrane prepared by breath figure method. Polymer Testing 2017, 60, 124–131. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Yang, G.J.; Li, X.Y.; Zhang, S.M.; Chen, R.J.; Wang, X.F.; Gao, Y.R.; Wang, Z.X.; Chen, L.Q. Polymer electrolytes based on interactions between [solvent-Li+] complex and solvent-modified polymer. Energy Storage Mater. 2022, 51, 443–452. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Wu, Q.; Zhang, Y.; Li, Z.; Liu, Z.; Huo, S.; Dong, J.; Zeng, D.; Cheng, H. A facile non-solvent induced phase separation process for preparation of highly porous polybenzimidazole separator for lithium metal battery application. Sci. Rep. 2019, 9, 19320. [Google Scholar] [CrossRef]
- Luo, R.P.; Wang, C.; Zhang, Z.X.; Lv, W.Q.; Wei, Z.H.; Zhang, Y.N.; Luo, X.Y.; He, W.D. Three-Dimensional Nanoporous Polyethylene-Reinforced PVDF-HFP Separator Enabled by Dual-Solvent Hierarchical Gas Liberation for Ultrahigh-Rate Lithium Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 921–927. [Google Scholar] [CrossRef]
- Qiu, G.; Shi, Y.; Huang, B. A highly ionic conductive succinonitrile-based composite solid electrolyte for lithium metal batteries. Nano Res. 2022, 15, 5153–5160. [Google Scholar] [CrossRef]
- Chen, G.H.; Zhang, F.; Zhou, Z.M.; Li, J.R.; Tang, Y.B. A Flexible Dual-Ion Battery Based on PVDF-HFP-Modified Gel Polymer Electrolyte with Excellent Cycling Performance and Superior Rate Capability. Adv. Energy Mater. 2018, 8, 1801219. [Google Scholar] [CrossRef]
- Li, X.; Cong, L.; Ma, S.; Shi, S.; Li, Y.; Li, S.; Chen, S.; Zheng, C.; Sun, L.; Liu, Y.; et al. Low Resistance and High Stable Solid–Liquid Electrolyte Interphases Enable High-Voltage Solid-State Lithium Metal Batteries. Adv. Funct. Mater. 2021, 31, 201061. [Google Scholar] [CrossRef]
- Li, Z.; Yu, R.; Weng, S.; Zhang, Q.; Wang, X.; Guo, X. Tailoring polymer electrolyte ionic conductivity for production of low- temperature operating quasi-all-solid-state lithium metal batteries. Nat. Commun. 2023, 14, 482. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.W.; Shi, D.Y.; Liu, F.; Zheng, L.P.; Nie, J.M.; Feng, W.F.; Huang, X.J.; Armand, M.; Zhou, Z.B. Single lithium-ion conducting polymer electrolytes based on poly[(4-styrenesulfonyl)(trifluoromethanesulfonyl)imide] anions. Electrochim. Acta 2013, 93, 254–263. [Google Scholar] [CrossRef]
- Yu, T.; Liang, J.; Luo, L.; Wang, L.; Zhao, F.; Xu, G.; Bai, X.; Yang, R.; Zhao, S.; Wang, J.; et al. Superionic Fluorinated Halide Solid Electrolytes for Highly Stable Li-Metal in All-Solid-State Li Batteries. Adv. Energy Mater. 2021, 11, 2101915. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, P.H.; Fang, Q.X.; Jing, M.X.; Shen, X.Q.; Yang, L.Z. A novel solid PEO/LLTO-nanowires polymer composite electrolyte for solid-state lithium-ion battery. Electrochim. Acta 2018, 292, 718–726. [Google Scholar] [CrossRef]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, Y.; Song, Y.; Ma, T.; Zhang, L.; Zhang, S. Highly Conductive Single-Ion Polymeric Electrolyte for Long-Cycle-Life Lithium Metal Batteries. Energies 2024, 17, 3398. https://doi.org/10.3390/en17143398
Yang Y, Zhang Y, Song Y, Ma T, Zhang L, Zhang S. Highly Conductive Single-Ion Polymeric Electrolyte for Long-Cycle-Life Lithium Metal Batteries. Energies. 2024; 17(14):3398. https://doi.org/10.3390/en17143398
Chicago/Turabian StyleYang, Yuying, Yabin Zhang, Yuxin Song, Tingbin Ma, Luqing Zhang, and Shuxiang Zhang. 2024. "Highly Conductive Single-Ion Polymeric Electrolyte for Long-Cycle-Life Lithium Metal Batteries" Energies 17, no. 14: 3398. https://doi.org/10.3390/en17143398
APA StyleYang, Y., Zhang, Y., Song, Y., Ma, T., Zhang, L., & Zhang, S. (2024). Highly Conductive Single-Ion Polymeric Electrolyte for Long-Cycle-Life Lithium Metal Batteries. Energies, 17(14), 3398. https://doi.org/10.3390/en17143398





