Unraveling the Degradation Mechanisms of Lithium-Ion Batteries
Abstract
1. Introduction
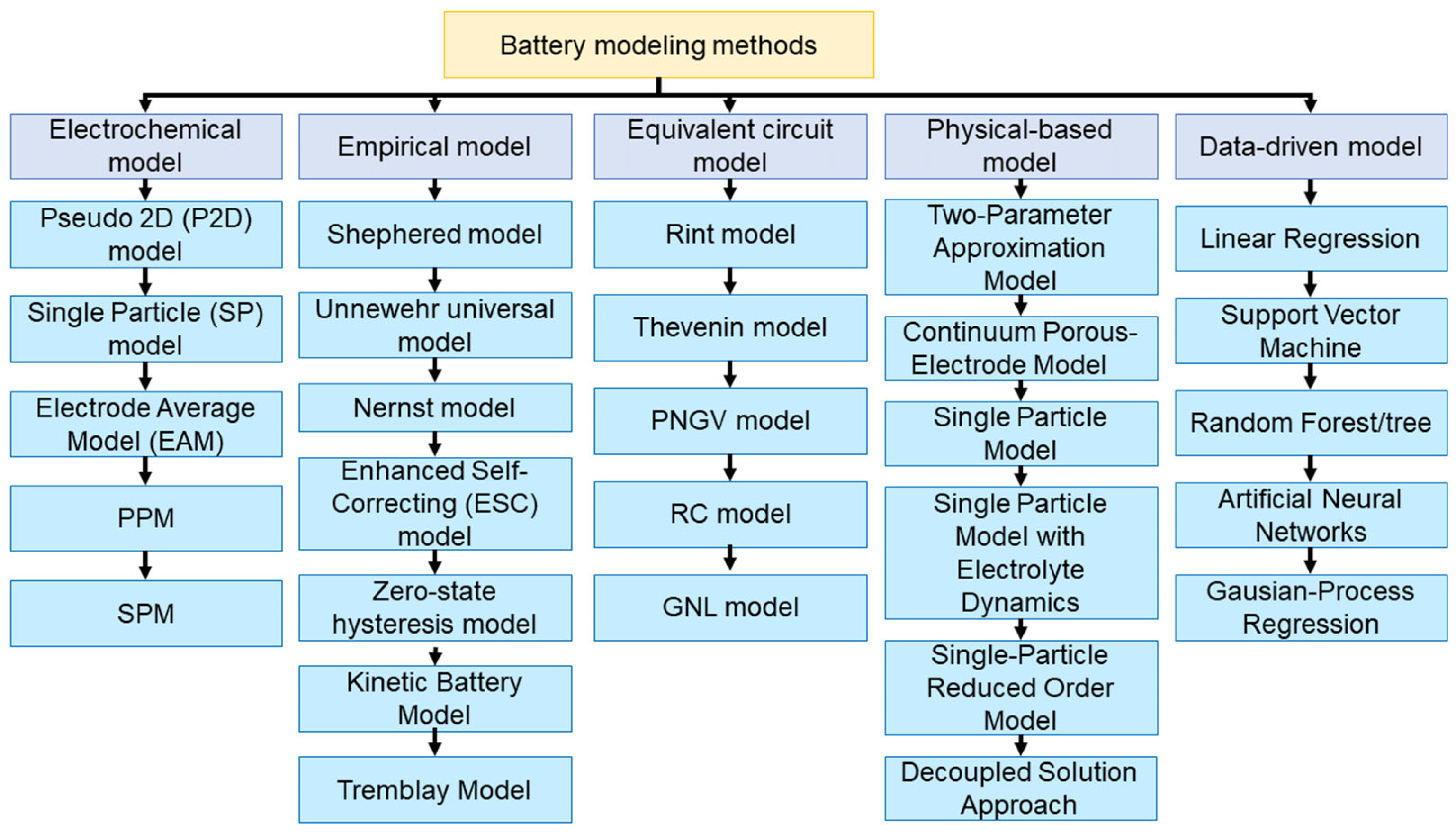
2. Diagnostic Methods
2.1. Electrochemical Models
2.2. Empirical Models
2.3. Equivalent Circuit Model
2.4. Physical-Based Models
2.5. Data-Driven Models
2.5.1. Linear Regression
2.5.2. Support Vector Machine
2.5.3. Random Forest/Tree
2.5.4. Artificial Neural Networks (ANNs)
- is the output of the neuron,
- is the weight that connects the input to neuron j,
- is the bias associated with the neuron j, and
- is the activation function.
- y is the final forecast, for example, the value of SOH or RUL,
- are the weights that connect the hidden layer to the output layer,
- is the bias of the output layer, and
- is the final activation function.
2.5.5. Gausian-Process Regression
2.6. Discussion on Battery Modelling
| Reference | Method | Features | Metrics | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neural Network | Support-Vector Machine | Gausian/Bayesian | Regression | Random Forest/Tree | Kalman Filter | Voltage | Current | Temperature | Cycle Number | Capacity | Power | Geometry | EIS | P2D Models Parameters | SOC | SOH | RUL | MAE [%] | MSE | RMSE [%] | R2 [%] | Percentage Error | |
| [17] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 9.1 | ||||||||||||||||
| [107] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ~8 × 10−4 | 6.4 | |||||||||||||||
| [108] | ✔ | ✔ | ✔ | ✔ | ✔ | 97 | 8.7 | ||||||||||||||||
| [109] | ✔ | ✔ | ✔ | ✔ | 7 | ||||||||||||||||||
| [110] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 0.1 | 0.1 | |||||||||||||||
| [111] | ✔ | ✔ | ✔ | ✔ | ✔ | 1.1 a | 2.4 | ||||||||||||||||
| [112] | ✔ | ✔ | ✔ | ✔ | ✔ | 9.27 × 10−7 | 1.3 | ||||||||||||||||
| [113] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 3.3 | ||||||||||||||||
| [114] | ✔ | ✔ | ✔ | ✔ | ✔ | 3 | |||||||||||||||||
| [115] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 12.41 | 6.7 | |||||||||||||
| [116] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 3.427 b | 0.6 | |||||||||||||||
| [117] | ✔ | ✔ | ✔ | ✔ | ✔ | 0.55 c | 0.28 d | 0.8 | |||||||||||||||
| [118] | ✔ | ✔ | ✔ | ✔ | ✔ | 97 | 12.2 | ||||||||||||||||
| [119] | ✔ | ✔ | ✔ | ✔ | ✔ | 3.8 | |||||||||||||||||
| [120] | ✔ | ✔ | ✔ | ✔ | ✔ | 3 e | |||||||||||||||||
| [121] | ✔ | ✔ | ✔ | ✔ | 5 f | 1.7 | |||||||||||||||||
| [122] | ✔ | ✔ | ✔ | ✔ | ✔ | 3 | |||||||||||||||||
| [123] | ✔ | ✔ | ✔ | ✔ | 0.45 g | 0.42 h | 5 | ||||||||||||||||
| [124] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 0.92 | 2.1 | |||||||||||||||
| [125] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 0.98 i | 1.3 i | 98 i | 1.6 | |||||||||||
| [126] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 4.86 j | 3.2 | |||||||||||||||
| [127] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 0.4 k | ||||||||||||||||
| [128] | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 3.93 l | |||||||||||||||
| [129] | ✔ | ✔ | ✔ | ✔ | ✔ | 0.0280 m | 98.8 m | 0.2 | |||||||||||||||
| [130] | ✔ | ✔ | ✔ | ✔ | ✔ | 0.0074 n | 99.9 n | 1.5 | |||||||||||||||
| [131] | ✔ | ✔ | ✔ | ✔ | 8 | ||||||||||||||||||
| [132] | ✔ | ✔ | ✔ | ✔ | ✔ | 3 | |||||||||||||||||
| [103] | ✔ | ✔ | ✔ | 8.57 | 96 o | ||||||||||||||||||
3. Motivation
4. Basic Structure of LIBs
4.1. General Overview of LIBs
4.2. Positive Electrodes of LIBs
4.3. Negative Electrodes of LIBs
5. Degradation of LIBs
5.1. Degradation Process at the Negative Electrode
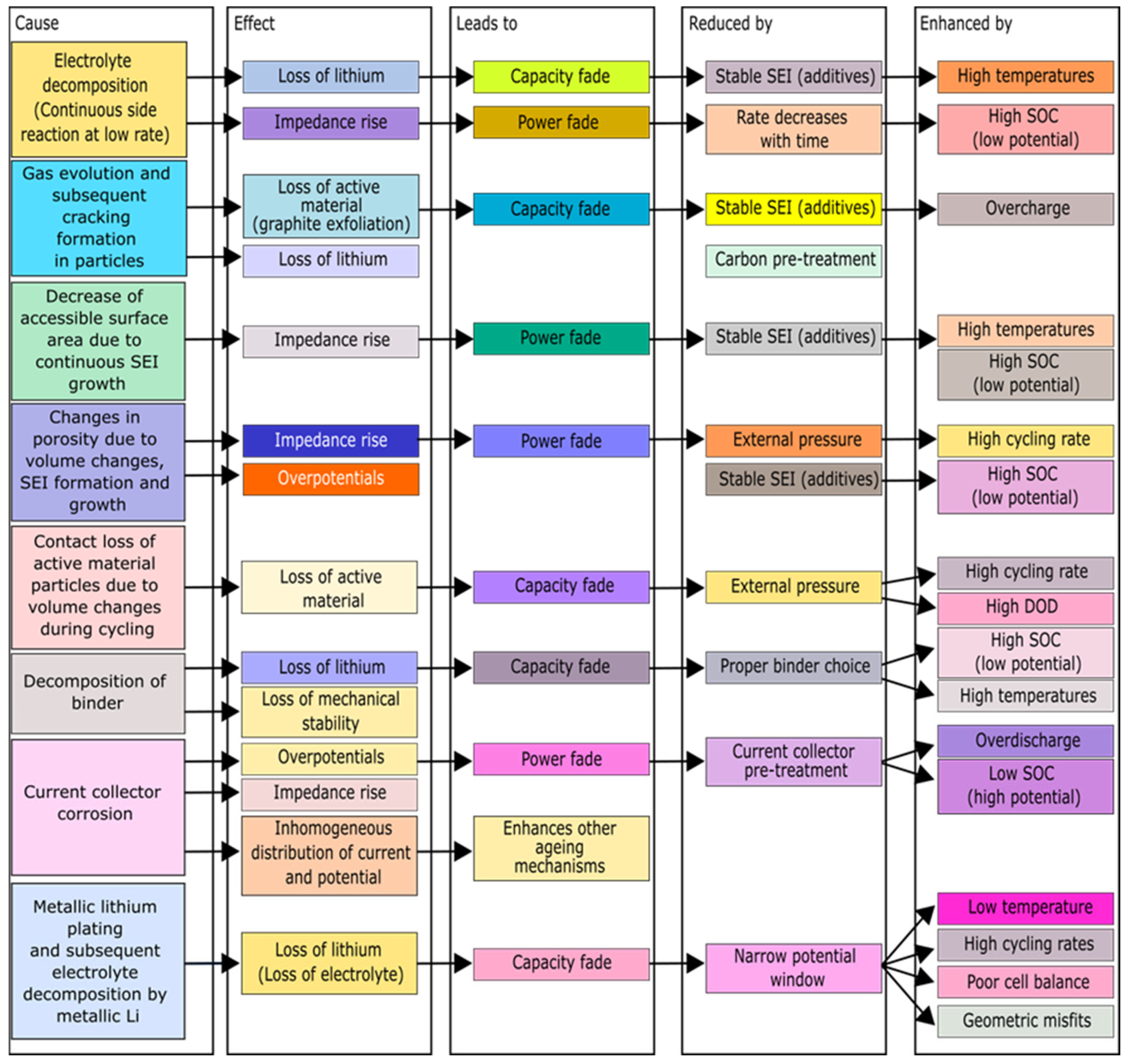
5.2. Degradation Process at the Positive Electrode
5.3. Degradation Process at the Electrolyte
5.4. Degradation Process in the Separator
5.5. Degradation of Large-Format LIBs
6. Discussion
6.1. Main Findings
- Battery charging type: slower battery charging provides a lower rate of battery degradation.
- Battery composition and chemical properties: battery characteristics such as voltage level, chemistry, performance, and efficiency can influence the battery’s degradation process.
- Climate: when exposed to low or high temperatures, batteries degrade quickly.
6.2. Comparison with Other Studies
6.3. Implication and Explanation of Findings
6.4. Strengths and Limitations
6.5. Current Problems and Future Research Directions
- Elucidating the degradation mechanisms: battery degradation mechanisms are still not fully understood. Developing accurate models and simulation tools that can explain the physical and chemical processes responsible for degradation is a crucial research problem.
- Developing advanced battery materials: novel materials with high stability and degradation resistance are required to enhance battery performance and durability. Advanced cathode materials and solid-state electrolytes are currently being studied for this purpose.
- Developing effective BMSs: BMSs are crucial to ensure safe and optimal battery operation. Developing new algorithms and control strategies to optimise battery performance and mitigate degradation is a pressing research problem.
- Developing reliable testing methodologies: accurate measurement of battery degradation is critical to developing effective strategies to combat it. Developing testing methods that provide accurate and dependable battery performance and degradation measurements is a critical research problem.
- Developing predictive models: predictive models anticipating battery performance and degradation are needed to create effective maintenance and replacement strategies. Developing models that can account for various parameters that influence battery degradation, such as temperature, cycling frequency, and SOC, is an essential research problem.
- Studying the effects of fast charging: fast charging is becoming increasingly popular, but it can also accelerate battery degradation. Researchers are investigating the influence of fast charging on different types of batteries and analysing how it affects battery degradation. Researchers aim to develop new charging strategies to minimise battery degradation by studying the fundamental mechanisms of fast charging.
- Investigating the effects of ageing on batteries: researchers have explored advanced characterisation techniques to gain more precise insights into the formation and composition of the SEI layer, co-intercalation phenomena and Li+ diffusion from the electrolyte to graphite bulk, and principles for designing anode materials, electrolytes, and cellular structure. Researchers are exploring the mechanisms behind ageing and developing models to predict how batteries degrade over time. Then, researchers can develop strategies to extend battery life by understanding the factors that contribute to battery ageing.
- Developing recycling and second-life strategies: battery recycling is an important issue, as batteries contain valuable materials that can be reused [22]. However, the degradation of these materials can make recycling difficult. Researchers are developing new recycling strategies that can recover valuable materials from degraded batteries and are exploring second-life strategies that can extend the batteries lifespan.
- Investigating the effects of extreme temperatures: temperature significantly impacts battery degradation, and extreme temperatures can accelerate the degradation process. Researchers are studying the mechanisms behind temperature-induced battery degradation and developing strategies to mitigate its effects. Researchers can develop new battery materials and cooling strategies to minimise temperature-related degradation by analysing how temperature affects the chemical reactions within batteries.
- Developing machine-learning models for predicting battery degradation: machine-learning models are an alternative to predicting battery degradation and optimising battery performance. Researchers are developing new machine-learning models that can account for various factors contributing to battery degradation, such as temperature, cycling frequency, and SOC. Researchers can develop effective maintenance and replacement strategies by accurately predicting battery degradation.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid Inorganic-Organic Proton-Conducting Membranes Based on SPEEK Doped with WO3 Nanoparticles for Application in Vanadium Redox Flow Batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Song, K.; Lan, Y.; Zhang, X.; Jiang, J.; Sun, C.; Yang, G.; Yang, F.; Lan, H. A Review on Interoperability of Wireless Charging Systems for Electric Vehicles. Energies 2023, 16, 1653. [Google Scholar] [CrossRef]
- Yang, F.; Xie, Y.; Deng, Y.; Yuan, C. Predictive Modeling of Battery Degradation and Greenhouse Gas Emissions from U.S. State-Level Electric Vehicle Operation. Nat. Commun. 2018, 9, 2429. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, E.L.; Kahn, M.E. The Greenness of Cities: Carbon Dioxide Emissions and Urban Development. J. Urban Econ. 2010, 67, 404–418. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Wanitschke, A.; Hoffmann, S. Are Battery Electric Vehicles the Future? An Uncertainty Comparison with Hydrogen and Combustion Engines. Environ. Innov. Soc. Transit. 2020, 35, 509–523. [Google Scholar] [CrossRef]
- Zhou, W.; Cleaver, C.J.; Dunant, C.F.; Allwood, J.M.; Lin, J. Cost, Range Anxiety and Future Electricity Supply: A Review of How Today’s Technology Trends May Influence the Future Uptake of BEVs. Renew. Sustain. Energy Rev. 2023, 173, 113074. [Google Scholar] [CrossRef]
- EC Regulation (EU) 2019/631 of the 2019/631 of the European Parliament and of the Council of 17 April 2019 Setting CO2 Emission Performance Standards for New Passenger Cars and for New Light Commercial Vehicles, and Repealing Regulations (EC) No 443/2009 and (EU) No 510/2011. Off. J Eur. Union L 2021, 111, 13.
- Greene, D.L.; Park, S.; Liu, C. Public Policy and the Transition to Electric Drive Vehicles in the U.S.: The Role of the Zero Emission Vehicles Mandates. Energy Strat. Rev. 2014, 5, 66–77. [Google Scholar] [CrossRef]
- Li, C.; Negnevitsky, M.; Wang, X.; Yue, W.L.; Zou, X. Multi-Criteria Analysis of Policies for Implementing Clean Energy Vehicles in China. Energy Policy 2019, 129, 826–840. [Google Scholar] [CrossRef]
- Åhman, M. Government Policy and the Development of Electric Vehicles in Japan. Energy Policy 2006, 34, 433–443. [Google Scholar] [CrossRef]
- Liu, Z.; Qian, Q.; Hu, B.; Shang, W.-L.; Li, L.; Zhao, Y.; Zhao, Z.; Han, C. Government Regulation to Promote Coordinated Emission Reduction among Enterprises in the Green Supply Chain Based on Evolutionary Game Analysis. Resour. Conserv. Recycl. 2022, 182, 106290. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, Y.; Zhang, Z.; Shang, W.-L.; Han, C.; Zhao, Y. The Impact of Carbon Emission Trading Policy on Firms’ Green Innovation in China. Financ. Innov. 2022, 8, 55. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Nykvist, B.; Nilsson, M. Rapidly Falling Costs of Battery Packs for Electric Vehicles. Nat. Clim. Change 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and Cost of Materials for Lithium-Based Rechargeable Automotive Batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Severson, K.A.; Attia, P.M.; Jin, N.; Perkins, N.; Jiang, B.; Yang, Z.; Chen, M.H.; Aykol, M.; Herring, P.K.; Fraggedakis, D.; et al. Data-Driven Prediction of Battery Cycle Life before Capacity Degradation. Nat. Energy 2019, 4, 383–391. [Google Scholar] [CrossRef]
- Guo, J.; Yang, J.; Cao, W.; Serrano, C. Evaluation of EV Battery Degradation under Different Charging Strategies and V2G Schemes. In Proceedings of the 8th Renewable Power Generation Conference (RPG 2019), Institution of Engineering and Technology, Shanghai, China, 24–25 October 2019. [Google Scholar]
- Chen, H.; Shen, J. A Degradation-Based Sorting Method for Lithium-Ion Battery Reuse. PLoS ONE 2017, 12, e0185922. [Google Scholar] [CrossRef] [PubMed]
- Lucu, M.; Martinez-Laserna, E.; Gandiaga, I.; Camblong, H. A Critical Review on Self-Adaptive Li-Ion Battery Ageing Models. J. Power Sources 2018, 401, 85–101. [Google Scholar] [CrossRef]
- Rufino Júnior, C.A.; Riva Sanseverino, E.; Gallo, P.; Koch, D.; Kotak, Y.; Schweiger, H.-G.; Zanin, H. Towards a Business Model for Second-Life Batteries—Barriers, Opportunities, Uncertainties, and Technologies. J. Energy Chem. 2023, 78, 507–525. [Google Scholar] [CrossRef]
- Rufino Júnior, C.A.; Riva Sanseverino, E.; Gallo, P.; Koch, D.; Diel, S.; Walter, G.; Trilla, L.; Ferreira, V.J.; Pérez, G.B.; Kotak, Y.; et al. Towards to Battery Digital Passport: Reviewing Regulations and Standards for Second-Life Batteries. Batteries 2024, 10, 115. [Google Scholar] [CrossRef]
- Naseri, F.; Schaltz, E.; Stroe, D.-I.; Gismero, A.; Farjah, E. An Enhanced Equivalent Circuit Model with Real-Time Parameter Identification for Battery State-of-Charge Estimation. IEEE Trans. Ind. Electron. 2022, 69, 3743–3751. [Google Scholar] [CrossRef]
- Harper, G.D.J.; Kendrick, E.; Anderson, P.A.; Mrozik, W.; Christensen, P.; Lambert, S.; Greenwood, D.; Das, P.K.; Ahmeid, M.; Milojevic, Z.; et al. Roadmap for a Sustainable Circular Economy in Lithium-Ion and Future Battery Technologies. J. Phys. Energy 2023, 5, 021501. [Google Scholar] [CrossRef]
- Júnior, C.A.R.; Sanseverino, E.R.; Gallo, P.; Koch, D.; Schweiger, H.G.; Zanin, H. Blockchain Review for Battery Supply Chain Monitoring and Battery Trading. Renew. Sustain. Energy Rev. 2022, 157, 112078. [Google Scholar] [CrossRef]
- Rauhala, T. Electrochemical Studies on Degradation Mechanisms of Electrode Materials in Lithium-Ion Batteries; School of Chemical Technology, Aalto University: Espoo, Finland, 2020. [Google Scholar]
- Liu, D.; Shadike, Z.; Lin, R.; Qian, K.; Li, H.; Li, K.; Wang, S.; Yu, Q.; Liu, M.; Ganapathy, S.; et al. Review of Recent Development of in Situ/Operando Characterization Techniques for Lithium Battery Research. Adv. Mater. 2019, 31, e1806620. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, J.W. Corrosion of Lithium-Ion Battery Current Collectors. J. Electrochem. Soc. 1999, 146, 448. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Cyclable Lithium and Capacity Loss in Li-Ion Cells. J. Electrochem. Soc. 2005, 152, A818. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing Mechanisms in Lithium-Ion Batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Singh, S.; Weeber, M.; Birke, K.P. Implementation of Battery Digital Twin: Approach, Functionalities and Benefits. Batteries 2021, 7, 78. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Sun, Z.; Wang, L.; Xu, R.; Li, M.; Chen, Z. A Comprehensive Review of Battery Modeling and State Estimation Approaches for Advanced Battery Management Systems. Renew. Sustain. Energy Rev. 2020, 131, 110015. [Google Scholar] [CrossRef]
- Meng, J.; Luo, G.; Ricco, M.; Swierczynski, M.; Stroe, D.-I.; Teodorescu, R. Overview of Lithium-Ion Battery Modeling Methods for State-of-Charge Estimation in Electrical Vehicles. Appl. Sci. 2018, 8, 659. [Google Scholar] [CrossRef]
- Falconi, A. Electrochemical Li-Ion Battery Modeling for Electric Vehicles. Doctoral Dissertation, Communaute Universite Grenoble Alpes, Saint-Martin-d’Hères, France, 2017. [Google Scholar]
- Wu, L.; Pang, H.; Geng, Y.; Liu, X.; Liu, J.; Liu, K. Low-complexity State of Charge and Anode Potential Prediction for Lithium-ion Batteries Using a Simplified Electrochemical Model-based Observer under Variable Load Condition. Int. J. Energy Res. 2022, 46, 11834–11848. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Wang, W.; Yang, K.; Zhang, Z.; Liang, X.; Chen, S.; Yang, S.; Li, J.; Liu, X. Lithium-Ion Battery Multi-Scale Modeling Coupled with Simplified Electrochemical Model and Kinetic Monte Carlo Model. iScience 2023, 26, 107661. [Google Scholar] [CrossRef] [PubMed]
- Louis, S.-Y.; Siriwardane, E.M.D.; Joshi, R.P.; Omee, S.S.; Kumar, N.; Hu, J. Accurate Prediction of Voltage of Battery Electrode Materials Using Attention-Based Graph Neural Networks. ACS Appl. Mater. Interfaces 2022, 14, 26587–26594. [Google Scholar] [CrossRef] [PubMed]
- Trembacki, B.L.; Vadakkepatt, A.; Roberts, S.A.; Murthy, J.Y. Volume-Averaged Electrochemical Performance Modeling of 3D Interpenetrating Battery Electrode Architectures. J. Electrochem. Soc. 2020, 167, 013507. [Google Scholar] [CrossRef]
- Chen, Z.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Porous Electrode Modeling and Its Applications to Li-ion Batteries. Adv. Energy Mater. 2022, 12, 2201506. [Google Scholar] [CrossRef]
- Zhu, G.; Wu, Z.; Ren, X.; Wang, J.V.; Kang, J.; Wang, Q.; Deng, X. A Self-Correction Single Particle Model of Lithium-Ion Battery Based on Multi-Population Genetic Algorithm. J. Energy Storage 2023, 71, 108005. [Google Scholar] [CrossRef]
- Wett, C.; Ganuza, C.; Ayerbe, E.; Turan, B.; Schwunk, S. Method of Lines for Flexible Coupling of the Single Particle Model for Lithium-Ion Batteries Demonstrated by Thermal Modelling. J. Energy Storage 2023, 68, 107459. [Google Scholar] [CrossRef]
- Trivella, A.; Corno, M.; Radrizzani, S.; Savaresi, S.M. Non-Invasive Experimental Identification of a Single Particle Model for LiFePO4 Cells. arXiv 2023, arXiv:2306.14195. [Google Scholar] [CrossRef]
- Chundru, V.R.; Downing, W.D.; Sarlashkar, J.; Surampudi, B. Extension of Single Particle Model with Electrolyte and Temperature (SPMeT) for Real-Time Performance and Safety Monitoring of Battery Energy Storage Systems (BESS) in Grid Service. In Proceedings of the 2023 IEEE International Systems Conference (SysCon), Vancouver, BC, Canada, 17–20 April 2023; IEEE: Piscataway, NJ, USA, 2023. [Google Scholar]
- Elmahallawy, M.; Elfouly, T.; Alouani, A.; Massoud, A.M. A Comprehensive Review of Lithium-Ion Batteries Modeling, and State of Health and Remaining Useful Lifetime Prediction. IEEE Access 2022, 10, 119040–119070. [Google Scholar] [CrossRef]
- Xu, B.; Oudalov, A.; Ulbig, A.; Andersson, G.; Kirschen, D.S. Modeling of Lithium-Ion Battery Degradation for Cell Life Assessment. IEEE Trans. Smart Grid 2018, 9, 1131–1140. [Google Scholar] [CrossRef]
- Zheng, Y.; Qin, C.; Lai, X.; Han, X.; Xie, Y. A Novel Capacity Estimation Method for Lithium-Ion Batteries Using Fusion Estimation of Charging Curve Sections and Discrete Arrhenius Aging Model. Appl. Energy 2019, 251, 113327. [Google Scholar] [CrossRef]
- Moore, S.; Eshani, M. An Empirically Based Electrosource Horizon Lead-Acid Battery Model. In Proceedings of the SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 1996. [Google Scholar]
- Manwell, J.; Mcgowan, J.G. Extension of the Kinetic Battery Model for Wind/Hybrid Power Systems. Proc. EWEC 1994, 3, 284–289. [Google Scholar]
- Unnewehr, L.E.; Nasar, S.A. Electric Vehicle Technology; Wiley: Hoboken, NJ, USA, 1982; ISBN 9780471083788. [Google Scholar]
- Fang, H.; Zhao, X.; Wang, Y.; Sahinoglu, Z.; Wada, T.; Hara, S.; de Callafon, R.A. State-of-Charge Estimation for Batteries: A Multi-Model Approach. In Proceedings of the 2014 American Control Conference, Portland, OR, USA, 4–6 June 2014; IEEE: Piscataway, NJ, USA, 2014. [Google Scholar]
- Manwell, J.F.; McGowan, J.G. Lead Acid Battery Storage Model for Hybrid Energy Systems. Sol. Energy 1993, 50, 399–405. [Google Scholar] [CrossRef]
- Tremblay, O.; Dessaint, L.-A. Experimental Validation of a Battery Dynamic Model for EV Applications. World Electric Veh. J. 2009, 3, 289–298. [Google Scholar] [CrossRef]
- Tremblay, O.; Dessaint, L.-A.; Dekkiche, A.-I. A Generic Battery Model for the Dynamic Simulation of Hybrid Electric Vehicles. In Proceedings of the 2007 IEEE Vehicle Power and Propulsion Conference, Arlington, TX, USA, 9–12 September 2007; IEEE: Piscataway, NJ, USA, 2007. [Google Scholar]
- Zhang, Y.; Lyden, S.; de la Barra, B.A.L.; Haque, M.E. Optimization of Tremblay’s Battery Model Parameters for Plug-in Hybrid Electric Vehicle Applications. In Proceedings of the 2017 Australasian Universities Power Engineering Conference (AUPEC), Melbourne, VIC, Australia, 19–22 November 2017; IEEE: Piscataway, NJ, USA, 2017. [Google Scholar]
- Hu, X.; Sun, F.; Zou, Y.; Peng, H. Online Estimation of an Electric Vehicle Lithium-Ion Battery Using Recursive Least Squares with Forgetting. In Proceedings of the 2011 American Control Conference, San Francisco, CA, USA, 29 June–1 July 2011; IEEE: Piscataway, NJ, USA, 2011. [Google Scholar]
- Plett, G. Battery Management Systems, Volume I: Battery Modeling; Artech: Norwood, MA, USA, 2015. [Google Scholar]
- Seitl, C.; Kathan, J.; Lauss, G.; Lehfuss, F. Power Hardware-in-the-Loop Implementation and Verification of a Real Time Capable Battery Model. In Proceedings of the 2014 IEEE 23rd International Symposium on Industrial Electronics (ISIE), Istanbul, Turkey, 1–4 June 2014; IEEE: Piscataway, NJ, USA, 2014. [Google Scholar]
- Seitl, C.; Kathan, J.; Lauss, G.; Lehfuss, F. Selection and Implementation of a Generic Battery Model for PHIL Applications. In Proceedings of the IECON 2013—39th Annual Conference of the IEEE Industrial Electronics Society, Vienna, Austria, 10–13 November 2013; IEEE: Piscataway, NJ, USA, 2013. [Google Scholar]
- Plett, G.L. Extended Kalman Filtering for Battery Management Systems of LiPB-Based HEV Battery Packs. J. Power Sources 2004, 134, 262–276. [Google Scholar] [CrossRef]
- Ecker, M.; Gerschler, J.B.; Vogel, J.; Käbitz, S.; Hust, F.; Dechent, P.; Sauer, D.U. Development of a Lifetime Prediction Model for Lithium-Ion Batteries Based on Extended Accelerated Aging Test Data. J. Power Sources 2012, 215, 248–257. [Google Scholar] [CrossRef]
- Attidekou, P.S.; Milojevic, Z.; Muhammad, M.; Ahmeid, M.; Lambert, S.; Das, P.K. Methodologies for Large-Size Pouch Lithium-Ion Batteries End-of-Life Gateway Detection in the Second-Life Application. J. Electrochem. Soc. 2020, 167, 160534. [Google Scholar] [CrossRef]
- Guo, J.; Che, Y.; Pedersen, K.; Stroe, D.-I. Battery Impedance Spectrum Prediction from Partial Charging Voltage Curve by Machine Learning. J. Energy Chem. 2023, 79, 211–221. [Google Scholar] [CrossRef]
- Hosen, M.S.; Pirooz, A.; Kalogiannis, T.; He, J.; Van Mierlo, J.; Berecibar, M. A Strategic Pathway from Cell to Pack-Level Battery Lifetime Model Development. Appl. Sci. 2022, 12, 4781. [Google Scholar] [CrossRef]
- Wu, G.; Lu, R.; Zhu, C.; Chan, C.C. An Improved Ampere-Hour Method for Battery State of Charge Estimation Based on Temperature, Coulomb Efficiency Model and Capacity Loss Model. In Proceedings of the 2010 IEEE Vehicle Power and Propulsion Conference, Lille, France, 1–3 September 2010; IEEE: Piscataway, NJ, USA, 2010. [Google Scholar]
- Zhang, X.; Hou, J.; Wang, Z.; Jiang, Y. Study of SOC Estimation by the Ampere-Hour Integral Method with Capacity Correction Based on LSTM. Batteries 2022, 8, 170. [Google Scholar] [CrossRef]
- Xiao, F.; Li, C.; Fan, Y.; Yang, G.; Tang, X. State of Charge Estimation for Lithium-Ion Battery Based on Gaussian Process Regression with Deep Recurrent Kernel. Int. J. Electr. Power Energy Syst. 2021, 124, 106369. [Google Scholar] [CrossRef]
- Ren, X.; Liu, S.; Yu, X.; Dong, X. A Method for State-of-Charge Estimation of Lithium-Ion Batteries Based on PSO-LSTM. Energy 2021, 234, 121236. [Google Scholar] [CrossRef]
- Liu, Z.; Zhe, L.; Zhang, J. Alternate Adaptive Extended Kalman Filter and Ampere-Hour Counting Method to Estimate the State of Charge. In Proceedings of the 2018 IEEE International Power Electronics and Application Conference and Exposition (PEAC), Shenzhen, China, 4–7 November 2018; IEEE: Piscataway, NJ, USA, 2018. [Google Scholar]
- Huang, C.-S. A Lithium-Ion Batteries Fault Diagnosis Method for Accurate Coulomb Counting State-of-Charge Estimation. J. Electr. Eng. Technol. 2024, 19, 433–442. [Google Scholar] [CrossRef]
- Zine, B.; Bia, H.; Benmouna, A.; Becherif, M.; Iqbal, M. Experimentally Validated Coulomb Counting Method for Battery State-of-Charge Estimation under Variable Current Profiles. Energies 2022, 15, 8172. [Google Scholar] [CrossRef]
- Zine, B.; Marouani, K.; Becherif, M.; Yahmedi, S. Estimation of Battery Soc for Hybrid Electric Vehicle Using Coulomb Counting Method. Int. J. Emerg. Electr. Power Syst. 2018, 19, 20170181. [Google Scholar] [CrossRef]
- Movassagh, K.; Raihan, A.; Balasingam, B.; Pattipati, K. A Critical Look at Coulomb Counting Approach for State of Charge Estimation in Batteries. Energies 2021, 14, 4074. [Google Scholar] [CrossRef]
- Wang, S.; Fernandez, C.; Yu, C.; Fan, Y.; Cao, W.; Stroe, D.-I. A Novel Charged State Prediction Method of the Lithium Ion Battery Packs Based on the Composite Equivalent Modeling and Improved Splice Kalman Filtering Algorithm. J. Power Sources 2020, 471, 228450. [Google Scholar] [CrossRef]
- Feng, X.; Weng, C.; He, X.; Wang, L.; Ren, D.; Lu, L.; Han, X.; Ouyang, M. Incremental Capacity Analysis on Commercial Lithium-Ion Batteries Using Support Vector Regression: A Parametric Study. Energies 2018, 11, 2323. [Google Scholar] [CrossRef]
- Tang, X.; Liu, K.; Lu, J.; Liu, B.; Wang, X.; Gao, F. Battery Incremental Capacity Curve Extraction by a Two-Dimensional Luenberger–Gaussian-Moving-Average Filter. Appl. Energy 2020, 280, 115895. [Google Scholar] [CrossRef]
- Kato, H.; Kobayashi, Y.; Miyashiro, H. Differential Voltage Curve Analysis of a Lithium-Ion Battery during Discharge. J. Power Sources 2018, 398, 49–54. [Google Scholar] [CrossRef]
- Lewerenz, M.; Marongiu, A.; Warnecke, A.; Sauer, D.U. Differential Voltage Analysis as a Tool for Analyzing Inhomogeneous Aging: A Case Study for LiFePO4|Graphite Cylindrical Cells. J. Power Sources 2017, 368, 57–67. [Google Scholar] [CrossRef]
- Sihvo, J.; Roinila, T.; Stroe, D.-I. Novel Fitting Algorithm for Parametrization of Equivalent Circuit Model of Li-Ion Battery from Broadband Impedance Measurements. IEEE Trans. Ind. Electron. 2021, 68, 4916–4926. [Google Scholar] [CrossRef]
- Sui, X.; He, S.; Stroe, D.-I.; Huang, X.; Meng, J.; Teodorescu, R. A Review of Sliding Mode Observers Based on Equivalent Circuit Model for Battery SoC Estimation. In Proceedings of the 2019 IEEE 28th International Symposium on Industrial Electronics (ISIE), Vancouver, BC, Canada, 12–14 June 2019; IEEE: Piscataway, NJ, USA, 2019. [Google Scholar]
- Guo, R.; Shen, W. A Review of Equivalent Circuit Model Based Online State of Power Estimation for Lithium-Ion Batteries in Electric Vehicles. Vehicles 2021, 4, 1–31. [Google Scholar] [CrossRef]
- Brosa Planella, F.; Ai, W.; Boyce, A.M.; Ghosh, A.; Korotkin, I.; Sahu, S.; Sulzer, V.; Timms, R.; Tranter, T.G.; Zyskin, M.; et al. A Continuum of Physics-Based Lithium-Ion Battery Models Reviewed. Prog. Energy 2022, 4, 042003. [Google Scholar] [CrossRef]
- Mckay, M.B.; Wetton, B.; Gopaluni, R.B. Learning Physics Based Models of Lithium-Ion Batteries. IFAC-PapersOnLine 2021, 54, 97–102. [Google Scholar] [CrossRef]
- Pozzato, G.; Onori, S. Combining Physics-Based and Machine Learning Methods to Accelerate Innovation in Sustainable Transportation and beyond: A Control Perspective. arXiv 2023, arXiv:2305.04840. [Google Scholar]
- Liu, X.; Zhang, X.-Q.; Chen, X.; Zhu, G.-L.; Yan, C.; Huang, J.-Q.; Peng, H.-J. A Generalizable, Data-Driven Online Approach to Forecast Capacity Degradation Trajectory of Lithium Batteries. J. Energy Chem. 2022, 68, 548–555. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, T. Deep Learning-Based Battery State of Charge Estimation: Enhancing Estimation Performance with Unlabelled Training Samples. J. Energy Chem. 2023, 80, 48–57. [Google Scholar] [CrossRef]
- Xiong, R.; Tian, J.; Shen, W.; Lu, J.; Sun, F. Semi-Supervised Estimation of Capacity Degradation for Lithium Ion Batteries with Electrochemical Impedance Spectroscopy. J. Energy Chem. 2023, 76, 404–413. [Google Scholar] [CrossRef]
- Chen, C.; Xiong, R.; Yang, R.; Li, H. A Novel Data-Driven Method for Mining Battery Open-Circuit Voltage Characterization. Green Energy Intell. Transp. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Liu, M.; Xu, J.; Jiang, Y.; Mei, X. Multi-Dimensional Features Based Data-Driven State of Charge Estimation Method for LiFePO4 Batteries. Energy 2023, 274, 127407. [Google Scholar] [CrossRef]
- Seh, Z.W. Interpretable Hybrid Machine Learning Demystifies the Degradation of Practical Lithium–Sulfur Batteries. J. Energy Chem. 2023, 79, 54–55. [Google Scholar] [CrossRef]
- Ando, K.; Matsuda, T.; Imamura, D. Degradation Diagnosis of Lithium-Ion Batteries Using AC Impedance Technique in Fixing the State of Charge of an Electrode. J. Energy Chem. 2021, 53, 285–289. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, J.; Lyu, Z.; You, H.; Zhou, Y.; Gu, L.; Qu, J.; Xia, Z.; Zhang, Z.; Dai, H. Deep Learning Enhanced Lithium-Ion Battery Nonlinear Fading Prognosis. J. Energy Chem. 2023, 78, 565–573. [Google Scholar] [CrossRef]
- Galatro, D.; Silva, C.D.; Romero, D.A.; Trescases, O.; Amon, C.H. Challenges in Data-based Degradation Models for Lithium-ion Batteries. Int. J. Energy Res. 2020, 44, 3954–3975. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, M. Lithium-Ion Batteries Remaining Useful Life Prediction Based on a Mixture of Empirical Mode Decomposition and ARIMA Model. Microelectron. Reliab. 2016, 65, 265–273. [Google Scholar] [CrossRef]
- Cao, L.; Xu, R.; Bi, Y. Research on Life Prediction of Lithium-Ion Battery Based on WEMD-ARIMA Model. In Proceedings of the 2022 34th Chinese Control and Decision Conference (CCDC), Hefei, China, 15–17 August 2022; IEEE: Piscataway, NJ, USA, 2022. [Google Scholar]
- Che, Y.; Zheng, Y.; Wu, Y.; Sui, X.; Bharadwaj, P.; Stroe, D.-I.; Yang, Y.; Hu, X.; Teodorescu, R. Data Efficient Health Prognostic for Batteries Based on Sequential Information-Driven Probabilistic Neural Network. Appl. Energy 2022, 323, 119663. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.; Du, D.; Zhu, C.; Zheng, M. A Sparse Least Squares Support Vector Machine Used for SOC Estimation of Li-Ion Batteries. IFAC-PapersOnLine 2019, 52, 256–261. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, K.; Liu, K.; Lin, X.; Dey, S.; Onori, S. Advanced Fault Diagnosis for Lithium-Ion Battery Systems:A review of fault mechanisms, fault features, and diagnosis procedures. IEEE Ind. Electron. Mag. 2020, 14, 65–91. [Google Scholar] [CrossRef]
- Guo, W.; He, M. An Optimal Relevance Vector Machine with a Modified Degradation Model for Remaining Useful Lifetime Prediction of Lithium-Ion Batteries. Appl. Soft Comput. 2022, 124, 108967. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, Q.; Zhao, H.; Feng, W.; Guan, X. Prognostics of Remaining Useful Life for Lithium-Ion Batteries Based on a Feature Vector Selection and Relevance Vector Machine Approach. In Proceedings of the 2017 IEEE International Conference on Prognostics and Health Management (ICPHM), Dallas, TX, USA, 19–21 June 2017; IEEE: Piscataway, NJ, USA, 2017. [Google Scholar]
- Hassini, M.; Redondo-Iglesias, E.; Venet, P. Lithium–Ion Battery Data: From Production to Prediction. Batteries 2023, 9, 385. [Google Scholar] [CrossRef]
- Tang, R.; Dore, J.; Ma, J.; Leong, P.H.W. Interpolating High Granularity Solar Generation and Load Consumption Data Using Super Resolution Generative Adversarial Network. Appl. Energy 2021, 299, 117297. [Google Scholar] [CrossRef]
- Hauck, B.; Wang, W.; Xue, Y. On the Model Granularity and Temporal Resolution of Residential PV-Battery System Simulation. Dev. Built Environ. 2021, 6, 100046. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Zhang, Y.; Wang, J.; Stimming, U.; Lee, A.A. Identifying Degradation Patterns of Lithium Ion Batteries from Impedance Spectroscopy Using Machine Learning. Nat. Commun. 2020, 11, 1706. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.-F.; Zhao, J.; Yan, Q.; Conduit, G.J.; Seh, Z.W. Predicting the State of Charge and Health of Batteries Using Data-Driven Machine Learning. Nat. Mach. Intell. 2020, 2, 161–170. [Google Scholar] [CrossRef]
- Geslin, A.; van Vlijmen, B.; Cui, X.; Bhargava, A.; Asinger, P.A.; Braatz, R.D.; Chueh, W.C. Selecting the Appropriate Features in Battery Lifetime Predictions. Joule 2023, 7, 1956–1965. [Google Scholar] [CrossRef]
- van Vlijmen, B.; Asinger, P.A.; Lam, V.; Cui, X.; Ganapathi, D.; Sun, S.; Herring, P.K.; Gopal, C.B.; Geise, N.; Deng, H.D.; et al. Interpretable Data-Driven Modeling Reveals Complexity of Battery Aging. ChemRxiv 2023. [Google Scholar] [CrossRef]
- Nuhic, A.; Terzimehic, T.; Soczka-Guth, T.; Buchholz, M.; Dietmayer, K. Health Diagnosis and Remaining Useful Life Prognostics of Lithium-Ion Batteries Using Data-Driven Methods. J. Power Sources 2013, 239, 680–688. [Google Scholar] [CrossRef]
- Guo, J.; Li, Z.; Pecht, M. A Bayesian Approach for Li-Ion Battery Capacity Fade Modeling and Cycles to Failure Prognostics. J. Power Sources 2015, 281, 173–184. [Google Scholar] [CrossRef]
- Wu, B.; Han, S.; Shin, K.G.; Lu, W. Application of Artificial Neural Networks in Design of Lithium-Ion Batteries. J. Power Sources 2018, 395, 128–136. [Google Scholar] [CrossRef]
- Zahid, T.; Xu, K.; Li, W.; Li, C.; Li, H. State of Charge Estimation for Electric Vehicle Power Battery Using Advanced Machine Learning Algorithm under Diversified Drive Cycles. Energy 2018, 162, 871–882. [Google Scholar] [CrossRef]
- Chemali, E.; Kollmeyer, P.J.; Preindl, M.; Emadi, A. State-of-Charge Estimation of Li-Ion Batteries Using Deep Neural Networks: A Machine Learning Approach. J. Power Sources 2018, 400, 242–255. [Google Scholar] [CrossRef]
- Jiménez-Bermejo, D.; Fraile-Ardanuy, J.; Castaño-Solis, S.; Merino, J.; Álvaro-Hermana, R. Using Dynamic Neural Networks for Battery State of Charge Estimation in Electric Vehicles. Procedia Comput. Sci. 2018, 130, 533–540. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Karvelis, P.; Georgoulas, G.; Nikolakopoulos, G. Remaining Useful Battery Life Prediction for UAVs Based on Machine Learning. IFAC-PapersOnLine 2017, 50, 4727–4732. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Z.; Zhao, Z.; Wang, L.; Lai, C.S.; Wang, D. Robustness Evaluation of Extended and Unscented Kalman Filter for Battery State of Charge Estimation. IEEE Access 2018, 6, 27617–27628. [Google Scholar] [CrossRef]
- Ren, L.; Zhao, L.; Hong, S.; Zhao, S.; Wang, H.; Zhang, L. Remaining Useful Life Prediction for Lithium-Ion Battery: A Deep Learning Approach. IEEE Access 2018, 6, 50587–50598. [Google Scholar] [CrossRef]
- Khumprom, P.; Yodo, N. A Data-Driven Predictive Prognostic Model for Lithium-Ion Batteries Based on a Deep Learning Algorithm. Energies 2019, 12, 660. [Google Scholar] [CrossRef]
- Sahinoglu, G.O.; Pajovic, M.; Sahinoglu, Z.; Wang, Y.; Orlik, P.V.; Wada, T. Battery State-of-Charge Estimation Based on Regular/Recurrent Gaussian Process Regression. IEEE Trans. Ind. Electron. 2018, 65, 4311–4321. [Google Scholar] [CrossRef]
- Álvarez Antón, J.C.; García Nieto, P.J.; de Cos Juez, F.J.; Sánchez Lasheras, F.; González Vega, M.; Roqueñí Gutiérrez, M.N. Battery State-of-Charge Estimator Using the SVM Technique. Appl. Math. Model. 2013, 37, 6244–6253. [Google Scholar] [CrossRef]
- Tong, S.; Lacap, J.H.; Park, J.W. Battery State of Charge Estimation Using a Load-Classifying Neural Network. J. Energy Storage 2016, 7, 236–243. [Google Scholar] [CrossRef]
- Kang, L.; Zhao, X.; Ma, J. A New Neural Network Model for the State-of-Charge Estimation in the Battery Degradation Process. Appl. Energy 2014, 121, 20–27. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.E.; Yang, Y. Advanced Machine Learning Approach for Lithium-Ion Battery State Estimation in Electric Vehicles. IEEE Trans. Transp. Electrif. 2016, 2, 140–149. [Google Scholar] [CrossRef]
- Wu, T.; Wang, M.; Xiao, Q.; Wang, X. The SOC Estimation of Power Li-Ion Battery Based on ANFIS Model. Smart Grid Renew. Energy 2012, 03, 51–55. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Zhang, X.; Chen, Z. A Novel State of Health Estimation Method of Li-Ion Battery Using Group Method of Data Handling. J. Power Sources 2016, 327, 457–464. [Google Scholar] [CrossRef]
- Hu, C.; Jain, G.; Schmidt, C.; Strief, C.; Sullivan, M. Online Estimation of Lithium-Ion Battery Capacity Using Sparse Bayesian Learning. J. Power Sources 2015, 289, 105–113. [Google Scholar] [CrossRef]
- Berecibar, M.; Devriendt, F.; Dubarry, M.; Villarreal, I.; Omar, N.; Verbeke, W.; Van Mierlo, J. Online State of Health Estimation on NMC Cells Based on Predictive Analytics. J. Power Sources 2016, 320, 239–250. [Google Scholar] [CrossRef]
- Richardson, R.R.; Osborne, M.A.; Howey, D.A. Gaussian Process Regression for Forecasting Battery State of Health. J. Power Sources 2017, 357, 209–219. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, R.; He, H.; Liu, Z. A LSTM-RNN Method for the Lithuim-Ion Battery Remaining Useful Life Prediction. In Proceedings of the 2017 Prognostics and System Health Management Conference (PHM-Harbin), Harbin, China, 9–12 July 2017; IEEE: Piscataway, NJ, USA, 2017. [Google Scholar]
- Hu, J.N.; Hu, J.J.; Lin, H.B.; Li, X.P.; Jiang, C.L.; Qiu, X.H.; Li, W.S. State-of-Charge Estimation for Battery Management System Using Optimized Support Vector Machine for Regression. J. Power Sources 2014, 269, 682–693. [Google Scholar] [CrossRef]
- Tseng, K.-H.; Liang, J.-W.; Chang, W.; Huang, S.-C. Regression Models Using Fully Discharged Voltage and Internal Resistance for State of Health Estimation of Lithium-Ion Batteries. Energies 2015, 8, 2889–2907. [Google Scholar] [CrossRef]
- Hussein, A.A. Kalman Filters versus Neural Networks in Battery State-of-Charge Estimation: A Comparative Study. Int. J. Mod. Nonlinear Theory Appl. 2014, 03, 199–209. [Google Scholar] [CrossRef][Green Version]
- Yang, D.; Wang, Y.; Pan, R.; Chen, R.; Chen, Z. A Neural Network Based State-of-Health Estimation of Lithium-Ion Battery in Electric Vehicles. Energy Procedia 2017, 105, 2059–2064. [Google Scholar] [CrossRef]
- Sin, S.; Cho, S.; Lee, P.; Abbas, M.; Lee, S.; Kim, J. Data-Driven Prediction of Battery Degradation Using EIS-Based Robust Features. In Proceedings of the 2022 IEEE Energy Conversion Congress and Exposition (ECCE), Detroit, MI, USA, 9–13 October 2022; IEEE: Piscataway, NJ, USA, 2022. [Google Scholar]
- Li, Y.; Sogaard, A.J.; Sorensen, J.I.; Guo, J.; Stroe, D.-I.; Pedersen, K.; Gurevich, L. Aging Mechanisms of Electrodes in LiFePO4/Graphite Batteries. In Proceedings of the 2022 IEEE Energy Conversion Congress and Exposition (ECCE), Detroit, MI, USA, 9–13 October 2022; IEEE: Piscataway, NJ, USA, 2022. [Google Scholar]
- Fang, C.; Tran, T.-N.; Zhao, Y.; Liu, G. Electrolyte Decomposition and Solid Electrolyte Interphase Revealed by Mass Spectrometry. Electrochim. Acta 2021, 399, 139362. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, W.; Liu, Q.; Wang, Y.; Yao, X.; Qing, F.; Li, E.; Yang, T.; Zhang, L.; Li, J. Stable, Fast and High-Energy-Density LiCoO2 Cathode at High Operation Voltage Enabled by Glassy B2O3 Modification. J. Power Sources 2017, 362, 131–139. [Google Scholar] [CrossRef]
- Stenzel, Y.; Horsthemke, F.; Winter, M.; Nowak, S. Chromatographic Techniques in the Research Area of Lithium Ion Batteries: Current State-of-the-Art. Separations 2019, 6, 26. [Google Scholar] [CrossRef]
- Aalund, R.; Endreddy, B.; Pecht, M. How Gas Generates in Pouch Cells and Affects Consumer Products. Front. Chem. Eng. 2022, 4, 828375. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.; Lee, K.; Yang, G.J.; Lee, S.S.; Kim, Y. Self-Assembly of Core–Shell Structures Driven by Low Doping Limit of Ti in LiCoO2: First-Principles Thermodynamic and Experimental Investigation. Phys. Chem. Chem. Phys. 2017, 19, 4104–4113. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, V.; Pfrang, A.; Kriston, A.; Omar, N.; Van den Bossche, P.; Boon-Brett, L. A Review of International Abuse Testing Standards and Regulations for Lithium Ion Batteries in Electric and Hybrid Electric Vehicles. Renew. Sustain. Energy Rev. 2018, 81, 1427–1452. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Huang, J.; Fu, T.; Sun, G.; Lai, S.; Zhou, R.; Li, K.; Zhao, J. The High-Temperature and High-Humidity Storage Behaviors and Electrochemical Degradation Mechanism of LiNi0.6Co0.2Mn0.2O2 Cathode Material for Lithium Ion Batteries. J. Power Sources 2017, 363, 168–176. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Kim, D.-H.; Yoon, C.S.; Myung, S.-T.; Prakash, J.; Amine, K. A Novel Cathode Material with a Concentration-Gradient for High-Energy and Safe Lithium-Ion Batteries. Adv. Funct. Mater. 2010, 20, 485–491. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, L.; He, C. Long Cycle Life Lithium Ion Battery with Lithium Nickel Cobalt Manganese Oxide (NCM) Cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- Kong, J.-Z.; Zhai, H.-F.; Ren, C.; Gao, M.-Y.; Zhang, X.; Li, H.; Li, J.-X.; Tang, Z.; Zhou, F. Synthesis and Electrochemical Performance of Macroporous LiNi0.5Co0.2Mn0.3O2 by a Modified Sol–Gel Method. J. Alloys Compd. 2013, 577, 507–510. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Chen, Y.-F.; Pai, C.-T.; Mo, C.-Y. Synthesis of Lithium Nickel Cobalt Manganese Oxide Cathode Materials by Infrared Induction Heating. J. Power Sources 2014, 269, 31–36. [Google Scholar] [CrossRef]
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.J.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode Degradation in Lithium-Ion Batteries. ACS Nano 2020, 14, 1243–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luo, X.; Zhi, H.; Yang, X.; Xing, L.; Liao, Y.; Xu, M.; Li, W. Structural Exfoliation of Layered Cathode under High Voltage and Its Suppression by Interface Film Derived from Electrolyte Additive. ACS Appl. Mater. Interfaces 2017, 9, 12021–12034. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Qin, C.; Liu, Z.; Feng, L.; Su, X.; Chen, Y.; Xia, L.; Xia, Y.; Liu, Z. Enhanced High Voltage Cyclability of LiCoO2 Cathode by Adopting Poly[Bis-(Ethoxyethoxyethoxy)Phosphazene] with Flame-Retardant Property as an Electrolyte Additive for Lithium-Ion Batteries. Appl. Surf. Sci. 2017, 403, 260–266. [Google Scholar] [CrossRef]
- Yu, J.; Han, Z.; Hu, X.; Zhan, H.; Zhou, Y.; Liu, X. Solid-State Synthesis of LiCoO2/LiCo0.99Ti0.01O2 Composite as Cathode Material for Lithium Ion Batteries. J. Power Sources 2013, 225, 34–39. [Google Scholar] [CrossRef]
- Kalluri, S.; Yoon, M.; Jo, M.; Park, S.; Myeong, S.; Kim, J.; Dou, S.X.; Guo, Z.; Cho, J. Li-Ion Cells: Surface Engineering Strategies of Layered LiCoO2 Cathode Material to Realize High-Energy and High-Voltage Li-Ion Cells (Adv. Energy Mater. 1/2017). Adv. Energy Mater. 2017, 7, 170006. [Google Scholar] [CrossRef]
- Kalluri, S.; Yoon, M.; Jo, M.; Liu, H.K.; Dou, S.X.; Cho, J.; Guo, Z. Feasibility of Cathode Surface Coating Technology for High-energy Lithium-ion and Beyond-lithium-ion Batteries. Adv. Mater. 2017, 29, 1605807. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Jeong, S.; Cho, J. Roles of Surface Chemistry on Safety and Electrochemistry in Lithium Ion Batteries. Acc. Chem. Res. 2013, 46, 1161–1170. [Google Scholar] [CrossRef]
- Fu, L.J.; Liu, H.; Li, C.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Surface Modifications of Electrode Materials for Lithium Ion Batteries. Solid State Sci. 2006, 8, 113–128. [Google Scholar] [CrossRef]
- Keil, P. Aging of Lithium-Ion Batteries in Electric Vehicles. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2017. [Google Scholar]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation Diagnostics for Lithium Ion Cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novák, P. Insertion Electrode Materials for Rechargeable Lithium Batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.G.; Cao, G. Understanding Electrochemical Potentials of Cathode Materials in Rechargeable Batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Rowden, B.; Garcia-Araez, N. A Review of Gas Evolution in Lithium Ion Batteries. Energy Rep. 2020, 6, 10–18. [Google Scholar] [CrossRef]
- Teichert, P.; Jahnke, H.; Figgemeier, E. Degradation Mechanism of Monocrystalline Ni-Rich Li[NixMnyCoz]O2 (NMC) Active Material in Lithium Ion Batteries. J. Electrochem. Soc. 2021, 168, 090532. [Google Scholar] [CrossRef]
- Zhuang, G.V.; Chen, G.; Shim, J.; Song, X.; Ross, P.N.; Richardson, T.J. Li2CO3 in LiNi0.8Co0.15Al0.05O2 Cathodes and Its Effects on Capacity and Power. J. Power Sources 2004, 134, 293–297. [Google Scholar] [CrossRef]
- Oh, P.; Song, B.; Li, W.; Manthiram, A. Overcoming the Chemical Instability on Exposure to Air of Ni-Rich Layered Oxide Cathodes by Coating with Spinel LiMn1.9Al0.1O4. J. Mater. Chem. A Mater. Energy Sustain. 2016, 4, 5839–5841. [Google Scholar] [CrossRef]
- Shizuka, K.; Kiyohara, C.; Shima, K.; Takeda, Y. Effect of CO2 on Layered Li1+zNi1−x−yCoxMyO2 (M=Al, Mn) Cathode Materials for Lithium Ion Batteries. J. Power Sources 2007, 166, 233–238. [Google Scholar] [CrossRef]
- Liu, W.; Hu, G.; Du, K.; Peng, Z.; Cao, Y. Enhanced Storage Property of LiNi0.8Co0.15Al0.05O2 Coated with LiCoO2. J. Power Sources 2013, 230, 201–206. [Google Scholar] [CrossRef]
- Eom, J.; Kim, M.G.; Cho, J. Storage Characteristics of LiNi0.8Co0.1+xMn0.1−xO2 (X = 0, 0.03, and 0.06) Cathode Materials for Lithium Batteries. J. Electrochem. Soc. 2008, 155, A239. [Google Scholar] [CrossRef]
- Li, J.; Zheng, J.M.; Yang, Y. Studies on Storage Characteristics of LiNi0.4Co0.2Mn0.4O2 as Cathode Materials in Lithium-Ion Batteries. J. Electrochem. Soc. 2007, 154, A427. [Google Scholar] [CrossRef]
- Zhou, W.; Hao, F.; Fang, D. The Effects of Elastic Stiffening on the Evolution of the Stress Field within a Spherical Electrode Particle of Lithium-Ion Batteries. Int. J. Appl. Mech. 2013, 05, 1350040. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, C.; Yu, S.; Ge, D.; Zhou, H. Status and Challenges Facing Representative Anode Materials for Rechargeable Lithium Batteries. J. Energy Chem. 2022, 66, 260–294. [Google Scholar] [CrossRef]
- Wu, Y.P.; Rahm, E.; Holze, R. Carbon Anode Materials for Lithium Ion Batteries. J. Power Sources 2003, 114, 228–236. [Google Scholar] [CrossRef]
- Yoo, H.D.; Markevich, E.; Salitra, G.; Sharon, D.; Aurbach, D. On the Challenge of Developing Advanced Technologies for Electrochemical Energy Storage and Conversion. Mater. Today 2014, 17, 110–121. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material—Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode Materials for Lithium-Ion Batteries: A Review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Piper, D.M.; Yersak, T.A.; Son, S.-B.; Kim, S.C.; Kang, C.S.; Oh, K.H.; Ban, C.; Dillon, A.C.; Lee, S.-H. Conformal Coatings of Cyclized-PAN for Mechanically Resilient Si Nano-composite Anodes. Adv. Energy Mater. 2013, 3, 697–702. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, N.; Monje, I.E.; Bélanger, D.; Camargo, P.H.C.; Torresi, R.M. High Rate and Long-Term Cycling of Silicon Anodes with Phosphonium-Based Ionic Liquids as Electrolytes for Lithium-Ion Batteries. Electrochim. Acta 2023, 439, 141680. [Google Scholar] [CrossRef]
- Araño, K.; Mazouzi, D.; Kerr, R.; Lestriez, B.; Le Bideau, J.; Howlett, P.C.; Dupré, N.; Forsyth, M.; Guyomard, D. Editors’ Choice—Understanding the Superior Cycling Performance of Si Anode in Highly Concentrated Phosphonium-Based Ionic Liquid Electrolyte. J. Electrochem. Soc. 2020, 167, 120520. [Google Scholar] [CrossRef]
- Sujith, R.; Gangadhar, J.; Greenough, M.; Bordia, R.K.; Panda, D.K. A Review of Silicon Oxycarbide Ceramics as next Generation Anode Materials for Lithium-Ion Batteries and Other Electrochemical Applications. J. Mater. Chem. A Mater. Energy Sustain. 2023, 11, 20324–20348. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Wang, X. The Anode Materials for Lithium-ion and Sodium-ion Batteries Based on Conversion Reactions: A Review. ChemElectroChem 2023, 10, e202201151. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, E.; Chen, H.; Hou, J.; Li, C.; Wan, H. High-Performance of LaCoO3/Co3O4 Nanocrystal as Anode for Lithium-Ion Batteries. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127265. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.-H.; Dong, P.-P.; Nan, J.-M. Conversion of α-Fe2O3 from Spindle Nanorods to Nanotubes, and Their Lithium-Storage Performance. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2015, 202, 15–24. [Google Scholar] [CrossRef]
- Xiong, R.; Ma, S.; Li, H.; Sun, F.; Li, J. Toward a Safer Battery Management System: A Critical Review on Diagnosis and Prognosis of Battery Short Circuit. iScience 2020, 23, 101010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, J.; Zhu, X.; Wang, H.; Huang, L.; Wang, Y.; Xu, S. Overcharge-to-Thermal-Runaway Behavior and Safety Assessment of Commercial Lithium-Ion Cells with Different Cathode Materials: A Comparison Study. J. Energy Chem. 2021, 55, 484–498. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bai, J. Influences of Multi Factors on Thermal Runaway Induced by Overcharging of Lithium-Ion Battery. J. Energy Chem. 2022, 70, 531–541. [Google Scholar] [CrossRef]
- Liu, S.; Ma, T.; Wei, Z.; Bai, G.; Liu, H.; Xu, D.; Shan, Z.; Wang, F. Study about Thermal Runaway Behavior of High Specific Energy Density Li-Ion Batteries in a Low State of Charge. J. Energy Chem. 2021, 52, 20–27. [Google Scholar] [CrossRef]
- Yun, F.; Liu, S.; Gao, M.; Bi, X.; Zhao, W.; Chang, Z.; Yuan, M.; Li, J.; Shen, X.; Qi, X.; et al. Investigation on Step Overcharge to Self-Heating Behavior and Mechanism Analysis of Lithium Ion Batteries. J. Energy Chem. 2023, 79, 301–311. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, M.; Weng, J.; Wang, K.; Wang, J.; Wang, Z. Exploring the Thermal Stability of Lithium-Ion Cells via Accelerating Rate Calorimetry: A Review. J. Energy Chem. 2023, 81, 543–573. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A Review of Lithium Ion Battery Failure Mechanisms and Fire Prevention Strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Zhou, H.; Fear, C.; Jeevarajan, J.A.; Mukherjee, P.P. State-of-Electrode (SOE) Analytics of Lithium-Ion Cells under Overdischarge Extremes. Energy Storage Mater. 2023, 54, 60–74. [Google Scholar] [CrossRef]
- Zhitao, E.; Guo, H.; Yan, G.; Wang, J.; Feng, R.; Wang, Z.; Li, X. Evolution of the Morphology, Structural and Thermal Stability of LiCoO2 during Overcharge. J. Energy Chem. 2021, 55, 524–532. [Google Scholar] [CrossRef]
- Wang, L.; Chen, B.; Ma, J.; Cui, G.; Chen, L. Reviving Lithium Cobalt Oxide-Based Lithium Secondary Batteries-toward a Higher Energy Density. Chem. Soc. Rev. 2018, 47, 6505–6602. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A.; Milojevic, Z.; Wise, M.S.; Ahmeid, M.; Attidekou, P.S.; Mrozik, W.; Dickmann, N.A.; Restuccia, F.; Lambert, S.M.; Das, P.K. Thermal and Mechanical Abuse of Electric Vehicle Pouch Cell Modules. Appl. Therm. Eng. 2021, 189, 116623. [Google Scholar] [CrossRef]
- Yokoshima, T.; Mukoyama, D.; Maeda, F.; Osaka, T.; Takazawa, K.; Egusa, S.; Naoi, S.; Ishikura, S.; Yamamoto, K. Direct Observation of Internal State of Thermal Runaway in Lithium Ion Battery during Nail-Penetration Test. J. Power Sources 2018, 393, 67–74. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal Runaway of Commercial 18650 Li-Ion Batteries with LFP and NCA Cathodes—Impact of State of Charge and Overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
- Chang, H.; Wu, Y.-R.; Han, X.; Yi, T.-F. Recent Developments in Advanced Anode Materials for Lithium-Ion Batteries. Energy Mater 2022, 1, 100003. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, H.; Guo, J.; Zhao, Y.; Yang, H.; Li, H.; Li, W.-J.; Chou, S.; Jiang, Y.; Zhang, Z. Research on Carbon-Based and Metal-Based Negative Electrode Materials via DFT Calculation for High Potassium Storage Performance: A Review. Energymater 2023, 3, 300044. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on Modeling of the Anode Solid Electrolyte Interphase (SEI) for Lithium-Ion Batteries. NPJ Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Majasan, J.O.; Robinson, J.B.; Owen, R.E.; Maier, M.; Radhakrishnan, A.N.P.; Pham, M.; Tranter, T.G.; Zhang, Y.; Shearing, P.R.; Brett, D.J.L. Recent Advances in Acoustic Diagnostics for Electrochemical Power Systems. J. Phys. Energy 2021, 3, 032011. [Google Scholar] [CrossRef]
- Bulla, M.; Schmandt, C.; Kolling, S.; Kisters, T.; Sahraei, E. An Experimental and Numerical Study on Charged 21700 Lithium-Ion Battery Cells under Dynamic and High Mechanical Loads. Energies 2022, 16, 211. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, C.; Qian, W.; Xie, Q.; Zheng, C.; Kong, C.; Wei, F. Carbon Nanotube Production and Application in Energy Storage. Asia-Pac. J. Chem. Eng. 2013, 8, 234–245. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Pedersen, K.; Stroe, D.-I. Lithium-Ion Battery Operation, Degradation, and Aging Mechanism in Electric Vehicles: An Overview. Energies 2021, 14, 5220. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature Effect and Thermal Impact in Lithium-Ion Batteries: A Review. Prog. Nat. Sci. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B.M. Advances in Lithium-Ion Batteries; van Schalkwijk, W., Scrosati, B., Eds.; Springer: New York, NY, USA, 2007; Volume 1, ISBN 9780306475085. [Google Scholar]
- Danzer, M.A.; Liebau, V.; Maglia, F. Aging of Lithium-Ion Batteries for Electric Vehicles. In Advances in Battery Technologies for Electric Vehicles; Elsevier: Amsterdam, The Netherlands, 2015; pp. 359–387. ISBN 9781782423775. [Google Scholar]
- Wiemers-Meyer, S.; Winter, M.; Nowak, S. Mechanistic Insights into Lithium Ion Battery Electrolyte Degradation—A Quantitative NMR Study. Phys. Chem. Chem. Phys. 2016, 18, 26595–26601. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.; Weng, J.; Chen, M.; Wang, J. Impact of High-Temperature Environment on the Optimal Cycle Rate of Lithium-Ion Battery. J. Energy Storage 2020, 28, 101242. [Google Scholar] [CrossRef]
- Stroe, D.-I.; Swierczynski, M.; Kar, S.K.; Teodorescu, R. Degradation Behavior of Lithium-Ion Batteries during Calendar Ageing—The Case of the Internal Resistance Increase. IEEE Trans. Ind. Appl. 2018, 54, 517–525. [Google Scholar] [CrossRef]
- Gao, T.; Bai, J.; Ouyang, D.; Wang, Z.; Bai, W.; Mao, N.; Zhu, Y. Effect of Aging Temperature on Thermal Stability of Lithium-Ion Batteries: Part A—High-Temperature Aging. Renew. Energy 2023, 203, 592–600. [Google Scholar] [CrossRef]
- Aiken, C.P.; Self, J.; Petibon, R.; Xia, X.; Paulsen, J.M.; Dahn, J.R. A Survey of in Situ Gas Evolution during High Voltage Formation in Li-Ion Pouch Cells. J. Electrochem. Soc. 2015, 162, A760–A767. [Google Scholar] [CrossRef]
- Abada, S.; Marlair, G.; Lecocq, A.; Petit, M.; Sauvant-Moynot, V.; Huet, F. Safety Focused Modeling of Lithium-Ion Batteries: A Review. J. Power Sources 2016, 306, 178–192. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Huang, W.; Wang, H.; Zhou, W.; Li, Y.; Li, Y.; Xu, J.; Huang, W.; Chiu, W.; et al. Cathode-Electrolyte Interphase in Lithium Batteries Revealed by Cryogenic Electron Microscopy. Matter 2021, 4, 302–312. [Google Scholar] [CrossRef]
- Liu, T.; Lin, L.; Bi, X.; Tian, L.; Yang, K.; Liu, J.; Li, M.; Chen, Z.; Lu, J.; Amine, K.; et al. In Situ Quantification of Interphasial Chemistry in Li-Ion Battery. Nat. Nanotechnol. 2019, 14, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Pinson, M.B.; Bazant, M.Z. Theory of SEI Formation in Rechargeable Batteries: Capacity Fade, Accelerated Aging and Lifetime Prediction. arXiv 2012, arXiv:1210.3672. [Google Scholar]
- Williard, N.D. Degradation Analysis and Health Monitoring of Lithium-Ion Batteries. Master’s Thesis, University of Maryland, College Park, MD, USA, 2011. [Google Scholar]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium Ion Battery Degradation: What You Need to Know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, C. Batteries: Widening Voltage Windows. Nat. Energy 2016, 1, 16161. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Cesar, R.; Moreira, C.M.R.; Santos, J.H.M.; De Souza, L.G.; Pires, B.M.; Vicentini, R.; Nunes, W.; Zanin, H. Reviewing the Fundamentals of Supercapacitors and the Difficulties Involving the Analysis of the Electrochemical Findings Obtained for Porous Electrode Materials. Energy Storage Mater. 2020, 27, 555–590. [Google Scholar] [CrossRef]
- Hendricks, C.; Williard, N.; Mathew, S.; Pecht, M. A Failure Modes, Mechanisms, and Effects Analysis (FMMEA) of Lithium-Ion Batteries. J. Power Sources 2015, 297, 113–120. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A Review on Lithium-Ion Battery Ageing Mechanisms and Estimations for Automotive Applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef]
- Koltypin, M.; Aurbach, D.; Nazar, L.; Ellis, B. More on the Performance of LiFePO4 Electrodes—The Effect of Synthesis Route, Solution Composition, Aging, and Temperature. J. Power Sources 2007, 174, 1241–1250. [Google Scholar] [CrossRef]
- Horstmann, B.; Single, F.; Latz, A. Review on Multi-Scale Models of Solid-Electrolyte Interphase Formation. Curr. Opin. Electrochem. 2019, 13, 61–69. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L., III. The State of Understanding of the Lithium-Ion-Battery Graphite Solid Electrolyte Interphase (SEI) and Its Relationship to Formation Cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Zhang, S.S. A Review on Electrolyte Additives for Lithium-Ion Batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Ogumi, Z.; Wang, H. Carbon Anode Materials. In Lithium-Ion Batteries; Yoshio, M., Brodd, R.J., Kozawa, A., Eds.; Springer: New York, NY, USA, 2009; Volume 1, pp. 49–73. ISBN 9780387344447. [Google Scholar]
- Ramanujapuram, A.; Gordon, D.; Magasinski, A.; Ward, B.; Nitta, N.; Huang, C.; Yushin, G. Degradation and Stabilization of Lithium Cobalt Oxide in Aqueous Electrolytes. Energy Environ. Sci. 2016, 9, 1841–1848. [Google Scholar] [CrossRef]
- Ahmadi, L.; Young, S.B.; Fowler, M.; Fraser, R.A.; Achachlouei, M.A. A Cascaded Life Cycle: Reuse of Electric Vehicle Lithium-Ion Battery Packs in Energy Storage Systems. Int. J. Life Cycle Assess. 2017, 22, 111–124. [Google Scholar] [CrossRef]
- Casals, L.C.; Amante García, B.; Canal, C. Second Life Batteries Lifespan: Rest of Useful Life and Environmental Analysis. J. Environ. Manag. 2019, 232, 354–363. [Google Scholar] [CrossRef]
- Woody, M.; Arbabzadeh, M.; Lewis, G.M.; Keoleian, G.A.; Stefanopoulou, A. Strategies to Limit Degradation and Maximize Li-Ion Battery Service Lifetime—Critical Review and Guidance for Stakeholders. J. Energy Storage 2020, 28, 101231. [Google Scholar] [CrossRef]
- Wissler, M. Graphite and Carbon Powders for Electrochemical Applications. J. Power Sources 2006, 156, 142–150. [Google Scholar] [CrossRef]
- Ng, S.H.; Vix-Guterl, C.; Bernardo, P.; Tran, N.; Ufheil, J.; Buqa, H.; Dentzer, J.; Gadiou, R.; Spahr, M.E.; Goers, D.; et al. Correlations between Surface Properties of Graphite and the First Cycle Specific Charge Loss in Lithium-Ion Batteries. Carbon 2009, 47, 705–712. [Google Scholar] [CrossRef]
- Lin, Y.-X.; Liu, Z.; Leung, K.; Chen, L.-Q.; Lu, P.; Qi, Y. Connecting the Irreversible Capacity Loss in Li-Ion Batteries with the Electronic Insulating Properties of Solid Electrolyte Interphase (SEI) Components. J. Power Sources 2016, 309, 221–230. [Google Scholar] [CrossRef]
- Wohlfahrt-Mehrens, M.; Vogler, C.; Garche, J. Aging Mechanisms of Lithium Cathode Materials. J. Power Sources 2004, 127, 58–64. [Google Scholar] [CrossRef]
- Dai, K.; Wang, Z.; Ai, G.; Zhao, H.; Yuan, W.; Song, X.; Battaglia, V.; Sun, C.; Wu, K.; Liu, G. The Transformation of Graphite Electrode Materials in Lithium-Ion Batteries after Cycling. J. Power Sources 2015, 298, 349–354. [Google Scholar] [CrossRef]
- Andriunas, I.; Milojevic, Z.; Wade, N.; Das, P.K. Impact of Solid-Electrolyte Interphase Layer Thickness on Lithium-Ion Battery Cell Surface Temperature. J. Power Sources 2022, 525, 231126. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li Plating as Unwanted Side Reaction in Commercial Li-Ion Cells—A Review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium Plating in a Commercial Lithium-Ion Battery—A Low-Temperature Aging Study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Liu, Q.; Du, C.; Shen, B.; Zuo, P.; Cheng, X.; Ma, Y.; Yin, G.; Gao, Y. Understanding Undesirable Anode Lithium Plating Issues in Lithium-Ion Batteries. RSC Adv. 2016, 6, 88683–88700. [Google Scholar] [CrossRef]
- Collins, G.A.; Geaney, H.; Ryan, K.M. Alternative Anodes for Low Temperature Lithium-Ion Batteries. J. Mater. Chem. A Mater. Energy Sustain. 2021, 9, 14172–14213. [Google Scholar] [CrossRef]
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Staniewicz, R.J. Main Aging Mechanisms in Li Ion Batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Zhang, C. Deciphering Electrolyte Degradation. Nat. Energy 2019, 4, 1006. [Google Scholar] [CrossRef]
- Weber, W.; Kraft, V.; Grützke, M.; Wagner, R.; Winter, M.; Nowak, S. Identification of Alkylated Phosphates by Gas Chromatography-Mass Spectrometric Investigations with Different Ionization Principles of a Thermally Aged Commercial Lithium Ion Battery Electrolyte. J. Chromatogr. A 2015, 1394, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Henschel, J.; Peschel, C.; Klein, S.; Horsthemke, F.; Winter, M.; Nowak, S. Clarification of Decomposition Pathways in a State-of-the-Art Lithium Ion Battery Electrolyte through 13C-Labeling of Electrolyte Components. Angew. Chem. Int. Ed. 2020, 59, 6128–6137. [Google Scholar] [CrossRef] [PubMed]
- Dose, W.M.; Li, W.; Temprano, I.; O’Keefe, C.A.; Mehdi, B.L.; De Volder, M.F.L.; Grey, C.P. Onset Potential for Electrolyte Oxidation and Ni-Rich Cathode Degradation in Lithium-Ion Batteries. ChemRxiv 2022, 7, 3524–3530. [Google Scholar] [CrossRef]
- Li, A.; Yuen, A.C.Y.; Wang, W.; De Cachinho Cordeiro, I.M.; Wang, C.; Chen, T.B.Y.; Zhang, J.; Chan, Q.N.; Yeoh, G.H. A Review on Lithium-Ion Battery Separators towards Enhanced Safety Performances and Modelling Approaches. Molecules 2021, 26, 478. [Google Scholar] [CrossRef] [PubMed]
- Abaza, A.; Ferrari, S.; Wong, H.K.; Lyness, C.; Moore, A.; Weaving, J.; Blanco-Martin, M.; Dashwood, R.; Bhagat, R. Experimental Study of Internal and External Short Circuits of Commercial Automotive Pouch Lithium-Ion Cells. J. Energy Storage 2018, 16, 211–217. [Google Scholar] [CrossRef]
- Liu, L.; Feng, X.; Rahe, C.; Li, W.; Lu, L.; He, X.; Sauer, D.U.; Ouyang, M. Internal Short Circuit Evaluation and Corresponding Failure Mode Analysis for Lithium-Ion Batteries. J. Energy Chem. 2021, 61, 269–280. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal Runaway Mechanism of Lithium Ion Battery for Electric Vehicles: A Review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Yuan, Z.; Xue, N.; Xie, J.; Xu, R.; Lei, C. Separator Aging and Performance Degradation Caused by Battery Expansion: Cyclic Compression Test Simulation of Polypropylene Separator. J. Electrochem. Soc. 2021, 168, 030506. [Google Scholar] [CrossRef]
- Kim, G.-H.; Pesaran, A.; Spotnitz, R. A Three-Dimensional Thermal Abuse Model for Lithium-Ion Cells. J. Power Sources 2007, 170, 476–489. [Google Scholar] [CrossRef]
- Kim, H.-K.; Kim, C.-J.; Kim, C.-W.; Lee, K.-J. Numerical Analysis of Accelerated Degradation in Large Lithium-Ion Batteries. Comput. Chem. Eng. 2018, 112, 82–91. [Google Scholar] [CrossRef]
- Arunachala, R.; Parthasarathy, C.; Jossen, A.; Garche, J. Inhomogeneities in Large Format Lithium Ion Cells: A Study by Battery Modelling Approach. ECS Trans. 2016, 73, 201–212. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, S.; Li, R.; Ren, D.; Yi, M.; Xu, C.; Han, X.; Lu, L.; Friess, B.; Offer, G.; et al. Inhomogeneous Degradation Induced by Lithium Plating in a Large-Format Lithium-Ion Battery. J. Power Sources 2022, 542, 231753. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, X.; Chen, C.; Chen, M.; Kong, F.; Qiao, Y.; Wang, J. Uncovering the Degradation Mechanism Induced by Ion-Diffusion Kinetics in Large-Format Lithium-Ion Pouch Cells. J. Energy Chem. 2023, 83, 98–105. [Google Scholar] [CrossRef]
- Li, X.; Zeng, T.; Qin, H.; Huo, R.; Liu, Y.; Wei, D.; Ding, X. Investigation of Inhomogeneous Degradation in Large-Format Lithium-Ion Batteries. J. Energy Storage 2021, 42, 103113. [Google Scholar] [CrossRef]
- Zhu, X.; Revilla, R.I.; Jaguemont, J.; Van Mierlo, J.; Hubin, A. Insights into Cycling Aging of LiNi0.80Co0.15Al0.05O2 Cathode Induced by Surface Inhomogeneity: A Post-Mortem Analysis. J. Phys. Chem. C Nanomater. Interfaces 2019, 123, 30046–30058. [Google Scholar] [CrossRef]
- Sieg, J.; Schmid, A.U.; Rau, L.; Gesterkamp, A.; Storch, M.; Spier, B.; Birke, K.P.; Sauer, D.U. Fast-Charging Capability of Lithium-Ion Cells: Influence of Electrode Aging and Electrolyte Consumption. Appl. Energy 2022, 305, 117747. [Google Scholar] [CrossRef]
- Li, R.; Ren, D.; Wang, S.; Xie, Y.; Hou, Z.; Lu, L.; Ouyang, M. Non-Destructive Local Degradation Detection in Large Format Lithium-Ion Battery Cells Using Reversible Strain Heterogeneity. J. Energy Storage 2021, 40, 102788. [Google Scholar] [CrossRef]
- Hou, M.; Hu, Y.; Zhang, J.; Cao, H.; Wang, Z. Development of Electrochemical-Thermal Modelling for Large-Format Li-Ion Battery. Electrochim. Acta 2020, 347, 136280. [Google Scholar] [CrossRef]
- Song, M.; Hu, Y.; Choe, S.-Y.; Garrick, T.R. Modeling and Analysis of Heat Generation Rate of a Large Format Pouch-Type Lithium-Ion Battery Considering Degradation. J. Electrochem. Soc. 2022, 169, 070502. [Google Scholar] [CrossRef]
- Kim, G.-H.; Smith, K.; Lee, K.-J.; Santhanagopalan, S.; Pesaran, A. Multi-Domain Modeling of Lithium-Ion Batteries Encompassing Multi-Physics in Varied Length Scales. J. Electrochem. Soc. 2011, 158, A955. [Google Scholar] [CrossRef]
- Sturm, J.; Spingler, F.B.; Rieger, B.; Rheinfeld, A.; Jossen, A. Non-Destructive Detection of Local Aging in Lithium-Ion Pouch Cells by Multi-Directional Laser Scanning. J. Electrochem. Soc. 2017, 164, A1342–A1351. [Google Scholar] [CrossRef]
- Sauerteig, D.; Ivanov, S.; Reinshagen, H.; Bund, A. Reversible and Irreversible Dilation of Lithium-Ion Battery Electrodes Investigated by in-Situ Dilatometry. J. Power Sources 2017, 342, 939–946. [Google Scholar] [CrossRef]
- Li, R.; Ren, D.; Guo, D.; Xu, C.; Fan, X.; Hou, Z.; Lu, L.; Feng, X.; Han, X.; Ouyang, M. Volume Deformation of Large-Format Lithium Ion Batteries under Different Degradation Paths. J. Electrochem. Soc. 2019, 166, A4106–A4114. [Google Scholar] [CrossRef]
- Chen, H.; Fan, J.; Zhang, M.; Feng, X.; Zhong, X.; He, J.; Ai, S. Mechanism of Inhomogeneous Deformation and Equal-Stiffness Design of Large-Format Prismatic Lithium-Ion Batteries. Appl. Energy 2023, 332, 120494. [Google Scholar] [CrossRef]
- Truchot, C.A. Study of State-Of-Charge and Degradation in Lithium Ion Battery Pack. Ph.D. Thesis, University of Hawaii at Manoa, Honolulu, HI, USA, 2012. [Google Scholar]
- Martinez-Laserna, E.; Sarasketa-Zabala, E.; Stroe, D.-I.; Swierczynski, M.; Warnecke, A.; Timmermans, J.M.; Goutam, S.; Rodriguez, P. Evaluation of Lithium-Ion Battery Second Life Performance and Degradation. In Proceedings of the 2016 IEEE Energy Conversion Congress and Exposition (ECCE), Milwaukee, WI, USA, 18–22 September 2016; pp. 1–7. [Google Scholar]
- Martinez-Laserna, E.; Sarasketa-Zabala, E.; Villarreal Sarria, I.; Stroe, D.-I.; Swierczynski, M.; Warnecke, A.; Timmermans, J.-M.; Goutam, S.; Omar, N.; Rodriguez, P. Technical Viability of Battery Second Life: A Study from the Ageing Perspective. IEEE Trans. Ind. Appl. 2018, 54, 2703–2713. [Google Scholar] [CrossRef]
- Sarasketa-Zabala, E.; Gandiaga, I.; Rodriguez-Martinez, L.M.; Villarreal, I. Calendar Ageing Analysis of a LiFePO4/Graphite Cell with Dynamic Model Validations: Towards Realistic Lifetime Predictions. J. Power Sources 2014, 272, 45–57. [Google Scholar] [CrossRef]
- Olsson, L.; Fallahi, S.; Schnurr, M.; Diener, D.; Van Loon, P. Circular Business Models for Extended EV Battery Life. Batteries 2018, 4, 57. [Google Scholar] [CrossRef]
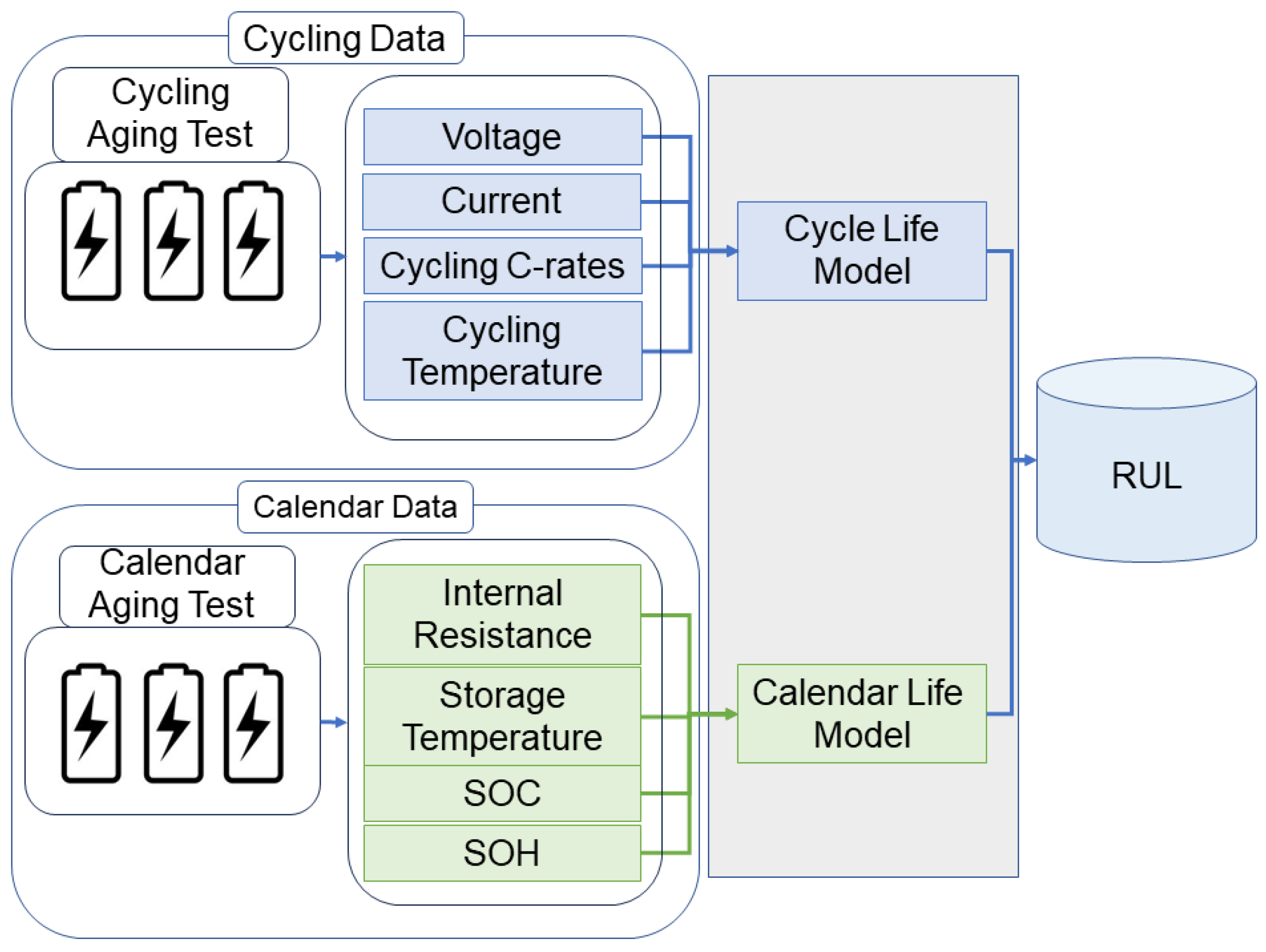
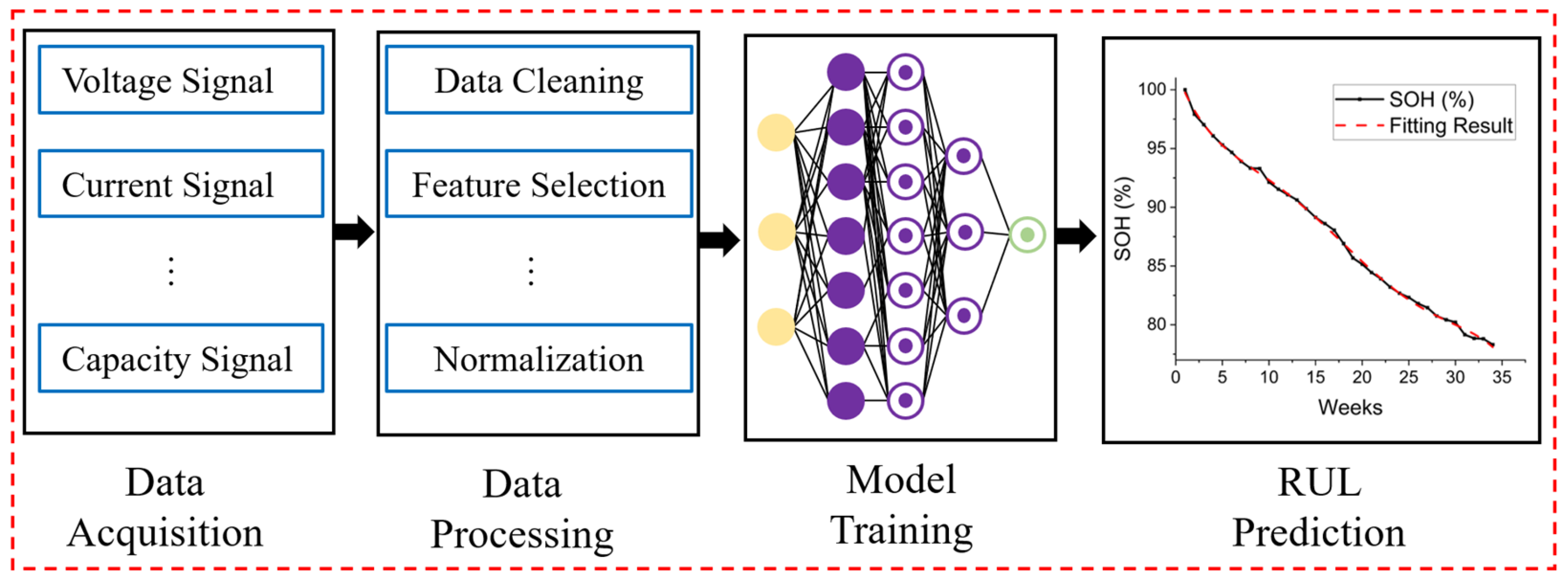
- Li, Y.; Liu, K.; Foley, A.M.; Zülke, A.; Berecibar, M.; Nanini-Maury, E.; Van Mierlo, J.; Hoster, H.E. Data-Driven Health Estimation and Lifetime Prediction of Lithium-Ion Batteries: A Review. Renew. Sustain. Energy Rev. 2019, 113, 109254. [Google Scholar] [CrossRef]
- Bonfitto, A.; Ezemobi, E.; Amati, N.; Feraco, S.; Tonoli, A.; Hegde, S. State of Health Estimation of Lithium Batteries for Automotive Applications with Artificial Neural Networks. In Proceedings of the 2019 AEIT International Conference of Electrical and Electronic Technologies for Automotive (AEIT AUTOMOTIVE), Turin, Italy, 2–4 July 2019; IEEE: Piscataway, NJ, USA, 2019. [Google Scholar]
- Hossain Lipu, M.; Karim, T.; Ansari, S.; Miah, M.; Rahman, M.; Meraj, S.; Elavarasan, R.; Vijayaraghavan, R. Intelligent SOX Estimation for Automotive Battery Management Systems: State-of-the-Art Deep Learning Approaches, Open Issues, and Future Research Opportunities. Energies 2022, 16, 23. [Google Scholar] [CrossRef]
- Shrivastava, P.; Soon, T.K.; Idris, M.Y.I.B.; Mekhilef, S.; Adnan, S.B.R.S. Model-based State of X Estimation of Lithium-ion Battery for Electric Vehicle Applications. Int. J. Energy Res. 2022, 46, 10704–10723. [Google Scholar] [CrossRef]
- Li, S.; Fang, H.; Shi, B. Remaining Useful Life Estimation of Lithium-Ion Battery Based on Interacting Multiple Model Particle Filter and Support Vector Regression. Reliab. Eng. Syst. Saf. 2021, 210, 107542. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, Y.; Wang, T.; Wang, Y. A Method for Predicting the Remaining Useful Life of Lithium-Ion Batteries Based on Particle Filter Using Kendall Rank Correlation Coefficient. Energies 2020, 13, 4183. [Google Scholar] [CrossRef]
- Hell, S.M.; Kim, C.D. Development of a Data-Driven Method for Online Battery Remaining-Useful-Life Prediction. Batteries 2022, 8, 192. [Google Scholar] [CrossRef]
- Gao, K.; Xu, J.; Li, Z.; Cai, Z.; Jiang, D.; Zeng, A. A Novel Remaining Useful Life Prediction Method for Capacity Diving Lithium-Ion Batteries. ACS Omega 2022, 7, 26701–26714. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; An, J.; Wang, H.; Zhang, M.; Pan, H. Remaining Useful Life Prediction for Lithium-Ion Battery by Combining an Improved Particle Filter with Sliding-Window Gray Model. Energy Rep. 2020, 6, 2086–2093. [Google Scholar] [CrossRef]
- Zhang, D.; Dey, S.; Perez, H.E.; Moura, S.J. Remaining Useful Life Estimation of Lithium-Ion Batteries Based on Thermal Dynamics. In Proceedings of the 2017 American Control Conference (ACC), Seattle, WA, USA, 24–26 May 2017; IEEE: Piscataway, NJ, USA, 2017. [Google Scholar]
- Wu, Y.; Li, W.; Wang, Y.; Zhang, K. Remaining Useful Life Prediction of Lithium-Ion Batteries Using Neural Network and Bat-Based Particle Filter. IEEE Access 2019, 7, 54843–54854. [Google Scholar] [CrossRef]
- Su, C.; Chen, H.J. A Review on Prognostics Approaches for Remaining Useful Life of Lithium-Ion Battery. IOP Conf. Ser. Earth Environ. Sci. 2017, 93, 012040. [Google Scholar] [CrossRef]
- Chen, L.; Xu, L.; Zhou, Y. Novel Approach for Lithium-Ion Battery on-Line Remaining Useful Life Prediction Based on Permutation Entropy. Energies 2018, 11, 820. [Google Scholar] [CrossRef]
- Pan, C.; Huang, A.; He, Z.; Lin, C.; Sun, Y.; Zhao, S.; Wang, L. Prediction of Remaining Useful Life for Lithium-ion Battery Based on Particle Filter with Residual Resampling. Energy Sci. Eng. 2021, 9, 1115–1133. [Google Scholar] [CrossRef]
- Wang, S.; Jin, S.; Deng, D.; Fernandez, C. A Critical Review of Online Battery Remaining Useful Lifetime Prediction Methods. Front. Mech. Eng. 2021, 7, 719718. [Google Scholar] [CrossRef]
- Hu, X.; Xu, L.; Lin, X.; Pecht, M. Battery Lifetime Prognostics. Joule 2020, 4, 310–346. [Google Scholar] [CrossRef]
- Matsuda, T.; Ando, K.; Myojin, M.; Matsumoto, M.; Sanada, T.; Takao, N.; Imai, H.; Imamura, D. Investigation of the Influence of Temperature on the Degradation Mechanism of Commercial Nickel Manganese Cobalt Oxide-Type Lithium-Ion Cells during Long-Term Cycle Tests. J. Energy Storage 2019, 21, 665–671. [Google Scholar] [CrossRef]
- Pelletier, S.; Jabali, O.; Laporte, G.; Veneroni, M. Battery Degradation and Behaviour for Electric Vehicles: Review and Numerical Analyses of Several Models. Trans. Res. Part B Methodol. 2017, 103, 158–187. [Google Scholar] [CrossRef]
- Preger, Y.; Barkholtz, H.M.; Fresquez, A.; Campbell, D.L.; Juba, B.W.; Romàn-Kustas, J.; Ferreira, S.R.; Chalamala, B. Degradation of Commercial Lithium-Ion Cells as a Function of Chemistry and Cycling Conditions. J. Electrochem. Soc. 2020, 167, 120532. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, C.; Xu, Y.; Jin, Y.; Shui, J. Performance Improvement of Lithium-Ion Battery by Pulse Current. J. Energy Chem. 2020, 46, 208–214. [Google Scholar] [CrossRef]


| Model | Assumption | Advantages | Remarks |
|---|---|---|---|
| P2D model |
|
|
|
| Electrode Average Model (EAM) |
|
|
|
| Porous electrode with Polynomial Model (PPM) |
|
|
|
| Single Particle Model (SPM) |
|
|
|
| SPM |
|
|
|
| Model Type | Assumptions | Advantages | Disadvantages | Limitations | Equations | Applications | Reference |
|---|---|---|---|---|---|---|---|
| Shepherd Model | Linearity under certain loading/unloading conditions | Simplicity of implementation, performs well on lead–acid batteries | Does not capture non-linear dynamics | Requires frequent calibration | SOC forecast, load capacity forecast | [47] | |
| Unnewehr universal model | Empirical relationship based on charge/discharge profile | Versatile for different charging and discharging states | Moderate computational complexity | Less accurate for extreme charge/discharge rates | SOC forecast, SOH forecast | [48,49] | |
| Nernst Model | Relationship between electromotive force and ion concentration | Accurate physicochemical model for SOC prediction | Requires detailed knowledge of electrochemical properties | Difficult to obtain accurate parameters for each battery | SOC forecast, load capacity forecast | [50] | |
| KiBaM (Kinetic Battery Model) | The model concept is derived from the kinetic model of charge and discharge | Considers the effect of discharge rate for lithium-ion | Complexity to define the parameters | Limitations in representing modern batteries | SOC forecast, load capacity forecast | [51] | |
| Tremblay Model | Empirical dynamic response model, adjustable | Easy to implement for lithium-ion batteries | Variable accuracy for different types of batteries | Requires specific performance tests for calibration | SOC forecast, SOH forecast | [52,53,54] |
| Model Equation | Reference |
|---|---|
Q represents the battery capacity, A represents the amplitude of the exponential zone, B represents the inverse of the time constant of the exponential zone, and K represents the polarization voltage. | [53] |
| Discharge: Charge: represents the current that has been filtered through the polarization resistance. | [52] |
is the filtered current through the polarization resistance. | [57,58] |
and are the two additional constants. | [50] |
is a small positive number, is the correction term. | [55] |
and are the parameters estimated based on test data. | [56,59] |
| Battery Model | Equation | Features | Advantage | Drawbacks | Circuit Schematic |
|---|---|---|---|---|---|
| Rint | UOC is the open circuit voltage, R is the internal ohmic resistance, U is the terminal voltage, I actual current. | Simple structure, Parameters easy to calculate. | Unable to describe the dynamic process, it will be fine when the current is too large. The temperature is relatively poor, which is particularly harmful to the battery. | 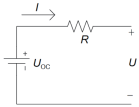 | |
| Thevenin | R1 and C are used to describe the polarization effect resistance and capacitance. | Takes into account the polarization of the battery effect on battery characteristics. Better simulation, in actual work. Used more in engineering applications. | Battery aging, temperature. Changes in the accuracy of the model have a greater impact. | 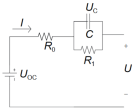 | |
| PNGV | UQ is the equivalent capacitance. | It is easy for the model to consider the temperature degree of influence on the battery. Good applicability to different working conditions. Provides good accuracy. | Series capacitance accumulation. Good reaction polarization phenomenon. | 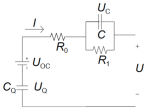 | |
| RC | R2 and C2 are rich, respectively. Differential impedance and concentration difference capacitance. | The level of computation is moderate. Enhanced accuracy, more closely resembling actual battery behaviour. | Calculation of structure and parameters. More complex. | 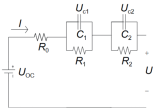 | |
| GNL | RS is self-discharge resistance. | Considering the effect of self-discharge, It considers the self-discharge effect and has high simulation accuracy. | The model is complex and the parameters are comprehensive. It is challenging to determine and complex to calculate. |  |
| Model | Assumptions | Advantages | Disadvantages | Limitations | Application |
|---|---|---|---|---|---|
| Two-Parameter Approximation Model | Linear approximation of battery characteristics | Simplicity and ease of implementation | Does not capture complex dynamic characteristics | Requires frequent calibration | SOC and SOH forecast |
| Continuum Porous-Electrode Model | Continuous model for porous electrodes | Detailed physical approach | Computational complexity | Requires precise knowledge of system parameters | SOH forecast, remaining life forecast |
| Single Particle Model | Approximation of homogeneous electrodes | Good accuracy with less complexity | Limitations in modelling electrolyte diffusion | Not suitable for electrodes with significant non-homogeneity | SOH forecast, remaining life forecast |
| Single Particle Model with Electrolyte Dynamics | Includes electrolyte dynamics | Improved accuracy for predicting kinetics | Increased computational complexity | Assumptions about homogeneous electrode properties | Prediction of SOC, SOH, RUL |
| Single-Particle Reduced Order Model | Simplifying equations to reduce order | Good accuracy with lower computational cost | May not capture all dynamics | Requires adjustment to capture desired behaviour | SOC and SOH forecast |
| Decoupled Solution Approach | Separation of physical components for separate solution | More flexibility for different mechanisms | Simplified assumptions can impact the accuracy | Difficult to adapt to new systems with complex configurations | SOH forecast, RUL |
| Model | Assumptions | Advantages | Disadvantages | Limitations |
|---|---|---|---|---|
| Multilayer Neural Network (MNN) | Battery data can be divided into sub-tasks | High efficiency for specific battery analysis tasks | High design complexity, requires specific training | Effective problem decomposition can be challenging for complex battery behaviour |
| FeedForward Neural Network (FFNN) | Battery data are static | Flexible, good for classification of battery faults | Prone to overfitting with limited battery data and black-box nature | Requires large battery data sets, challenging to interpret |
| SONNs | Battery data have meaningful clusters | Effective for clustering battery state-of-health data | Grid size determination can be difficult | Limited to clustering and visualization of battery data |
| RBF | Battery data are sensitive to local variances | Effective for capturing local variations in battery data | Sensitive to noisy battery data, requires radial centre tuning | Not scalable for high-dimensional battery data analysis |
| Hopfield Neural Network (HNN) | Symmetric weight matrix in battery data | Suitable for pattern recognition in battery diagnostics | Converges to local minima in training | Limited to small-scale pattern recognition in battery data |
| Recurrent Neural Network (RNN) | Battery data exhibit temporal dependencies | Effective for sequential battery data analysis | Gradient vanishing/explosion, difficult training | Limited in capturing long-term dependencies in battery data |
| Long Short-Term Memory (LSTM) | Long-term dependence on battery data | Effective in retaining long-term dependencies in battery data | High computational complexity, prone to overfitting | Requires extensive training data, not easily interpretable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rufino Júnior, C.A.; Sanseverino, E.R.; Gallo, P.; Amaral, M.M.; Koch, D.; Kotak, Y.; Diel, S.; Walter, G.; Schweiger, H.-G.; Zanin, H. Unraveling the Degradation Mechanisms of Lithium-Ion Batteries. Energies 2024, 17, 3372. https://doi.org/10.3390/en17143372
Rufino Júnior CA, Sanseverino ER, Gallo P, Amaral MM, Koch D, Kotak Y, Diel S, Walter G, Schweiger H-G, Zanin H. Unraveling the Degradation Mechanisms of Lithium-Ion Batteries. Energies. 2024; 17(14):3372. https://doi.org/10.3390/en17143372
Chicago/Turabian StyleRufino Júnior, Carlos Antônio, Eleonora Riva Sanseverino, Pierluigi Gallo, Murilo Machado Amaral, Daniel Koch, Yash Kotak, Sergej Diel, Gero Walter, Hans-Georg Schweiger, and Hudson Zanin. 2024. "Unraveling the Degradation Mechanisms of Lithium-Ion Batteries" Energies 17, no. 14: 3372. https://doi.org/10.3390/en17143372
APA StyleRufino Júnior, C. A., Sanseverino, E. R., Gallo, P., Amaral, M. M., Koch, D., Kotak, Y., Diel, S., Walter, G., Schweiger, H.-G., & Zanin, H. (2024). Unraveling the Degradation Mechanisms of Lithium-Ion Batteries. Energies, 17(14), 3372. https://doi.org/10.3390/en17143372










