An Experimental Investigation of Surfactant-Stabilized CO2 Foam Flooding in Carbonate Cores in Reservoir Conditions
Abstract
1. Introduction
2. Methodology
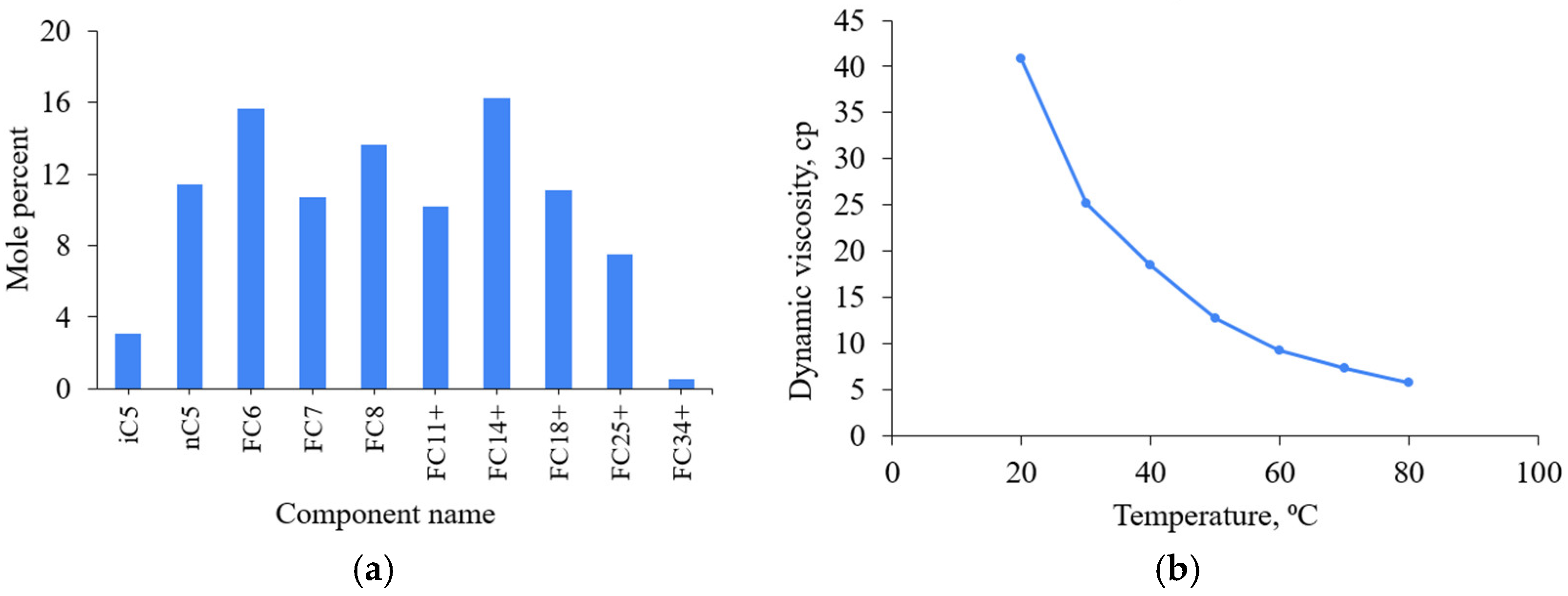
2.1. Materials
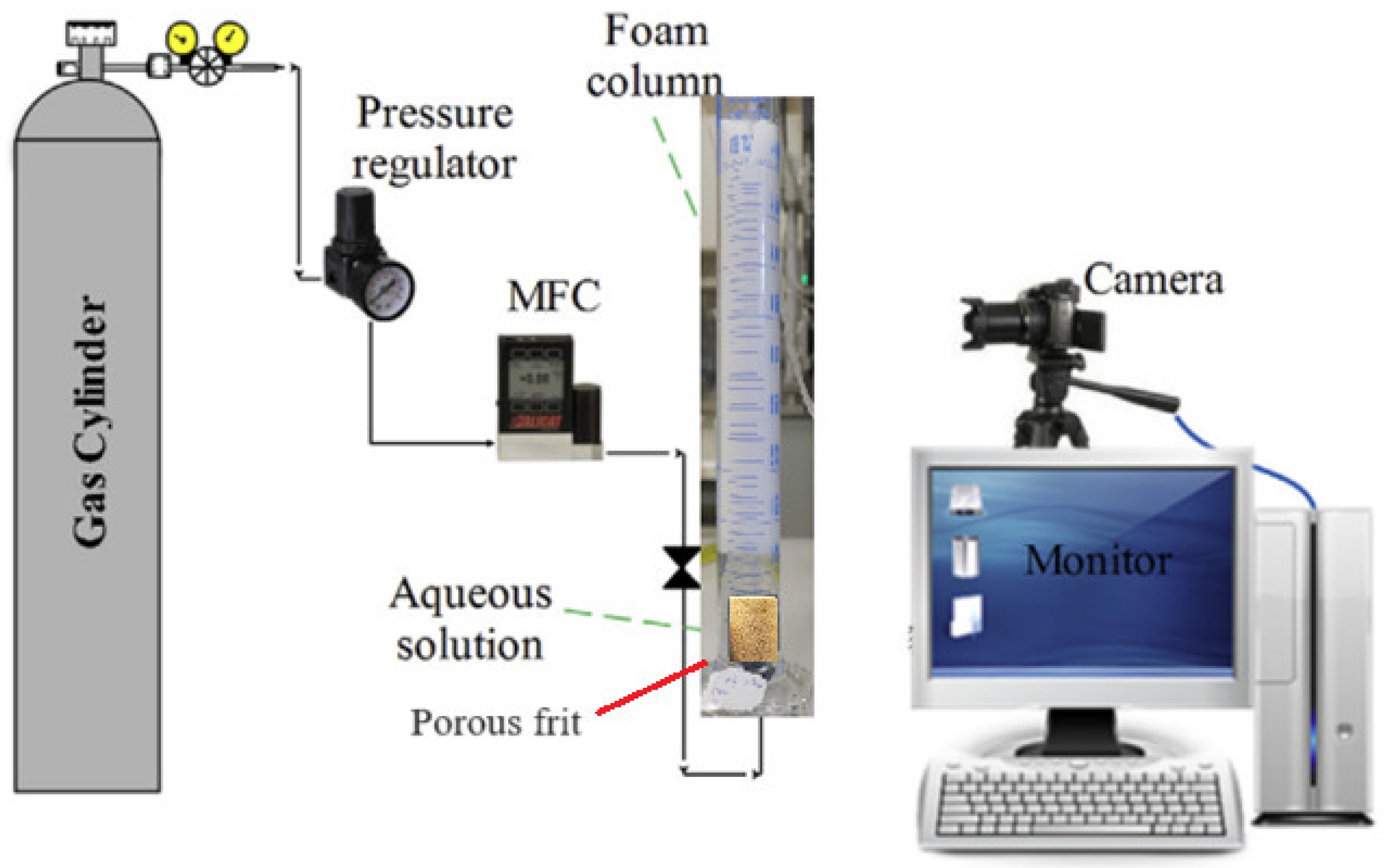
2.2. Bulk Foam Stability Tests
2.3. Foam Dynamic Tests
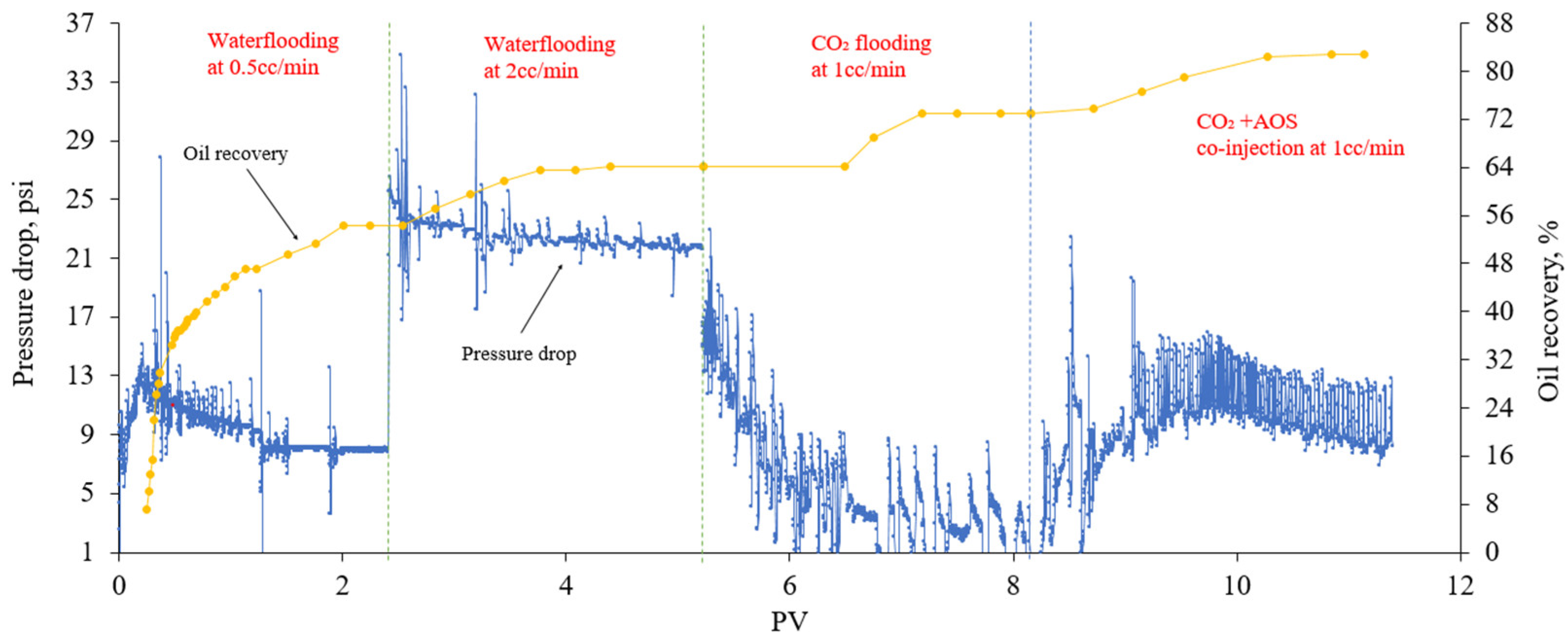
2.4. Oil Recovery Experiments
3. Results
3.1. Foam Stability Tests
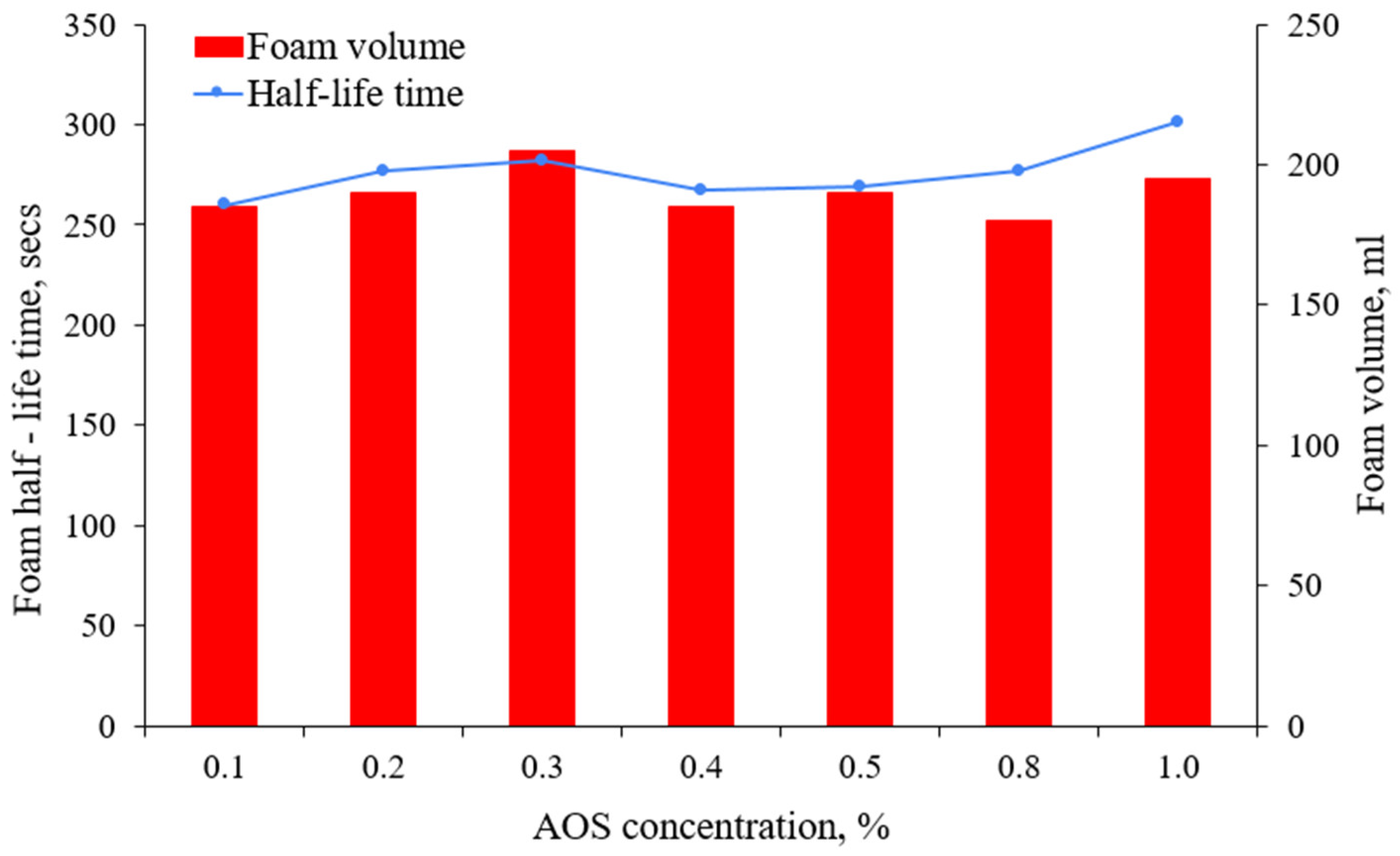
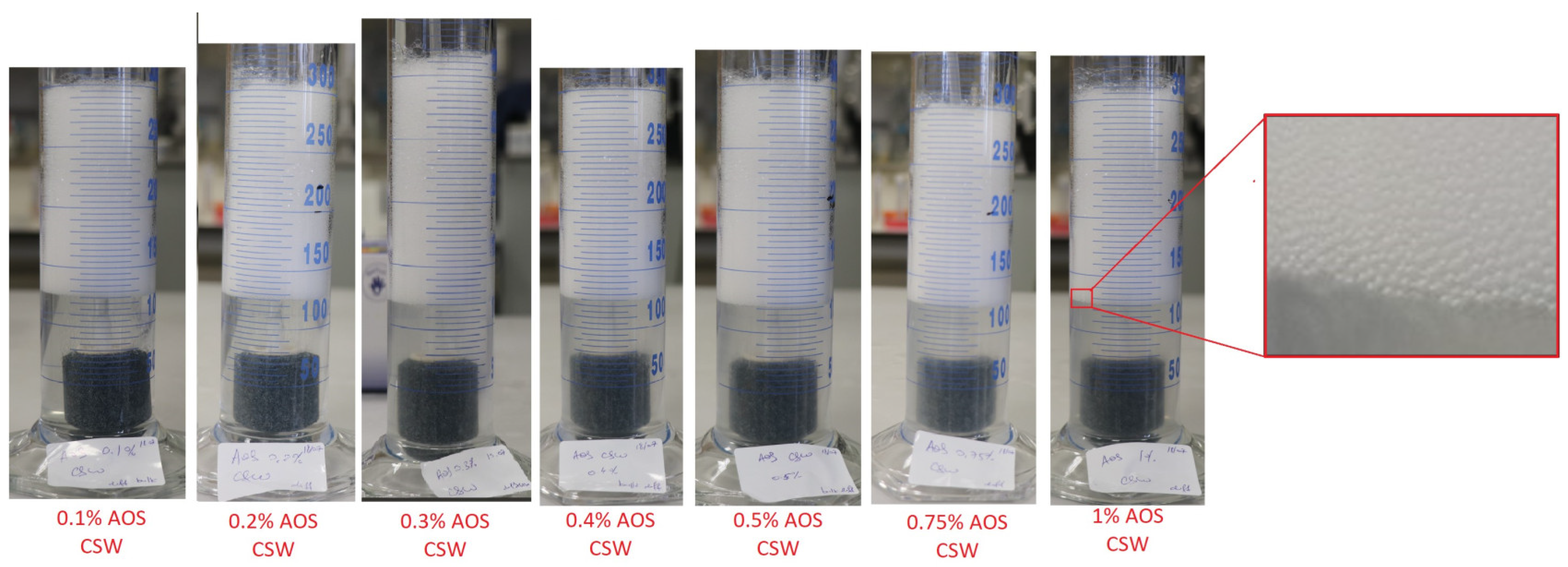
3.1.1. The Effect of Surfactant Concentration
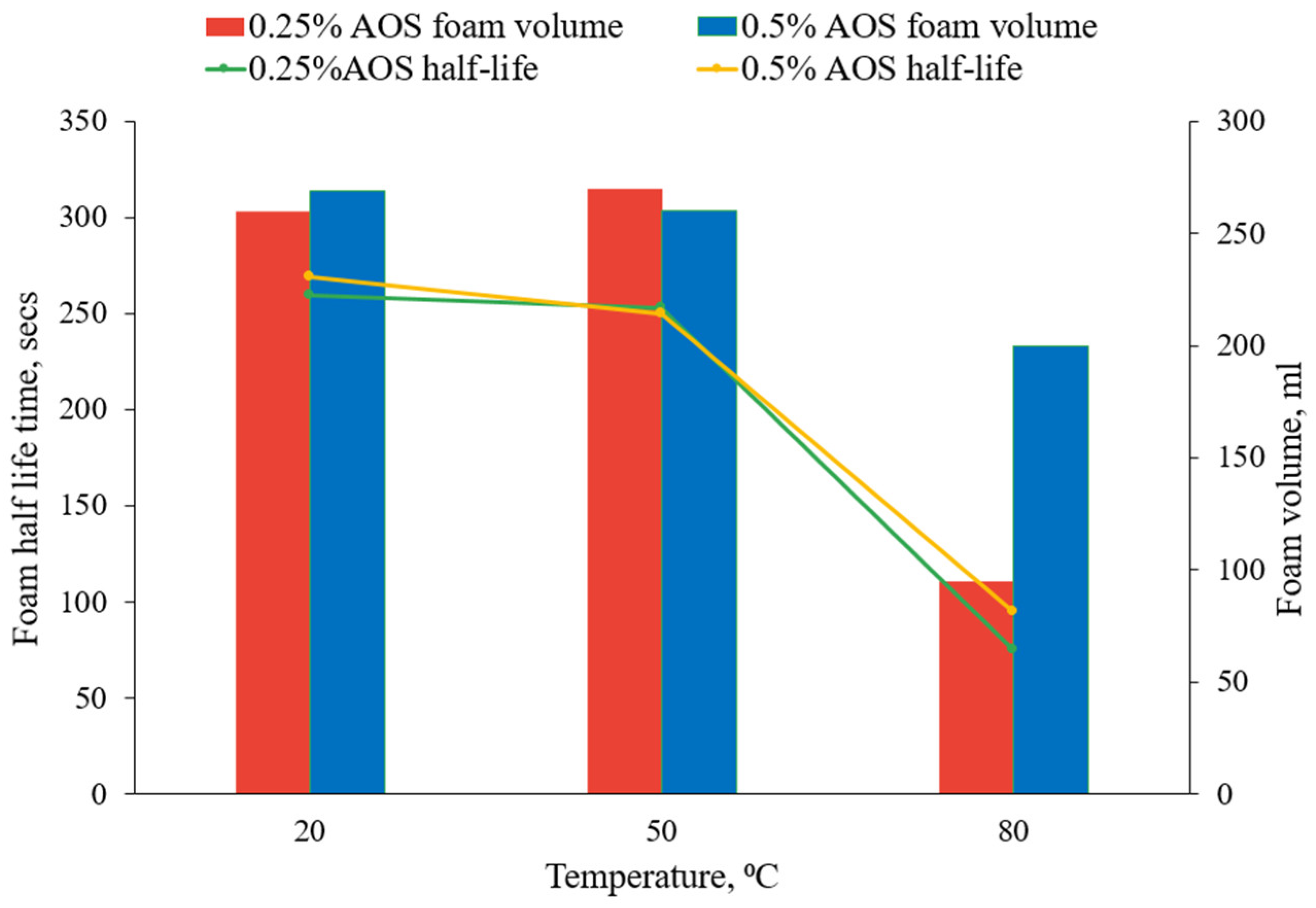
3.1.2. The Effect of Temperature
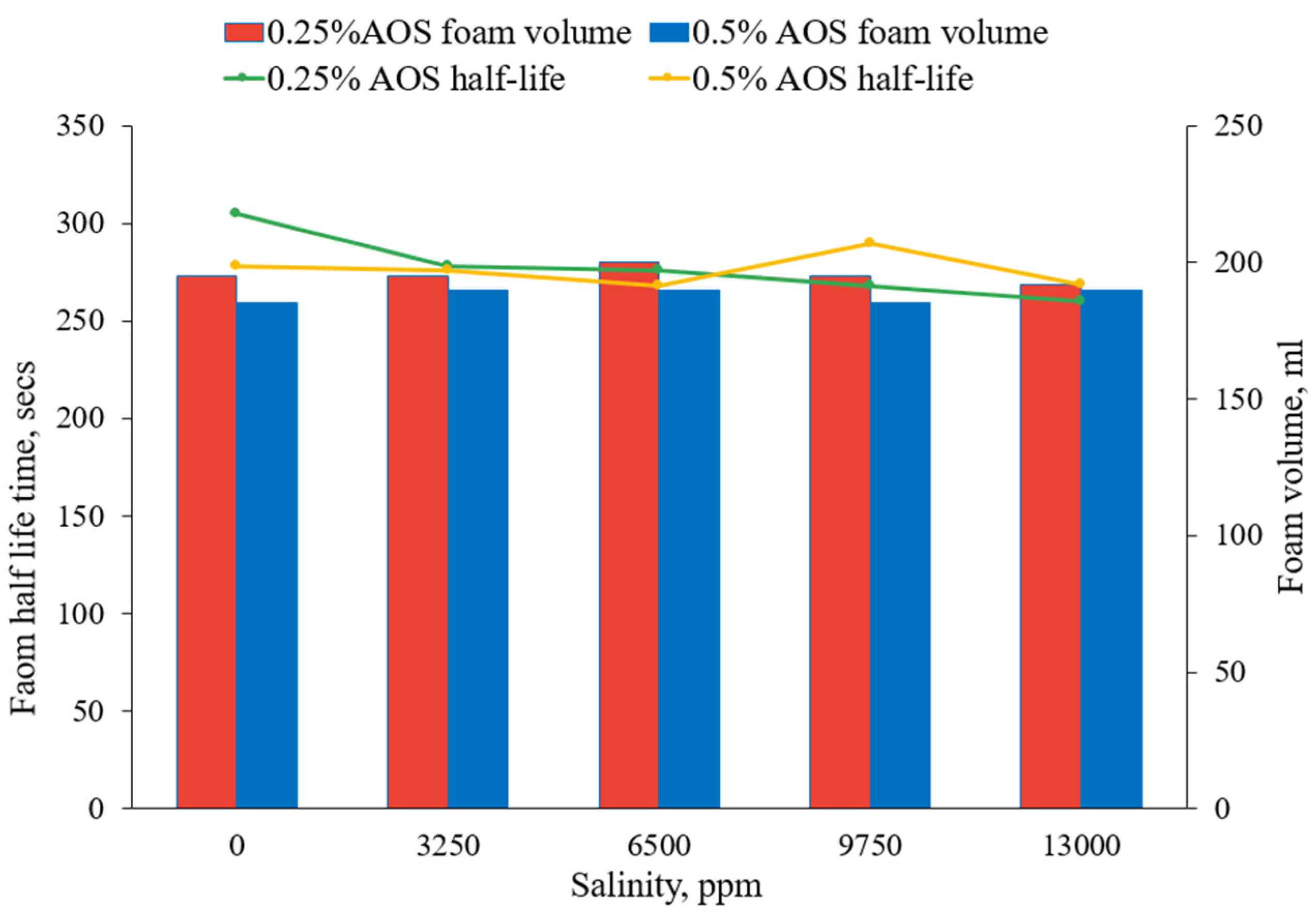
3.1.3. The Effect of Brine Salinity
3.2. Foam Performance in Porous Media in the Absence of Oil
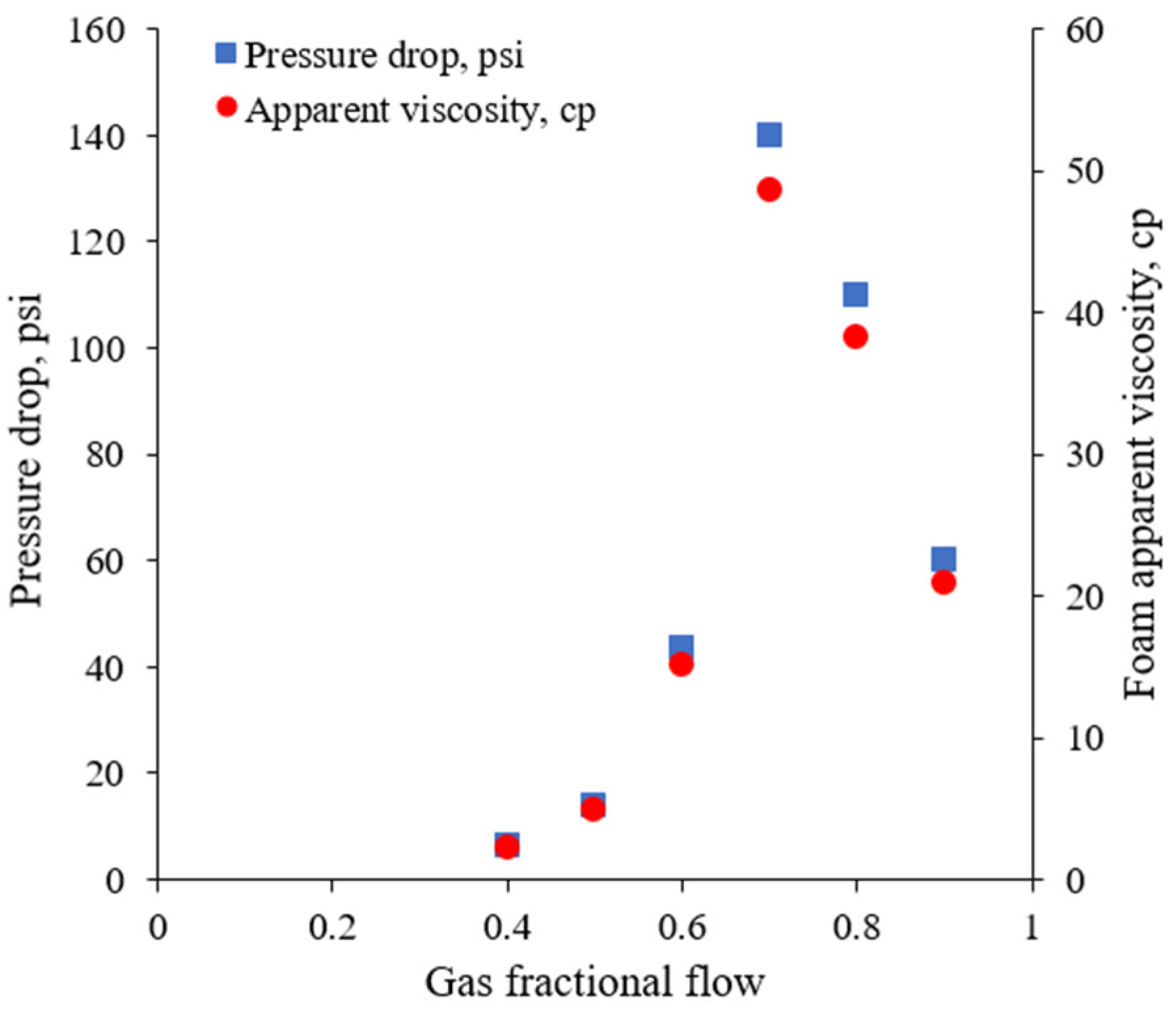
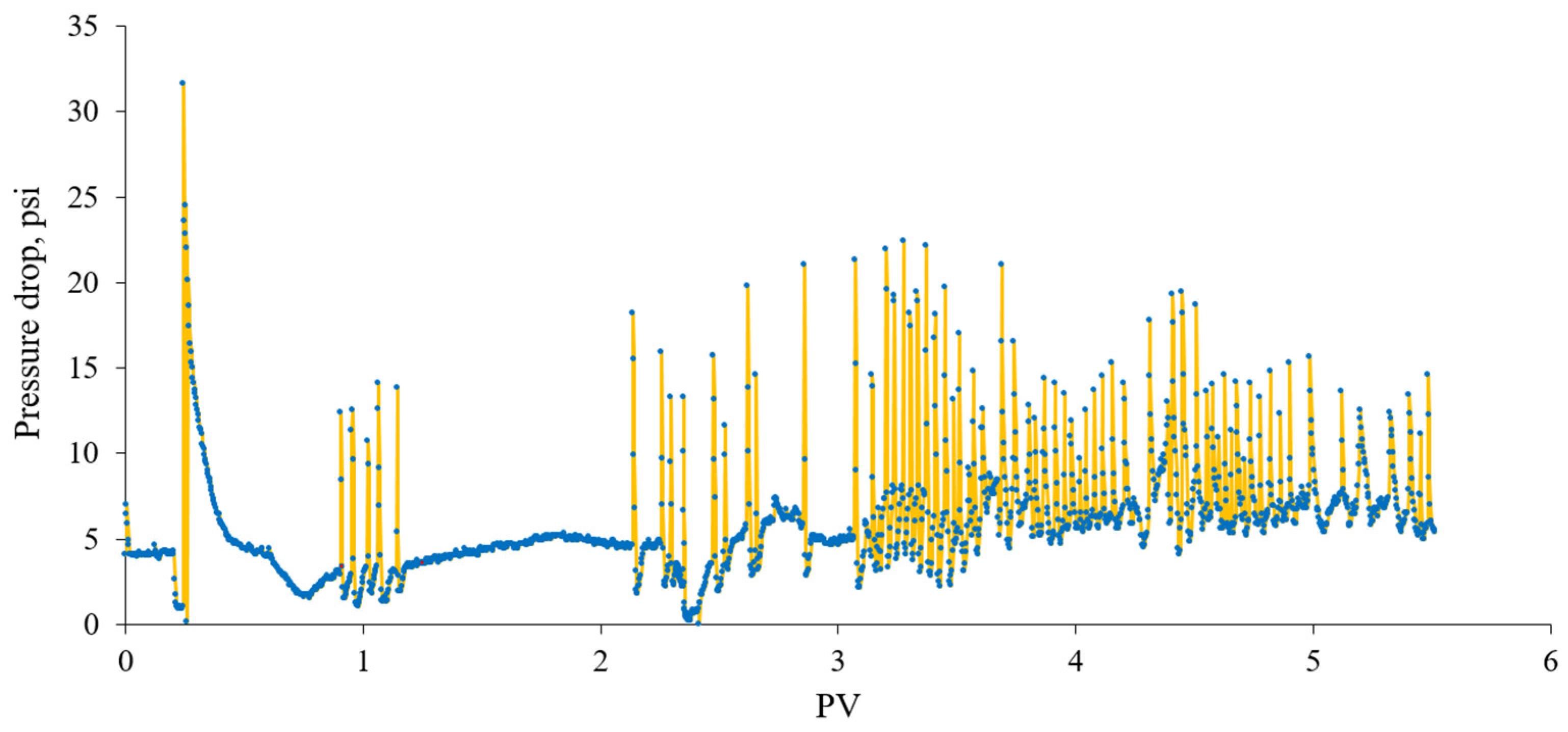
3.2.1. Foam Quality Scan
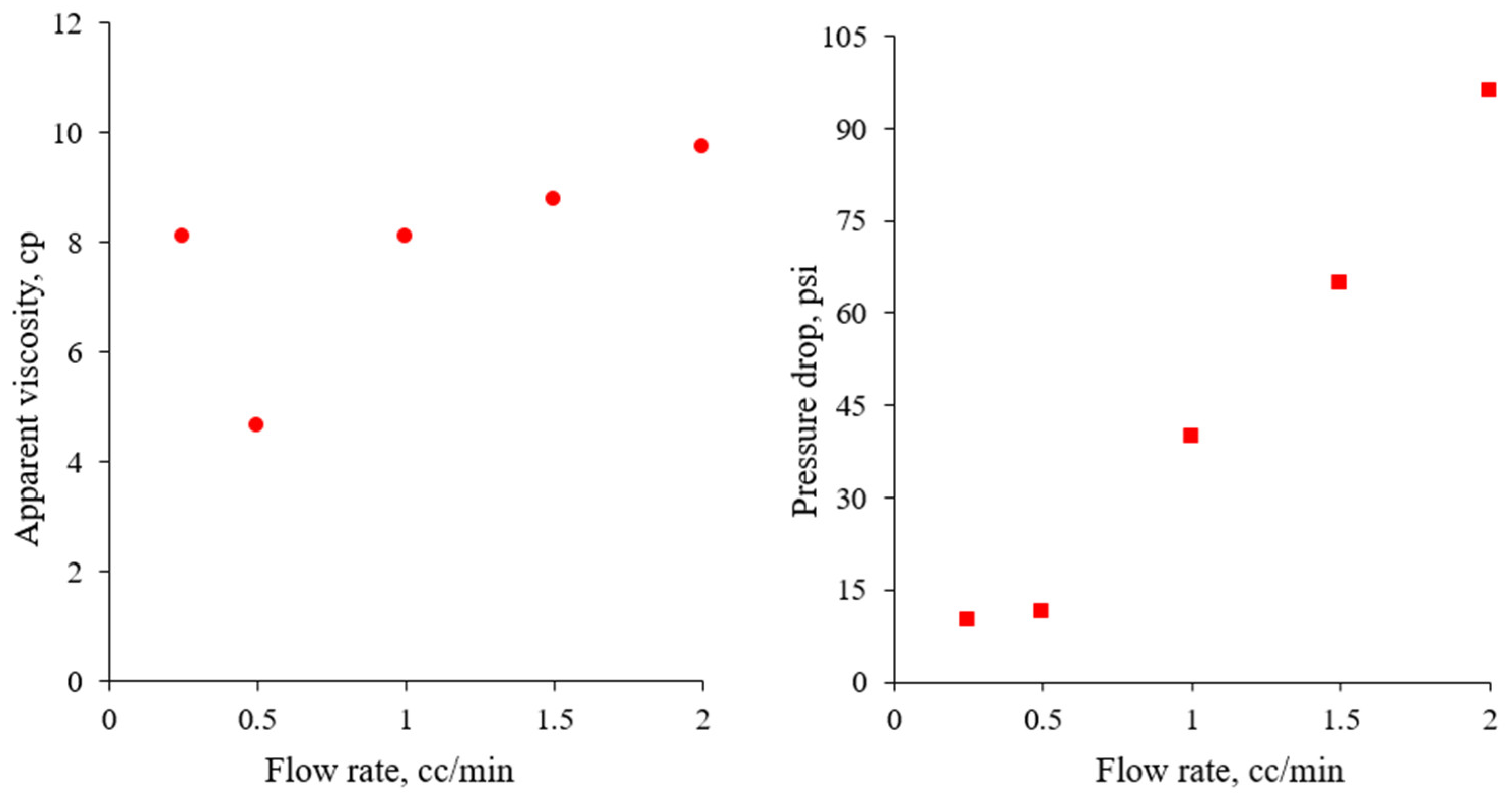
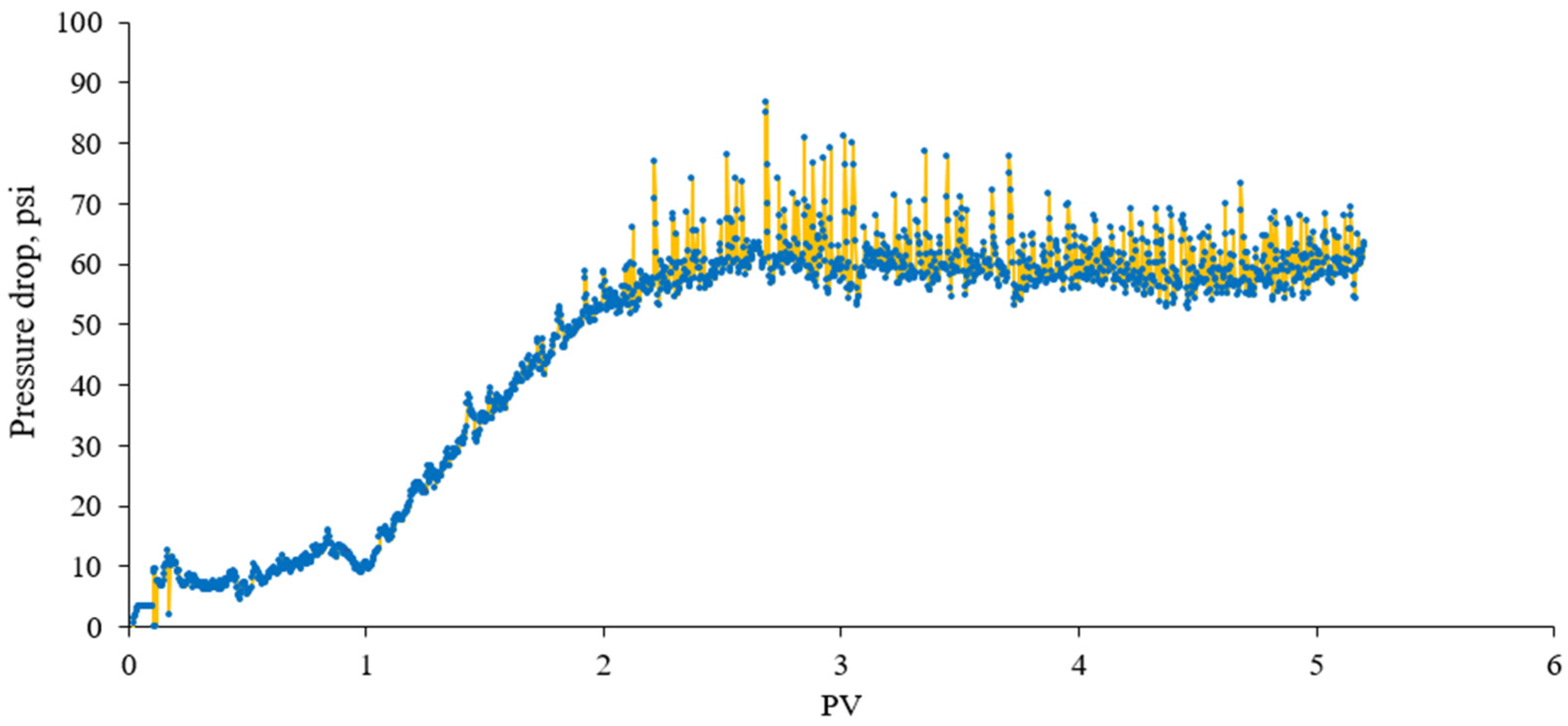
3.2.2. Flow Rate Tests
3.2.3. In Situ Foam Generation Test by Co-Injection
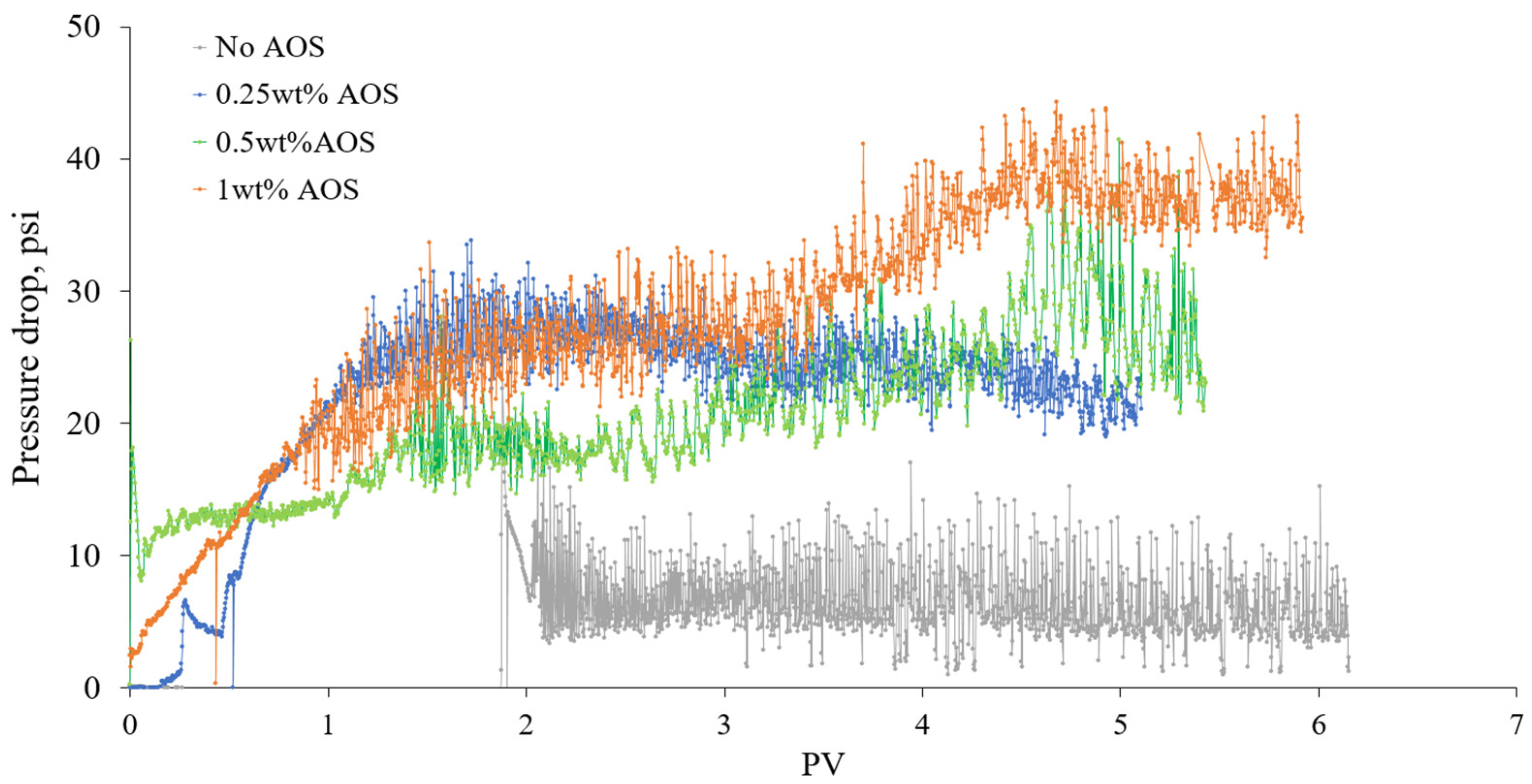
3.2.4. Effect of Surfactant Concentration on Foam Strength in Porous Media
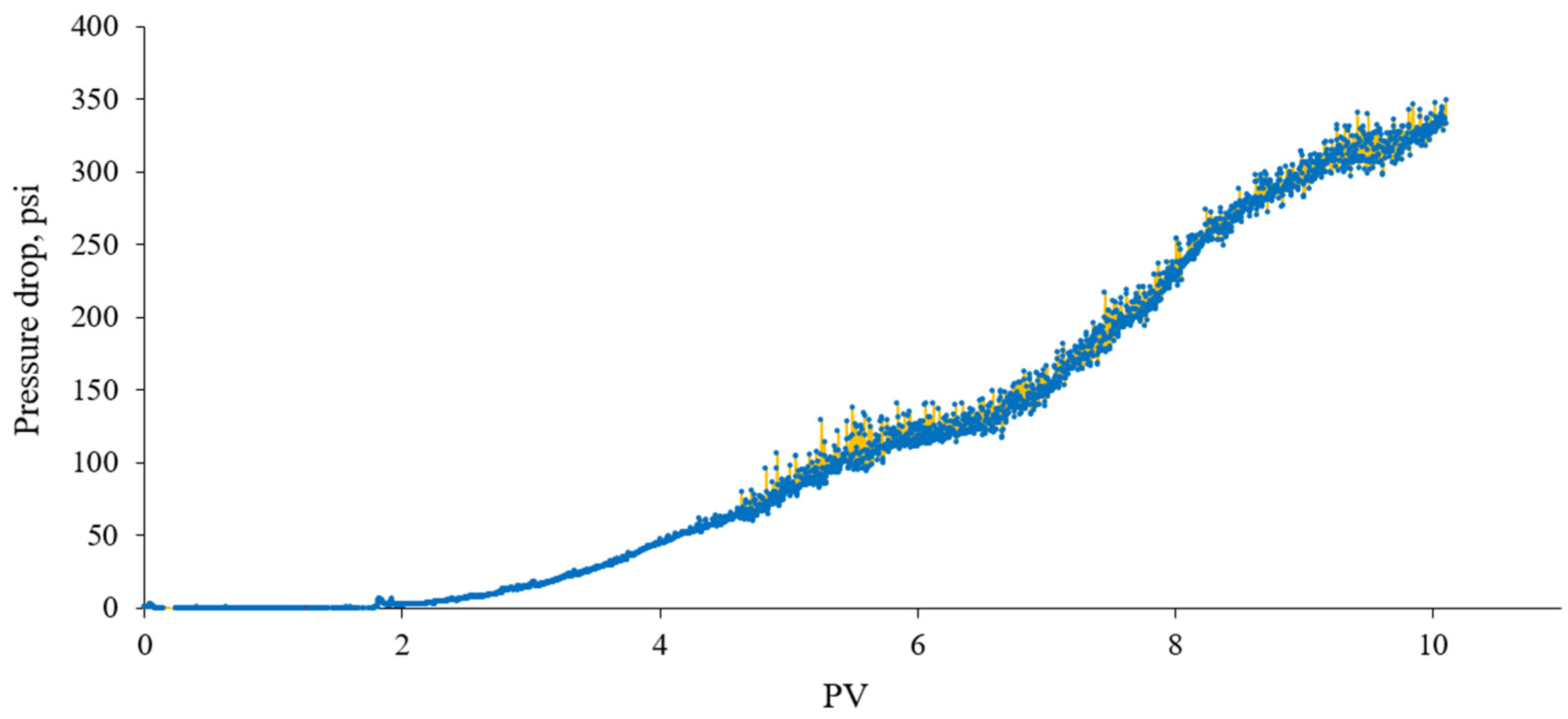
3.3. Oil Recovery Experiments
4. Conclusions
- Above a threshold value, increasing the AOS concentration had an insignificant effect in bulk foam screening tests at the measured foam volume and half-life at ambient pressure, suggesting an economic optimum surfactant concentration.
- Foam stability decreased with increasing temperature during bulk foam tests at ambient pressure. This effect was stronger at a low surfactant concentration, showing the importance of screening at elevated temperatures.
- Foam behavior was insensitive to salinity over the range of salinities tested with this surfactant.
- In high-pressure CO2 core flood experiments in carbonate outcrop cores, the foam apparent viscosity became higher with increasing gas fractional flow until the transition point of 70%. Above the transition point, the apparent viscosity of the foam declined. This supports the concept of a critical capillary pressure for foam instability.
- Temperature had a detrimental effect on the foam transport behavior in porous media in the absence of oil. The foam stability and strength decreased dramatically with increasing reservoir temperature beyond a threshold, limiting the effectiveness at or above 80 °C.
- Foam flooding tests showed that the pressure drop across cores increased with increasing AOS concentration, which was more noticeable after the injection of a sufficient amount of the surfactant. This is important in the termination of the minimum chemical requirement.
- The co-injection of CO2 and brine alone at a fixed gas fractional flow prior during two oil recovery experiments was able to produce around 10% of the OOIP, but this method still left significant unrecovered oil. This is a characteristic of the recovery efficiency of immiscible gas displacement processes.
- Core floods in a limestone core with crude oil showed that the addition of a surfactant demonstrated to support transient CO2 foam stability was able to enhance oil recovery. The addition of a foam-forming surfactant as a tertiary CO2 flooding method following waterflooding, CO2 flooding, and the co-injection of CO2/brine improved oil recovery by about 6–10% of the OOIP. The ability to increase the microscopic displacement efficiency is attributed to mitigation of heterogeneity on the core scale and sweep improvement offered by foam.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirasaki, G.J.; Miller, C.A.; Puerto, M. Recent advances in surfactant EOR. SPE J. 2011, 16, 889–907. [Google Scholar] [CrossRef]
- Worthen, A.J.; Bagaria, H.G.; Chen, Y.; Bryant, S.L.; Huh, C.; Johnston, K.P. Nanoparticle-stabilized carbon dioxide-in-water foams with fine texture. J. Colloid Interface Sci. 2013, 391, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, Y.; Yu, X.; Wang, C.; Yao, B.; Wang, S.; Winterfeld, P.H.; Wang, X.; Yang, Z.; Wang, Y.; et al. Advances in improved/enhanced oil recovery technologies for tight and shale reservoirs. Fuel 2017, 210, 425–445. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S. An experimental investigation of nanoparticle-stabilized CO2 foam used in enhanced oil recovery. Fuel 2016, 186, 430–442. [Google Scholar] [CrossRef]
- Koyanbayev, M.; Wang, L.; Wang, Y.; Hashmet, M.R.; Hazlett, R.D. Impact of gas composition and reservoir heterogeneity on miscible sour gas flooding—A simulation study. Fuel 2023, 346, 128267. [Google Scholar] [CrossRef]
- Schramm, L.L.; Mannhardt, K. The effect of wettability on foam sensitivity to crude oil in porous media. J. Pet. Sci. Eng. 1996, 15, 101–113. [Google Scholar] [CrossRef]
- Enick, R.M.; Olsen, D.; Ammer, J.; Schuller, W. Mobility and conformance control for CO2 EOR via thickeners, foams, and gels-a literature review of 40 years of research and pilot tests. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12 April 2012. [Google Scholar]
- Talebian, S.H.; Masoudi, R.; Tan, I.M.; Zitha, P.L.J. Foam assisted CO2-EOR: A review of concept, challenges, and future prospects. J. Pet. Sci. Eng. 2014, 120, 202–215. [Google Scholar] [CrossRef]
- Worthen, A.; Taghavy, A.; Aroonsri, A.; Kim, I.; Johnston, K.; Huh, C.; Bryant, S.; DiCarlo, D. Multi-scale Evaluation of Nanoparticle-stabilized CO2-in-water Foams: From the Benchtop to the Field. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 28 September 2015. [Google Scholar]
- Aryana, S.A.; Kovscek, A.R. Experiments and analysis of drainage displacement processes relevant to carbon dioxide injection. Phys. Rev. E 2012, 86, 066310. [Google Scholar] [CrossRef]
- Sanders, A.W.; Nguyen, Q.P.; Nguyen, N.M.; Adkins, S.S.; Johnston, K.P. Twin-tailed surfactants for creating CO2-in-water macroemulsions for sweep enhancement in CO2-EOR. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 1 November 2010. [Google Scholar]
- Chakravarthy, D.; Muralidharan, V.; Putra, E.; Schechter, D.S. Application of X-ray CT for investigation of CO2 and WAG injection in fractured reservoirs. In Proceedings of the Canadian International Petroleum Conference, Calgary, Alberta, 8 June 2004. [Google Scholar]
- Dalland, M.; Hanssen, J.E. Enhanced foams for efficient gas influx control. In Proceeding of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 18 February 1997. [Google Scholar]
- Lake, L.W.; Johns, R.T.; Rossen, R.W.; Pope, G.A. Fundamentals of Enhanced Oil Recovery, 2nd ed.; Society of Petroleum Engineers: Richardson, TX, USA, 2014; pp. 150–186. [Google Scholar]
- Bond, D.C.; Holbrook, O.C. Gas Drive Oil Recovery Process. U.S. Patent 2,866,507, 30 December 1958. [Google Scholar]
- Zhao, J.; Torabi, F.; Yang, J. The synergistic role of silica nanoparticle and anionic surfactant on the static and dynamic CO2 foam stability for enhanced heavy oil recovery: An experimental study. Fuel 2021, 287, 119443. [Google Scholar] [CrossRef]
- Solbakken, J.S.; Aarra, M.G. CO2 mobility control improvement using N2-foam at high pressure and high temperature conditions. Int. J. Greenh. Gas Control 2021, 109, 103392. [Google Scholar] [CrossRef]
- Yu, J.; An, C.; Mo, D.; Liu, N.; Lee, R. Foam mobility control for nanoparticle-stabilized CO2 foam. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14 April 2012. [Google Scholar]
- Kovscek, A.R.; Radke, C.J. Fundamentals of Foam Transport in Porous Media. In Foams: Fundamentals and Application in the Petroleum Industry; American Chemical Society: Washington, DC, USA, 1994; pp. 115–163. [Google Scholar]
- Rognmo, A.U.; Heldal, S.; Fernø, M.A. Silica nanoparticles to stabilize CO2-foam for improved CO2 utilization: Enhanced CO2 storage and oil recovery from mature oil reservoirs. Fuel 2018, 216, 621–626. [Google Scholar] [CrossRef]
- Chou, S.I.; Vasicek, S.L.; Pisio, D.L.; Jasek, D.E.; Goodgame, J.A. CO2 foam field trial at north ward-estes. In Proceedings of the SPE Annual Technical Conference and Exhibition, Washington, DC, USA, 4 October 1992. [Google Scholar]
- Patil, P.D.; Knight, T.; Katiyar, A.; Vanderwal, P.; Scherlin, J.; Rozowski, P.; Nguyen, Q.P. CO2 foam field pilot test in sandstone reservoir: Complete analysis of foam pilot response. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14 April 2018. [Google Scholar]
- Simjoo, M.; Dong, Y.; Andrianov, A.; Talanana, M.; Zitha, P.L. Novel insight into foam mobility control. SPE J. 2013, 18, 416–427. [Google Scholar] [CrossRef]
- Aarra, M.G.; Murad, A.M.; Solbakken, J.S.; Skauge, A. Foam dynamics in limestone carbonate cores. ACS Omega 2020, 5, 23604–23612. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, R.; Krastev, R.; Zitha, P.L.J. Foam films stabilized with alpha olefin sulfonate (AOS). Colloids and Surfaces A: Physicochem. Eng. Asp. 2008, 324, 35–40. [Google Scholar] [CrossRef]
- Aarra, M.G.; Skauge, A.; Martinsen, H.A. FAWAG: A Breakthrough for EOR in the North Sea. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 29 September 2002. [Google Scholar]
- Blaker, T.; Aarra, M.G.; Skauge, A.; Rasmussen, L.; Celius, H.K.; Martinsen, H.A.; Vassenden, F. Foam for Gas Mobility Control in the Snorre Field: The FAWAG Project. SPE Reservoir Eval. Eng. 2002, 5, 317–323. [Google Scholar] [CrossRef]
- Ocampo, A.; Restrepo, A.; Cifuentes, H.; Hester, J.; Orozco, N.; Gil, C.; Gonzalez, C. Successful foam EOR pilot in a mature volatile oil reservoir under miscible gas injection. In Proceedings of the International Petroleum Technology Conference, Beijing, China, 26 March 2013. [Google Scholar]
- Ahmed, S.; Elraies, K.A.; Forooozesh, J.; Bt Mohd Shafian, S.R.; Hashmet, M.R.; Hsia, I.C.C.; Almansour, A. Experimental investigation of immiscible supercritical carbon dioxide foam rheology for improved oil recovery. J. Earth Sci. 2017, 28, 835–841. [Google Scholar] [CrossRef]
- Føyen, T.; Alcorn, Z.P.; Fernø, M.A.; Barrabino, A.; Holt, T. CO2 mobility reduction using foam stabilized by CO2-and water-soluble surfactants. J. Pet. Sci. Eng. 2021, 196, 107651. [Google Scholar] [CrossRef]
- Bello, A.; Ivanova, A.; Cheremisin, A. Enhancing N2 and CO2 foam stability by surfactants and nanoparticles at high temperature and various salinities. J. Pet. Sci. Eng. 2022, 215, 110720. [Google Scholar] [CrossRef]
- Abdelall, A.; Gajbhiye, R.; AlShehri, D.; Mahmoud, M.; Patil, S.; Zhou, X. Improvement of supercritical carbon dioxide foam performance for EOR in sandstone reservoirs: An experimental and optimization study. Gas Sci. Eng. 2023, 110, 204859. [Google Scholar] [CrossRef]
- Aarra, M.G.; Ormehaug, P.A.; Skauge, A.; Masalmeh, S.K. Experimental Study of CO2- and Methane-Foam Using Carbonate Core Material at Reservoir Conditions. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25 September 2011. [Google Scholar]
- Skauge, A.; Solbakken, J.; Ormehaug, P.A.; Aarra, M.G. Foam generation, propagation and stability in porous medium. Transp. Porous Media 2020, 131, 5–21. [Google Scholar] [CrossRef]
- Mannhardt, K.; Schramm, L.L.; Novosad, J.J. Adsorption of anionic and amphoteric foam-forming surfactants on different rock types. Colloids Surf. 1992, 68, 37–53. [Google Scholar] [CrossRef]
- Ghosh, P.; Mohanty, K.K. Novel application of cationic surfactants for foams with wettability alteration in oil-wet low-permeability carbonate rocks. SPE J. 2018, 23, 2218–2231. [Google Scholar] [CrossRef]
- Shepherd, M. Carbonate Reservoirs. In Oil Field Production Geology, 1st ed.; American Association of Petroleum Geologists: Tulsa, OK, USA, 2009; pp. 301–309. [Google Scholar]
- Boeije, C.S.; Rossen, W.R. SAG foam flooding in carbonate rocks. J. Pet. Sci. Eng. 2017, 1, 1–18. [Google Scholar]
- Skauge, A.; Aarra, M.G.; Ormehaug, P.A.; Solbakken, J.S.; Mogensen, K.; Masalmeh, S. Preparations for Foam Gas Shut-off In Carbonate Reservoirs. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 11 November 2019. [Google Scholar]
- Shakeel, M.; Samanova, A.; Pourafshary, P.; Hashmet, M.R. Experimental analysis of oil displacement by hybrid engineered water/chemical EOR approach in carbonates. J. Pet. Sci. Eng. 2021, 207, 109297. [Google Scholar] [CrossRef]
- Beheshti, E.; Riahi, S.; Riazi, M. Impacts of oil components on the stability of aqueous bulk CO2 foams: An experimental study. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 648, 129328. [Google Scholar] [CrossRef]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Houston, TX, USA, 2011; pp. 79–100. [Google Scholar]
- Majeed, T.; Sølling, T.I.; Kamal, M.S. Foamstability: The interplay between salt-, surfactant-and critical micelle concentration. J. Pet. Sci. Eng. 2020, 187, 106871. [Google Scholar] [CrossRef]
- AlSumaiti, A.M.; Hashmet, M.R.; AlAmeri, W.S.; Anto-Darkwah, E. Laboratory study of CO2 foam flooding in high temperature, high salinity carbonate reservoirs using Co-injection Technique. Energy Fuels 2018, 32, 1416–1422. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Rivas, H.J.; Rossen, W.R. Unified model for steady-state foam behavior at high and low foam qualities. SPE J. 2001, 6, 325–333. [Google Scholar] [CrossRef]
- Khatib, Z.I.; Hirasaki, G.J.; Falls, A.H. Effects of capillary pressure on coalescence and phase mobilities in foams flowing through porous media. SPE Reserv. Eng. 1988, 3, 919–926. [Google Scholar] [CrossRef]
- Li, B.F.; Zhang, M.Y.; Li, Z.M.; Kovscek, A.; Xin, Y.; Li, B.L. Flow characteristics and regime transition of aqueous foams in porous media over a wide range of quality, velocity, and surfactant concentration. Pet. Sci. 2023, 20, 1044–1052. [Google Scholar] [CrossRef]
- Rossen, W.R. Minimum pressure gradient for foam flow in porous media: Effect of interactions with stationary lamellae. J. Colloid Interface Sci. 1990, 139, 457–468. [Google Scholar] [CrossRef]
- Lotfollahi, M.; Kim, I.; Beygi, M.R.; Worthen, A.J.; Huh, C.; Johnston, K.P.; Wheeler, M.F.; DiCarlo, D.A. Foam Generation Hysteresis in Porous Media: Experiments and New Insights. Transp. Porous Media 2017, 116, 687–703. [Google Scholar] [CrossRef]
- Kahrobaei, S.; Bonnieu, S.V.; Farajzadeh, R. Experimental Study of Hysteresis Behavior of Foam in Porous Media. Sci. Rep. 2017, 2017, 1–11. [Google Scholar]




| Diameter, mm | Length, mm | Porosity, % | Kabs, mD |
|---|---|---|---|
| 38 | 151 | 15.3 | 120 |
| 38 | 151 | 15.9 | 100 |
| 38 | 147 | 18.3 | 66 |
| 38 | 154 | 18.3 | 45 |
| 38 | 147 | 19.8 | 175 |
| 38 | 150 | 18.9 | 630 |
| Ions | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | Total |
|---|---|---|---|---|---|---|
| SSW, ppm | 3240 | 350 | 740 | 5440 | 3010 | 13,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyanbayev, M.; Hazlett, R.D.; Wang, L.; Hashmet, M.R. An Experimental Investigation of Surfactant-Stabilized CO2 Foam Flooding in Carbonate Cores in Reservoir Conditions. Energies 2024, 17, 3353. https://doi.org/10.3390/en17133353
Koyanbayev M, Hazlett RD, Wang L, Hashmet MR. An Experimental Investigation of Surfactant-Stabilized CO2 Foam Flooding in Carbonate Cores in Reservoir Conditions. Energies. 2024; 17(13):3353. https://doi.org/10.3390/en17133353
Chicago/Turabian StyleKoyanbayev, Madiyar, Randy Doyle Hazlett, Lei Wang, and Muhammad Rehan Hashmet. 2024. "An Experimental Investigation of Surfactant-Stabilized CO2 Foam Flooding in Carbonate Cores in Reservoir Conditions" Energies 17, no. 13: 3353. https://doi.org/10.3390/en17133353
APA StyleKoyanbayev, M., Hazlett, R. D., Wang, L., & Hashmet, M. R. (2024). An Experimental Investigation of Surfactant-Stabilized CO2 Foam Flooding in Carbonate Cores in Reservoir Conditions. Energies, 17(13), 3353. https://doi.org/10.3390/en17133353






