Study on the Applicability of Autothermic Pyrolysis In Situ Conversion Process for Low-Grade Oil Shale: A Case Study of Tongchuan, Ordos Basin, China
Abstract
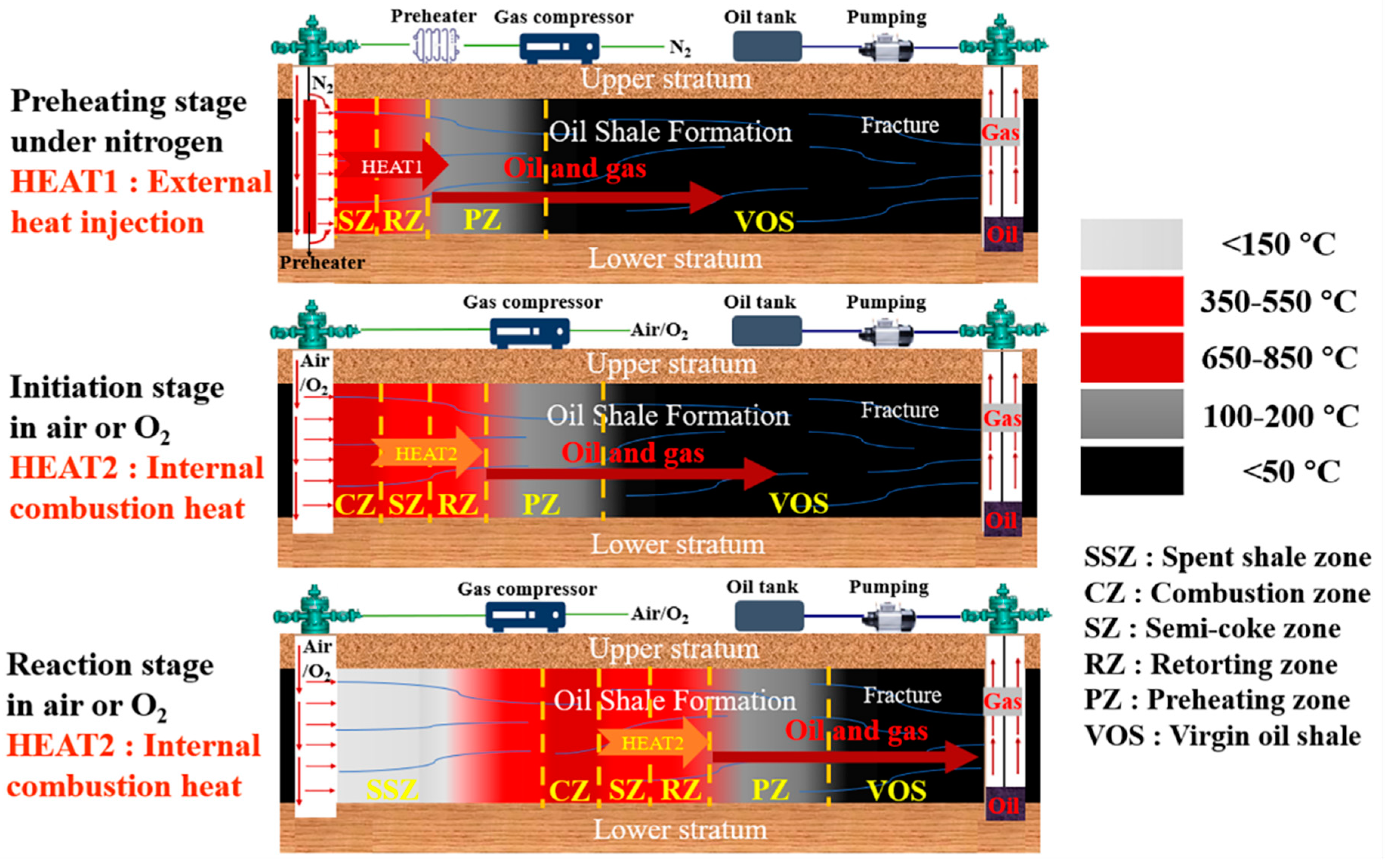
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
2.3. Analytical Methods
3. Results and Discussion
3.1. The Pyrolysis Behavior of Tongchuan OS
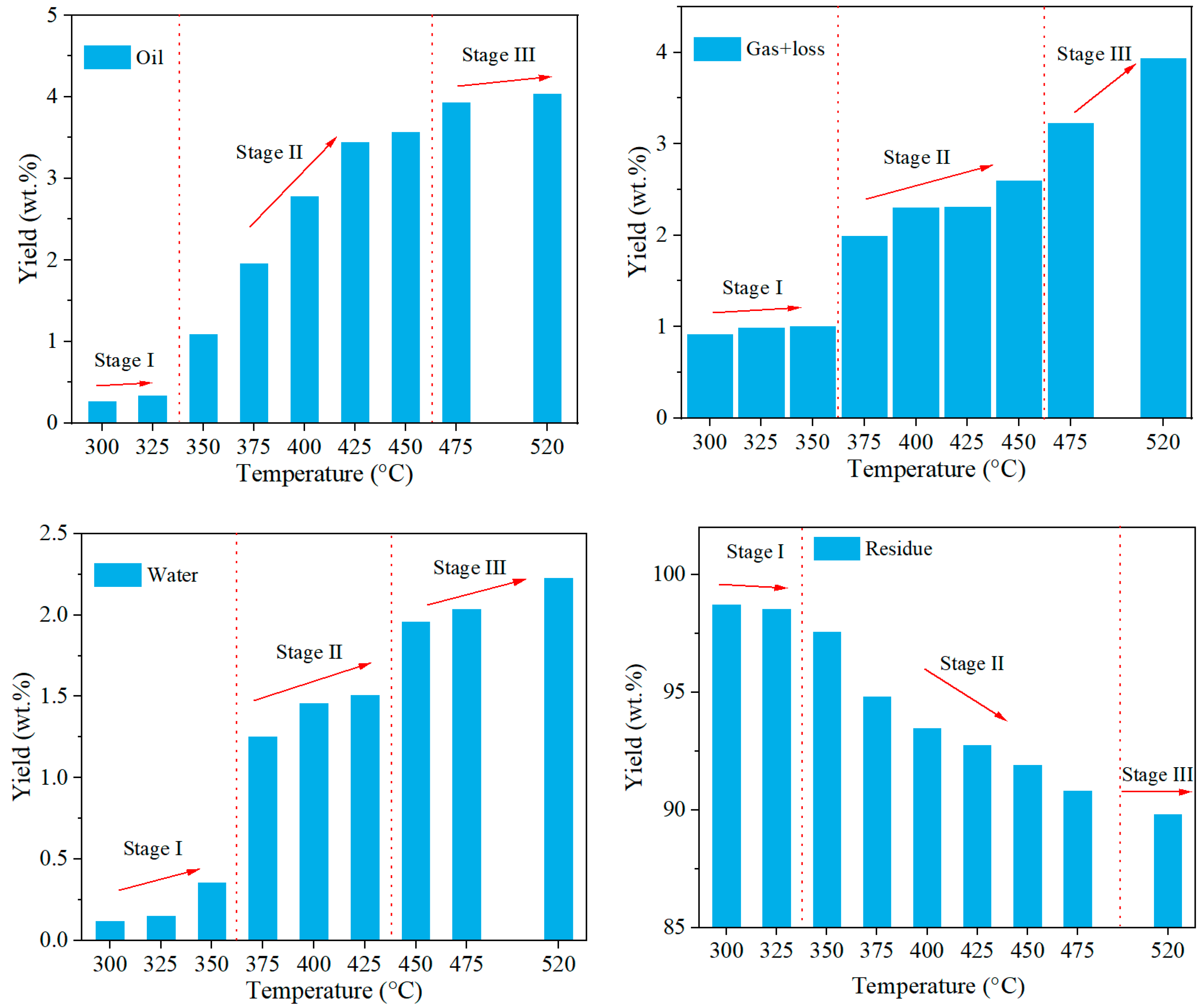
3.1.1. Yields of Pyrolysis Products
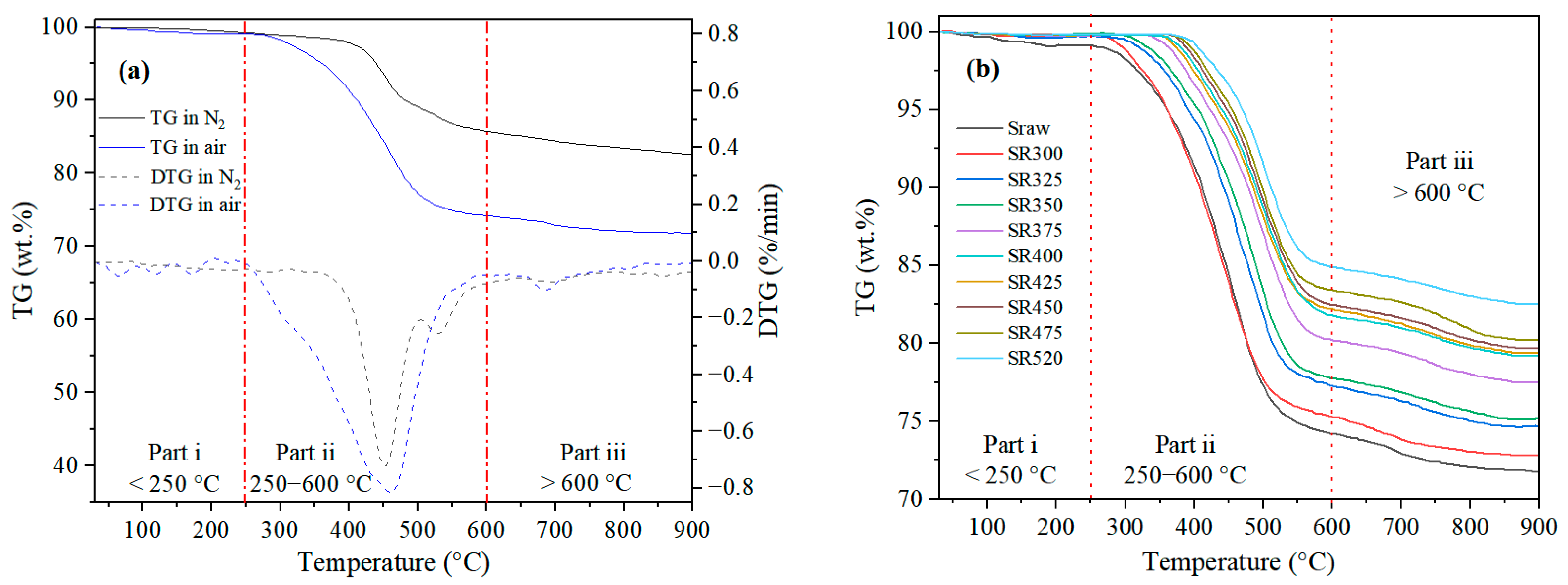
3.1.2. Residual Organic Matter in Residues
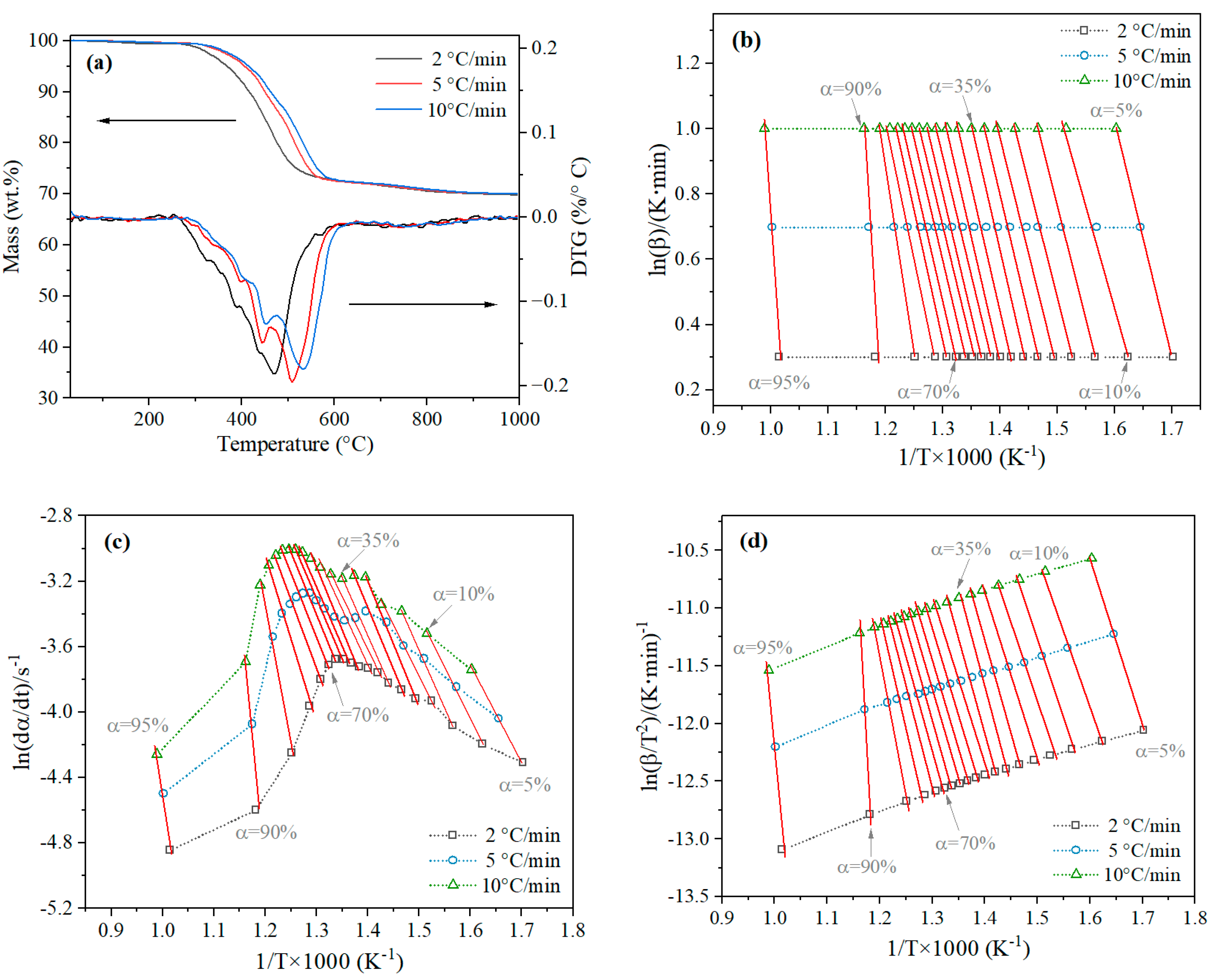
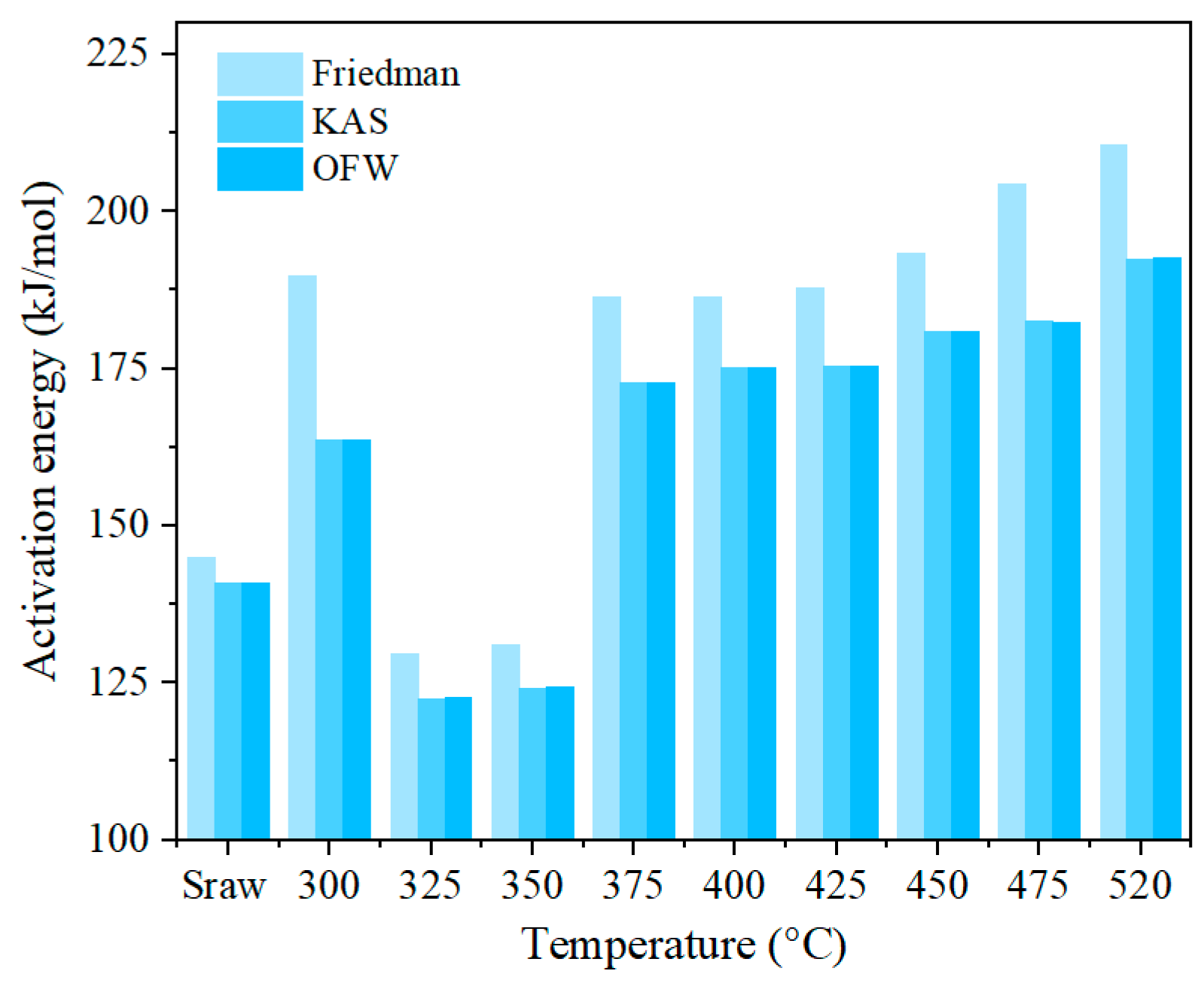
3.2. Analysis of Reaction Activation Energy
3.2.1. Kinetic Model
3.2.2. Reaction Activation Energy
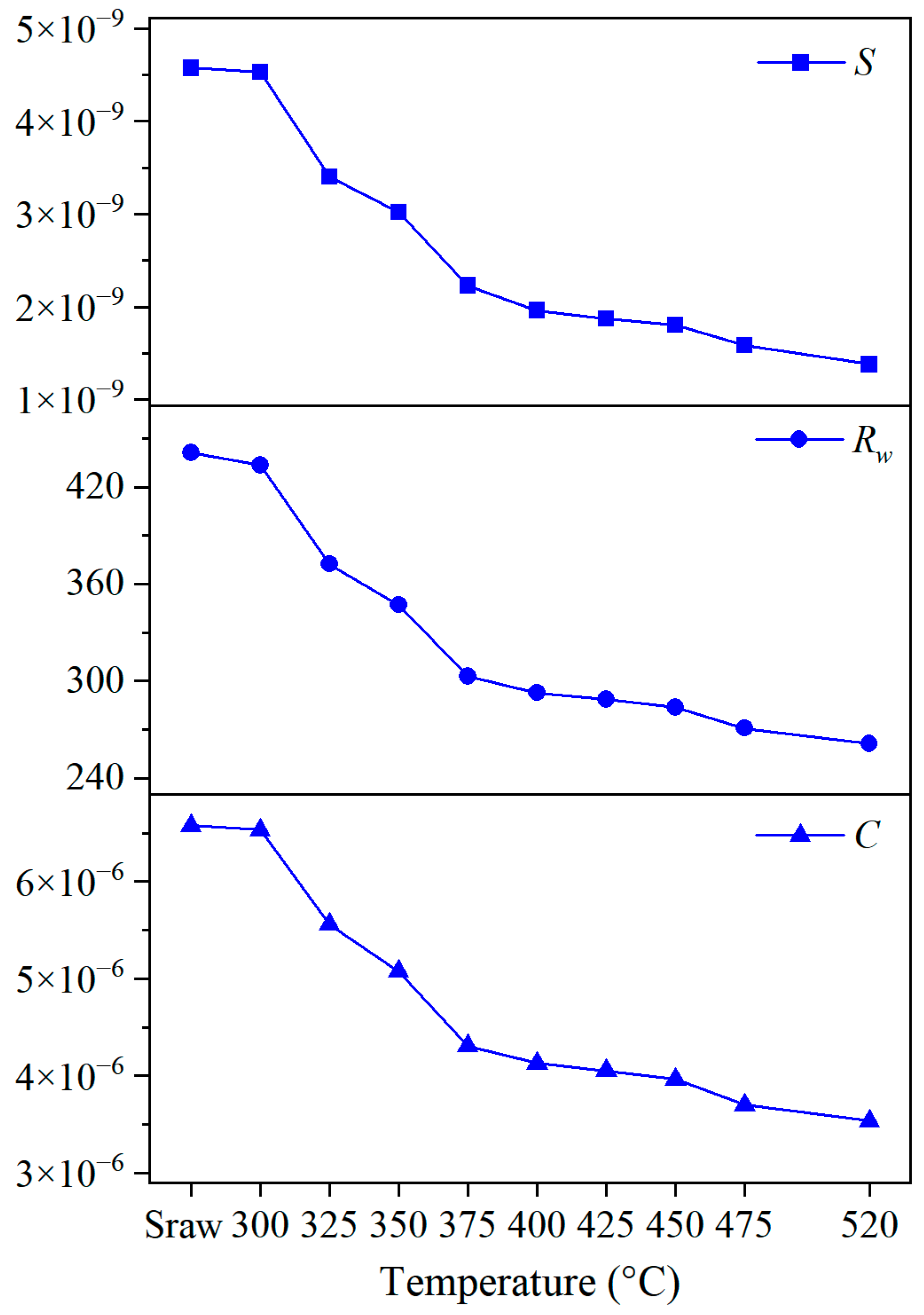
3.3. Analysis of Combustion Properties
3.3.1. Parameter Acquisition and Calculation
3.3.2. Combustion Property Parameters
3.3.3. Combustion Property Indices Analysis
3.3.4. Release Characteristics Analysis of Combustion Products
3.4. Optimization of Extraction Temperature Parameters
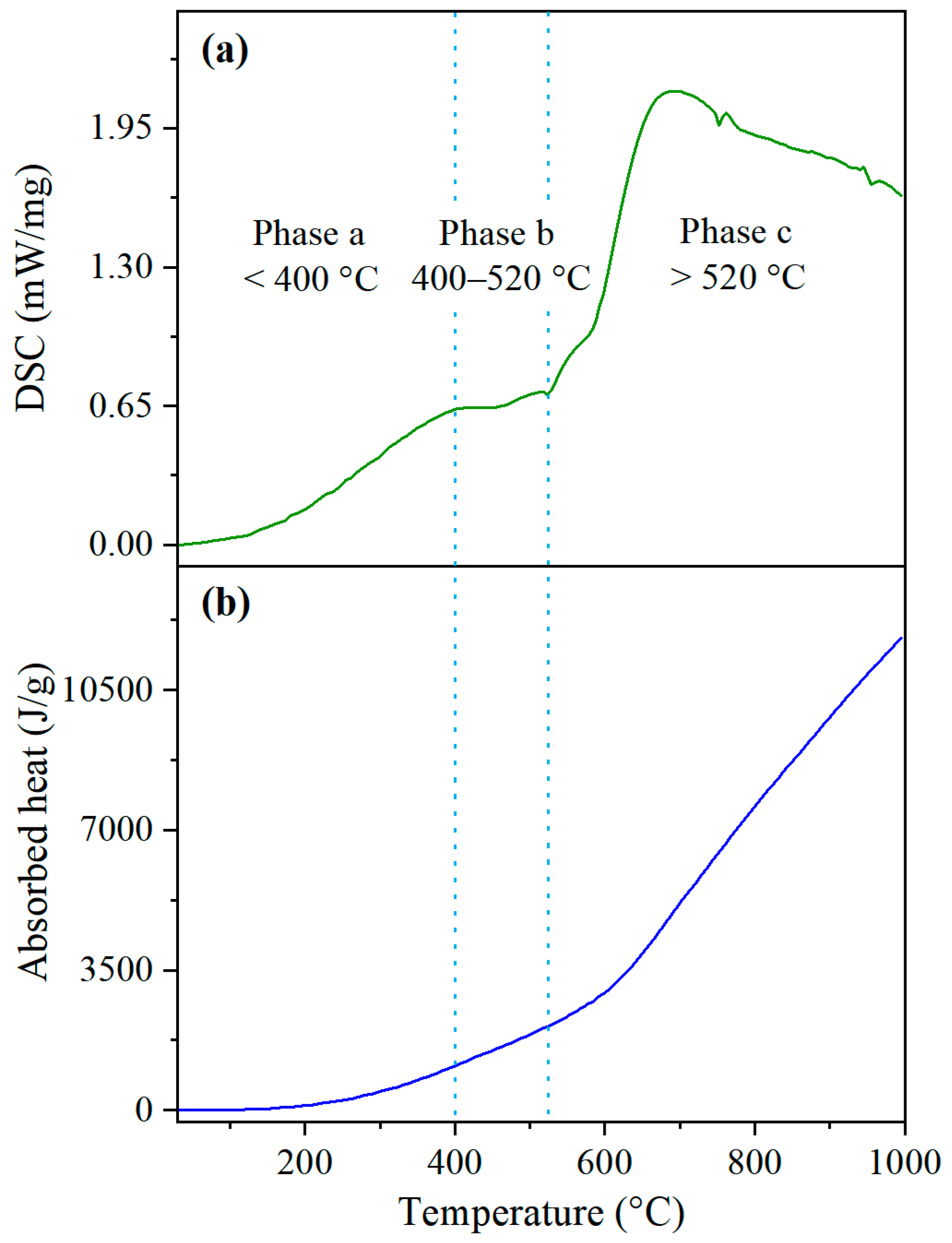
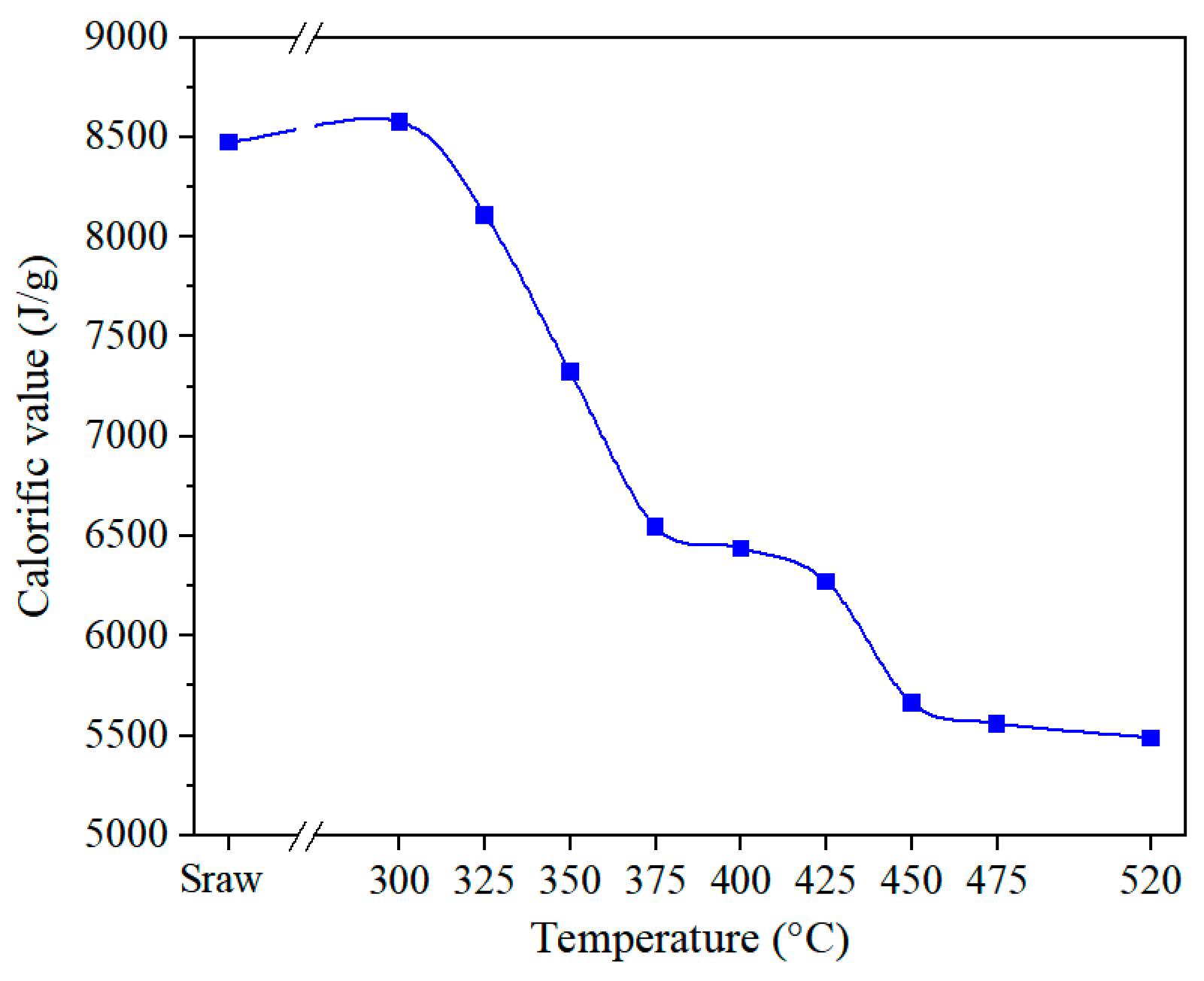
3.4.1. Heat Absorption for OS Pyrolysis
3.4.2. Combustion Heat of the Residue
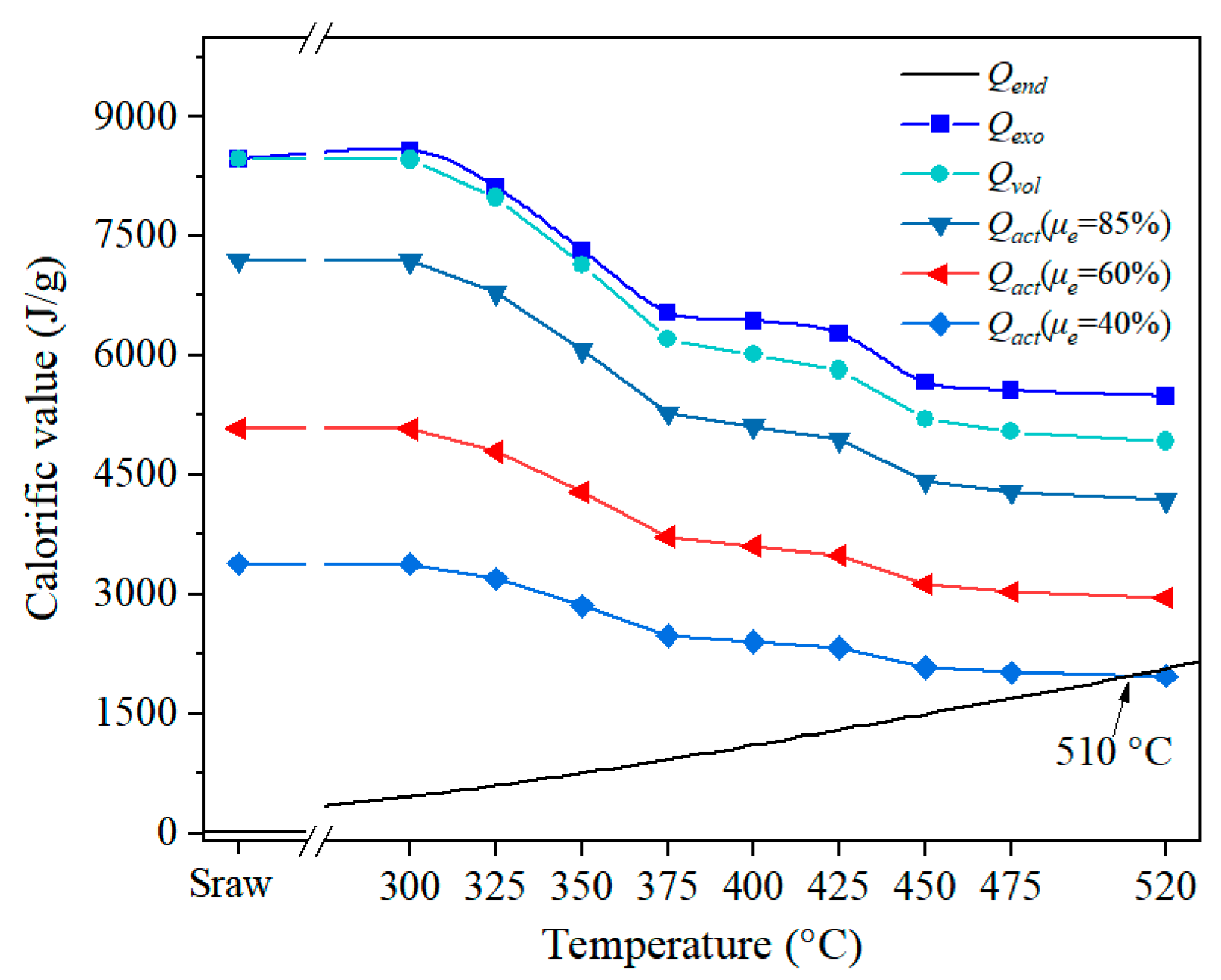
3.4.3. Analysis of Heat Supply and Demand
3.4.4. Optimization for Temperature Parameters
- Homogeneity in the layer’s regions concerning OS properties such as oil yield and density;
- OS distribution, as illustrated in Figure 1, uniformly spreads in the horizontal plane;
- The targeted OS layer has undergone reservoir stimulation pre-exploitation, nullifying the impact of large fractures and subsurface water, ensuring the ATS process normalcy.
- Exceeding the residue’s ignition temperature after OS pyrolysis at T0;
- Residue obtained at T0 has a lower activation energy;
- Residue obtained at T0 has better combustion performance;
- Residue obtained at T0 has better product release characteristics;
- T0 does not have to be lower than Tact.
- Residue obtained at T1 has a lower activation energy;
- Residue obtained at T1 has better combustion performance;
- Residue obtained at T1 has better product release characteristics;
- T1 should be less or equal to Tact.
4. Conclusions
- The product yield distribution during Tongchuan OS pyrolysis exhibited a three-stage pattern. Temperatures ranging from 350 to 425 °C favored oil production by facilitating kerogen pyrolysis, while temperatures from 450 to 520 °C resulted in a higher rate of gaseous product generation.
- The pyrolysis residue of Tongchuan OS displayed significantly increased combustion activation energy at 300 °C. This result can be attributed to pore plugging caused by bitumen volume expansion and bitumen coking during ignition. These effects negatively impact the residue’s ignition characteristics, thus elevating its combustion activation energy.
- The presence of substantial flammable bitumen, generated during heat treatment at 300 °C, led to significantly lowered ignition and burnout temperatures of the pyrolysis residue. As the pyrolysis temperature increased from 300 to 520 °C, the amount of organic matter residue decreased while the proportion of heavy products and residual carbon by-products increased. These changes adversely affected the combustion behavior, leading to a transition from homogeneous to non-homogeneous combustion.
- Tongchuan OS exhibited a slightly higher calorific value between 425 and 520 °C compared to Huadian OS, owing to its higher fixed carbon content (10.79%).
- The recommended preheating temperature for Tongchuan OS was found to be 425 °C, based on the ideal temperature screening method described in this study, and the ideal control temperature for the retorting zone was found to be 510 °C when taking a 40% heat utilization rate into account.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhai, Y.; Zhu, Y.; Cui, S.; Tao, Y.; Kai, X.; Yang, T. Study on the co-pyrolysis of oil shale and corn stalk: Pyrolysis characteristics, kinetic and gaseous product analysis. J. Anal. Appl. Pyrolysis 2022, 163, 105456. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, S.; Wang, Z.; Li, S.; Zhao, F.; Yang, J.; Zhou, L.; Deng, Y.; Sun, G.; Yu, H. Highly efficient catalytic pyrolysis of oil shale by CaCl2 in subcritical water. Energy 2023, 274, 127343. [Google Scholar] [CrossRef]
- Hrayshat, E. Oil shale-an alternative energy source for Jordan. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 30, 1915–1920. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, W.; Sun, Y.; Li, Q.; Deng, S.; Wang, Y.; Cui, G. Reaction mechanism and reservoir simulation study of the high-temperature nitrogen injection in-situ oil shale process: A case study in Songliao Basin, China. Fuel 2022, 316, 123164. [Google Scholar] [CrossRef]
- Li, J.; Shan, X.; Song, X.; He, W. Evaluation of the organic matter product of Huadian oil shale during pyrolysis using multiple approaches: Guidance for the in situ conversion of oil shale. J. Anal. Appl. Pyrolysis 2022, 167, 105656. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Y.; Lü, X.; Yang, Q.; Li, Q.; Wang, Z.; Guo, M. Effects of composition and pore evolution on thermophysical properties of Huadian oil shale in retorting and oxidizing pyrolysis. Fuel 2021, 305, 121565. [Google Scholar] [CrossRef]
- Amer, M.; Alhesan, J.; Marshall, M.; Fei, Y.; Jackson, W.; Chaffee, A. Energy efficient method of supercritical extraction of oil from oil shale. Energy Convers. Manag. 2022, 252, 115108. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, S.; Wang, S.; He, L.; Guo, W.; Li, Q.; Deng, S. Subcritical water extraction of Huadian oil shale at 300 °C. Energy Fuels 2019, 33, 2106–2114. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, X.; Deng, S.; Sun, Y.; Han, J.; Bai, F.; Kang, S.; He, W. Enhanced pyrolysis of Huadian oil shale at high temperature in the presence of water and air atmosphere. J. Pet. Sci. Eng. 2022, 215, 110623. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, S.; Yang, Q.; Lai, C.; Guo, M. Oxidizing pyrolysis of Huadian oil shale and its product distribution. J. China Univ. Pet. (Ed. Nat. Sci.) 2021, 45, 149–156. [Google Scholar]
- Dyni, J. Geology and resources of some world oil shale deposits. Oil Shale 2003, 20, 193–252. [Google Scholar] [CrossRef]
- Bai, F.; Zhao, J.; Liu, Y. An investigation into the characteristics and kinetics of oil shale oxy-fuel combustion by thermogravimetric analysis. Oil Shale 2019, 36, 1–18. [Google Scholar] [CrossRef]
- Jaber, T.; Apostolos, K. A comprehensive review of microwave application on the oil shale: Prospects for shale oil production. Fuel 2021, 305, 121519. [Google Scholar]
- He, W.; Sun, Y.; Shan, X. Organic matter evolution in pyrolysis experiments of oil shale under high pressure: Guidance for in situ conversion of oil shale in the Songliao Basin. J. Anal. Appl. Pyrolysis 2021, 155, 105091. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, Q.; Dong, Q.; Zhu, J.; Guo, W.; Ye, S.; Liu, R.; Jia, J. Characteristics and resource potential of oil shale in China. Oil Shale 2017, 34, 15–41. [Google Scholar] [CrossRef]
- Sun, P.; Li, W.; Liu, Z.; Niu, D.; Wu, X.; Tao, L.; Wang, Z.; Luan, Z. Selection of favourable targets for the in-situ conversion of continental oil shale in China. Oil Shale 2023, 40, 177–193. [Google Scholar] [CrossRef]
- Guo, H.; Peng, S.; Lin, J.; Chang, J.; Lei, S.; Fan, T.; Liu, Y. Retorting oil shale by a self-heating route. Energy Fuels 2013, 27, 2445–2451. [Google Scholar] [CrossRef]
- Guo, W.; Yang, Q.; Sun, Y.; Xu, S.; Kang, S.; Lai, C.; Guo, M. Characteristics of low temperature co-current oxidizing pyrolysis of Huadian oil shale. J. Anal. Appl. Pyrolysis 2020, 146, 104759. [Google Scholar] [CrossRef]
- Guo, W.; Yang, Q.; Zhang, X.; Xu, S.; Deng, S.; Li, Q. Thermal behavior of oil shale pyrolysis under low-temperature co-current oxidizing conditions. ACS Omega 2021, 6, 18074–18083. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Y.; Yang, Q.; Wang, H.; Kang, S.; Guo, W.; Shan, X.; He, W. Product migration and regional reaction characteristics in the autothermic pyrolysis in-situ conversion process of low-permeability Huadian oil shale core. Energy 2023, 283, 128525. [Google Scholar] [CrossRef]
- Kang, Z.; Zhao, Y.; Yang, D. Review of oil shale in-situ conversion technology. Appl. Energy 2020, 269, 115121. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Ignashev, N.E.; Krapivnitskaya, T.O.; Peskov, N.Y.; Glyavin, M.Y.; Bulanova, S.A.; Slavkina, O.V.; Schekoldin, K.A. Microwave radiation impact on heavy oil upgrading from carbonate deposits in the presence of nano-sized magnetite. Processes 2021, 9, 2021. [Google Scholar] [CrossRef]
- Djimasbe, R.; Ilyasov, I.R.; Kwofie, M.; Khelkhal, M.A.; Emelianov, D.A.; Al-Muntaser, A.A.; Suwaid, M.A.; Varfolomeev, M.A. Direct hydrogen production from extra-heavy crude oil under supercritical water conditions using a catalytic (ni-co/al2o3) upgrading process. Catalysts 2022, 12, 1183. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, W.; Xu, S.; Zhu, C. The autothermic pyrolysis in-situ conversion process for oil shale recovery: Effect of gas injection parameters. Energy 2023, 283, 129134. [Google Scholar] [CrossRef]
- Guo, W.; Li, Q.; Deng, S.; Wang, Y.; Zhu, C. Mechanism and reservoir simulation study of the autothermic pyrolysis in-situ conversion process for oil shale recovery. Pet. Sci. 2022, 20, 1053–1067. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Xu, S.; Wang, Z.; Guo, W. Low-temperature co-current oxidizing pyrolysis of oil shale: Study on the physicochemical properties, reactivity and exothermic characters of residue as heat generation donor. J. Pet. Sci. Eng. 2022, 216, 110726. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Y.; Guo, W.; Yang, Q.; Li, Q.; Guo, M.; Bai, F.; Zhu, C.; Deng, S. Regulating the oxidative assisted pyrolysis of Huadian oil shale by preheating temperature and oxygen flow rate. Energy 2023, 262, 125602. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, W.; Deng, S. The status and development trend of in-situ conversion and drilling exploitation technology for oil shale. Explor. Eng. (Rock Soil Drill. Tunneling) 2021, 48, 57–67. [Google Scholar]
- He, W.; Sun, Y.; Guo, W.; Shan, X. Controlling the in-situ conversion process of oil shale via geochemical methods: A case study on the Fuyu oil shale, China. Fuel Process. Technol. 2021, 219, 106876. [Google Scholar] [CrossRef]
- Xu, S.; Lü, X.; Sun, Y.; Guo, W.; Li, Q.; Liu, L.; Kang, S.; Deng, S. Optimization of temperature parameters for the autothermic pyrolysis in-situ conversion process of oil shale. Energy 2023, 264, 126309. [Google Scholar] [CrossRef]
- Guo, W.; Yang, Q.; Deng, S.; Li, Q.; Sun, Y.; Su, J.; Zhu, C. Experimental study of the autothermic pyrolysis in-situ conversion process (ATS) for oil shale recovery. Energy 2022, 258, 124878. [Google Scholar] [CrossRef]
- Bai, F.; Sun, Y.; Liu, Y. Thermogravimetric analysis of Huadian oil shale combustion at different oxygen concentrations. Energy Fuels 2016, 30, 4450–4456. [Google Scholar] [CrossRef]
- Bai, F.; Sun, Y.; Liu, Y.; Li, Q.; Guo, M. Thermal and kinetic characteristics of pyrolysis and combustion of three oil shales. Energy Convers. Manag. 2015, 97, 374–381. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.; Varfolomeev, M.; Yuan, C.; Xu, C. Influence of water on thermo-oxidative behavior and kinetic triplets of shale oil during combustion. Fuel 2022, 318, 123690. [Google Scholar] [CrossRef]
- Ifticene, M.; Yuan, C.; Al-Muntaser, A.; Onishchenko, Y.; Emelianov, D.; Varfolomeev, M. Behavior and kinetics of the conversion/combustion of oil shale and its components under air condition. Fuel 2022, 324, 124597. [Google Scholar] [CrossRef]
- Bolotov, A.; Yuan, C.; Varfolomeev, M.; Taura, U.; Al-Wahaibi, Y.; Minkhanov, I.; Derevyanko, V.; Al-Bahry, S.; Joshi, S.; Tazeev, A.; et al. In-situ combustion technique for developing fractured low permeable oil shale: Experimental evidence for synthetic oil generation and successful propagation of combustion front. Fuel 2023, 344, 127995. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, M.; Guo, W. Effects of associated minerals on the co-current oxidizing pyrolysis of oil shale in a low-temperature stage. ACS Omega 2021, 6, 23988–23997. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Dai, F.; Huang, J.; Liu, S.; Li, G. Study of the effect of mineral matters on the thermal decomposition of Jimsar oil shale using TG-MS. Thermochim. Acta 2016, 627, 31–38. [Google Scholar] [CrossRef]
- GB/T 212-2008; Proximate Analysis of Coal. Standard Press of China: Beijing, China, 2008.
- SH/T 0508-1992; Determination of Oil Content of Oil Shale (Low Temperature Retorting Method). Petrochemical Industry Standard of the People’s Republic of China, Fushun Petrochemical Research Institute: Fushun, China, 1992.
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Simon, P. Isoconversional methods. J. Therm. Anal. Calorim. 2004, 76, 123–132. [Google Scholar] [CrossRef]
- Flynn, J. The isoconversional method for determination of energy of activation at constant heating rates. J. Therm. Anal. Calorim. 1983, 27, 95–102. [Google Scholar] [CrossRef]
- Burnham, A.K.; Dinh, L. A comparison of isoconversional and model-fitting approaches to kinetic parameter estimation and application predictions. J. Therm. Anal. Calorim. 2007, 89, 479–490. [Google Scholar] [CrossRef]
- Han, X.; Jiang, X.; Yan, J.; Liu, J. Effects of retorting factors on combustion properties of shale char. 2. pore structure. Energy Fuels 2011, 25, 97–102. [Google Scholar] [CrossRef]
- James, L.; Thomas, H.; Mark, S.; Ronald, J. Characterization of macromolecular structure of pyrolysis products from a colorado green river oil shale. Ind. Eng. Chem. Res. 2013, 52, 15522–15532. [Google Scholar]
- Olga, P.; Vladimir, K.; Julia, K.; Allan, N.; Andres, S. Co-pyrolysis of estonian oil shale with polymer wastes. ACS Omega 2021, 6, 31658–31666. [Google Scholar]
- Han, J.; Sun, Y.; Guo, W.; Deng, S.; Hou, C.; Qu, L.; Li, Q. Non-isothermal thermogravimetric analysis of pyrolysis kinetics of four oil shales using Sestak-Berggren method. J. Therm. Anal. Calorim. 2019, 135, 2287–2296. [Google Scholar] [CrossRef]
- Zhan, H.; Qin, F.; Chen, S.; Chen, R.; Meng, Z.; Miao, X.; Zhao, K. Two-step pyrolysis degradation mechanism of oil shale through comprehensive analysis of pyrolysis semi-cokes and pyrolytic gases. Energy 2022, 241, 122871. [Google Scholar] [CrossRef]
- Xu, Y.; Lun, Z.; Wang, H.; Zhou, X.; Zhao, C.; Zhang, G.; Zhang, D. Influences of controlled microwave field irradiation on occurrence space and state of shale oil: Implications for shale oil production. J. Pet. Sci. Eng. 2022, 219, 111067. [Google Scholar] [CrossRef]
- Yang, D.; Wang, G.; Kang, Z.; Zhao, J.; Lv, Y. Experimental investigation of anisotropic thermal deformation of oil shale under high temperature and triaxial stress based on mineral and micro-fracture characteristics. Nat. Resour. Res. 2020, 29, 3987–4002. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Yang, C.; Jiang, W.; Li, Y.; Xiong, Y.; Peng, P. Evolution of mechanical properties of kerogen with thermal maturity. Mar. Pet. Geol. 2022, 145, 105906. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Zheng, G.; Yu, P.; Wang, W.; Jin, Y.; Chen, G. Effects of parent well spacing on the poroelastic behaviors in the infill zone in shale oil reservoirs: A case study in Jimsar shale oil, China. Energy Sci. Eng. 2022, 10, 1043–1054. [Google Scholar] [CrossRef]



| Proximate Analysis (wt.%) a | Sd b | Ultimate Analysis (wt.%) c | Sd b | ||
|---|---|---|---|---|---|
| Moisture | 0.78 | 0.01 | C | 16.83 | 0.13 |
| Volatile matter | 16.10 | 0.02 | H | 1.67 | 0.04 |
| Ash | 72.33 | 0.14 | N | 1.42 | 0.03 |
| Fixed carbon | 10.79 | 0.14 | O | 7.97 | 0.04 |
| S | 6.61 | 0.17 | |||
| Fischer Assay Analysis (wt.%) a | |||||
| Shale oil | 4.04 | 0.06 | |||
| Water | 2.22 | 0.06 | |||
| Residue | 89.81 | 0.11 | |||
| Gases + loss | 3.93 | 0.07 | |||
| Products | Stage I | Stage II | Stage III | |||
|---|---|---|---|---|---|---|
| Yield (wt.%) | Temp. (°C) | Yield (wt.%) | Temp. (°C) | Yield (wt.%) | Temp. (°C) | |
| Oil | 0.27–0.34 | 300–325 | 0.34–3.56 | 325–475 | 3.56–4.04 | 475–520 |
| Gas + loss | 0.91–1.00 | 300–350 | 1.00–3.22 | 375–450 | 3.22–3.93 | 475–520 |
| Water | 0.12–0.36 | 300–350 | 0.36–1.96 | 375–425 | 1.96–2.22 | 450–520 |
| Residue | 98.70–98.53 | 300–325 | 98.53–89.81 | 325–520 | 89.81 | 520 |
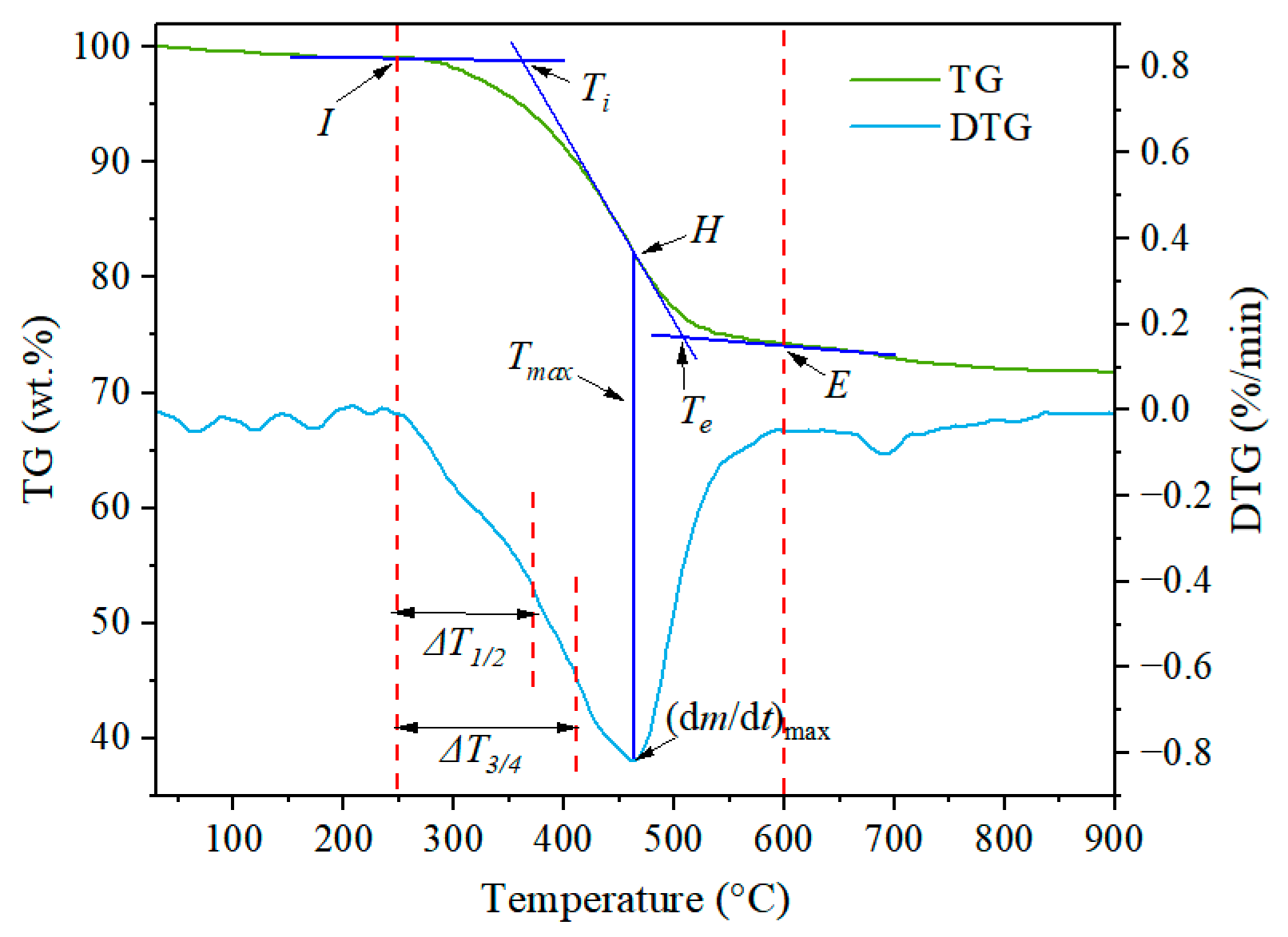
| Sample | Ti (°C) | Te (°C) | ΔT a (°C) | Tmax b (°C) | (dm/dt)max c (%/min) | (dm/dt)mean d (%/min) |
|---|---|---|---|---|---|---|
| Sraw e | 361 | 512 | 151 | 462 | 0.8169 | 0.3560 |
| SR300 | 348 | 498 | 150 | 451 | 0.8057 | 0.3509 |
| SR325 | 379 | 523 | 144 | 486 | 0.7985 | 0.3199 |
| SR350 | 395 | 531 | 136 | 496 | 0.7918 | 0.3161 |
| SR375 | 407 | 540 | 133 | 497 | 0.7134 | 0.2796 |
| SR400 | 411 | 541 | 130 | 498 | 0.6978 | 0.2567 |
| SR425 | 414 | 542 | 128 | 499 | 0.6944 | 0.2506 |
| SR450 | 417 | 544 | 127 | 500 | 0.6894 | 0.2477 |
| SR475 | 427 | 545 | 118 | 501 | 0.6748 | 0.2339 |
| SR520 | 436 | 546 | 110 | 507 | 0.6725 | 0.2130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, D.; Wang, Z.; Yang, F.; Zeng, H.; Lü, C.; Wang, H.; Wang, S.; Xu, S. Study on the Applicability of Autothermic Pyrolysis In Situ Conversion Process for Low-Grade Oil Shale: A Case Study of Tongchuan, Ordos Basin, China. Energies 2024, 17, 3225. https://doi.org/10.3390/en17133225
Ren D, Wang Z, Yang F, Zeng H, Lü C, Wang H, Wang S, Xu S. Study on the Applicability of Autothermic Pyrolysis In Situ Conversion Process for Low-Grade Oil Shale: A Case Study of Tongchuan, Ordos Basin, China. Energies. 2024; 17(13):3225. https://doi.org/10.3390/en17133225
Chicago/Turabian StyleRen, Dazhong, Zhendong Wang, Fu Yang, Hao Zeng, Chenyuan Lü, Han Wang, Senhao Wang, and Shaotao Xu. 2024. "Study on the Applicability of Autothermic Pyrolysis In Situ Conversion Process for Low-Grade Oil Shale: A Case Study of Tongchuan, Ordos Basin, China" Energies 17, no. 13: 3225. https://doi.org/10.3390/en17133225
APA StyleRen, D., Wang, Z., Yang, F., Zeng, H., Lü, C., Wang, H., Wang, S., & Xu, S. (2024). Study on the Applicability of Autothermic Pyrolysis In Situ Conversion Process for Low-Grade Oil Shale: A Case Study of Tongchuan, Ordos Basin, China. Energies, 17(13), 3225. https://doi.org/10.3390/en17133225







