Biocarbon Production Using Three-Stage Pyrolysis and Its Preliminary Suitability to the Iron and Steel Industry
Abstract
1. Introduction
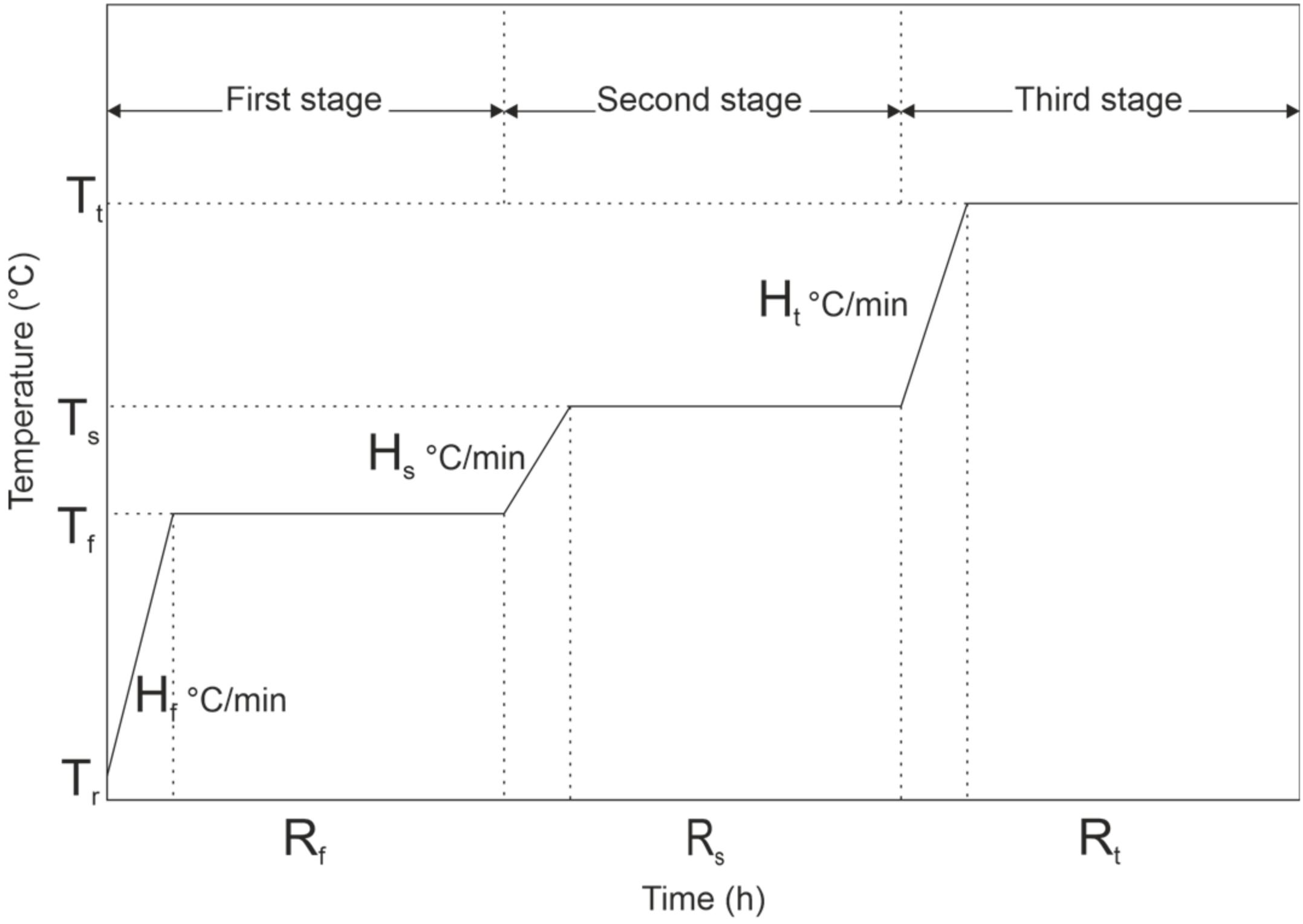
- Heating at a constant rate to the first target temperature.
- Holding stage at the target temperature.
- Heating at a constant rate to the second target temperature.
- Holding stage at the target temperature.
2. Materials and Methods
2.1. Materials
2.2. Charazterization Methodology
2.2.1. Thermogravimetric Analysis and Mass Spectrometry
2.2.2. Elemental Analysis
2.2.3. Biocarbon Yield
2.2.4. Fixed Carbon Yield
2.2.5. Bulk Density
2.2.6. Higher Heating Value
2.2.7. Energy Density
2.3. Grinding
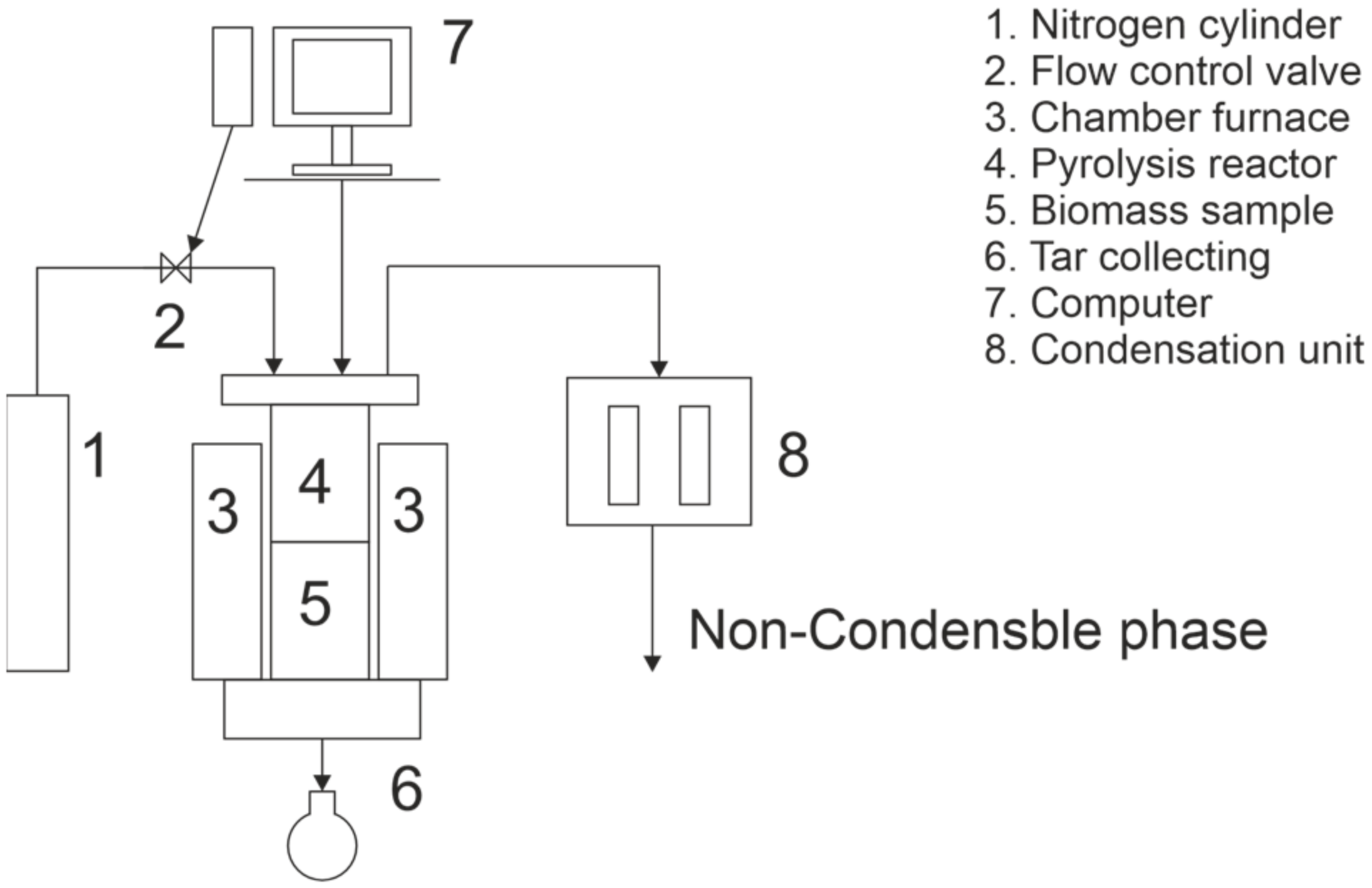
2.4. Pyrolysis Experiments
3. Results and Discussion
3.1. Proximate Analysis of Feedstock
3.2. Pyrolysis Temperatures for Segmented Pyrolysis
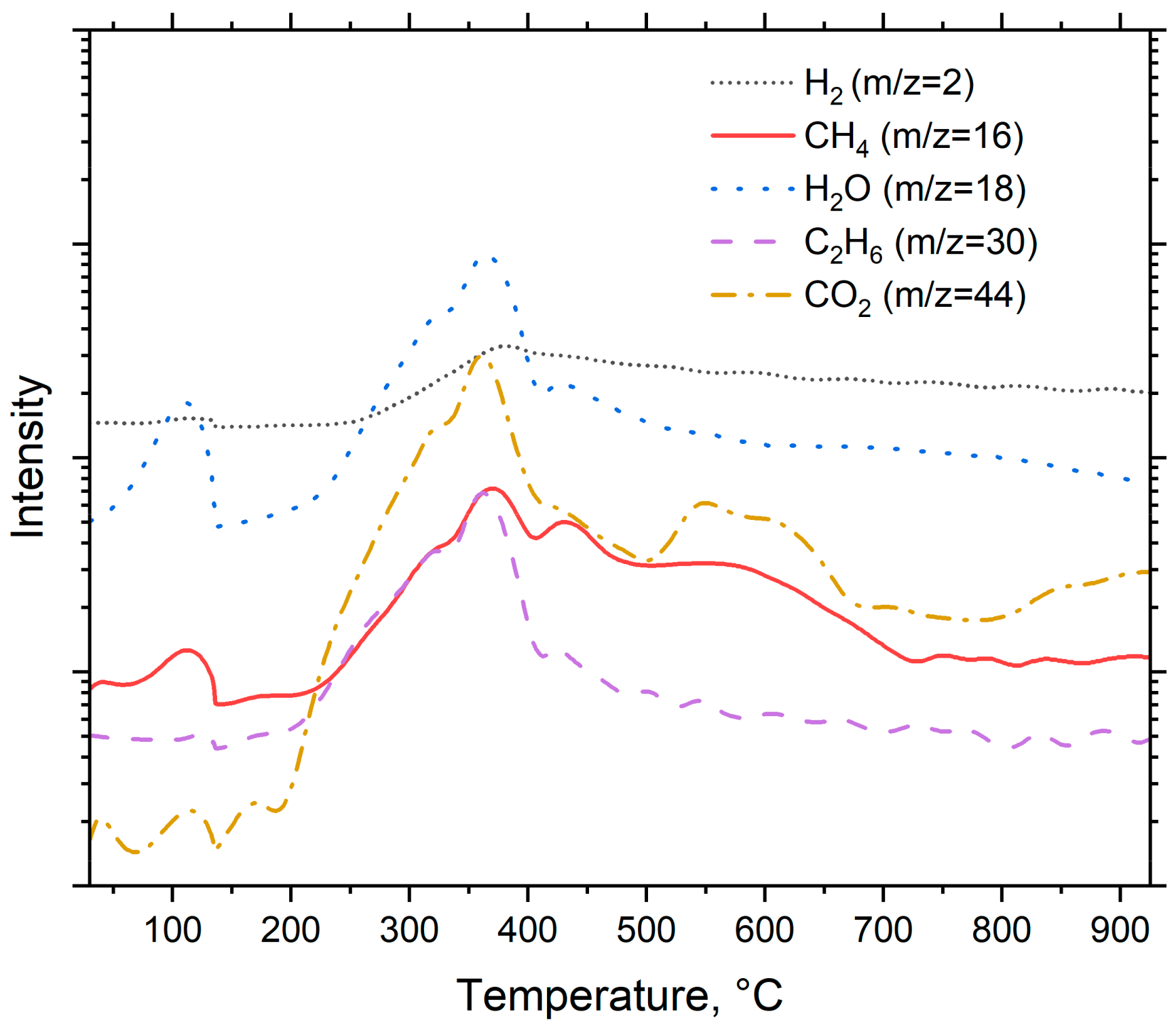
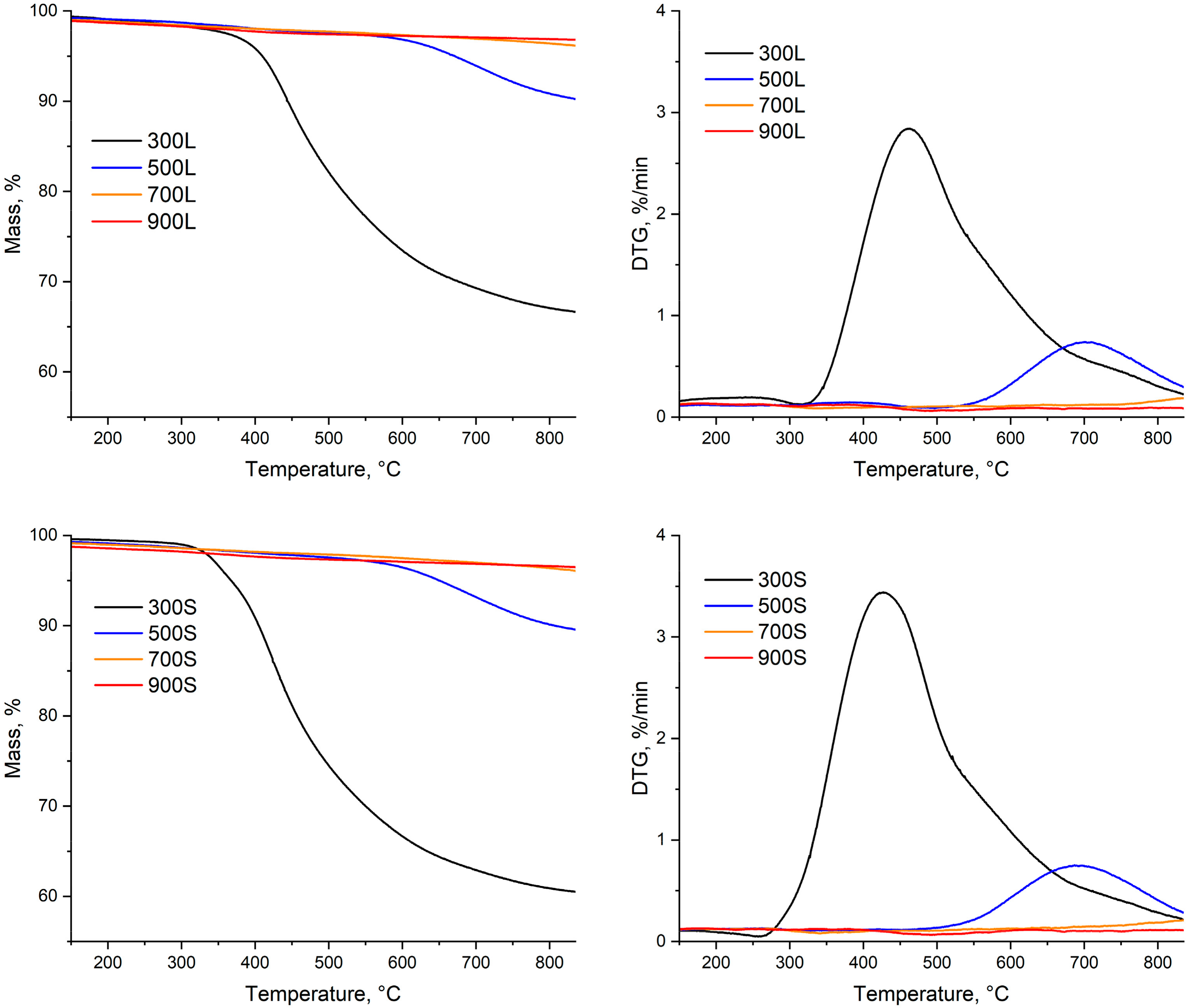
3.3. Thermal Analysis of Pyrolysis
- The temperature for the first stage should be close to feedstock’s decomposition temperature, regardless of the final pyrolysis temperature. For spruce, the peak decomposition temperature is around 360 °C.
- The temperature for the second stage should be between the temperatures of the first stage and final pyrolysis temperature. As seen in Figure 5, at pyrolysis temperatures over 400 °C, the decomposition has mainly taken place already. This further enables use of higher heating rates in the final stage, and possibly reduces the total process time compared to conventional pyrolysis.
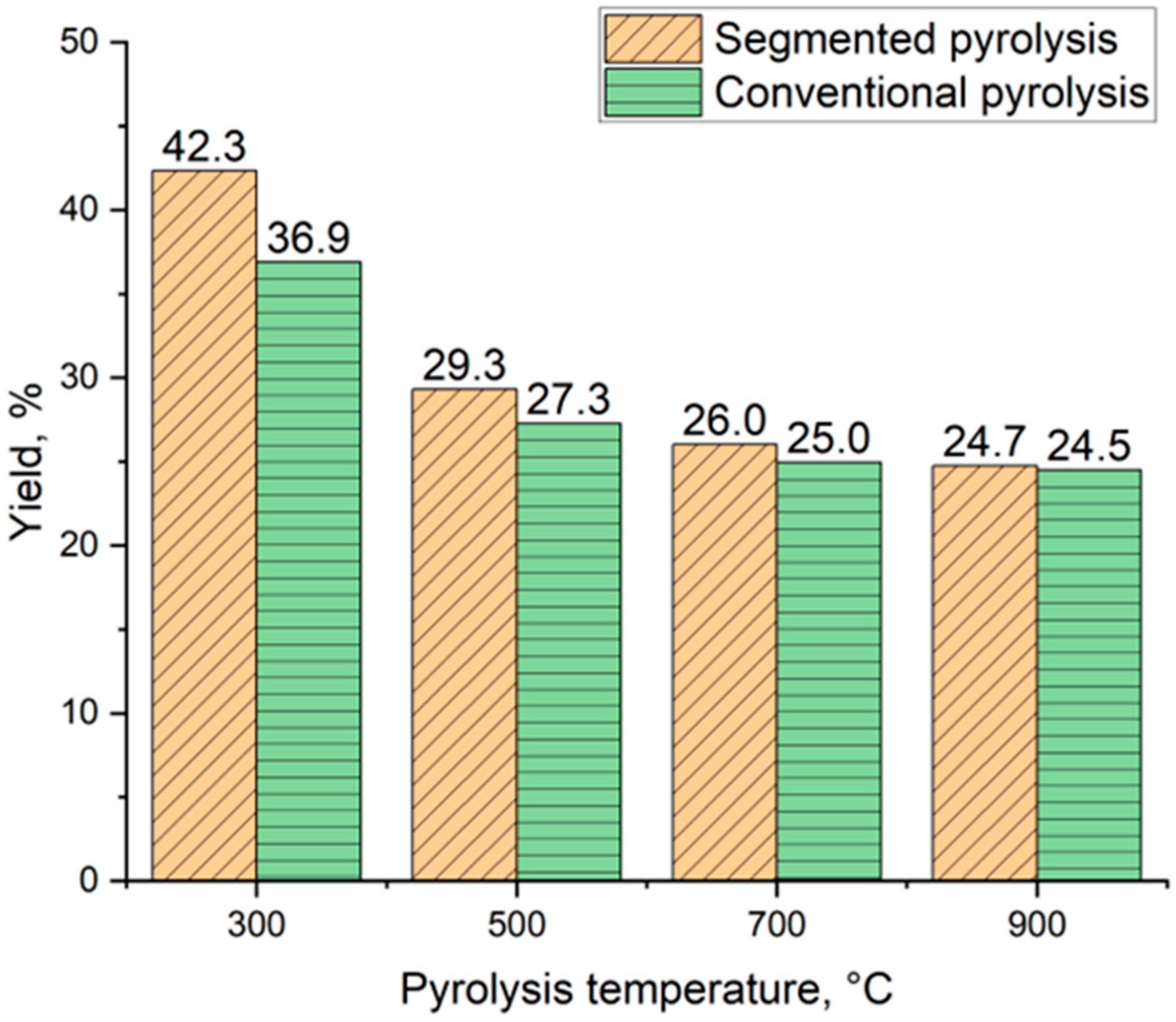
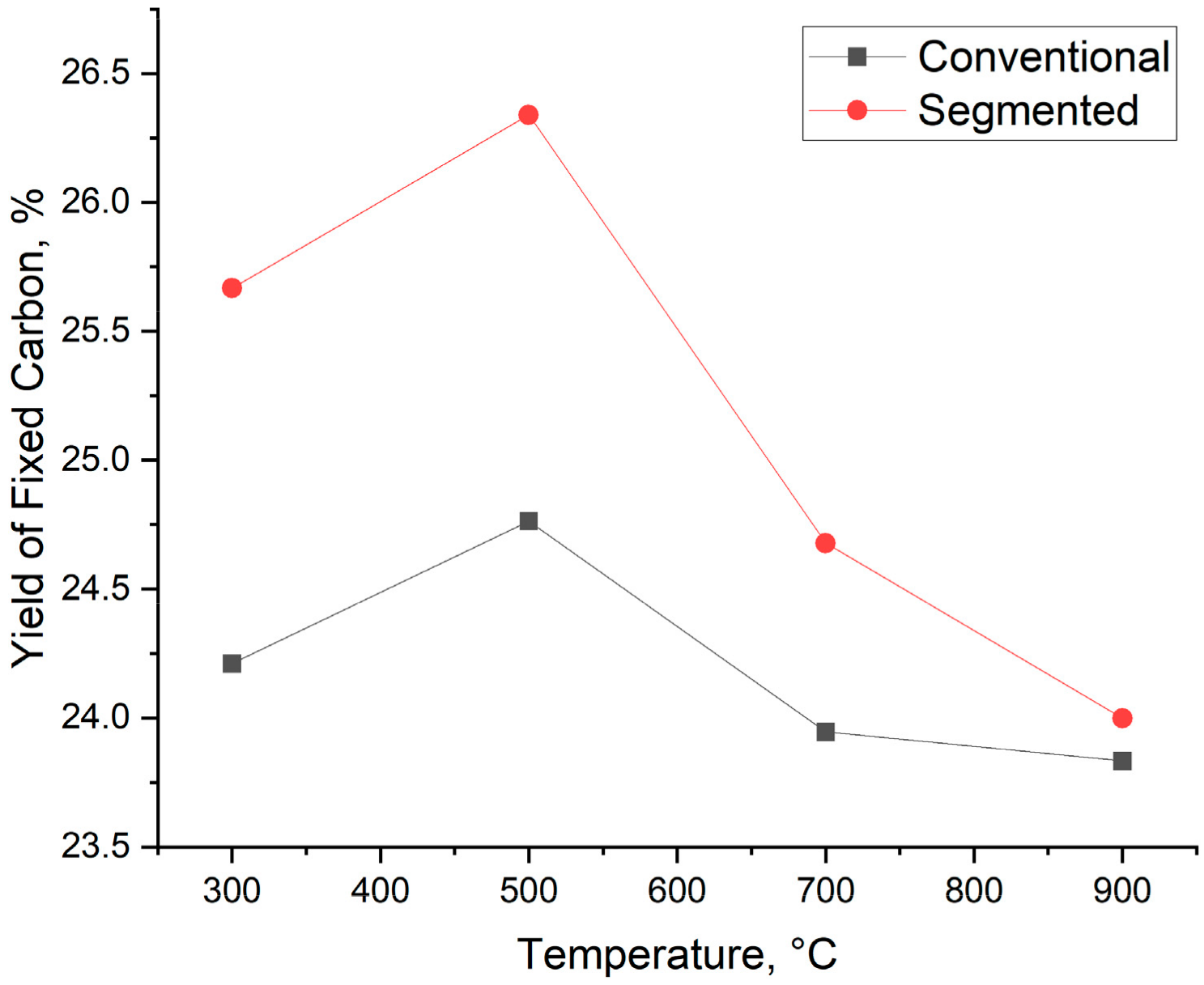
3.4. Biocarbon Yield
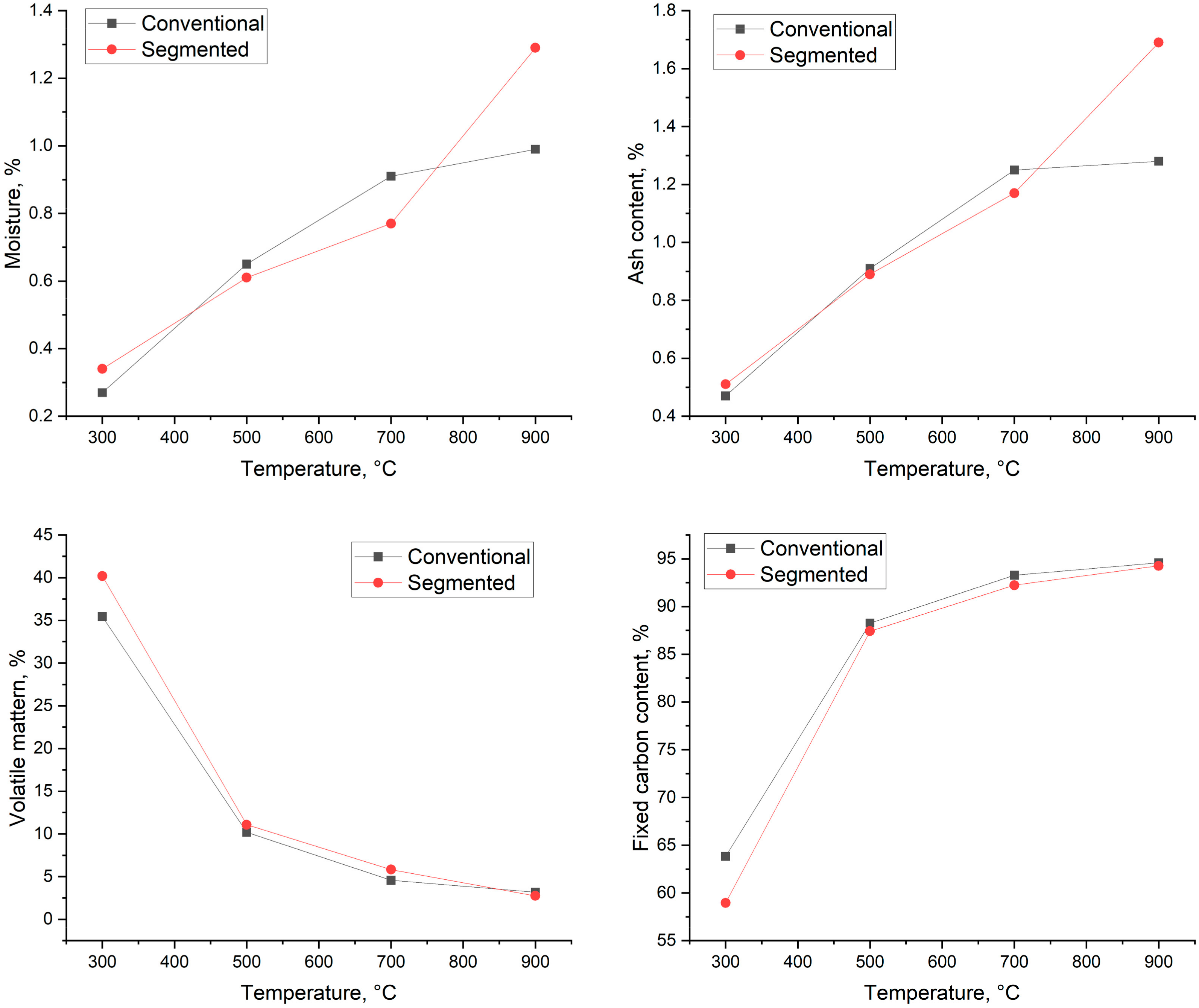
3.5. Proximate and Thermal Analysis of Biocarbon
3.5.1. Proximate Analysis of Produced Biocarbons
3.5.2. Thermal Analysis of Produced Biocarbons
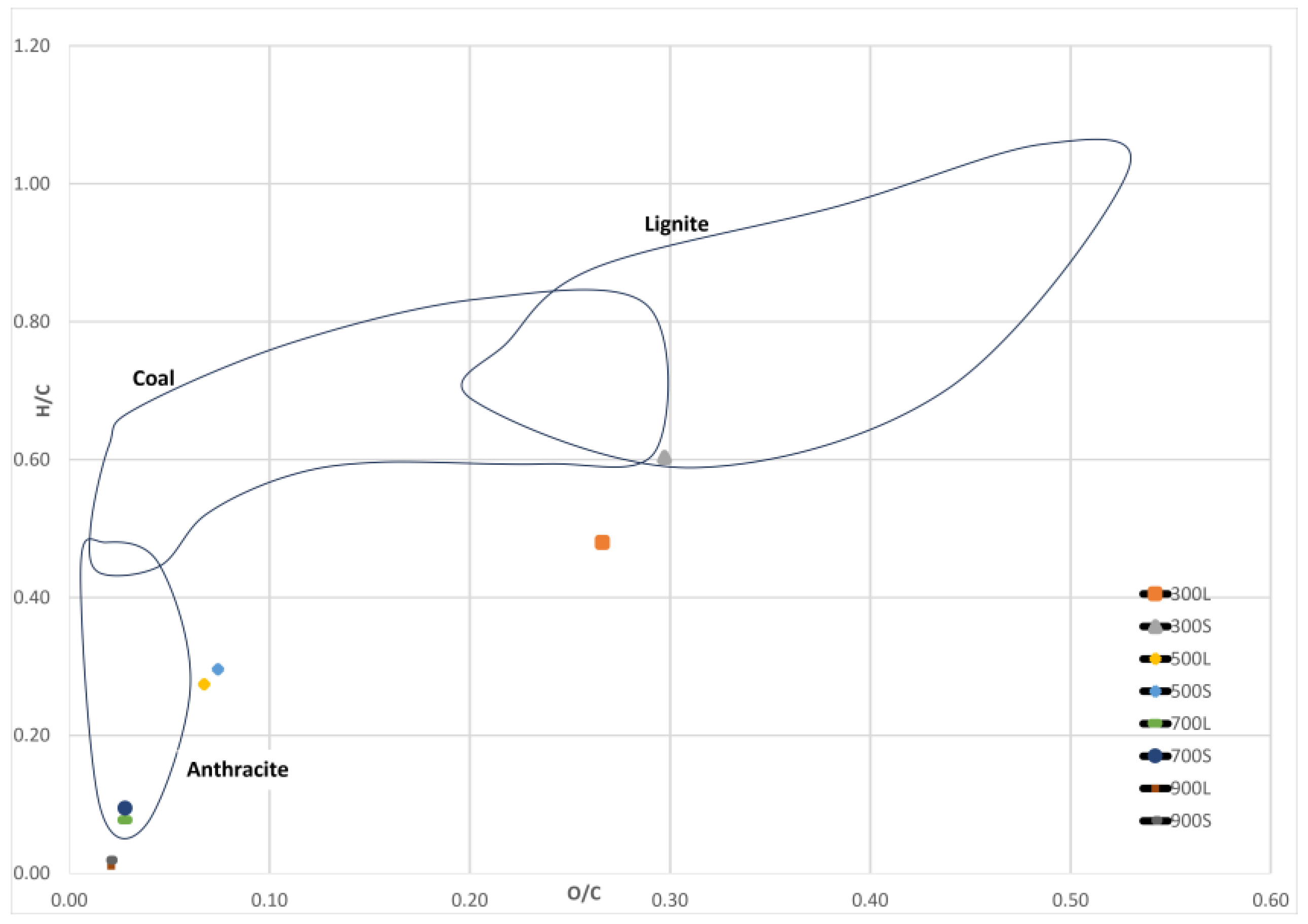
3.6. Ultimate Analysis
3.7. Density and Higher Heating Value of Biocarbons
3.7.1. Density
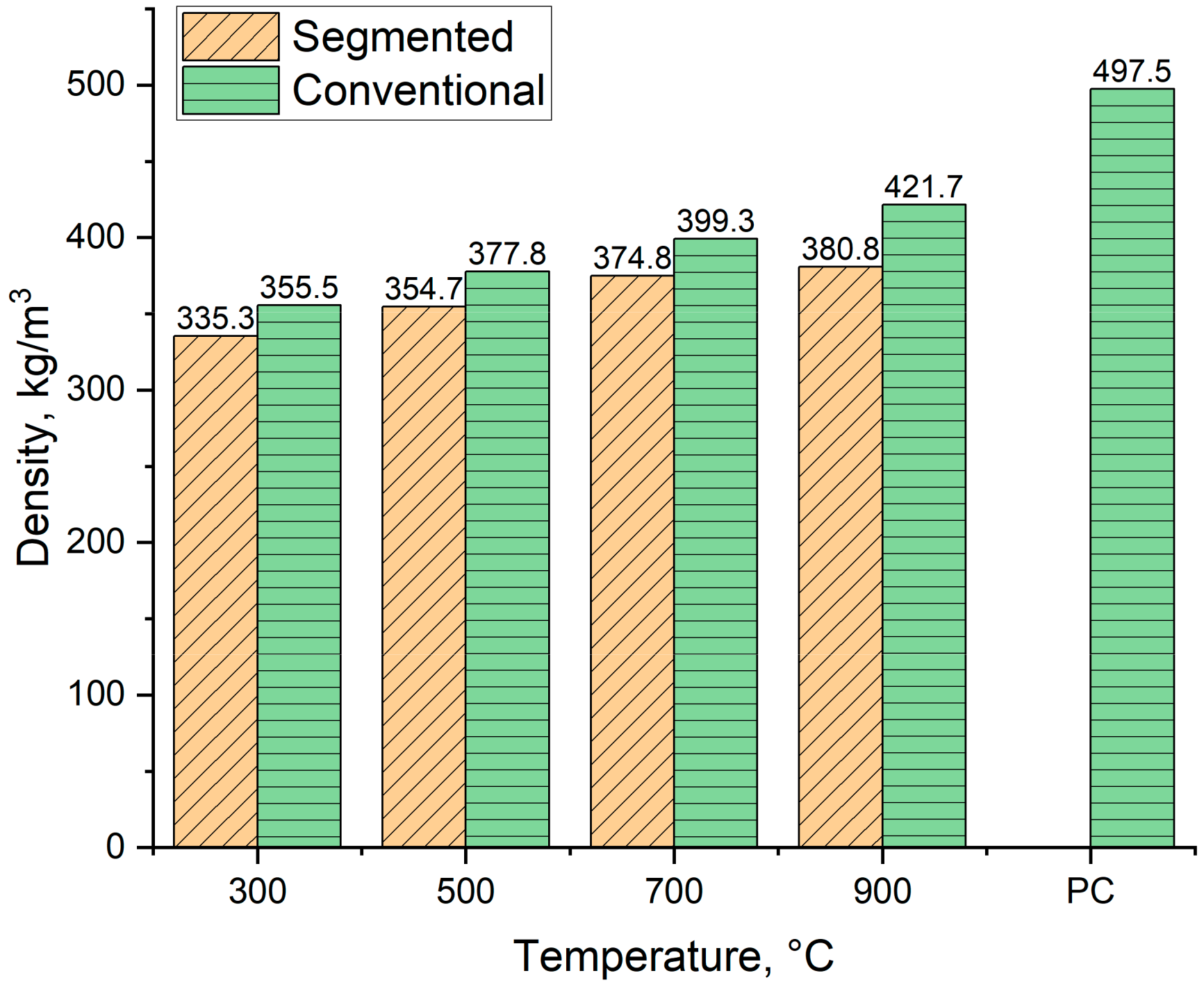
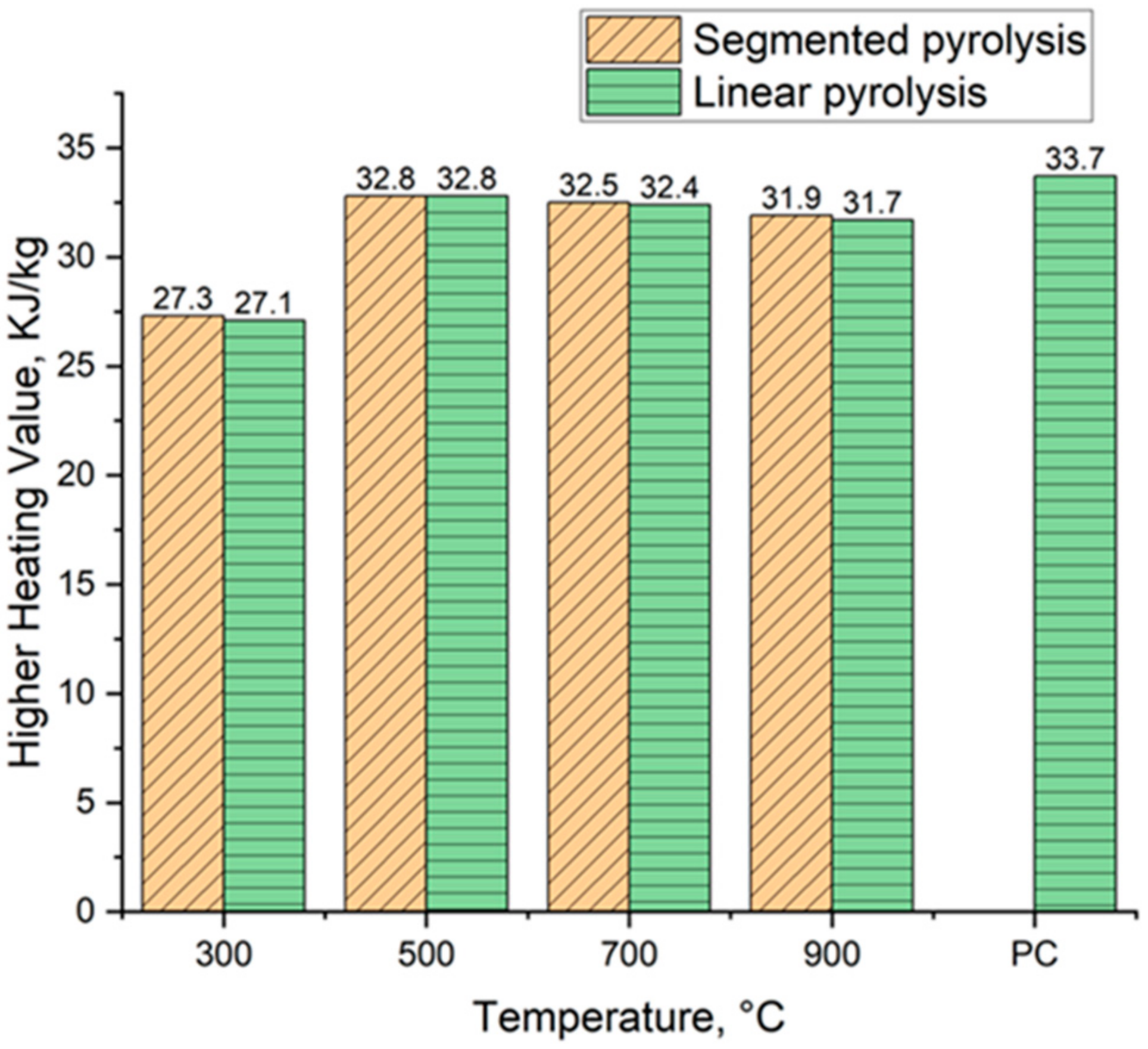
3.7.2. Higher Heating Value
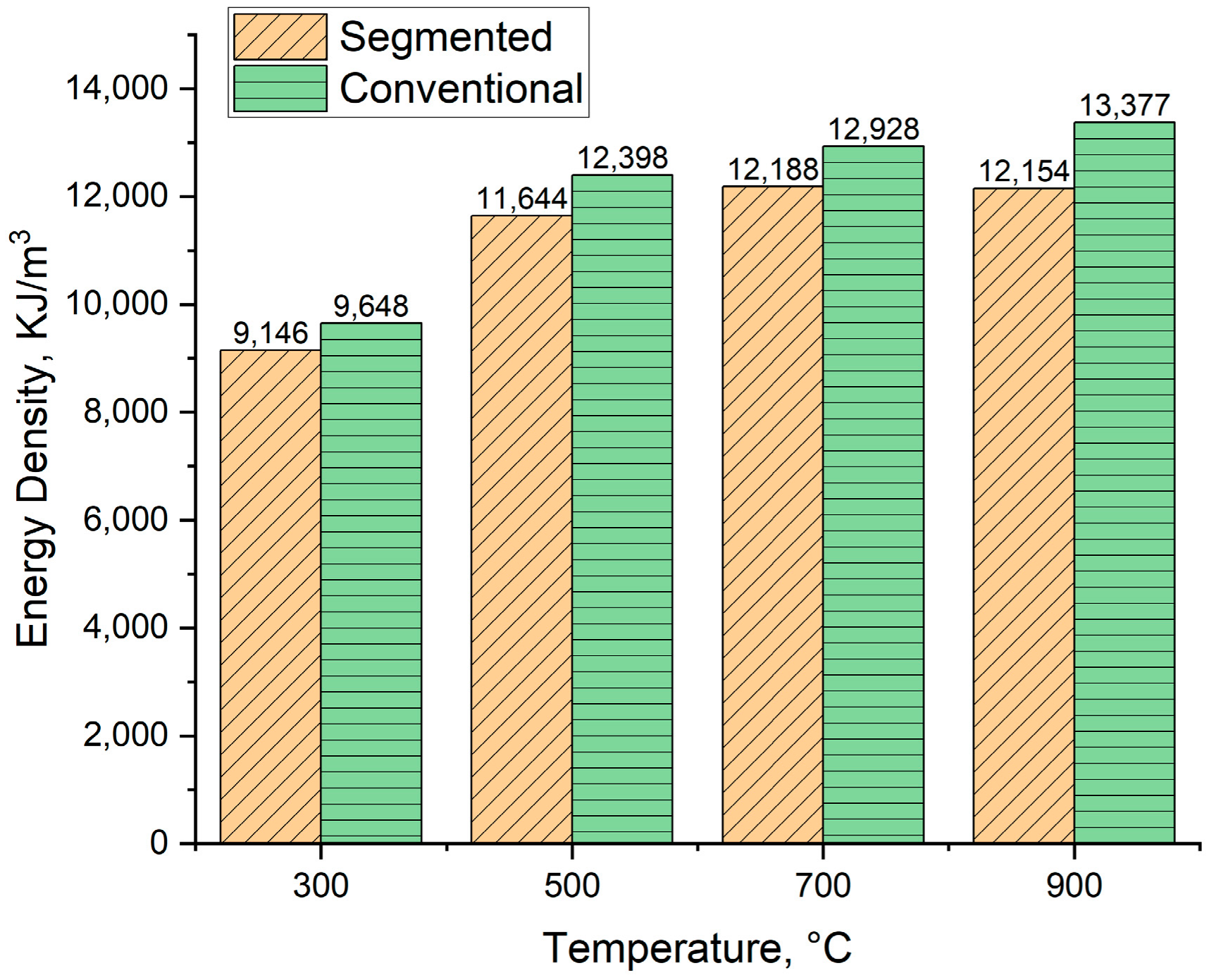
3.7.3. Energy Density
4. Conclusions
- High biocarbon yield and yield of fixed carbon. Segmented pyrolysis led to 1% higher biocarbon yield compared to conventional pyrolysis with increased thermal stability and yield of fixed carbon.
- High carbon content. Carbon content was 94.55% when segmented pyrolysis took place. This was slightly lower compared to conventional pyrolysis, but still high enough to fulfill the requirements of processes in the iron and steel industry.
- Shorter process time compared to pyrolysis at higher temperatures. Biocarbons that were produced at higher temperatures showed similar characteristics to those produced at 700 °C, but because of the shorter process time and greater energy needed to heat the feedstock in higher temperatures, pyrolysis at 700 °C is more suitable.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kieush, L.; Rieger, J.; Schenk, J.; Brondi, C.; Rovelli, D.; Echterhof, T.; Cirilli, F.; Thaler, C.; Jaeger, N.; Snaet, D.; et al. A Comprehensive Review of Secondary Carbon Bio-Carriers for Application in Metallurgical Processes: Utilization of Torrefied Biomass in Steel Production. Metals 2022, 12, 2005. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive Review of the Opportunities to Use Biomass-Based Fuels in Iron and Steelmaking Processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Digiovanni, C.; Li, D.; Ng, K.W.; Huang, X.; Digiovanni, C.; Li, D.; Ng, K.W.; Huang, X. Ranking of Injection Biochar for Slag Foaming Applications in Steelmaking. Metals 2023, 13, 1003. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. The Potential of Using Biomass-Based Reducing Agents in the Blast Furnace: A Review of Thermochemical Conversion Technologies and Assessments Related to Sustainability. Renew. Sustain. Energy Rev. 2013, 25, 511–528. [Google Scholar] [CrossRef]
- Sriram, N.; Shahidehpour, M. Renewable Biomass Energy. In Proceedings of the 2005 IEEE Power Engineering Society General Meeting, San Francisco, CA, USA, 16 June 2005; Volume 1, pp. 612–617. [Google Scholar] [CrossRef]
- Echterhof, T. Review on the Use of Alternative Carbon Sources in EAF Steelmaking. Metals 2021, 11, 222. [Google Scholar] [CrossRef]
- Khanna, R.; Li, K.; Wang, Z.; Sun, M.; Zhang, J.; Mukherjee, P.S. Biochars in Iron and Steel Industries. In Char and Carbon Materials Derived from Biomass: Production, Characterization and Applications; Jeguirim, M., Limousy, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 429–446. ISBN 9780128148945. [Google Scholar]
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass Applications in Iron and Steel Industry: An Overview of Challenges and Opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and Characterization of Slow Pyrolysis Biochar: Influence of Feedstock Type and Pyrolysis Conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of Slow and Fast Pyrolysis for Converting Biomass into Fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Nanda, S.; Mohammad, J.; Reddy, S.N.; Kozinski, J.A.; Dalai, A.K. Pathways of Lignocellulosic Biomass Conversion to Renewable Fuels. Biomass Convers. Biorefin. 2014, 5, 157–191. [Google Scholar] [CrossRef]
- Babinszki, B.; Sebestyén, Z.; Jakab, E.; Kőhalmi, L.; Bozi, J.; Várhegyi, G.; Wang, L.; Skreiberg, Ø; Czégény, Z. Effect of Slow Pyrolysis Conditions on Biocarbon Yield and Properties: Characterization of the Volatiles. Bioresour. Technol. 2021, 338, 125567. [Google Scholar] [CrossRef]
- Patra, B.R.; Nanda, S.; Dalai, A.K.; Meda, V. Slow Pyrolysis of Agro-Food Wastes and Physicochemical Characterization of Biofuel Products. Chemosphere 2021, 285, 131431. [Google Scholar] [CrossRef]
- Qing, M.; Long, Y.; Liu, L.; Yi, Y.; Li, W.; He, R.; Yin, Y.; Tian, H.; He, J.; Cheng, S.; et al. Pyrolysis of the Food Waste Collected from Catering and Households under Different Temperatures: Assessing the Evolution of Char Structure and Bio-Oil Composition. J. Anal. Appl. Pyrolysis 2022, 164, 105543. [Google Scholar] [CrossRef]
- Cheung, K.Y.; Lee, K.L.; Lam, K.L.; Chan, T.Y.; Lee, C.W.; Hui, C.W. Operation Strategy for Multi-Stage Pyrolysis. J. Anal. Appl. Pyrolysis 2011, 91, 165–182. [Google Scholar] [CrossRef]
- Lam, K.-L.; Lee, C.-W.; Hui, C.-W. Multi-Stage Waste Tyre Pyrolysis: An Optimisation Approach. Chem. Eng. Trans. 2010, 21, 853. [Google Scholar] [CrossRef]
- Nachenius, R.W.; Ronsse, F.; Venderbosch, R.H.; Prins, W. Biomass Pyrolysis. In Advances in Chemical Engineering; Marin, G.B., West, D.H., Li, J., Narasimhan, S., Eds.; Academic Press Inc.: San Diego, CA, USA, 2013; Volume 42, pp. 75–139. ISBN 9780123865052. [Google Scholar]
- Oyedun, A.O.; Lam, K.-L.; Gebreegziabher, T.; Lee, H.K.; Hui, C.-W. Optimisation of Operating Parameters in Multi-Stage Pyrolysis. Chem. Eng. Trans. 2012, 29, 655–660. [Google Scholar] [CrossRef]
- Cai, W.; Liu, Q.; Shen, D.; Wang, J. Py-GC/MS Analysis on Product Distribution of Two-Staged Biomass Pyrolysis. J. Anal. Appl. Pyrolysis 2019, 138, 62–69. [Google Scholar] [CrossRef]
- Jian, J.; Lu, Z.; Yao, S.; Li, Y.; Liu, Z.; Lang, B.; Chen, Z. Effects of Thermal Conditions on Char Yield and Char Reactivity of Woody Biomass in Stepwise Pyrolysis. J. Anal. Appl. Pyrolysis 2019, 138, 211–217. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Lam, K.L.; Hui, C.W. Charcoal Production via Multistage Pyrolysis. Chin. J. Chem. Eng. 2012, 20, 455–460. [Google Scholar] [CrossRef]
- Han, K.; Wang, Q.; Zhao, J.; Luo, K.H.; Li, H.; Chen, Y.; Lu, C. Combustion Pattern, Characteristics, and Kinetics of Biomass and Chars from Segmented Heating Carbonization. Asia-Pac. J. Chem. Eng. 2016, 11, 812–822. [Google Scholar] [CrossRef]
- Qi, J.; Zhao, J.; Xu, Y.; Wang, Y.; Han, K. Segmented Heating Carbonization of Biomass: Yields, Property and Estimation of Heating Value of Chars. Energy 2018, 144, 301–311. [Google Scholar] [CrossRef]
- Grigiante, M.; Ischia, M.; Baratieri, M.; Maschio, R.D.; Ragazzi, M. Pyrolysis Analysis and Solid Residue Stabilization of Polymers, Waste Tyres, Spruce Sawdust and Sewage Sludge. Waste Biomass Valorization 2010, 1, 381–393. [Google Scholar] [CrossRef]
- Smołka-Danielowska, D.; Jabłońska, M. Chemical and Mineral Composition of Ashes from Wood Biomass Combustion in Domestic Wood-Fired Furnaces. Int. J. Environ. Sci. Technol. 2022, 19, 5359–5372. [Google Scholar] [CrossRef]
- Cassel, B.; Menard, K. Proximate Analysis of Coal and Coke Using the STA 8000 Simultaneous Thermal Analyzer; PerkinElmer, Inc.: Shelton, CT, USA, 2012; Available online: https://resources.perkinelmer.com/lab-solutions/resources/docs/app_proximate_analysis_coal_coke.pdf (accessed on 12 October 2022).
- Ingemarsson, Å.; Nilsson, U.; Nilsson, M.; Pedersen, J.R.; Olsson, J.O. Slow Pyrolysis of Spruce and Pine Samples Studied with GC/MS and GC/FTIR/FID. Chemosphere 1998, 36, 2879–2889. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Z.; Jian, J.; Bao, Z.; Cai, J.; Yao, S. Effect of Torrefaction on Yield, Reactivity and Physicochemical Properties of Pyrolyzed Char from Three Major Biomass Constituents. J. Anal. Appl. Pyrolysis 2023, 173, 106104. [Google Scholar] [CrossRef]
- Elyounssi, K.; Blin, J.; Halim, M. High-Yield Charcoal Production by Two-Step Pyrolysis. J. Anal. Appl. Pyrolysis 2010, 87, 138–143. [Google Scholar] [CrossRef]
- Abdullah, E.C.; Geldart, D. The Use of Bulk Density Measurements as Flowability Indicators. Powder Technol. 1999, 102, 151–165. [Google Scholar] [CrossRef]
- Chevanan, N.; Womac, A.R.; Bitra, V.S.P.; Igathinathane, C.; Yang, Y.T.; Miu, P.I.; Sokhansanj, S. Bulk Density and Compaction Behavior of Knife Mill Chopped Switchgrass, Wheat Straw, and Corn Stover. Bioresour. Technol. 2010, 101, 207–214. [Google Scholar] [CrossRef]
- Theegala, C.S.; Midgett, J.S. Hydrothermal Liquefaction of Separated Dairy Manure for Production of Bio-Oils with Simultaneous Waste Treatment. Bioresour. Technol. 2012, 107, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Suresh Babu, K.K.B.; Nataraj, M.; Tayappa, M.; Vyas, Y.; Mishra, R.K.; Acharya, B. Production of Biochar from Waste Biomass Using Slow Pyrolysis: Studies of the Effect of Pyrolysis Temperature and Holding Time on Biochar Yield and Properties. Mater. Sci. Energy Technol. 2024, 7, 318–334. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Waters, C.L.; Janupala, R.R.; Mallinson, R.G.; Lobban, L.L. Staged Thermal Fractionation for Segregation of Lignin and Cellulose Pyrolysis Products: An Experimental Study of Residence Time and Temperature Effects. J. Anal. Appl. Pyrolysis 2017, 126, 380–389. [Google Scholar] [CrossRef]
- Zou, X.; Debiagi, P.; Amjed, M.A.; Zhai, M.; Faravelli, T. Impact of High-Temperature Biomass Pyrolysis on Biochar Formation and Composition. J. Anal. Appl. Pyrolysis 2024, 179, 106463. [Google Scholar] [CrossRef]
- Iurchenkova, A.; Kobets, A.; Ahaliabadeh, Z.; Kosir, J.; Laakso, E.; Virtanen, T.; Siipola, V.; Lahtinen, J.; Kallio, T. The Effect of the Pyrolysis Temperature and Biomass Type on the Biocarbons Characteristics. ChemSusChem 2024, 17, e202301005. [Google Scholar] [CrossRef] [PubMed]
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of Biomass in Integrated Steelmaking—Status Quo, Future Needs and Comparison to Other Low-CO2 Steel Production Technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef]
- Varsally, Z.B.; Tripathi, N.; Weldekidan, H.; Rodriguez-Uribe, A.; Das, O.; Mohanty, A.K.; Misra, M. A Sustainable Approach for Developing Biocarbon from Lignin and Its Utilization in Recycled Ocean Nylon Based Biocomposites. Compos. Part C Open Access 2023, 12, 100376. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass Pyrolysis—A Review of Modelling, Process Parameters and Catalytic Studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Wang, L.; Buvarp, F.; Skreiberg, Ø.; Khalil, R. Impact of Storage Time and Conditions on Properties of Biocarbon. Chem. Eng. Trans. 2018, 65, 715–720. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Bachmann, R.T.; Rafiq, M.T.; Shang, Z.; Joseph, S.; Long, R.L. Influence of Pyrolysis Temperature on Physico-Chemical Properties of Corn Stover (Zea mays L.) Biochar and Feasibility for Carbon Capture and Energy Balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Tripathi, N.; Rodriguez Uribe, A.; Weldekidan, H.; Misra, M.; Mohanty, A.K. Upcycling of Waste Jute Biomass to Advanced Biocarbon Materials: The Effect of Pyrolysis Temperature on Their Physicochemical and Electrical Properties. Mater. Adv. 2022, 3, 9071–9082. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A Review on Pyrolysis of Biomass Constituents: Mechanisms and composition of the Products Obtained from the Conversion of Cellulose, Hemicelluloses and Lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Bamboriya, O.P.; Thakur, L.S.; Parmar, H.; Varma, A.K.; Hinge, V.K. A Review on Mechanism and Factors Affecting Pyrolysis of Biomass. Int. J. Res. Advent Technol. 2019, 7, 1014–1024. [Google Scholar]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an Exceptional Bioresource for Energy, Agronomy, Carbon Sequestration, Activated Carbon and Specialty Materials. Waste Biomass Valorization 2015, 7, 201–235. [Google Scholar] [CrossRef]
- Kurose, R.; Ikeda, M.; Makino, H.; Kimoto, M.; Miyazaki, T. Pulverized Coal Combustion Characteristics of High-Fuel-Ratio Coals. Fuel 2004, 83, 1777–1785. [Google Scholar] [CrossRef]
- Miller, B.G.; Tillman, D.A. Coal Characteristics. In Combustion Engineering Issues for Solid Fuel Systems; Miller, B., Tillman, D., Eds.; Academic Press: Amsterdam, The Netherlands, 2008; pp. 33–81. ISBN 9780123736116. [Google Scholar]
- Poudel, J.; Karki, S.; Oh, S.C. Valorization of Waste Wood as a Solid Fuel by Torrefaction. Energies 2018, 11, 1641. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New Approaches to Measuring Biochar Density and Porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Farrokh, N.T.; Suopajärvi, H.; Mattila, O.; Sulasalmi, P.; Fabritius, T. Characteristics of Wood-Based Biochars for Pulverized Coal Injection. Fuel 2020, 265, 117017. [Google Scholar] [CrossRef]
- Farrokh, N.T.; Suopajärvi, H.; Mattila, O.; Umeki, K.; Phounglamcheik, A.; Romar, H.; Sulasalmi, P.; Fabritius, T. Slow Pyrolysis of By-Product Lignin from Wood-Based Ethanol Production—A Detailed Analysis of the Produced Chars. Energy 2018, 164, 112–123. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, G.; Xu, X.; Shen, D.; Jin, B. Study on Carbonization of Lignin by TG-FTIR and High-Temperature Carbonization Reactor. Fuel Process. Technol. 2013, 106, 41–47. [Google Scholar] [CrossRef]
- Yao, M.; Bi, X.; Wang, Z.; Yu, P.; Dufresne, A.; Jiang, C. Recent Advances in Lignin-Based Carbon Materials and Their Applications: A Review. Int. J. Biol. Macromol. 2022, 223, 980–1014. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Guo, X.; Liu, Y.; Gong, X. Effect of Particle Size on Flow Mode and Flow Characteristics of Pulverized Coal. KONA Powder Part. J. 2015, 32, 143–153. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Fabritius, T. Towards More Sustainable Ironmaking—An Analysis of Energy Wood Availability in Finland and the Economics of Charcoal Production. Sustainability 2013, 5, 1188–1207. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Wang, C.; Mellin, P.; Lövgren, J.; Nilsson, L.; Yang, W.; Salman, H.; Hultgren, A.; Larsson, M. Biomass as Blast Furnace Injectant—Considering Availability, Pretreatment and Deployment in the Swedish Steel Industry. Energy Convers. Manag. 2015, 102, 217–226. [Google Scholar] [CrossRef]
- Babich, A.; Senk, D.; Fernandez, M. Charcoal Behaviour by Its Injection into the Modern Blast Furnace. ISIJ Int. 2010, 50, 81–88. [Google Scholar] [CrossRef]


| Spruce Cutter Flakes | ||
|---|---|---|
| Parameter | Unit | Value |
| Moisture | (m%) | 1.70 |
| Ash | (m%) | 2.78 |
| Volatiles | (m%) | 79.07 |
| Fixed carbon | (m%) | 16.45 |
| The First Stage | The Second Stage | The Third Stage | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Tf (°C) | Hf (°C/min) | Rt (min) | Ts (°C) | Hs (°C/min) | Rs (min) | Tt (°C) | Ht (°C/min) | Rt (min) |
| 300S | 150 | 7.0 | 160 | 230 | 4.8 | 160 | 300 | 3.2 | 160 |
| 500S | 240 | 6.7 | 160 | 330 | 2.8 | 160 | 500 | 5.2 | 160 |
| 700S | 300 | 6.1 | 160 | 360 | 1.3 | 160 | 700 | 7.4 | 160 |
| 900S | 360 | 5.9 | 160 | 510 | 2.6 | 160 | 900 | 6.8 | 160 |
| Ultimate Analyses (m%) | |||||||
|---|---|---|---|---|---|---|---|
| Sample | O | N | C | H | S | H/C | O/C |
| 300L | 19.91 | 0 | 74.79 | 3.59 | 0.25 | 0.48 | 0.27 |
| 300S | 21.61 | 0 | 72.69 | 4.38 | 0 | 0.60 | 0.30 |
| 500L | 6.07 | 0 | 90.08 | 2.47 | 0 | 0.27 | 0.07 |
| 500S | 6.65 | 0 | 89.61 | 2.65 | 0 | 0.30 | 0.07 |
| 700L | 2.65 | 0 | 94.72 | 0.73 | 0 | 0.08 | 0.03 |
| 700S | 2.65 | 0 | 94.55 | 0.89 | 0 | 0.09 | 0.03 |
| 900L | 2.00 | 0 | 95.25 | 0.10 | 0 | 0.01 | 0.02 |
| 900S | 1.95 | 0 | 95.49 | 0.18 | 0 | 0.02 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. Biocarbon Production Using Three-Stage Pyrolysis and Its Preliminary Suitability to the Iron and Steel Industry. Energies 2024, 17, 3131. https://doi.org/10.3390/en17133131
Pahnila M, Koskela A, Sulasalmi P, Fabritius T. Biocarbon Production Using Three-Stage Pyrolysis and Its Preliminary Suitability to the Iron and Steel Industry. Energies. 2024; 17(13):3131. https://doi.org/10.3390/en17133131
Chicago/Turabian StylePahnila, Mika, Aki Koskela, Petri Sulasalmi, and Timo Fabritius. 2024. "Biocarbon Production Using Three-Stage Pyrolysis and Its Preliminary Suitability to the Iron and Steel Industry" Energies 17, no. 13: 3131. https://doi.org/10.3390/en17133131
APA StylePahnila, M., Koskela, A., Sulasalmi, P., & Fabritius, T. (2024). Biocarbon Production Using Three-Stage Pyrolysis and Its Preliminary Suitability to the Iron and Steel Industry. Energies, 17(13), 3131. https://doi.org/10.3390/en17133131