Large-Scale Experimental Investigation of Hydrate-Based Carbon Dioxide Sequestration
Abstract
1. Introduction
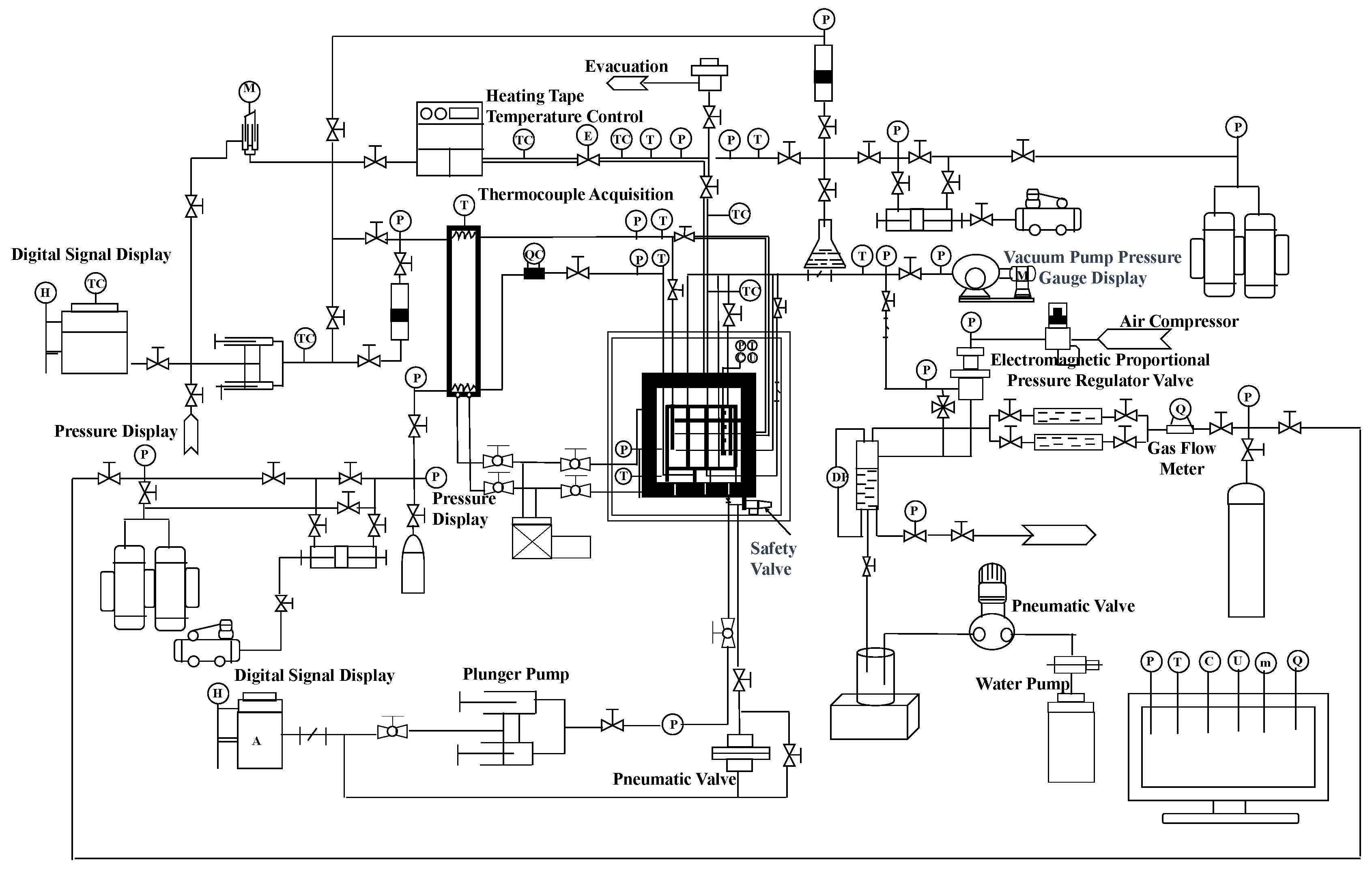
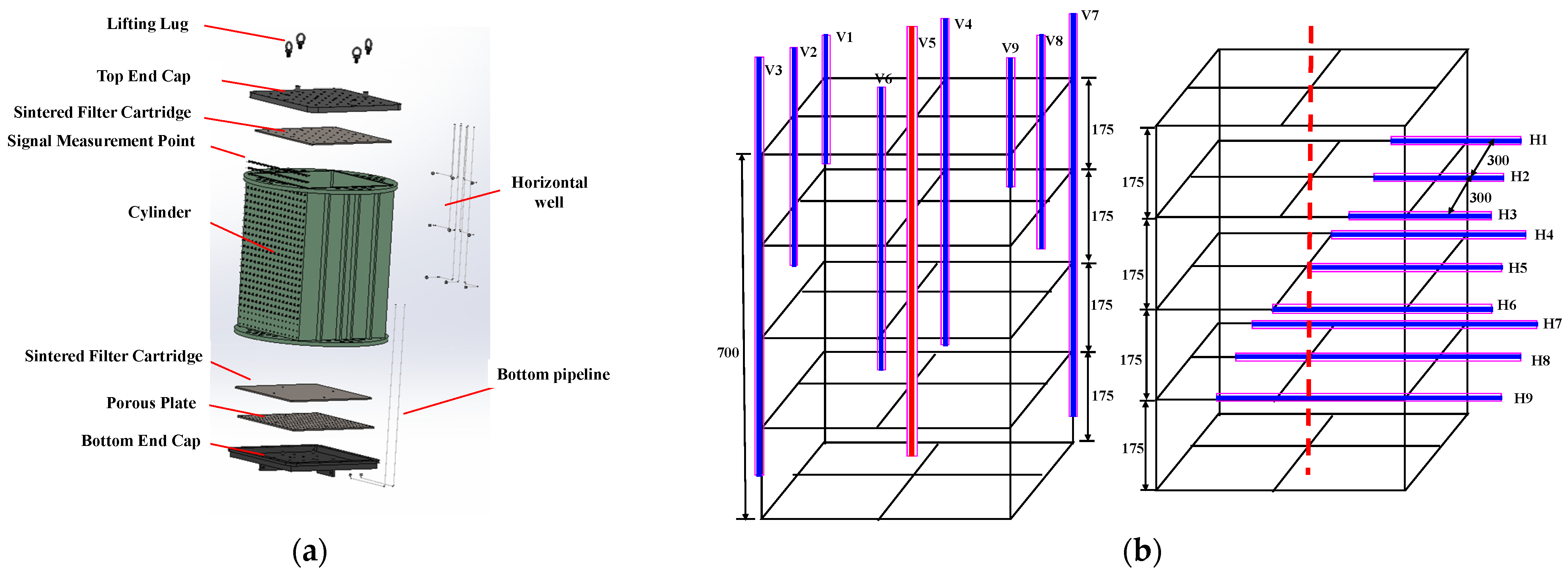
2. Experimental Setup and Procedure
2.1. Experimental Setup
2.2. Experimental Procedure
- (1)
- A certain amount of sand with a particle size of 120 mesh was first prepared and mixed with water for sand washing. Afterward, the sand was placed into the incubator for drying and was selected a second time to ensure the consistency of the sand particle mesh.
- (2)
- The sieved sand and water were mixed again in the stainless steel to make all the sand wet, and the water between the sand particles was discharged. The sand with the remaining water was then evenly stirred to ensure that the water on the sand surface was as uniform as possible.
- (3)
- The wet sand was filled into the internal model and compacted evenly layer by layer. After sealing, the internal model was turned over and kept standing to ensure the uniform distribution of water between the sand particles. The internal model was then placed into the large reactor vessel with a volume of 1695 L.
- (4)
- Nitrogen was then injected into the internal model from the bottom until the pressure reached 13.5 MPa, which was maintained for 7 h to test the seal of the system.
- (5)
- After the seal check, water was injected into the internal model from the bottom to displace the nitrogen. The top valve was then closed, and water injection continued until the pressure in the internal model reached 11 MPa.
- (6)
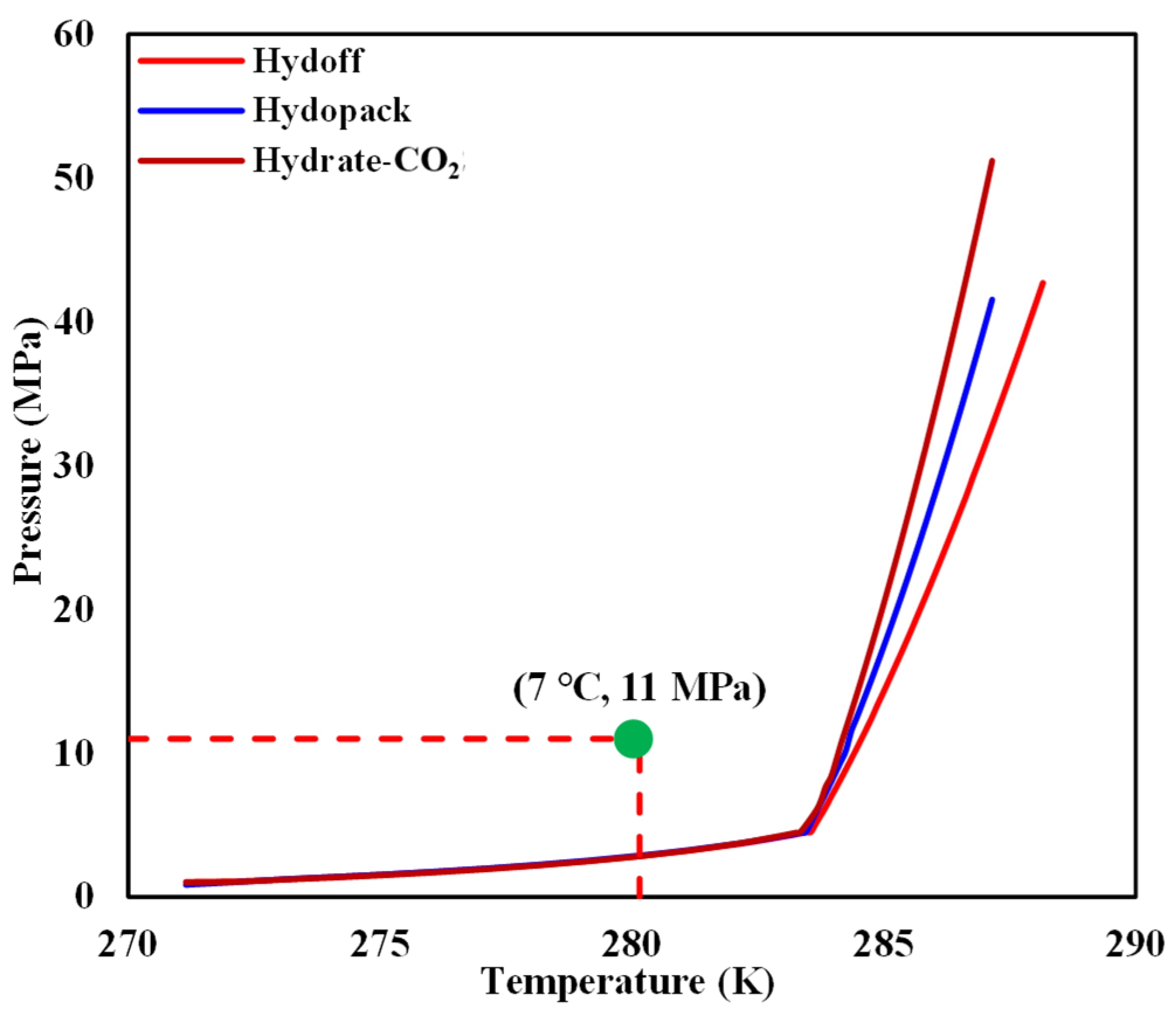
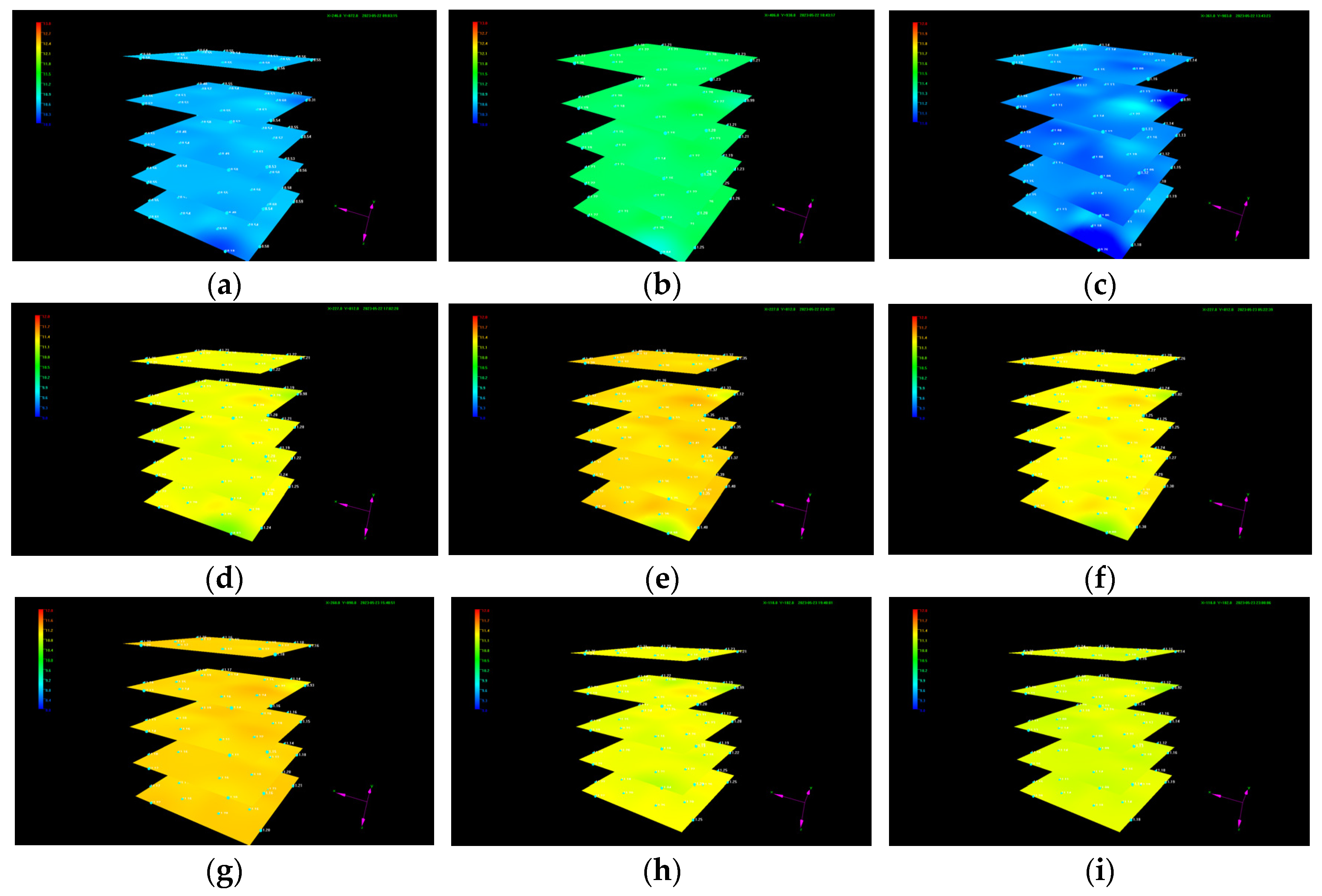
- The chiller and cold-water jacket were then opened to adjust the temperature of the internal model to 7 °C. It can be seen from Figure 4 that the reshaped sediment satisfied the condition for hydrate-based CO2 sequestration.
- (7)
- Afterward, liquid CO2 was injected into the internal model at a constant flux of 10 mL/min through an intermediate vertical well labeled as V5. To avoid an exceedingly high pressure in the inlet model, an outlet was set at the bottom with a back pressure of 12 MPa.
- (8)
- The water and CO2 produced were recorded once the outlet was opened due to a pressure difference. When one tank of liquid CO2 injection was completed, another tank was opened until a large amount of CO2 was released from the outlet.
- (9)
- The cumulative sequestered CO2 and proportion of CO2 in the hydrate and the fluid were calculated.
3. Results and Analysis
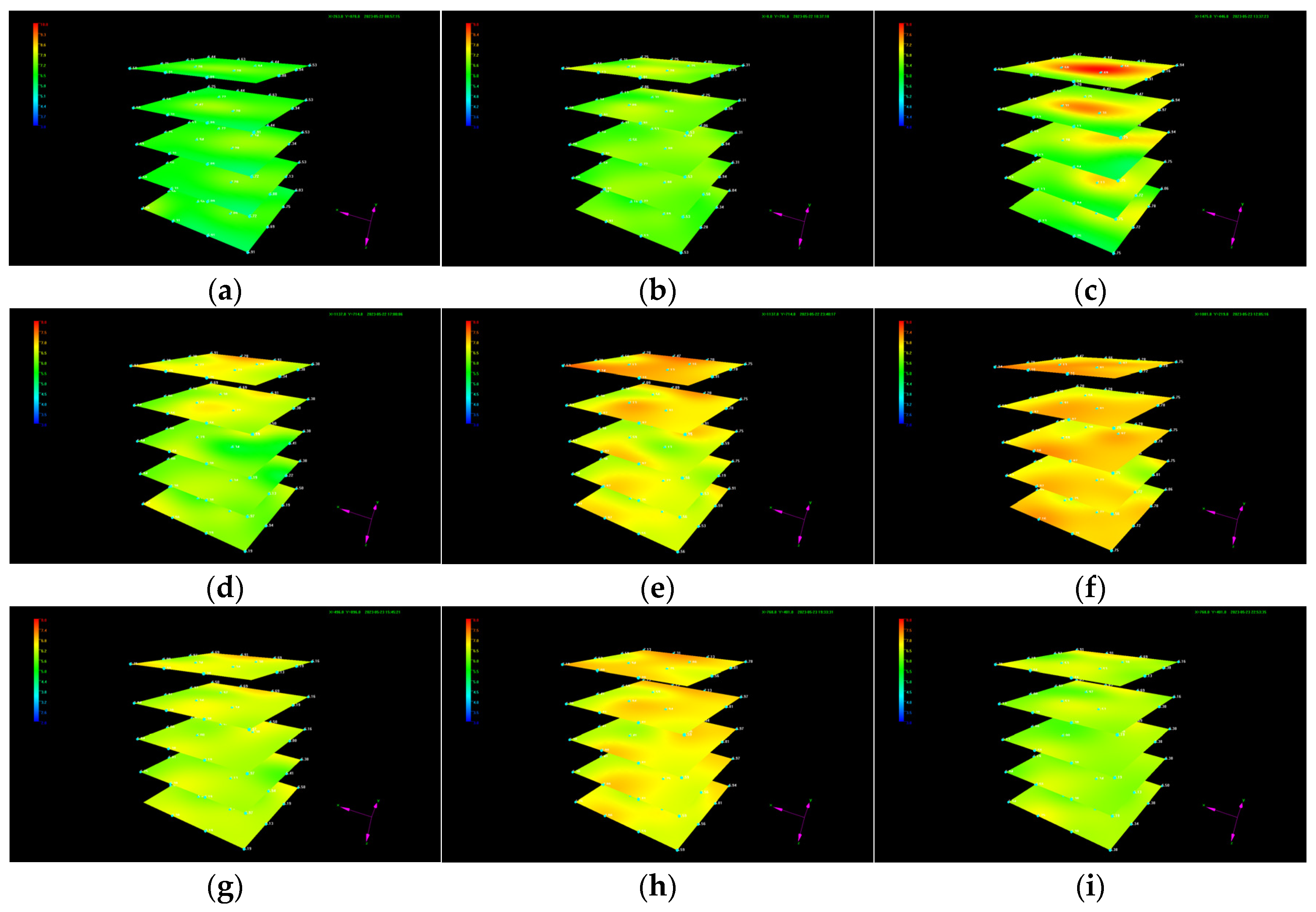
3.1. Evolution of the Temperature and Pressure during Liquid CO2 Injection
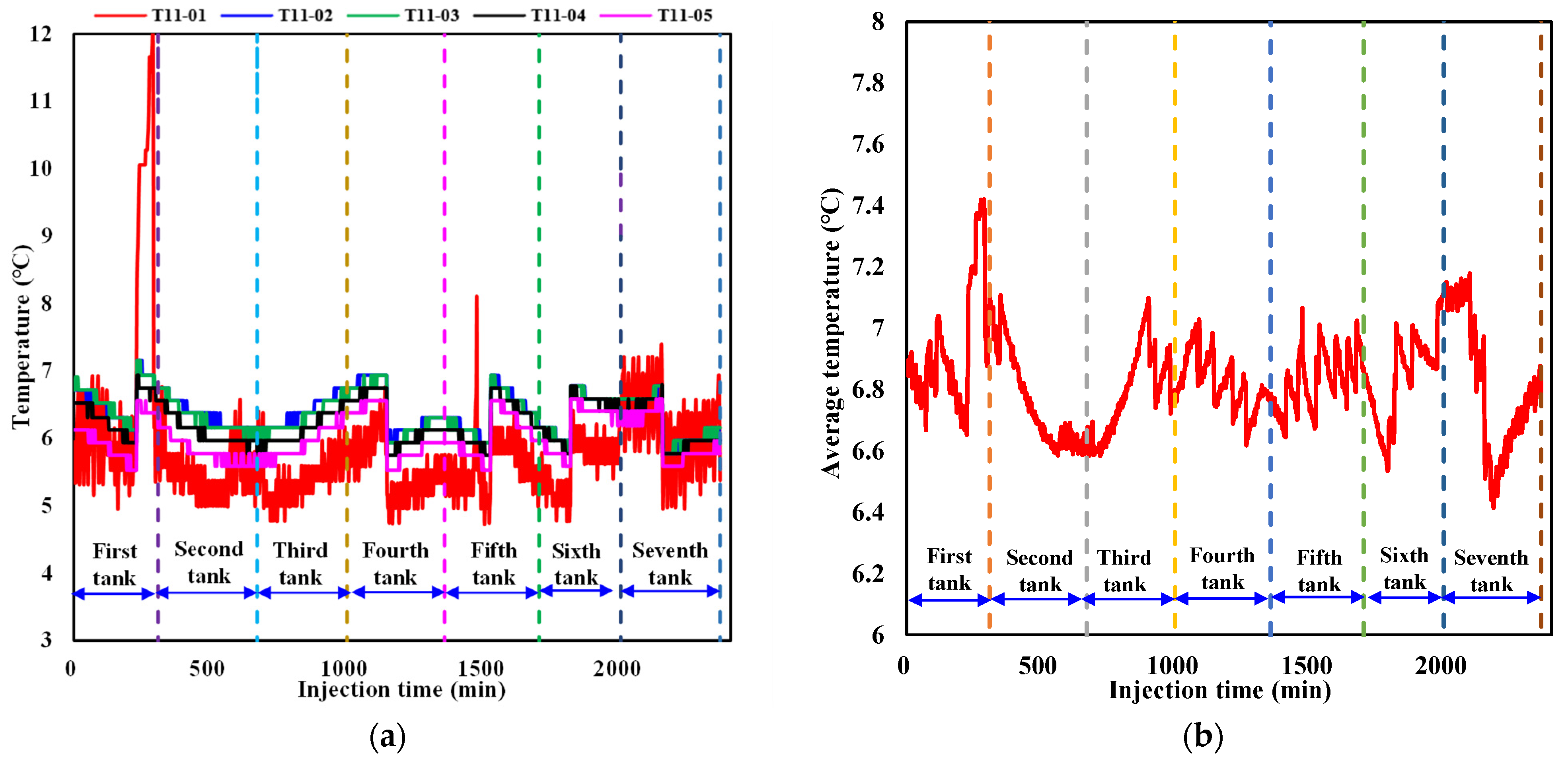
3.1.1. Evolution of the Temperature
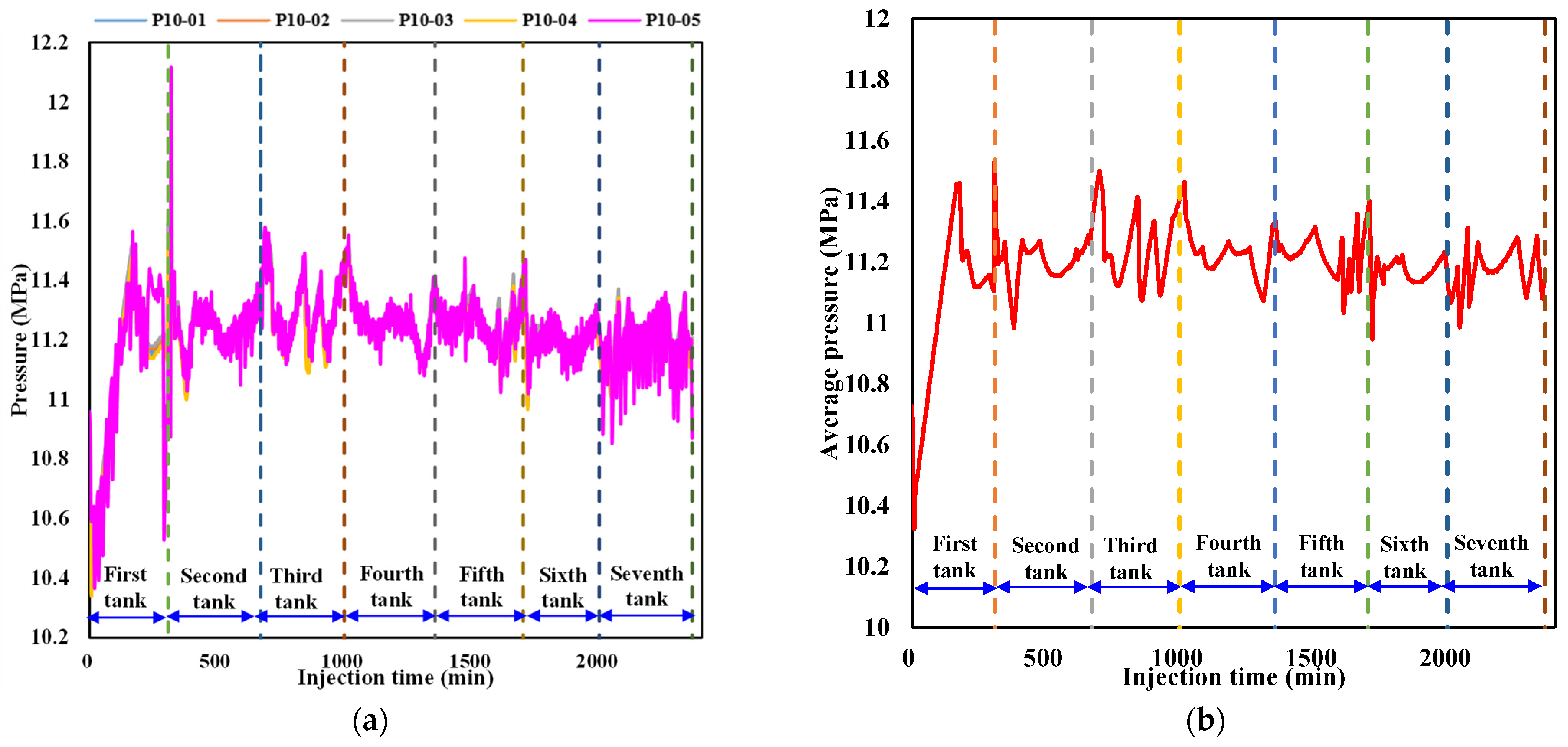
3.1.2. Evolution of the Pressure
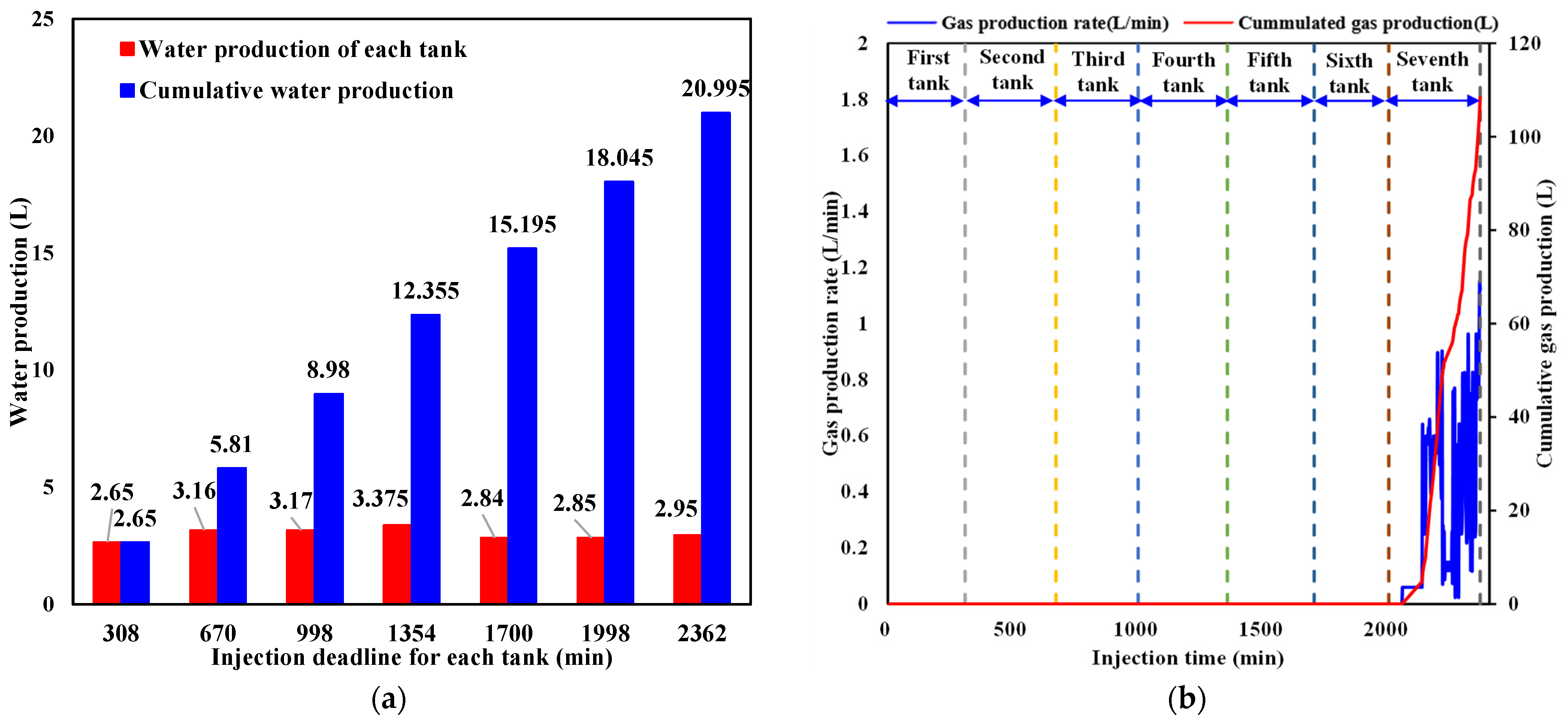
3.2. Water and Gas Production Behavior during Liquid CO2 Injection
3.3. Sequestration Calculation in Hydrate-Based CO2 Storage
3.3.1. Cumulative CO2 Sequestration
3.3.2. CO2 Sequestration in Hydrate and Liquid Phases
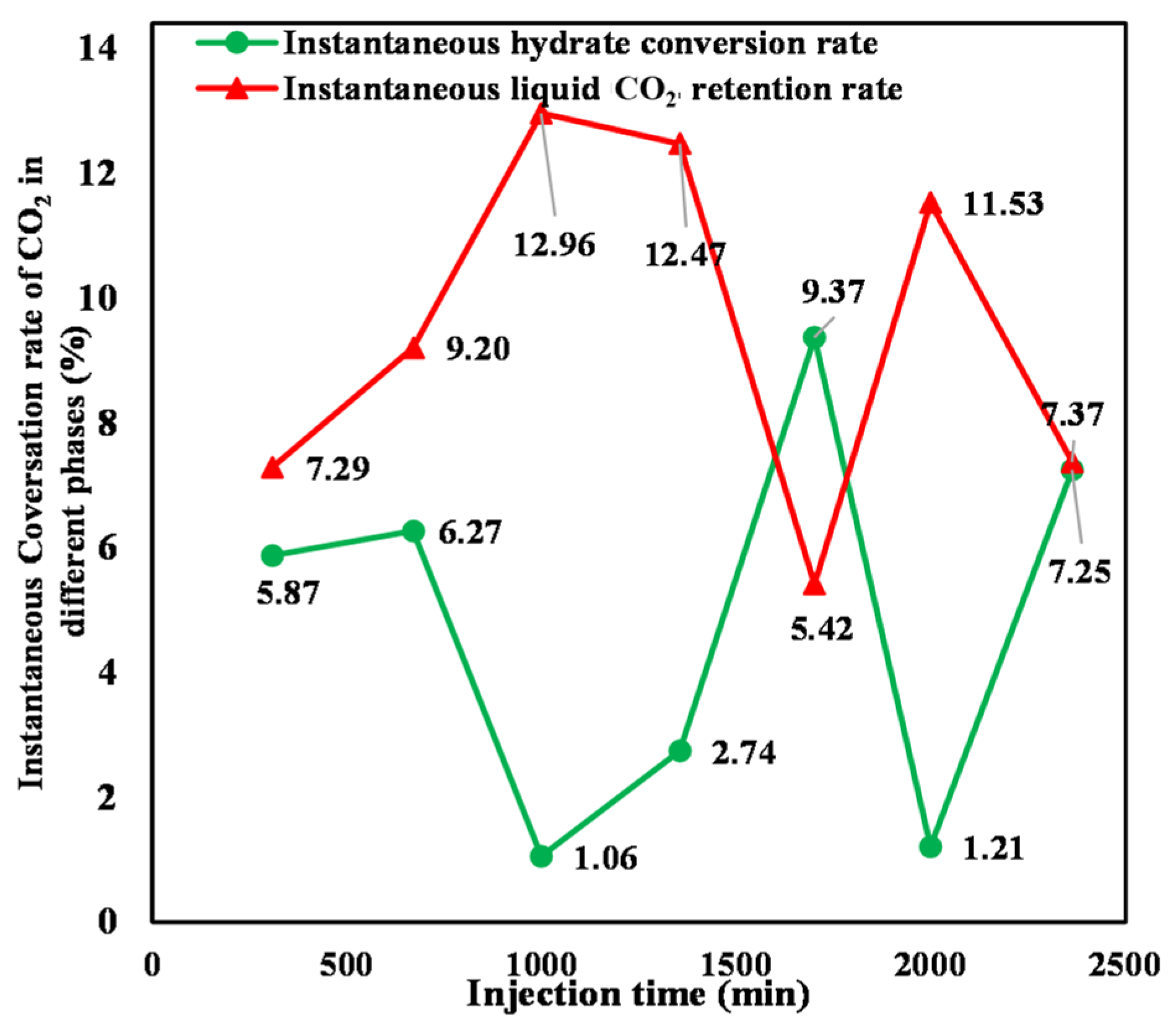
3.3.3. Instantaneous Hydrate Conversion and Liquid CO2 Retention Rates
4. Conclusions
- (1)
- Irregular variations in the temperature and pressure at a certain point and in each layer during CO2 injection confirm the generation of CO2 hydrate as well as its heterogeneous spatial distribution.
- (2)
- When CO2 is injected into the deposit at a constant rate and no large amount of gas is expelled from the outlet, the cumulative sequestered CO2 increases approximately linearly with continuous CO2 injection, while hydrate saturation is always larger than liquid CO2.
- (3)
- CO2 hydrate aggravates the heterogeneity and anisotropy of the sediment, resulting in low CO2 sweep efficiency and small cumulative sequestration in the sediment when a large amount of CO2 is released from the outlet.
- (4)
- The instantaneous hydrate conversion rate and liquid CO2 retention rate both exhibit irregular fluctuations due to the consumption of formation water by CO2, variation in the effective contact area between liquid CO2 and water, and heterogeneity aggravation of the sediment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Salzton, UK, 2020; pp. 3–28. [Google Scholar]
- Lee, Z.H.; Sethupathi, S.; Lee, K.T.; Bhatia, S.; Mohamed, A.R. An overview on global warming in Southeast Asia: CO2 emission status, efforts done, and barriers. Renew. Sustain. Energy Rev. 2013, 28, 71–81. [Google Scholar] [CrossRef]
- Quadrelli, R.; Peterson, S. The energy–Climate challenge: Recent trends in CO2 emissions from fuel combustion. Energy Policy 2007, 35, 5938–5952. [Google Scholar] [CrossRef]
- Nejat, P.; Jomehzadeh, F.; Taheri, M.M.; Gohari, M.; Abd Majid, M.Z. A global review of energy consumption, CO2 emissions and policy in the residential sector (with an overview of the top ten CO2 emitting countries). Renew. Sustain. Energy Rev. 2015, 43, 843–862. [Google Scholar] [CrossRef]
- Albertz, M.; Stewart, S.A.; Goteti, R. Perspectives on geologic carbon storage. Front. Energy Res. 2023, 10, 1–13. [Google Scholar] [CrossRef]
- Yang, B.; Shao, C.; Hu, X.; Ngata, M.R.; Aminu, M.D. Advances in Carbon Dioxide Storage Projects: Assessment and Perspectives. Energy Fuels 2023, 37, 1757–1776. [Google Scholar]
- Lin, Q.; Zhang, X.; Wang, T.; Zheng, C.; Gao, X. Technical Perspective of Carbon Capture, Utilization, and Storage. Engineering 2022, 14, 27–32. [Google Scholar] [CrossRef]
- de Coninck, H.; Benson, S.M. Carbon Dioxide Capture and Storage: Issues and Prospects. Annu. Rev. Environ. Resour. 2014, 39, 243–270. [Google Scholar] [CrossRef]
- Ringrose, P.S.; Furre, A.-K.; Gilfillan, S.M.V.; Krevor, S.; Landrø, M.; Leslie, R.; Meckel, T.; Nazarian, B.; Zahid, A. Storage of Carbon Dioxide in Saline Aquifers: Physicochemical Processes, Key Constraints, and Scale-Up Potential. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 471–494. [Google Scholar] [CrossRef]
- Wei, N.; Li, X.; Jiao, Z.; Stauffer, P.H.; Liu, S.; Ellett, K.; Middleton, R.S. A Hierarchical Framework for CO2 Storage Capacity in Deep Saline Aquifer Formations. Front. Earth Sci. 2022, 9, 1–21. [Google Scholar] [CrossRef]
- Ismail, I.; Gaganis, V. Carbon Capture, Utilization, and Storage in Saline Aquifers: Subsurface Policies, Development Plans, Well Control Strategies and Optimization Approaches—A Review. Clean Technol. 2023, 5, 609–637. [Google Scholar] [CrossRef]
- Worden, R.H. Carbon Dioxide Capture and Storage (CCS) in Saline Aquifers versus Depleted Gas Fields. Geosciences 2024, 14, 146. [Google Scholar] [CrossRef]
- Williams, G.A.; Chadwick, R.A. Influence of reservoir-scale heterogeneities on the growth, evolution and migration of a CO2 plume at the Sleipner Field, Norwegian North Sea. Int. J. Greenh. Gas Control 2021, 106, 103260. [Google Scholar] [CrossRef]
- Wen, G.; Li, Z.; Long, Q.; Azizzadenesheli, K.; Anandkumar, A.; Benson, S.M. Real-time high-resolution CO2 geological storage prediction using nested Fourier neural operators. Energy Environ. Sci. 2023, 16, 1732–1741. [Google Scholar] [CrossRef]
- Akai, T.; Kuriyama, T.; Kato, S.; Okabe, H. Numerical modelling of long-term CO2 storage mechanisms in saline aquifers using the Sleipner benchmark dataset. Int. J. Greenh. Gas Control 2021, 110, 103405. [Google Scholar] [CrossRef]
- Luo, A.; Li, Y.; Chen, X.; Zhu, Z.; Peng, Y. Review of CO2 sequestration mechanism in saline aquifers. Nat. Gas Ind. B 2022, 9, 383–393. [Google Scholar] [CrossRef]
- Bickle, M.J. Geological carbon storage. Nat. Geosci. 2009, 2, 815–818. [Google Scholar] [CrossRef]
- Bradshaw, J.; Bachu, S.; Bonijoly, D.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Issues and development of standards. Int. J. Greenh. Gas Control 2007, 1, 62–68. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Liu, S.; Xu, J.; Liu, J.; Wang, X. Risk evaluation of CO2 leakage through fracture zone in geological storage reservoir. Fuel 2023, 342, 127896. [Google Scholar] [CrossRef]
- Katsuki, D.; Ohmura, R.; Ebinuma, T.; Narita, H. Formation, growth and ageing of clathrate hydrate crystals in a porous medium. Philos. Mag. 2006, 86, 1753–1761. [Google Scholar] [CrossRef]
- Sadeq, D.; Iglauer, S.; Lebedev, M.; Rahman, T.; Zhang, Y.; Barifcani, A. Experimental pore-scale analysis of carbon dioxide hydrate in sandstone via X-Ray micro-computed tomography. Int. J. Greenh. Gas Control 2018, 79, 73–82. [Google Scholar] [CrossRef]
- Circone, S.; Stern, L.A.; Kirby, S.H.; Durham, W.B.; Chakoumakos, B.C.; Rawn, C.J.; Rondinone, A.J.; Ishii, Y. CO2 Hydrate: Synthesis, Composition, Structure, Dissociation Behavior, and a Comparison to Structure I CH4 Hydrate. J. Phys. Chem. B 2003, 107, 5529–5539. [Google Scholar] [CrossRef]
- Kim, H.-S.; Cho, G.-C. Experimental Simulation of the Self-Trapping Mechanism for CO2 Sequestration into Marine Sediments. Minerals 2019, 9, 579. [Google Scholar] [CrossRef]
- Almenningen, S.; Gauteplass, J.; Fotland, P.; Aastveit, G.; Barth, T.; Ersland, G. Visualization of hydrate formation during CO2 storage in water-saturated sandstone. Int. J. Greenh. Gas Control 2018, 79, 272–278. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F.; Lackner, K.S. Permanent carbon dioxide storage in deep-sea sediments. Proc. Natl. Acad. Sci. USA 2006, 103, 12291–12295. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Yang, J.; Salehabadi, M.; Anderson, R.; Chapoy, A. CO2 Hydrates Could Provide Secondary Safety Factor in Subsurface Sequestration of CO2. Environ. Sci. Technol. 2010, 44, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Gauteplass, J.; Almenningen, S.; Ersland, G.; Barth, T.; Yang, J.; Chapoy, A. Multiscale investigation of CO2 hydrate self-sealing potential for carbon geo-sequestration. Chem. Eng. J. 2020, 381, 122646. [Google Scholar] [CrossRef]
- Sun, Z.; Li, N.; Jia, S.; Cui, J.; Yuan, Q.; Sun, C.; Chen, G. A novel method to enhance methane hydrate exploitation efficiency via forming impermeable overlying CO2 hydrate cap. Appl. Energy 2019, 240, 842–850. [Google Scholar] [CrossRef]
- Cui, J.; Sun, Z.; Kan, J.; Jia, S.; Sun, C.; Chen, G.; Wang, X.; Yuan, Q.; Li, N. Study on the factors affecting the sealing performance and mechanical stability of CO2 hydrate cap during gas production from methane hydrate. J. Nat. Gas Sci. Eng. 2021, 93, 104050. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Wang, J.; Zhao, J.; Dong, H.; Yang, M.; Liu, Y.; Song, Y. Enhanced CH4 recovery and CO2 storage via thermal stimulation in the CH4/CO2 replacement of methane hydrate. Chem. Eng. J. 2017, 308, 40–49. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.; Lee, J.; Lee, H.; Seo, Y. Experimental Verification of Methane–Carbon Dioxide Replacement in Natural Gas Hydrates Using a Differential Scanning Calorimeter. Environ. Sci. Technol. 2013, 47, 13184–13190. [Google Scholar] [CrossRef]
- Liu, T.; Wu, P.; Chen, Z.; Li, Y. Review on Carbon Dioxide Replacement of Natural Gas Hydrate: Research Progress and Perspectives. Energy Fuels 2022, 36, 7321–7336. [Google Scholar] [CrossRef]
- Wei, W.; Li, B.; Gan, Q.; Li, Y. Research progress of natural gas hydrate exploitation with CO2 replacement: A review. Fuel 2022, 312, 122873. [Google Scholar] [CrossRef]
- Ndlovu, P.; Babaee, S.; Naidoo, P. Review on CH4-CO2 replacement for CO2 sequestration and CH4/ CO2 hydrate formation in porous media. Fuel 2022, 320, 123795. [Google Scholar] [CrossRef]
- Jia, W.; Song, S.; Li, C.; Wu, X. Predictions on CH4 recovery factors using the CO2 replacement method to develop natural gas hydrate resources. J. CO2 Util. 2020, 41, 101238. [Google Scholar] [CrossRef]
- Hyodo, M.; Li, Y.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Kajiyama, S.; Nishimura, A.; Song, Y. A comparative analysis of the mechanical behavior of carbon dioxide and methane hydrate-bearing sediments. Am. Miner. 2014, 99, 178–183. [Google Scholar] [CrossRef]
- Ersland, G.; Husebø, J.; Graue, A.; Kvamme, B. Transport and storage of CO2 in natural gas hydrate reservoirs. Energy Procedia 2009, 1, 3477–3484. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, M.; Wang, L.; Zhu, Y.; Li, Z.; Sun, C.; Chen, G. Gas hydrate exploitation and carbon dioxide sequestration under maintaining the stiffness of hydrate-bearing sediments. Energy 2020, 194, 116869. [Google Scholar] [CrossRef]
- Kvamme, B.; Graue, A.; Buanes, T.; Kuznetsova, T.; Ersland, G. Storage of CO2 in natural gas hydrate reservoirs and the effect of hydrate as an extra sealing in cold aquifers. Int. J. Greenh. Gas Control 2007, 1, 236–246. [Google Scholar] [CrossRef]
- Castellani, B.; Rossetti, G.; Tupsakhare, S.; Rossi, F.; Nicolini, A.; Castaldi, M.J. Simulation of CO2 storage and methane gas production from gas hydrates in a large scale laboratory reactor. J. Pet. Sci. Eng. 2016, 147, 515–527. [Google Scholar] [CrossRef]
- Baldwin, B.A.; Stevens, J.; Howard, J.J.; Graue, A.; Kvamme, B.; Aspenes, E.; Ersland, G.; Husebø, J.; Zornes, D.R. Using magnetic resonance imaging to monitor CH4 hydrate formation and spontaneous conversion of CH4 hydrate to CO2 hydrate in porous media. Magn. Reson. Imaging 2009, 27, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, S.; Li, S.; Wang, X.; Peng, S. Hydrate formation from clay bound water for CO2 storage. Chem. Eng. J. 2021, 406, 126872. [Google Scholar] [CrossRef]
- Cao, X.; Wang, H.; Yang, K.; Wu, S.; Chen, Q.; Bian, J. Hydrate-based CO2 sequestration technology: Feasibilities, mechanisms, influencing factors, and applications. J. Pet. Sci. Eng. 2022, 219, 111121. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Yang, H.; Li, J.; Li, Y.; Wu, Q. Experimental study on the formation characteristics of CO2 hydrate in porous media below the freezing point: Influence of particle size and temperature on the formation process and storage capacity. Energy Sci. Eng. 2022, 10, 1164–1176. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Yang, M.; Zhao, Y.; Zhao, J.; Song, Y. CO2 sequestration in depleted methane hydrate sandy reservoirs. J. Nat. Gas Sci. Eng. 2018, 49, 428–434. [Google Scholar] [CrossRef]
- Zheng, J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar] [CrossRef]
- Zhang, K.; Lau, H.C. Sequestering CO2 as CO2 hydrate in an offshore saline aquifer by reservoir pressure management. Energy 2022, 239, 122231. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, L.; Zheng, Y. Enhanced CO2 sequestration based on hydrate technology with pressure oscillation in porous medium using NMR. Energy 2022, 252, 124082. [Google Scholar] [CrossRef]
- Zhao, G.; Zheng, J.; Gong, G.; Chen, B.; Yang, M.; Song, Y. Formation characteristics and leakage termination effects of CO2 hydrate cap in case of geological sequestration leakage. Appl. Energy 2023, 351, 121896. [Google Scholar] [CrossRef]
- Castellani, B. Potential Pathway for Reliable Long-Term CO2 Storage as Clathrate Hydrates in Marine Environments. Energies 2023, 16, 2856. [Google Scholar] [CrossRef]
- Junji Yamaguchi, A.; Sato, T.; Tobase, T.; Wei, X.; Huang, L.; Zhang, J.; Bian, J.; Liu, T.-Y. Multiscale numerical simulation of CO2 hydrate storage using machine learning. Fuel 2023, 334, 126678. [Google Scholar] [CrossRef]
- Zatsepina, O.Y.; Pooladi-Darvish, M. Storage of CO2 as Hydrate in Depleted Gas Reservoirs. SPE Reserv. Eval. Eng. 2012, 15, 98–108. [Google Scholar] [CrossRef]
- De-Galda, V.; Rahmanian, N.; Batrshin, D. Assessment of CO2 Storage as Hydrates in Saline Aquifers using Machine Learning Algorithms. Chem. Eng. Trans. 2021, 86, 517–522. [Google Scholar]
- Nordbotten, J.M.; Celia, M.A.; Bachu, S. Injection and Storage of CO2 in Deep Saline Aquifers: Analytical Solution for CO2 Plume Evolution During Injection. Transp. Porous Media 2005, 58, 339–360. [Google Scholar] [CrossRef]
- Anwar, S.; Carroll, J.J. Density (kg/m3) of Carbon Dioxide as a Function of Temperature and Pressure. In Carbon Dioxide Thermodynamic Properties Handbook; Scrivener Publishing LLC: Beverly, MA, USA, 2016; pp. 9–148. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, W.; Ge, Y.; Chen, M.; Zhang, X.; Wen, H.; Fu, Q.; Lei, X.; Li, Q.; Zhou, S. Large-Scale Experimental Investigation of Hydrate-Based Carbon Dioxide Sequestration. Energies 2024, 17, 3103. https://doi.org/10.3390/en17133103
Pang W, Ge Y, Chen M, Zhang X, Wen H, Fu Q, Lei X, Li Q, Zhou S. Large-Scale Experimental Investigation of Hydrate-Based Carbon Dioxide Sequestration. Energies. 2024; 17(13):3103. https://doi.org/10.3390/en17133103
Chicago/Turabian StylePang, Weixin, Yang Ge, Mingqiang Chen, Xiaohan Zhang, Huiyun Wen, Qiang Fu, Xin Lei, Qingping Li, and Shouwei Zhou. 2024. "Large-Scale Experimental Investigation of Hydrate-Based Carbon Dioxide Sequestration" Energies 17, no. 13: 3103. https://doi.org/10.3390/en17133103
APA StylePang, W., Ge, Y., Chen, M., Zhang, X., Wen, H., Fu, Q., Lei, X., Li, Q., & Zhou, S. (2024). Large-Scale Experimental Investigation of Hydrate-Based Carbon Dioxide Sequestration. Energies, 17(13), 3103. https://doi.org/10.3390/en17133103





