Abstract
The combustion of briquettes made from organic and industrial residues in small boilers requires researchers to consider the characteristics of this type of fuel and methods of its combustion. For the efficient combustion of fuel briquettes, a layered combustion method with the ability to regulate the supply of combustion air is better suited. The purpose of this research is to study the thermal technical conditions of briquetted fuel combustion. In order to carry this out, a stand was created, which made it possible to determine the combustion efficiency of this type of fuel. Two types of briquettes were studied: one with 30% sunflower husks and 70% leaves, and one with and 70% sunflower husks and 30% coke breeze. The combustion results of the briquettes show that heat loss from chemical under-burning was no more than 6.25%. To determine the temperature distribution in the fuel layer, a model of unsteady heat transfer in a fixed layer was used. A calculation of the temperature fields in the layer of burned fuel briquettes was carried out, which showed that the most favorable conditions for burning briquettes were created with a layer about 15–20 cm thick for both burned briquette options. The temperature was in the range of 450–750 °C, which on the one hand corresponds to experimental data and on the other hand provides a combustion regime that occurs with a relatively low loss to the environment. This installation and mathematical model will help future studies based on the processes of other types of organic waste combustion with a grate system.
1. Introduction
The use of biomass- and industrial-residue-based briquettes as a fuel for small boilers and thermal technology installations requires specific features of both the fuel itself and the methods used for its combustion to be considered. Firstly, briquettes differ from classic solid fuels, for example, coal, in certain characteristics such as the composition ratio of biomass and industrial residues [1]. This ratio determines the overall carbon content of the fuel, which can vary over a wide range. Accordingly, the fuel’s elemental composition may change, particularly its ash content, as well as the yield of volatile substances, whose values affect the reactivity of the fuel and the quality of combustion. If we consider the fuel briquettes to have almost the same size, then it is possible to obtain a fairly uniform distribution of air across the cross-section of the layer and, therefore, improve the characteristics of the combustion processes. Another important characteristic of a briquette is its density. Since briquettes are formed at high pressures [2], the density value affects both the structure of the layer and the combustion heat produced [3].
The energy efficiency of burning briquettes is assessed using the following parameters: water boiling time (WBT), water evaporation time (WET), mass of briquettes used (MB), burning rate (BR), and maximum temperature reached in the furnace (Tmax) [4,5,6]. These indicators are determined by burning fuel in a furnace and heating a small volume of water. A more objective indicator of biofuel combustion in a boiler unit will be its efficiency.
The efficiency of boilers burning bio-briquettes differs slightly, as a rule, from the efficiency of boilers burning coal. Thus, some researchers [7] have provided data on the efficiency of burning fuel briquettes made from Palm Branches, Screw Pine, Saw Dust, Indian Bdellium, and Coconut Coir. This article does not indicate the type and power of the boiler unit. The efficiency values obtained ranged from 74% to 80.79%.
In another article [8], the authors compared the operation of a boiler burning briquettes and the operation of a furnace oil-fired steam boiler. It has been shown that the efficiency of a boiler operating on briquettes is 66.5%. The efficiency of the boiler operating on liquid fuel was 73.4%. The results of the study show that an oil-fired boiler is more efficient than a briquette-burning boiler. It is proposed to increase the productivity of a boiler using briquettes by introducing an automatic fuel supply system, as well as by reconstructing the heating surfaces of the boiler.
For sawdust briquettes, the efficiency calculated by an indirect method using Indian boiler efficiency standard IS 8753 [9] was 68.80% [10]. When using a briquette of coconut leaves with sawdust as a binder, the efficiency was 61.17%. The amounts of heat loss due to the chemically incomplete combustion of fuel were 2.78% and 3.58%, respectively.
The reason for the higher boiler efficiency, according to the authors, is the higher calorific value of sawdust briquettes (18.63 MJ/kg) compared to the coconut leaf briquettes (15.37 MJ/kg). The authors propose to increase the efficiency of burning fuel briquettes by introducing additives to increase the calorific value of the fuel.
Thus, the efficiency of boilers when burning biomass is slightly lower than when burning coal or liquid fuel. Increasing the efficiency of biomass combustion involves increasing the caloric content of the fuel or reconstructing the boiler.
There are several methods for burning solid fuels [11,12]. We will review some of them, considering the specific features of fuel briquettes.
One heat generation method involves the combustion of biomass- and industrial-residue-based briquettes or pellets in furnaces with grate firing [13,14]. The advantages of this type of heat generation are operational simplicity and reliability, the possibility of using fuel with a significant percentage of non-combustible components, and relatively low financial and operating costs [15,16]. The disadvantages of units that implement grate firing are their relatively low efficiency due to significant heat losses with flue gases and mechanical under-burning [17,18,19], as well as the need to limit the temperature of combustion products. This is necessary to prevent the melting of mineral fractions of ash and the sticking of slag to the heating surfaces and to the grate [14,20].
The combustion of fuel briquettes in a fluidized bed boiler makes it possible to increase the completeness of fuel combustion and, accordingly, increase the efficiency of the whole installation. When operating such boilers, problems arise if the percentage of components in the briquettes changes, since in this case it is necessary to carry out routine adjustments to the boiler [21].
A promising method for burning fuel is to use grate firing, with a regulated air supply under the grate. This method allows the completeness of combustion to be increased, on the one hand, and makes it possible to control the height of the layer above the grate, on the other. The disadvantages of this method include increased risks of fuel particles being carried away from the combustion zone when their size decreases [22,23].
The supply of fuel and air when using a grate with air control is managed by a crossflow and counterflow configuration, which is standard for various types of boilers with a grate. The cross-supply of fuel and air allows the uniform combustion of fuel along the grate.
The counterflow configuration ensures a uniform combustion of fuel over the entire grate area. Air is supplied from below through the grate, which also promotes uniform combustion and a uniform temperature profile across the grate.
Both schemes have their advantages and disadvantages, and the choice of air supply scheme depends on the type of boiler, the type of fuel, and the characteristics of its combustion process.
The analysis conducted shows that, for an efficient combustion of fuel briquettes, a method with the ability to regulate the supply of combustion air is the most suitable [24]. However, when burning fuel in a layer, a few problems can arise:
- -
- The uneven bulk density of the layer can be accompanied by different aerodynamic resistances both over the cross section and in the layer plane, changing not only the flow rate of particles but also the ratio of the supplied combustion air. As a result, combustion conditions change and, therefore, the quality of the whole combustion process changes.
- -
- Directly related to this problem is the instability of the height of the fuel layer, since changes in the air supply speed affect not only the height of the layer but also the stability of redox reactions.
- -
- The ratio of volatile matter release and the formation of coke residue depend on the percentage composition of the main components of the fuel briquette, and they affect all stages of combustion.
- -
- There are certain restrictions on the temperature of the layer. An increase in temperature not only increases the combustion efficiency but also leads to an increase in the yield of nitrogen oxides [25,26].
A large number of scientific works have been devoted to studying the emission of harmful substances when burning biomass [27,28,29]. The overall result of the research is the conclusion that biomass can hardly be considered a truly environmentally friendly fuel. Thus, authors [30] have noted that, in many cases, emissions from the combustion of some types of biomasses are higher than for coal, particularly total organic compounds. With significantly lower sulfur dioxide (SO2) emissions, biomass combustion releases significant amounts of organic micropollutants, including more toxic compounds such as polycyclic aromatic hydrocarbons (PAHs) and polychlorinated dibenzo-p-dioxins and dibenzofurans PCDD/Fs.
A significant number of scientific works are devoted to the efficiency of biofuel combustion, considering the incomplete combustion of fuel. Despite this, the influence of layer thickness on combustion efficiency has not received sufficient attention. The thickness of the layer is an indicator of the uniform distribution of air flow over the combustion area. The correct choice of layer thickness can be assessed by the temperature distribution in the layer. The temperature values indicate the amount of air that is sufficient for combustion and its uniform distribution.
In conclusion, for the efficient combustion of briquettes from agricultural and industrial residues, it is necessary to study the influence of the factors above on the processes of heat generation in the layer of fuel.
The purpose of this article is to study thermal conditions for burning briquetted fuel.
2. Materials and Methods
Two types of briquettes were taken as the fuel under study, in the following compositions: 30% sunflower husks and 70% leaves, and 70% sunflower husks and 30% coke breeze (Figure 1). Coke breeze is a waste product from the calcination of petroleum coke.

Figure 1.
Briquettes from organic and industrial residues. (a) 70% sunflower husks and 30% coke breeze; (b) 30% sunflower husks and 70% leaves.
The characteristics of the briquettes are presented in Table 1.

Table 1.
The characteristics of the briquettes.
The technological process for manufacturing fuel briquettes consisted of the following stages:
- -
- Cleaning raw materials from foreign inclusions (glass, plastic, metal, etc.);
- -
- Drying raw materials in the open air to an air-dry state;
- -
- Grinding raw materials to a size of no more than three millimeters;
- -
- Preparing a homogeneous mixture from crushed raw materials;
- -
- Loading into the press and pressing fuel briquettes at a pressure of 25 MPa;
- -
- Drying the resulting fuel briquettes indoors to an air-dry state.
When producing briquettes, the compression pressure was 25 MPa, the holding time under pressure was 30 s, and the pressing temperature was 20 °C.
A fire bench was created to evaluate the efficiency of burning fuel briquettes made from plant and industrial waste, the diagram of which is presented in Figure 2.
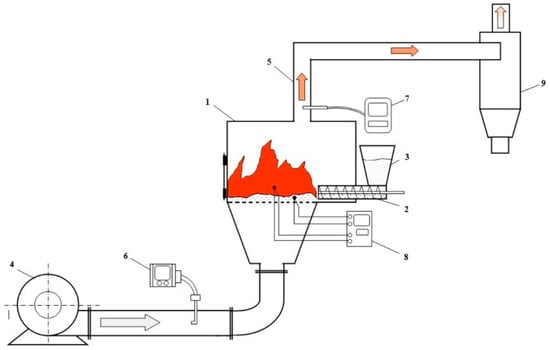
Figure 2.
Installation diagram for determining the combustion efficiency of fuel briquettes. 1. Firebox; 2. combustion zone; 3. loading hopper; 4. medium pressure radial fan; 5. chimney; 6. differential pressure gauge; 7. gas analyzer; 8. secondary device; 9. ash catcher.
The installation includes a firebox (1) (dimensions: length 360 mm, height 400 mm, width 200 mm) designed for burning fuel in a layer. There is a screw conveyor for supplying fuel to the combustion zone (2) (with pipe diameter 80 mm) and a loading hopper (3) (dimensions: width 150 mm, height 90 mm).
Combustion air is supplied under the grate using a medium-pressure radial fan (4) (Nevatom VR-280-46, «NEVATOM» company, Novosibirsk, Russia) (via a 100 mm diameter air duct), the speed of which can vary within a wide range. Flue gases are removed from the combustion zone through a chimney (5) (pipe diameter 100 mm).
The installation’s operating parameters are monitored by the following instruments: (6) Differential pressure gauge with a pitot tube (accuracy class 0.3) is used for measuring the volume of air supplied for combustion. The measurement location is in a horizontal section of the air supply duct to the combustion zone. The differential pressure gauge’s brand is DT-889 (Group of companies «Teplopribor», Moscow, Russia). To measure the flue gases’ composition and temperature, a gas analyzer (7) of the brand Testo 340 (first class accuracy) is used. The installation location of the gas sampling probe of the gas analyzer is the end-section of the chimney.
Before the measurements are carried out, the gas analyzer and probe are automatically purged. The purging time should not exceed two minutes. The combustion air temperature was measured using the thermocouple readings of the sampling probe during zeroing. During measurements, the gas sampling probe was positioned in such a way that the flow of gases freely entered the thermocouple through the holes in the probe. In this case, the probe tip was located in the center of the gas flow (the area with the maximum temperature).
The CO, SO2, NO2, NO and O2 values can be calibrated according to the operating instructions for the Testo 340 gas analyzer.. This is undertaken if it is obvious that the device is displaying incorrect values. For this purpose, a reference gas with a concentration below the established cell protection limit was used.
The measurement of the flame temperature and the temperature in the fuel layer during the combustion process was carried out by contact method, using chromel–alumel thermocouples TChA (1 tolerance class, according to ASTM E 230) [31] and a secondary device OVEN-2TRM0 (accuracy class 0.5).
The combustion process was assessed by the amount of under-burning, which was determined during the combustion process by a gas analyzer and was regulated by changing the amount of air supplied by a blower fan. To ensure α = 1.4 in the firebox, air was supplied, the speed and flow of which were measured with a DT-8890 differential pressure gauge.
When stable combustion and minimal under-burning were achieved, the readings were recorded, and the efficiency of the combustion process was calculated.
The combustion of fuel briquettes was carried out on a layer of ash and slag particles. The standing fuel thickness in both cases was 0.3 m (Figure 3).

Figure 3.
Combustion of briquettes.
The temperature of the layer, recorded by thermocouples, varied within a range from 250 °C to 650 °C, depending on the stage of combustion. The temperature above the layer was in the range of 1200 to 1250 °C.
3. Results and Discussion
The test results for briquettes made from agricultural residues and a mixture of agricultural residues and coke breeze are shown in Table 2 and Table 3.

Table 2.
Results of burning agricultural residues.

Table 3.
Results of the combustion of a mixture of agricultural residues and coke breeze.
The combustion efficiency of briquettes was determined based on the determination of heat loss from the chemical under-burning of fuel q3 (%). Gases leaving the firebox may contain products of incomplete combustion of fuel, the calorific value of which was not used in the firebox. The total heat of combustion of these gases causes chemical under-burning.
The amount of heat loss from the chemical under-burning of briquettes was calculated using the following formula:
where CO, H2, and CH4 are the volumetric contents of products of incomplete combustion of fuel in dry combustion products, %; VDCP is the volume of dry combustion products, m3/kg; and represents low calorific value, kJ/kg.
The efficiency of the boiler was calculated using the indirect method given in [7].
After the release and burnout of volatiles above the layer, a sufficiently long combustion of coke residue in the layer occurs on the grate, the magnitude of which depends on the composition of the burned briquettes and the carbon content in the solid phase. From the long-term experience of burning solid fuel in a layer, it is known that there is practically no oxygen in even a thin layer of about 30–35 mm above the fuel layer [32], which is all spent on the combustion of carbon in the layer. As a result, a certain amount of carbon monoxide CO is formed over a thin layer, i.e., as a reducing environment, which, at a relatively low temperature in the layer, contributes to low greenhouse gas emissions; in particular, the CO2 content is about 4%. As an example, Figure 4 shows the dynamics of the formation of combustion products of a mixture of agricultural residues and coke breeze.
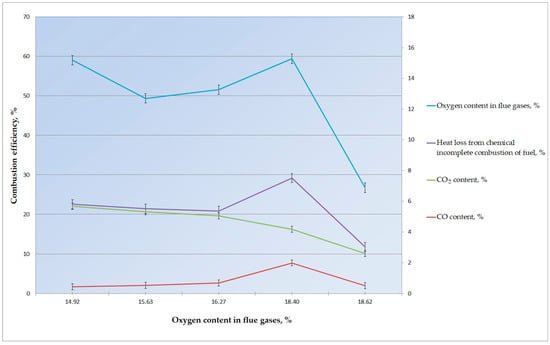
Figure 4.
Dynamics of the formation of combustion products made from a mixture of agricultural residues and coke breeze.
The analysis of data on the results of fuel combustion shows that for all measurements, heat loss from chemical under-burning is no more than 6.25%. The main parameter that determines the amount of chemical under-burning is the content of carbon monoxide, CO, in the flue gases.
The oxygen content in the flue gases does not reflect the completeness of fuel combustion. The reason for this is the uneven distribution of air flow over the horizontal area of combustion.
This indicates a significant influence of the air flow distribution uniformity factor over the combustion area. An uneven supply of air to the burning fuel layer leads to a situation in which part of the layer is lacking the oxidizer and where under-burning takes place, which is detected by the gas analyzer. On the other hand, in the part of the layer where excess air is supplied, combustion occurs due to the excess oxygen in the flue gases. Thus, there are several combustion modes in which a high oxygen content in the flue gases occurs with a high CO content. This is confirmed by the relationship between the simultaneous increase in two components of under-burning, CO and H2.
On the other hand, the factor of briquettes’ uniform distribution in a layer is directly related to the height of the layer. Therefore, it is of interest to study the influence of this parameter on the heat transfer in a layer of briquetted fuel.
To assess the nature of the temperature distribution in the layer, we use a model of unsteady heat transfer in a fixed layer, representing it as a semi-bounded body (Figure 5) [26].
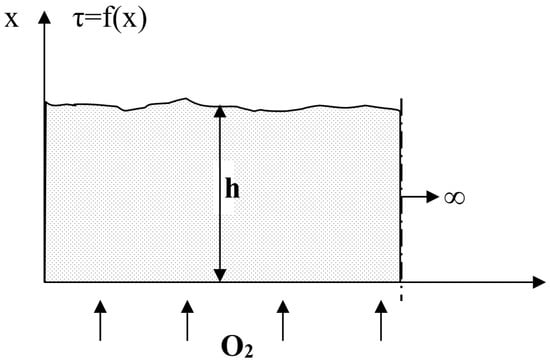
Figure 5.
Fuel layer diagram.
In this case, the problem is reduced to one dimension and is described by the following heat equation:
where c is the specific heat capacity of the material, kJ/(kg·°C).
- λ—thermal conductivity coefficient of the material, kJ/(m·°C).
- ρ—material density, kg/m3.
- a = —thermal diffusivity coefficient, m2/s.
Under boundary conditions,
T(x, 0) = To = const;
T(0, τ) = τc = const.
The solution to Equation (1) is found operationally. We will assume that the desired solution T(x, τ) for any fixed x is original in variable τ:
T(x, τ) = V(x, p)
Applying the Laplace transform to Equation (1) and using the differentiation theorem of the original [33], we proceed to the operator equation:
Thus, the partial differential equation for the original function of T(x, τ) is transformed into a second-order linear equation with constant coefficients for the function (V(x, p) − To/p). To solve Equation (5), we used the Euler method. Thus, the general solution to Equation (5) took the following form:
where To/p = T(x,0).
Constants c1 and c2 are determined from Condition (3) and require that the solution of T(x, t) is bounded, which follows from the physical meaning of the problem. It means that c1 = 0.
Thus,
At x = 0, V(0, p) = c2 + To/p. Moreover, according to condition (3), V(0, p) = Tc/p. Therefore,
The general solution to Problem (1) will be written in the following form:
To find the original function of T(x, τ), we use the correspondence table [34] and then
where is the Gaussian error function.
The proposed model allows the temperature distribution to be assessed quickly in the layer. The calculated temperatures agree well with those obtained during the experiments. More accurate calculations can be obtained if boundary conditions of the third kind are used on the external surface, but this significantly complicates the model without a significant gain in the estimation of temperature fields.
One of the limitations of this model is the representation of the layer as a continuous semi-bounded solid body. In this formulation of the problem, it is possible to obtain a fairly simple but informative model for assessing the thermal state of the fuel layer.
The temperature fields in the layer of burned fuel briquettes were calculated in accordance with the mathematical model presented.
Boundary conditions of the first kind were adopted in the mathematical model. The temperature of the layer at the initial moment was taken to be 20 °C. The temperature on the surface of the layer was taken to be equal to the ambient temperature in the firebox and was 1200 °C.
For research purposes, the characteristics of the following two types of fuel briquettes were taken: briquettes from a mixture of agricultural waste and briquettes from a mixture of agricultural and industrial waste (coke breeze). The thermal diffusivity coefficient of briquettes in the first case was a = 1.17·10−7 m2/s; in the second case, it was a = 2.64·10−7 m2/s.
Using Equation (6), we can find the temperature distribution according to the thickness of the layer. The temperature distribution in the layer for the first type of briquette during its combustion, 900 s after the start of combustion, is shown in Table 4.

Table 4.
Temperature distribution along the height of the briquette layer for the first type of briquette.
The temperature distribution in the layer for the second type of briquette during its combustion, 2100 s after the start of combustion, is shown in Table 5.

Table 5.
Temperature distribution along the height of the briquette layer for the second type of briquette.
Typical graphs of the temperature distribution in the layer are shown in Figure 6.
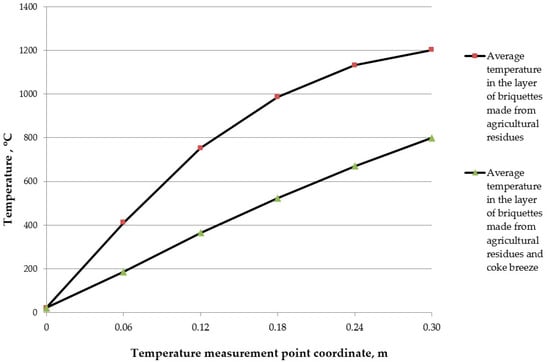
Figure 6.
Temperature distribution in the layer.
The dots in the figure indicate the results of experimental temperature measurements on a fire stand in a layer of burned fuel. A comparison of the temperature values obtained during the experiments (Figure 5) and the temperatures obtained based on mathematical modeling (Table 1 and Table 2) show that the difference between them does not exceed 13%.
It can also be seen that, with the given parameters, the most favorable conditions for burning briquettes are created with a layer about 15–20 cm thick for both options. The temperature lies in the range of 450–750 °C. On the one hand, this corresponds to experimental data. On the other hand, it means that combustion modes occur with relatively low losses of heat to the environment.
4. Conclusions and Future Research Directions
The research results revealed two important aspects that are interconnected and influence each other. The analysis of data on the results of fuel combustion shows that, for all measurements, heat loss from chemical under-burning is no more than 6.25%. The amount of under-burning is determined by the ratio of the main parameters, which are the amounts of carbon monoxide CO and oxygen in the flue gases.
Moreover, the oxygen content in the flue gases does not reflect the completeness of fuel combustion. The reason for this is the uneven distribution of air flow over the horizontal area of combustion.
This indicates a significant influence of the uniformity factor of air flow distribution over the combustion area. An uneven supply of air to the burning fuel layer leads to a situation where, in the part of the layer lacking oxidizer, under-burning occurs, which is then detected by a gas analyzer. On the other hand, in the part of the layer where excess air is supplied, combustion occurs due to the excess oxygen in the flue gases. Therefore, there are a few combustion modes in which a high oxygen content in the flue gases occurs with a significant CO content. This is confirmed by the relationship between the simultaneous increase in two components of under-burning, CO and H2.
This is directly explained by the second aspect of the problem—the size of the burning layer. The results of both experimental and modeling studies clearly indicate the presence of an optimal layer thickness, which ranges from 15 to 20 cm.
The physical model (fire stand) and the mathematical model developed for these studies allow them to be used to study the processes of other types of biomass residue combustion processes in a grate firing furnace. The physical model (fire bench) and mathematical model developed for these studies allow them to be used to study the processes of other types of organic waste combustion on a grate. The fire stand allows the scope of application to be expanded to the following areas:
- -
- Experimental study of the influence of the thermophysical and thermal characteristics of briquettes on the combustion process.
- -
- Study of the influence of the design features of a firebox with a grate on the process of burning briquettes (with appropriate modernization of the stand).
Thus, there are potential opportunities to expand the range of scientific research on the use of fuel briquettes based on agricultural and industrial waste.
Author Contributions
Conceptualization, A.N., A.K. (Akmaral Kinzhibekova) and E.P.; methodology, A.N., A.K. (Akmaral Kinzhibekova) and E.P.; validation, A.K. (Akmaral Kinzhibekova) and E.P.; formal analysis, A.N.; investigation, E.P. and A.K. (Amangeldy Karmanov); resources, E.P., A.K. (Amangeldy Karmanov) and T.A.I.; data curation, A.N., T.A.I. and E.P.; writing—original draft preparation, A.N. and E.P.; writing—review and editing, E.P. and A.K. (Akmaral Kinzhibekova); visualization, E.P.; supervision, A.N.; project administration, A.K. (Akmaral Kinzhibekova). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee, Ministry of Science and Higher Education, Republic of Kazakhstan (Grant No. AP 14869152).
Data Availability Statement
Data are contained within this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nikiforov, A.; Kinzhibekova, A.; Prikhodko, E.; Karmanov, A.; Nurkina, S. Analysis of the Characteristics of Bio-Coal Briquettes from Agricultural and Coal Industry Waste. Energies 2023, 16, 3527. [Google Scholar] [CrossRef]
- Nurkina, S.; Kinzhibekova, A.; Prikhodko, E. Research and analysis of characteristics of fuel from organic and industrial waste. EUREKA Phys. Eng. 2022, 5, 43–54. [Google Scholar] [CrossRef]
- Akpenpuun, T.D.; Salau, R.A.; Adebayo, A.O.; Adebayo, O.; Salawu, J.; Durotoye, M. Physical and combustible properties of briquettes produced from a combination of groundnut shell, rice husk, sawdust and wastepaper using starch as a binder. J. Appl. Sci. Environ. Manag. 2020, 24, 171–177. [Google Scholar] [CrossRef]
- Kpelou, P.; Kongnine, D.; Kombate, S.; Mouzou, E.; Napo, K. Energy Efficiency of Briquettes Derived from Three Agricultural Waste’s Charcoal Using Two Organic Binders. J. Sustain. Bioenergy Syst. 2019, 9, 79–89. [Google Scholar] [CrossRef]
- Ana, G.R.; Fabunmi, V.T. Energy Efficiency Evaluation from the Combustion of Selected Briquettes-Derived Agro-Waste with Paper and Starch Binders. Int. J. Sustain. Green Energy 2016, 5, 71–79. [Google Scholar] [CrossRef]
- Abimbola, A.I.; Rapheal, I.A.; Musa, A. Combustion Quality Evaluation of Briquettes Produced from Sesame Hull as Source of Sustainable Energy. Asian J. Energy Transform. Conserv. 2020, 4, 30–39. [Google Scholar] [CrossRef]
- Kumar, G.S.; Rao, C.J.; Sreeramulu, D.; Madhavi, S.K. Evaluation of boiler efficiency of bio briquettes by indirect method. Int. J. Mech. Eng. Technol. (IJMET) 2016, 7, 624–633. [Google Scholar]
- Yashpal, A.; Kumar, D.; Anand, E.T. Efficiency Performance Analysis of Briquette & FO Boiler of Beverage Industry. Int. J. Res. Appl. Sci. Eng. Technol. 2021, 9, 284–290. [Google Scholar] [CrossRef]
- IS 8753; Code for Acceptance Tests on Stationary Steam Generators of the Power Station Type. Indian Standards Institution: New Delhi, India, 1977.
- Balakrishna, D.; Javaregowda, M.B.; Ramanaik, H.S.; Kuvenja, S.K. Comparative analysis of boiler efficiency between commercial sawdust briquettes and biomass briquettes. Proc. AIP Conf. Proc. 2020, 2236, 030010. [Google Scholar] [CrossRef]
- Khzmalyan, D.M.; Kagan, Y.A. Combustion Theory and Combustion Devices; Energy: Moscow, Russia, 1976; p. 484. (In Russian) [Google Scholar]
- Érces, N.; Kajtár, L. Operational Testing of a Solid Fuel Boiler with Different Fuels. Energies 2021, 14, 2966. [Google Scholar] [CrossRef]
- Goerner, K. Waste Incineration European State of the Art and New Developments. IFRF Combust. J. 2003, 32, 200303. Available online: https://ifrf.net/research/archive/waste-incineration-european-state-of-the-art-and-new-developments/ (accessed on 12 December 2023).
- Morrow, R.S. Renewable Fuel Grate firing Combustion Technology—The European Experience; Detroit Stoker Company: Monroe, MI, USA, 2005. [Google Scholar]
- Spliethoff, H.; Hein, K.R.G. Effect of co-combustion of biomass on emissions in pulverized fuel furnaces. Fuel Process. Technol. 1998, 54, 189–205. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Subramanian, A.K.; Marwaha, Y. Use of bagasse and other biomass fuels in high pressure travelling grate boilers. Int. Sugar J. 2006, 108, 6–9. Available online: https://www.researchgate.net/publication/284672399_Use_of_bagasse_and_other_biomass_fuels_in_high_pressure_Travelling_Grate_boilers (accessed on 5 January 2024).
- Nikiforov, A.; Prikhodko, E.; Kinzhibekova, A.; Nurkina, S. Modeling the influence of the characteristics of renewable organic materials on the energy performance of the boiler. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Borovets, Bulgaria, 26–29 November 2020; IOP Publishing: Bristol, UK, 2020; Volume 1032, p. 012035. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T. Sewage sludge combustion. Progr. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Sami, M.; Annamalai, K.; Wooldridge, M. Co-firing of coal and biomass fuel blends. Progr. Energy Combust. Sci. 2001, 27, 171–214. [Google Scholar] [CrossRef]
- Thunman, H.; Leckner, B. Ignition and propagation of a reaction front in cross-current bed combustion of wet biofuels. Fuel 2001, 80, 473–481. [Google Scholar] [CrossRef]
- Nikiforov, A.S.; Prikhodko, E.V.; Kinzhibekova, A.K.; Nurkina, S.M. Study of strength characteristics of fuel briquettes from organic waste. In Proceedings of the AIP Conference Proceedings, Tomsk, Russia, 9–11 October 2019; Volume 2212, p. 020044. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Xu, L.; Ma, M.; Huang, X.; Han, F.; Zhou, Y.; Du, C.; Da, Y.; Deng, L. Computational Particle Fluid Dynamics Simulation on Combustion Characteristics of Blended Fuels of Coal, Biomass, and Oil Sludge in a 130th−1 Circulating Fluidized Bed Boiler. Energies 2024, 17, 149. [Google Scholar] [CrossRef]
- Kraszkiewicz, A.; Przywara, A.; Anifantis, A.S. Impact of Ignition Technique on Pollutants Emission during the Combustion of Selected Solid Biofuels. Energies 2020, 13, 2664. [Google Scholar] [CrossRef]
- Kraszkiewicz, A.; Przywara, A.; Parafiniuk, S. Emission of Nitric Oxideduring the Combustion of Various Forms of Solid Biofuels in a Low-Power Heating Device. Energies 2022, 15, 5960. [Google Scholar] [CrossRef]
- Ozgen, S.; Cernuschi, S.; Caserini, S. An overview of nitrogen oxides emissions from biomass combustion for domestic heat production. Renew. Sustain. Energy Rev. 2021, 135, 110113. [Google Scholar] [CrossRef]
- Huang, H.; Gao, Y.; Chen, H.; Wu, Y.; Wang, J.; Yu, C.; Li, J.; Zou, C. Biomass briquette fuel, boiler types and pollutant emissions of industrial biomass boiler: A review. Particuology 2023, 77, 79–90. [Google Scholar] [CrossRef]
- Yue, T.; Tong, Y.; Gao, J.; Yuan, Y.; Wang, L.; Wei, H. High-precision spatial-temporal variations and future perspectives of multiple air pollutant emissions from Chinese biomass-fired industrial boilers. Sci. Total Environ. 2024, 907, 167982. [Google Scholar] [CrossRef] [PubMed]
- Pilusa, T.; Huberts, R.; Muzenda, E. Emissions analysis from combustion of ecofuel briquettes for domestic application. J. Energy South. Afr. 2013, 24, 30–36. [Google Scholar] [CrossRef]
- Wielgosiński, G.; Łechtańska, P.; Namiecińska, O. Emission of some pollutants from biomass combustion in comparison to hard coal combustion. J. Energy Inst. 2017, 90, 787–796. [Google Scholar] [CrossRef]
- ACTM E 230/E 230Ma:2023; Standard Specification and Temperature-Electromotive Force (EMF) Tables for Standardized Thermocouples. ASTM International: Washington, DC, USA, 2023.
- Knorre, G.F. Furnace Processes [Toposhnye Prosesy], 2nd ed.; Gosenergoizdat Publ: Moscow, Russia, 1959; p. 396. (In Russian) [Google Scholar]
- Panteleev, A.V.; Yakimova, A.S.; Bosov, A.V. Ordinary Differential Equations in Examples and Problems; Higher School: Moscow, Russia, 2001; p. 376. (In Russian) [Google Scholar]
- Aramanovich, I.G.; Lunts, G.L.; Elsgolts, L.E. Functions of a Complex Variable. Operational Calculus. Theory of Stability; Nauka: Moscow, Russia, 1968; 416p. (In Russian) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).