Methods and Validation Techniques of Chemical Kinetics Models in Waste Thermal Conversion Processes
Abstract
1. Introduction
2. Theoretical Background
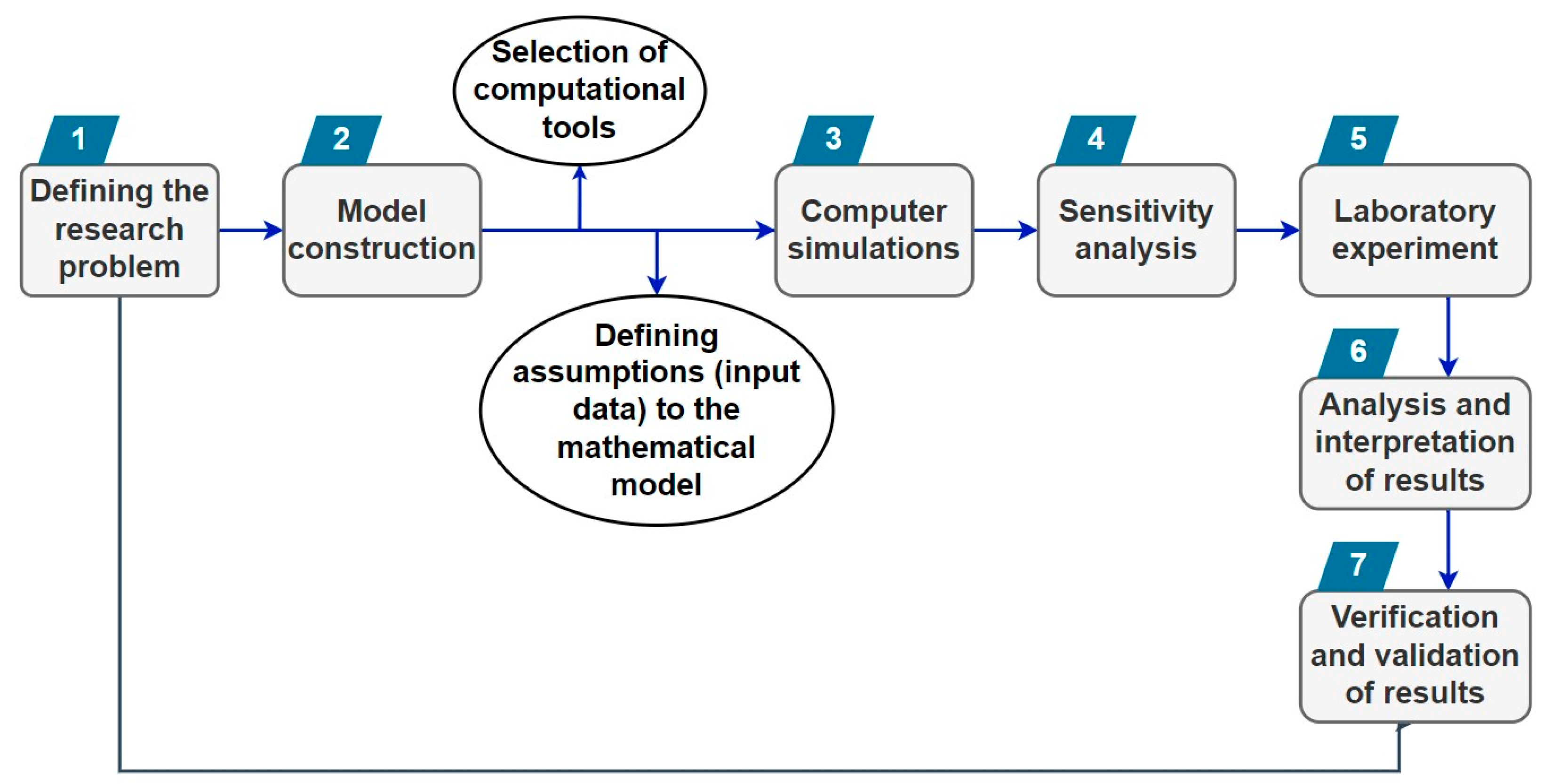
2.1. The Essence of Computer Simulations
- Too high costs of conducting experimental research in real conditions;
- The need to quickly obtain accurate data on the phenomenon under study;
- The need to obtain a range of information that is impossible to obtain exponentially, which will complement and enrich the existing state of knowledge;
- The need to avoid mistakes whose consequences may be serious;
- The lack of an actual research object or when it is only at the design stage.
2.2. Application of Numerical-Simulation Methods in the Analysis of Thermal Conversion Processes of Fuels and Waste
- i.
- ii.
- iii.
- i.
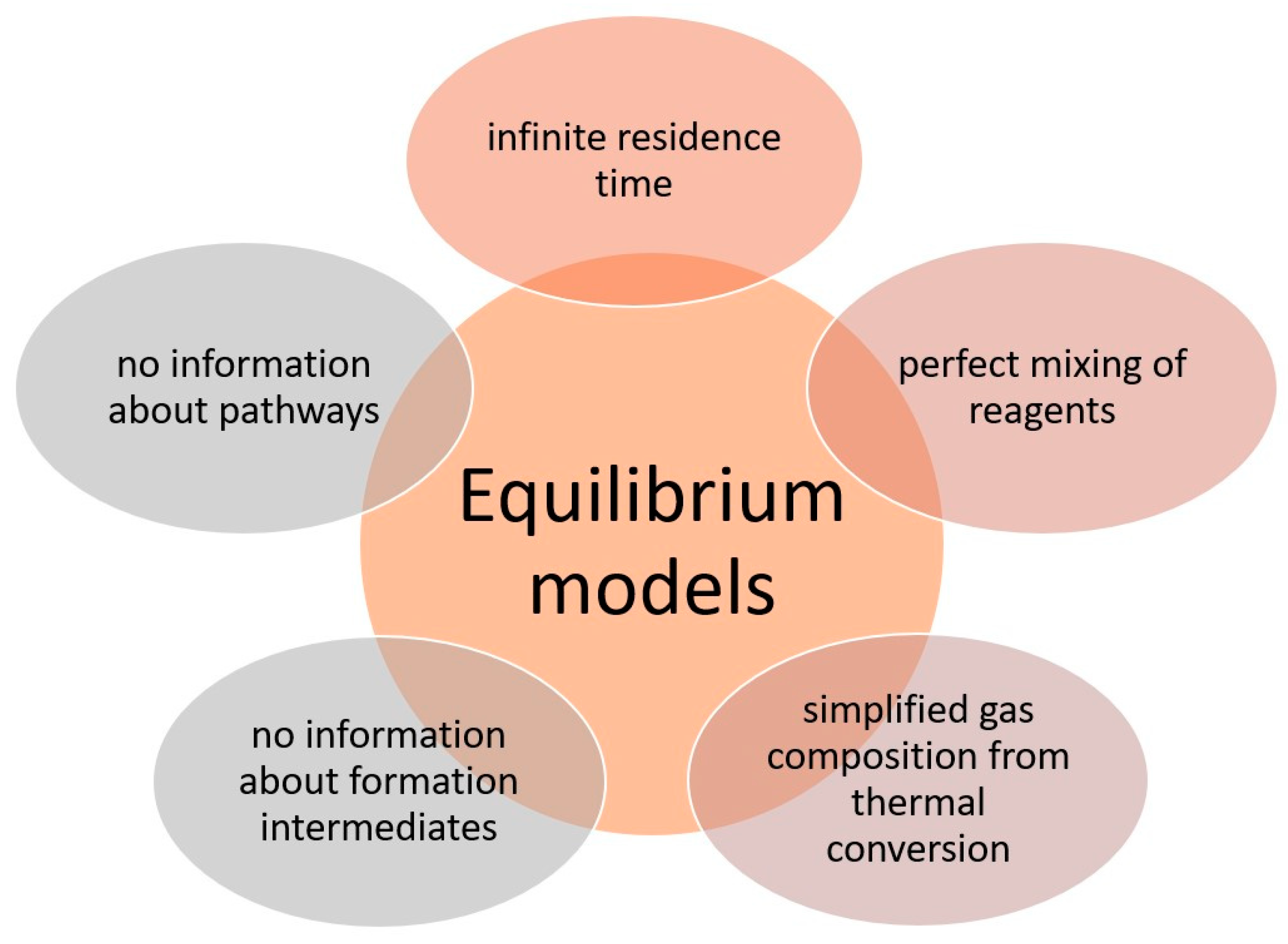
- Stoichiometric models;
- ii.
- Non-stoichiometric models.
2.3. Tools for Numerical Simulation of Thermal Conversion Processes of Fuels and Waste
2.4. Validation Techniques of Chemical Kinetics Models in Waste Thermal Conversion Processes
3. Results
4. Discussion
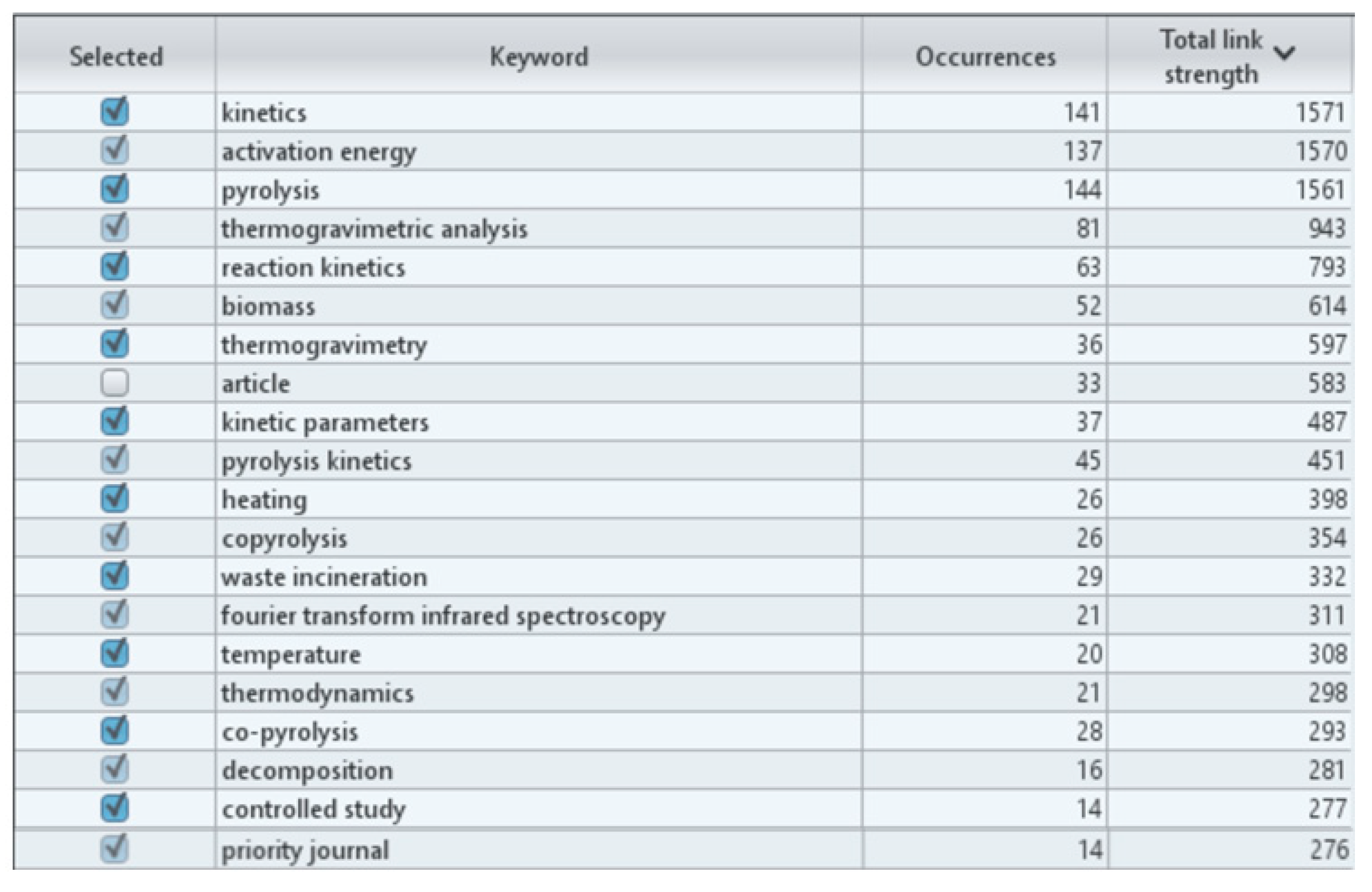
- The analysis carried out with the use of the VOSviewer software shows that, despite many scientific works published on this topic, there is still a research gap in the issues discussed in the article.
- The literature on the subject emphasizes that a number of factors are responsible for the proper use of computer simulation methods, and the most important advantage is the ability to simulate phenomena that are difficult or impossible to implement in real conditions.
- The results of numerical modeling using simplified chemical mechanisms (CFD modeling) describing the combustion process are subject to large errors (due to the long calculation time, simplifications of chemical kinetics are used in the computational procedure, namely reducing the chemistry of methane oxidation to a single-stage mechanism in which combustion products are CO2, H2O, O2 and N2), which is why they often do not coincide with actual measurements. The assumption of a simple combustion mechanism in the calculations results in significant discrepancies with the measurement results.
- Due to the possibility to create a multi-stage, complex model in the ANSYS Chemkin-Pro program, which does not use simplifications in thermodynamics and chemical kinetics, various waste thermal processing processes can be modeled with high accuracy.
- The cost of building a model is often much lower than conducting experimental research, resulting in significant savings in time and money.
- Computer simulations also have several disadvantages, the most important of which is the possibility of making an error when creating a model that omits parameters and chemical reactions that are important from the point of view of the analyzed process.
- Moreover, numerous works indicate difficulties and errors when interpreting the obtained simulation results. The above-mentioned defects are the responsibility of humans, who may not only incorrectly formulate the simulated problem and adjust the tool but may also draw incorrect conclusions from the performed simulations.
- It is crucial to validate the developed model and verify the obtained calculations through experimental means using statistical analysis (e.g., MATLAB, ANOVA) or based on literature data. Without such validation, the results of the model calculations remain hypothetical.
- The issue of managing environmentally burdensome gaseous products of thermal conversion of calorific waste is of an applied nature, as evidenced by the interest of both entrepreneurs from the waste and metallurgical industries. However, this direction requires extensive theoretical studies in the analysis of chemical mechanisms.
- Knowledge of chemical mechanisms describing the process of combustion or co-combustion of gases from the thermal conversion of waste will create the possibility of their energy utilization.
- Providing comprehensive knowledge about combustion kinetics will also enable taking appropriate actions to manage gases after thermal conversion of waste while maintaining the proper operation of the heating chamber and minimizing pollution.
- Currently, it is particularly important and problematic to properly identify the chemical composition of exhaust gases while, at the same time, focusing on activities aimed at minimizing harmful pollutants, such as PAHs.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuo, W.C.; Lasek, J.; Słowik, K.; Głód, K.; Jagustyn, B.; Li, Y.H.; Cygan, A. Low-Temperature Pre-Treatment of Municipal Solid Waste for Efficient Application in Combustion Systems. Energy Convers. Manag. 2019, 196, 525–535. [Google Scholar] [CrossRef]
- Lombardi, L.; Carnevale, E.; Corti, A. Analysis of Energy Recovery Potential Using Innovative Technologies of Waste Gasification. Waste Manag. 2012, 32, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, X.; Ge, X.; Chen, M. Thermochemical Treatment of Non-Metallic Residues from Waste Printed Circuit Board: Pyrolysis vs. Combustion. J. Clean. Prod. 2018, 176, 1045–1053. [Google Scholar] [CrossRef]
- Albergaria Campos, A.M.; Khozhanov, N.; Assis, P.S.; Tursunbaev, K.; Masatbayev, M. Economic and Environmental Analyses of Biomass Torrefaction for Injection as Pulverized Material in Blast Furnaces. REM-Int. Eng. J. 2021, 74, 471–482. [Google Scholar] [CrossRef]
- Pan, S.Y.; Du, M.A.; Huang, I.T.; Liu, I.H.; Chang, E.E.; Chiang, P.C. Strategies on Implementation of Waste-to-Energy (WTE) Supply Chain for Circular Economy System: A Review. J. Clean. Prod. 2015, 108, 409–421. [Google Scholar] [CrossRef]
- Rathore, P.; Sarmah, S.P. Economic, Environmental and Social Optimization of Solid Waste Management in the Context of Circular Economy. Comput. Ind. Eng. 2020, 145, 106510. [Google Scholar] [CrossRef]
- Lu, P.; Huang, Q.; Bourtsalas, A.C.; Themelis, N.J.; Chi, Y.; Yan, J. Review on Fate of Chlorine during Thermal Processing of Solid Wastes. J. Environ. Sci. 2019, 78, 13–28. [Google Scholar] [CrossRef]
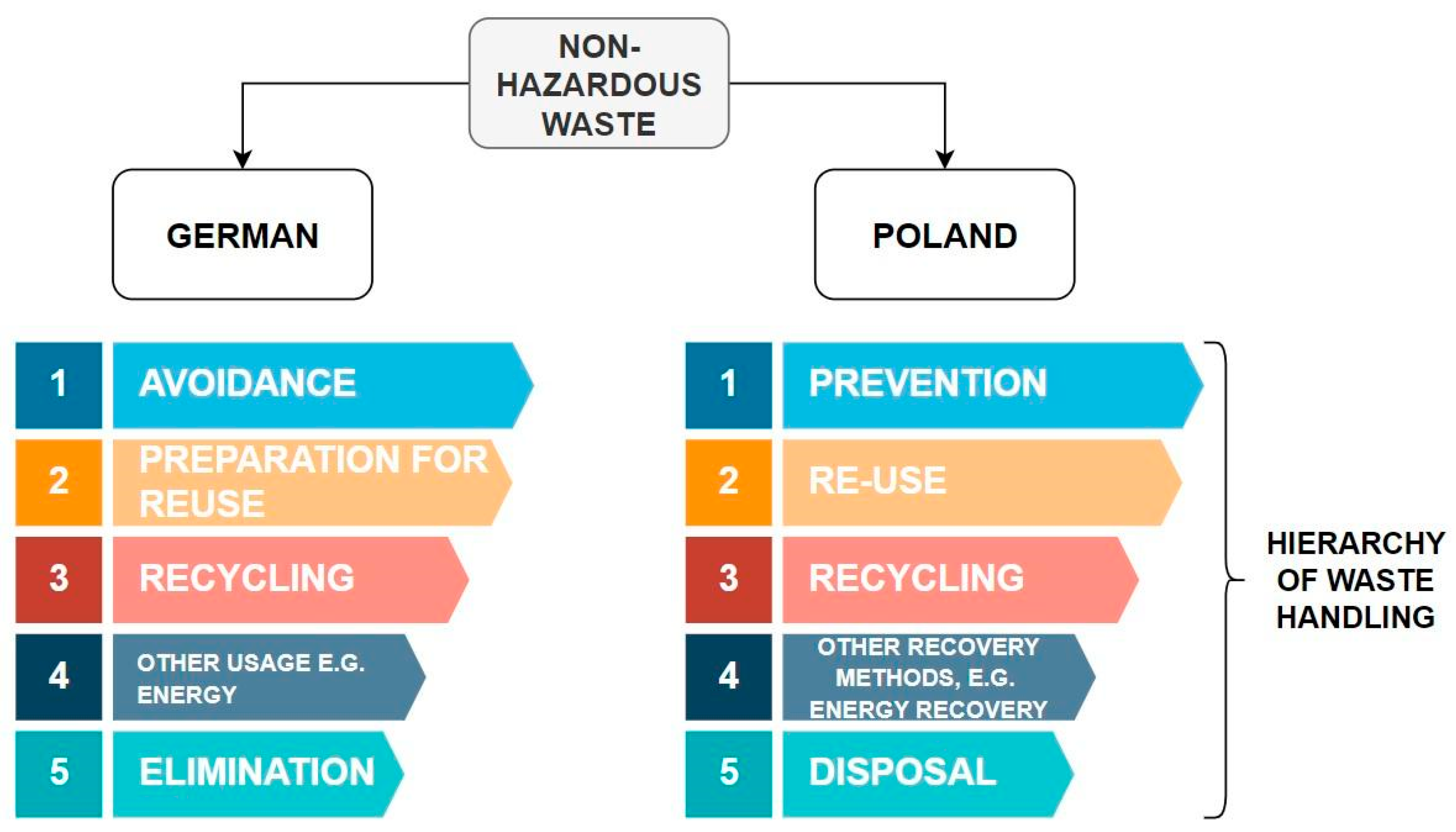
- Available online: https://www.Europarl.Europa.Eu/Topics/Pl/Article/20180328STO00751/Zrownowazone-Zarzadzanie-Odpadami-Dzialania-Ue (accessed on 22 May 2024).
- European Parliament; Council of the European Union. Dyrektywa Parlamentu Europejskiego i Rady 2008/98/WE Dnia 19 Listopada 2008 r.W Sprawie Odpadów Oraz Uchylająca Niektóre Dyrektywy. Dziennik Urzędowy Unii Europejskiej 2008, 22, 3–30. [Google Scholar]
- Available online: https://www.Bonnorange.de/Nachhaltigkeit/Klimarechner/Abfallhierarchie (accessed on 23 May 2024).
- Available online: https://www.Retech-Germany.Net/Themen/Der-Weg-Zur-Modernen-Abfallwirtschaft/Prinzipien-Nachhaltiger-Abfallwirtschaft (accessed on 23 May 2024).
- Li, Y.; Fu, Z.; Li, J. Assessing the Policy Benefits of Constructing “Zero-Waste Cities” in China: From the Perspective of Hazardous Waste Lifecycle Management. Sci. Total. Environ. 2024, 918, 170184. [Google Scholar] [CrossRef]
- Zhang, Z.; Malik, M.Z.; Khan, A.; Ali, N.; Malik, S.; Bilal, M. Environmental Impacts of Hazardous Waste, and Management Strategies to Reconcile Circular Economy and Eco-Sustainability. Sci. Total. Environ. 2022, 807, 150856. [Google Scholar] [CrossRef]
- Xi, B.; Yang, T.; Zhao, R.; Jing, L.; Gong, T.; Huang, Q.; Hou, L. Hazardous Waste Management in the Guangdong–Hong Kong–Macao Greater Bay Area. Engineering 2022, 8, 25–28. [Google Scholar] [CrossRef]
- Andrade, L.C.; Míguez, C.G.; Gómez, M.C.T.; Bugallo, P.M.B. Management Strategy for Hazardous Waste from Atomised SME: Application to the Printing Industry. J. Clean. Prod. 2012, 35, 214–229. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Monteiro, E.; Rouboa, A. Hazardous Waste Management in Portugal: An Overview. Energy Procedia 2013, 36, 607–611. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Moustakas, K. Household Hazardous Waste Management: A Review. J. Environ. Manag. 2015, 150, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Ranzi, E.; Corbetta, M.; Manenti, F.; Pierucci, S. Kinetic Modeling of the Thermal Degradation and Combustion of Biomass. Chem. Eng. Sci. 2014, 110, 2–12. [Google Scholar] [CrossRef]
- Chavando, J.A.M.; Silva, V.; Puig-gamero, M.; Cardoso, J.S.; Tarelho, L.A.C.; Eusébio, D. Simulation of Biomass to Syngas: Pyrolysis and Gasification Processes; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323918794. [Google Scholar]
- Li, J.; Suvarna, M.; Li, L.; Pan, L.; Pérez-Ramírez, J.; Ok, Y.S.; Wang, X. A Review of Computational Modeling Techniques for Wet Waste Valorization: Research Trends and Future Perspectives. J. Clean. Prod. 2022, 367, 133025. [Google Scholar] [CrossRef]
- Escalante, J.; Chen, W.H.; Tabatabaei, M.; Hoang, A.T.; Kwon, E.E.; Andrew Lin, K.Y.; Saravanakumar, A. Pyrolysis of Lignocellulosic, Algal, Plastic, and Other Biomass Wastes for Biofuel Production and Circular Bioeconomy: A Review of Thermogravimetric Analysis (TGA) Approach. Renew. Sustain. Energy Rev. 2022, 169, 112914. [Google Scholar] [CrossRef]
- Khan, S.A.; Ali, I.; Naqvi, S.R.; Li, K.; Mehran, M.T.; Khoja, A.H.; Alarabi, A.A.; Atabani, A.E. Investigation of Slow Pyrolysis Mechanism and Kinetic Modeling of Scenedesmus Quadricauda Biomass. J. Anal. Appl. Pyrolysis 2021, 158, 105149. [Google Scholar] [CrossRef]
- Chen, Z.; Hammad, A.W.A. Mathematical Modelling and Simulation in Construction Supply Chain Management. Autom. Constr. 2023, 156, 105147. [Google Scholar] [CrossRef]
- Zajemska, M.; Poskart, A. Mozliwości Zastosowania Metod Numerycznych Do Przewidywania I Ograniczania Emisji Zanieczyszczeń z Instalacji Spalania Stosowanych w Przemyśle Chemicznym I Rafineryjnym. Przem. Chem. 2013, 92, 357–361. [Google Scholar]
- Kurebwa, J.; Mushiri, T. Design and Simulation of an Integrated Steering System for All-Purpose Sport Utility Vehicles (SUVs)—Case for Toyota. Procedia Manuf. 2019, 35, 56–74. [Google Scholar] [CrossRef]
- Hamlaci Baskaya, Y.; Kurt, G.; İlcioğlu, K.; Turan, Z. Assessment of Efficacy of Two Different Simulation Techniques Used in Breech Birth Management Training: A Randomized Controlled Study. Clin. Simul. Nurs. 2024, 87, 101499. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, S.; Zuo, H.; Lin, J.; Zheng, H.; Lei, H.; Yu, Q.; Wu, X.; Guo, Z. Freeze-drying pretreatment of watermelon peel to improve the efficiency of pectin extraction: RSM optimization, extraction mechanism, and characterization. Int. J. Biol. Macromol. 2023, 249, 125944. [Google Scholar] [CrossRef] [PubMed]
- Louback, E.; Biswas, A.; Machado, F.; Emadi, A. Review Article A Review of the Design Process of Energy Management Systems for Dual-Motor Battery Electric Vehicles. Renew. Sustain. Energy Rev. 2024, 193, 114293. [Google Scholar] [CrossRef]
- Baker, K.R.; Liljegren, J.; Valin, L.; Judd, L.; Szykman, J.; Millet, D.B.; Czarnetzki, A.; Whitehill, A.; Murphy, B.; Stanier, C. Photochemical Model Representation of Ozone and Precursors during the 2017 Lake Michigan Ozone Study (LMOS). Atmos. Environ. 2023, 293, 119465. [Google Scholar] [CrossRef]
- Tao, Z.; Kawa, S.R.; Jacob, J.P.; Liu, D.Y.; Collatz, G.J.; Wang, J.S.; Ott, L.E.; Chin, M. Application of NASA-Unified WRF Model to Carbon Dioxide Simulation- Model Development and Evaluation. Environ. Model. Softw. 2020, 132, 104785. [Google Scholar] [CrossRef]
- Kahre, M.A.; Haberle, R.M.; Wilson, R.J.; Urata, R.A.; Steakley, K.E.; Brecht, A.S.; Bertrand, T.; Kling, A.; Batterson, C.M.; Hartwick, V.; et al. The NASA Ames Legacy Mars Global Climate Model: Radiation Code Error Correction and New Baseline Water Cycle Simulation. Icarus 2023, 400, 115561. [Google Scholar] [CrossRef]
- Huff, J.L.; Poignant, F.; Rahmanian, S.; Khan, N.; Blakely, E.A.; Britten, R.A.; Chang, P.; Fornace, A.J.; Hada, M.; Kronenberg, A.; et al. Galactic Cosmic Ray Simulation at the NASA Space Radiation Laboratory—Progress, Challenges and Recommendations on Mixed-Field Effects. Life Sci. Space Res. 2023, 36, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Marschik, C.; Roland, W.; Löw-Baselli, B.; Steinbichler, G. Application of Hybrid Modeling in Polymer Processing. Annu. Tech. Conf.-ANTEC Conf. Proc. 2020, 2, 535–542. [Google Scholar]
- Zhang, Y.; Ji, Y.; Qian, H. Progress in Thermodynamic Simulation and System Optimization of Pyrolysis and Gasification of Biomass. Green Chem. Eng. 2021, 2, 266–283. [Google Scholar] [CrossRef]
- Vikram, S.; Rosha, P.; Kumar, S. Recent Modeling Approaches to Biomass Pyrolysis: A Review. Energy Fuels 2021, 35, 7406–7433. [Google Scholar] [CrossRef]
- Irfan, M.; Nabi, R.A.U.; Hussain, H.; Naz, M.Y.; Shukrullah, S.; Khawaja, H.A.; Rahman, S.; Ghanim, A.A.J.; Kruszelnicka, I.; Ginter-Kramarczyk, D.; et al. Response Surface Methodology Analysis of Pyrolysis Reaction Rate Constants for Predicting Efficient Conversion of Bulk Plastic Waste into Oil and Gaseous Fuels. Energies 2022, 15, 9594. [Google Scholar] [CrossRef]
- Ramanathan, A.; Begum, K.M.M.S.; Pereira, A.O.; Cohen, C. Biomass Pyrolysis System Based on Life Cycle Assessment and Aspen plus Analysis and Kinetic Modeling. In A Thermo-Economic Approach to Energy from Waste; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780128243572. [Google Scholar]
- Agh, O. Metody Symulacji Komputerowej. Available online: http://www.pi.zarz.agh.edu.pl/inne/odlewn/Wyklady/PSIZ%20Symulacja.pdf (accessed on 5 January 2024).
- Jąderko, K.; Białecka, B. Model Technologiczno-Logistyczny Logistyczny Procesu Energetycznego Procesu Energetycznego Wykorzystania Odpadów; 2016; ISBN 9788365265081. Available online: http://www.stegroup.pl/attachments/article/1/Monografia%20J.pdf (accessed on 6 January 2024).
- Zajemska, M. Wymagania Stawiane Technice Obliczeniowej W Zakresie Numerycznego Modelowania Składu Chemicznego Produktów Spalania. Model. Inżynierskie 2011, 41, 453–461. [Google Scholar]
- Jaiswal, S.; Sahani, S.; Shar, Y.C. Enviro-Benign Synthesis of Glycerol Carbonate Utilizing Bio-Waste Glycerol over Na-Ti Based Heterogeneous Catalyst: Kinetics and E- Metrics Studies. J. Environ. Chem. Eng. 2022, 10, 107485. [Google Scholar] [CrossRef]
- Xiao, H.; Li, Z.; Jia, X.; Ren, J. Waste to Energy in a Circular Economy Approach for Better Sustainability: A Comprehensive Review and SWOT Analysis. In Waste-to-Energy; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128163948. [Google Scholar]
- Zajemska, M.; Sajdak, M.; Iwaszko, J.; Skrzyniarz, M.; Biniek-Poskart, A.; Skibiński, A.; Maroszek, A. The Role of Calorific Waste in Transformation of Iron and Steel Industry towards Sustainable Production. Resour. Conserv. Recycl. 2023, 191, 2022–2024. [Google Scholar] [CrossRef]
- Yahya, S.A.; Iqbal, T.; Omar, M.M.; Ahmad, M. Techno-Economic Analysis of Fast Pyrolysis of Date Palm Waste for Adoption in Saudi Arabia. Energies 2021, 14, 6048. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A Review on Thermal and Catalytic Pyrolysis of Plastic Solid Waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Valin, S.; Cances, J.; Castelli, P.; Thiery, S.; Dufour, A.; Boissonnet, G.; Spindler, B. Upgrading Biomass Pyrolysis Gas by Conversion of Methane at High Temperature: Experiments and Modelling. Fuel 2009, 88, 834–842. [Google Scholar] [CrossRef]
- Lukáč, L.; Kizek, J.; Jablonský, G.; Karakash, Y. Defining the Mathematical Dependencies of NOx and CO Emission Generation after Biomass Combustion in Low-Power Boiler. Civ. Environ. Eng. Rep. 2019, 29, 153–163. [Google Scholar] [CrossRef]
- Dhahak, A.; Bounaceur, R.; Le Dreff-Lorimier, C.; Schmidt, G.; Trouve, G.; Battin-Leclerc, F. Development of a Detailed Kinetic Model for the Combustion of Biomass. Fuel 2019, 242, 756–774. [Google Scholar] [CrossRef]
- Chang, S.-L.; Zhou, C.Q. Combustion and Thermochemistry. Encycl. Energy 2004, 1, 595–603. [Google Scholar] [CrossRef]
- Dupont, C.; Boissonnet, G.; Seiler, J.M.; Gauthier, P.; Schweich, D. Study about the Kinetic Processes of Biomass Steam Gasification. Fuel 2007, 86, 32–40. [Google Scholar] [CrossRef]
- Mazaheri, N.; Akbarzadeh, A.H.; Madadian, E.; Lefsrud, M. Systematic Review of Research Guidelines for Numerical Simulation of Biomass Gasification for Bioenergy Production. Energy Convers. Manag. 2019, 183, 671–688. [Google Scholar] [CrossRef]
- Gambarotta, A.; Morini, M.; Zubani, A. A Non-Stoichiometric Equilibrium Model for the Simulation of the Biomass Gasification Process. Appl. Energy 2018, 227, 119–127. [Google Scholar] [CrossRef]
- Ren, R.; Wang, H.; Feng, X.; You, C. Techno-Economic Analysis of Auto-Thermal Gasification of Municipal Solid Waste with Ash Direct Melting for Hydrogen Production. Energy Convers. Manag. 2023, 292, 117401. [Google Scholar] [CrossRef]
- Ferreiro, A.I.; Segurado, R.; Costa, M. Modelling Soot Formation during Biomass Gasification. Renew. Sustain. Energy Rev. 2020, 134, 110380. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of Biomass Torrefaction Based on Three Major Components: Hemicellulose, Cellulose, and Lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X. The Thermochemical Conversion of Biomass into Biofuels; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081024263. [Google Scholar]
- Venturini, P.; Borello, D.; Iossa, C.; Lentini, D.; Rispoli, F. Modeling of Multiphase Combustion and Deposit Formation in a Biomass-Fed Furnace. Energy 2010, 35, 3008–3021. [Google Scholar] [CrossRef]
- Ji, W.; Richter, F.; Gollner, M.J.; Deng, S. Autonomous Kinetic Modeling of Biomass Pyrolysis Using Chemical Reaction Neural Networks. Combust. Flame 2022, 240, 111992. [Google Scholar] [CrossRef]
- Eke, J.; Onwudili, J.A.; Bridgwater, A.V. Physical Pretreatment of Biogenic-Rich Trommel Fines for Fast Pyrolysis. Waste Manag. 2017, 70, 81–90. [Google Scholar] [CrossRef][Green Version]
- Du, Y.; Ju, T.; Meng, Y.; Han, S.; Jiang, J. Pyrolysis Characteristics of Excavated Waste and Generation Mechanism of Gas Products. J. Clean. Prod. 2022, 370, 133489. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Bouadili, A. Thermal Degradation Behaviors of Polyethylene and Polypropylene. Part I: Pyrolysis Kinetics and Mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Till, Z.; Varga, T.; Sója, J.; Miskolczi, N.; Chován, T. Kinetic Modeling of Plastic Waste Pyrolysis in a Laboratory Scale Two-Stage Reactor. Comput. Aided Chem. Eng. 2018, 43, 349–354. [Google Scholar] [CrossRef]
- Martínez-Narro, G.; Royston, N.J.; Billsborough, K.L.; Phan, A.N. Kinetic Modelling of Mixed Plastic Waste Pyrolysis. Chem. Thermodyn. Therm. Anal. 2023, 9, 100105. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H.; McDonald, A.G. Evaluation of Pyrolysis Process Parameters on Polypropylene Degradation Products. J. Anal. Appl. Pyrolysis 2014, 109, 272–277. [Google Scholar] [CrossRef]
- Ateş, F.; Miskolczi, N.; Borsodi, N. Comparision of Real Waste (MSW and MPW) Pyrolysis in Batch Reactor over Different Catalysts. Part I: Product Yields, Gas and Pyrolysis Oil Properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, B.; Fernández, A.M.; Barriocanal, C. Identification of Polymers in Waste Tyre Reinforcing Fibre by Thermal Analysis and Pyrolysis. J. Anal. Appl. Pyrolysis 2015, 111, 224–232. [Google Scholar] [CrossRef]
- Policella, M.; Wang, Z.; Burra, K.G.; Gupta, A.K. Characteristics of Syngas from Pyrolysis and CO2-Assisted Gasification of Waste Tires. Appl. Energy 2019, 254, 113678. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, D.; Lv, P.; Liu, Z.; Cheng, T.; Wang, B. Fine particles removal of pyrolysis gasification flue gas from rural domestic waste: Laboratory research, molecular dynamics simulation, and applications. Environ. Res. 2023, 236, 116732. [Google Scholar] [CrossRef]
- Porshnov, D.; Ozols, V.; Ansone-Bertina, L.; Burlakovs, J.; Klavins, M. Thermal Decomposition Study of Major Refuse Derived Fuel Components. Energy Procedia 2018, 147, 48–53. [Google Scholar] [CrossRef]
- Jannelli, E.; Minutillo, M. Simulation of the Flue Gas Cleaning System of an RDF Incineration Power Plant. Waste Manag. 2007, 27, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Çepelioğullar; Haykiri-Açma, H.; Yaman, S. Kinetic Modelling of RDF Pyrolysis: Model-Fitting and Model-Free Approaches. Waste Manag. 2016, 48, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Bystrzejewski, M.; De Adhikari, A.; Huczko, A.; Wang, N. Methods for the Conversion of Biomass Waste into Value-Added Carbon Nanomaterials: Recent Progress and Applications. Prog. Energy Combust. Sci. 2022, 92, 101023. [Google Scholar] [CrossRef]
- Sabogal, O.S.; Valin, S.; Thiery, S.; Salvador, S. Pyrolysis of Solid Waste and Its Components in a Lab Scale Induction-Heating Reactor. Detritus 2021, 15, 107–112. [Google Scholar] [CrossRef]
- Xie, J.; Zhong, W.; Jin, B.; Shao, Y.; Liu, H. Simulation on Gasification of Forestry Residues in Fluidized Beds by Eulerian-Lagrangian Approach. Bioresour. Technol. 2012, 121, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Radmanesh, R.; Courbariaux, Y.; Chaouki, J.; Guy, C. A Unified Lumped Approach in Kinetic Modeling of Biomass Pyrolysis. Fuel 2006, 85, 1211–1220. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P. Kinetic Study of High Density Polyethylene (HDPE) Pyrolysis. Chem. Eng. Res. Des. 2010, 88, 1599–1606. [Google Scholar] [CrossRef]
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A Review of Thermochemical Conversion of Waste Biomass to Biofuels. Energies 2022, 15, 6352. [Google Scholar] [CrossRef]
- Liu, H.; Alhumade, H.; Elkamel, A. A Combined Scheme of Parallel-Reaction Kinetic Model and Multi-Layer Artificial Neural Network Model on Pyrolysis of Reed Canary. Chem. Eng. Sci. 2023, 281, 119109. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Pan, S.; Dong, N.; Wang, X.; Sun, S. Initial Pyrolysis Mechanism and Product Formation of Cellulose: An Experimental and Density Functional Theory(DFT) Study. Sci. Rep. 2020, 10, 3626. [Google Scholar] [CrossRef]
- Tang, X.; Xie, Q.; Qiu, R.; Yang, Y. Development of a Relationship between Kinetic Triplets and Heating Rates to Improve Pyrolysis Kinetic Modeling of Polymer. Polym. Degrad. Stab. 2018, 154, 10–26. [Google Scholar] [CrossRef]
- González-Vázquez, M.P.; Rubiera, F.; Pevida, C.; Pio, D.T.; Tarelho, L.A.C. Thermodynamic Analysis of Biomass Gasification Using Aspen plus: Comparison of Stoichiometric and Non-Stoichiometric Models. Energies 2021, 14, 189. [Google Scholar] [CrossRef]
- Moretti, L.; Arpino, F.; Cortellessa, G.; Di Fraia, S.; Di Palma, M.; Vanoli, L. Reliability of Equilibrium Gasification Models for Selected Biomass Types and Compositions: An Overview. Energies 2022, 15, 61. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, Z.; Zhang, B.; Xue, Z.; Guo, F.; Wang, J. Prediction of Kinetic Parameters of Biomass Pyrolysis Based on the Optimal Mixture Design Method. Clean. Technol. Environ. Policy 2016, 18, 1621–1629. [Google Scholar] [CrossRef]
- Xie, T.; Zhao, L.; Yao, Z.; Kang, K.; Jia, J.; Hu, T.; Zhang, X.; Sun, Y.; Huo, L. Co-Pyrolysis of Biomass and Polyethylene: Insights into Characteristics, Kinetic and Evolution Paths of the Reaction Process. Sci. Total. Environ. 2023, 897, 165443. [Google Scholar] [CrossRef] [PubMed]
- Mian, I.; Li, X.; Jian, Y.; Dacres, O.D.; Zhong, M.; Liu, J.; Ma, F.; Rahman, N. Kinetic Study of Biomass Pellet Pyrolysis by Using Distributed Activation Energy Model and Coats Redfern Methods and Their Comparison. Bioresour. Technol. 2019, 294, 122099. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of Wood. Part 1. Weight Loss Kinetics. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Hu, S.; Jess, A.; Xu, M. Kinetic Study of Chinese Biomass Slow Pyrolysis: Comparison of Different Kinetic Models. Fuel 2007, 86, 2778–2788. [Google Scholar] [CrossRef]
- Zou, J.; Hu, H.; Xue, Y.; Li, C.; Li, Y.; Yellezuome, D.; He, F.; Zhang, X.; Maksudur Rahman, M.; Cai, J. Exploring Kinetic Mechanisms of Biomass Pyrolysis Using Generalized Logistic Mixture Model. Energy Convers. Manag. 2022, 258, 115522. [Google Scholar] [CrossRef]
- Torres-Sciancalepore, R.; Asensio, D.; Nassini, D.; Fernandez, A.; Rodriguez, R.; Fouga, G.; Mazza, G. Assessment of the Behavior of Rosa Rubiginosa Seed Waste during Slow Pyrolysis Process towards Complete Recovery: Kinetic Modeling and Product Analysis. Energy Convers. Manag. 2022, 272, 116340. [Google Scholar] [CrossRef]
- Wissing, F.; Wirtz, S.; Scherer, V. Simulating Municipal Solid Waste Incineration with a DEM/CFD Method—Influences of Waste Properties, Grate and Furnace Design. Fuel 2017, 206, 638–656. [Google Scholar] [CrossRef]
- Kumar, U.; Paul, M.C. CFD Modelling of Biomass Gasification with a Volatile Break-up Approach. Chem. Eng. Sci. 2019, 195, 413–422. [Google Scholar] [CrossRef]
- Bieniek, A.; Reinmöller, M.; Küster, F.; Gräbner, M.; Jerzak, W.; Magdziarz, A. Investigation and Modelling of the Pyrolysis Kinetics of Industrial Biomass Wastes. J. Environ. Manag. 2022, 319, 115707. [Google Scholar] [CrossRef]
- Debiagi, P.; Nicolai, H.; Han, W.; Janicka, J.; Hasse, C. Machine Learning for Predictive Coal Combustion CFD Simulations—From Detailed Kinetics to HDMR Reduced-Order Models. Fuel 2020, 274, 117720. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A Review on the Pyrolysis of Woody Biomass to Bio-Oil: Focus on Kinetic Models. Renew. Sustain. Energy Rev. 2015, 52, 1580–1595. [Google Scholar] [CrossRef]
- Sommariva, S.; Grana, R.; Maffei, T.; Pierucci, S.; Ranzi, E. A Kinetic Approach to the Mathematical Model of Fixed Bed Gasifiers. Comput. Chem. Eng. 2011, 35, 928–935. [Google Scholar] [CrossRef]
- Baruah, D.; Baruah, D.C. Modeling of Biomass Gasification: A Review. Renew. Sustain. Energy Rev. 2014, 39, 806–815. [Google Scholar] [CrossRef]
- Ascher, S.; Watson, I.; You, S. Machine Learning Methods for Modelling the Gasification and Pyrolysis of Biomass and Waste. Renew. Sustain. Energy Rev. 2022, 155, 111902. [Google Scholar] [CrossRef]
- Grange, N.; Chetehouna, K.; Gascoin, N.; Coppalle, A.; Reynaud, I.; Senave, S. One-Dimensional Pyrolysis of Carbon Based Composite Materials Using FireFOAM. Fire Saf. J. 2018, 97, 66–75. [Google Scholar] [CrossRef]
- Díaz González, C.A.; de Oliveira, D.C.; Yepes, D.M.; Pacheco, L.E.; Silva, E.E. Aspen Plus Model of a Downdraft Gasifier for Lignocellulosic Biomass Adjusted by Principal Component Analysis. Energy Convers. Manag. 2023, 296, 117570. [Google Scholar] [CrossRef]
- Agu, C.E.; Pfeifer, C.; Eikeland, M.; Tokheim, L.A.; Moldestad, B.M.E. Measurement and Characterization of Biomass Mean Residence Time in an Air-Blown Bubbling Fluidized Bed Gasification Reactor. Fuel 2019, 253, 1414–1423. [Google Scholar] [CrossRef]
- Locaspi, A.; Pelucchi, M.; Mehl, M.; Faravelli, T. Towards a Lumped Approach for Solid Plastic Waste Gasification: Polyethylene and Polypropylene Pyrolysis. Waste Manag. 2023, 156, 107–117. [Google Scholar] [CrossRef]
- Safavi, A.; Richter, C.; Unnthorsson, R. Revisiting the Reaction Scheme of Slow Pyrolysis of Woody Biomass. Energy 2023, 280, 128123. [Google Scholar] [CrossRef]
- Yang, T.; Yuan, G.; Xia, M.; Mu, M.; Chen, S. Kinetic Analysis of the Pyrolysis of Wood/Inorganic Composites under Non-Isothermal Conditions. Eur. J. Wood Wood Prod. 2021, 79, 273–284. [Google Scholar] [CrossRef]
- Nawaz, A.; Kumar, P. Pyrolysis Behavior of Low Value Biomass (Sesbania Bispinosa) to Elucidate Its Bioenergy Potential: Kinetic, Thermodynamic and Prediction Modelling Using Artificial Neural Network. Renew. Energy 2022, 200, 257–270. [Google Scholar] [CrossRef]
- Kumar, M.; Shukla, S.K.; Upadhyay, S.N.; Mishra, P.K. Analysis of Thermal Degradation of Banana (Musa Balbisiana) Trunk Biomass Waste Using Iso-Conversional Models. Bioresour. Technol. 2020, 310, 123393. [Google Scholar] [CrossRef]
- Rasool, T.; Najar, I.; Srivastava, V.C.; Pandey, A. Pyrolysis of Almond (Prunus Amygdalus) Shells: Kinetic Analysis, Modelling, Energy Assessment and Technical Feasibility Studies. Bioresour. Technol. 2021, 337, 125466. [Google Scholar] [CrossRef]
- Alsulami, R.A.; El-sayed, S.A.; Eltaher, M.A.; Mohammad, A.; Almitani, K.H.; Mostafa, M.E. Pyrolysis Kinetics and Thermal Degradation Characteristics of Coffee, Date Seed, and Prickly Pear Wastes and Their Blends. Renew. Energy 2023, 216, 119039. [Google Scholar] [CrossRef]
- Aboulkas, A.; Makayssi, T.; Bilali, L.; El Harfi, K.; Nadifiyine, M.; Benchanaa, M. Co-Pyrolysis of Oil Shale and Plastics: Influence of Pyrolysis Parameters on the Product Yields. Fuel Process. Technol. 2012, 96, 209–213. [Google Scholar] [CrossRef]
- Ranganathan, P.; Gu, S. Computational Fluid Dynamics Modelling of Biomass Fast Pyrolysis in Fluidised Bed Reactors, Focusing Different Kinetic Schemes. Bioresour. Technol. 2016, 213, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Wang, Y.; Hu, M.; Xu, P.; Yuan, H.; Chen, Y. A Reactor Network of Biomass Gasification Process in an Updraft Gasifier Based on the Fully Kinetic Model. Energy 2023, 268, 126642. [Google Scholar] [CrossRef]
- Koçer, A.T.; Erarslan, A.; Özçimen, D. Pyrolysis of Aloe Vera Leaf Wastes for Biochar Production: Kinetics and Thermodynamics Analysis. Ind. Crops Prod. 2023, 204, 117354. [Google Scholar] [CrossRef]
- Potnuri, R.; Suriapparao, D.V.; Rao, C.S.; Kumar, T.H. Understanding the Role of Modeling and Simulation in Pyrolysis of Biomass and Waste Plastics: A Review. Bioresour. Technol. Rep. 2022, 20, 101221. [Google Scholar] [CrossRef]
- EL-Sayed, S.A. Review of Thermal Decomposition, Kinetics Parameters and Evolved Gases during Pyrolysis of Energetic Materials Using Different Techniques. J. Anal. Appl. Pyrolysis 2022, 161, 105364. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, H.; Qing, T.; Zhang, J.; Tian, K. Transformation and Kinetics of Chlorine-Containing Products during Pyrolysis of Plastic Wastes. Chemosphere 2021, 284, 131348. [Google Scholar] [CrossRef]
- Dorokhov, V.V.; Nyashina, G.S.; Strizhak, P.A. Thermogravimetric, Kinetic Study and Gas Emissions Analysis of the Thermal Decomposition of Waste-Derived Fuels. J. Environ. Sci. 2024, 137, 155–171. [Google Scholar] [CrossRef]
- Fakhrhoseini, S.M.; Dastanian, M. Predicting Pyrolysis Products of PE, PP, and PET Using NRTL Activity Coefficient Model. J. Chem. 2013, 2013, 7–9. [Google Scholar] [CrossRef]
- Locaspi, A.; Pelucchi, M.; Faravelli, T. Towards a Lumped Approach for Solid Plastic Waste Gasification: Polystyrene Pyrolysis. J. Anal. Appl. Pyrolysis 2023, 171, 105960. [Google Scholar] [CrossRef]
- An, S.; Jung, J.C. Kinetic Modeling of Thermal Reactor in Claus Process Using CHEMKIN-PRO Software. Case Stud. Therm. Eng. 2020, 21, 100694. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Alves, R.F.; Di Domenico, M. Kinetic Triplet and Thermodynamic Parameters of the Pyrolysis Reaction of Invasive Grass Eleusine Indica Biomass: A New Low-Cost Feedstock for Bioenergy Production. Biomass Convers. Biorefinery 2022, 1, 1–17. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; de Sena, R.F.; Machado, R.A.F.; Marangoni, C. Prospection of Catole Coconut (Syagrus cearensis) as a New Bioenergy Feedstock: Insights from Physicochemical Characterization, Pyrolysis Kinetics, and Thermodynamics Parameters. Renew. Energy 2022, 181, 207–218. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Domenico, M.D.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Investigation on Prospective Bioenergy from Pyrolysis of Butia Seed Waste Using TGA-FTIR: Assessment of Kinetic Triplet, Thermodynamic Parameters and Evolved Volatiles. Renew. Energy 2022, 191, 238–250. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Domenico, M.D.; Arias, S.; Pacheco, J.G.A.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Prospecting Pecan Nutshell Pyrolysis as a Source of Bioenergy and Bio-Based Chemicals Using Multicomponent Kinetic Modeling, Thermodynamic Parameters Estimation, and Py-GC/MS Analysis. Renew. Sustain. Energy Rev. 2022, 153, 111753. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Alves, R.F.; de Sena, R.F.; Machado, R.A.F.; Marangoni, C. Potential of Macauba Endocarp (Acrocomia Aculeate) for Bioenergy Production: Multi-Component Kinetic Study and Estimation of Thermodynamic Parameters of Activation. Thermochim. Acta 2022, 708, 179134. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Arias, S.; Pacheco, J.G.A.; Di Domenico, M.; Marangoni, C. Valorization of Royal Palm Tree Agroindustrial Waste via Pyrolysis with a Focus on Physicochemical Properties, Kinetic Triplet, Thermodynamic Parameters, and Volatile Products. Biomass Bioenergy 2023, 177, 106937. [Google Scholar] [CrossRef]
- Sharma, A.; Aravind Kumar, A.; Mohanty, B.; Sawarkar, A.N. Critical Insights into Pyrolysis and Co-Pyrolysis of Poplar and Eucalyptus Wood Sawdust: Physico-Chemical Characterization, Kinetic Triplets, Reaction Mechanism, and Thermodynamic Analysis. Renew. Energy 2023, 210, 321–334. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Ru, B.; Dai, G.; Wang, X.; Xiao, G.; Luo, Z. Kinetic Modeling of Biomass Components Pyrolysis Using a Sequential and Coupling Method. Fuel 2016, 185, 763–771. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Mumbach, G.D.; Alves, R.F.; Di Domenico, M.; Marangoni, C. Physicochemical Properties, Pyrolysis Kinetics, Thermodynamic Parameters of Activation, and Evolved Volatiles of Mango Seed Waste as a Bioenergy Feedstock: A Potential Exploration. Thermochim. Acta 2023, 725, 179519. [Google Scholar] [CrossRef]
- Xiong, Q.; Aramideh, S.; Passalacqua, A.; Kong, S.C. BIOTC: An Open-Source CFD Code for Simulating Biomass Fast Pyrolysis. Comput. Phys. Commun. 2014, 185, 1739–1746. [Google Scholar] [CrossRef]
- Mahmood, H.; Ramzan, N.; Shakeel, A.; Moniruzzaman, M.; Iqbal, T.; Kazmi, M.A.; Sulaiman, M. Kinetic Modeling and Optimization of Parameters for Biomass Pyrolysis: A Comparison of Different Lignocellulosic Biomass. Energy Sources Part. A Recover. Util. Environ. Eff. 2019, 41, 1690–1700. [Google Scholar] [CrossRef]
- Luo, H.; Wang, X.; Krochmalny, K.; Niedzwiecki, L.; Czajka, K.; Pawlak-Kruczek, H.; Wu, X.; Liu, X.; Xiong, Q. Assessments and Analysis of Lumped and Detailed Pyrolysis Kinetics for Biomass Torrefaction with Particle-Scale Modeling. Biomass Bioenergy 2022, 166, 106619. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling Chemical and Physical Processes of Wood and Biomass Pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Nakhaei, M.; Wu, H.; Grévain, D.; Jensen, L.S.; Glarborg, P.; Clausen, S.; Dam-Johansen, K. Experiments and Modeling of Single Plastic Particle Conversion in Suspension. Fuel Process. Technol. 2018, 178, 213–225. [Google Scholar] [CrossRef]
- Gao, N.; Chen, C.; Magdziarz, A.; Zhang, L.; Quan, C. Modeling and Simulation of Pine Sawdust Gasification Considering Gas Mixture Reflux. J. Anal. Appl. Pyrolysis 2021, 155, 105094. [Google Scholar] [CrossRef]
- Lee, Y.R.; Choi, H.S.; Park, H.C.; Lee, J.E. A Numerical Study on Biomass Fast Pyrolysis Process: A Comparison between Full Lumped Modeling and Hybrid Modeling Combined with CFD. Comput. Chem. Eng. 2015, 82, 202–215. [Google Scholar] [CrossRef]
- Akinnawo, O.O.; Nurhafizah, M.D.; Abdullah, N. Pyrolysis Kinetic Study of the Thermal Degradation of Pre-Treated Empty Fruit Bunches. Mater. Today Proc. 2023, 1, 2214. [Google Scholar] [CrossRef]
- Matta, J.; Bronson, B.; Gogolek, P.E.G.; Mazerolle, D.; Thibault, J.; Mehrani, P. Comparison of Multi-Component Kinetic Relations on Bubbling Fluidized-Bed Woody Biomass Fast Pyrolysis Reactor Model Performance. Fuel 2017, 210, 625–638. [Google Scholar] [CrossRef]
- Che, D.; Li, S.; Yang, W.; Jia, J.; Zheng, N. Application of Numerical Simulation on Biomass Gasification. Energy Procedia 2012, 17, 49–54. [Google Scholar] [CrossRef]
- Peters, J.F.; Banks, S.W.; Bridgwater, A.V.; Dufour, J. A Kinetic Reaction Model for Biomass Pyrolysis Processes in Aspen Plus. Appl. Energy 2017, 188, 595–603. [Google Scholar] [CrossRef]
- Granados, D.A.; Chejne, F.; Basu, P. A Two Dimensional Model for Torrefaction of Large Biomass Particles. J. Anal. Appl. Pyrolysis 2016, 120, 1–14. [Google Scholar] [CrossRef]
- Park, C.; Zahid, U.; Lee, S.; Han, C. Effect of Process Operating Conditions in the Biomass Torrefaction: A Simulation Study Using One-Dimensional Reactor and Process Model. Energy 2015, 79, 127–139. [Google Scholar] [CrossRef]
- Bates, R.B.; Ghoniem, A.F. Biomass Torrefaction: Modeling of Volatile and Solid Product Evolution Kinetics. Bioresour. Technol. 2012, 124, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ramos, A.; Monteiro, E.; Rouboa, A. Modeling and Simulation of a Fixed Bed Gasification Process for Thermal Treatment of Municipal Solid Waste and Agricultural Residues. Energy Rep. 2021, 7, 256–269. [Google Scholar] [CrossRef]
- Vaishnavi, M.; Vasanth, P.M.; Rajkumar, S.; Gopinath, K.P.; Devarajan, Y. A Critical Review of the Correlative Effect of Process Parameters on Pyrolysis of Plastic Wastes. J. Anal. Appl. Pyrolysis 2023, 170, 105907. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, W. Effect of Heat Transfer Model on the Prediction of Refuse-Derived Fuel Pyrolysis Process. Fuel 2015, 142, 46–57. [Google Scholar] [CrossRef]
- Aluri, S.; Syed, A.; Flick, D.W.; Muzzy, J.D.; Sievers, C.; Agrawal, P.K. Pyrolysis and Gasification Studies of Model Refuse Derived Fuel (RDF) Using Thermogravimetric Analysis. Fuel Process. Technol. 2018, 179, 154–166. [Google Scholar] [CrossRef]
- Zaini, I.N.; García López, C.; Pretz, T.; Yang, W.; Jönsson, P.G. Characterization of Pyrolysis Products of High-Ash Excavated-Waste and Its Char Gasification Reactivity and Kinetics under a Steam Atmosphere. Waste Manag. 2019, 97, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Chen, L.; Cances, J.; Commandre, J.M.; Cuoci, A.; Pierucci, S.; Ranzi, E. Biomass Pyrolysis: Kinetic Modelling and Experimental Validation under High Temperature and Flash Heating Rate Conditions. J. Anal. Appl. Pyrolysis 2009, 85, 260–267. [Google Scholar] [CrossRef]
- Ibrahimoglu, B.; Cucen, A.; Yilmazoglu, M.Z. Numerical Modeling of a Downdraft Plasma Gasification Reactor. Int. J. Hydrog. Energy 2017, 42, 2583–2591. [Google Scholar] [CrossRef]
- Sieradzka, M.; Rajca, P.; Zajemska, M.; Mlonka-Mędrala, A.; Magdziarz, A. Prediction of Gaseous Products from Refuse Derived Fuel Pyrolysis Using Chemical Modelling Software—Ansys Chemkin-Pro. J. Clean. Prod. 2020, 248, 119277. [Google Scholar] [CrossRef]
- Ślefarski, R.; Jójka, J.; Czyżewski, P.; Gołębiewski, M.; Jankowski, R.; Markowski, J.; Magdziarz, A. Experimental and Numerical-Driven Prediction of Automotive Shredder Residue Pyrolysis Pathways toward Gaseous Products. Energies 2021, 14, 1779. [Google Scholar] [CrossRef]
- Zeaiter, J. A Process Study on the Pyrolysis of Waste Polyethylene. Fuel 2014, 133, 276–282. [Google Scholar] [CrossRef]
- Adrados, A.; de Marco, I.; Caballero, B.M.; López, A.; Laresgoiti, M.F.; Torres, A. Pyrolysis of Plastic Packaging Waste: A Comparison of Plastic Residuals from Material Recovery Facilities with Simulated Plastic Waste. Waste Manag. 2012, 32, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, S.; Zhao, X.; Eiji, S. Pyrolysis Characteristics and Kinetics of Municipal Solid Waste. Trans. Tianjin Univ. 2005, 11, 353–359. [Google Scholar]
- Gautam, R.; Vinu, R. Unraveling the Interactions in Fast Co-Pyrolysis of Microalgae Model Compounds via Pyrolysis-GC/MS and Pyrolysis-FTIR Techniques. React. Chem. Eng. 2019, 4, 278–297. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Lun, L.; Li, Q.; Zhang, Y. Comparative Study on Synergistic Effects in Co-Pyrolysis of Tobacco Stalk with Polymer Wastes: Thermal Behavior, Gas Formation, and Kinetics. Bioresour. Technol. 2019, 292, 121970. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, J.; Zhang, Y. Analysis of Pyrolysis Characteristics and Kinetics of Euphausia Superba Shell Waste Using TG-FTIR and Distributed Activation Energy Model. Biomass Convers. Biorefinery 2018, 8, 329–337. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Yang, Y.; Zhang, Y.; Zhao, C.; Yu, Y.; Wang, S. Comparison of the Thermal Degradation Behaviors and Kinetics of Palm Oil Waste under Nitrogen and Air Atmosphere in TGA-FTIR with a Complementary Use of Model-Free and Model-Fitting Approaches. J. Anal. Appl. Pyrolysis 2018, 134, 12–24. [Google Scholar] [CrossRef]
- Rajca, P.; Poskart, A.; Chrubasik, M.; Sajdak, M.; Zajemska, M.; Skibiński, A.; Korombel, A. Technological and Economic Aspect of Refuse Derived Fuel Pyrolysis. Renew. Energy 2020, 161, 482–494. [Google Scholar] [CrossRef]
- Paraiso, K.; Sauvage, E.; Schuller, S.; Hocine, S.; Lemaitre, V.; Burov, E. Characterization and Modeling of Chemical Reactions Taking Place during the Vitrification of High Level Nuclear Waste. J. Nucl. Mater. 2022, 569, 153878. [Google Scholar] [CrossRef]
- Nawaz, A.; Singh, B.; Mishra, R.K.; Kumar, P. Pyrolysis of Low-Value Waste Trapa Natans Peels: An Exploration of Thermal Decomposition Characteristics, Kinetic Behaviour, and Pyrolytic Liquid Product. Sustain. Energy Technol. Assess. 2023, 56, 103128. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. Pyrolysis Kinetic Behaviour and TG-FTIR-GC–MS Analysis of Coronavirus Face Masks. J. Anal. Appl. Pyrolysis 2021, 156, 105118. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Pérez, J.M.; Cortez, L.A.B.; Marín-Mesa, H.R.; Rocha, J.D.; Peláez-Samaniego, M.R.; Cascarosa, E. A Statistical Analysis of the Auto Thermal Fast Pyrolysis of Elephant Grass in Fluidized Bed Reactor Based on Produced Charcoal. Appl. Therm. Eng. 2014, 65, 322–329. [Google Scholar] [CrossRef]
- Postawa, K.; Fałtynowicz, H.; Sczygieł, J.; Beran, E.; Kułażski, M. Analyzing the Kinetics of Waste Plant Biomass Pyrolysis via Thermogravimetry Modeling and Semi-Statistical Methods. Bioresour. Technol. 2022, 344, 126181. [Google Scholar] [CrossRef] [PubMed]
- Chomsamutr, K.; Jongprasithporn, S. Optimization Parameters of Tool Life Model Using the Taguchi Approach and Response Surface Methodology. Int. J. Comput. Sci. Issues 2012, 9, 120–125. [Google Scholar]
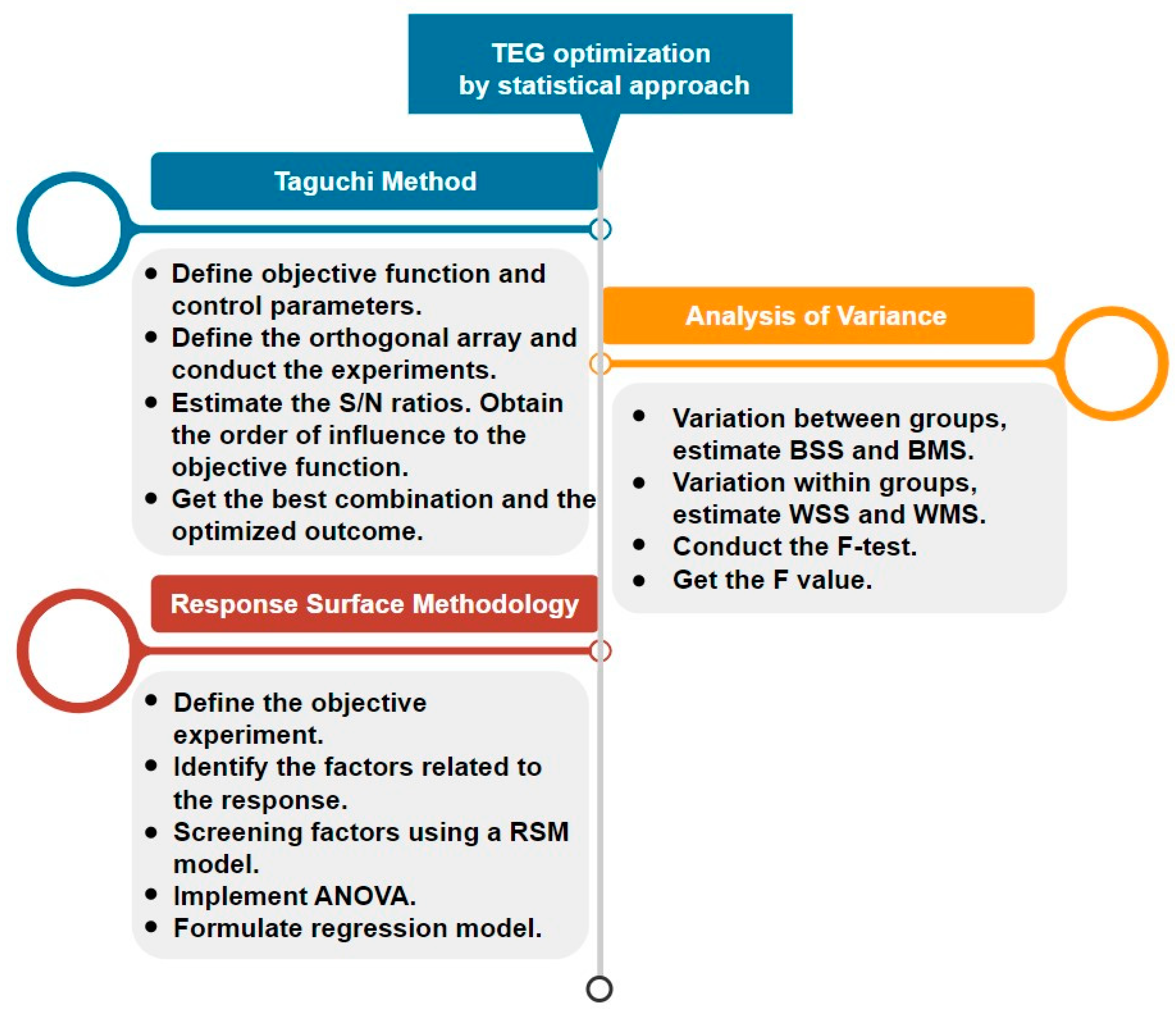
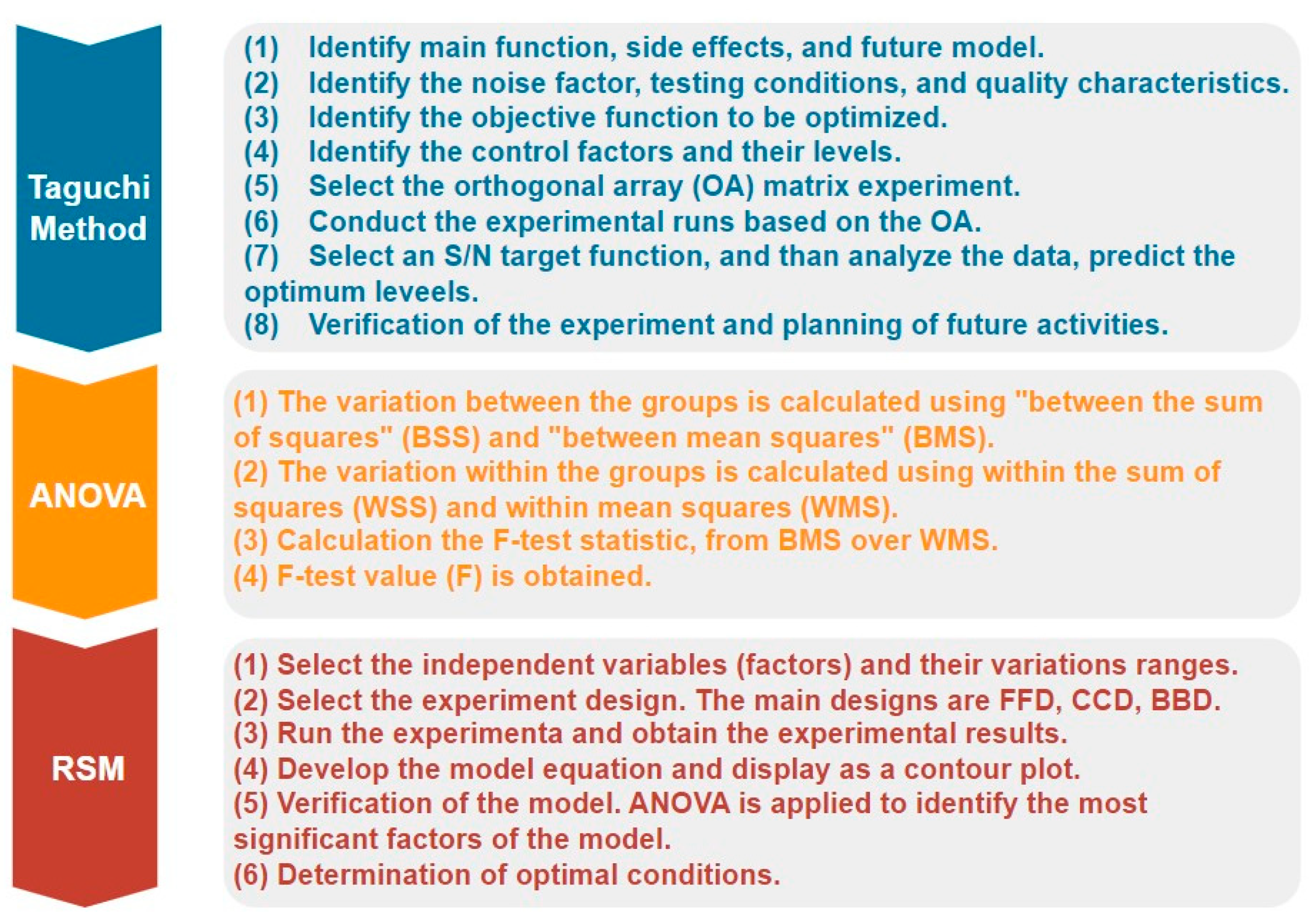
- Chen, W.H.; Carrera Uribe, M.; Kwon, E.E.; Lin, K.Y.A.; Park, Y.K.; Ding, L.; Saw, L.H. A Comprehensive Review of Thermoelectric Generation Optimization by Statistical Approach: Taguchi Method, Analysis of Variance (ANOVA), and Response Surface Methodology (RSM). Renew. Sustain. Energy Rev. 2022, 169, 112917. [Google Scholar] [CrossRef]
- Mazurek, I.; Skawińska, A.; Sajdak, M. Analysis of Chlorine Forms in Hard Coal and the Impact of Leaching Conditions on Chlorine Removal. J. Energy Inst. 2021, 94, 337–351. [Google Scholar] [CrossRef]
- Ajorloo, M.; Ghodrat, M.; Scott, J.; Strezov, V. Modelling and Statistical Analysis of Plastic Biomass Mixture Co-Gasification. Energy 2022, 256, 124638. [Google Scholar] [CrossRef]
- Jha, K.K.; Kannan, T.T.M.; Senthilvelan, N. Optimization of Catalytic Pyrolysis Process for Change of Plastic Waste into Fuel. Mater. Today Proc. 2020, 39, 708–711. [Google Scholar] [CrossRef]
- Alqarni, A.O.; Nabi, R.A.U.; Althobiani, F.; Naz, M.Y.; Shukrullah, S.; Khawaja, H.A.; Bou-Rabee, M.A.; Gommosani, M.E.; Abdushkour, H.; Irfan, M.; et al. Statistical Optimization of Pyrolysis Process for Thermal Destruction of Plastic Waste Based on Temperature-Dependent Activation Energies and Pre-Exponential Factors. Processes 2022, 10, 1559. [Google Scholar] [CrossRef]
- Chen, W.H.; Pratim Biswas, P.; Kwon, E.E.; Park, Y.K.; Rajendran, S.; Gnanasekaran, L.; Chang, J.S. Optimization of the Process Parameters of Catalytic Plastic Pyrolysis for Oil Production Using Design of Experiment Approaches: A Review. Chem. Eng. J. 2023, 471, 144695. [Google Scholar] [CrossRef]
- Ali, D.A.; Gadalla, M.A.; Abdelaziz, O.Y.; Hulteberg, C.P.; Ashour, F.H. Co-Gasification of Coal and Biomass Wastes in an Entrained Flow Gasifier: Modelling, Simulation and Integration Opportunities. J. Nat. Gas Sci. Eng. 2017, 37, 126–137. [Google Scholar] [CrossRef]

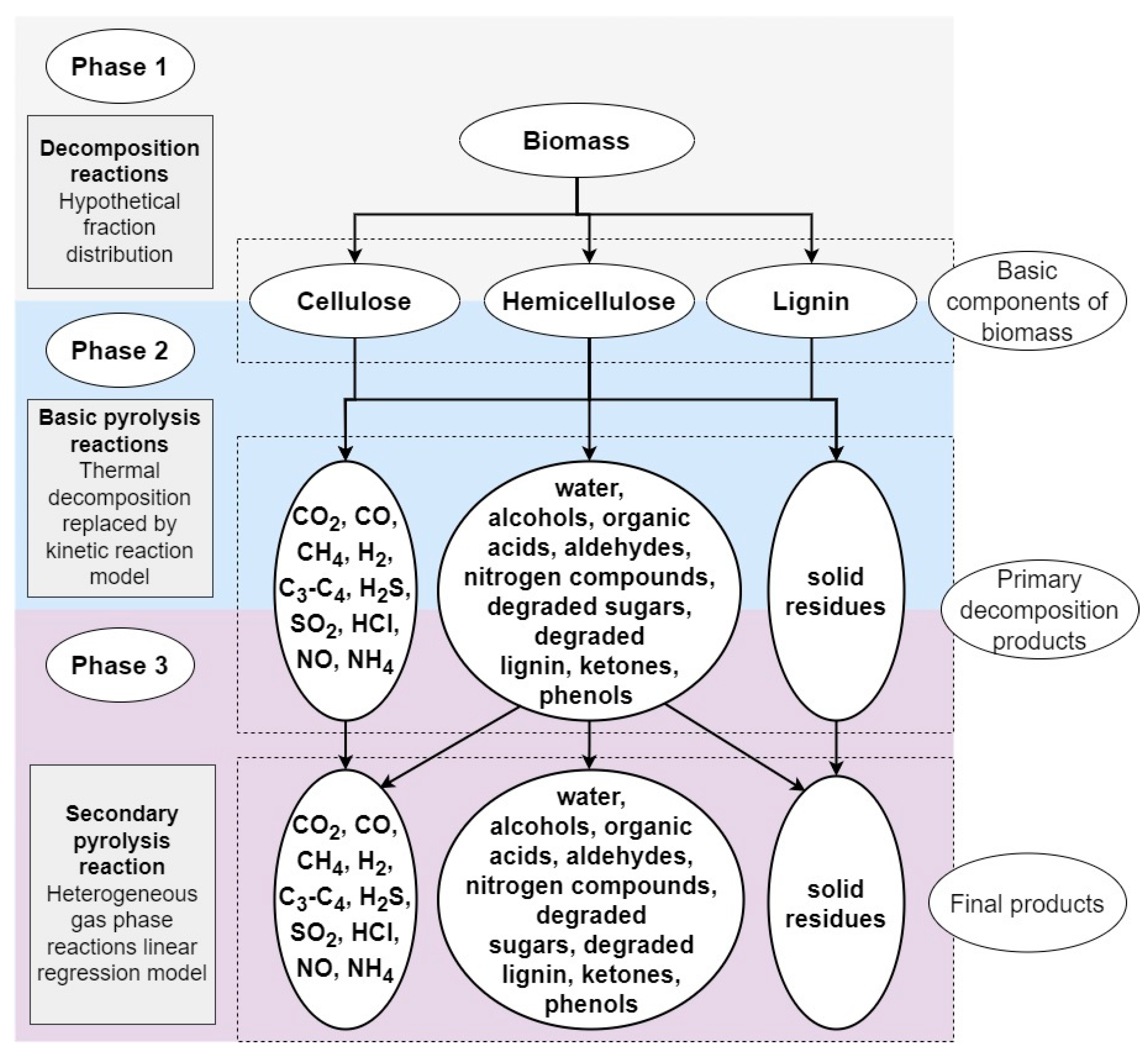

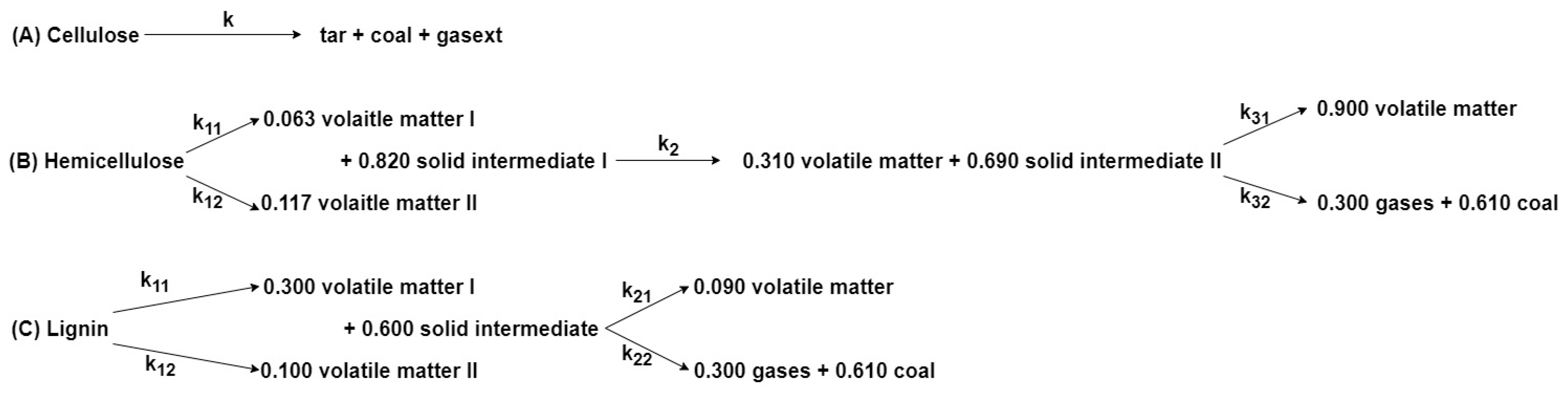
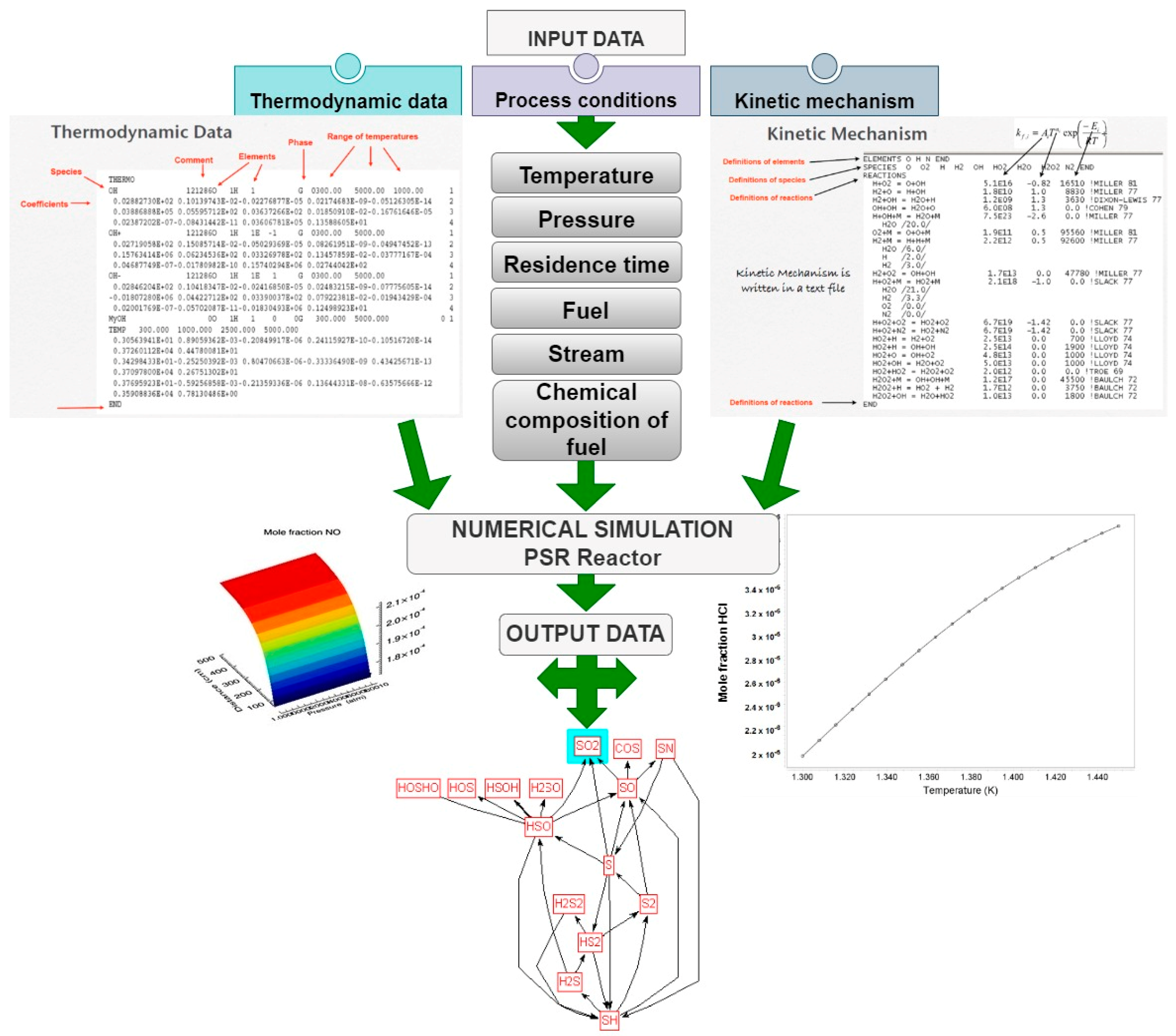
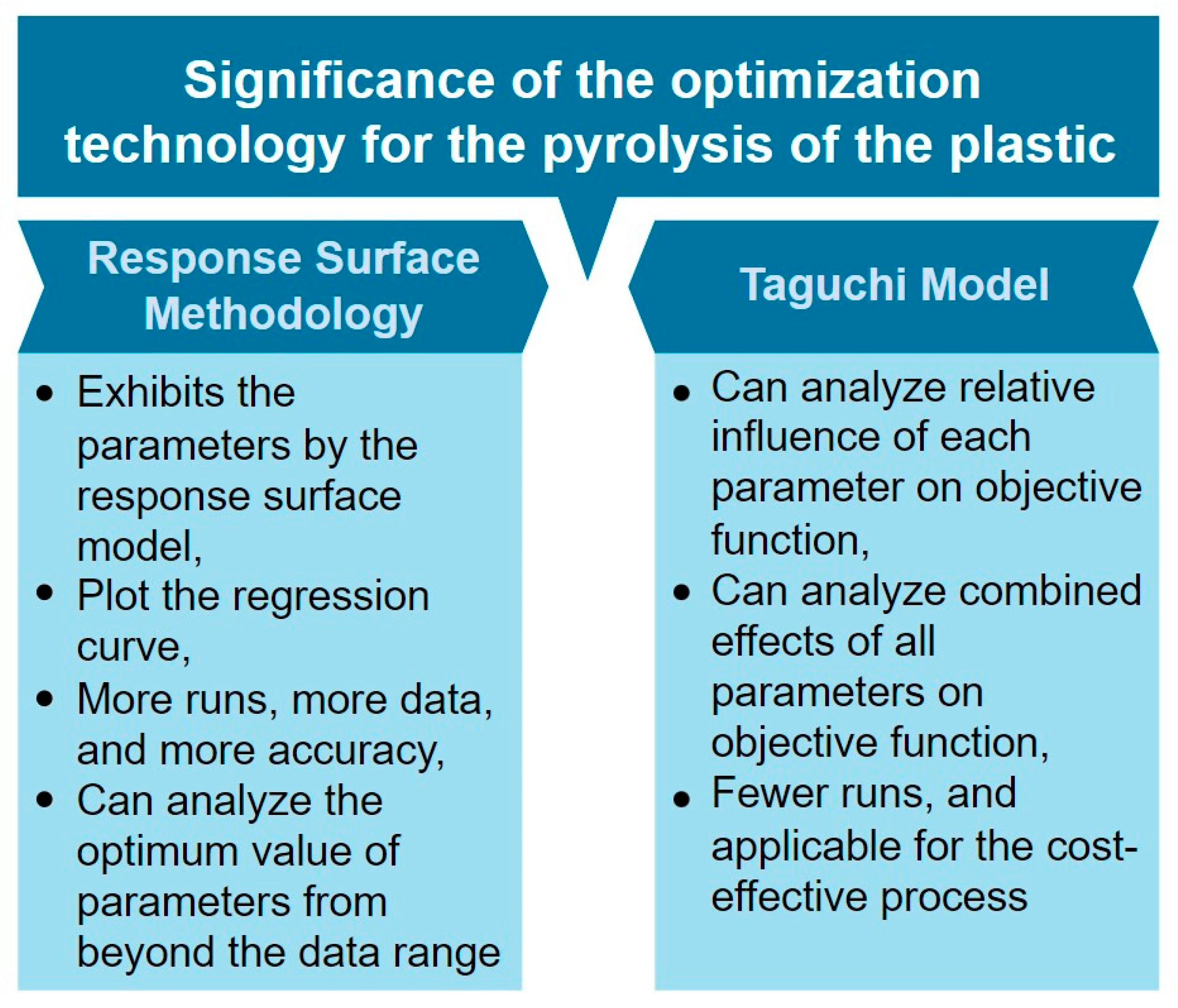
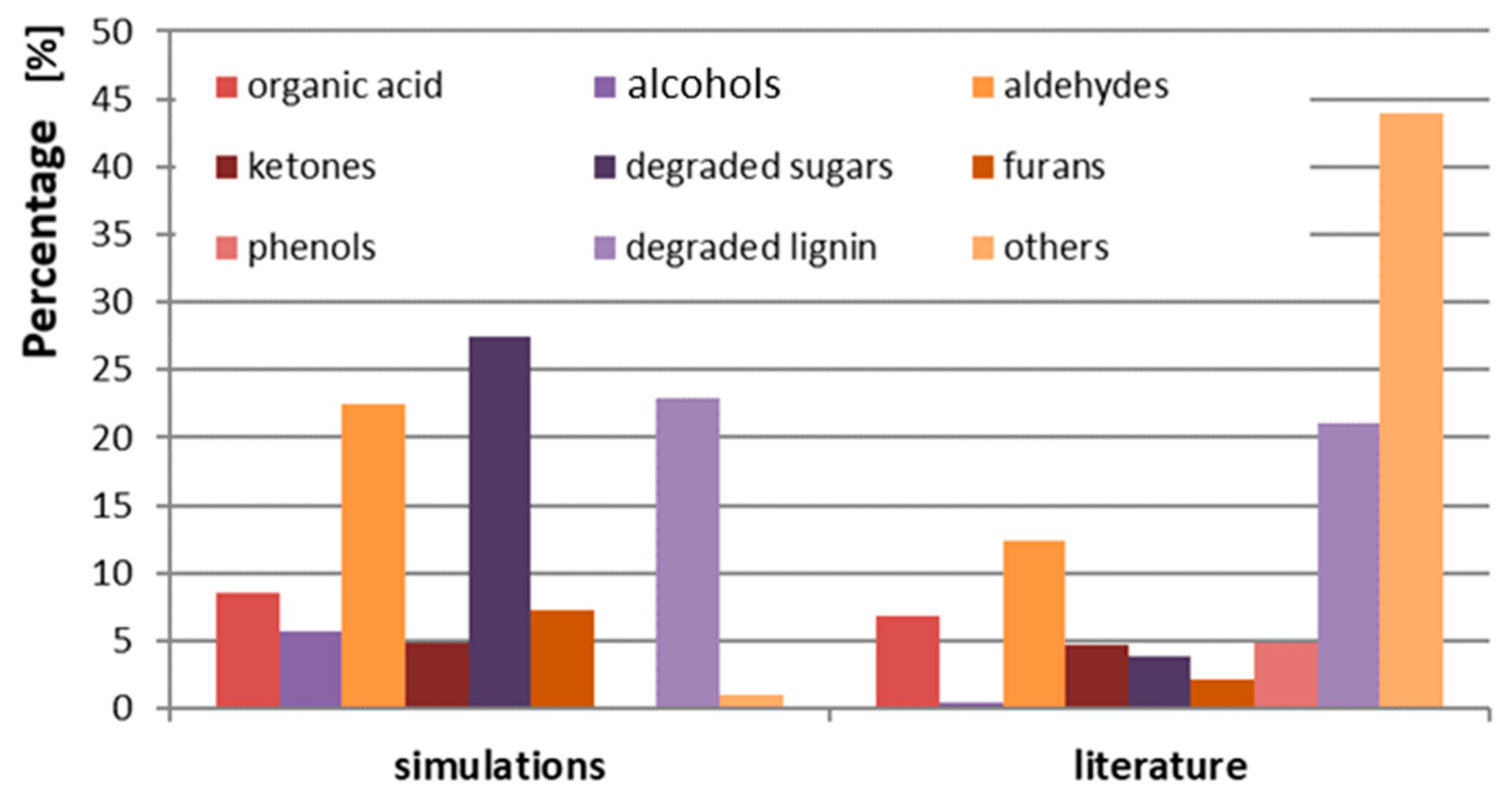
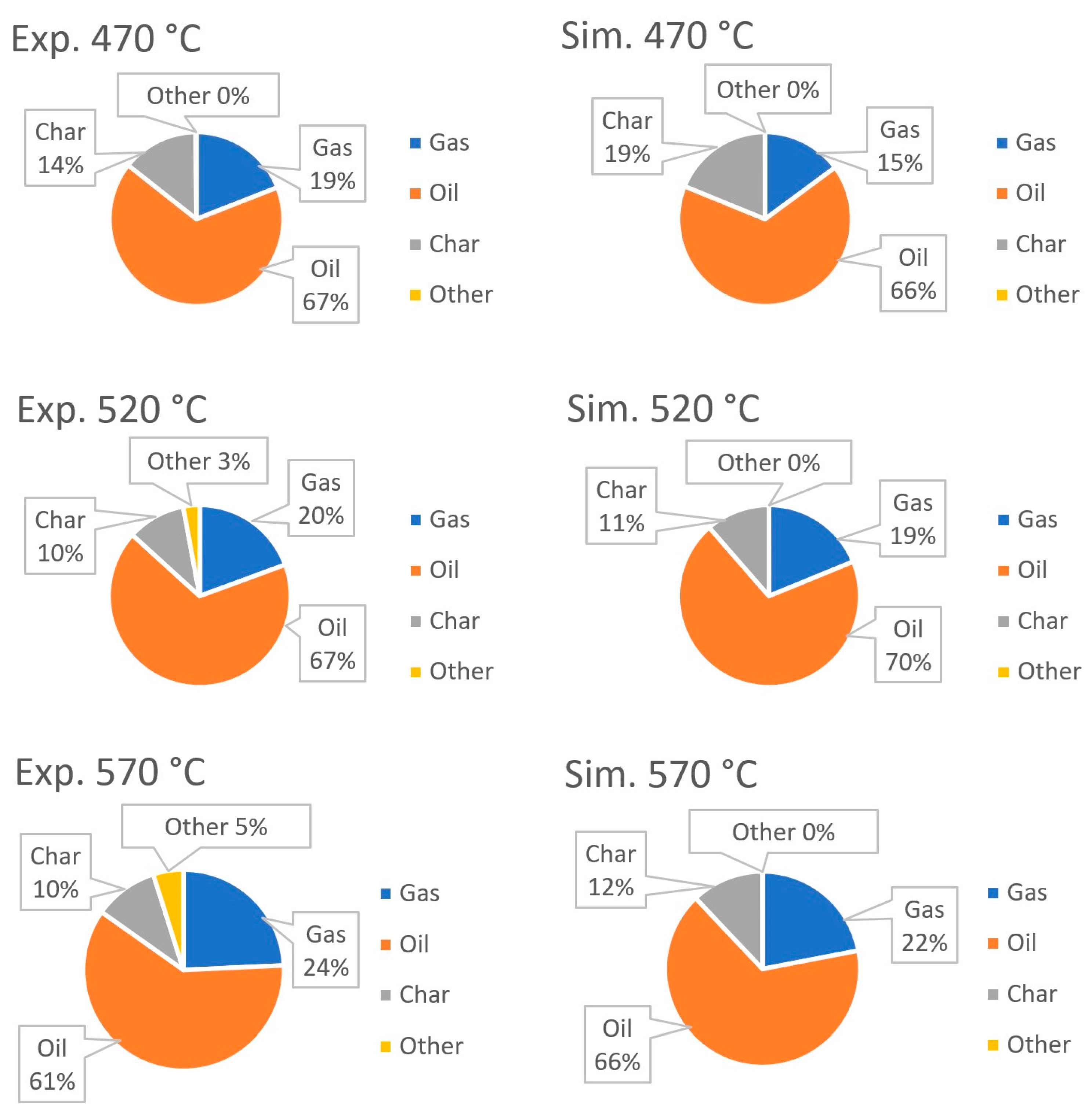

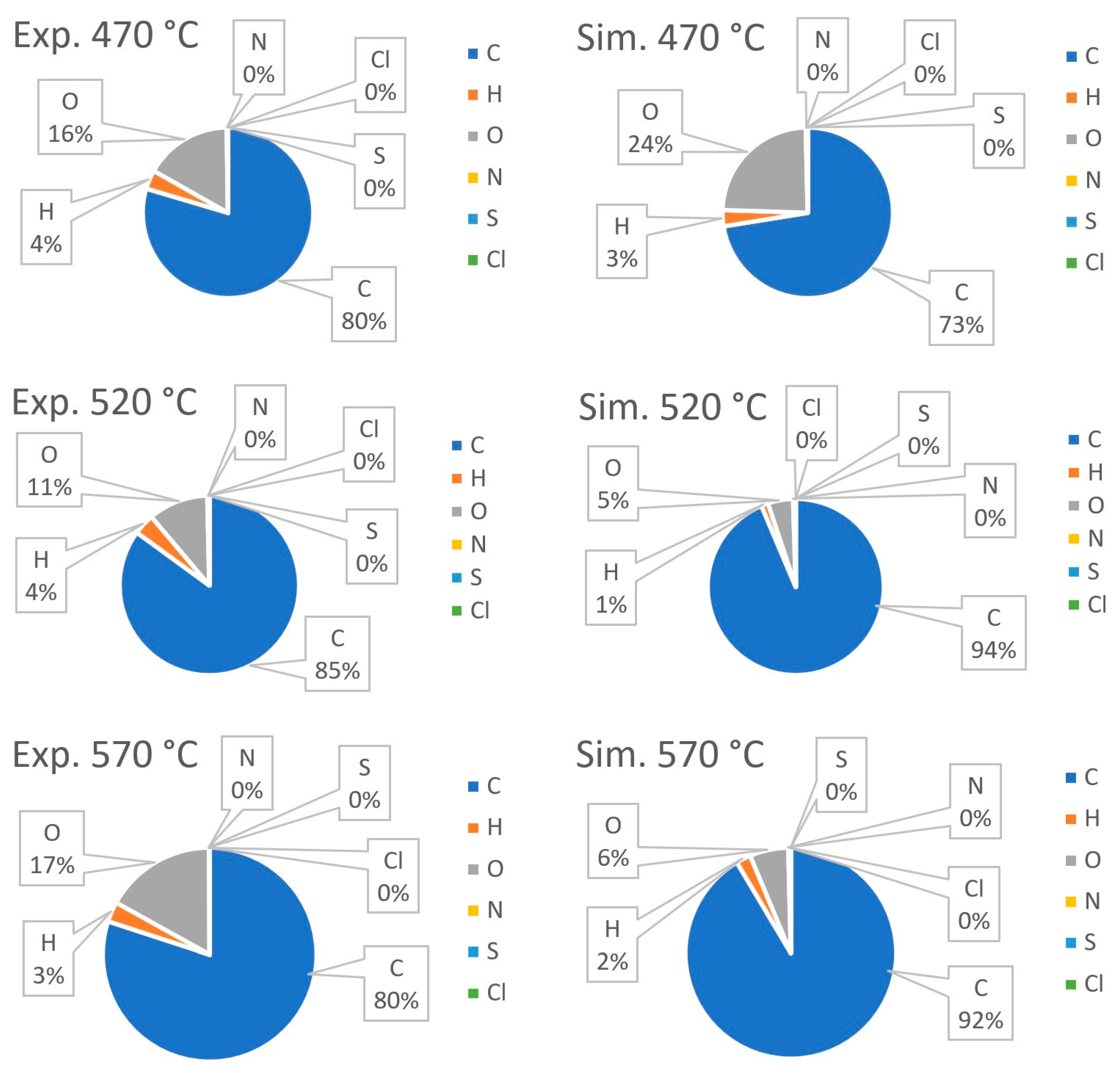
| Process | Feedstock Type | Kinetic Mechanism | Res. |
|---|---|---|---|
| Pyrolysis Single-step and multi-step thermos kinetic study multi-step mechanism reaction | Biomass: grass Eleusine indica | [119] | |
| Kinetic triplets | [120] | ||
| Butia seed waste (BSW) | [121] | ||
| Pecan nutshell | [122] | ||
| Macauba endocarp (Acrocomia aculeate) | [123] | ||
| Pyrolysis | Royal palm tree waste | [124] | |
| Pyrolysis | Poplar and eucalyptus wood sawdust | The kinetic parameters were computed via model-fitting (inflection point and multiple linear regression) and model-free (OFW and KAS) methods. | [125] |
| Thermal conversion | Bambusa vulgaris dust (BD) and delonix regia pods peels (DRP) | Model-free methods, like Kissinger–Akahira–Sunose (KAS) and Flynn–Wall-Ozawa (FWO), were applied for the determination of kinetic parameters. | [126] |
| Pyrolysis | Sesbania bispinosa | The three-pseudo-component model is made up of hemicellulose, cellulose and lignin. Artificial neural networks (ANN) are models based on the operation of the human brain. | [104] |
| Combustion | Biomass |
| [48] |
| Pyrolysis | [127] | ||
| Pyrolysis | Virgin |  | [128] |
| Pyrolysis three-independent parallel reactions model | Beech wood, Rice husk | A three-independent-parallel-reactions model is used to model the kinetics of total devolatilization. | [75] |
| The two-step kinetic model proposed by Koufopanos et al. for lignocellulosic biomass pyrolysis | Wood sawdust, bagasse, peanut hull, douglas fir bark, and rice husk | [129] | |
| Torrefaction process | Beechwood |
| [130] |
| Pyrolysis | 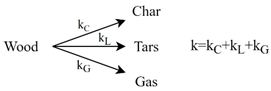 One-component mechanism of primary wood pyrolysis proposed by Shafizadeh and Chin. Multi-component (or multi-stage) mechanisms of wood/biomass pyrolysis. | [131] | |
| Pyrolysis | Biomass | 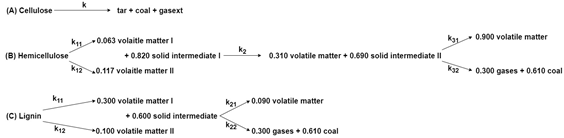 Comprehensive kinetic models for pyrolysis of cellulose, hemicellulose and lignin. | [126] |
| Combustion | Polyethylene | A Non-isothermal 1D model | [132] |
| Gasification process | Pine sawdust | Comprehensive model was developed by Aspen Plus. | [133] |
| Pyrolysis | Wood, grass, and crops | Chemical reaction neural networks (CRNN) model | [58] |
| Pyrolysis | Biomass | Two-stage semi-global mechanism (CFD)  | [134] |
| Gasification | Main kinetic schemes for wood gasification:
| [51] | |
| Pyrolysis | Polietylen, polipropylen, politereftalan etylenu |  | [116] |
| Pyrolysis | Empty fruit bunch | A simplified first-order gasification reaction model | [135] |
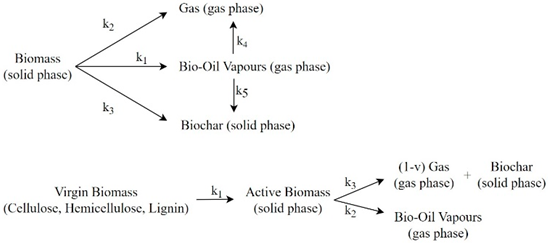 | [136] | ||
| Torrefaction | Willow | 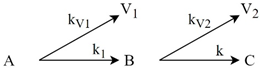 | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrzyniarz, M.; Sajdak, M.; Biniek-Poskart, A.; Skibiński, A.; Krakowiak, M.; Piotrowski, A.; Krasoń, P.; Zajemska, M. Methods and Validation Techniques of Chemical Kinetics Models in Waste Thermal Conversion Processes. Energies 2024, 17, 3067. https://doi.org/10.3390/en17133067
Skrzyniarz M, Sajdak M, Biniek-Poskart A, Skibiński A, Krakowiak M, Piotrowski A, Krasoń P, Zajemska M. Methods and Validation Techniques of Chemical Kinetics Models in Waste Thermal Conversion Processes. Energies. 2024; 17(13):3067. https://doi.org/10.3390/en17133067
Chicago/Turabian StyleSkrzyniarz, Magdalena, Marcin Sajdak, Anna Biniek-Poskart, Andrzej Skibiński, Marlena Krakowiak, Andrzej Piotrowski, Patrycja Krasoń, and Monika Zajemska. 2024. "Methods and Validation Techniques of Chemical Kinetics Models in Waste Thermal Conversion Processes" Energies 17, no. 13: 3067. https://doi.org/10.3390/en17133067
APA StyleSkrzyniarz, M., Sajdak, M., Biniek-Poskart, A., Skibiński, A., Krakowiak, M., Piotrowski, A., Krasoń, P., & Zajemska, M. (2024). Methods and Validation Techniques of Chemical Kinetics Models in Waste Thermal Conversion Processes. Energies, 17(13), 3067. https://doi.org/10.3390/en17133067