The Effect of Carpinus betulus Ash on the Maize as an Energy Crop and the Enzymatic Soil Properties
Abstract
1. Introduction
2. Materials and Methods
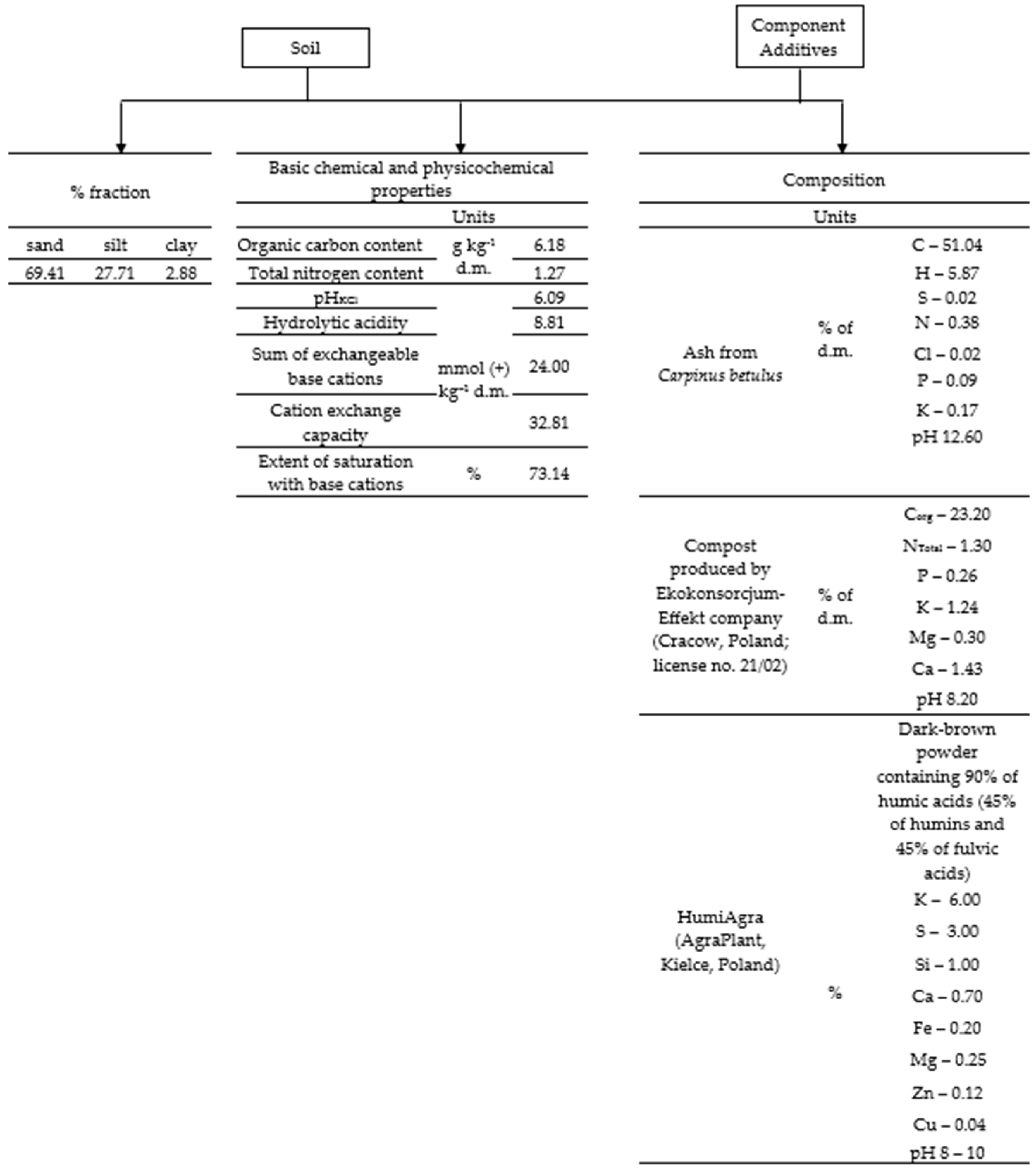
2.1. Characteristics of Soil, Wood Ash, Compost, and HumiAgra
2.2. Pot Experiment
- Control soil;
- Soil + ash from Carpinus betulus;
- Soil + compost;
- Soil + ash from Carpinus betulus + compost;
- Soil + HumiAgra;
- Soil + ash from Carpinus betulus + HumiAgra.
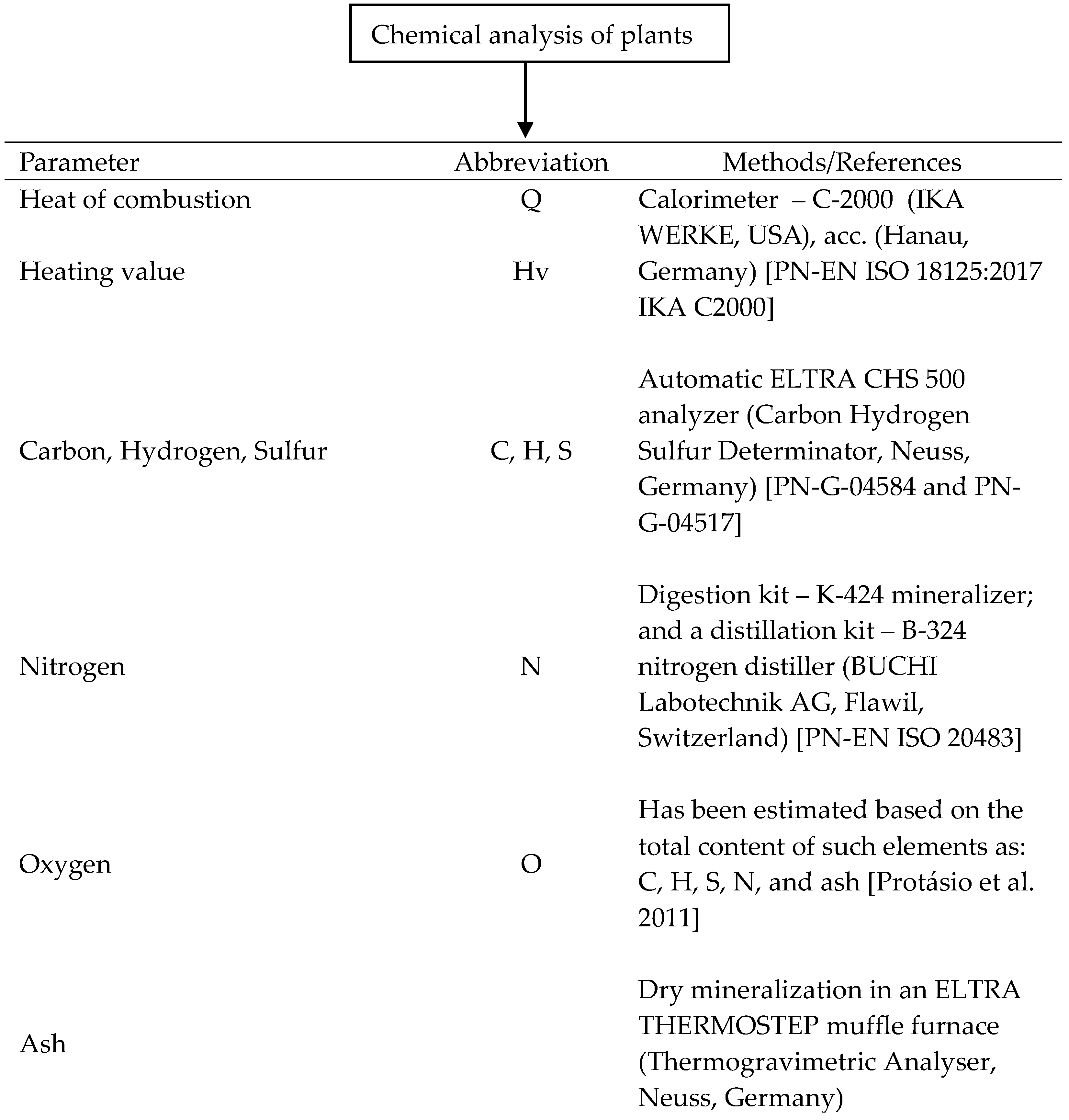
2.3. Chemical Analyses of Plants
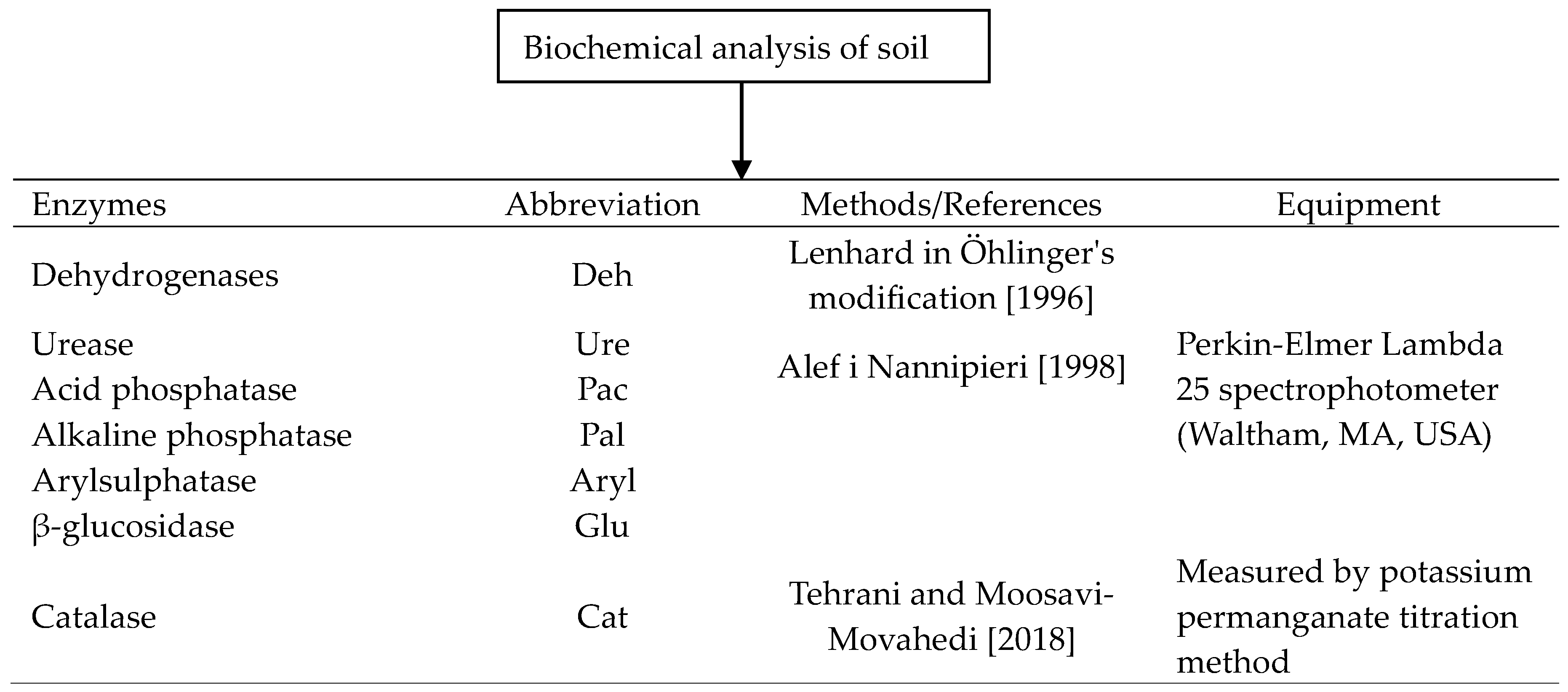
2.4. Soil Biochemical Analyses
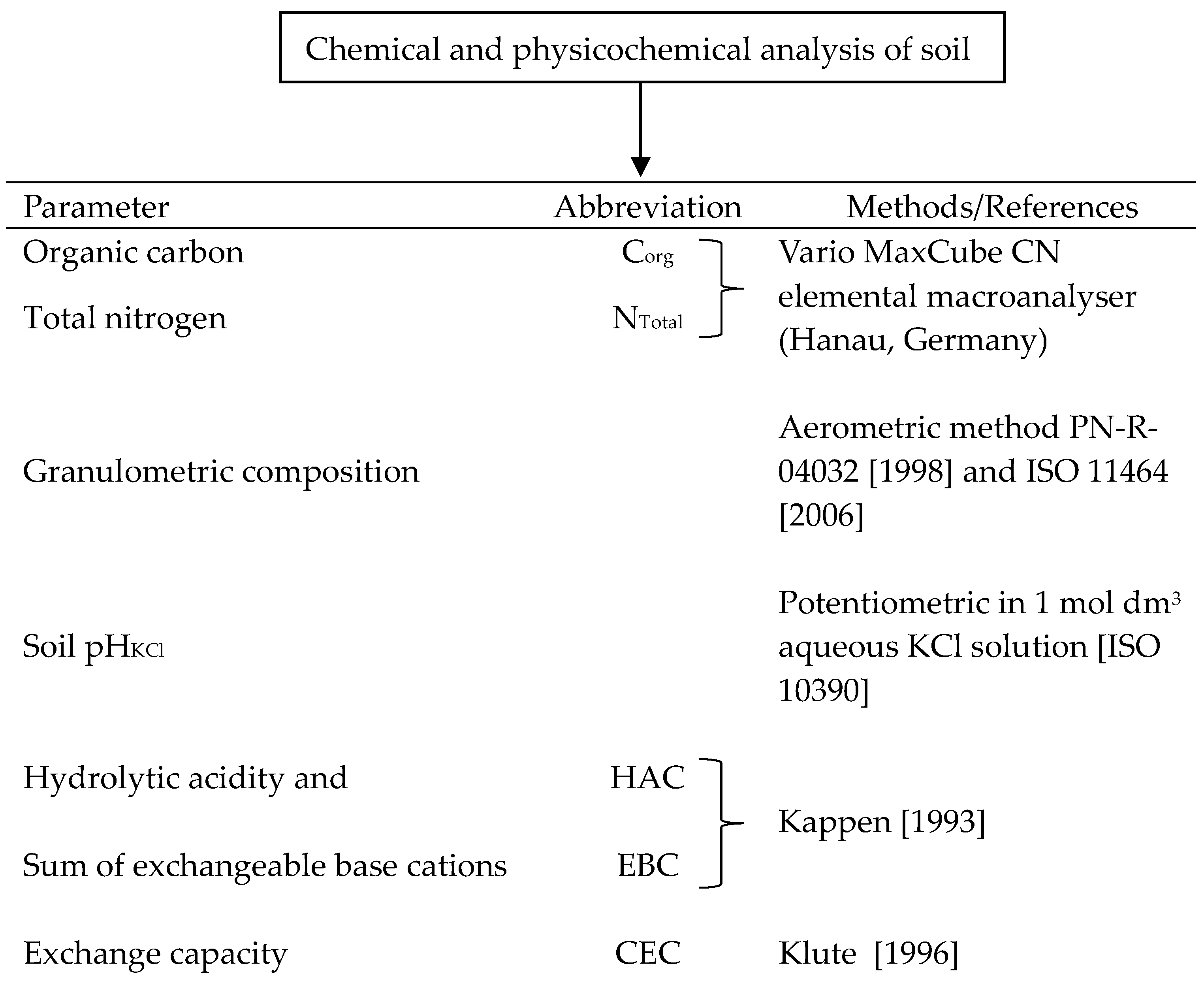
2.5. Soil Chemical and Physicochemical Analyses
2.6. Statistical Analysis
3. Results
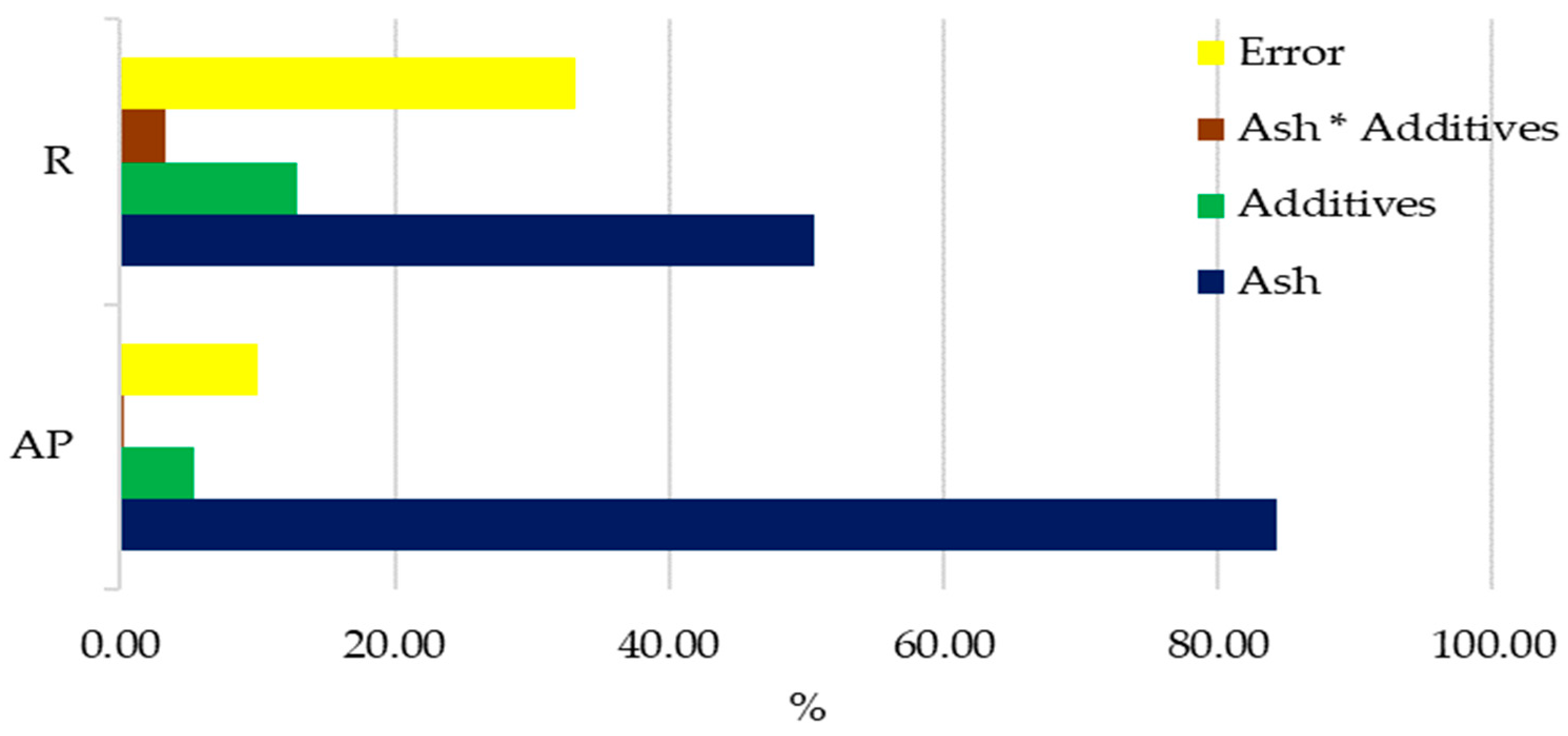
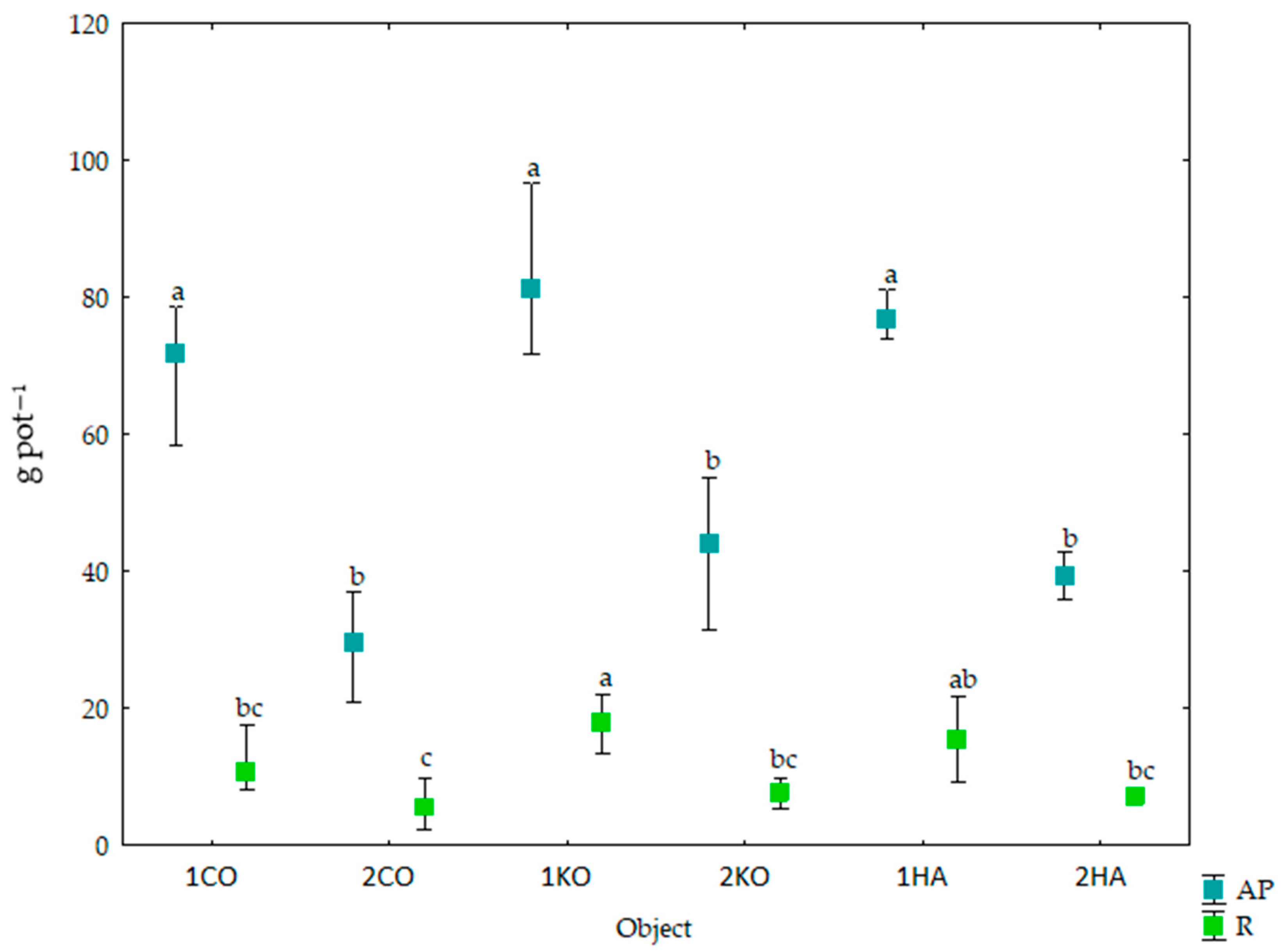
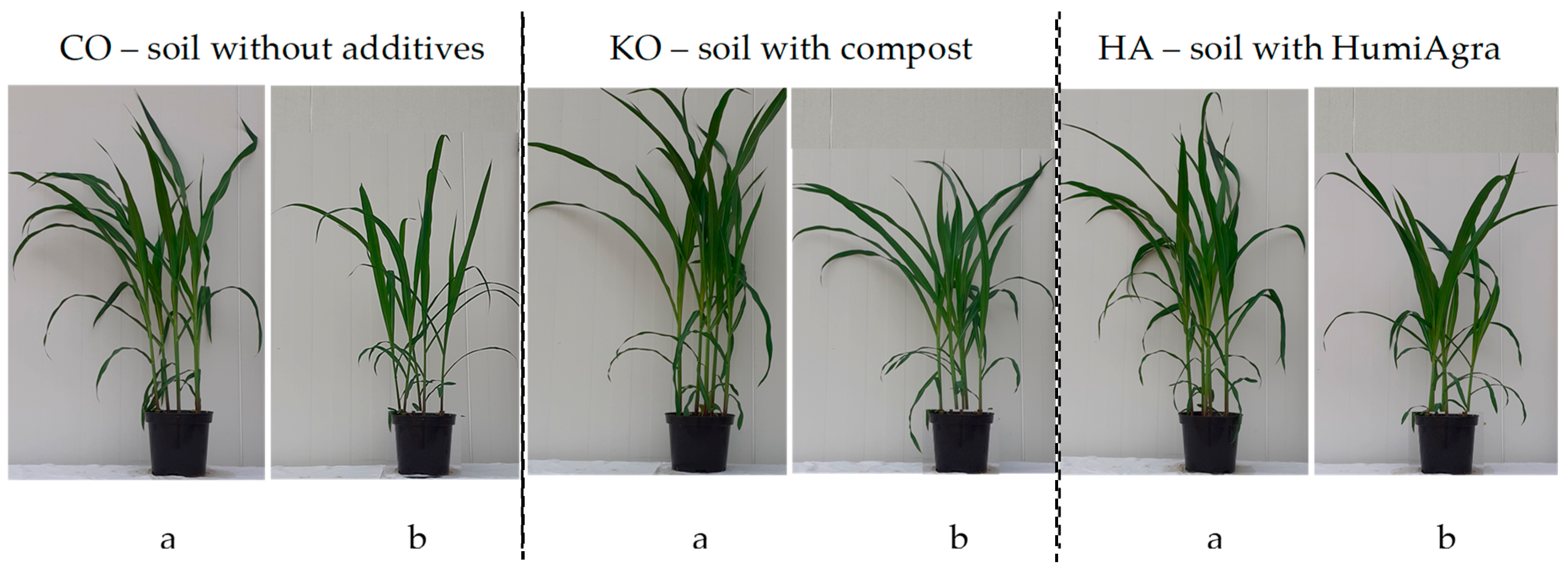
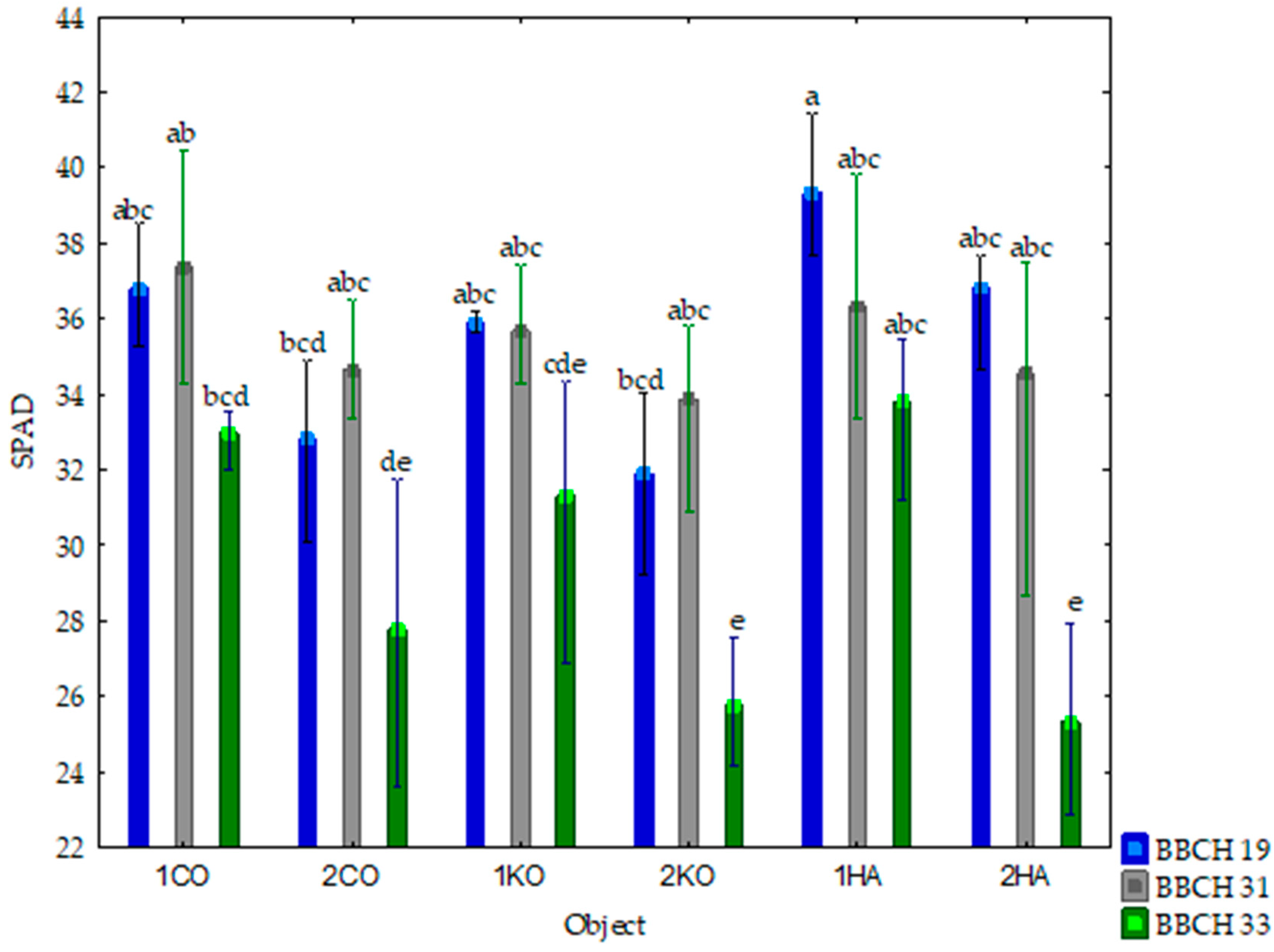
3.1. Response of Mazie to Ash from Carpinus betulus
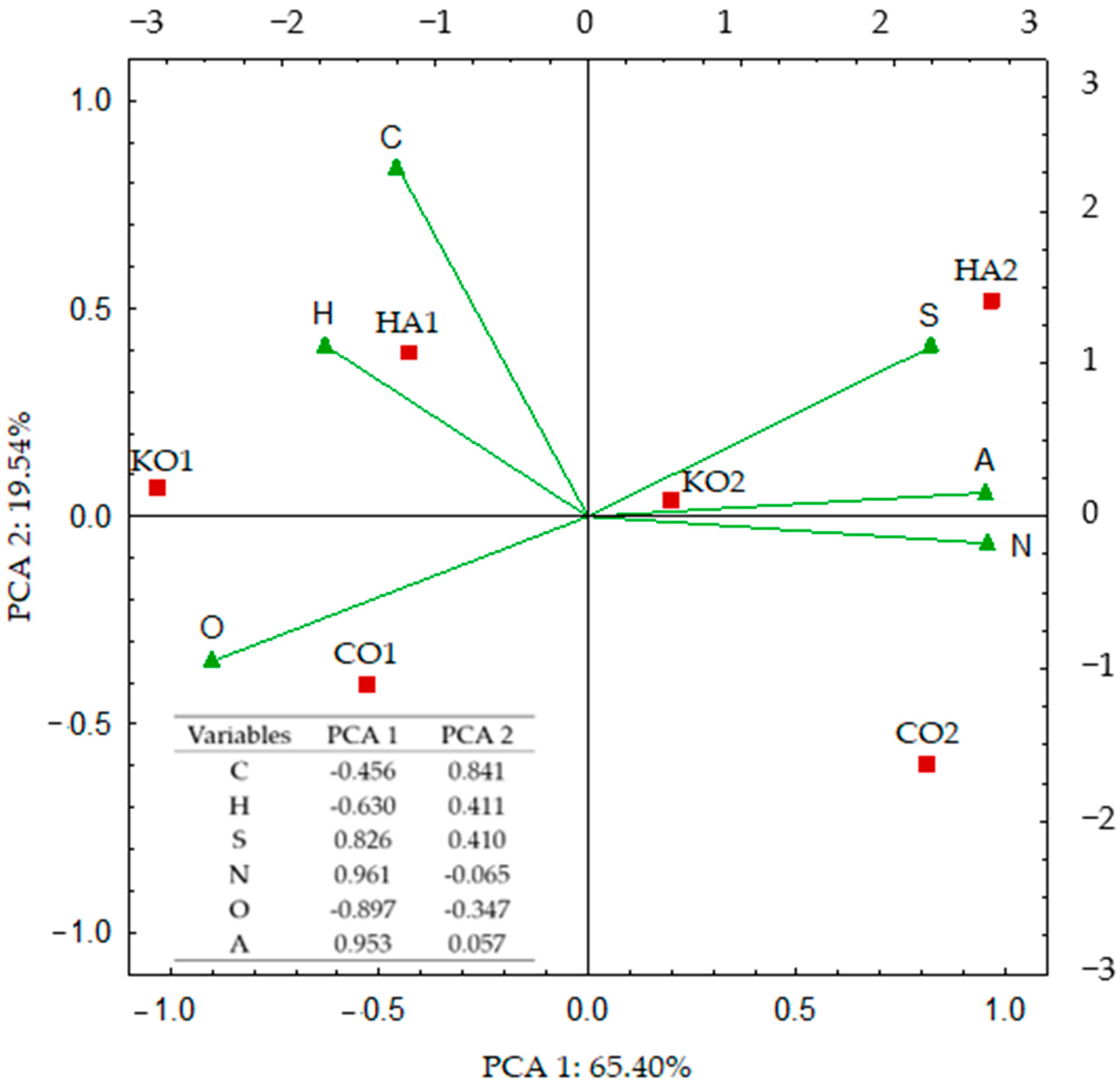
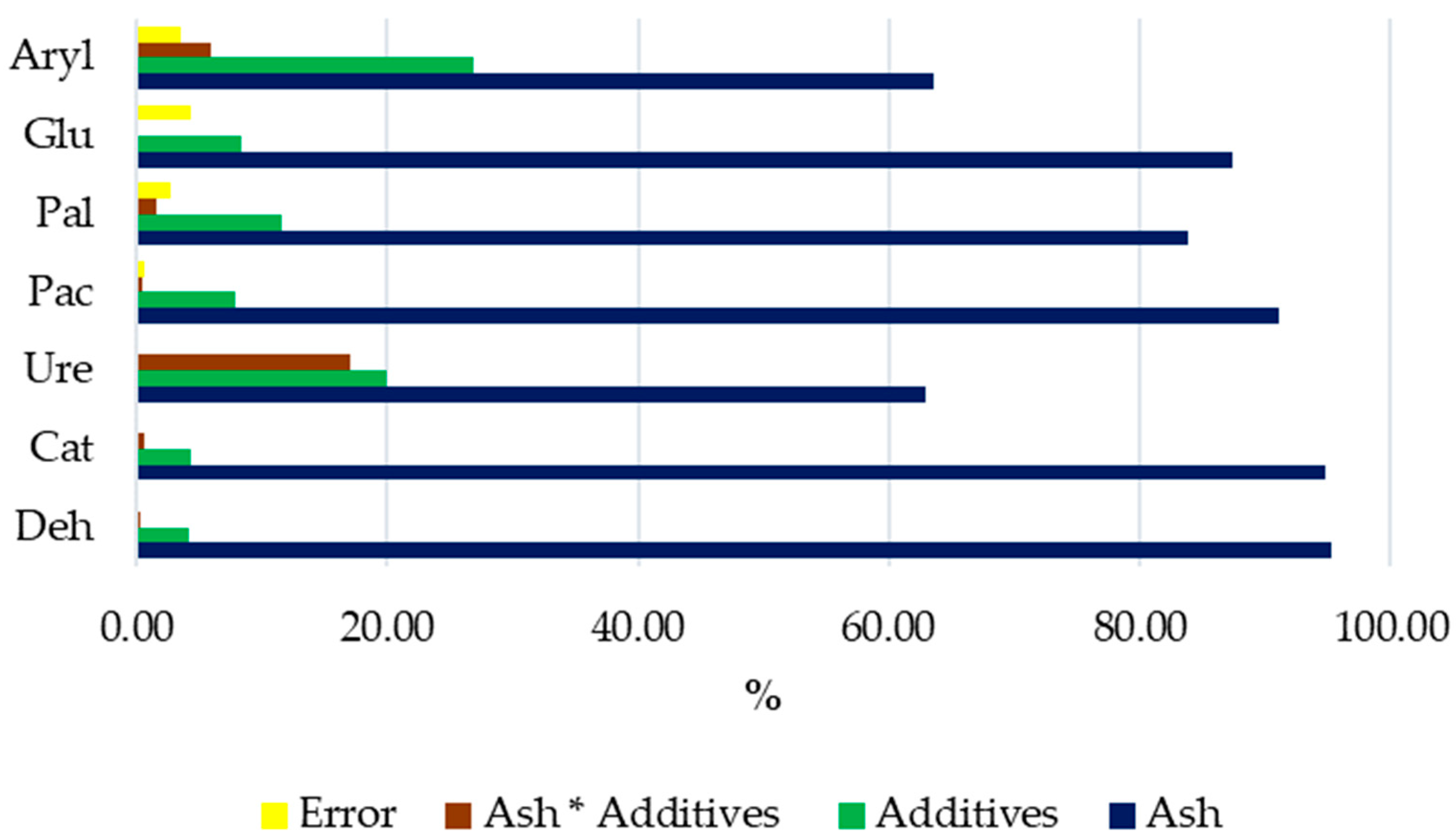
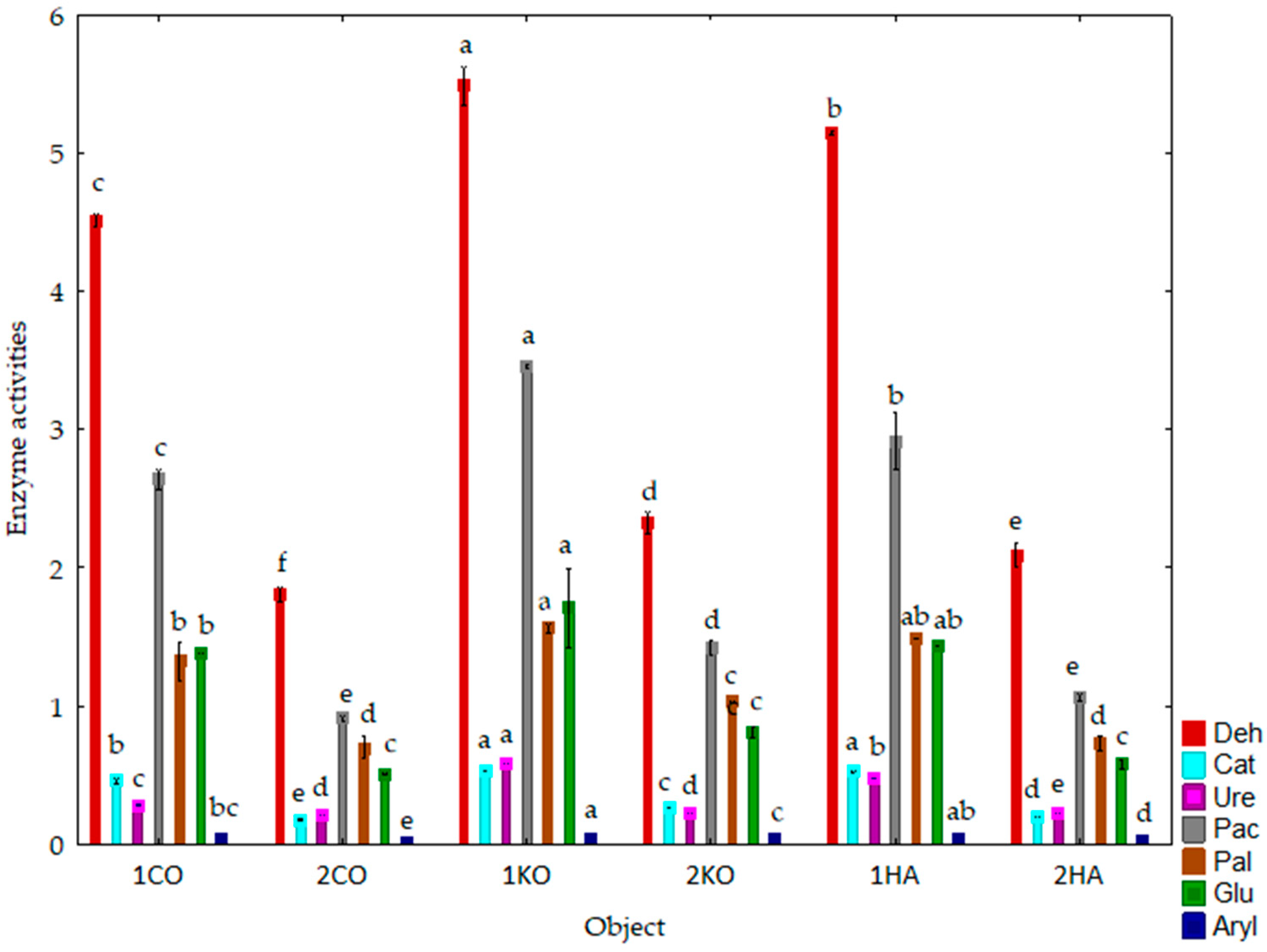
3.2. Response of Soil Enzymes and Certain Chemical and Physicochemical Properties of the Soil to Its Amendment with Ash from Carpinus betulus
4. Discussion
4.1. Response of Maize to Ash from Carpinus betulus
4.2. Response of Soil Enzymes and Certain Chemical and Physicochemical Properties of the Soil to Soil Amendment with Ash from Carpinus betulus
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morales-Máximo, C.N.; López-Sosa, L.B.; Rutiaga-Quiñones, J.G.; Corral-Huacuz, J.C.; Aguilera-Mandujano, A.; Pintor-Ibarra, L.F.; López-Miranda, A.; Delgado-Domínguez, S.N.; Rodríguez-Magallón, M.d.C.; Morales-Máximo, M. Characterization of agricultural residues of Zea mays for their application as solid biofuel: Case study in San Francisco Pichátaro, Michoacán, Mexico. Energies 2022, 15, 6870. [Google Scholar] [CrossRef]
- Skoufogianni, E.; Solomou, A.; Charvalas, G.; Danalatos, N. Maize as Energy Crop. In Maize—Production and Use; Hossain, A., Ed.; Intech Open: London, UK, 2019; pp. 1–16. [Google Scholar] [CrossRef]
- Greinert, A.; Mrówczyńska, M.; Grech, R.; Szefner, W. The use of plant biomass pellets for energy production by combustion in dedicated furnaces. Energies 2020, 13, 463. [Google Scholar] [CrossRef]
- Cheba, K.; Bak, I. Environmental Production Efficiency in the European Union Countries as a Tool for the Implementation of Goal 7 of the 2030 Agenda. Energies 2021, 14, 4593. [Google Scholar] [CrossRef]
- Sabine, C.L.; Feely, R.A. Climate and climate change. Carbon Dioxide. In Encyclopedia of Atmospheric Sciences; Pyle, J., Zhang, F., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 10–17. [Google Scholar]
- Moreira, H.; Pereira, S.I.A.; Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Mine land valorization through energy maize production enhanced by the application of plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. 2016, 23, 6940–6950. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; He, J.; Zhao, H.; Zhang, Q. Investigation of the absorption coefficient of methane in pollution gas measurement. Optik 2015, 126, 1385–1388. [Google Scholar] [CrossRef]
- Morales-Máximo, M.; Rutiaga-Quiñones, J.G.; Masera, O.; Ruiz-García, V.M. Briquettes from Pinus spp. residues: Energy savings and emissions mitigation in the rural sector. Energies 2022, 15, 3419. [Google Scholar] [CrossRef]
- Hauptvogl, M.; Kotrla, M.; Prčík, M.; Pauková, Ž.; Kováčik, M.; Lošák, T. Phytoremediation potential of fast-growing energy plants: Challenges and Perspectives—A Review. Pol. J. Environ. Stud. 2020, 29, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, W.; Rosentrater, K.A. Anhydrous ammonia pretreatment of corn stover and enzymatic hydrolysis of glucan from pretreated corn stover. Fermentation 2017, 3, 9. [Google Scholar] [CrossRef]
- Karimi, S.; Karri, R.R.; Yaraki, M.T.; Koduru, J.R. Processes and separation technologies for the production of fuel-grade bioethanol: A review. Environ. Chem. Lett. 2021, 19, 2873–2890. [Google Scholar] [CrossRef]
- Van Fan, Y.; Romanenko, S.; Gai, L.; Kupressova, E.; Varbanov, P.S.; Klemeš, J.J. Biomass integration for energy recovery and efficient use of resources: Tomsk Region. Energy 2021, 235, 121378. [Google Scholar] [CrossRef]
- Perzon, M. Emissions of organic compounds from the combustion of oats—A comparison with softwood pellets. Biomass Bioenergy 2010, 34, 828–837. [Google Scholar] [CrossRef]
- Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical characteristics of selected grass species from Polish Meadows and their potential utilization for energy generation purposes. Energies 2021, 14, 1669. [Google Scholar] [CrossRef]
- Kraszkiewicz, A.; Niedziółka, I. Selected parameters of maize straw briquettes combustion. BIO Web Conf. 2018, 10, 5. [Google Scholar] [CrossRef]
- Klopfenstein, T.J.; Erickson, G.E.; Berger, L.L. Maize is a critically important source of food, feed, energy and forage in the USA. Field Crops Res. 2013, 153, 5–11. [Google Scholar] [CrossRef]
- OECD. Proceedings of Biomass and Agriculture—Sustainability Markets and Policies; OECD: Paris, France, 2015; ISBN 92-64-10555-7. [Google Scholar]
- Muhaji; Sutjahjo, D.H. The characteristics of bioethanol fuel made of vegetable raw materials. The Consortium of Asia-Pacific Education Universities (Cape U). IOP Conf. Ser. Mater. Sci. Eng. 2018, 296, 1–7. [Google Scholar] [CrossRef]
- Romdhane, L.; Ebinezer, L.B.; Panozzo, A.; Barion, G.; Cortivo, C.D.; Radhouane, L.; Vamerali, T. Effects of soil amendment with wood ash on transpiration, growth, and metal uptake in two contrasting maize (Zea mays L.) hybrids to drought tolerance. Front. Plant Sci. 2021, 12, 661909. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, J.; Wu, G.; Shen, H.; Fu, G.; Wang, Y. Combined effects of biochar and chicken manure on maize (Zea mays L.) growth, lead uptake and soil enzyme activities under lead stress. Peer J. 2021, 9, e11754. [Google Scholar] [CrossRef]
- Ondrasek, G.; Kranjčec, F.; Filipović, L.; Filipović, V.; Bubalo Kovačić, M.; Jelovica Badovinac, I.; Peter, R.; Petravić, M.; Macan, J.; Rengel, Z. Biomass bottom ash & dolomite similarly ameliorate an acidic low-nutrient soil, improve phytonutrition and growth, but increase Cd accumulation in radish. Sci. Total Environ. 2021, 753, 141902. [Google Scholar] [CrossRef]
- Füzesi, I.; Heil, B.; Kovács, G. Effects of wood ash on the chemical properties of soil and crop vitality in small plot experiments. Acta Silv. Lign. Hung. 2015, 11, 55–64. [Google Scholar] [CrossRef]
- Błońska, E.; Prażuch, W.; Boroń, P.; Lasota, J. Effects of wood ash on the soil properties and fungal community structure in a beech forest in Poland. Geoderma Reg. 2023, 34, e00676. [Google Scholar] [CrossRef]
- Smenderovac, E.; Emilson, E.; Porter, C.T.; Morris, D.; Hazlett, P.; Diochon, A.; Basiliko, N.; Bélanger, N.; Markham, J.; Rutherford, P.M.; et al. Forest soil biotic communities show few responses to wood ash applications at multiple sites. Sci. Rep. 2022, 12, 4171. [Google Scholar] [CrossRef] [PubMed]
- Uliasz-Bocheńczyk, A.; Pawluk, A.; Pyzalski, M. Characteristics of ash from the combustion of biomass in fluidized bed boilers. Gospod. Surow. Miner. Miner. Resour. Manag. 2016, 32, 149–162. [Google Scholar] [CrossRef]
- Yang, J.S.; Yang, F.L.; Yang, Y.; Xing, G.L.; Deng, C.P.; Shen, Y.T.; Luo, L.Q.; Li, B.Z.; Yuan, H.L. A proposal of “core enzyme” bioindicator in long-term Pb-Zn ore pollution areas based on topsoil property analysis. Environ. Pollut. 2016, 213, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.S.; Kim, J.G.; Kim, S.O. Use of soil enzymes as indicators for contaminated soil monitoring and sustainable management. Sustainability 2020, 12, 8209. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, Z.; Shi, M.; Cheng, L. Growth and physicochemical changes of Carpinus betulus L. influenced by salinity treatments. Forests 2018, 9, 354. [Google Scholar] [CrossRef]
- Sikkema, R.; Caudullo, G.; Rigo, D.D. Carpinus betulus in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; Publications Office of the European Union: Luxembourg, 2016; pp. 73–75. [Google Scholar]
- Fodor, F.; Németh, R.; Lankveld, C.; Hofmann, T. Effect of acetylation on the chemical composition of hornbeam (Carpinus betulus L.) in relation with the physical and mechanical properties. Wood Mat. Sci. Eng. 2018, 13, 271–278. [Google Scholar] [CrossRef]
- Sierant, D.; Antczak, A. Comparison of the chemical composition of domestic common hornbeam (Carpinus betulus L.) wood and exotic yakal (Shorea astylosa Foxw.) wood. Ann. WULS—SGGW For. Wood Technol. 2021, 212, 5–10. [Google Scholar] [CrossRef]
- Sierant, D.; Szadkowska, D. Analysis of quantitative and qualitative extractive components extracted from hornbeam (Carpinus betulus L.) and yakal (Shorea astylosa Foxw.) wood. Ann. WULS—SGGW For. Wood Technol. 2022, 117, 63–73. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Effect of ash from Salix viminalis on the biomass and heating value of Zea mays and on the biochemical and physicochemical properties of soils. Energies 2023, 16, 8037. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Boros-Lajszner, E.; Kucharski, J. Calorific value of Festuca rubra biomass in the phytostabilization of soil contaminated with nickel, cobalt and cadmium which disrupt the microbiological and biochemical properties of soil. Energies 2022, 15, 3445. [Google Scholar] [CrossRef]
- PN-EN ISO 18125:2017-07; Solid Biofuels—Determination of Calorific Value. Polish Standardization Committee: Warsaw, Poland, 2017.
- PN-G-04584; Oznaczanie Zawartości Siarki Całkowitej i Popiołowej Automatycznymi Analizatorami. Determination of Total Sulphur and Ash Sulphur in Automatic Analyzers. National Standards Body in Poland: Warszaw, Poland, 2001. (In Polish)
- PN-G-04517; Węgiel Kamienny-Oznaczanie Wskaźników Dylatometrycznych. Bituminous Coal. Determination of Dilatometric Features. National Standards Body in Poland: Warszaw, Poland, 1981. (In Polish)
- PN-EN ISO 20483; Oznaczanie Zawartości Azotu i Przeliczanie na Zawartość Białka Surowego—Metoda Kjeldahla. Determination of Dilatometric Features. National Standards Body in Poland: Warsaw, Poland, 2014. (In Polish)
- Protásio, T.P.; Bufalino, L.; Tonoli, G.H.D.; Couto, A.M.; Trugilho, P.F.; Guimarães, M., Jr. Relação entre o poder calorífico superior e os componentes elementares e minerais da biomassa vegetal. Pesq. Flor. Bras. 2011, 31, 113–122. [Google Scholar] [CrossRef]
- Öhlinger, R. Dehydrogenase activity with the substrate TTC. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 241–243. [Google Scholar]
- Alef, K.; Nannipieri, P. Enzyme Activities. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press Harcourt Brace & Company Publishers: London, UK, 1998; pp. 316–365. [Google Scholar]
- Tehrani, H.S.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The usability of sorbents in restoring enzymatic activity in soils polluted with petroleum-derived products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef] [PubMed]
- PN-R-04032; Soil and Mineral Materials—Sampling and Determination of Particle Size Distribution. Polish Committee for Standardization: Warsaw, Poland, 1998.
- ISO 11464; Soil Quality—Pre-Treatment of Samples for Physico-Chemical Analysis. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 10390; In Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- Carter, M.R. Soil Sampling and Methods of Analysis; Canadian Society of Soil Science: London, UK; Lewis Publishers: London, UK, 1993. [Google Scholar]
- Klute, A. Methods of Soil Analysis; Agronomy Monograph 9; American Society of Agronomy: Madison, WI, USA, 1996. [Google Scholar]
- TIBCO Software Inc. Data Analysis Software System; Statistica, Version 13; Tibco Software Inc.: Palo Alto, CA, USA, 2021; Available online: https://www.statistica.com (accessed on 23 January 2024).
- Menardo, S.; Airoldi, G.; Cacciatore, V.; Balsari, P. Potential biogas and methane yield of maize stover fractions and evaluation of some possible stover harvest chains. Biosyst. Eng. 2015, 129, 352–359. [Google Scholar] [CrossRef]
- Sarkar, N.; Gosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Zhao, Y.; Damgaard, A.; Christensen, T.H. Bioethanol from corn stover—A review and technical assessment of alternative biotechnologies. Prog. Energy Combust. Sci. 2018, 67, 275–291. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Dubis, B.; Budzyński, W.S.; Bórawski, P.; Bułkowska, K. Energy efficiency of crops grown for biogas production in a large-scale farm in Poland. Energy 2016, 109, 277–286. [Google Scholar] [CrossRef]
- Konieczna, A.; Roman, K.; Roman, M.; Śliwiński, D.; Roman, M. Energy efficiency of maize production technology: Evidence from Polish farms. Energies 2021, 14, 170. [Google Scholar] [CrossRef]
- Muylle, H.; Van Hullea, S.; de Vliegher, A.; Baert, J.; Van Bockstaele, E.; Roldán-Ruiz, I. Yield and energy balance of annual and perennial lignocellulosic crops for bio-refinery use: A 4-year field experiment in Belgium. Eur. J. Agron. 2015, 63, 62–70. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H. Influence of biochar on soil nutrient transformations, nutrient leaching and crop yield. Adv. Plants Agric. Res. 2016, 4, 00150. [Google Scholar] [CrossRef]
- Hale, S.E.; Nurida, N.L.; Jubaedah; Mulder, J.; Sørmo, E.; Silvani, L.; Abiven, S.; Joseph, S.; Taherymoosavi, S.; Cornelissen, G. The effect of biochar, lime and ash on maize yield in a long-term field trial in a Ultisol in the humid tropics. Sci. Total Environ. 2020, 719, 137455. [Google Scholar] [CrossRef]
- Saletnik, B.; Zagula, G.; Bajcar, M.; Czernicka, M.; Puchalski, C. Biochar and Biomass Ash as a Soil Ameliorant: The Effect on Selected Soil Properties and Yield of Giant Miscanthus (Miscanthus × giganteus). Energies 2018, 11, 2535. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Wyszkowska, J.; Kordala, N.; Borowik, A. Effects of coal and sewage sludge ashes on macronutrient content in maize (Zea mays L.) grown on soil contaminated with Eco-Diesel oil. Materials 2022, 15, 525. [Google Scholar] [CrossRef] [PubMed]
- Pazalja, M.; Salihović, M.; Sulejmanović, J.; Smajović, A.; Begić, S.; Špirtović-Halilović, S.; Sher, F. Heavy metals content in ashes of wood pellets and the health risk assessment related to their presence in the environment. Sci. Rep. 2021, 11, 17952. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical characteristics of biomass ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Biochar sequestration in terrestrial ecosystems—A review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 395–419. [Google Scholar] [CrossRef]
- Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y. Soil nitrogen sorption using charcoal and wood ash. Agronomy 2021, 11, 1801. [Google Scholar] [CrossRef]
- Nabeela, F.; Murad, W.; Khan, I.; Mian, I.A.; Rehman, H.; Adnan, M.; Azizullah, A. Effect of wood ash application on the morphological, physiological and biochemical parameters of Brassica Napus L. Plant Physiol. Biochem. 2015, 95, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zhang, Y.; Zhuo, Y.; Lestander, T.; Geladi, P. Variations in fuel characteristics of corn (Zea mays) stovers: General spatial patterns and relationships to soil properties. Renew. Energy 2010, 35, 1185–1191. [Google Scholar] [CrossRef]
- Lizotte, P.-L.; Savoie, P.; De Champlain, A. Ash content and calorific energy of corn stover components in Eastern Canada. Energies 2015, 8, 4827–4838. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Pawłowski, A.; Przybył, J. Energy comparison of corn stover fraction. In Farm Machinery and Processes Management in Sustainable Agriculture. FMPMSA 2022. Lecture Notes in Civil Engineering, 289; Pascuzzi, S., Santoro, F., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Gömöryová, E.; Pichler, V.; Tóthová, S.; Gömöry, D. Changes of chemical and biological properties of distinct forest floor layers after wood ash application in a norway spruce stand. Forests 2016, 7, 108. [Google Scholar] [CrossRef]
- Bang-Andreasen, T.; Nielsen, J.T.; Voriskova, J.; Heise, J.; Ronn, R.; Kjoller, R.; Hansen, H.C.; Jacobsen, C.S. Wood ash induced ph changes strongly affect soil bacterial numbers and community composition. Front. Microbiol. 2017, 8, 1400. [Google Scholar] [CrossRef] [PubMed]
- Symanowicz, B.; Becher, M.; Jaremko, D.; Skwarek, K. Possibilities for the use of wood ashes in agriculture. J. Ecol. Eng. 2018, 19, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Szostek, M.; Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Ilek, A. Short-Term effect of fly ash from biomass combustion on spring rape plants growth, nutrient, and trace elements accumulation, and soil properties. Int. J. Environ. Res. Public Health 2023, 20, 455. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Hovmand, M.F.; Ekelund, F.; Rønn, R.; Christensen, S.; de Groot, G.A.; Mortensen, L.H.; Skov, S.; Krogh, P.H. Wood ash application increases pH but does not harm the soil mesofauna. Environ. Pollut. 2017, 224, 581–589. [Google Scholar] [CrossRef] [PubMed]
- An, J.Y.; Park, B.B. Effects of wood ash and N fertilization on soil chemical properties and growth of Zelkova Serrata across soil types. Sci. Rep. 2021, 11, 14489. [Google Scholar] [CrossRef] [PubMed]
- Meller, E.; Bilenda, E. Effects of biomass ash on the physicochemical properties of light soil. Energy Policy J. 2012, 15, 287–292. [Google Scholar]
- Rolka, E.; Żołnowski, A.C.; Wyszkowski, M.; Zych, W.; Skorwider-Namiotko, A. Wood biomass ash (WBA) from the heat production process as a mineral amendment for improving selected soil properties. Energies 2023, 16, 5110. [Google Scholar] [CrossRef]
- Rolka, E.; Wyszkowski, M.; Żołnowski, A.C.; Skorwider-Namiotko, A. Role of woody biomass ash material in immobilization of cadmium, lead and zinc in soil. Materials 2024, 17, 2206. [Google Scholar] [CrossRef]
- Park, B.B.; Yanai, R.D.; Sahm, J.M.; Lee, D.K.; Abrahamson, L.P. Wood ash effects on plant and soil in a willow bioenergy plantation. Biomass Bioenergy 2005, 28, 355–365. [Google Scholar] [CrossRef]
- Bang-Andreasen, T.; Peltre, M.; Ellegaard-Jensen, L.; Hansen, L.H.; Ingerslev, M.; Rønn, R.; Jacobsen, C.S.; Kjøller, R. Application of wood ash leads to strong vertical gradients in soil pH changing prokaryotic community structure in forest top Soil. Sci. Rep. 2021, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Stankowski, S.; Sobolewska, M.; Jaroszewska, A.; Gibczyńska, M. Influence of biomass ash, lime and gypsum fertilization on macro-and microelement contents in the soil and grains of spring wheat. Soil Sci. Annu. 2018, 69, 177–183. [Google Scholar] [CrossRef]
- Thomas, S.; Anand, A.; Chinnusamy, V.; Dahuja, A.; Basu, S. Magnetopriming circumvents the effect of salinity stress on germination in chickpea seeds. Acta Physiol. Plant. 2013, 35, 3401–3411. [Google Scholar] [CrossRef]
- Ram, L.C.; Masto, R.E. Fly ash for soil amelioration: A review on the influence of ash blending with inorganic and organic amendments. Earth-Sci. Rev. 2014, 128, 52–74. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Paramisparam, P.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y.; Johan, P.D.; Hamidi, N.H. Co-Application of charcoal and wood ash to improve potassium availability in tropical mineral acid soils. Agronomy 2021, 11, 2081. [Google Scholar] [CrossRef]
- Mindari, W.; Sasongko, P.E.; Kusuma, Z.; Syekhfani; Ain, N. Efficiency of various sources and doses of humic acid on physical and chemical properties of saline soil and growth and yield of rice. AIP Conf. Proc. 2018, 2019, 030001. [Google Scholar] [CrossRef]
- Tiwari, J.; Ramanathan, A.; Bauddh, K.; Korstad, J. Humic substances: Structure, function and benefits for agroecosystems—A review. Pedosphere 2023, 33, 237–249. [Google Scholar] [CrossRef]
- Kishor, P.; Ghosh, A.K.; Kumar, D. Use of flyash in agriculture: A way to improve soil fertility and its productivity. Asian J. Agric. Res. 2010, 4, 1–14. [Google Scholar] [CrossRef]
- Johansen, J.L.; Nielsen, M.L.; Vestergård, M.; Mortensen, L.H.; Cruz-Paredes, C.; Rønn, R.; Kjøller, R.; Hovmand, M.; Christensen, S.; Ekelund, F. The complexity of wood ash fertilization disentangled: Effects on soil pH, nutrient status, plant growth and cadmium accumulation. Environ. Exp. Bot. 2021, 185, 104424. [Google Scholar] [CrossRef]
| Ash g kg−1 d.m. Soil | Heat of Combustion | Heating Value | Energy Production |
|---|---|---|---|
| MJ kg−1 Air-Dried Plant Matter | |||
| Control | |||
| 0 | 18.189 b ± 0.014 | 16.291 b ± 0.200 | 0.333 b ± 0.020 |
| 20 | 18.065 c ± 0.001 | 16.066 c ± 0.100 | 0.136 c ± 0.010 |
| Compost | |||
| 0 | 18.453 a ± 0.004 | 16.594 a ± 0.200 | 0.384 a ± 0.020 |
| 20 | 18.162 b ± 0.035 | 16.339 b ± 0.100 | 0.205 b ± 0.010 |
| HumiAgra | |||
| 0 | 18.218 b ± 0.037 | 16.462 b ± 0.200 | 0.360 b ± 0.020 |
| 20 | 18.186 b ± 0.015 | 16.402 b ± 0.100 | 0.184 b ± 0.010 |
| Ash g kg−1 d.m. Soil | pHKCl | Total Organic Carbon | Total Nitrogen | Hydrolytic Acid | Total Exchangeable Base Cations | Total Cation Exchange Capacity of the Soil | Base Cation Saturation Ratio in the Soil |
|---|---|---|---|---|---|---|---|
| (g kg−1) | (mmol(+) kg−1 Soil) | % | |||||
| Control | |||||||
| 0 | 4.200 e ± 0.02 | 7.896 d ± 0.179 | 1.311 f ± 0.001 | 17.213 b ± 0.037 | 43.700 f ± 0.300 | 60.913 f ± 0.337 | 71.742 d ± 0.095 |
| 20 | 7.800 c ± 0.05 | 12.039 b ± 0.097 | 1.453 c ± 0.004 | 2.363 c ± 0.038 | 310.000 c ± 0.200 | 312.363 c ± 0.238 | 99.244 a ± 0.011 |
| Compost | |||||||
| 0 | 4.550 d ± 0.02 | 8.617 c ± 0.135 | 1.432 d ± 0.001 | 18.038 a ± 0.039 | 71.000 d ± 0.300 | 89.038 d ± 0.237 | 79.726 b ± 0.077 |
| 20 | 8.000 a ± 0.01 | 12.992 a ± 0.112 | 1.734 a ± 0.002 | 2.438 c ± 0.033 | 322.000 a ± 1.000 | 324.438 a ± 0.113 | 99.249 a ± 0.034 |
| HumiAgra | |||||||
| 0 | 4.500 d ± 0.05 | 7.965 d ± 0.075 | 1.373 e ± 0.002 | 17.325 b ± 0.035 | 49.000 e ± 0.300 | 66.325 e ± 0.317 | 73.875 c ± 0.031 |
| 20 | 7.900 b ± 0.05 | 12.854 a ± 0.226 | 1.522 b ± 0.008 | 2.400 c ± 0.037 | 315.000 b ± 1.000 | 317.400 b ± 0.925 | 99.244 a ± 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. The Effect of Carpinus betulus Ash on the Maize as an Energy Crop and the Enzymatic Soil Properties. Energies 2024, 17, 3031. https://doi.org/10.3390/en17123031
Boros-Lajszner E, Wyszkowska J, Kucharski J. The Effect of Carpinus betulus Ash on the Maize as an Energy Crop and the Enzymatic Soil Properties. Energies. 2024; 17(12):3031. https://doi.org/10.3390/en17123031
Chicago/Turabian StyleBoros-Lajszner, Edyta, Jadwiga Wyszkowska, and Jan Kucharski. 2024. "The Effect of Carpinus betulus Ash on the Maize as an Energy Crop and the Enzymatic Soil Properties" Energies 17, no. 12: 3031. https://doi.org/10.3390/en17123031
APA StyleBoros-Lajszner, E., Wyszkowska, J., & Kucharski, J. (2024). The Effect of Carpinus betulus Ash on the Maize as an Energy Crop and the Enzymatic Soil Properties. Energies, 17(12), 3031. https://doi.org/10.3390/en17123031