An Air Over-Stoichiometry Dependent Voltage Model for HT-PEMFC MEAs
Abstract
1. Introduction
2. Experimental Setup
- -
- MEA produced by Advent Technologies Inc., model PBI MEA (previously BASF P1100W), called AdventPBI in the following parts (cell area 45.2 cm2);
- -
- MEA manufactured by Advent Technologies Inc., model TPS® MEA, called AdventTPS in the following parts (45 cm2);
- -
- MEA manufactured by Danish Power Systems, model Dapozol®, called DPSPBI in the following parts (44.8 cm2).
3. Experimental Results
3.1. AME Comparison at Different Operating Temperatures
3.2. AME Comparison at Different Gas Oxidant
3.3. AME Comparison at Different Gas Over-Stoichiometry
4. Model Development
4.1. Basic Model
4.1.1. Governing Equations
4.1.2. Model Validation
4.2. Final Model
4.2.1. Governing Equations
4.2.2. Model Validation
5. Modelling Results
5.1. Model Accuracy on Full Sensibility Study Data
5.2. Capacity of the Model to Predict Fuel Cell Performance
5.3. Advantages and Drawbacks of the Proposed Method
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rigal, S.; Jaafar, A.; Turpin, C.; Hordé, T.; Jollys, J.-B.; Kreczanik, P. Steady-state voltage modelling of a HT-PEMFC under various operating conditions. Energies 2024, 17, 573. [Google Scholar] [CrossRef]
- FCB. BASF acquires PEMEAS. Fuel Cells Bulletin 2, 24 February 2007; p. 3. [Google Scholar]
- Carter, D. A Turning Point for High-Temperature PEM Fuel Cells. Fuel Cell Today Analysis 2013. Carter D. A turning point for high-temperature PEM fuel cells. Fuel Cell Today 28 August 2013. Available online: http://www.fuelcelltoday.com/analysis/analyst-views/2013/13-08-28-a-turning-point-for-high-temperature-pem-fuel-cells (accessed on 16 January 2024).
- Hensche, C. Celtec-P Series Membrane Electrode Assembly Technical Information Brochure; BASF Fuel Cells GmbH: Frankfurt am Main, Germany, 2010. [Google Scholar]
- Wainright, J.S.; Wang, J.T.; Weng, D.; Savinell, R.F.; Litt, M. Acid-doped polybenzimidazoles: A new polymer electrolyte. J. Electrochem. Soc. 1995, 142, 121–123. [Google Scholar] [CrossRef]
- Rahim, Y.; Janßen, H.; Lehnert, W. Characterizing Membrane Electrode Assemblies for High Temperature Polymer Electrolyte Membrane Fuel Cells Using Design of Experiments. Int. J. Hydrogen Energy 2017, 42, 1189–1202. [Google Scholar] [CrossRef]
- Pessot, A.; Turpin, C.; Jaafar, A.; Soyez, E.; Rallières, O.; Gager, G.; d’Arbigny, J. Contribution to the Modelling of a Low Temperature PEM Fuel Cell in Aeronautical Conditions by Design of Experiments. Math. Comput. Simul. 2019, 158, 179–198. [Google Scholar] [CrossRef]
- Kanani, H.; Shams, M.; Hasheminasab, M.; Bozorgnezhad, A. Model Development and Optimization of Operating Conditions to Maximize PEMFC Performance by Response Surface Methodology. Energy Convers. Manag. 2015, 93, 9–22. [Google Scholar] [CrossRef]
- Giner-Sanz, J.J.; Ortega, E.M.; Pérez-Herranz, V. Statistical Analysis of the Effect of the Temperature and Inlet Humidities on the Parameters of a PEMFC Model. Fuel Cells 2015, 15, 479–493. [Google Scholar] [CrossRef]
- Cheng, S.-J.; Miao, J.-M.; Wu, S.-J. Investigating the Effects of Operational Factors on PEMFC Performance Based on CFD Simulations Using a Three-Level Full-Factorial Design. Renew. Energy 2012, 39, 250–260. [Google Scholar] [CrossRef]
- Wahdame, B.; Candusso, D.; François, X.; Harel, F.; Kauffmann, J.-M.; Coquery, G. Design of Experiment Techniques for Fuel Cell Characterisation and Development. Int. J. Hydrogen Energy 2009, 34, 967–980. [Google Scholar] [CrossRef]
- Yu, W.-L.; Wu, S.-J.; Shiah, S.-W. Parametric Analysis of the Proton Exchange Membrane Fuel Cell Performance Using Design of Experiments. Int. J. Hydrogen Energy 2008, 33, 2311–2322. [Google Scholar] [CrossRef]
- Santarelli, M.G.; Torchio, M.F. Experimental Analysis of the Effects of the Operating Variables on the Performance of a Single PEMFC. Energy Convers. Manag. 2007, 48, 40–51. [Google Scholar] [CrossRef]
- Kregar, A.; Zelič, K.; Kravos, A.; Katrašnik, T. Educational Scale-Bridging Approach towards Modelling of Electric Potential, Electrochemical Reactions, and Species Transport in PEM Fuel Cell. Catalysts 2023, 13, 1131. [Google Scholar] [CrossRef]
- Li, S.; Wei, R.; Lv, X.; Ye, W.; Shen, Q.; Yang, G. Numerical Analysis on the Effect of Stoichiometric Ratio on Fuel Utilization and Performance of High Temperature Proton Exchange Membrane Fuel Cells. Int. J. Electrochem. Sci. 2020, 15, 7407–7416. [Google Scholar] [CrossRef]
- Oono, Y.; Fukuda, T.; Sounai, A.; Hori, M. Influence of Operating Temperature on Cell Performance and Endurance of High Temperature Proton Exchange Membrane Fuel Cells. J. Power Sources 2010, 195, 1007–1014. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Song, C.; Zhang, J. Polybenzimidazole-Membrane-Based PEM Fuel Cell in the Temperature Range of 120–200 °C. J. Power Sources 2007, 172, 163–171. [Google Scholar] [CrossRef]
- Taccani, R.; Zuliani, N. Effect of Flow Field Design on Performances of High Temperature PEM Fuel Cells: Experimental Analysis. Int. J. Hydrogen Energy 2011, 36, 10282–10287. [Google Scholar] [CrossRef]
- Singdeo, D.; Dey, T.; Gaikwad, S.; Andreasen, S.J.; Ghosh, P.C. A New Modified-Serpentine Flow Field for Application in High Temperature Polymer Electrolyte Fuel Cell. Appl. Energy 2017, 195, 13–22. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; Gladen, A.C. Comparative and Sensitivity Analysis of Operating Conditions on the Performance of a High Temperature PEM Fuel Cell. Int. J. Hydrogen Energy 2024, 50, 1239–1256. [Google Scholar] [CrossRef]
- Waller, M.G.; Walluk, M.R.; Trabold, T.A. Performance of High Temperature PEM Fuel Cell Materials. Part 1: Effects of Temperature, Pressure and Anode Dilution. Int. J. Hydrogen Energy 2016, 41, 2944–2954. [Google Scholar] [CrossRef]
- Xu, B.; Li, D.; Ma, Z.; Zheng, M.; Li, Y. Thermodynamic Optimization of a High Temperature Proton Exchange Membrane Fuel Cell for Fuel Cell Vehicle Applications. Mathematics 2021, 9, 1792. [Google Scholar] [CrossRef]
- Søndergaard, S.; Cleemann, L.N.; Jensen, J.O.; Bjerrum, N.J. Influence of Oxygen on the Cathode in HT-PEM Fuel Cells. Int. J. Hydrogen Energy 2019, 44, 20379–20388. [Google Scholar] [CrossRef]
- Bayat, M.; Özalp, M.; Gürbüz, H. Comprehensive Performance Analysis of a High-Temperature PEM Fuel Cell under Different Operating and Design Conditions. Sustain. Energy Technol. Assess. 2022, 52, 102232. [Google Scholar] [CrossRef]
- Haghighi, M.; Sharifhassan, F. Exergy Analysis and Optimization of a High Temperature Proton Exchange Membrane Fuel Cell Using Genetic Algorithm. Case Stud. Therm. Eng. 2016, 8, 207–217. [Google Scholar] [CrossRef]
- Ye, L.; Jiao, K.; Du, Q.; Yin, Y. Exergy Analysis of High-Temperature Proton Exchange Membrane Fuel Cell Systems. Int. J. Green Energy 2015, 12, 917–929. [Google Scholar] [CrossRef]
- KP, V.B.; Varghese, G.; Joseph, T.V.; Chippar, P. Sensitivity Analysis of Operational Parameters of a High Temperature-Proton Exchange Membrane Fuel Cell. J. Electrochem. Soc. 2023, 170, 124513. [Google Scholar]
- Baudy, M.; Rondeau, O.; Jaafar, A.; Turpin, C.; Abbou, S.; Grignon, M. Voltage Readjustment Methodology According to Pressure and Temperature Applied to a High Temperature PEM Fuel Cell. Energies 2022, 15, 3031. [Google Scholar] [CrossRef]
- Wang, X.C.; Chen, J.Z.; Quan, S.W.; Wang, Y.X.; He, H.W. Hierarchical model predictive control via deep learning vehicle speed predictions for oxygen stoichiometry regulation of fuel cells. Appl. Energy 2020, 276, 115460. [Google Scholar] [CrossRef]
- Ziogou, C.; Voutetakis, S.; Georgiadis, M.C.; Papadopoulou, S. Model predictive control (MPC) strategies for PEM fuel cell systems—A comparative experimental demonstration. Chem. Eng. Res. Des. 2018, 131, 656–670. [Google Scholar] [CrossRef]
- Sisworahardjo, N.S.; Yalcinoz, T.; El-Sharkh, M.Y.; Alam, M.S. Neural network model of 100 W portable PEM fuel cell and experimental verification. Int. J. Hydrogen Energy 2010, 35, 9104–9109. [Google Scholar] [CrossRef]
- In-Su, H.; Chang-Bock, C. Performance prediction and analysis of a PEM fuel cell operating on pure oxygen using data-driven models: A comparison of artificial neural network and support vector machine. Int. J. Hydrogen Energy 2016, 41, 10202–10211. [Google Scholar]
- Rigal, S.; Turpin, C.; Jaafar, A.; Hordé, T.; Jollys, J.B.; Chadourne, N. Ageing Tests at Constant Currents and Associated Modeling of High Temperature PEMFC MEAs. Fuel Cells 2020, 20, 272–284. [Google Scholar] [CrossRef]
- Fontès, G.; Turpin, C.; Saisset, R.; Meynard, T.; Astier, S. Interactions Between Fuel Cells and Power Converters: Influence of Current Harmonics on a Fuel Cell Stack. IEEE Trans. Power Electron. 2007, 22, 670–678. [Google Scholar] [CrossRef]
- Labach, I.; Rallières, O.; Turpin, C. Steady state Semi-empirical Model of a Single Proton Exchange Membrane Fuel Cell (PEMFC). at Varying Operating Conditions. Fuel Cells 2017, 17, 166–177. [Google Scholar] [CrossRef]
- Rigal, S.; Turpin, C.; Jaafar, A.; Chadourne, N.; Hordé, T.; Jollys, J.-B. Steady-state modelling of a HT-PEMFC under various operating conditions. In Proceedings of the 2019 IEEE 12th International Symposium on Diagnostics for Electrical Machines, Power Electronics and Drives (SDEMPED), Toulouse, France, 27–30 August 2019; pp. 439–445. [Google Scholar]
- Qi, Z.; Buelte, S. Effect of open circuit voltage on performance and degradation of high temperature PBI–H3PO4 fuel cells. J. Power Sources 2006, 161, 1126–1132. [Google Scholar] [CrossRef]
- Mainka, J.; Maranzana, G.; Dillet, J.; Didierjean, S.; Lottin, O. On the Estimation of High Frequency Parameters of Proton Exchange Membrane Fuel Cells via Electrochemical Impedance Spectroscopy. J. Power Sources 2014, 253, 381–391. [Google Scholar] [CrossRef]
- Labach, I. Caractérisation et Modélisation de Piles à Combustible et D’électrolyseurs PEM a Conditions Opératoires Variables en Vue de Leur Association. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2016. [Google Scholar]
- Neyerlin, K.C.; Gu, W.; Jorne, J.; Gasteiger, H.A. Determination of Catalyst Unique Parameters for the Oxygen Reduction Reaction in a PEMFC. J. Electrochem. Soc. 2006, 153, 1955–1963. [Google Scholar] [CrossRef]
- Subramanian, N.P.; Greszler, T.A.; Zhang, J.; Gu, W.; Makharia, R. Pt-Oxide Coverage-Dependent Oxygen Reduction Reaction (ORR) Kinetics. J. Electrochem. Soc. 2012, 159, 531–540. [Google Scholar] [CrossRef]
- Kim, M.; Kang, T.; Kim, J.; Sohn, Y.J. One-dimensional modeling and analysis for performance degradation of high temperature proton exchange high fuel cell using PA doped PBI membrane. Solid State Ion. 2014, 262, 319–323. [Google Scholar] [CrossRef]
- Shamardina, O.; Chertovich, A.; Kulikovsky, A.A.; Khokhlov, A.R. A Simple Model of a High Temperature PEM Fuel Cell. Int. J. Hydrogen Energy 2010, 35, 9954–9962. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, H.; Zhai, Y.; Liu, G.; Yi, B. 500 h Continuous aging life test on PBI/H3PO4 high-temperature PEMFC. Int. J. Hydrogen Energy 2006, 31, 1855–1862. [Google Scholar] [CrossRef]
- Hu, J.; Zhai, Y.; Liu, G.; Hu, J.; Yi, B. Performance degradation studies on PBI/H3PO4 high temperature PEMFC and one-dimensional numerical analysis. Electrochim. Acta 2006, 52, 394–401. [Google Scholar] [CrossRef]
- Scott, K.; Pilditch, S.; Mamlouk, M. Modelling and experimental validation of a high temperature polymer electrolyte fuel cell. J. Appl. Electrochem. 2007, 37, 1245–1259. [Google Scholar] [CrossRef]
- Chippar, P.; Ju, H. Numerical modeling and investigation of gas crossover effects in high temperature proton exchange membrane (PEM) fuel cells. Int. J. Hydrogen Energy 2013, 38, 7704–7714. [Google Scholar] [CrossRef]
- Zeidan, M. Etude Expérimentale et Modélisation d’une Micropile à Combustible à Respiration. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2011. [Google Scholar]
- Park, J.; Min, K. A quasi-three-dimensional non-isothermal dynamic model of a high-temperature proton exchange membrane fuel cell. J. Power Sources 2012, 216, 152–161. [Google Scholar] [CrossRef]
- Wang, Y. Modeling of Two-Phase Transport in the Diffusion Media of Polymer Electrolyte Fuel Cells. J. Power Sources 2008, 185, 261–271. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X. Analysis of the Reaction Rates in the Cathode Electrode of Polymer Electrolyte Fuel Cells II. Dual-Layer Electrodes. J. Electrochem. Soc. 2009, 156, B403–B409. [Google Scholar] [CrossRef]
- Vang, J.R. HTPEM Fuel Cell Impedance: Mechanistic Modelling and Experimental Characterisation. Ph.D. Thesis, Aalborg University, Allborg, Danemark, 2014. [Google Scholar]
- Kriksunov, L.; Bunakova, L.; Zabusova, S.; Krishtalik, L. Anodic oxygen evolution reaction at high temperatures in acid solutions at platinum. Electrochim. Acta 1994, 39, 137–142. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, M.; Li, T.; Zhang, L.Y.; Jing, M.; Yuan, W.; Li, C.M. In situ self-assembled N-rich carbon on pristine graphene as a highly effective support and cocatalyst of short Pt nanoparticle chains for superior electrocatalytic activity toward methanol oxidation. Nanoscale 2021, 13, 18332–18339. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, Y.; Shen, P.K.; Li, C.M.; Jiang, S.P. Significance of wall number on the carbon nanotube support-promoted electrocatalytic activity of Pt NPs towards methanol/formic acid oxidation reactions in direct alcohol fuel cells. J. Mater. Chem. A 2015, 3, 1961–1971. [Google Scholar] [CrossRef]
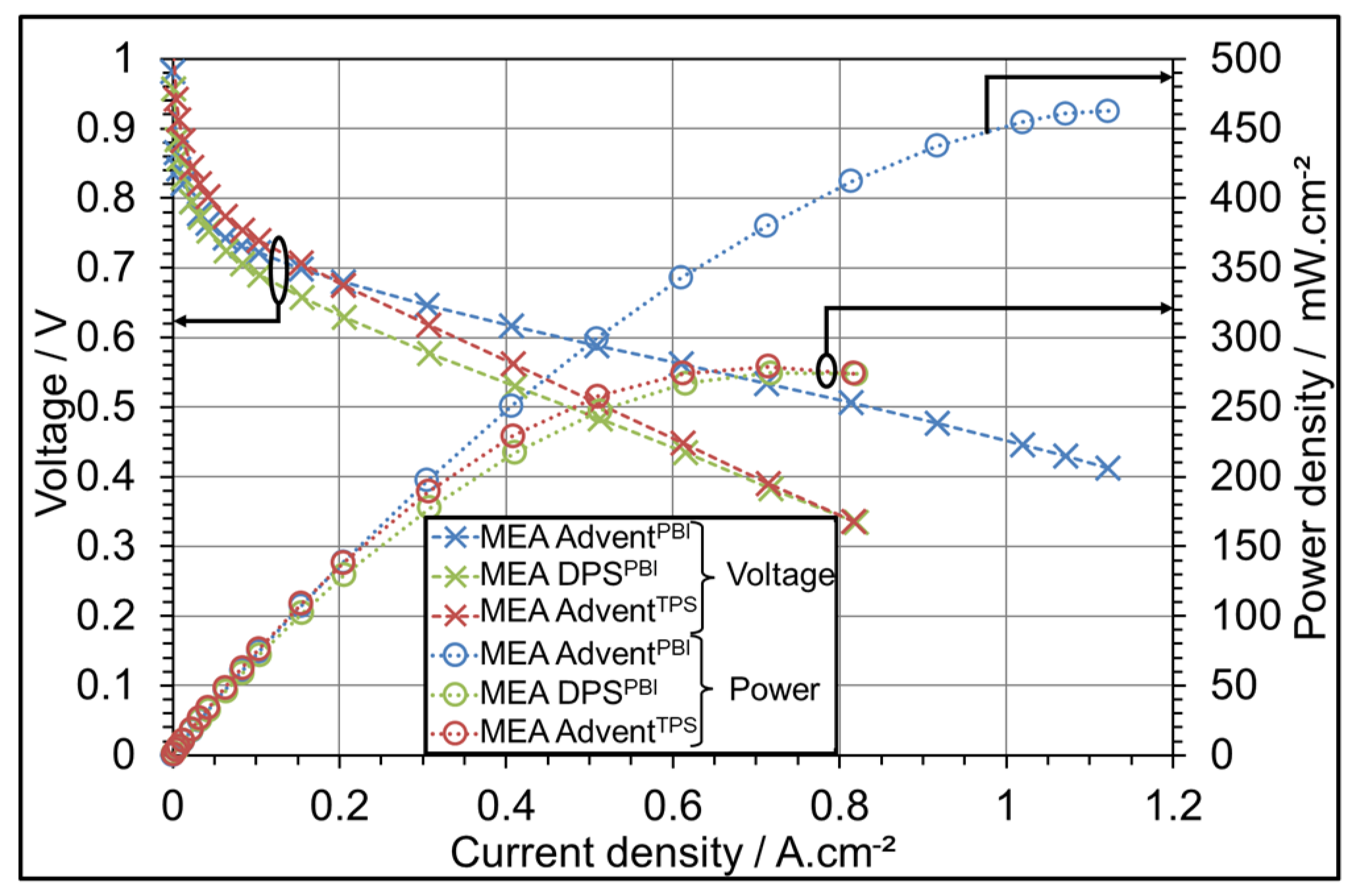
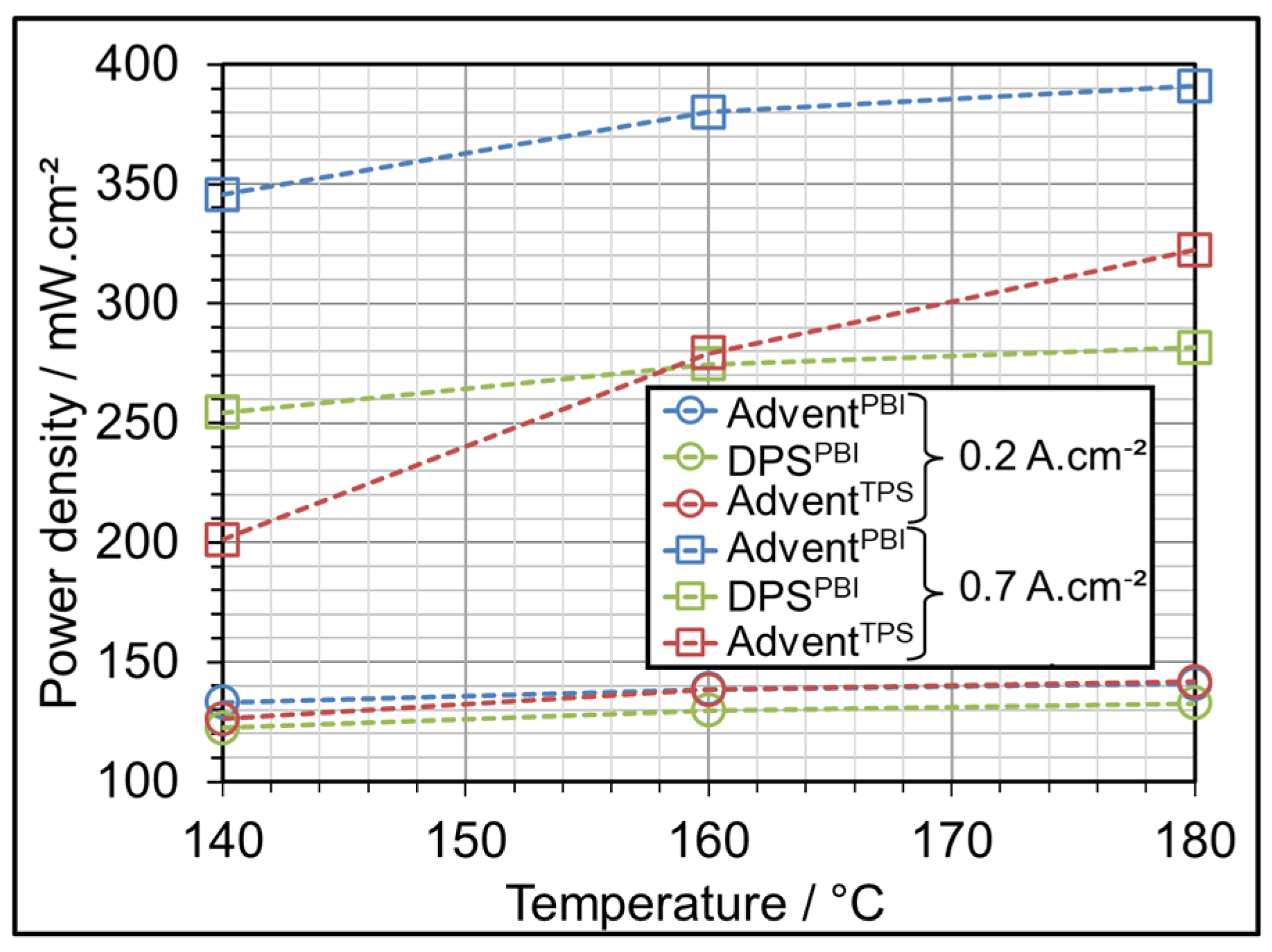

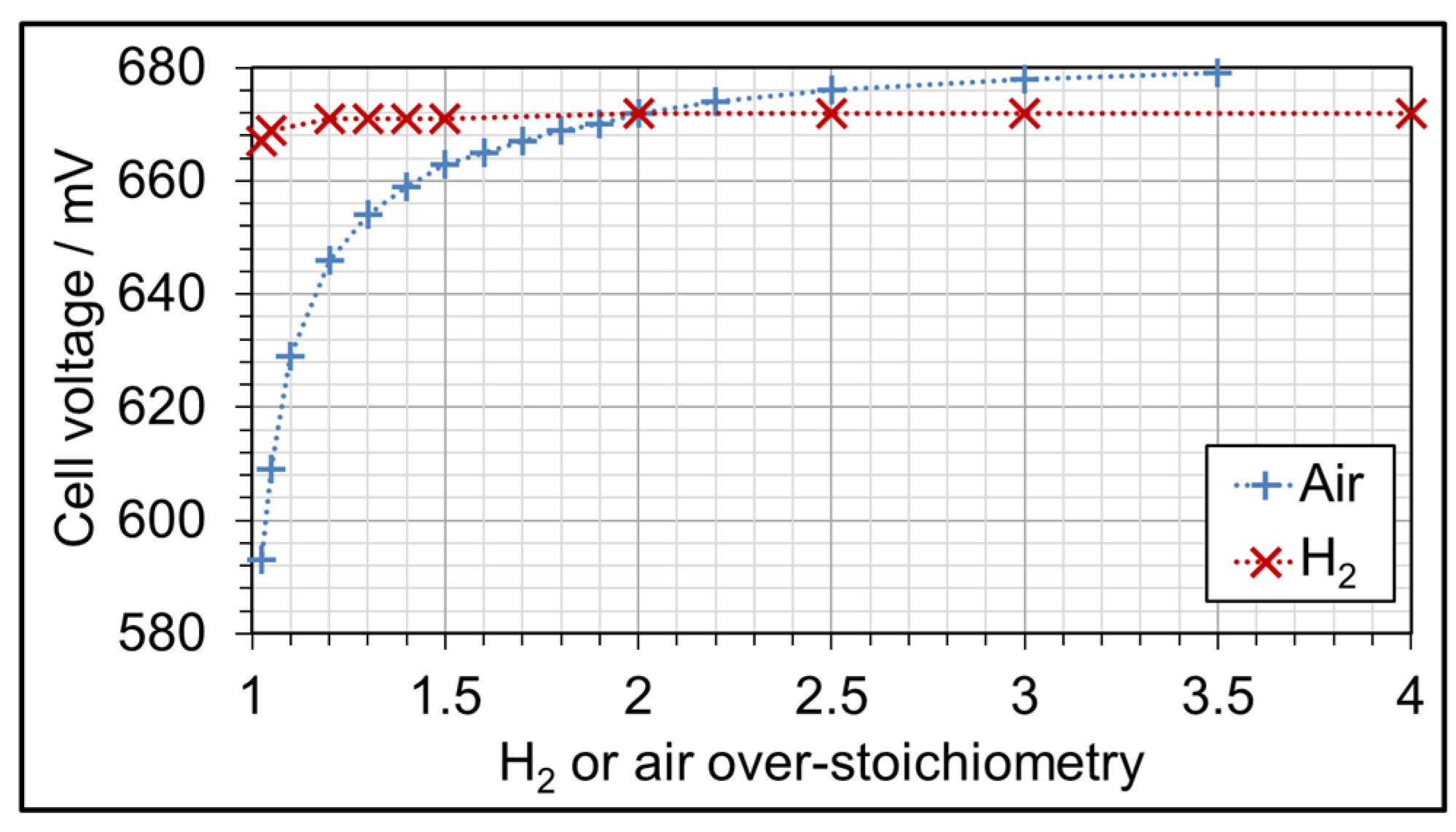
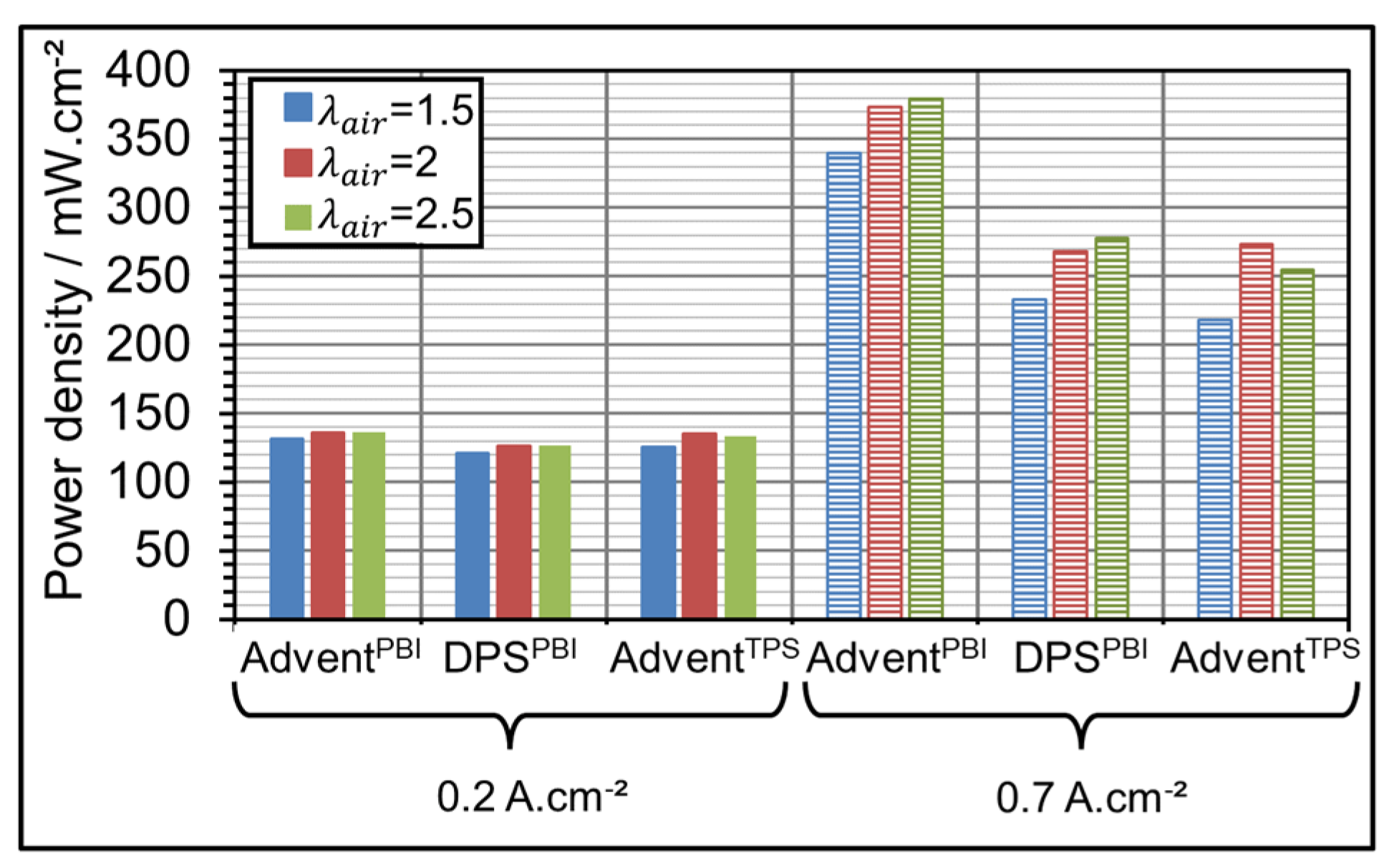
| Parameter Symbol | Minimum Value | Medium Value | Maximum Value |
|---|---|---|---|
| λH2 | 1.05 | 1.2 | 1.35 |
| λair | 1.5 | 2 | 2.5 |
| T | 140 °C (413.15 K) | 160 °C (433.15 K) | 180 °C (453.15 K) |
| Parameter | Type | Value |
|---|---|---|
| Variable | Calculated | |
| Constant | 2 | |
| Constant | 96,485 C·mol−1 | |
| Constant | 8.314 J·mol−1·K | |
| Variable | Measured | |
| Constant | Measured: 102,100 Pa absolute | |
| Constant | Measured: 21,400 Pa absolute | |
| Variable | Measured | |
| Fitting Parameter | OCs dependent | |
| Fitting Parameter | non-OCs dependent | |
| Variable | Measured | |
| Fitting Parameter | OCs dependent | |
| Fitting Parameter | non-OCs dependent |
| Error | AdventPBI | DPSPBI | AdventTPS |
|---|---|---|---|
| Overall error a (%) | 1.42 | 1.07 | 2.11 |
| Maximum error b (%) | 18/15 c | 6 | 18 |
| Fit | AdventPBI | DPSPBI | AdventTPS |
|---|---|---|---|
| Best fit | 160 °C—λ = 1.05/2.5 | 140 °C—λ = 1.35/2 | 180 °C—λ = 1.2/2.5 |
| Worst fit a | 140 °C—λ = 1.2/1.5 | 140 °C—λ = 1.2/1.5 | 140 °C—λ = 1.05/2.5 |
| Parameter | AdventPBI | DPSPBI | AdventTPS | Literature |
|---|---|---|---|---|
| 0.46 | 0.34 | 0.40 | 0.5 [38] | |
| 0.47 | 0.34 | 0.70 | 0.37–0.56 [39,40,41] | |
| (J·mol−1) | 8.42 × 104 | 6.17 × 104 | 7.43 × 104 | 5.8 × 104–6.7 × 104 [39,40] |
| (A) | 5.62 × 10−4 | 1.27 × 10−3 | 6.31 × 10−4 | 5.25 × 10−5–4.85 × 10−4 [39] |
| 3.60 | 1.21 × 10−1 | 1.00 × 10−1 | / | |
| 1.08 × 10−1 | 8.31 × 10−2 | 2.55 × 10−2 | / | |
| (cm2·s−1) | 5.61 × 10−3 | 2.37 × 10−7 | 1.06 × 10−6 | 1.2 × 10−2–2.4 × 10−1 [42,43,44,45] |
| 1.97 | 1.24 | 5.98 | 1.5–2.07 [46,47,48,49,50,51,52] | |
| 4.32 | 8.24 | 4.15 | / |
| Error | AdventPBI | DPSPBI | AdventTPS |
|---|---|---|---|
| Overall error a (%) | 1.46 | 1.29 | 4.02 |
| Maximum error b (%) | 30/8 c | 7 | 21 |
| Parameter | AdventPBI | DPSPBI | AdventTPS | Literature |
|---|---|---|---|---|
| 0.47 | 0.35 | 0.65 | 0.5 [38] | |
| 0.44 | 0.27 | 1.20 | 0.37–0.56 [39,40,41] | |
| 8.60 × 104 | 5.86 × 104 | 1.94 × 104 | 5.8 × 104–6.7 × 104 [39,40] | |
| 7.30 × 10−4 | 3.24 × 10−3 | 2.39 × 10−4 | 5.25 × 10−5–4.85 × 10−4 [39] | |
| 8.83 | 3.70 | 2.08 × 10−1 | / | |
| 1.04 × 10−1 | 7.96 × 10−2 | 3.98 × 10−3 | / | |
| 5.66 × 10−3 | 4.96 × 10−1 | 1.98 × 10−6 | 1.2 × 10−2–2.4 × 10−1 [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] | |
| 4.40 | 1.75 | 6.10 | 1.5–2.07 [46,47,48,49,50,51,52] | |
| 1.80 | 10.57 | 26.02 | / |
| Topic | 2D-3D Model | Final Model |
|---|---|---|
| Parameter consistency | + | − |
| Accuracy | + | − |
| Fast calculation | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigal, S.; Jaafar, A.; Turpin, C.; Hordé, T.; Jollys, J.-B.; Kreczanik, P. An Air Over-Stoichiometry Dependent Voltage Model for HT-PEMFC MEAs. Energies 2024, 17, 3002. https://doi.org/10.3390/en17123002
Rigal S, Jaafar A, Turpin C, Hordé T, Jollys J-B, Kreczanik P. An Air Over-Stoichiometry Dependent Voltage Model for HT-PEMFC MEAs. Energies. 2024; 17(12):3002. https://doi.org/10.3390/en17123002
Chicago/Turabian StyleRigal, Sylvain, Amine Jaafar, Christophe Turpin, Théophile Hordé, Jean-Baptiste Jollys, and Paul Kreczanik. 2024. "An Air Over-Stoichiometry Dependent Voltage Model for HT-PEMFC MEAs" Energies 17, no. 12: 3002. https://doi.org/10.3390/en17123002
APA StyleRigal, S., Jaafar, A., Turpin, C., Hordé, T., Jollys, J.-B., & Kreczanik, P. (2024). An Air Over-Stoichiometry Dependent Voltage Model for HT-PEMFC MEAs. Energies, 17(12), 3002. https://doi.org/10.3390/en17123002






