Abstract
The aim of this review is to understand the progress in waste material management through pyrolysis to produce eco-energy. The growing demand for energy, combined with the depletion of traditional fossil fuels and their contribution to environmental problems, has led to the search for waste-to-energy technologies in pursuit of carbon neutrality. While municipal residues are only part of the waste management problem, the impact of discarded plastics on the environment and landfills is significant. Plastics not only take centuries to decompose, but also seriously pollute the oceans. Pyrolysis is a thermochemical process that allows for the thermal decomposition of waste in the absence of oxygen. There are several types of pyrolytic reactors, including batch and continuous ones. Batch reactors are preferred to process polymeric waste, with studies highlighting the importance of optimizing parameters, i.e., type of feedstock, heating rate, and pyrolysis temperature. Moreover, the choice of reactor type can influence the yield and structure of the final compounds. Furthermore, various studies have highlighted the gas heating value obtained through waste pyrolysis and how the composition of the liquid fraction is influenced by the type of polyethylene used. Though scientific interest in pyrolysis is remarkable, as publications have increased in recent years, kinetics studies are scarce. Overall, pyrolysis is a promising technique for managing waste materials to produce energy. Ongoing research and development in this area offer significant potential for improving the sustainability of waste management systems.
1. Introduction
Global energy demand is steadily increasing due to economic and population growth. At the same time, traditional fossil fuels, such as oil and coal, are being depleted and significantly contributing to environmental problems, such as climate change. It is therefore necessary to look for new renewable fuels. In this context, waste-to-energy technologies may help in meeting energy demand while reducing dependence on fossil fuels. Governments around the world are taking steps to encourage research and development of these technologies, i.e., establishing policies and investment programs. In addition, to reduce greenhouse gas emissions while achieving higher energy efficiency, long-term goals and targets are also being set. The United Nations Framework Convention on Climate Change (UNFCCC) is an international treaty, signed in 1992, to address the problem of climate change. The treaty, which has been ratified by nearly all countries, establishes a framework for reducing greenhouse gas emissions and mitigating their effects. The goal of the UNFCCC is to stabilize greenhouse gas concentrations in the atmosphere at a level that will prevent dangerous human interference with the climate system. To oversee the implementation of the convention, it also established the Conference of the Parties (COP) as its supreme body [1]. In recent years, to address climate change while reducing greenhouse gas emissions, several international agreements have been reached. Some of the most prominent include the following:
- The Paris Agreement: Adopted in 2015 during the UN Conference of the Parties on Climate Change (COP21), it aims to limit global temperature rise to less than 2 °C above pre-industrial levels. Moreover, it works towards a more ambitious target of 1.5 °C [2].
- Glasgow Accord: This agreement was reached at the UN COP26 on climate change, in 2021, and aims to increase efforts to achieve carbon neutrality by 2050, reducing greenhouse gas emissions in all sectors [3].
- Environment and Economy Partnership Agreement: Reached at COP26, this is a partnership between governments, businesses, and non-governmental organizations. It promotes collaboration and commitment to address climate change and accelerate the transition to a low-carbon economy [4].
Approximately 1.9 billion tonnes of municipal solid waste (MSW) is produced annually worldwide, and almost 30% is not collected by municipal waste management systems [5]. In addition, MSW generation in 2050 is projected to increase to 3.4 billion tonnes. In this context, the EU has included sustainable resource use as a priority area in the European Green Pact. Moreover, the EU has reiterated its commitment to implement the 2030 Agenda for Sustainable Development, with the aim of protecting the environment, reducing land degradation, and preventing biodiversity loss. This includes reducing the EU’s dependence on the use of natural resources from fossil origin [6]. Excessive and uncontrolled consumption leads to large generation of waste, and consumption extends to fuels. The transport sector is a major consumer of fossil fuels. The road transport sector accounts for 72% of total greenhouse gas emissions in transport [7]. Through the correct treatment of waste, it is possible to obtain eco-fuels, contributing to mitigating the problem. In the field of thermochemical processes, pyrolysis occupies a unique position for the conversion of coal and biomass into both energy and non-energy applications [8]. In this sense, pyrolysis plays an important role in potential resource and waste management pathways.
Municipal waste is only part of the problem in waste management. Discarded plastics have a major environmental and landfill impact. Accelerated consumption has led to an increase in single-use products, which, coupled with slow growth in recycling, results in a high rate of accumulation of plastic waste [9,10]. A recent study by Zhao et al. [11] analyzed plastic imports and exports from 1990 to 2019. The plastic trade has been growing steadily since 1990 (84,500 t of imports), but it is between 2000 and 2016 that the highest growth was experienced. The highest point of consumption was achieved in 2012, with 11,386,200 t of imported plastic waste. It is from 2016 onwards that trade has slowed down sharply. China has been the largest importer and exporter of plastic waste in the world for decades. However, in 2017 it announced a zero plastic waste import policy, significantly reducing the volume of the plastic trade, although the export volume has not changed [12]. The massive use of plastics has a negative impact on the environment. Despite its versatility and durability, non-biodegradable plastic takes centuries to decompose and ends up polluting the oceans. The full extent of plastics in the oceans and seas is not yet known, but the impact on marine wildlife is notorious, as they ingest the waste, which can then be consumed by humans [13].
Biomass refers to any organic matter that is derived from living organisms, including plants and animals. Biomass can be used as a source of energy and is considered to be a renewable energy source, because it can be replenished relatively quickly. Examples of biomass include wood, crops, and agricultural waste, such as straw, as well as organic waste from the food and paper industries. Biomass can be converted into a variety of energy sources, e.g., heat, electricity, and biofuels. Biofuels mainly include ethanol, methanol, and biodiesel, which can be used as a replacement or supplement to traditional fossil fuels. Hydrocarbons are compounds that consist of hydrogen and carbon atoms. Some hydrocarbons, such as those found in fossil fuels like coal, oil, and natural gas, are non-renewable resources. However, biomass is mainly composed of carbohydrates; some examples include the following:
- Cellulose: A complex carbohydrate that is the main structural component of plants’ cell walls.
- Hemicellulose: A complex carbohydrate found in plants’ cell walls, often found alongside cellulose.
- Lignin: An amorphous phenol-based organic polymer, which provides strength and rigidity to wood [14].
- Lipids: Compounds present in plants and animals, insoluble in water but soluble in organic solvents. They include gums, oils, and waxes, and their structures may consist of long, straight hydrocarbon chains or isoprene units [1].
- Sugars: A class of simple carbohydrates that include glucose, fructose, and sucrose, which can be found in many plants [15].
Pyrolysis is a thermochemical cracking process that breaks down long-chain hydrocarbons into molecules with lower molecular weight [16]. The process is carried out under oxygen-free conditions and in temperature ranges between 300 and 700 °C. The final products depend on various factors, i.e., thermal decomposition rate, feedstock, particle size, and temperature [17]. The word “pyrolysis” is derived from the Greek words “pyro”, meaning fire, and “lysis”, meaning decomposition. It has been used for centuries for charcoal production and, in ancient Egypt, for making tar and embalming agents [18].
Early work on biomass pyrolysis is known from the 18th century. During the 1980s, scientists discovered that the yield of pyrolysis liquid could be increased by fast pyrolysis, where the feedstock is heated rapidly and the resulting vapors are also rapidly condensed [19]. Since then, and to date, the discoveries and advances in the use of pyrolysis have been significant. The commercial applications offered by pyrolysis promote the creation of systems to exploit this technology. One of the most recent patents, made in 2022 in Japan, proposes a process and a system for converting biomass into high-carbon bioreagents, suitable for various commercial applications [20].
The purpose of this review is to understand the possibilities of using pyrolysis for waste treatment and to create a workflow for pyrolysis experimentation. It is therefore essential to know which feedstocks can be pyrolyzed and what results can be obtained from them. Moreover, it is essential to have a comprehensive understanding of the various types of pyrolytic reactors, as well as the operating conditions and the effects of catalysis.
2. Raw Materials
The selection of the pre-treatment method and its feasibility are highly influenced by the type and humidity of the raw material. For this reason, it is crucial to classify the raw material for the desired production. Some of the most common feedstocks for pyrolysis include the following:
- Agricultural and forestry industry residues: Pyrolysis can be used to convert them into biofuels [21,22]. These feedstocks include pine pruning waste, rice straw, corn stover, sunflower waste, and olive waste, among others. Chen et al. [23] carried out pyrolysis at different temperatures with pine needles. The pine needles were pre-treated by cleaning, air-drying, and baking for subsequent grinding. In this case, the authors sought to avoid burning and charring. Garcia-Perez et al. [24] used two types of pine chips, of different origins and sizes, in different pyrolysis reactors. This allowed for a comparative analysis, finding similar results, with slightly better liquid fraction yields in the auger-type reactor compared to the batch one. Another work, by Yildiz et al. [25], analyzed the product composition of catalytic and non-catalytic fast pyrolysis of pine wood, itself a low-ash feedstock. The ash that accumulates can affect the catalyst efficiency by influencing the composition of the resulting pyrolysis vapors. The authors concluded that ash accumulation has an impact comparable to that of other catalyst problems that can affect the pyrolysis process, e.g., catalyst deactivation. Nam et al. [17] experimented with the possibilities of rice residue pyrolysis with different reactors. The rice residue was subjected to pre-treatment by air drying, and then it was chopped into smaller particles. The authors emphasized the importance of the moisture content, and they observed better yields in slow processes (auger and batch) for biochar, in addition to higher bio-oil quantity in fluidized bed reactors. Huang et al. [26] discussed the recovery of rice straw into resources and energy, using microwave-induced pyrolysis. They sought constant moisture by tanning the rice residue for 10 days, before it was crushed and sieved. The efficiency of this process depended on the microwave power and the size of the rice straw particles. They concluded that, for a satisfactory result using very small particles, a lower microwave power would be necessary. Zabaniotou et al. [27] compared the results of pyrolysis of different agricultural-based materials, i.e., maize, sunflower, and olive residues. Using fixed-bed reactors with and without catalysis, cellulose- and hemicellulose-based wastes produced higher amounts of hydrogen-rich gas than those based on lignin. Ren et al. [28] investigated the integration of microwave torrefaction and pyrolysis of corn stover. Torrefaction oils are noted for their high-value-added chemicals (furans and phenols), making them potentially interesting as a fuel source. The authors highlighted the use of torrefaction as a pre-treatment, combined with pyrolysis, to improve bio-oil quality. Colantoni et al. [29] based their study on the pyrolysis of grape and sunflower residues in the search for sustainable alternative fuels. The results demonstrated that torrefaction and pyrolysis of pelletized agricultural residues was an effective method to produce high-calorific-value biochar. Lajili et al. [30] studied olive residue pyrolysis, implementing biomass gasification, although the results were inconclusive. Kabakci et al. [31], in addition to studying the characteristics of olive residue pyrolysis, also investigated pyrolysis kinematics. The results were compared with those from refuse-derived fuel (RDF) pyrolysis, finding that olive residue decomposition started at lower temperatures but showed a higher maximum temperature; the temperature range of olive residue devolatilization was larger than that for RDF.
- Old and discarded furniture can also be processed by pyrolysis to produce biofuels and other chemicals. Uzun and Kanmaz [32] found that pine sawdust was a promising feedstock for bio-oil production, with maximum production rates of 42% (w/w). The importance of particle size is highlighted in both the above study and the one carried out by Heo et al. [33]. In the latter study, with respect to furniture sawdust pyrolysis, it was found that a higher gas flow, together with a higher feed rate, was favorable for bio-oil production, as vapor residence times were reduced.
- Plastic waste is an important source of feedstock for pyrolysis, as it contains carbons and can be converted into fuels, e.g., diesel fuel and natural gas [10]. In fact, liquids with a high calorific value can be obtained from the pyrolysis of plastics. In other words, they can be useful as fuels. Some plastics commonly used for pyrolysis include polyethylene (PE), low-density polyethylene (LDPE), linear low-density polyethylene (LLDPE), high-density polyethylene (HDPE), polystyrene (PS), and polypropylene (PP) [34]. Gaurch and Pramanik [35] studied the aromatization of PE plastic waste with fly ash (FA) as a catalyst. From this work, potential interest in and application of the catalytic pyrolysis process as an option to produce aromatics (benzene, toluene, ethylbenzene, and xylene (BTEX)) can be extracted. LDPE has been used as a raw material in numerous works, from which various conclusions can be drawn. Aguado et al. [36] highlighted the increase in the conversion rate by integrating catalysts in LDPE pyrolysis in a continuous screw reactor. Marcilla et al. [37] also analyzed the impact of catalysts. In their study, the authors investigated the polymer structure of products obtained from thermal and catalytic pyrolysis of LDPE and HDPE, in the presence of HZSM5 and HUSY zeolites. They focused on analyzing the composition of the gaseous and liquid fractions and found that the liquid fraction contained higher amounts of 1-olefins and n-paraffins. Investigations by Alonso-Morales et al. [38], on LDPE pyrolysis in batch feed reactors with slow and fast heating, did not provide optimal results in terms of solid char production, despite the use of activation additives and pyrolysis materials. However, the use of a semi-continuous feed reactor with fast heating achieved a high yield (15–52%, w/w) of solid coals due to the longer residence times of the pyrolysis products. In addition, pyrolysis in a metal-free quartz reactor produced very high solid carbon yields (15–43%, w/w). Fan et al. [39] focused their investigation on LLDPE conversion using both continuous-stirred microwave pyrolysis (CSMP) and batch microwave pyrolysis (BMP) systems. Reactions took place in the presence and absence of an ex situ catalytic bed with HZSM-5. The authors observed significant differences in product yields for the non-catalytic processes, where CSMP produced a higher condensate yield and a lower gas yield compared to BMP. Pyrolysis of HDPE was studied by Sogancioglu et al. [40], together with pyrolysis of LDPE, characterizing the resulting fractions. The char obtained was analyzed for its use as an additive in epoxy composites. Epoxy composites with HDPE carbon additives at 300 °C showed improved elongation at break and tensile strength performance. Kim et al. [41] performed kinetics tests on the pyrolysis of a mixture of waste automobile lubricating oil (WALO) and PS using thermogravimetric analysis (TGA). From this study, the analysis of the carbon number distribution of the oil produced at different heating rates is noteworthy. Decreasing the heating rates resulted in a slight shift in the carbon number of the produced oil towards light hydrocarbons. Park et al. [42] studied PP pyrolysis with a novel activator-assisted process. Increasing the activator temperature and the bubbling zone significantly increased the gas and oil yields, respectively. The use of nitrogen and a short residence time were found to increase the olefin yield. Degradation of activated PP molecules took place through different mechanisms. In this context, Kasar and Ahmaruzzaman [43] found that co-pyrolysis of crude oil with PP produced 80% pyrolytic oil. Furthermore, homogeneous catalysis has been proposed as an alternative for plastic waste treatment and high-value chemical production [44]. Aside from everything mentioned so far, plastics are ideal for pyrolysis because of their abundance, low density, and calorific value, among other properties. In summary, plastic waste, once considered to be an environmental problem, has become a valuable pyrolysis raw material.
- Other industrial waste, such as paper and wood, can also be processed by pyrolysis to produce biofuels and other chemicals. Potential landfill waste can include newsprint and cardboard, which contain nitrogen, sulfur, and oxygen, as highlighted by Fekhar et al. [45], who also highlighted the difference in moisture content between plastics and paper waste. The latter contains considerably more moisture than plastics, which hardly include any moisture at all. It is important to take this characteristic into account before a pre-treatment process is selected. After pyrolysis, the authors noted that the liquid product from newsprint and cardboard resulted in water and various oxygenated compounds. Ahmed and Gupta [46] investigated the gasification and pyrolysis of paper, underlining that gasification offers better results in terms of higher material destruction, hydrogen production, and chemical energy. Yao et al. [47] focused on the treatment of paper sludge, emphasizing the problems that it poses in terms of industrial pollution in China. Moreover, sludges do not come with paper alone; they also contain heterocyclic compounds, polycyclic aromatic hydrocarbons, amino acids, and organic fluorinated compounds. The authors proposed pyrolysis treatment of this waste to reduce air pollution and carbon emissions, compared to direct burning of waste. Determining the pyrolysis temperature is important for optimal results. Kim et al. [48] sought the optimal temperature to achieve the maximum bio-oil yield from the pyrolysis of construction wood waste. Carlson et al. [49], in their study on the production of aromatics and olefins from wood in a fluidized bed reactor, showed that propylene is more reactive than ethylene and produces higher quantities of aromatics. They also noted that the lower the temperature, the lower the methane production. The study of experimental kinetics is interesting in pyrolysis studies. Slopiecka et al. [50] conducted a kinetics study of the devolatilization of aspen wood, finding that its thermal decomposition proceeds in three stages.
- Waste tires are an important source of raw materials for pyrolysis, as they contain rubber and steel, which can be recycled into fuels and other products. In the research of Berrueco et al. [51] on tire pyrolysis, it was pointed out that gas production is favored by long residence times at high temperatures. Tires are notable for their contents of hydrocarbons and gaseous fractions, i.e., H2, CO, and CO2. However, the calorific value of the gas obtained from the pyrolysis was lower than expected, although it was still valid for use in gas engines. According to Williams [52], the oil from tire pyrolysis is chemically complex and contains aliphatic, aromatic, heteroatomic, and polar compounds. This oil’s properties allow its use as a fuel, as its properties are similar to those of diesel fuel or light fuel oil.
3. Pyrolysis Reactors
Understanding the different pyrolysis techniques and available reactors is essential. The choice of the right reactor is linked to economic availability, reaction time, and the desired results. Pyrolytic reactors are divided into two main groups: batch and fluid reactors [48]. However, some changes in the cycle allow for differentiation between subtypes of pyrolytic processes. Some preliminary concepts that must be understood before addressing the different types of reactors are developed below. To create an oxygen-free environment, the reactor is purged with nitrogen. In some cases, the flow of N is maintained only at the beginning of the process [53], while in others, the flow is kept throughout the entire process [37]. The main parameters affecting the yield and final product properties are reaction time, pressure, temperature, particle size, heating rates, and feedstock initial moisture content [54,55]. During pyrolysis, the feedstock is subjected to high temperatures and fragmented into a series of simpler chemical compounds. These compounds can be divided into different fractions, each of which is made up of a specific set of chemical compounds. Some of the common fractions that can be obtained during pyrolysis include the following [56]:
- Gas: The gaseous fraction, like all fractions, depends on the composition of the pyrolyzed material and the type of reactor. Biomass pyrolysis results in gases such as CO2, CO, or hydrocarbons, but gases like acetic acid, methanol, furfural, acetaldehyde, ethanol, propane, or hydroxymethylfural (HMF) can also be released. The increase in CO2 content indicates further degradation of cellulosic and hemicellulosic components. Also, the presence of CH4 and CO suggests secondary cracking of the volatile compounds released during the process. Nam et al. [17] demonstrated that the composition of the resulting gases depends on the reactor. Hydrogen formation is characteristic of biomass containing paper and cardboard, whereas in the case of pyrolysis of plastics, mainly hydrocarbons can be identified [45]. In the study by Marcilla et al. [37], pyrolysis was carried out with LDPE and HDPE waste, resulting in the production of 1-olefins, n-paraffins, olefins, iso-paraffins, and aromatics.
- Liquid: The liquid fraction resulting from pyrolysis depends on the type of pyrolyzed material and other parameters, such as temperature or the type of pyrolysis reactor. As highlighted in their research, Uzun and Kanmaz [32] found that the liquid product from biomass pyrolysis was a mixture of multiple organic compounds. It consisted of two phases: an aqueous phase, containing low-molecular-weight oxygenated organic compounds (acetic acid, methanol, and acetone), and a non-aqueous phase, containing aromatic hydrocarbons and organic compounds (aliphatic alcohols, carbonyls, acids, phenols, cresols, benzenediols, guaiacol, and its alkylated derivatives). Aromatic hydrocarbons include single-ring aromatic compounds (such as benzene, toluene, indene, and alkylated derivatives) and polycyclic aromatic hydrocarbons (PAH, such as naphthalene, furans, phenanthrene, and their alkylated derivatives). Water is also released from biomass pyrolysis [33]. On the other hand, the liquid fraction resulting from the pyrolysis of polymers results in aromatic hydrocarbons as the main compound [42]. In the liquid fraction resulting from the pyrolysis of polymers, iso-paraffins, aromatics, n-paraffins, and 1-olefins were also observed. The n-paraffins are more frequent at low temperatures, while 1-olefins are more frequent at high temperatures [37].
- Solid: The solid fraction of pyrolysis mostly consists of charcoal and other residues, e.g., ash. If the pyrolyzed residue is biomass, it is called vegetable charcoal. As with the other fractions, the pyrolysis temperature influences this final fraction. It has been found that the higher the temperature in the pyrolysis of plastics, the higher the amount of charcoal [38]. Secondary repolymerization reactions are responsible for carbon formation. Slight hydrogen production is also observed. In all cases, pyrolysis solids are carbon-rich materials with a high calorific value that can be used as a substitute for solid fossil fuels [57]. The pyrolysis of cellulose-containing biomass results in the formation of dehydrated saccharides, furans, furanones, benzenes, and cyclopentanones. The highest yield in cellulose pyrolysis is obtained from the saccharides [58].
It is important to note that the exact composition of the fractions obtained during pyrolysis will depend on the process conditions and the organic material being used. As mentioned, pyrolysis reactors mainly differ in how the feedstock is fed and how the process conditions are controlled. The batch reactor loads a fixed amount of material, whereby the process is stopped at the end of the reaction. This leads to cleaning, emptying, and reloading for another batch. In contrast, in a continuous reactor, the material is continuously fed into the reactor and is constantly processed, without interruption. As for the process conditions, in a batch reactor, they must be controlled for the whole batch, while in a continuous reactor the conditions can be adjusted as the reactor is fed. From these differences, it follows that the continuous reactor allows for greater process control and optimization. In addition, the time required to load, process, and unload the material in a batch reactor restricts the production capacity and efficiency compared to a continuous reactor. The main advantage of the continuous reactor over the batch reactor is its ability to process homogeneous materials and small-scale processes. The different reactor types are further developed in the following sections.
3.1. Batch Reactor
Batch reactors are strictly discontinuous. A batch reactor is characterized as a simplified process with the capacity to process all types of polymeric waste. However, the batch reactor is irregular in the products of each batch, labor-intensive, and highly dependent on the heating and cooling times [45].
The reactor can be modified to allow the addition of reagents to the process, making it a semi-batch reactor. Another variant is the batch microwave reactor, which is widely used but has some problems. It makes homogeneous reaction difficult, and due to its low thermal and mass efficiency the pyrolytic products are negatively affected. But it also shortens the reaction time by improving energy consumption and the quality of the resulting fuel [39].
Batch-type stirred vessels are characterized by the agitation of the material during the heating process, at a stable speed and temperature [59]. In contrast, the static-bed batch reactor keeps the raw material immobile during the process [51]. Semi-continuous reactors consist of a tube reactor in a vertical position, with an automatic raw material loader. Quartz and Hastelloy are used for the tube [38].
Table 1 lists the key parameters in studies on pyrolysis in batch reactors. The char yield mainly depends on three parameters: feedstock type, heating rate, and pyrolysis temperature [60]. The optimal pyrolysis temperature will depend on the reactor type and the pyrolysis feedstock. With increasing temperature, a decrease in solid-phase yield is observed, except for the LDPE pyrolysis carried out by Alonso-Morales et al. [38]. The yield of the liquid fraction increases with temperature, as can be seen in Table 1. It can also be observed that the increase in yield slows down and starts to decrease from a maximum temperature. As shown in Table 1, in studies carried out with waste tires, polanga, construction wood waste, and softwood sawdust, the temperature at which the yield of the liquid fraction started to decrease was around 500–550 °C. The gaseous fraction, however, increased with temperature in all cases. It can be seen from Table 1 that the studies carried out, irrespective of the pyrolyzed waste, tended to pyrolyze small particles. A small particle size can facilitate the homogeneity of the pyrolysis.

Table 1.
Characteristics of pyrolysis considering various types of batch reactors.
In the study by Kim J. et al. [48], the maximum oil yield from construction waste wood pyrolysis was achieved at a temperature of 500 °C (54.2%, w/w). They also found that, at higher temperatures, the decomposition reaction produced higher gas-phase yields, rather than liquid-phase yields. This deduction can be extrapolated to the pyrolysis of other feedstocks, and it can be stated that pyrolysis should ideally be carried out at the optimal temperature for each feedstock. Sometimes, not all of the liquid fraction from pyrolysis is useful. This mainly depends on the pyrolysis feedstock. In the study of Fekhar et al. [45], the liquid yield consisted of two parts: light oil, and water. In another study by Shadangi and Singh [62], pyrolysis of polanga seed oil was carried out. The results showed that this pyrolytic oil contained other bio-oils, such as oleic acid, hexadecanoic acid, and octadecanonic acid, in addition to presenting a characteristic functional group. It was found that this oil has a high calorific value and can be used as a fuel substitute, as well as in other applications, i.e., as an adsorbent and solid fuel. Garcia-Perez et al. [24] pyrolyzed pine chips and found it difficult to produce crude bio-oils with appropriate fuel properties. In any case, the combination with other fuels, i.e., biodiesel, could provide great economic and environmental improvements. Furthermore, it was stated that biodiesel’s properties, after mixing, do not seem to be highly altered by the soluble fractions of bio-oils, although neutralization with a weak base, e.g., NaHCO3, is required to remove the soluble organic acids from biodiesel.
Some of the studies listed in Table 1 performed pyrolysis on feedstocks such as plastics and synthetic waste. Several conclusions can be drawn from these studies. Berrueco et al. [51] pyrolyzed tire waste, highlighting the heating value of the obtained gas, between 5.5 and 9.0 MJ/m3, valid for use in gas engines. Alonso-Morales et al. [38] studied LDPE pyrolysis in reactors with different fabrication materials: one with Hastelloy (cobalt–chromium–nickel–molybdenum alloy) and the other with quartz. The use of a semi-continuous loading reactor with a fast heating process can significantly increase the yield of carbonaceous solids, due to extended residence times. It has also been found that the material used in the construction of the reactor has an important influence on the yield and structure of the obtained solids. The use of a Hastelloy tube instead of quartz can increase the structural order of the resulting solids. Marcilla et al. [37] compared LDPE and HDPE pyrolysis. From this comparison, they highlighted that a higher proportion of gases was produced in the pyrolysis of HDPE than in that of LDPE. The researchers also concluded that the composition of the liquid fraction is influenced by the type of PE used, and that the observed disparities in results seemed to be more related to the polymer and zeolite properties than to the experimental conditions. The study by Kim et al. [59] examined the isothermal pyrolysis of PS at low temperatures using a batch-operated stirred tank. The main liquid products were single- and double-aromatic ring species, with styrene being the main product, with a yield of 70% (w/w). Other studies, such as the one carried out by Lopez-Urionabarrenechea et al. [57], focused on the influence of temperature and time on the products obtained in the pyrolysis of plastic waste. This study established an optimal reaction time of 15–30 min; longer times had no effect on conversion or on the characteristics of the obtained products. Gaurh and Pramanik [64] investigated the thermal and catalytic pyrolysis of PE in a nitrogen medium, using three different types of catalysts. The possibilities and benefits exposed by this article on the implementation of catalysts will be discussed later, but the authors concluded that the same process can be purely thermal (without catalysis) and still yield valuable products, protecting the environment and saving energy.
From the research collected so far, it is possible to observe the involvement of researchers in the search for alternatives for the management of these wastes, the possibilities of the compounds of the fractions produced by pyrolysis, and how it is important to take into account the parameters affecting pyrolysis, such as reactor type, raw material, particle size, and temperature.
3.2. Continuous Reactors
The fluidized bed reactor is noted for its high efficiency due to its high particle heating rate. In fact, it maintains constant particle movement, which increases the heat transfer of the particles. The appropriate mass and heat transfer between individual particles and gas ensures the high uniformity of the product quality [65].
The auger reactor is a variant for conveying feedstock to the reaction vessel and evacuating solid residues. To improve particle mixing and heat transfer between solid heat carriers and reactants, an appropriate design is essential. The material transport in the auger reactor allows for a good axial dispersion, which favors uniformity during the application of the thermal conditions [66]. A variant can be configured with two screws intercalated inside the reactor, known as a twin-auger or double-screw pyrolyzer. This configuration favors a constant undulation and agitation of the raw material, due to the interference of the propellers of one screw with those of the other [67].
In the case of fast pyrolysis, the most common alternative to the fluidized bed reactor for biomass and waste pyrolysis is the conical spouted bed reactor (CSBR). The particles’ cyclic movement results in high rates of mass and heat transfer between phases. This characteristic makes the reactor able to handle fine materials, sticky solids, and particles with irregular texture. Energy consumption tends to be exponential with particle size; however, a spouted bed reactor can operate with larger particle diameters. The size of these reactors is smaller than that of a fluidized bed reactor, while keeping the same capacity, and without the need for a gas distribution plate [68,69].
The bubbling fluidized bed reactor (BFB) is a common alternative to the fluidized bed reactor, due to its simple operation/design and high heat and mass transfer efficiency to biomass particles. However, it has drawbacks in heat transfer to the bed [69]. The bubbles play a key role in particle mixing and the thermal decomposition of the biomass [70].
A circulating bed reactor (CFB) is a reactor characterized by operating at high gas velocities. It also allows for the recovery of light solids and recirculation of solids that have been dragged to the bottom of the bed. Its operation is pseudo-homogeneous, with high mass and heat transfer rates. Continuous recirculation of hot solids at high rates promotes temperature uniformity throughout the bed [71].
Table 2 shows the clear tendency of the gaseous fraction to increase with temperature. Park et al. [68] determined that the reaction temperature in fast pyrolysis processes is proportional to the decomposition heat required to break biomass bonds. Moreover, the higher the reaction temperature, the higher the biomass conversion efficiency. With increasing temperature, the gaseous fraction content is affected. In the research of Haydary et al. [72], the contents of hydrogen and CO increased, but the contents of light hydrocarbons decreased, except for methane.

Table 2.
Characteristics of pyrolysis in various types of continuous reactors.
As can be seen in Table 1 and Table 2, a slight improvement in the yields of pyrolysis liquid fractions can be observed in continuous reactors, in comparison to batch reactors. From a quick glance at Table 2, it can be seen that the tendency is to pyrolyze small particles, in search of homogeneity, to improve the results. Temperatures range from 400 to 800 °C, with 500 and 550 °C being the ones that, in most cases, provide the best results in terms of the desired end products. Jung et al. [78] reaffirmed that the ideal pyrolysis temperature is around 400–550 °C, depending on the pyrolyzed material. This research also highlighted the influence of feed size and speed, with better bio-oil results for smaller feed size and higher speed. In the same way, Heo et al. [33] found that increasing the gas flow and feed rate resulted in more effective bio-oil production, due to decreased vapor residence time. In addition, the use of non-condensable gas as a fluidization medium showed significant potential to improve bio-oil production, achieving a maximum yield of 65% (w/w). However, from Table 2, it is not so evident that the yield of the liquid fraction is directly proportional to the temperature increase. Kim et al. [48], in addition to the pyrolysis of construction wood waste in a batch reactor, used a fluidized bed reactor. For both reactors, the maximum oil yield was reached at 500 °C. When the fluidized bed reactor was used, an increase in oil yield and a decrease in its temperature sensitivity were observed. Thus, it can be concluded that the fluidized bed reactor is an advantageous option for bio-oil production.
Nam et al. [17] carried out the experiment with three different reactors (batch, bed, and auger). The results of rice straw pyrolysis with the batch reactor are listed in Table 1, while the rest are depicted in Table 2. The authors found that the choice of reactor type, among other factors, may influence the final product. In this sense, the highest biochar yields were achieved with auger and batch reactors. Also, the highest amount of bio-oil was obtained in the fluidized bed reactor. The qualities of the products will also depend on the type of reactor. The bio-oil composition varied according to reactor type, while the biochar composition was similar in the three reactors. Based on the results, the authors suggested using the batch and auger reactors for biochar production. The interest in pyrolysis of waste tires has been mentioned above, but there are other automotive waste products that are also of interest. Haydary et al. [72] studied the suitability of ASR (automotive waste) for thermal processing to recover valuable materials and energy. It is possible to estimate the parameters required to design a thermal process from those associated with individual ASR components.
Some studies have been carried out in pilot plants, seeking the optimal design. Martinez et al. [74] carried out a study of the pyrolysis of tires in an auger-type pilot reactor. One of the most interesting conclusions drawn from this experimentation was that the gaseous fraction of the tire gas was the most efficient. They also highlighted the importance of CO2 emissions produced during the process, which were lower than those generated in the direct combustion of used tires, which is a point in favor of the pyrolytic process. Fernandez-Akarregi et al. [69] used a conical bed pilot reactor, which is characterized by a non-porous draft tube that allows its operation at low gas flow rates and under stable conditions, ensuring high bio-oil yields. Similarly, the arrangement of the reactor’s hydrodynamic system enables uninterrupted char suppression and ensures a high heat transfer rate, preventing the accumulation of residues in the bed and ensuring temperature uniformity. Temperature uniformity is desirable for a homogeneous process. The system studied by Yang et al. [76] successfully generated intermediate pyrolysis oils, gases, and coals. They used wood pellets and barley straw as feedstocks in two types of reactors (twin co-axial and auger), with very similar results. The authors focused their study on determining the efficiency of the process once the energy yields of the pyrolysis products were known. Determining the efficiency of a process is an essential point in knowing its viability before it can be used on a commercial scale.
In summary, the ideal temperature is mainly sought to obtain bio-oil. To determine viability and yields, without forgetting to consider speed and the size of the reactor feed, reactors and waste are compared. For this, studies in pilot reactors are highlighted as an alternative prior to industrial use.
3.3. Other Types of Reactors
In addition to the abovementioned reactors, there are variants that do not completely fit into the above classification. Some of these reactors have been studied in recent years, the results of which are given in Table 3.
The wire mesh reactor (WMR) is characterized by the heating process using an electric current flowing through a mesh. It features minimized side reactions between primary volatile particles, along with relative control of the particles in terms of time and temperature [80]. The latter authors noted that the minimal side reaction results in a purer tar. The WMR also minimizes secondary reactions of the primary volatiles in the vapor phase [81].
Vacuum reactors can be used in batch or continuous conditions. This is a promising technology for the production of bio-oil [82]. Vacuum conditions are produced with a mechanical pump and by diffusion [83].
The drop-tube reactor stands out for its versatility to work with all types of fuels, and in having good control over mass balances and residence times [84]. It is a reactor that operates at high pressures. As observed in [85], the char yield increased with pressure, while the tar yield decreased. At the same time, the liquid and gas yields remained relatively stable. The higher coal yield was due to the fact that the vapor pressure of tar and liquid is lower than the experimental pressure, causing them to remain solid.
The horizontal tubular reactor is a pyrolyzer that consists of a horizontal tube where the feedstock to be pyrolyzed is introduced. The horizontal arrangement of the reactor allows for uniform heat distribution and higher efficiency in the decomposition of the materials [86]. The combination of different systems is being studied to obtain better results. Chen et al. [87] integrated coal pyrolysis and volatiles optimization in an integrated reactor with two sections to improve tar quality. Their article compares the results of three cases, the results of which are shown in Table 3. In the first case, only the drop tube was used. In the second case, the pyrolysis time was prolonged, favoring the release of volatiles. They observed the decrease in the solid fraction and the increase in the liquid and gaseous fractions. Finally, the third case optimized the moving bed inside the reactor. The volatiles generated during pyrolysis in the drop tube passed through the carbon bed to be upgraded in the isothermal zone. A lower fraction of solids and a higher fraction of liquids were obtained.

Table 3.
Characteristics of pyrolysis in other types of reactors.
Table 3.
Characteristics of pyrolysis in other types of reactors.
| Reactor Type | Feedstock | Sample (g) | Particle Size (mm) | Heating Rate (°C/min) | Pyrolysis Temperature (°C) | Solid (%, w/w) | Liquid (%, w/w) | Gas (%, w/w) | References |
|---|---|---|---|---|---|---|---|---|---|
| Wire mesh | PVC | 0.02–0.20 | 0.106–0.150 | n.r. | 500 | n.r. | 6.13 | n.r. | [80] |
| 600 | 17.50 | ||||||||
| 700 | 22.50 | ||||||||
| 800 | 27.79 | ||||||||
| Cellulose | n.r. | n.r. | n.r. | 500 | 2.00 | 80.00 | 18.00 * | [81] | |
| Vacuum | Palm oil decanter cake (PDC) | n.r. | 0.850–2.000 | 15.0 | 400 | 48.07 | 34.52 | 17.41 | [82] |
| 500 | 38.57 | 41.31 | 20.06 | ||||||
| Drop-tube reactor | Coal (Naomachu sub-bituminous) | n.r. | 0.200–1.000 | n.r. | 500 | 94.00 | 3.00 * | 3.00 | [88] |
| 550 | 79.00 | 16.00 * | 5.00 | ||||||
| 600 | 68.00 | 25.50 * | 6.50 | ||||||
| 700 | 59.00 | 28.00 * | 13.00 | ||||||
| Coal | n.r. | 0.200–0.400 | n.r. | 600 | 80.10 | 6.40 | 4.00 | [87] | |
| 600 | 71.70 | 7.00 | 9.70 | ||||||
| 600 | 68.90 | 6.20 | 13.10 | ||||||
| Horizontal tubular reactor | Municipal plastic waste | 750.00 | 5.000 | 8.0 | 550–560 | 5.0–6.0 | 85.0–87.0 | 8.0–9.0 | [86] |
(*) By difference; (n.r.) not reported; (PVC) polyvinylchloride.
In short, Table 3 includes alternatives to traditional pyrolysis aiming to minimize secondary reactions, providing more versatile reactors, as well as better yields and efficiencies in the decompositions.
4. Types of Pyrolysis Depending on the Operating Conditions
There are two main types of pyrolysis, depending on the operating conditions, namely, fast and slow pyrolysis. Each has its advantages and disadvantages, and they are used for different purposes. In the following sections, the types of pyrolysis, along with their characteristics and applications, are discussed.
4.1. Slow Pyrolysis
Slow or conventional pyrolysis is considered to be an efficient conversion technology for energy production, producing liquid and solid char, which facilitates its storage [89]. The biochar yield from slow pyrolysis is higher than that from fast pyrolysis; that is, slow pyrolysis has a lower degree of ablation of the feedstock [90]. Slow pyrolysis can work at lower temperatures, between 350 and 450 °C, as well as longer residence times for larger biomass particle sizes [89]. The low temperatures, together with the slow heating rate, mainly produce charcoal [91]. In terms of the economic cost of the system, slow pyrolysis reactors are less expensive [92].
4.2. Fast Pyrolysis
Fast pyrolysis is an economically viable type of thermochemical conversion for the transformation of organic materials into liquid fuels. It requires fast heating rates and short volatile residence times. Vacuum pyrolysis, screw, and fluidized bed reactors are fast pyrolysis reactors [65].
The advantages of fast pyrolysis are the simplicity of the process, operation at atmospheric pressure, low production cost, high thermal efficiency, low fossil fuel inputs leading to CO2 neutrality, and the production of a main liquid product that is easily stored and transported. At the same time, it is a process with very specific requirements, such as a suitable pyrolysis temperature, fast heating rate of biomass particles, fast condensation, and short residence time of volatiles in the reactor. The heating rate of the particles must be high enough for fast pyrolysis, thus increasing the gaseous products [19,91]. The residence time of volatiles must be limited to minimize their cracking to non-condensable gases or carbon resulting from secondary reactions [78]. In fast pyrolysis, at high temperatures, secondary cracking reactions of volatile compounds dominate, leading to a reduction in pyrolysis oil yield and, subsequently, increasing the gas yield [93]. In Table 2, the results of fast pyrolysis of sawdust reflect an improvement in oil yield from 400 °C to 500 °C. However, from 550 °C onwards, the opposite effect is shown, due to secondary cracking of volatiles [68].
4.3. Soft Pyrolysis or Dry Torrefaction
Dry torrefaction, or soft pyrolysis, is a process in which biomass is heated to a temperature of 200–300 °C, in an inert atmosphere, for a period from 30 min to several hours [54]. In pyrolysis processes, torrefaction is useful to reduce the oxygen content and improve the bio-oil quality. It is carried out as a raw material pre-treatment [94].
As a result, three types of products are generated, namely, solid products, permanent gases (H2, CO, CO2, and CH4), and a condensable mixture containing water, organic compounds, and lipids. The solid product is the main product, representing approximately 70% of the mass and 90% of the energy of the raw biomass. The results indicate that torrefaction with CO2 and O2 instead of N2 reduces the number of obtained solids. However, if H2O is added, the negative effects of non-inert gases may be reduced [95].
The effects of both roasting and pyrolysis of corn stover have also been studied [28]. Torrefied biomass had similar calorific values to coal and better than those of raw biomass. Moreover, torrefied oils contained valuable chemical compounds, such as furans and phenols. Although torrefaction reduced the pyrolytic oil yield, the total bio-oil yield obtained through torrefaction and pyrolysis was similar to that obtained through pyrolysis of unprocessed biomass.
Other authors have investigated the influence of roasting on hemicellulose, cellulose, and lignin pyrolysis [96]. The results indicated that, during torrefaction, O-acetyl and pentose units present in the hemicellulose were thermally degraded into acetic acid and furfural. As a result, acetic acid and furfural acid levels significantly decreased in the pyrolysis of torrefied hemicellulose. However, the impact of torrefaction on cellulose is small, due to the high thermal stability of its crystalline structure. Torrefaction at a temperature of 300 °C has a large impact on lignin pyrolysis. Torrefied lignin leads to an increase in the quantity of aromatic compounds during pyrolysis.
5. Catalytic Pyrolysis
The presence of catalysts in pyrolysis processes has been discussed by many authors. The aim of this section is to find out how catalysts may influence each fraction’s yield. In addition, their influence is compared and assessed in comparison to non-catalytic processes.
Catalytic pyrolysis of biomass has been studied in depth in recent years, focusing mainly on evaluating the influence of zeolitic catalysts, which have a strong presence in the petrochemical industry, as they promote cracking, deoxygenation, and aromatization reactions [61]. To remove oxygen from organic compounds and convert them to hydrocarbons, vapor-enhanced pyrolysis using zeolite catalysis is a potentially promising approach. ZSM-5 has been shown to be particularly effective in converting methanol to hydrocarbons in the gasoline range [97]. Fluid catalytic cracking (FCC) is the most used process in refineries to convert heavy crude oil into gasoline and other hydrocarbons [98].
Compared to thermal cracking, catalytic pyrolysis has a higher yield of the gaseous fraction and a lower liquid fraction. This is due to the properties of the ZSM-5 catalyst (based on silica and alumina), which, due to its strong acidity and microporous crystalline structure, exhibits excellent performance in catalytic cracking efficiency, isomerization, and aromatization of larger hydrocarbon molecules [35,64]. Yildiz et al. [24] studied the effects of alkali and alkaline earth metals (AAEMs) as intrinsic catalysts for the thermal decomposition of biomass. AAEMs influenced cracking and repolymerization that occurred with biomass devolatilization. AAEMs with 0.5% (w/w) ash contents have been shown to be sufficient for the large alteration of pyrolysis products, affecting chemical speciation.
Catalytic fast pyrolysis (CFP) is a promising technology to directly convert solid biomass into gasoline-range aromatics that are compatible with current market gasoline. Carlson et al. [49] demonstrated that CFP can be carried out in a continuous fluidized bed reactor with real biomass feed.
Table 4 shows the results of the use of catalysts for pyrolysis. The coexistence of fractions, together with the presence of coke, can be observed. Inayat et al. [53] found small amounts of coke on the catalyst surface, less than 0.5% (w/w). It was also found that the implementation of a layered catalyst (feedstock and catalyst separated with quartz wool) and the use of a semi-batch reactor had a positive impact on the recovery of oil and styrene during thermal and catalytic pyrolysis of PS. These findings could be of great use in improving the efficiency of the thermocatalytic pyrolysis process in the treatment of plastic waste. As mentioned for thermal pyrolysis without catalysts, Celikgogus and Karaduman [63] verified that the optimal temperature was around 500 °C. Fekhar et al. [45] demonstrated that catalysts could increase the yield of volatile compounds by improving the properties of the final product to be used as fuel. Table 4 shows that the gaseous fractions are higher than the data reported in Table 1 for reactions without catalysts. Efika et al. [75] evaluated several nickel-based catalysts, with similar results in terms of phase yields. However, substantial differences were found in the yields of the phase compounds. Results on the composition of the pyrolysis products are discussed in the following section.

Table 4.
Characteristics of catalyst-modified pyrolysis.
The HZSM-5 and ZSM-5 zeolite catalysts have been used in numerous studies, as shown in Table 4. Marcilla et al. [37] compared them with a hierarchical Y-type zeolite catalyst (HUSY). They highlighted the increase in gas generation when the catalysts were used, especially with HZSM-5. In the case of HUSY, the increases in liquids and coke deposition were more remarkable. Fan et al. [39], in addition to comparing a continuous microwave pyrolysis reactor with a batch reactor, compared results with or without the presence of the HZSM-5 catalyst. Significant differences in product yields between catalytic and non-catalytic processes were found (Table 1, Table 2 and Table 4). The continuous-stirred microwave pyrolysis (CSMP) process was found to be more effective in condensate generation and less effective in gas production, compared to batch microwave pyrolysis (BMP). Subsequent catalytic upgrading reduced the carbon number distribution and facilitated the formation of aromatics. Compared to BMP, CSMP in the downstream catalytic configuration demonstrated a narrower carbon number distribution, a higher selectivity of hydrocarbons in the gasoline range, and a larger higher heating value (HHV). Persson and Yang [61] carried out a catalytic batch pyrolysis of demineralized biomass. Using a bench scale at higher temperatures, higher yields of aromatic hydrocarbons were obtained. The findings indicated that secondary reactions of demineralized biomass pyrolysis produced a vapor composition conducive to aromatic hydrocarbon generation in the presence of HZSM-5. Table 4 shows how temperature affected the coke yield for these cases. The yield decreased as the temperature increased, but a much greater increase in coke yield was observed at higher temperatures. Kim et al. [48] reaffirmed that the use of the HZSM-5 catalyst increased the yield of aromatics. In addition, an increase in light phenols was also observed, improving the quality of the produced oil.
Gaurh and Pramanik [35] carried out an investigation on the pyrolysis and aromatization of PE plastic waste (present in municipal solid waste) using an FA catalyst, which was synthesized in two different arrangements. The results showed that the FA-800 catalyst was very efficient in the aromatization of the pyrolysis product in the reactor, obtaining the highest amount of aromatics/BTEX (22.10%, w/w). Additionally, the study showed that this process can be scalable to treat large amounts of municipal PE waste and convert it into energy. In another investigation on the thermal and catalytic pyrolysis of PE in a nitrogen medium, three catalysts were used [64]. It was found that the ZSM-5 catalyst showed a high yield in the aromatization of the pyrolysis product in the reactor. In addition, pyrolysis with the multiphase catalyst (liquid and vapor phases) resulted in the highest number of aromatics (35%, w/w). It was also observed that the pyrolysis oil had physicochemical properties that made it suitable as an alternative fuel and source of valuable chemicals, such as benzene, toluene, or xylene. The gas produced during pyrolysis may be used to supply energy in the process industry, while the surplus can be used to generate additional energy. Wang et al. [79], when applying a Ni-Fe catalyst in the pyrolysis of sawdust, observed that the gaseous fraction increased remarkably, while the liquid and solid fractions decreased significantly.
6. Pyrolysis Composition
The composition of the individual fractions depends, among other things, on the type of pyrolysis and the pyrolyzed feedstock. The knowledge of the composition of the elements resulting from pyrolysis is crucial for finding possible applications. Nevertheless, it is difficult to obtain an overview of the individual compounds and sub-compounds, due to their wide variety. Considering the research mentioned above, Table 5, Table 6 and Table 7 reflect the chemical characteristics of the different pyrolyzed raw materials.

Table 5.
Product gas composition for different feedstock and pyrolysis conditions.

Table 6.
Product liquid composition for different feedstock and pyrolysis conditions. Chemical selectivity of the liquid fraction.

Table 7.
Product liquid composition for different feedstock and pyrolysis conditions. Elemental contents of the liquid fraction.
Table 5 shows that the use of catalysts considerably increases the formation of aromatics. According to Fan et al. [39], products generated by batch pyrolysis show a higher selectivity towards short carbon chains, while those from continuous pyrolysis tend to have longer carbon chains. By applying ex situ catalytic upgrading processes, a narrower carbon number distribution and a higher formation of aromatic compounds were achieved. When comparing the catalytic configuration applied to the two processes, a tighter carbon number distribution, higher selectivity of gasoline-range hydrocarbons, and higher calorific value in the condensate products were observed in the continuous process. In addition, continuous processes seem to perform better for fuel production. In the pyrolysis of plastics, in the liquid fraction (Table 6), aromatic hydrocarbons are present. The presence of toluene and benzene is of interest as fuel-quality-enhancing additives, as they have a high combustion capacity. However, the toxicity of these compounds must be considered.
The influence of temperature on the resulting compounds is also shown in Table 5. Based on the investigations of Haydary et al. [73], the H2 and CO contents in the gas increased with temperature, while the HCx content decreased. The use of catalysts has a significant impact on the liquid fraction (tar) content and the composition of the pyrolytic gases, especially for temperatures above 750 °C. The increase in phenols with temperature can be a sign of good-quality oil, and this is more common in continuous reactors. Kim et al. [48] found that the contents of oxygenates, acids, and N-compounds in bio-oil decreased as the temperature increased. In contrast, the phenol content increased, which was associated with a higher quality of the produced oil. In addition, the phenolic contents in the continuous reactor were found to be higher than those in the batch reactor, suggesting that the former is more effective in breaking down lignin. As indicated by Lopez-Urionabarrenechea et al. [57], gases produced during pyrolysis can be used to supply the energy demand of the process, and the surplus can be used to produce additional energy. In other words, pyrolysis, especially of plastic waste, is an energetically sustainable process. Jung et al. [78], in the pyrolysis of rice straw and bamboo, also assessed the possibility of using waste charcoal as an energy source, thus making the process more sustainable as a waste treatment method. In the same research, some bio-oil compounds were highlighted, namely, phenolics, ketones, and aldehydes. Phenolics and aldehydes can be used in the manufacture of resins, while ketones can be used as solvents. Considering bio-oil from the pyrolysis of furniture sawdust residues, Heo et al. [33] also identified the presence of phenols.
The higher heating value is one of the main quality guarantees of a pyrolytic fuel, as it determines its energy value [32]. However, the presence of products that interfere with combustion must be considered. Higher carbon and hydrogen contents translate into higher combustion energy and, as demonstrated in Table 7, this combustion capacity is intrinsically related to the HHV. In cases where cellulose is present, the presence of hydrogen is common, as shown in the research of Fekhar et al. [45]. When the aim of pyrolysis is to obtain gaseous products to be used as fuels, the presence of hydrogen is beneficial, as it is a flammable gas with a high HHV. However, if the objective is to obtain liquid or solid products, the presence of hydrogen may decrease the quality and properties of these products. Results from Shadangi and Singh [62] indicate that pyrolytic polanga oil possesses suitable liquid fuel properties. The presence of a higher amount of oxygen in bio-oils produced by pyrolysis indicates the presence of oxygenated chemical compounds, as shown by Nam et al. [17]. A high oxygen content in the biofuel can have several drawbacks, such as low calorific value, or instability and immiscibility with other hydrocarbons.
7. Workflow for Pyrolysis Experimentation
Figure 1 gives an overview of what the workflow in pyrolysis experimentation might look like. For this scheme design, previous sections have been considered, as well as the experiments carried out in the literature. First, it is important to select the feedstock to be pyrolyzed. For this purpose, an analysis of the feedstock properties, including moisture content, sample homogeneity, and particle size, must be carried out. At this point, the TGA technique provides very useful information. TGA is fundamental in this type of research. It highlights the importance of a good selection of reactor type, together with the assessment of pyrolysis temperatures in the reactor, to provide a favorable eco-energy production yield. Although TGA has been mentioned above, there are other uses of this technique that should be mentioned. Özsin and Pütün used TGA to perform a kinematic study of the co-pyrolysis of cherry seeds and PVC [99]. Slopiecka et al. [50] also carried out a kinematic study with TGA to analyze the slow pyrolysis of tree residues. In the study conducted by Sun et al. [100], a kinetics study of the decomposition of waste printed circuit boards was carried out. To discuss the heating rates and the influence of pyrolysis on the thermal decomposition kinetics, TGA was used. Sometimes, TGA is carried out in conjunction with other techniques, as in the case of the study by Yao et al. [47], which investigated the weight loss characteristics, combustion kinetics parameters, and pyrolysis and gasification processes, and identified the gases using Fourier-transform infrared (FTIR) spectrometry.
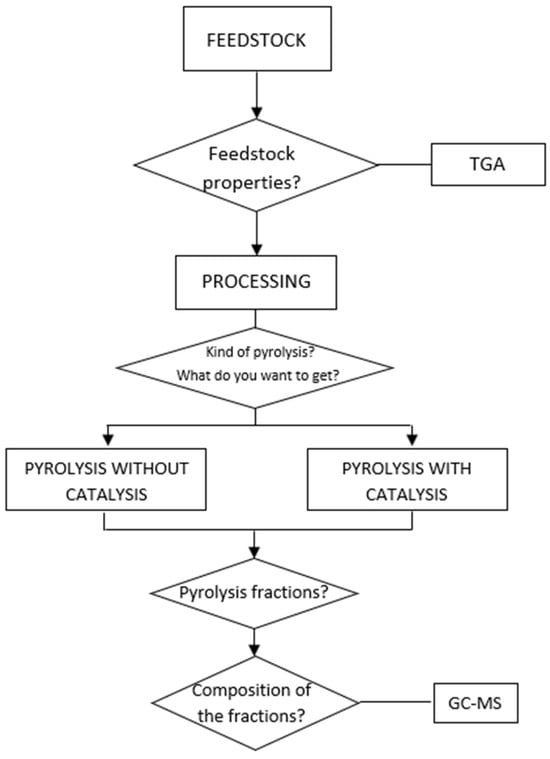
Figure 1.
Workflow for pyrolysis.
Once the feedstock has been analyzed, the sample must be processed. In other words, the material to be pyrolyzed must be crushed, cut, dried, or any other pre-treatment required to provide the best results. Next, the type of pyrolytic reactor must be selected beforehand. Then, there would be two possible routes, pyrolysis with or without a catalyst. Regardless of the process followed, the next step would be to analyze the obtained fractions. The analysis of the compounds derived from pyrolysis is mostly carried out by gas chromatography–mass spectrometry (GC-MS), as shown in Table 5, Table 6 and Table 7. As an example of the use of GC-MS, Table 7 includes the study of polanga pyrolysis by Shadangi and Singh, which, in addition to making use of TGA, also performed an analysis of the chemical composition with GC-MS [62]. Another example of the use of GC-MS can be seen in the research of Jung et al. [78], where two different materials (rice straw and bamboo) were characterized, thus being very useful to differentiate the chemical properties resulting from pyrolysis under similar conditions from different materials.
8. Conclusions
This manuscript provides an overview of pyrolysis techniques and reactors, highlighting the importance of choosing the right reactor for achieving the desired results. Residues used in pyrolysis include agricultural and forestry residues, old furniture, plastic waste, industrial (paper, wood) residues, and waste tires. Pre-treatment effectiveness significantly impacts the pyrolysis efficiency and yield, highlighting the need for adapted strategies for each type of feedstock. Agricultural residues require specific pre-treatments, such as air-drying and grinding, to improve yields. Moreover, pre-treatment of paper and wood, which have high moisture contents, is critical. Plastic waste, due to its carbon content, is suitable for conversion to high-calorific-value fuels. Waste tires, rich in hydrocarbons, are preferred for gas and oil production.
The two main types of pyrolytic reactors are batch and continuous ones, with variations within each category. Parameters such as reaction time, pressure, temperature, particle size, heating rates, and the initial moisture content of the feedstock play a crucial role in determining the yield and properties of the final products. During the pyrolysis process, the feedstock is fragmented into a series of simpler chemical compounds that can be divided into different fractions, namely, gas, liquid, and solid. The composition of these fractions depends on the type of material being pyrolyzed, the process conditions, and the type of reactor. In the case of biomass pyrolysis, the resulting gaseous fraction typically includes CO2, CO, and hydrocarbons, but it may also include other compounds, e.g., acetic acid, methanol, and furfural. Hydrogen formation is characteristic of biomass containing paper and cardboard, while in the case of plastics, hydrocarbons are mainly identified. The liquid fraction resulting from biomass pyrolysis consists of multiple organic compounds, including aliphatic alcohols, carbonyls, acids, phenols, cresols, benzenediols, guaiacol and its alkylated derivatives, and aromatic hydrocarbons. In contrast, the liquid fraction resulting from polymer pyrolysis is dominated by aromatic hydrocarbons. The solid fraction mostly consists of charcoal and other residues, e.g., ash, with the composition depending on the process conditions and the organic material being used.
The type of pyrolysis significantly impacts the efficiency and generated products. Slow pyrolysis is effective for cost-efficient biochar production. Fast pyrolysis excels in converting organic materials into liquid fuels, offering simplicity, high thermal efficiency, and low production costs. However, minimizing secondary reactions that reduce oil yields is necessary. Additionally, torrefaction provides a beneficial pre-treatment, improving the bio-oil quality. It also generates high-energy solid products and valuable chemicals, despite potentially reducing the overall oil yield. The choice of pyrolysis type should align with the production goals and operational conditions, maximizing efficiency and product quality.
Previous studies reveal that increasing the reaction temperature improves the biomass conversion efficiency. The choice of reactor type, feed size, and speed are also crucial factors affecting the yield and quality of the final product. Studies also show that different waste materials require different reactors for optimal conversion to bio-oil or biochar. The fluidized bed reactor has been found to be an advantageous option for bio-oil production, while batch and auger reactors are suggested for biochar production. The pyrolysis of automotive waste and used tires is also a suitable process for the recovery of energy and valuable materials. The efficiency of the pyrolysis process is crucial in determining the process’s commercial viability.
The use of catalysts has been shown to significantly increase the formation of aromatics, which have a high combustion capacity and are, therefore, of interest as fuel-quality-enhancing additives. However, the toxicity of some of these compounds must be considered. The influence of temperature on the resulting compounds exhibits different behavior, either increasing or decreasing. Previous research highlights the importance of a fuel’s calorific value, which is determined by its carbon and hydrogen contents. The presence of hydrogen can be beneficial for gaseous products, but it may decrease the quality of liquid or solid products.
Catalysts present significant potential for improving waste pyrolysis, opening up future lines of research. The development of new, more efficient and specific catalysts may help in optimizing the conversion and selectivity of the desired products, e.g., high-quality bio-oil. In addition, exploring multifunctional, sustainable, and cost-effective catalysts, as well as studying their stability and durability, is crucial. The integration of catalytic processes may maximize energy efficiency, thus reducing operating costs. This research could lead to significant advances, making waste pyrolysis efficient and economically viable.
Also, it is worth noting that pyrolysis may be an energy-sustainable process when used to supply energy in the process industry. Overall, research demonstrates the potential of pyrolysis as a waste treatment method that can generate valuable products, including energy (bio-oil, biochar) and material recovery. Further research is needed to optimize the process parameters for different waste materials and reactor types. Further kinetics studies should also be implemented.
Funding
The authors wish to express their gratitude to the Spanish Ministry of Science and Innovation for funding this research (PID2019-105936RB-C21 and TED2021-130596B-C22).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Metzger, P.; Largeau, C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 2005, 66, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Kuyper, J.; Schroeder, H.; Linnér, B.-O. The Evolution of the UNFCCC. Annu. Rev. Environ. Resour. 2018, 43, 343–368. [Google Scholar] [CrossRef]
- Lennan, M.; Morgera, E. Current legal developments climate change the Glasgow climate conference (COP26). Int. J. Mar. Coast. Law 2022, 37, 137–151. [Google Scholar] [CrossRef]
- Chaytor, B. Environmental Issues in Economic Partnership Agreements: Implications for Developing Countries; International Centre for Trade and Sustainable Development (ICTSD): Geneva, Switzerland, 2009; p. 64. [Google Scholar]
- Nanda, S.; Berruti, F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard. Mater. 2021, 403, 16. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.A. European environment policy for the circular economy: Implications for business and industry stakeholders. Sustain. Dev. 2020, 28, 1804–1812. [Google Scholar] [CrossRef]
- Watrobski, J. Temporal PROMETHEE II-New multi-criteria approach to sustainable management of alternative fuels consumption. J. Clean. Prod. 2023, 413, 26. [Google Scholar] [CrossRef]
- Bieniek, A.; Jerzak, W.; Gajek, M.; Magdziarz, A. Numerical investigations of biomass pyrolysis with partial oxidation in a drop tube reactor. J. Clean. Prod. 2023, 401, 136774. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 11. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Zhao, C.P.; Liu, M.R.; Du, H.Z.; Gong, Y. The evolutionary trend and impact of global plastic waste trade network. Sustainability 2021, 13, 3662. [Google Scholar] [CrossRef]
- Wen, Z.G.; Xie, Y.L.; Chen, M.H.; Dinga, C.D. China’s plastic import ban increases prospects of environmental impact mitigation of plastic waste trade flow worldwide. Nat. Commun. 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L.; Annual, R. Plastics in the marine environment. In Annual Review of Marine Science; Annual Reviews: Palo Alto, CA, USA, 2017; Volume 9, pp. 205–229. [Google Scholar]
- Han, X.W.; Guo, Y.; Liu, X.H.; Xia, Q.N.; Wang, Y.Q. Catalytic conversion of lignocellulosic biomass into hydrocarbons: A mini review. Catal. Today 2019, 319, 2–13. [Google Scholar] [CrossRef]
- Davis, R.; Tao, L.; Tan, E.C.D.; Biddy, M.J.; Beckham, G.T.; Scarlata, C.; Jacobson, J.; Cafferty, K.; Ross, J.; Lukas, J.; et al. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Biological Conversion of Sugars to Hydrocarbons; Idaho National Lab. (INL): Idaho Falls, ID, USA, 2013. [Google Scholar]
- Lechleitner, A.E.; Schubert, T.; Hofer, W.; Lehner, M. Lumped kinetic modeling of polypropylene and polyethylene co-pyrolysis in tubular reactors. Processes 2021, 9, 34. [Google Scholar] [CrossRef]
- Nam, H.; Capareda, S.C.; Ashwath, N.; Kongkasawan, J. Experimental investigation of pyrolysis of rice straw using bench-scale auger, batch and fluidized bed reactors. Energy 2015, 93, 2384–2394. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis. A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Mennell James, A.; Despen Daniel, J. Systems and Apparatus for the Production of High-Carbon Bioreagents; WIPO: Geneva, Switzerland, 2022. [Google Scholar]
- Fu, P.; Hu, S.; Xiang, J.; Li, P.S.; Huang, D.; Jiang, L.C.; Zhang, A.Y.; Zhang, J. FTIR study of pyrolysis products evolving from typical agricultural residues. J. Anal. Appl. Pyrolysis 2010, 88, 117–123. [Google Scholar] [CrossRef]
- Zanzi, R.; Sjostrom, K.; Bjornbom, E. Rapid pyrolysis of agricultural residues at high temperature. Biomass Bioenergy 2002, 23, 357–366. [Google Scholar] [CrossRef]
- Chen, B.L.; Zhou, D.D.; Zhu, L.Z. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Garcia-Perez, M.; Adams, T.T.; Goodrum, J.W.; Geller, D.P.; Das, K.C. Production and fuel properties of pine chip Bio-oil/Biodiesel blends. Energy Fuels 2007, 21, 2363–2372. [Google Scholar] [CrossRef]
- Yildiz, G.; Ronsse, F.; Venderbosch, R.; van Duren, R.; Kersten, S.R.A.; Prins, W. Effect of biomass ash in catalytic fast pyrolysis of pine wood. Appl. Catal. B Environ. 2015, 168, 203–211. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Total recovery of resources and energy from rice straw using microwave-induced pyrolysis. Bioresour. Technol. 2008, 99, 8252–8258. [Google Scholar] [CrossRef] [PubMed]
- Zabaniotou, A.; Ioannidou, O.; Antonakou, E.; Lappas, A. Experimental study of pyrolysis for potential energy, hydrogen and carbon material production from lignocellulosic biomass. Int. J. Hydrogen Energy 2008, 33, 2433–2444. [Google Scholar] [CrossRef]
- Ren, S.J.; Lei, H.W.; Wang, L.; Yadavalli, G.; Liu, Y.P.; Julson, J. The integrated process of microwave torrefaction and pyrolysis of corn stover for biofuel production. J. Anal. Appl. Pyrolysis 2014, 108, 248–253. [Google Scholar] [CrossRef]
- Colantoni, A.; Evic, N.; Lord, R.; Retschitzegger, S.; Proto, A.R.; Gallucci, F.; Monarca, D. Characterization of biochars produced from pyrolysis of pelletized agricultural residues. Renew. Sustain. Energy Rev. 2016, 64, 187–194. [Google Scholar] [CrossRef]
- Lajili, M.; Guizani, C.; Sanz, F.J.E.; Jeguirim, M. Fast pyrolysis and steam gasification of pellets prepared from olive oil mill residues. Energy 2018, 150, 61–68. [Google Scholar] [CrossRef]
- Kabakci, S.B.; Aydemir, H. Pyrolysis of olive pomace and copyrolysis of olive pomace with refuse derived fuel. Environ. Prog. Sustain. Energy 2014, 33, 649–656. [Google Scholar] [CrossRef]
- Uzun, B.B.; Kanmaz, G. Effect of operating parameters on bio-fuel production from waste furniture sawdust. Waste Manag. Res. 2013, 31, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.S.; Park, H.J.; Park, Y.K.; Ryu, C.; Suh, D.J.; Suh, Y.W.; Yim, J.H.; Kim, S.S. Bio-oil production from fast pyrolysis of waste furniture sawdust in a fluidized bed. Bioresour. Technol. 2010, 101, S91–S96. [Google Scholar] [CrossRef]
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769. [Google Scholar] [CrossRef]
- Gaurh, P.; Pramanik, H. Production of benzene/toluene/ethyl benzene/xylene (BTEX) via multiphase catalytic pyrolysis of hazardous waste polyethylene using low cost fly ash synthesized natural catalyst. Waste Manag. 2018, 77, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Aguado, J.; Serrano, D.P.; Escola, J.M.; Garagorri, E. Catalytic conversion of low-density polyethylene using a continuous screw kiln reactor. Catal. Today 2002, 75, 257–262. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltran, M.I.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86. [Google Scholar] [CrossRef]
- Alonso-Morales, N.; Gilarranz, M.A.; Heras, F.; Eser, S.; Rodriguez, J.J. Effects of reactor configuration on the yield of solid carbon from pyrolysis of low-density polyethylene. Energy Fuels 2009, 23, 6095–6101. [Google Scholar] [CrossRef]
- Fan, L.L.; Liu, L.; Xiao, Z.G.; Su, Z.Y.; Huang, P.; Peng, H.Y.; Lv, S.; Jiang, H.W.; Ruan, R.; Chen, P.; et al. Comparative study of continuous-stirred and batch microwave pyrolysis of linear low-density polyethylene in the presence/absence of HZSM-5. Energy 2021, 228, 120612. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, J.; Jeon, J.K.; Park, Y.K.; Park, C.J. Non-isothermal pyrolysis of the mixtures of waste automobile lubricating oil and polystyrene in a stirred batch reactor. Renew. Energy 2013, 54, 241–247. [Google Scholar] [CrossRef]
- Park, K.B.; Jeong, Y.S.; Kim, J.S. Activator-assisted pyrolysis of polypropylene. Appl. Energy 2019, 253, 10. [Google Scholar] [CrossRef]
- Kasar, P.; Ahmaruzzaman, M. Characterization of liquid products obtained from catalytic binary co-cracking of residual fuel oil with various waste plastics. Sci. Rep. 2022, 12, 10987. [Google Scholar] [CrossRef]
- Chen, X.R.; Cheng, L.L.; Gu, J.; Yuan, H.R.; Chen, Y. Chemical recycling of plastic wastes via homogeneous catalysis: A review. Chem. Eng. J. 2024, 479, 147853. [Google Scholar] [CrossRef]
- Fekhar, B.; Zsinka, V.; Miskolczi, N. Thermo-catalytic co-pyrolysis of waste plastic and paper in batch and tubular reactors for in-situ product improvement. J. Environ. Manag. 2020, 269, 110741. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Gupta, A.K. Syngas yield during pyrolysis and steam gasification of paper. Appl. Energy 2009, 86, 1813–1821. [Google Scholar] [CrossRef]
- Yao, Z.L.; Ma, X.Q.; Wu, Z.D.; Yao, T.T. TGA-FTIR analysis of co-pyrolysis characteristics of hydrochar and paper sludge. J. Anal. Appl. Pyrolysis 2017, 123, 40–48. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, H.W.; Lee, I.G.; Jeon, J.K.; Ryu, C.; Park, S.H.; Jung, S.C.; Park, Y.K. Influence of reaction conditions on bio-oil production from pyrolysis of construction waste wood. Renew. Energy 2014, 65, 41–48. [Google Scholar] [CrossRef]
- Carlson, T.R.; Cheng, Y.T.; Jae, J.; Huber, G.W. Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ. Sci. 2011, 4, 145–161. [Google Scholar] [CrossRef]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Berrueco, C.; Esperanza, E.; Mastral, F.J.; Ceamanos, J.; Garcia-Bacaicoa, P. Pyrolysis of waste tyres in an atmospheric static-bed batch reactor: Analysis of the gases obtained. J. Anal. Appl. Pyrolysis 2005, 74, 245–253. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Inayat, A.; Klemencova, K.; Grycova, B.; Sokolova, B.; Lestinsky, P. Thermo-catalytic pyrolysis of polystyrene in batch and semi-batch reactors: A comparative study. Waste Manag. Res. 2021, 39, 260–269. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Demirbas, A. Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J. Anal. Appl. Pyrolysis 2004, 72, 243–248. [Google Scholar] [CrossRef]
- Inguanzo, M.; Dominguez, A.; Menendez, J.A.; Blanco, C.G.; Pis, J.J. On the pyrolysis of sewage sludge: The influence of pyrolysis conditions on solid, liquid and gas fractions. J. Anal. Appl. Pyrolysis 2002, 63, 209–222. [Google Scholar] [CrossRef]
- Lopez-Urionabarrenechea, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Chen, L.M.; Liao, Y.F.; Guo, Z.G.; Cao, Y.W.; Ma, X.Q. Products distribution and generation pathway of cellulose pyrolysis. J. Clean. Prod. 2019, 232, 1309–1320. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hwang, G.C.; Bae, S.Y.; Yi, S.C.; Moon, S.K.; Kumazawa, H. Pyrolysis of polystyrene in a batch-type stirred vessel. Korean J. Chem. Eng. 1999, 16, 161–165. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.; Yang, W. Catalytic pyrolysis of demineralized lignocellulosic biomass. Fuel 2019, 252, 200–209. [Google Scholar] [CrossRef]
- Shadangi, K.P.; Singh, R.K. Thermolysis of polanga seed cake to bio-oil using semi batch reactor. Fuel 2012, 97, 450–456. [Google Scholar] [CrossRef]
- Celikgogus, C.; Karaduman, A. Thermal-catalytic pyrolysis of polystyrene waste foams in a semi-batch reactor. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 2507–2513. [Google Scholar] [CrossRef]
- Gaurh, P.; Pramanik, H. A novel approach of solid waste management via aromatization using multiphase catalytic pyrolysis of waste polyethylene. Waste Manag. 2018, 71, 86–96. [Google Scholar] [CrossRef]
- Xue, Q.; Heindel, T.J.; Fox, R. A CFD model for biomass fast pyrolysis in fluidized-bed reactors. Chem. Eng. Sci. 2011, 66, 2440–2452. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martinez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Marmur, B.L.; Heindel, T.J. Effect of particle size, density, and concentration on granular mixing in a double screw pyrolyzer. Powder Technol. 2016, 302, 222–235. [Google Scholar] [CrossRef]
- Park, H.C.; Lee, B.K.; Yoo, H.S.; Choi, H.S. Influence of operating conditions for fast pyrolysis and pyrolysis oil production in a conical spouted-bed reactor. Chem. Eng. Technol. 2019, 42, 2493–2504. [Google Scholar] [CrossRef]
- Fernandez-Akarregi, A.R.; Makibar, J.; Lopez, G.; Amutio, M.; Olazar, M. Design and operation of a conical spouted bed reactor pilot plant (25 kg/h) for biomass fast pyrolysis. Fuel Process. Technol. 2013, 112, 48–56. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, H.C.; Choi, H.S. Numerical study on fast pyrolysis of lignocellulosic biomass with varying column size of bubbling fluidized bed. ACS Sustain. Chem. Eng. 2017, 5, 2196–2204. [Google Scholar] [CrossRef]
- Hastaoglu, M.A.; Hassam, M.S. Application of a general gas-solid reaction model to flash pyrolysis of wood in a circulating fluidized bed. Fuel 1995, 74, 697–703. [Google Scholar] [CrossRef]
- Haydary, J.; Susa, D.; Gelinger, V.; Cacho, F. Pyrolysis of automobile shredder residue in a laboratory scale screw type reactor. J. Environ. Chem. Eng. 2016, 4, 965–972. [Google Scholar] [CrossRef]
- Haydary, J.; Susa, D.; Dudas, J. Pyrolysis of aseptic packages (tetrapak) in a laboratory screw type reactor and secondary thermal/catalytic tar decomposition. Waste Manag. 2013, 33, 1136–1141. [Google Scholar] [CrossRef]
- Martinez, J.D.; Murillo, R.; Garcia, T.; Veses, A. Demonstration of the waste tire pyrolysis process on pilot scale in a continuous auger reactor. J. Hazard. Mater. 2013, 261, 637–645. [Google Scholar] [CrossRef]
- Efika, C.E.; Wu, C.F.; Williams, P.T. Syngas production from pyrolysis-catalytic steam reforming of waste biomass in a continuous screw kiln reactor. J. Anal. Appl. Pyrolysis 2012, 95, 87–94. [Google Scholar] [CrossRef]
- Yang, Y.; Brammer, J.G.; Mahmood, A.S.N.; Hornung, A. Intermediate pyrolysis of biomass energy pellets for producing sustainable liquid, gaseous and solid fuels. Bioresour. Technol. 2014, 169, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Sirijanusorn, S.; Sriprateep, K.; Pattiya, A. Pyrolysis of cassava rhizome in a counter-rotating twin screw reactor unit. Bioresour. Technol. 2013, 139, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Kang, B.S.; Kim, J.S. Production of bio-oil from rice straw and bamboo sawdust under various reaction conditions in a fast pyrolysis plant equipped with a fluidized bed and a char separation system. J. Anal. Appl. Pyrolysis 2008, 82, 240–247. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhao, B.F.; Liu, S.X.; Zhu, D.; Huang, F.Y.; Yang, H.J.; Guan, H.B.; Song, A.G.; Xu, D.; Sun, L.Z.; et al. Catalytic pyrolysis of biomass with Ni/Fe-CaO-based catalysts for hydrogen-rich gas: DFT and experimental study. Energy Convers. Manag. 2022, 254, 115246. [Google Scholar] [CrossRef]
- Gui, B.; Qiao, Y.; Wan, D.; Liu, S.; Han, Z.N.; Yao, H.; Xu, M.H. Nascent tar formation during polyvinylchloride (PVC) pyrolysis. Proc. Combust. Inst. 2013, 34, 2321–2329. [Google Scholar] [CrossRef]
- Gong, X.; Yu, Y.; Gao, X.P.; Qiao, Y.; Xu, M.H.; Wu, H.W. Formation of anhydro-sugars in the primary volatiles and solid residues from cellulose fast pyrolysis in a wire-mesh reactor. Energy Fuels 2014, 28, 5204–5211. [Google Scholar] [CrossRef]
- Dewayanto, N.; Isha, R.; Nordin, M.R. Use of palm oil decanter cake as a new substrate for the production of bio-oil by vacuum pyrolysis. Energy Convers. Manag. 2014, 86, 226–232. [Google Scholar] [CrossRef]
- Ruan, J.J.; Huang, J.X.; Qin, B.J.; Dong, L.P. Heat transfer in vacuum pyrolysis of decomposing hazardous plastic wastes. ACS Sustain. Chem. Eng. 2018, 6, 5424–5430. [Google Scholar] [CrossRef]
- Lehto, J. Determination of kinetic parameters for Finnish milled peat using drop tube reactor and optical measurement techniques. Fuel 2007, 86, 1656–1663. [Google Scholar] [CrossRef]
- Matsuoka, K.; Ma, Z.X.; Akiho, H.; Zhang, Z.G.; Tomita, A.; Fletcher, T.H.; Wojtowicz, M.A.; Niksa, S. High-pressure coal pyrolysis in a drop tube furnace. Energy Fuels 2003, 17, 984–990. [Google Scholar] [CrossRef]
- Fekhar, B.; Zsinka, V.; Miskolczi, N. Value added hydrocarbons obtained by pyrolysis of contaminated waste plastics in horizontal tubular reactor: In situ upgrading of the products by chlorine capture. J. Clean. Prod. 2019, 241, 118166. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wang, D.M.; Li, C.M.; Yang, H.; Wang, D.L.; Lai, D.G.; Yu, J.; Gao, S.Q. A tandem pyrolysis-upgrading strategy in an integrated reactor to improve the quality of coal tar. Energy Convers. Manag. 2020, 220, 113065. [Google Scholar] [CrossRef]
- Wang, D.M.; Chen, Z.H.; Li, C.M.; Wang, D.L.; Li, Y.J.; Yang, H.; Liu, Z.E.; Yu, J.; Gao, S.Q. High-quality tar production from coal in an integrated reactor: Rapid pyrolysis in a drop tube and downstream volatiles upgrading over char in a moving bed. Fuel 2021, 285, 119156. [Google Scholar] [CrossRef]
- Cai, N.; Zhang, H.L.; Nie, J.P.; Deng, Y.M.; Baeyens, J. Biochar from biomass slow pyrolysis. In Proceedings of the 2nd International Conference on Environment Sciences and Renewable Energy (ESRE), Vienna, Austria, 18–21 May 2020. [Google Scholar]
- Yuan, T.; He, W.J.; Yin, G.J.; Xu, S. Comparison of bio-chars formation derived from fast and slow pyrolysis of walnut shell. Fuel 2020, 261, 116450. [Google Scholar] [CrossRef]
- Duman, G.; Okutucu, C.; Ucar, S.; Stahl, R.; Yanik, J. The slow and fast pyrolysis of cherry seed. Bioresour. Technol. 2011, 102, 1869–1878. [Google Scholar] [CrossRef]
- Snyder, B.F. Costs of biomass pyrolysis as a negative emission technology: A case study. Int. J. Energy Res. 2019, 43, 1232–1244. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Wu, N.N.; Niu, Q.; Pieters, J.; Ronsse, F. Influence of torrefaction as pretreatment on the fast pyrolysis of sugarcane trash. Energy Convers. Manag. 2023, 291, 117291. [Google Scholar] [CrossRef]
- Bach, Q.V.; Trinh, T.N.; Tran, K.Q.; Ngoc, B.D.T. Pyrolysis characteristics and kinetics of biomass torrefied in various atmospheres. Energy Convers. Manag. 2017, 141, 72–78. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, C.W.; Ong, H.C.; Show, P.L.; Hsieh, T.H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Corma, A.; Huber, G.W.; Sauvanaud, L.; O’Connor, P. Processing biomass-derived oxygenates in the oil refinery: Catalytic cracking (FCC) reaction pathways and role of catalyst. J. Catal. 2007, 247, 307–327. [Google Scholar] [CrossRef]
- Özsin, G.; Pütün, A.E. TGA/MS/FT-IR study for kinetic evaluation and evolved gas analysis of a biomass/PVC co-pyrolysis process. Energy Convers. Manag. 2019, 182, 143–153. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.L.; Liu, Z.; Ma, Q.L.; Zhao, C.; Ma, C.Y. Kinetic study of the pyrolysis of waste printed circuit boards subject to conventional and microwave heating. Energies 2012, 5, 3295–3306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).