3.1. Results for Natural Gas
The radiative heat flux measurements for natural gas were carried out at a thermal load of 310 kW, a swirl number of 0.96, and a global stoichiometric ratio of 1.4. The oxyfuel cases were performed by enriching the recirculated flue gas to an oxygen content of 28 vol% (Oxy28), 29 vol% (Oxy29) and 31 vol% (Oxy31) at a temperature of 50 °C.
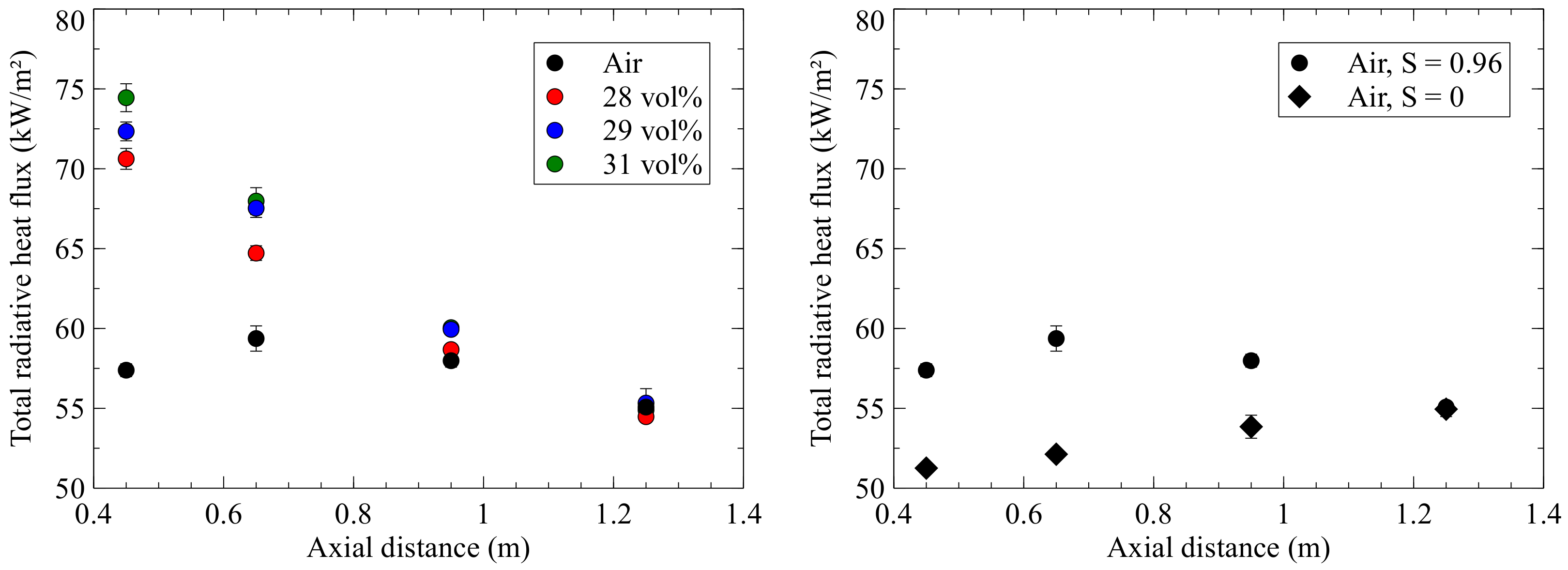
Figure 3 shows the heat flux measurement plotted against the axial distance to the quarl.
On the left-hand chart, a maximum heat flux of 60 kW/m
2 is measured for air-firing, which is reached at a position of 0.65 m. For oxyfuel combustion it can be seen that the heat flux in the upper part of the combustion chamber increases with an increasing oxygen concentration. Compared to the air case, the oxyfuel points already reach their maximum at 0.45 m with up to 75 kW/m
2 and decrease continuously with further axial distance. The adiabatic flame temperature increases with increasing oxygen content in the oxidant (cf. Corrêa da Silva et al. [
2]), therefore higher radiative heat flux is measured at higher oxygen concentrations. From a distance of 0.95 m, all measuring points are close together. The end of the flame is possibly located here, and the background radiation of the combustion chamber is measured. In the chart on the right, the air flame with a swirl number of 0.96 is compared to a non-swirled air flame with the same conditions otherwise. As expected, the short, highly swirled air flame has stronger emissions than the non-swirled jet flame. Due to the high swirl, the reaction zones and, thus, the heat release are concentrated on a smaller local area than with the jet flame, where the reaction zones extend over a longer distance. All three operating points are met at the 1.25 m position, since this position marks the end of the flame. The length of the flame can also be seen through windows installed at the same height as the measuring ports. As described in [
7], the emissivity of the flame decreases with a lower oxygen concentration, as the increased proportion of CO
2 during combustion reduces the gas temperature. In addition, a higher CO
2 volume flow results in an unchanged equivalence ratio, due to a lower oxygen concentration in the oxidant. In the case of oxyfuel firing, the radiative heat flux decreases with increasing distance from the burner quarl, whereas the combustion with air first increases and then decreases. One reason for the maximum close to the burner could be the higher O
2 concentration near the burner, which leads to a stronger reaction of the mixture in this zone and, thus, to higher heat release rates, higher temperatures and faster ignition. The near-wall gas temperatures inside the combustion chamber for the various flames are shown in
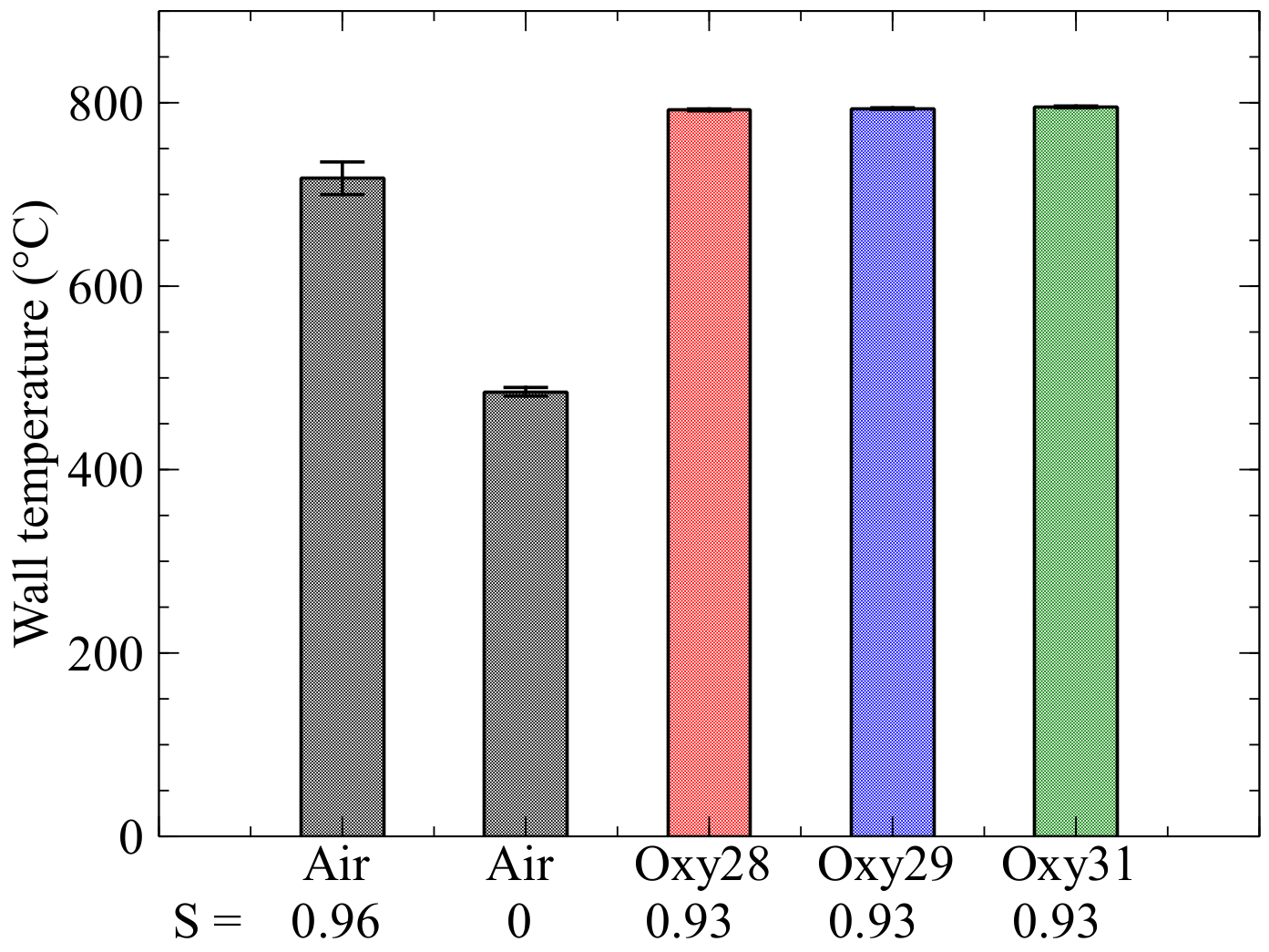
Figure 4 at position T
2.
While the oxyfuel cases have almost the same wall temperature of approx. 800 °C, the value for the air case is below the values of the oxyfuel cases, as with the radiative heat flux measurement. The non-swirled air flame has the lowest temperature here, at just under 500 °C.
3.2. Results for Walnut Shells
The pulverised WS are burned at a thermal load of 500 kW, which corresponds to a mass flow of 110 kg/h. The swirl number is 0.95, and the global stoichiometric ratio is approx. 1.4 for air and 1.6 for oxyfuel combustion. Lower ratios show a significant increase in CO emissions for WS. The local equivalence ratio is changed between 0.8 and 1.0 for air combustion and 1.0 to 1.4 for oxyfuel. The oxygen concentration in the recirculated flue gas is enriched to 27 vol% (Oxy27), 30 vol% (Oxy30) and 33 vol% (Oxy33). The left-hand diagram in
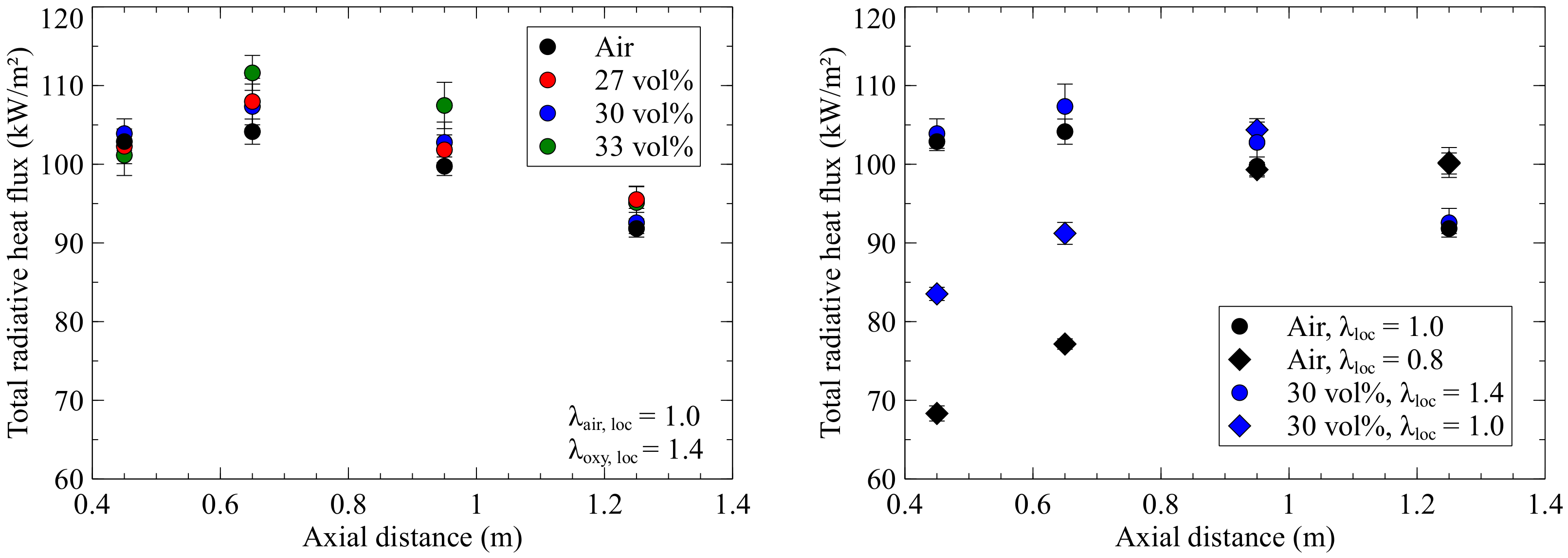
Figure 5 shows the results of the radiative wall heat flux measurements plotted against the axial distance to the quarl for air combustion and oxyfuel.
It is immediately noticeable that the shape of the curves does not fall continuously over the axial distance to the quarl, as in the oxyfuel cases for natural gas, but first rises and then falls again as in the air-fired natural gas case. Since particle radiation dominates for pulverised firing, the soot concentration probably has a smaller effect than in natural gas combustion. The highest incident radiation of 112 kW/m
2 was measured at the 0.65 m position for Oxy33, due to highest O
2 concentration and gas temperatures. In contrast to the investigations with natural gas, deviations of the oxyfuel operations from the air point are significantly lower, whereby Oxy27 and Oxy30 are closest to the values of the air point. At the lowest measuring port, the results for the different flames do not overlap, as is the case of natural gas combustion, as the dust flame is longer than the gas flame. The right-hand diagram in
Figure 5 shows the comparison of an air point and Oxy30 with low and high local stoichiometric ratios. The global equivalence ratio is 1.6 for all points. The results from the diagram on the left are also shown for comparison. The sub-stoichiometric condition reduces the NO emissions for the air point from 94 to 61 ppm. CO emissions, on the other hand, increase from 580 to approx. 4900 ppm, as the lower velocities in the flame lead to poor mixing and combustion. For the oxyfuel case, NO is reduced from 87 to 48 ppm, and the CO emissions increase from 4800 to 8800 ppm. In this case, a lower radiative heat flux and temperature is measured than in the comparable stoichiometric case. The low conversion of carbon monoxide, which indicates poor combustion, may leads to lower heat release. Furthermore, a long flame is generated which distributes the heat radiation over a larger area. Both the air and the oxyfuel case show significantly lower values compared to the flames with a higher local equivalence ratio, but otherwise the same boundary conditions. The change in the local equivalence ratio has a stronger effect on the differences in thermal radiation in the air case than in the oxyfuel case. However, the difference between the local equivalence ratio in the air case is 0.2, while in the oxyfuel case, the difference is 0.4.
The difference in heat release can also be seen from the near-wall gas temperatures at T
2 in
Figure 6. The air case with an stoichiometric local equivalence ratio reaches the highest temperature, although the lowest radiative heat flux is measured on average over the burner axis under air conditions. As the equivalence ratio for air firing is slightly lower than for oxyfuel firing, the adiabatic flame temperature is higher. Since particle radiation dominates for radiative heat exchange, it is assumed that this does not have a significant effect on heat transfer by radiation. The temperature for the same operation point at
= 0.8 is approx. 250 K lower. The reduced velocity near the burner for small local stoichiometric ratios may lead to a poor mixing of fuel and oxidiser and, thus, to a lower heat release rate, which is reflected in the temperature.
3.3. Results for Rhenish Lignite
The RBK is burnt with a thermal output of 500 kW, a combustion equivalence ratio of approx. 1.6, and a swirl number of 0.95. The oxygen content in the oxyfuel operation is 26 vol% (Oxy26) and 30 vol% (Oxy30).
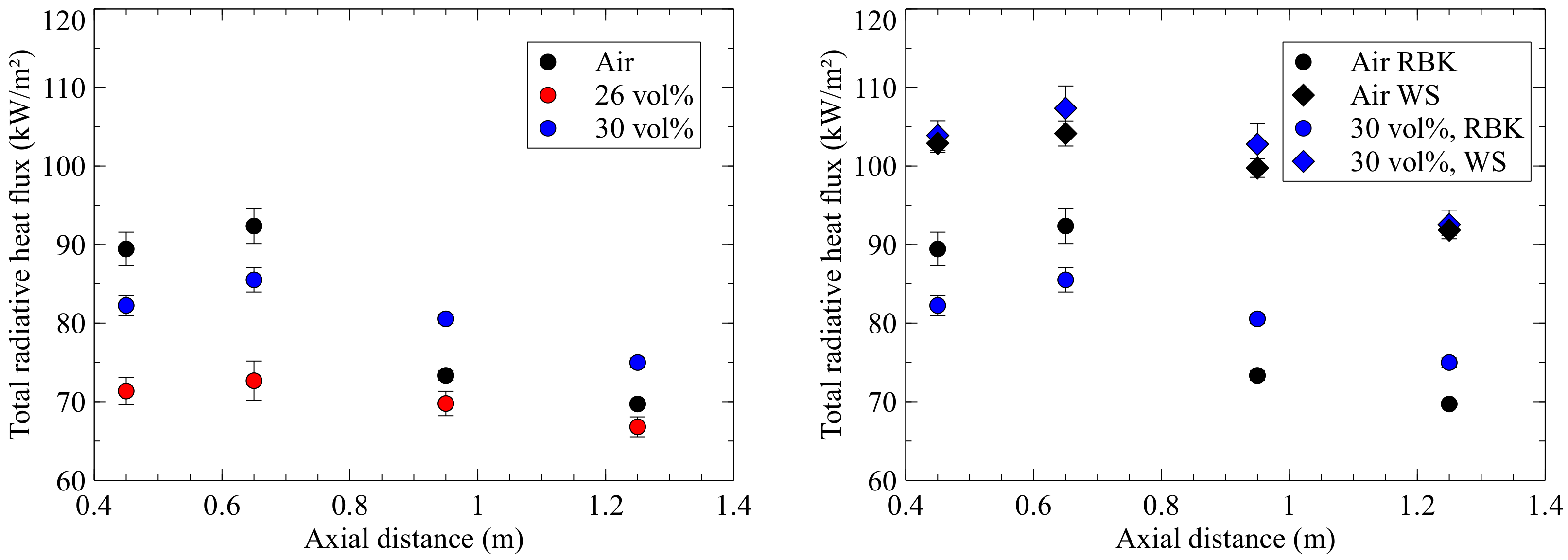
Figure 7 shows the radiative heat flux plotted against the axial distance to the quarl in the diagram on the left-hand side for air and oxyfuel combustion. The course of the measuring results along the axial direction are similar for all operating conditions, except for the air-fired case, where there is a significant drop in radiative heat flux from 0.95 m. The reason for this drop is fluctuations in fuel feeding. These are caused by the discontinuous feeding of fuel into the dosing system which, in some cases, leads to fuel blockages. This was also noticeable in the drop of the wall temperatures. The maximum incident radiative heat flux of 92 kW/m
2 is measured for the lignite at 0.65 m. Both oxyfuel cases (Oxy30 and Oxy26) are below the values of air-firing. A higher oxygen concentration >30 vol% is therefore necessary for the lignite in order to obtain values similar to those of the air case. In comparison with the WS, which are close to the air-fired case for all oxygen concentrations in oxyfuel operation, higher deviations are recognisable here. In
Figure 7, on the right-hand diagram, the radiative heat flux of the lignite for air and oxyfuel operation is compared with the measurements of the WS. It can be seen for both the air and the oxyfuel case that the measured incident radiation is significantly higher for the WS than for the lignite. Zabrodiec et al. [
4], who investigated RBK and torrefied biomass, came to the same result. Their assumption is that radiation emissions from the flame are larger due to the higher particle load of the biomass, which is necessary to achieve the same thermal output due to the lower calorific value of biomass. However, it is also possible that the higher volatile content of the WS results in faster ignition and, thus, higher local temperatures.
Figure 8 shows the near-wall gas temperature of RBK for air and oxyfuel firing. One would expect the Oxy30 case to be closer to the air case if the radiative heat flux in
Figure 7 is considered. However, since fluctuations in the fuel supply occurred during this experiment, as already mentioned, the temperature dropped, so these two cases produce similar temperatures. In addition, the adiabatic flame temperature decreases with decreasing oxygen concentration in the oxidant in an oxyfuel atmosphere. Due to the high heat capacity of CO
2, the temperature is lower than the adiabatic flame temperature of air, which has an oxygen concentration of 21 vol% in the oxidant. This results in the lowest temperature for Oxy26 in the investigation of the RBK.
Despite different boundary conditions, in the study of Corrêa da Silva and Krautz [
2] a coal-fired oxyfuel combustion chamber with water-cooled membrane walls also shows the best agreement with a comparable air-fired case at an oxygen concentration of 31 vol%. Overall, the values for the radiative wall heat flux in their work are higher than the values measured here, despite a lower thermal power of less than 400 kW. This may be due to the fact that the coal has a higher calorific value, different properties of the chamber walls or cooling, higher flame temperatures, and different mixing conditions. In general, it is difficult to determine the radiative heat transfer in absolute terms due to reflective walls. Therefore, an absolute comparison with most measurements in the literature, such as [
4,
7], is not possible, as combustion chambers have refractory-lined walls, which have different absorption and emission properties than water-cooled steel walls. Other authors, such as Zabrodiec et al. [
4], therefore only make comparisons in relative terms.
Since the oxyfuel burner used for this study is an up-scaled version of the 40 kW
th burner used by Zabrodiec et al. [
4] in their work, the results for the radiative heat flux measurements should be compared to each other. As the combustion chambers have different dimensions, and the measurements were taken at different heights, the distance to the quarl is presented in dimensionless values (
) and the position of the maximum measured radiative heat flux is indicated. The measurement data are difficult to compare, since Zabrodiec et al. [
4] used a combustion chamber with heated, refractory-lined walls, whereas in the current study, the combustion chamber has cooled steel walls. For this purpose, all absolute values are normalised by the maximum measured value. The RBK used in both studies is approx. identical.
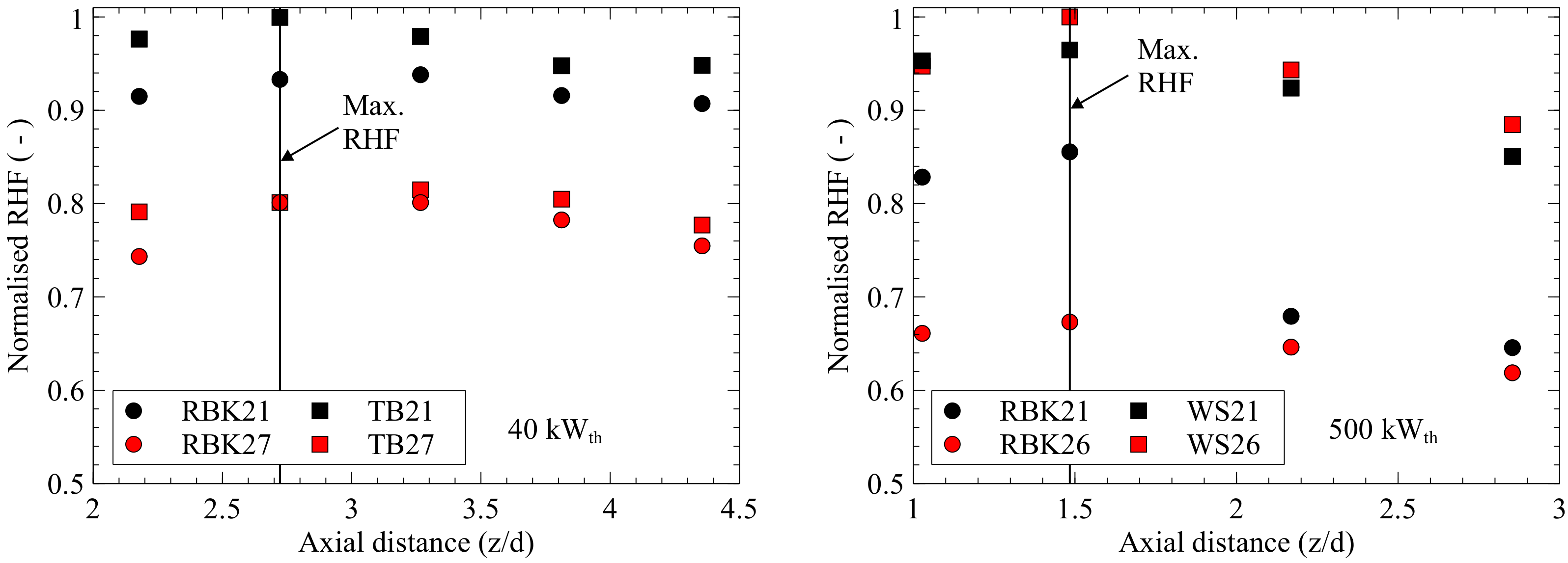
Figure 9 shows the results in terms of normalised radiative heat flux (RHF) of Zabrodiec et al. [
4], and those of the current study plotted against the dimensionless axial distance to the quarl. The torrefied biomass (TB) used by Zabrodiec et al. [
4] has a higher calorific value than the WS, so their results for RBK and TB are closer than RBK and WS in the current work. Major differences in absolute values may caused by the refractory-lined walls of the reference combustion chamber. At position 0.45, the measurements differ for RBK by 0.17 between air and oxyfuel and, at position 0.65, by 0.13 for the 40 kW
th case. For the 500 kW
th case, the first two positions of RBK differ by 0.17 and 0.18 in oxyfuel and air mode. Thus, the differences in thermal radiation in relative values near the maximum for RBK show good agreement for both of the combustion chambers with different thermal loads. The remaining points beyond the maximum value cannot be compared because, as already mentioned, the radiative heat flux measurement for the 500 kW
th case with RBK is distorted by fluctuations in fuel feeding. TB differs by 0.14–0.2 between air and oxyfuel for the 40 kW
th case, while the 500 kW
th cases differ by 0–0.04 between air and oxyfuel for WS. Since the carbon content of TB is higher than that of WS, the endothermic Boudouard reaction at high temperatures may lead to lower local temperatures and radiation intensity of the TB flame. Therefore, the difference in RHF between the air and oxyfuel flame could be greater for TB in the 40 kW
th case than WS at 500 kW
th. Another reason could be the worse combustion of TB under oxyfuel conditions. Both theories are supported by the significantly increased CO concentration measured by Zabrodiec and co-workers during TB combustion with 27 vol% oxygen in the oxidant.