Uncalcined Zn/Al Carbonate LDH and Its Calcined Counterpart for Treating the Wastewater Containing Anionic Congo Red Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZAC-LDH and Its Calcined Counterpart CZA-LDH
2.2. Characterization
2.3. Method of Adsorbate Solution Preparation
2.4. Batch Adsorption Experiments
3. Result and Discussion
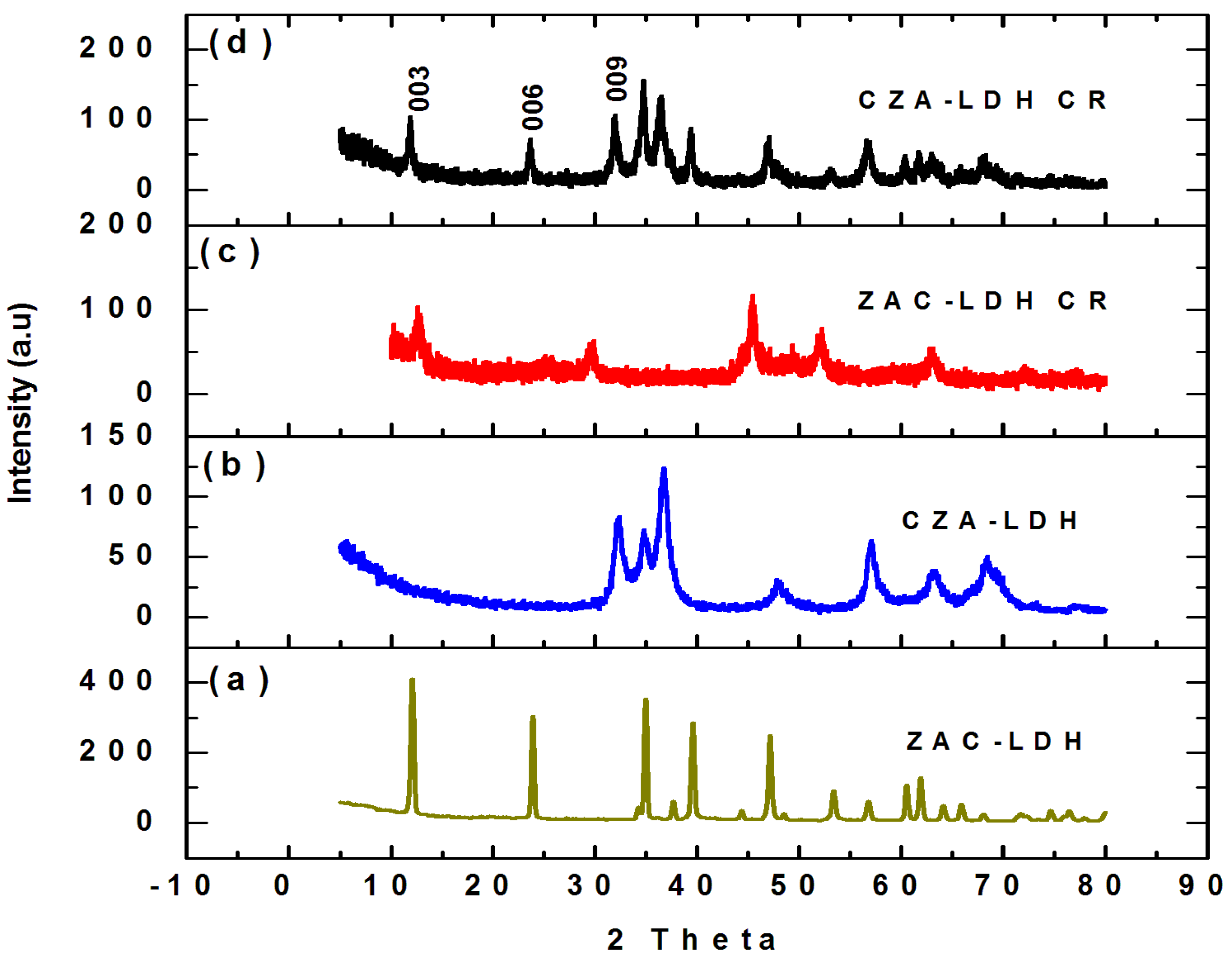
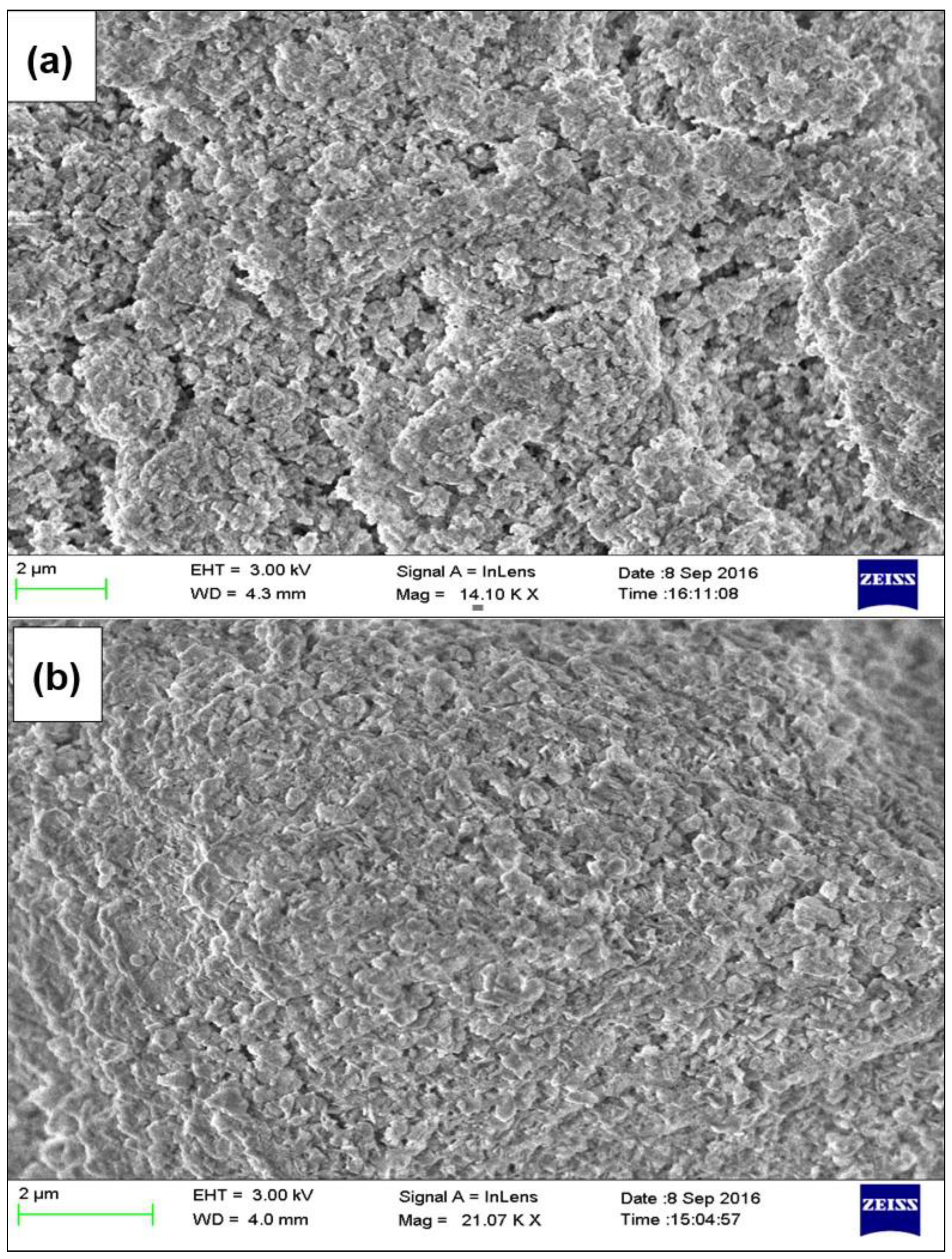
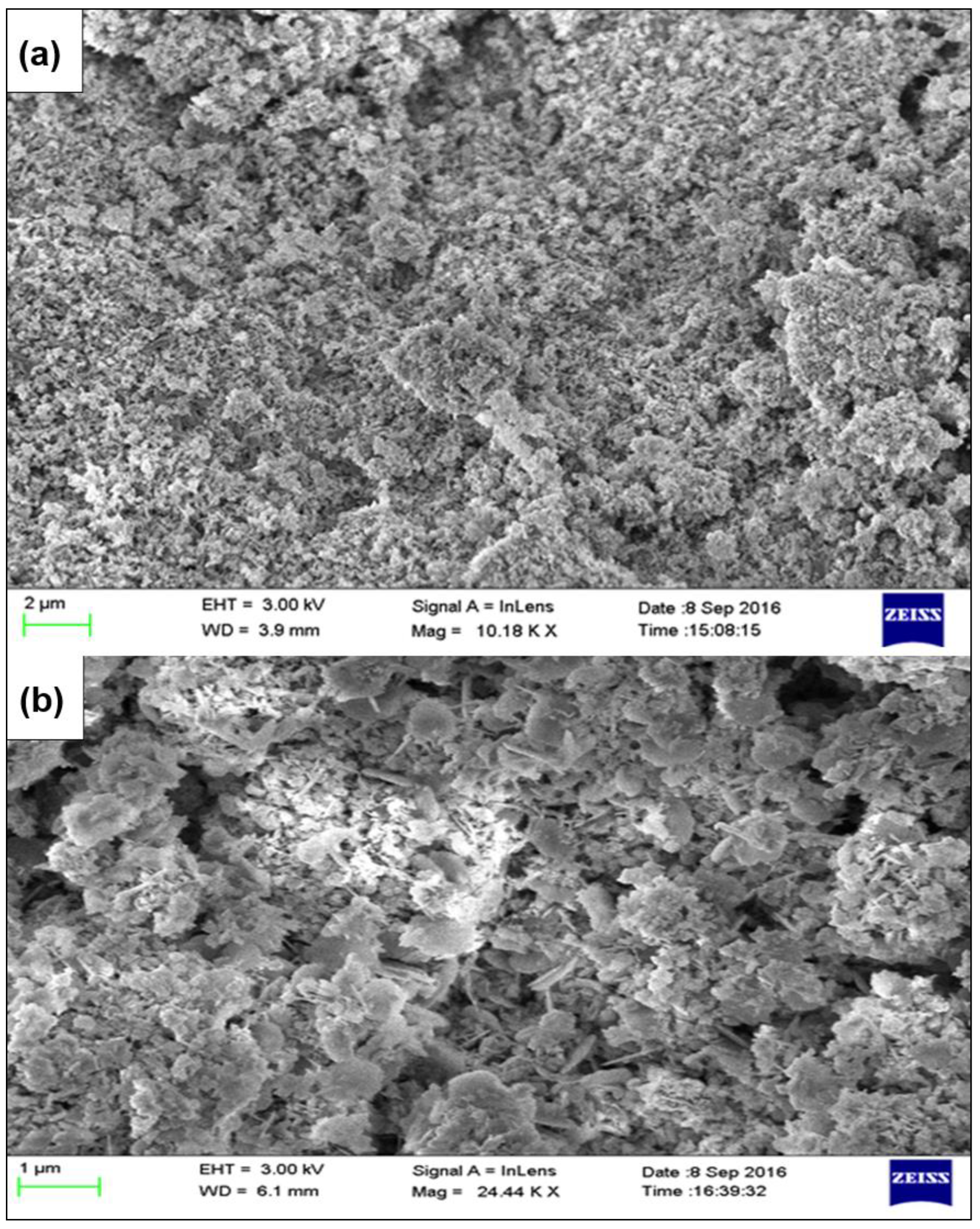
3.1. Characterization of Materials
3.2. The Influence of IDC and Contact Time
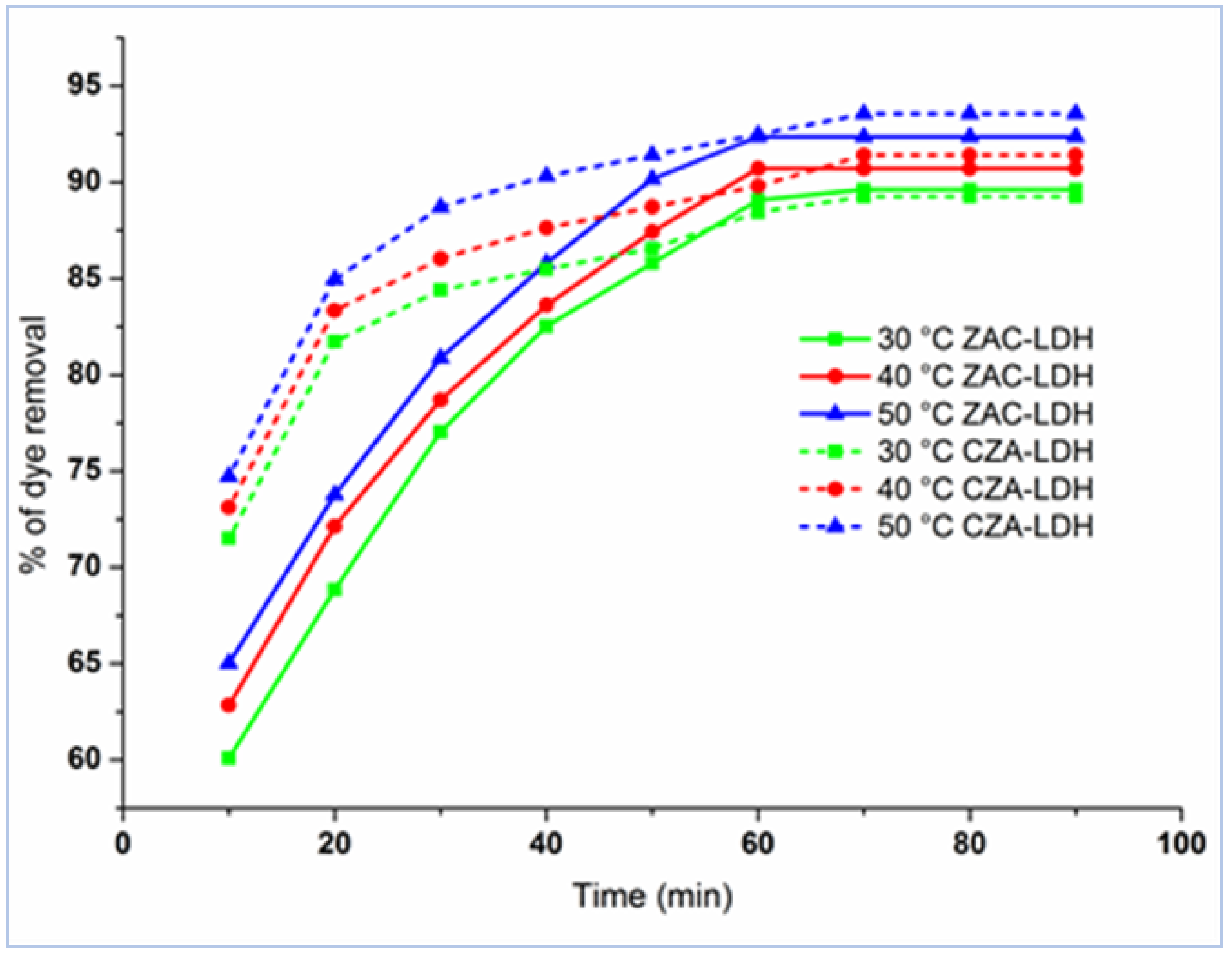
3.3. The Influence of Temperature
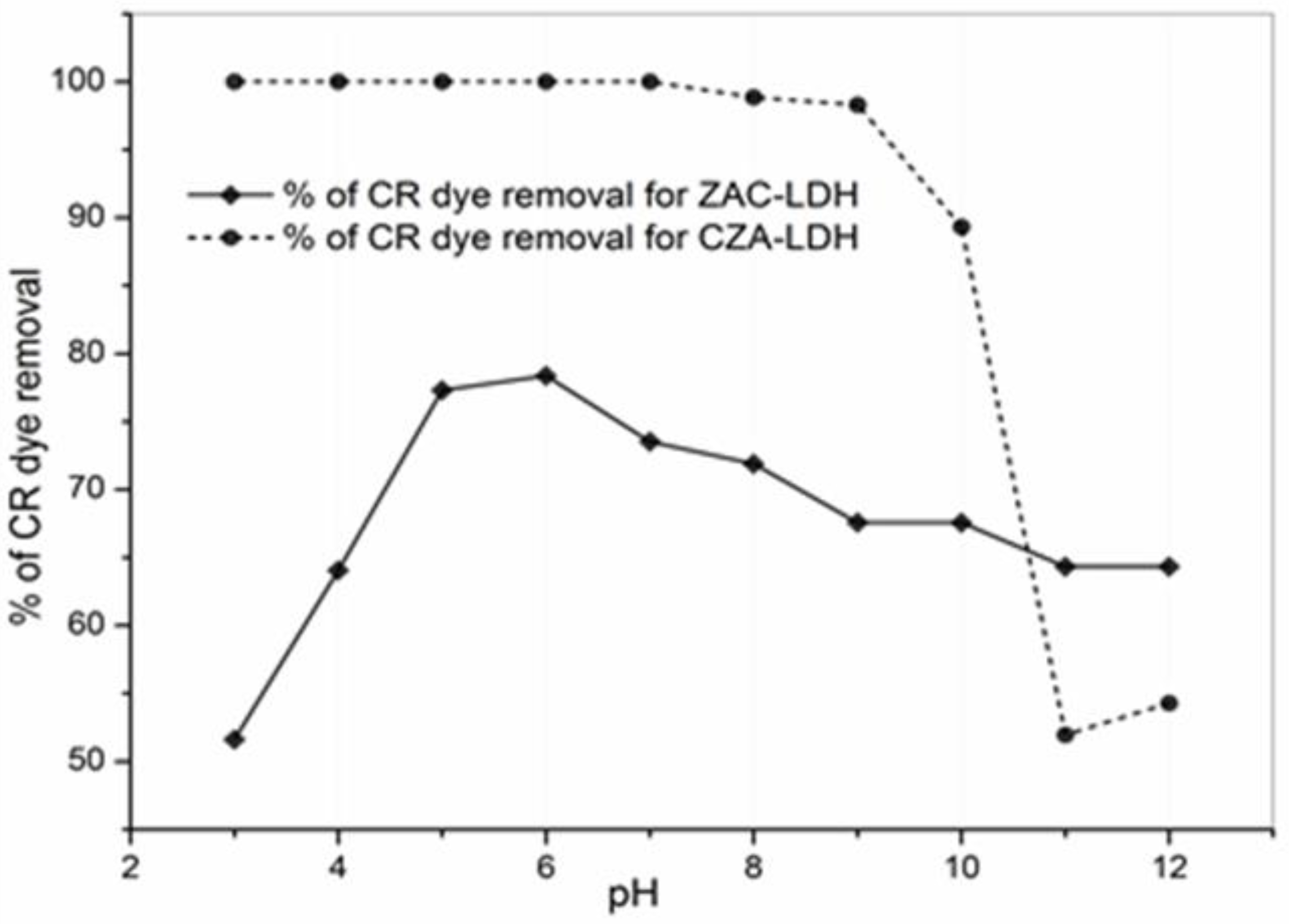
3.4. The Implication of pH
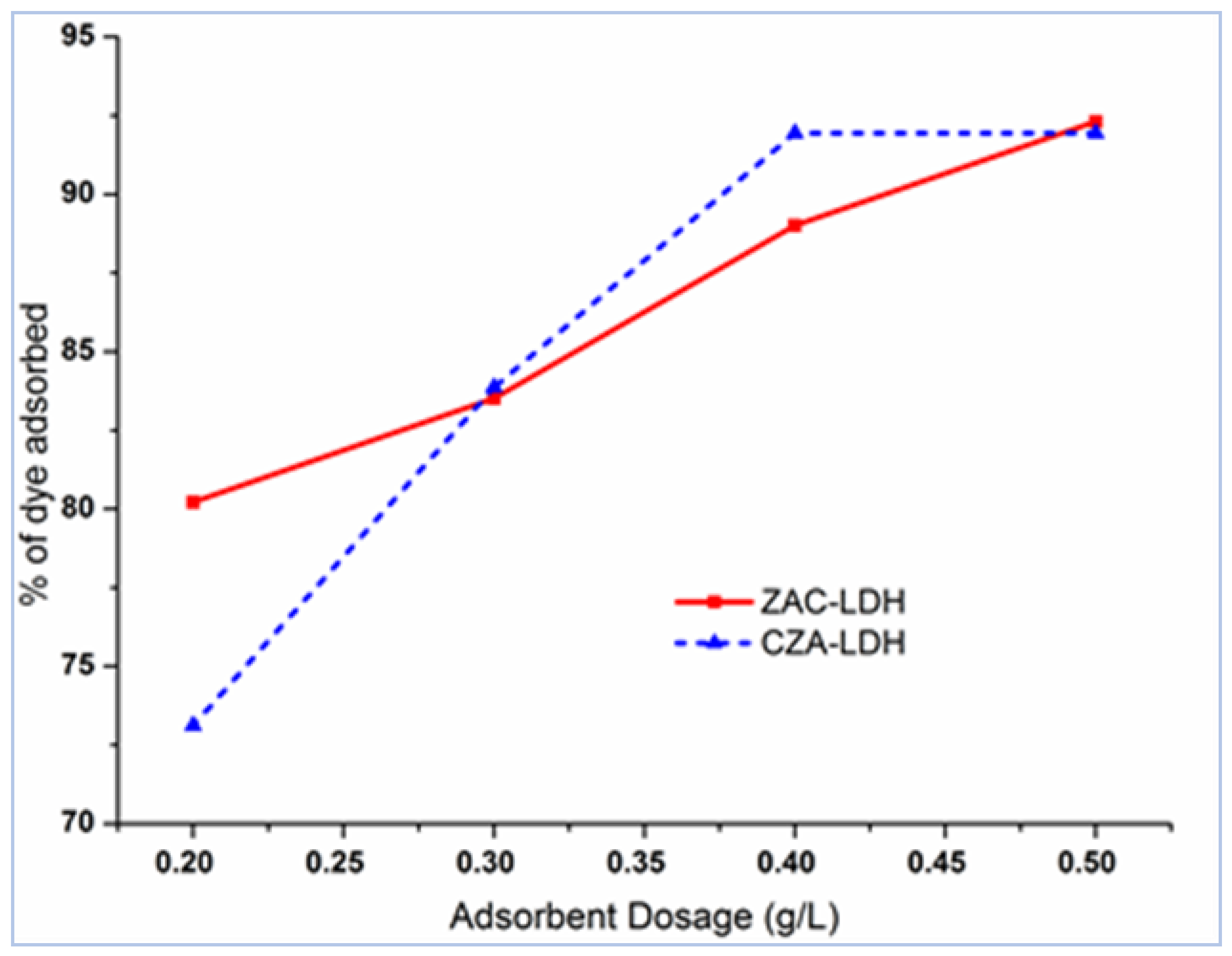
3.5. The Implications of Adsorbent Dose
3.6. Kinetic Studies
3.6.1. Pseudo First Order (PFO) Model
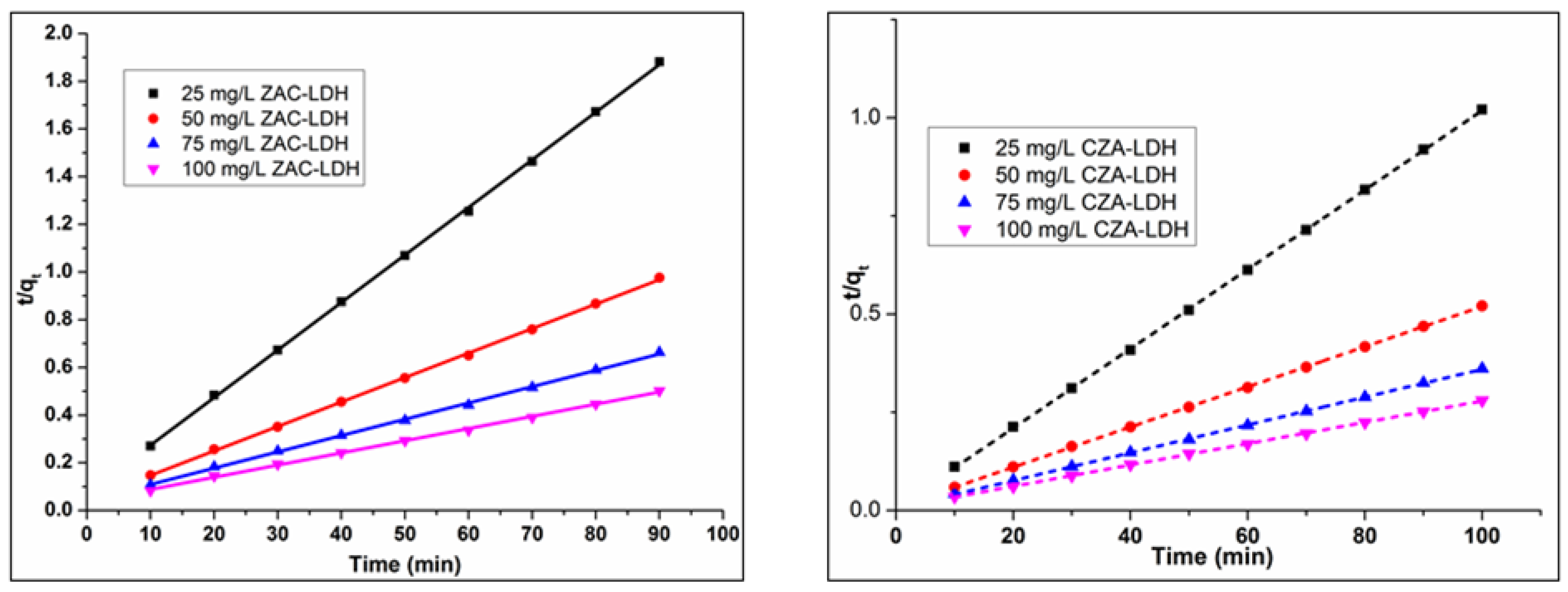
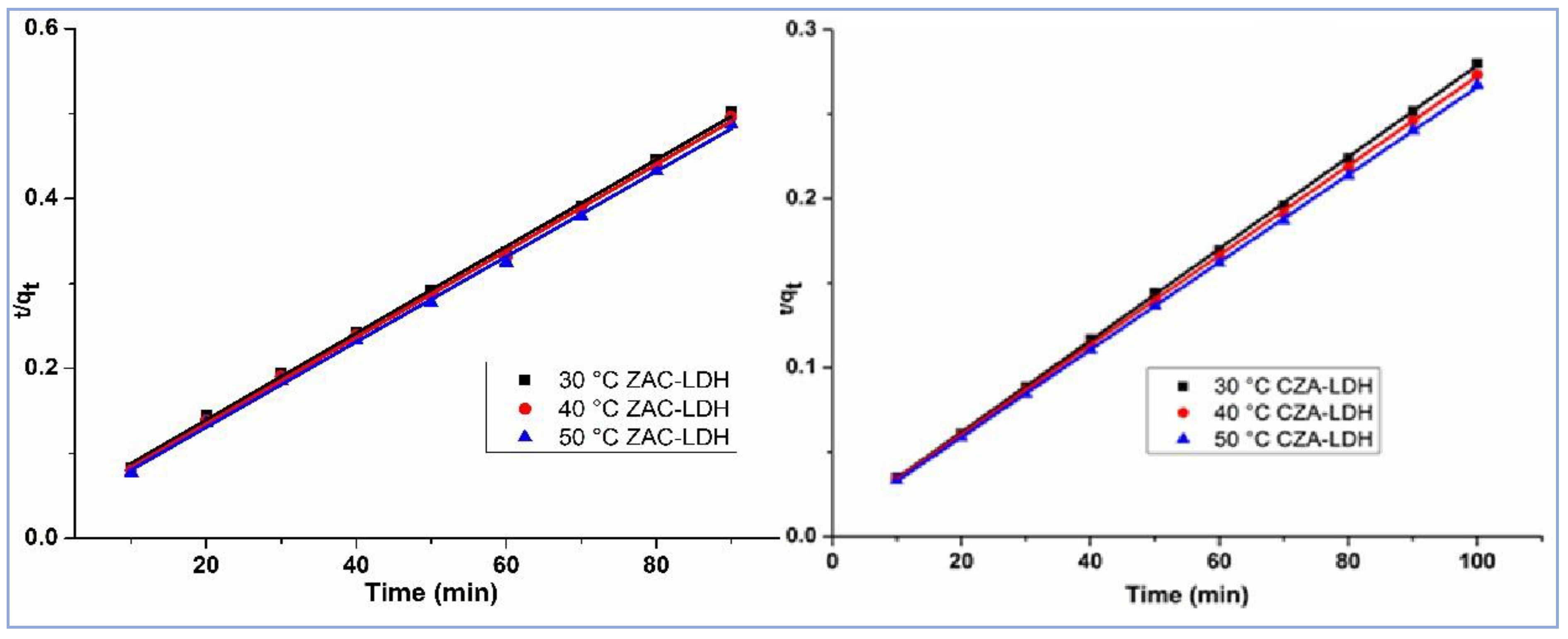
3.6.2. PSO Model
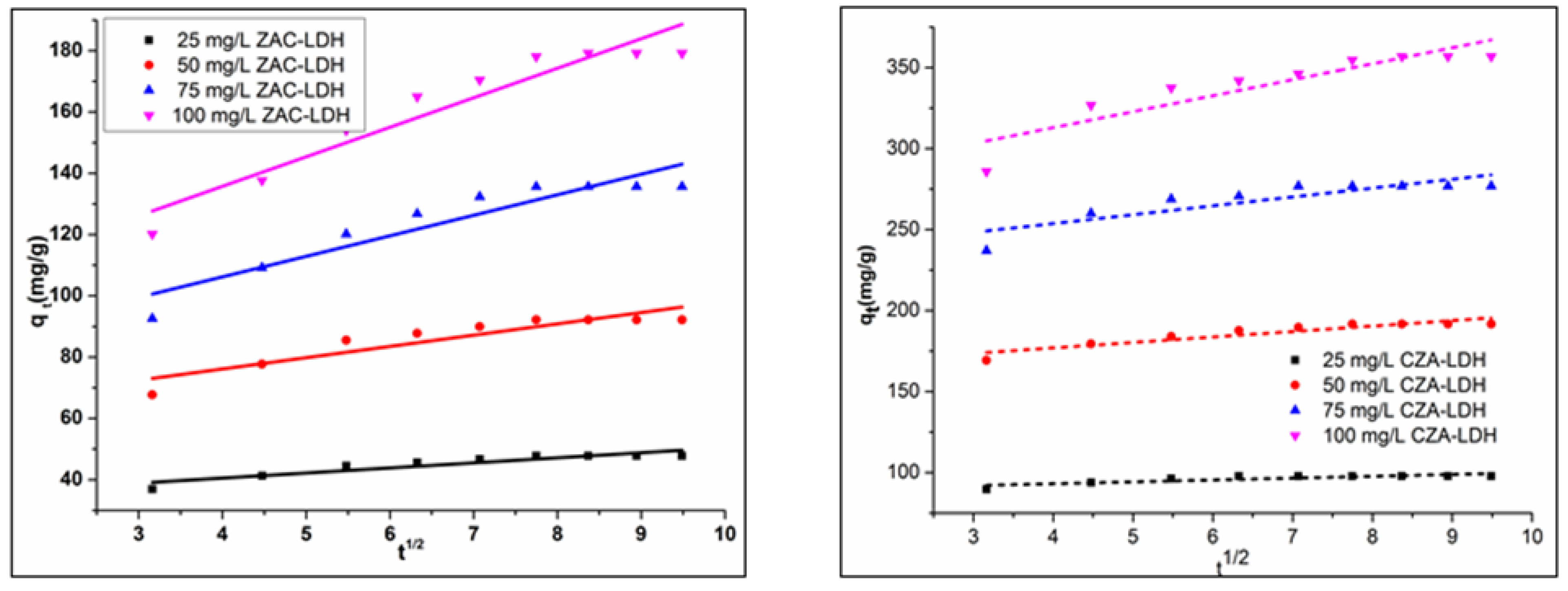
3.6.3. IPDM (Intra-Particle Diffusion Model)
3.7. Adsorption Isotherm
3.7.1. Langmuir Isotherm
3.7.2. Freundlich Model
3.8. Thermodynamic Parameters
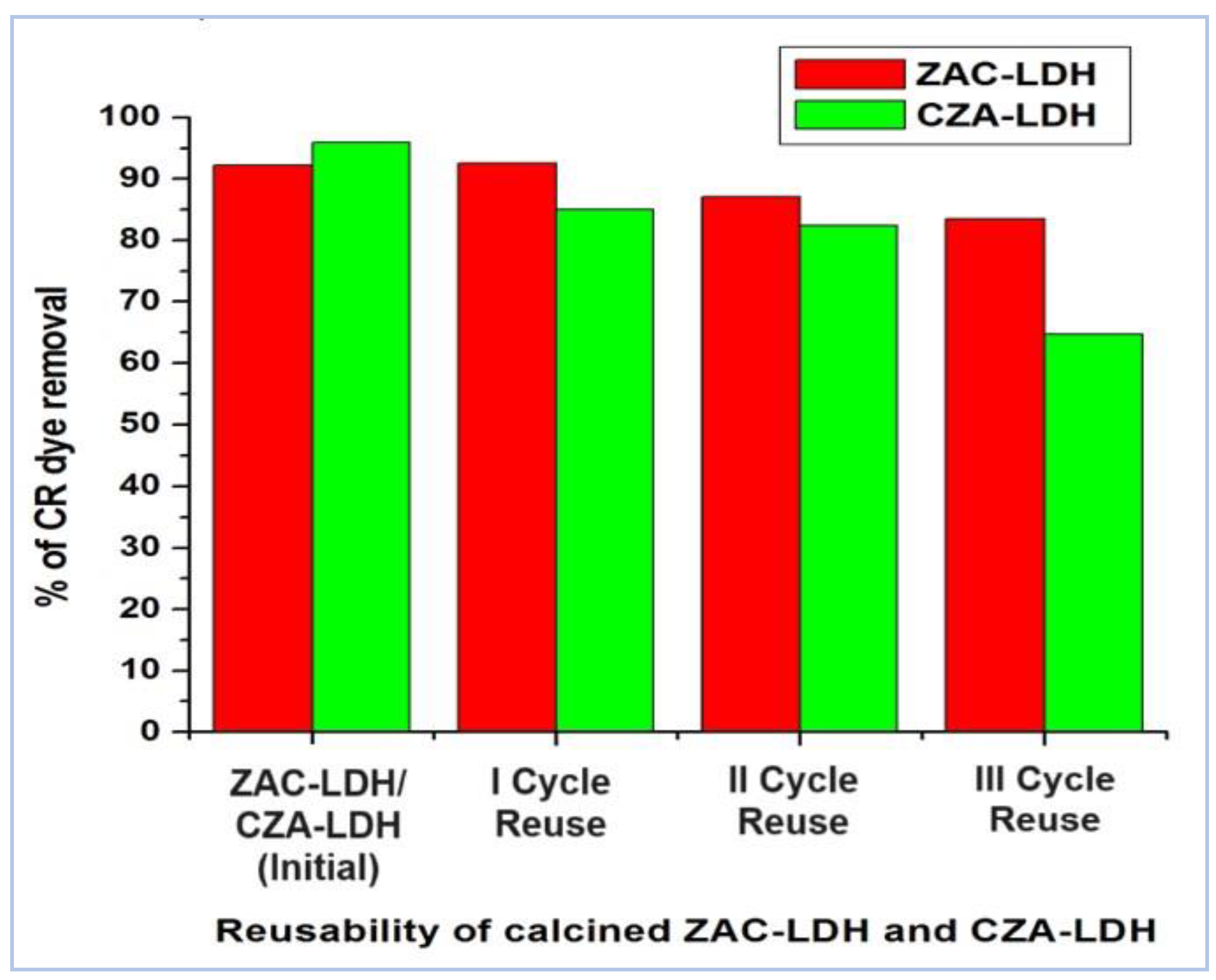
3.9. Reusability of the Adsorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| BET | Brunauer–Emmett–Teller |
| CLDH | calcined layered double hydroxide |
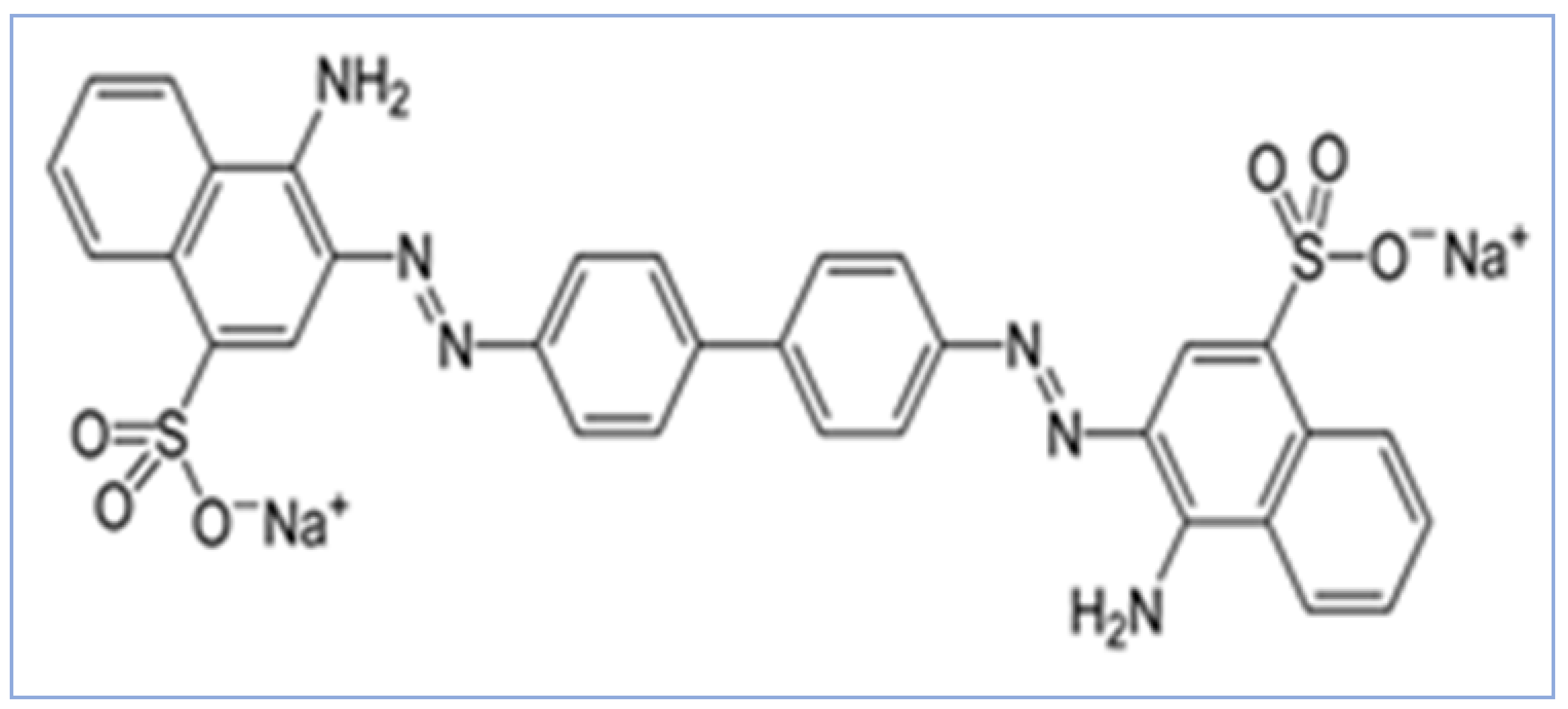
| CR | Congo red |
| CRD | Congo red dye |
| CTAB | cetyltrimethylammonium bromide |
| CZA-LDH | calcined Zn/Al layered double hydroxide |
| DC | Dye Concentration |
| FESEM | Field Emission Scanning Electron Microscope |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| IDC | Initial Dye Concentration |
| IPDM | intra-particle diffusion model |
| KP | kinetic parameter |
| LDH | layered double hydroxide |
| PFO | pseudo-first order |
| PSO | pseudo-second order |
| XRD | X-ray diffraction |
| ZAC-LDH | Zn/Al carbonate layered double hydroxide |
References
- Smetana, G.; Grosser, A. The Application of an Upflow Anaerobic Sludge Blanket Reactor in the Treatment of Brewery and Dairy Wastewater: A Critical Review. Energies 2024, 17, 1504. [Google Scholar] [CrossRef]
- Smol, M. Circular Economy in Wastewater Treatment Plant—Water, Energy and Raw Materials Recovery. Energies 2023, 16, 3911. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, X.; Zhan, Y.; Ding, X.; Wang, M.; Wang, X. In situ growth of ZIF-8 nanoparticles on chitosan to form the hybrid nanocomposites for high-efficiency removal of Congo Red. Int. J. Biol. Macromol. 2019, 137, 77–86. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, J.; Wang, B.; Xie, J.; Ying, G.; Li, J.; Cheng, Z.; Li, J.; Chen, K. Highly efficient and rapid purification of organic dye wastewater using lignin-derived hierarchical porous carbon. J. Colloid Interface Sci. 2022, 625, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Kafshgari, L.A.; Ghorbani, M.; Azizi, A. Fabrication and investigation of MnFe2O4/MWCNTs nanocomposite by hydrothermal technique and adsorption of cationic and anionic dyes. Appl. Surf. Sci. 2017, 419, 70–83. [Google Scholar] [CrossRef]
- Wei, Y.; Hao, J.-G.; Zhang, J.-L.; Huang, W.-Y.; Ouyang, S.-b.; Yang, K.; Lu, K.-Q. Integrating Co(OH)2 nanosheet arrays on graphene for efficient noble-metal-free EY-sensitized photocatalytic H2 evolution. Dalton Trans. 2023, 52, 13923–13929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, B.; Jiang, X.; Ji, Y. Dual function magnetic hydroxyapatite nanopowder for removal of malachite green and Congo red from aqueous solution. Powder Technol. 2016, 302, 207–214. [Google Scholar] [CrossRef]
- Lan, D.; Zhu, H.; Zhang, J.; Li, S.; Chen, Q.; Wang, C.; Wu, T.; Xu, M. Adsorptive removal of organic dyes via porous materials for wastewater treatment in recent decades: A review on species, mechanisms and perspectives. Chemosphere 2022, 293, 133464. [Google Scholar] [CrossRef]
- Zhang, P.; Qian, G.; Shi, H.; Ruan, X.; Yang, J.; Frost, R.L. Mechanism of interaction of hydrocalumites (Ca/Al-LDH) with methyl orange and acidic scarlet GR. J. Colloid Interface Sci. 2012, 365, 110–116. [Google Scholar] [CrossRef]
- Włodarczyk-Makuła, M.; Myszograj, S.; Włodarczyk, M. Removal of Organic Micro-Pollutants from Wastewater in Electrochemical Processes—Review. Energies 2023, 16, 5591. [Google Scholar] [CrossRef]
- Sherino, B.; Abdul Halim, S.N.; Shahabuddin, S.; Mohamad, S. Simultaneous removal of carcinogenic anionic and cationic dyes from environmental water using a new Zn-based metal–organic framework. Sep. Sci. Technol. 2021, 56, 330–343. [Google Scholar] [CrossRef]
- Wekoye, J.N.; Wanyonyi, W.C.; Wangila, P.T.; Tonui, M.K. Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environ. Chem. Ecotoxicol. 2020, 2, 24–31. [Google Scholar] [CrossRef]
- Arab, C.; El Kurdi, R.; Patra, D. Zinc curcumin oxide nanoparticles for enhanced adsorption of Congo red: Kinetics and adsorption isotherms study. Mater. Today Chem. 2022, 23, 100701. [Google Scholar] [CrossRef]
- Kaur, N.; Kaushal, J.; Mahajan, P.; Mantri, A. Phytoremediation of methylene blue dye (triarylmethane) and Congo red (diazo) by T. ammi L.: Kinetic studies. Int. J. Environ. Sci. Technol. 2024, 21, 1697–1714. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Ding, Z. Synthesis of Superparamagnetic Fe3O4 Nano-Adsorbent Using an Energy-Saving and Pollution-Reducing Strategy for the Removal of Xylenol Orange Dye in Water. Energies 2022, 15, 7378. [Google Scholar] [CrossRef]
- Abas, K.M.; Fathy, N.A. Sodalite zeolitic materials produced from coal fly ash for removal of congo red dye from aqueous solutions. Int. J. Environ. Sci. Technol. 2024, 21, 5165–5184. [Google Scholar] [CrossRef]
- Abbas, S.H.; Ridha, A.M.; Rashid, K.H.; Khadom, A.A. Biosorption of Congo red dye removal from aqueous solution using fennel seed spent and garlic peel. Int. J. Environ. Sci. Technol. 2023, 20, 13845–13858. [Google Scholar] [CrossRef]
- Mielcarek, A.; Bryszewski, K.Ł.; Rodziewicz, J.; Kłobukowska, K.; Janczukowicz, W. Phosphorus Removal Rate and Efficiency in an Electrochemical Sequencing Reactor for the Treatment of Wastewater with Low Organic Carbon Content. Energies 2024, 17, 1352. [Google Scholar] [CrossRef]
- Gusiatin, M.Z.; Pasieczna-Patkowska, S.; Bálintová, M.; Kuśmierz, M. Treatment of Wastewater from Soil Washing with Soluble Humic Substances Using Biochars and Activated Carbon. Energies 2023, 16, 4311. [Google Scholar] [CrossRef]
- Hu, M.; Yan, X.; Hu, X.; Zhang, J.; Feng, R.; Zhou, M. Ultra-high adsorption capacity of MgO/SiO2 composites with rough surfaces for Congo red removal from water. J. Colloid Interface Sci. 2018, 510, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, Y.; Cheng, B.; Yu, J.; Jiang, C. Chestnut husk-like nickel cobaltite hollow microspheres for the adsorption of Congo red. J. Alloys Compd. 2018, 735, 1041–1051. [Google Scholar] [CrossRef]
- Moosavi, S.; Lai, C.W.; Gan, S.; Zamiri, G.; Akbarzadeh Pivehzhani, O.; Johan, M.R. Application of efficient magnetic particles and activated carbon for dye removal from wastewater. ACS Omega 2020, 5, 20684–20697. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption properties and mechanisms of palygorskite for removal of various ionic dyes from water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Mansha, M.; Waheed, A.; Ahmad, T.; Kazi, I.W.; Ullah, N. Synthesis of a novel polysuccinimide based resin for the ultrahigh removal of anionic azo dyes from aqueous solution. Environ. Res. 2020, 184, 109337. [Google Scholar] [CrossRef]
- Ohemeng-Boahen, G.; Sewu, D.D.; Tran, H.N.; Woo, S.H. Enhanced adsorption of congo red from aqueous solution using chitosan/hematite nanocomposite hydrogel capsule fabricated via anionic surfactant gelation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126911. [Google Scholar] [CrossRef]
- Hashem, A.H.; Saied, E.; Hasanin, M.S. Green and ecofriendly bio-removal of methylene blue dye from aqueous solution using biologically activated banana peel waste. Sustain. Chem. Pharm. 2020, 18, 100333. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Yarramuthi, V.; Kim, Y.; Lee, K.M.; Kim, D.-S. Removal of anionic dyes (Reactive Black 5 and Congo Red) from aqueous solutions using Banana Peel Powder as an adsorbent. Ecotoxicol. Environ. Saf. 2018, 148, 601–607. [Google Scholar] [CrossRef]
- Bukhari, A.; Ijaz, I.; Zain, H.; Gilani, E.; Nazir, A.; Bukhari, A.; Raza, S.; Ansari, J.; Hussain, S.; Alarfaji, S.S.; et al. Removal of Eosin dye from simulated media onto lemon peel-based low cost biosorbent. Arab. J. Chem. 2022, 15, 103873. [Google Scholar] [CrossRef]
- Gomaa, H.; Hussein, M.A.; Motawea, M.M.; Aboraia, A.M.; Cheira, M.F.; Alotaibi, M.T.; El-Bahy, S.M.; Ali, H.M. A hybrid mesoporous CuO@barley straw-derived SiO2 nanocomposite for adsorption and photocatalytic degradation of methylene blue from real wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128811. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Chai, W.S.; Chen, S.-J.; Shih, J.-Y.; Koyande, A.K.; Liu, B.-L.; Chang, Y.-K. Removal of soluble microbial products and dyes using heavy metal wastes decorated on eggshell. Chemosphere 2021, 270, 128615. [Google Scholar] [CrossRef] [PubMed]
- Chikri, R.; Elhadiri, N.; Benchanaa, M.; Maguana, Y. Efficiency of sawdust as low-cost adsorbent for dyes removal. J. Chem. 2020, 2020, 8813420. [Google Scholar] [CrossRef]
- Malik, A.; Khan, A.; Anwar, N.; Naeem, M. A comparative study of the adsorption of Congo red dye on rice husk, rice husk char and chemically modified rice husk char from aqueous media. Bull. Chem. Soc. Ethiop. 2020, 34, 41–54. [Google Scholar] [CrossRef]
- Astuti, W.; Chafidz, A.; Wahyuni, E.T.; Prasetya, A.; Bendiyasa, I.M.; Abasaeed, A.E. Methyl violet dye removal using coal fly ash (CFA) as a dual sites adsorbent. J. Environ. Chem. Eng. 2019, 7, 103262. [Google Scholar] [CrossRef]
- Sarkar, S.; Tiwari, N.; Basu, A.; Behera, M.; Das, B.; Chakrabortty, S.; Sanjay, K.; Suar, M.; Adhya, T.K.; Banerjee, S.; et al. Sorptive removal of malachite green from aqueous solution by magnetite/coir pith supported sodium alginate beads: Kinetics, isotherms, thermodynamics and parametric optimization. Environ. Technol. Innov. 2021, 24, 101818. [Google Scholar] [CrossRef]
- Nehaba, S.S.; Abdullah, R.H.; Oda, A.M.; Omran, A.R.; Mottaleb, A.S. Evaluation of the efficiency of tea waste powder to remove the Safranin O dye compared to the activated carbon as adsorbent. Orient. J. Chem. 2019, 35, 1201–1207. [Google Scholar] [CrossRef]
- Tuli, F.; Hossain, A.; Kibria, A.F.; Tareq, A.; Mamun, S.M.; Ullah, A.A. Removal of methylene blue from water by low-cost activated carbon prepared from tea waste: A study of adsorption isotherm and kinetics. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100354. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Deng, Z.; Xu, X.; Qin, J.; Guo, X.; Bai, Z.; Chen, X.; Lu, Z. Highly efficient removal of Congo red on calcined MgAl-layered double hydroxide with microporous structure. Int. J. Environ. Sci. Technol. 2023, 20, 10141–10152. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Hao, J.-G.; Wei, Y.; Weng, B.; Ge, S.; Yang, K.; Lu, S.; Yang, M.-Q.; Liao, Y. Photocatalytic Conversion of Diluted CO2 into Tunable Syngas via Modulating Transition Metal Hydroxides. Inorg. Chem. 2024, 63, 795–802. [Google Scholar] [CrossRef]
- Bobde, P.; Sharma, A.K.; Panchal, D.; Sharma, A.; Patel, R.K.; Dhodapkar, R.S.; Pal, S. Layered double hydroxides (LDHs)-based photocatalysts for dye degradation: A review. Int. J. Environ. Sci. Technol. 2023, 20, 5733–5752. [Google Scholar] [CrossRef]
- Farghali, M.A.; Selim, A.M.; Khater, H.F.; Bagato, N.; Alharbi, W.; Alharbi, K.H.; Radwan, I.T. Optimized adsorption and effective disposal of Congo red dye from wastewater: Hydrothermal fabrication of MgAl-LDH nanohydrotalcite-like materials. Arab. J. Chem. 2022, 15, 104171. [Google Scholar] [CrossRef]
- Xie, J.; Yamaguchi, T.; Oh, J.-M. Synthesis of a mesoporous Mg–Al–mixed metal oxide with P123 template for effective removal of Congo red via aggregation-driven adsorption. J. Solid State Chem. 2021, 293, 121758. [Google Scholar] [CrossRef]
- Khalil, A.K.; Almanassra, I.W.; Chatla, A.; Ihsanullah, I.; Laoui, T.; Atieh, M.A. Insights into the adsorption of lead ions by Mg-Al LDH doped activated carbon composites: Implications for fixed bed column and batch applications. Chem. Eng. Sci. 2023, 281, 119192. [Google Scholar] [CrossRef]
- Manea, Y.K.; Khan, A.M.; Wani, A.A.; Saleh, M.A.; Qashqoosh, M.T.; Shahadat, M.; Rezakazemi, M. In-grown flower like Al-Li/Th-LDH@CNT nanocomposite for enhanced photocatalytic degradation of MG dye and selective adsorption of Cr (VI). J. Environ. Chem. Eng. 2022, 10, 106848. [Google Scholar] [CrossRef]
- Shirin, V.A.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.; Pramod, K. Advanced drug delivery applications of layered double hydroxide. J. Control. Release 2021, 330, 398–426. [Google Scholar] [CrossRef]
- El Khanchaoui, A.; Sajieddine, M.; Mansori, M.; Essoumhi, A. Anionic dye adsorption on ZnAl hydrotalcite-type and regeneration studies based on “memory effect”. Int. J. Environ. Anal. Chem. 2022, 102, 3542–3560. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhou, B.; Meng, F.; Wang, Y.; Wen, G. Recent Advance of Layered Double Hydroxides Materials: Structure, Properties, Synthesis, Modification and Applications of Wastewater Treatment. J. Environ. Chem. Eng. 2023, 11, 111191. [Google Scholar] [CrossRef]
- Manjula Rani, K.; Palanisamy, P.N. Synthesis and Characterization of Mesoporous, Nanostructured Zinc Aluminium Carbonate Layered Double Hydroxides (ZAC-LDHs) and Its Calcined Product (CZA-LDH). J. Inorg. Organomet. Polym. Mater. 2018, 28, 1127–1135. [Google Scholar] [CrossRef]
- Rani, K.M.; Palanisamy, P. Comparative studies on the adsorptive removal of Acid Violet-17 dye from aqueous solution by using zinc aluminium carbonate-LDH (ZAC-LDH) and modified LDH. Desalination Water Treat. 2017, 65, 337–345. [Google Scholar] [CrossRef]
- Tamilvanan, A.; Balamurugan, D.K.; Ponappa, D.K.; Kumar, B.M. Using Response Surface Methodology in Synthesis of Ultrafine Copper Nanoparticles by Electrolysis. Int. J. Nanosci. 2016, 15, 1650001. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Boutahala, M.; Djebri, N. Synthesis and characterization of ZnAl-layered double hydroxide and organo-K10 montmorillonite for the removal of diclofenac from aqueous solution. Adsorpt. Sci. Technol. 2017, 35, 20–36. [Google Scholar] [CrossRef]
- Ahmed, D.N.; Naji, L.A.; Faisal, A.A.; Al-Ansari, N.; Naushad, M. Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Sci. Rep. 2020, 10, 2042. [Google Scholar] [CrossRef]
- Mahmood, T.; Noreen, U.; Ali, R.; Ullah, A.; Naeem, A.; Aslam, M. Adsorptive removal of Congo red from aqueous phase using graphene–tin oxide composite as a novel adsorbent. Int. J. Environ. Sci. Technol. 2022, 19, 10275–10290. [Google Scholar] [CrossRef]
- Daud, M.; Hai, A.; Banat, F.; Wazir, M.B.; Habib, M.; Bharath, G.; Al-Harthi, M.A. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)–Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 2019, 288, 110989. [Google Scholar] [CrossRef]
- Rathee, G.; Awasthi, A.; Sood, D.; Tomar, R.; Tomar, V.; Chandra, R. A new biocompatible ternary Layered Double Hydroxide Adsorbent for ultrafast removal of anionic organic dyes. Sci. Rep. 2019, 9, 16225. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, W.; Zhao, T.-L.; Liu, M.; Yao, Q.-Z.; Zhou, G.-T. Efficient Removal of Congo Red, Methylene Blue and Pb(II) by Hydrochar–MgAlLDH Nanocomposite: Synthesis, Performance and Mechanism. Nanomaterials 2023, 13, 1145. [Google Scholar] [CrossRef]
- Wai, T.T.; Aung, E.M.; Kyaw, N.C. Potential of neem leaf powder as bio-adsorbents for dye colour removal. Int. J. Trend Sci. Res. Dev. 2019, 3, 2502–2505. [Google Scholar]
- Feng, L.; Zhang, Q.; Ji, F.; Jiang, L.; Liu, C.; Shen, Q.; Liu, Q. Phosphate removal performances of layered double hydroxides (LDH) embedded polyvinyl alcohol/lanthanum alginate hydrogels. Chem. Eng. J. 2022, 430, 132754. [Google Scholar] [CrossRef]
- Dovi, E.; Aryee, A.A.; Kani, A.N.; Mpatani, F.M.; Li, J.; Li, Z.; Qu, L.; Han, R. Functionalization of walnut shell by grafting amine groups to enhance the adsorption of Congo red from water in batch and fixed-bed column modes. J. Environ. Chem. Eng. 2021, 9, 106301. [Google Scholar] [CrossRef]
- Constantin, M.; Asmarandei, I.; Harabagiu, V.; Ghimici, L.; Ascenzi, P.; Fundueanu, G. Removal of anionic dyes from aqueous solutions by an ion-exchanger based on pullulan microspheres. Carbohydr. Polym. 2013, 91, 74–84. [Google Scholar] [CrossRef]
- Shaban, M.; Ashraf, A.M.; Abukhadra, M.R. TiO2 nanoribbons/carbon nanotubes composite with enhanced photocatalytic activity; fabrication, characterization, and application. Sci. Rep. 2018, 8, 781. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga svenska vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Singh, P.K.; Banerjee, S.; Srivastava, A.L.; Sharma, Y.C. Kinetic and equilibrium modeling for removal of nitrate from aqueous solutions and drinking water by a potential adsorbent, hydrous bismuth oxide. RSC Adv. 2015, 5, 35365–35376. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Zhang, C.; Yang, S.; Chen, H.; He, H.; Sun, C. Adsorption behavior and mechanism of reactive brilliant red X-3B in aqueous solution over three kinds of hydrotalcite-like LDHs. Appl. Surf. Sci. 2014, 301, 329–337. [Google Scholar] [CrossRef]
- Ahmed, M.; Mohamed, A. An efficient adsorption of indigo carmine dye from aqueous solution on mesoporous Mg/Fe layered double hydroxide nanoparticles prepared by controlled sol-gel route. Chemosphere 2017, 174, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Mensah, K.; Mahmoud, H.; Fujii, M.; Shokry, H. Novel nano-ferromagnetic activated graphene adsorbent extracted from waste for dye decolonization. J. Water Process Eng. 2022, 45, 102512. [Google Scholar] [CrossRef]
- Palapa, N.R.; Juleanti, N.; Normah; Taher, T.; Mohadi, R.; Rachmat, A.; Lesbani, A. Adsorptive Performance of Congo Red using Copper-Aluminum LDHS Load to Rice Husk Biochar. Sains Malays. 2022, 51, 161–173. [Google Scholar] [CrossRef]
- Brahma, D.; Priyom Nath, K.; Patgiri, M.; Saikia, H. Synthesis of Ternary CaNiAl-Layered Double Hydroxide as Potential Adsorbent for Congo Red Dye Removal in Aqueous Solution. Asian J. Chem. 2022, 34, 3215–3223. [Google Scholar] [CrossRef]
- Kong, X.-q.; Zhang, N.-n.; Lian, Y.-m.; Tang, Q.-j. Removal of Congo red using the chlorinated Ca-al layered double hydroxide produced from the desulfurization circulating wastewater. Desalination Water Treat. 2021, 238, 294–305. [Google Scholar] [CrossRef]

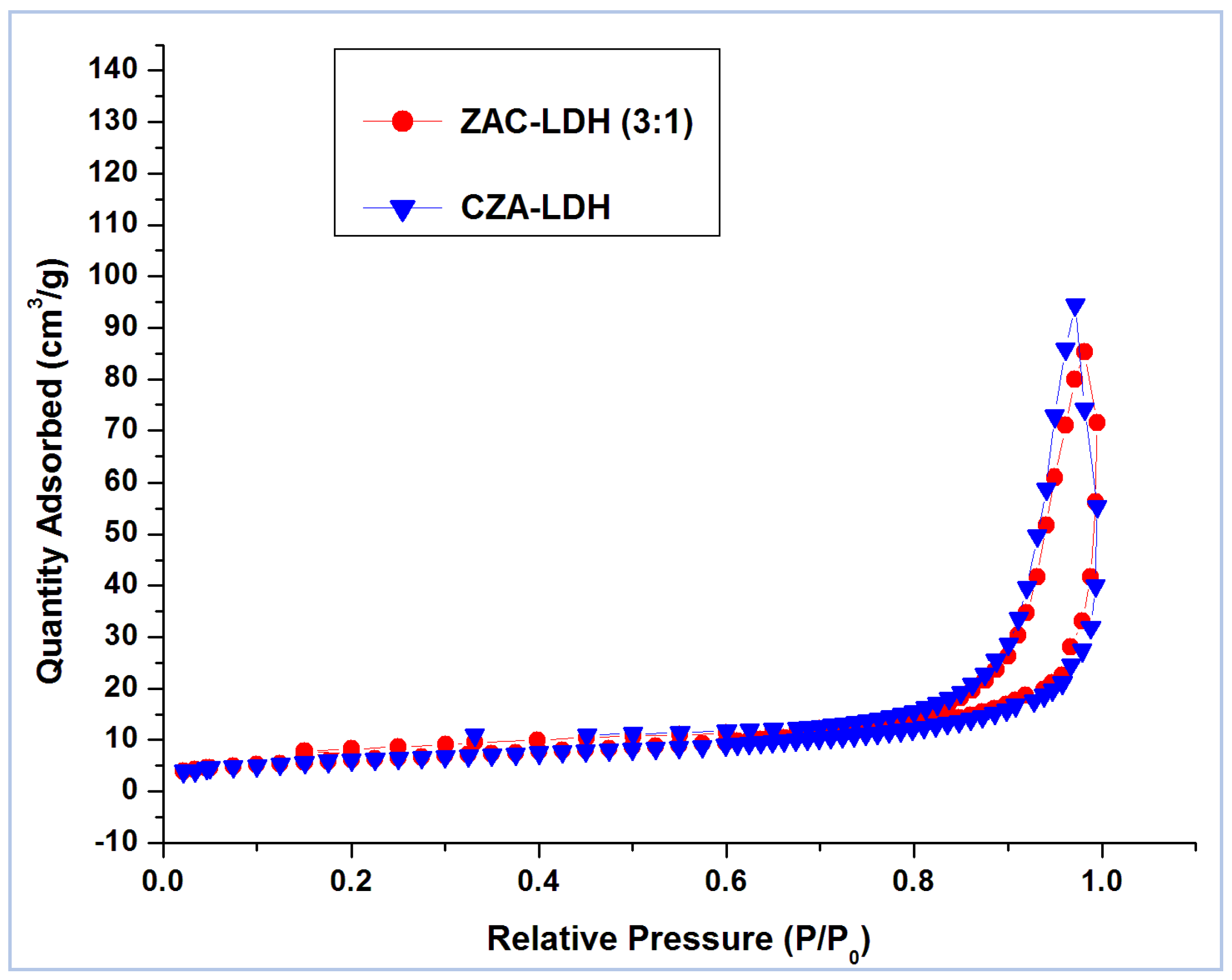
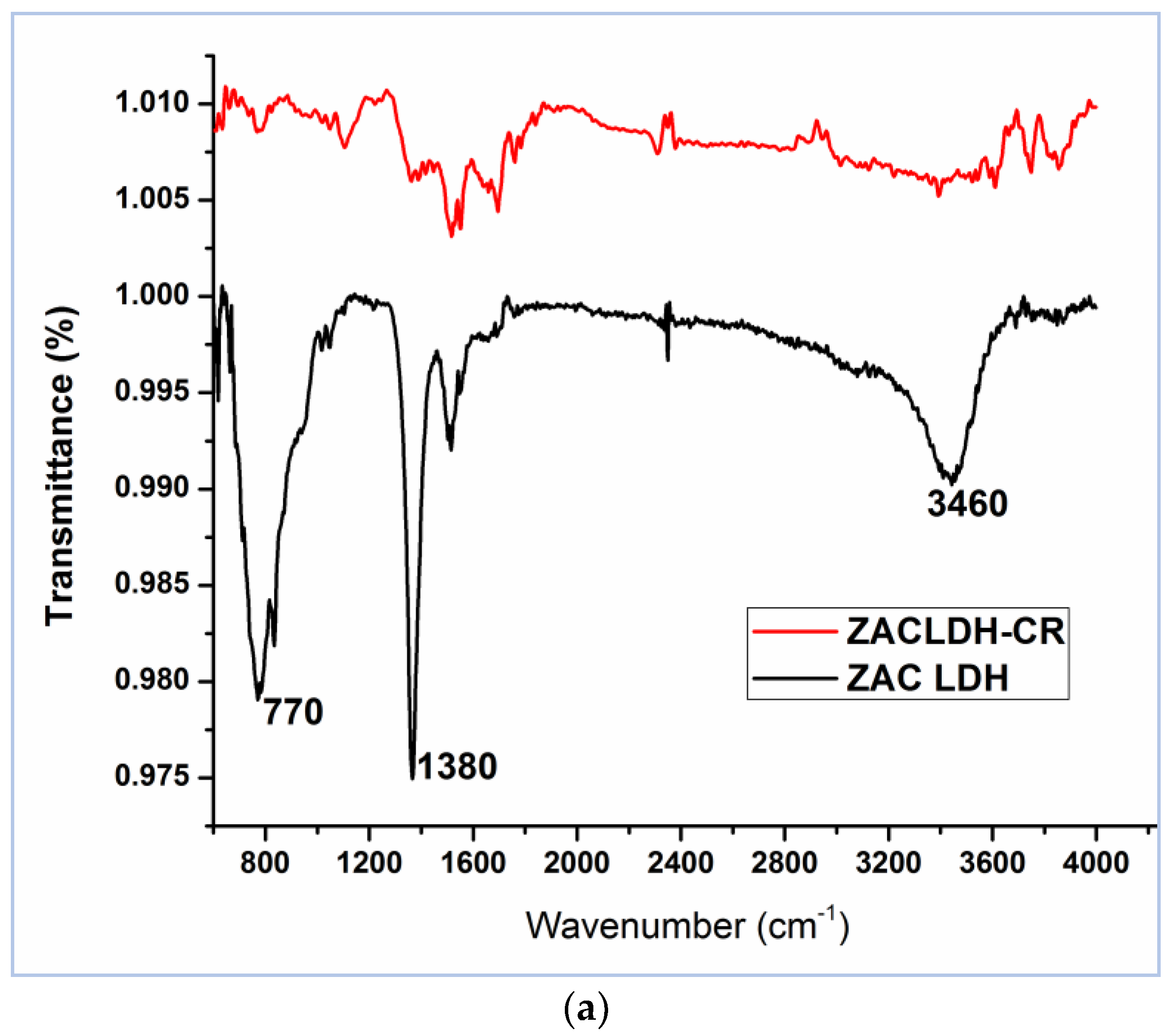
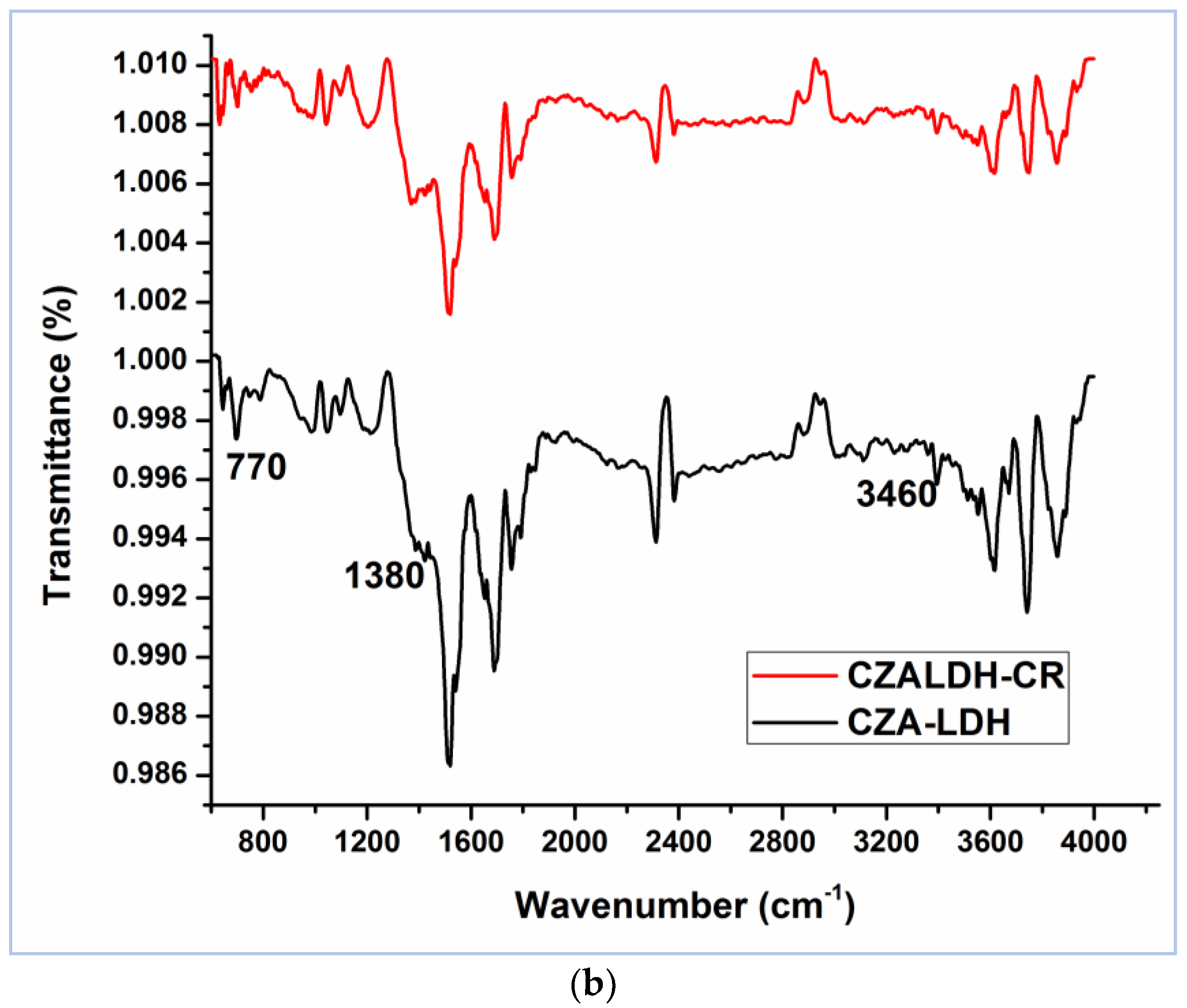
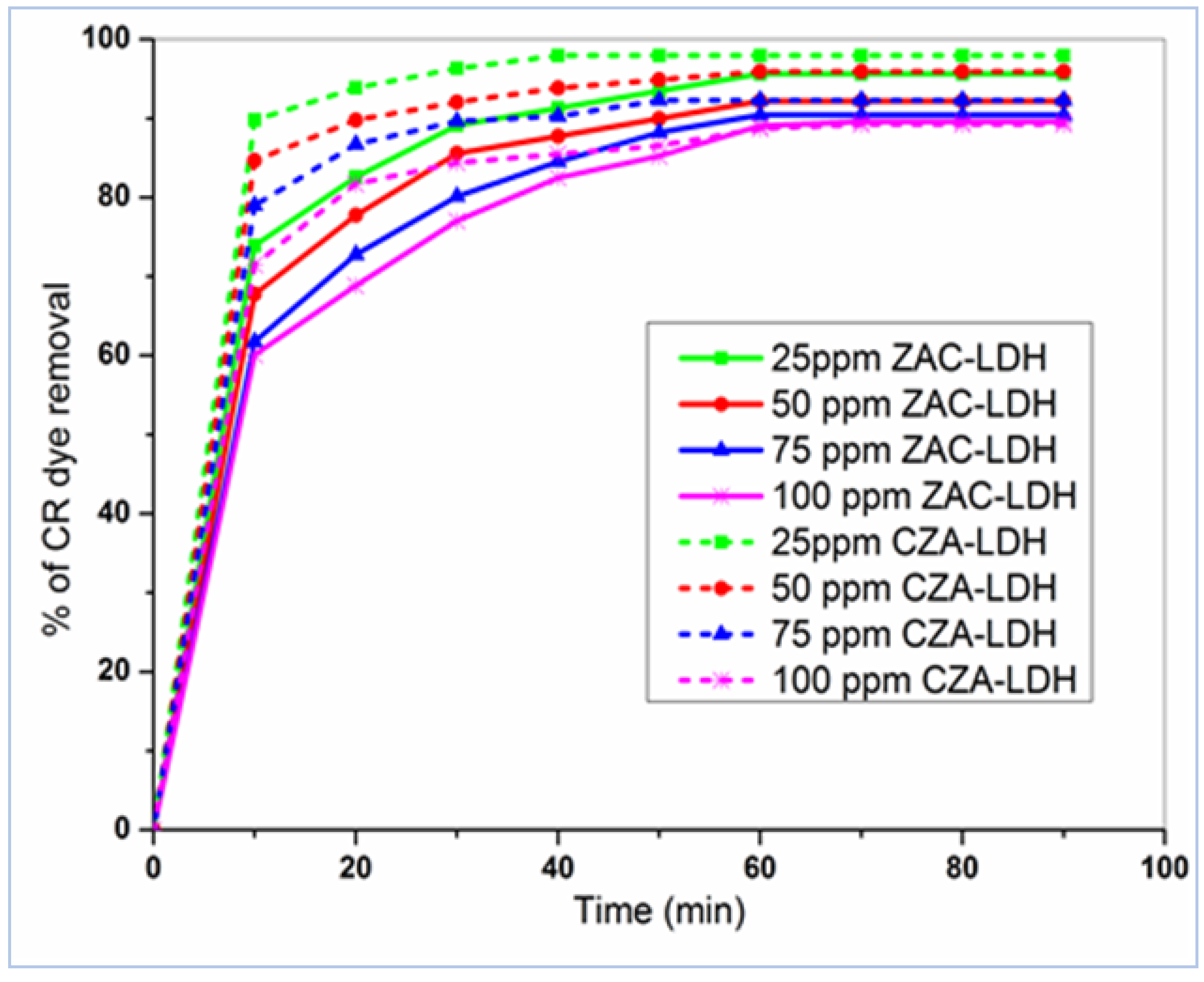
| Parameter | ZAC-LDH | CZA-LDH |
|---|---|---|
| Single point surface area (P/Po = 0.21) in (m2/g) | 22.0257 | 22.0469 |
| BET surface area (m2/g) | 22.5610 | 22.5963 |
| Dubinin–Astakhov—micropore surface area (m2/g) | 23.2209 | 23.2866 |
| Total pore volume of (P/Po = 0.99) (cm3/g) | 0.132013 | 0.145968 |
| t-Plot micropore volume (cm3/g) | 0.001020 | 0.002219 |
| t-Plot mesopore volume (cm3/g) | 0.130993 | 0.143749 |
| Average pore width (Å) | 233.7454 | 258.7366 |
| Adsorbents | Uncalcined LDH | Calcined LDH | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial Concentration (mg/L) | ||||||||
| Parameter | 25 | 50 | 75 | 100 | 25 | 50 | 75 | 100 |
| qe exp (mg/g) | 47.83 | 92.22 | 135.66 | 179.23 | 97.96 | 191.84 | 276.92 | 356.99 |
| PFO kinetic model | ||||||||
| k1 (min−1) | 0.062 | 0.070 | 0.068 | 0.072 | 0.114 | 0.081 | 0.093 | 0.071 |
| qe cal (mg/g) | 31.21 | 65.43 | 111.51 | 178.11 | 59.84 | 92.49 | 156.24 | 203.56 |
| R2 | 0.9552 | 0.9694 | 0.9795 | 0.9205 | 0.9114 | 0.9097 | 0.9035 | 0.919 |
| PSO kinetic model | ||||||||
| k2 (g/mg min−1) | 0.005 | 0.002 | 0.001 | 0.001 | 0.013 | 0.003 | 0.002 | 0.001 |
| h | 13.441 | 22.371 | 24.390 | 26.810 | 123.457 | 121.951 | 181.818 | 135.135 |
| qe cal (mg/g) | 50.25 | 97.09 | 147.06 | 196.08 | 99.01 | 196.08 | 285.71 | 370.37 |
| R2 | 0.9997 | 0.9996 | 0.9992 | 0.9989 | 0.9999 | 0.9999 | 0.9999 | 0.9999 |
| IPDM | ||||||||
| kid (mg/g/min½) | 0.604 | 0.272 | 0.149 | 0.104 | 0.991 | 0.331 | 0.206 | 0.113 |
| R2 | 0.8647 | 0.8491 | 0.8784 | 0.9173 | 0.6897 | 0.8282 | 0.7221 | 0.7989 |
| Adsorbents | Uncalcined LDH | Calcined LDH | ||||
|---|---|---|---|---|---|---|
| Temperature | ||||||
| Parameter | 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C |
| qe exp (mg/g) | 179.23 | 181.42 | 184.70 | 356.99 | 365.59 | 374.19 |
| PFO kinetic model | ||||||
| k1 (min−1) | 0.073 | 0.060 | 0.067 | 0.066 | 0.055 | 0.067 |
| qe cal (mg/g) | 179.80 | 139.06 | 150.42 | 189.37 | 173.66 | 196.20 |
| R2 | 0.9307 | 0.9693 | 0.9665 | 0.9199 | 0.8814 | 0.9367 |
| PSO kinetic model | ||||||
| k2 (g/mg min−1) | 0.00070 | 0.00079 | 0.00083 | 0.00097 | 0.00096 | 0.00097 |
| h | 26.954 | 30.395 | 33.003 | 133.333 | 131.579 | 142.857 |
| qe cal (mg/g) | 196.08 | 196.08 | 200.00 | 370.37 | 370.37 | 384.62 |
| R2 | 0.9989 | 0.999 | 0.999 | 0.9999 | 0.9999 | 0.9999 |
| IPDM | ||||||
| kid (mg/g/min½) | 0.104 | 0.111 | 0.113 | 0.113 | 0.109 | 0.106 |
| R2 | 0.9143 | 0.9123 | 0.9003 | 0.8009 | 0.8158 | 0.7917 |
| Adsorbent | Temperature (°C) | Langmuir Isotherm | Freundlich Isotherm | |||||
|---|---|---|---|---|---|---|---|---|
| Q0 | b | RL | R2 | n | kf | R2 | ||
| Uncalcined LDH | 30 | 769.23 | 0.0024 | 0.8914 | 0.9208 | 1.133 | 29.580 | 0.999 |
| 40 | 714.29 | 0.0026 | 0.8831 | 0.68 | 1.122 | 33.159 | 0.9915 | |
| 50 | 454.55 | 0.0042 | 0.8250 | 0.8712 | 1.412 | 53.407 | 0.9867 | |
| Calcined LDH | 30 | 526.32 | 0.0073 | 0.7335 | 0.9531 | 1.676 | 86.298 | 0.993 |
| 40 | 588.24 | 0.0065 | 0.7543 | 0.9637 | 1.745 | 97.521 | 0.9983 | |
| 50 | 625.00 | 0.0062 | 0.7646 | 0.92 | 1.703 | 106.955 | 0.9897 | |
| Adsorbent | Temperature (°C) | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/K/mol) |
|---|---|---|---|---|
| Uncalcined LDH | 30 | −5.43 | 13.59 | 62.65 |
| 40 | −5.93 | |||
| 50 | −6.69 | |||
| Calcined LDH | 30 | −5.33 | 22.72 | 92.67 |
| 40 | −6.33 | |||
| 50 | −7.18 |
| S. No. | Adsorbent Material | Quantity of Dye Adsorbed (mg/g) | Reference |
|---|---|---|---|
| 1. | Cabbage waste powder | 1.78 | [13] |
| 2. | Coal fly ash | 152.7 | [17] |
| 3. | Mg/Al-LDH | 769.23 | [41] |
| 4. | CuAl LDH | 47.619 | [69] |
| 5. | Ternary CaNiAl- LDH | 135.21 | [70] |
| 6. | CaAl-LDH Cl | 123.9 | [71] |
| 7. | ZAC-LDH | 179.23 | Present work |
| 8. | CZA-LDH | 356.99 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, K.M.; Palanisamy, P.N.; Kowshalya, V.N.; Tamilvanan, A.; Prabakaran, R.; Kim, S.C. Uncalcined Zn/Al Carbonate LDH and Its Calcined Counterpart for Treating the Wastewater Containing Anionic Congo Red Dye. Energies 2024, 17, 2698. https://doi.org/10.3390/en17112698
Rani KM, Palanisamy PN, Kowshalya VN, Tamilvanan A, Prabakaran R, Kim SC. Uncalcined Zn/Al Carbonate LDH and Its Calcined Counterpart for Treating the Wastewater Containing Anionic Congo Red Dye. Energies. 2024; 17(11):2698. https://doi.org/10.3390/en17112698
Chicago/Turabian StyleRani, Kuppusamy Manjula, Pachagoundanpalayam Nachimuthugounder Palanisamy, Vennila Nagamuthu Kowshalya, Ayyasamy Tamilvanan, Rajendran Prabakaran, and Sung Chul Kim. 2024. "Uncalcined Zn/Al Carbonate LDH and Its Calcined Counterpart for Treating the Wastewater Containing Anionic Congo Red Dye" Energies 17, no. 11: 2698. https://doi.org/10.3390/en17112698
APA StyleRani, K. M., Palanisamy, P. N., Kowshalya, V. N., Tamilvanan, A., Prabakaran, R., & Kim, S. C. (2024). Uncalcined Zn/Al Carbonate LDH and Its Calcined Counterpart for Treating the Wastewater Containing Anionic Congo Red Dye. Energies, 17(11), 2698. https://doi.org/10.3390/en17112698







