Energetic, Exergetic, and Techno-Economic Analysis of A Bioenergy with Carbon Capture and Utilization Process via Integrated Torrefaction–CLC–Methanation
Abstract
1. Introduction
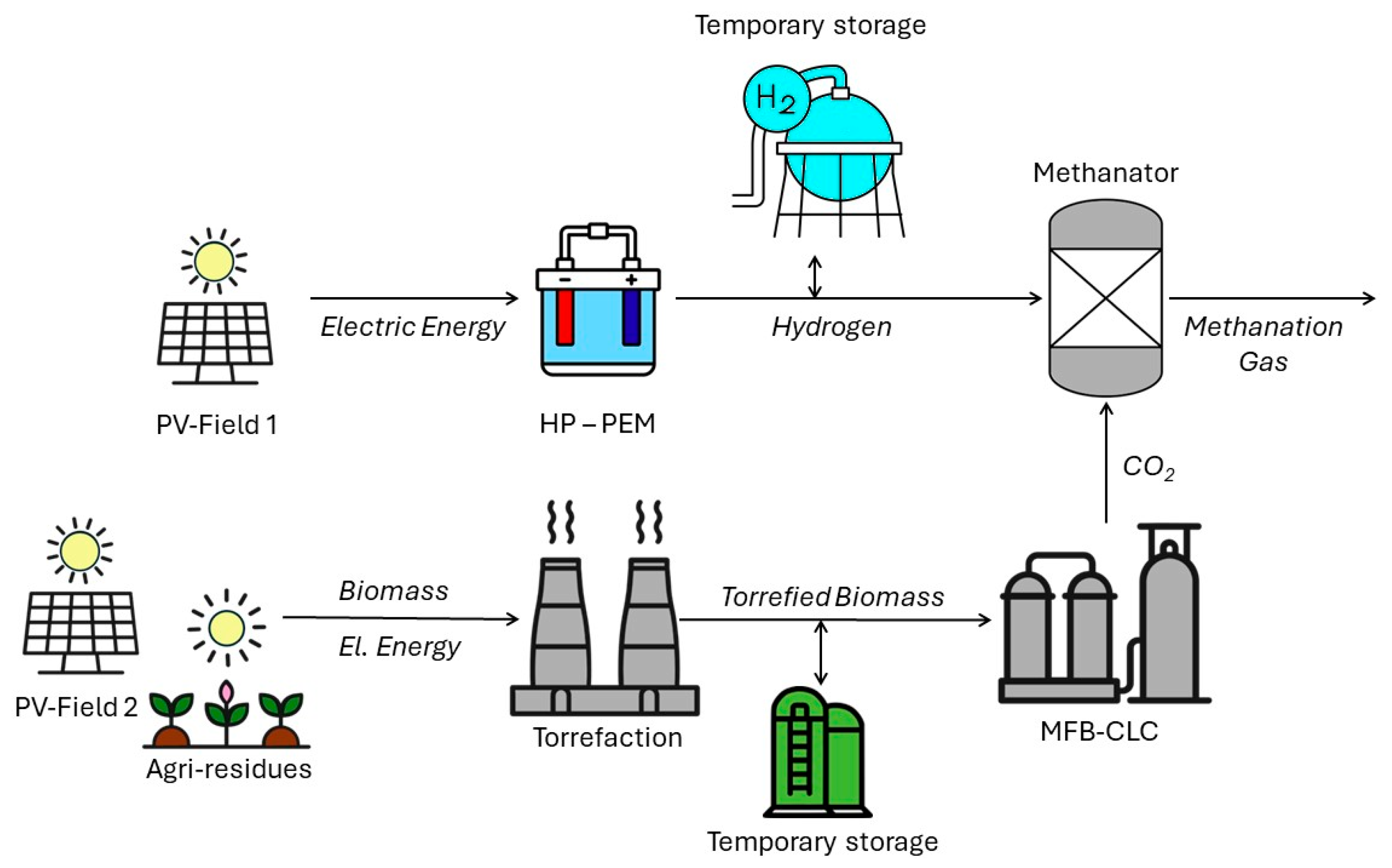
2. Process Description
3. Methodology
3.1. Energetic and Exergetic Analysis
3.2. Techno-Economic Analysis
- For the torrefaction, feeding (made of a front-end loader, hopper, and conveyor), drying, reaction, and storage units, plus the PV field required for the energy supply, and a heat exchanger/condenser to recover a share of the steam sensible/latent heat;
- For the chemical looping, reactors, cyclone, oxygen carrier, heat exchangers, and steam turbine;
- For the methanation, CO2 compressor, reactors, heat exchangers/condensers, and catalyst;
- For the H2 production, PV field, polymer electrolyte membrane (PEM) electrolyzer, and H2 temporary storage vessel.
4. Results
4.1. Energetic and Exergetic Analysis
4.2. Techno-Economic Analysis
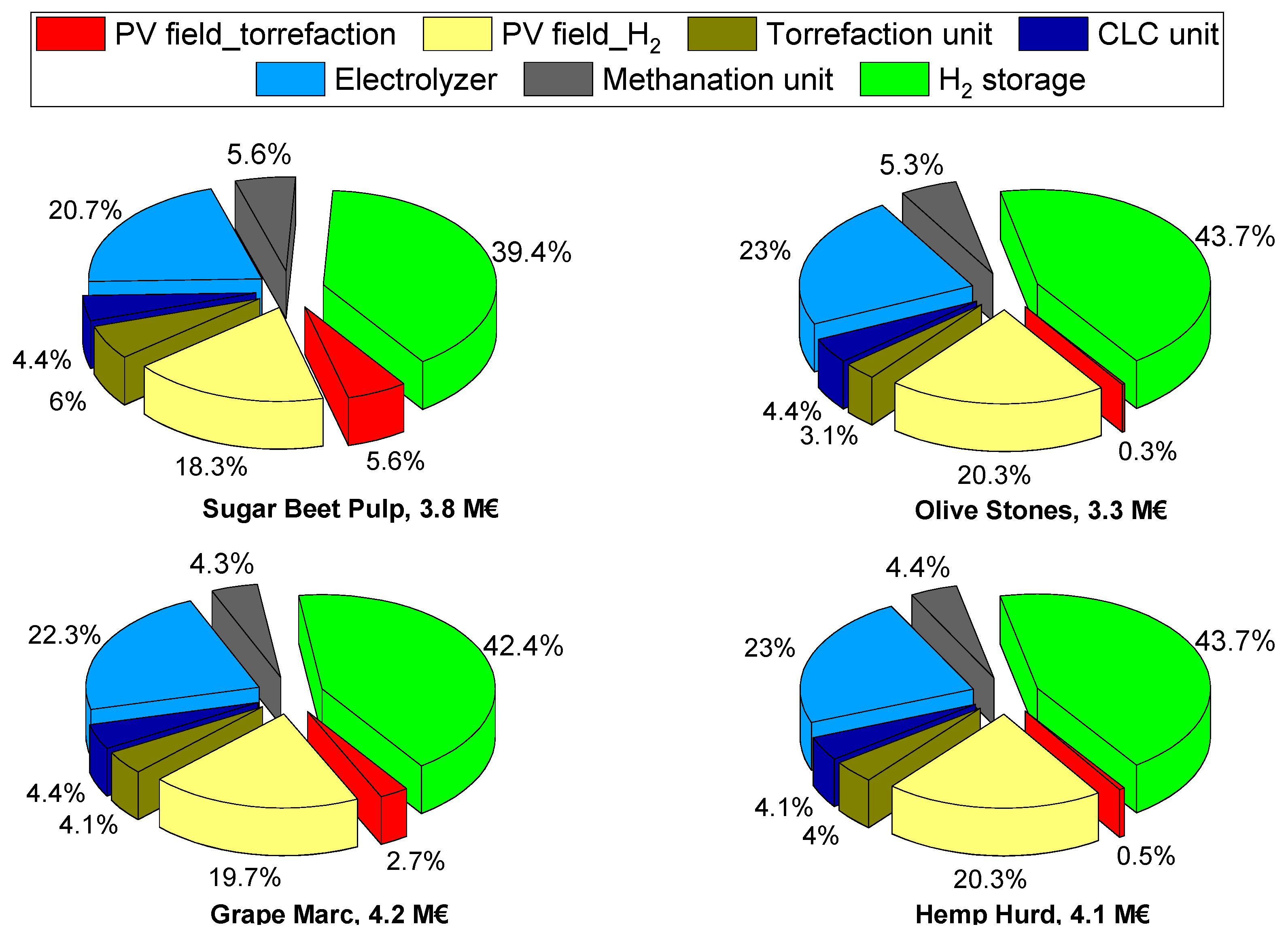
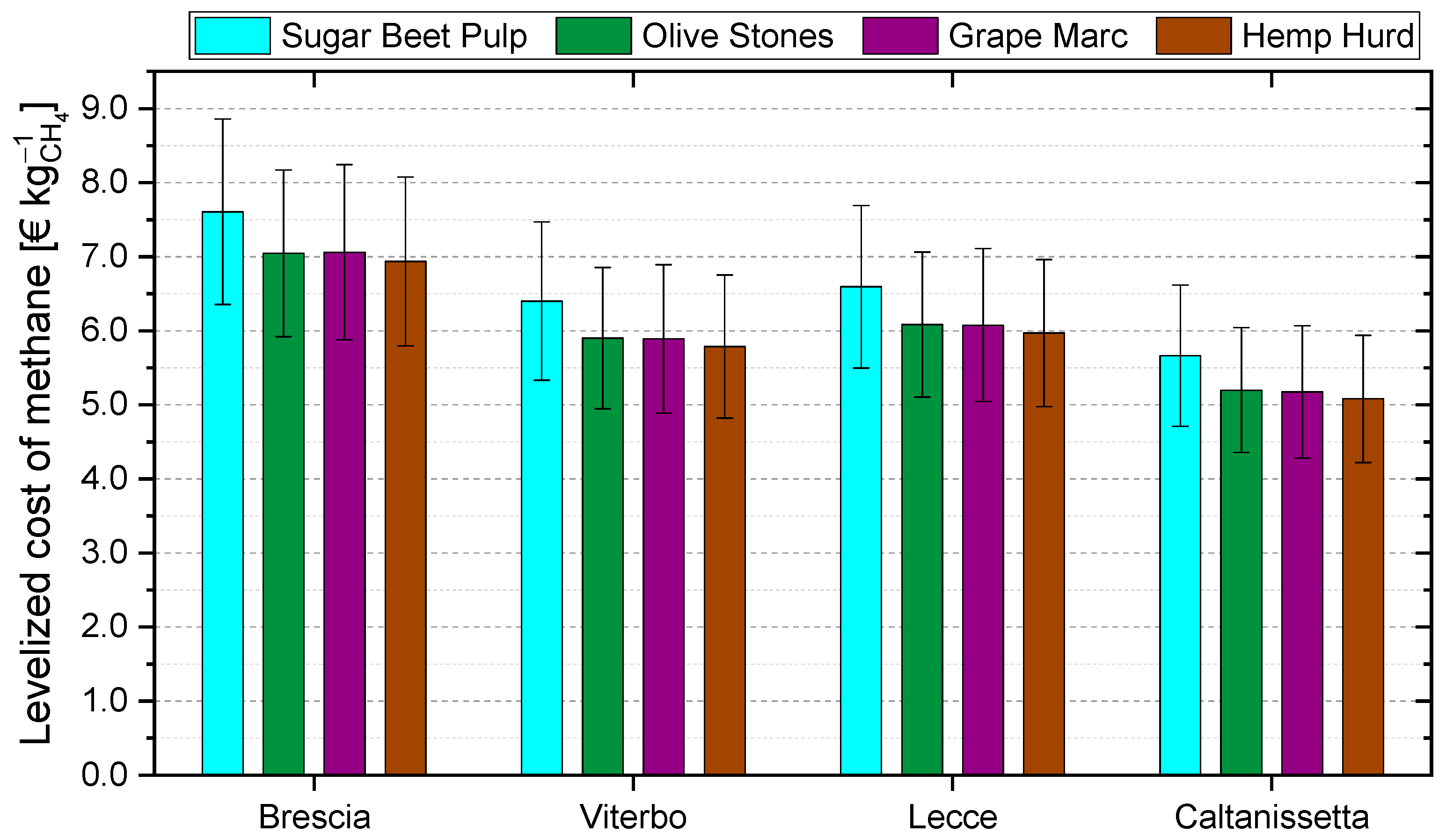
- Regardless of the site location, the use of SBP returns the highest levelized costs of methane. Values reduce by about 0.5–0.6 € kgCH4−1 when considering the other biomasses;
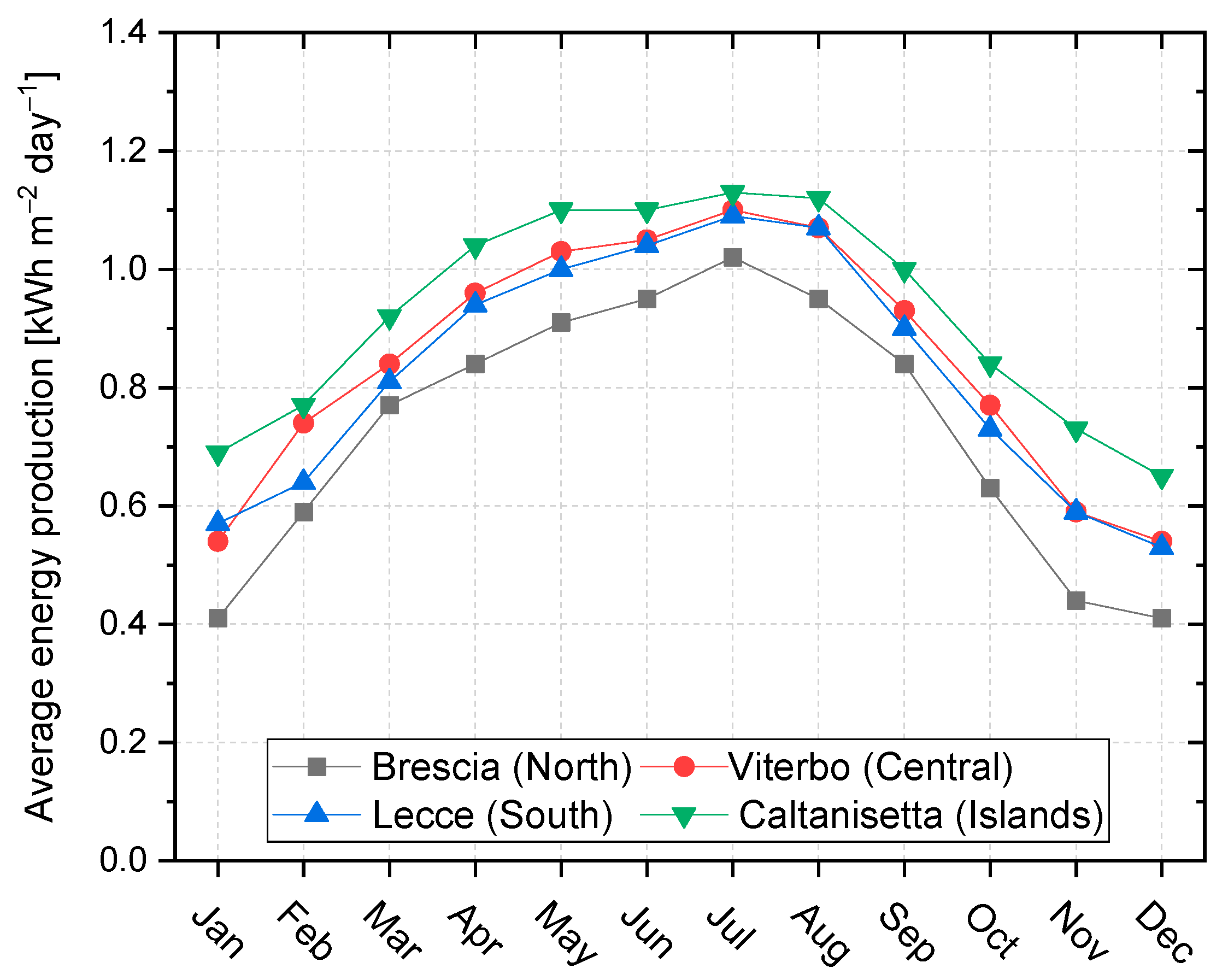
- Following the trend already observed in Figure 6, the levelized cost of methane decreases when the site location shifts towards the south of Italy. It decreases by about 1.0–1.2 € kgCH4−1 when moving from the north to the center and early south of Italy, and by 1.9 € kgCH4−1 when moving to the deep south;
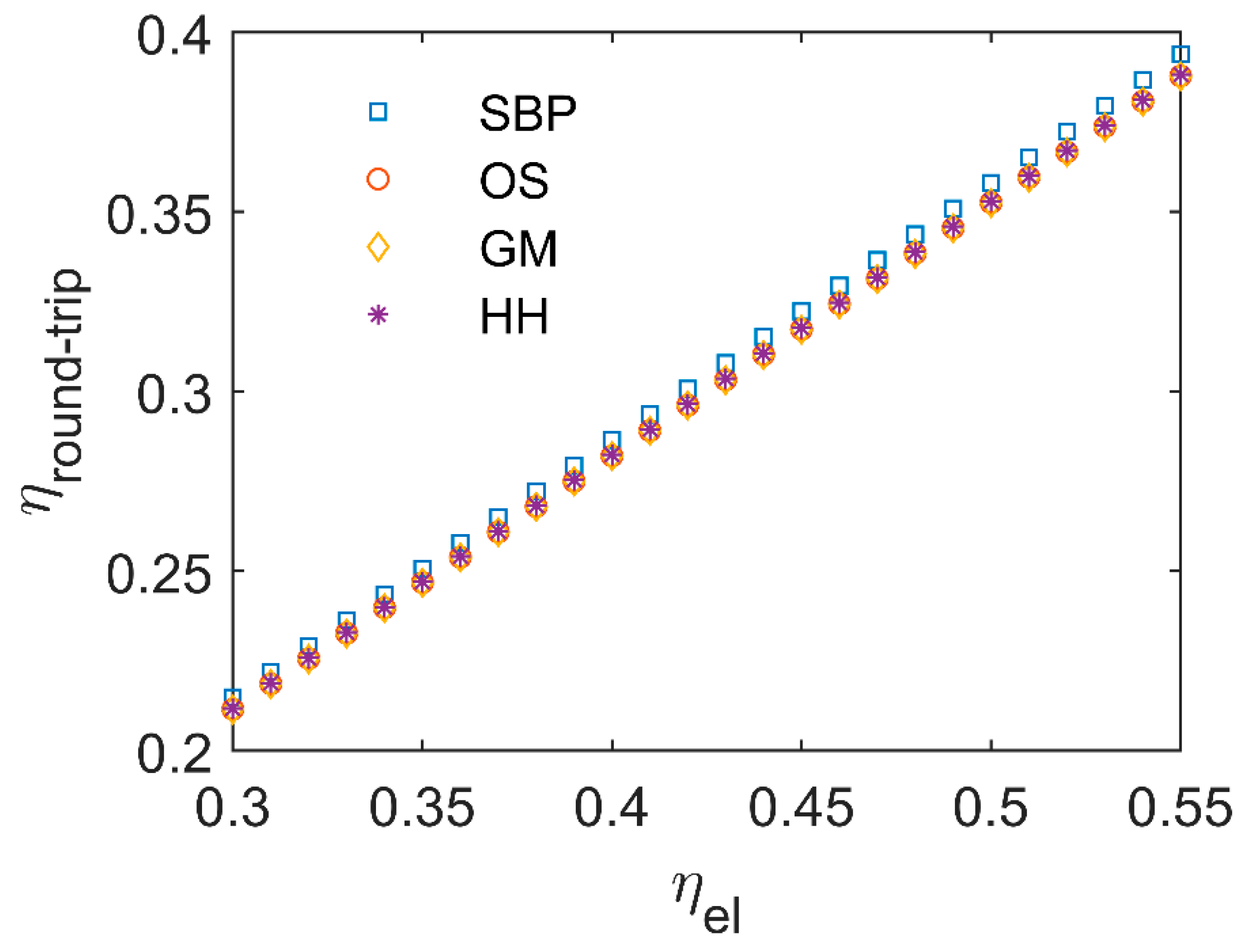
- By varying PIR and PoE values, the levelized cost of methane varies by about 0.9–1.3 € kgCH4−1 depending on the specific case. Lower values are obtained when the PIR is minimum and PoE is maximum, and vice versa;
- In any case, in all the considered scenarios, the levelized cost of methane is out of the market, as it ranges within 4.3–8.9 kgCH4−1. These values mostly originate from the capital expenditures and O&M fixed costs (i.e., first addend of Equation (9)), whereas the O&M variable costs account for about 0.54–0.68 € kgCH4−1, and the revenue determines a saving of about 0.23–0.50 € kgCH4−1 depending on the value of PoE considered.
- Store CO2 and operate the methanation reactors at different throughputs over the year according to the H2 availability;
- Sell a share of the energy produced during the sunniest months and buy it back during the months with less solar irradiation;
- Combine the utilization of different renewable energies, such as solar and wind power;
- Oversize the PV field and sell the extra energy to the market.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| BECCS | bioenergy with carbon capture and storage |
| BECCU | bioenergy with carbon capture and utilization |
| CEPCI | chemical engineering plant cost index |
| CLC | chemical looping combustion |
| GM | grape marc |
| HH | hemp hurd |
| IEA | international energy agency |
| LCOM | levelized cost of methane |
| O&M | operating and maintenance |
| OS | olive stones |
| PEM | polymer electrolyte membrane |
| PV | photovoltaic |
| SBP | sugar beet pulp |
References
- IEA Net Zero Roadmap: A Global Pathway to Keep the 1.5 °C Goal in Reach, IEA, Paris. 2023. Available online: https://www.iea.org/reports/net-zero-roadmap-a-global-pathway-to-keep-the-15-0c-goal-in-reach (accessed on 1 April 2024).
- European Commission; Directorate-General for Research and Innovation; Unit F—Bioeconomy. A Sustainable Bioeconomy for Europe—Strengthening the Connection between Economy, Society and the Environment; Updated Bioeconomy Strategy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- European Environment Agency. Towards EU Climate Neutrality—Progress, Policy Gaps and Opportunities; Assessment Report 2024; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- European Commission. Europe’s 2040 Climate Pathway—A Path to Climate Neutrality by 2050; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- IEA The Role of E-Fuels in Decarbonising Transport, IEA, Paris. 2023. Available online: https://www.iea.org/reports/the-role-of-e-fuels-in-decarbonising-transport (accessed on 1 April 2024).
- Koytsoumpa, E.I.; Magiri-Skouloudi, D.; Karellas, S.; Kakaras, E. Bioenergy with Carbon Capture and Utilization: A Review on the Potential Deployment towards a European Circular Bioeconomy. Renew. Sustain. Energy Rev. 2021, 152, 111641. [Google Scholar] [CrossRef]
- Sterman, J.D.; Siegel, L.; Rooney-Varga, J.N. Does Replacing Coal with Wood Lower CO2 Emissions? Dynamic Lifecycle Analysis of Wood Bioenergy. Environ. Res. Lett. 2018, 13, 15007. [Google Scholar] [CrossRef]
- Troiano, M.; Cammarota, A.; Tregambi, C.; Chirone, R.; Salatino, P.; Solimene, R. Fluidized Bed Combustion of Solid Lignin-Rich Residues from Bioethanol Production. Powder Technol. 2020, 371, 170–179. [Google Scholar] [CrossRef]
- Brachi, P.; Bareschino, P.; Tregambi, C.; Pepe, F.; Urciuolo, M.; Ruoppolo, G.; Mancusi, E. Assessing the Feasibility of an Integrated CLC-Methanation System Using Solar Dried and Torrefied Biomasses as a Feedstock. Fuel 2023, 331, 125951. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Yuan, T.Q.; Xu, F.; Sun, R.C. Understanding the Chemical and Structural Transformations of Lignin Macromolecule during Torrefaction. Appl. Energy 2014, 121, 1–9. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A State-of-the-Art Review of Biomass Torrefaction, Densification and Applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Brachi, P.; Chirone, R.; Miccio, M.; Ruoppolo, G. Fluidized Bed Torrefaction of Commercial Wood Pellets: Process Performance and Solid Product Quality. Energy Fuels 2018, 32, 9459–9469. [Google Scholar] [CrossRef]
- Mutlu, Ö.; Roy, P.; Zeng, T. Downstream Torrefaction of Wood Pellets in a Rotary Kiln Reactor—Impact on Solid Biofuel Properties and Torr-Gas Quality. Processes 2022, 10, 1912. [Google Scholar] [CrossRef]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Integration of Torrefaction and CHP Plant: Operational and Economic Analysis. Appl. Energy 2016, 183, 88–99. [Google Scholar] [CrossRef]
- Adánez, J.; De Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Palacios, J.M. Selection of Oxygen Carriers for Chemical-Looping Combustion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- Forero, C.R.; Gayán, P.; de Diego, L.F.; Abad, A.; García-Labiano, F.; Adánez, J. Syngas Combustion in a 500 WTh Chemical-Looping Combustion System Using an Impregnated Cu-Based Oxygen Carrier. Fuel Process. Technol. 2009, 90, 1471–1479. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B.; Mattisson, T. A Fluidized-Bed Combustion Process with Inherent CO2 Separation; Application of Chemical-Looping Combustion. Chem. Eng. Sci. 2001, 56, 3101–3113. [Google Scholar] [CrossRef]
- Noorman, S.; Van Sint Annaland, M.; Kuipers, H. Packed Bed Reactor Technology for Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2007, 46, 4212–4220. [Google Scholar] [CrossRef]
- Ciancio, A.; Lo Basso, G.; Pastore, L.M.; de Santoli, L. Carbon Abatement Cost Evolution in the Forthcoming Hydrogen Valleys by Following Different Hydrogen Pathways. Int. J. Hydrogen Energy 2024, 64, 80–97. [Google Scholar] [CrossRef]
- Schoeneberger, C.A.; McMillan, C.A.; Kurup, P.; Akar, S.; Margolis, R.; Masanet, E. Solar for Industrial Process Heat: A Review of Technologies, Analysis Approaches, and Potential Applications in the United States. Energy 2020, 206, 118083. [Google Scholar] [CrossRef]
- Tommasi, M.; Degerli, S.N.; Ramis, G.; Rossetti, I. Advancements in CO2 Methanation: A Comprehensive Review of Catalysis, Reactor Design and Process Optimization. Chem. Eng. Res. Des. 2024, 201, 457–482. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on Methanation—From Fundamentals to Current Projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A Technological and Economic Review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Bareschino, P.; Mancusi, E.; Tregambi, C.; Pepe, F.; Urciuolo, M.; Brachi, P.; Ruoppolo, G. Integration of Biomasses Gasification and Renewable-Energies-Driven Water Electrolysis for Methane Production. Energy 2021, 230, 120863. [Google Scholar] [CrossRef]
- Hervy, M.; Maistrello, J.; Brito, L.; Rizand, M.; Basset, E.; Kara, Y.; Maheut, M. Power-to-Gas: CO2 Methanation in a Catalytic Fluidized Bed Reactor at Demonstration Scale, Experimental Results and Simulation. J. CO2 Util. 2021, 50, 101610. [Google Scholar] [CrossRef]
- Bareschino, P.; Piso, G.; Pepe, F.; Tregambi, C.; Mancusi, E. Numerical Modelling of a Sorption-Enhanced Methanation System. Chem. Eng. Sci. 2023, 277, 118876. [Google Scholar] [CrossRef]
- Kreitz, B.; Wehinger, G.D.; Turek, T. Dynamic Simulation of the CO2 Methanation in a Micro-Structured Fixed-Bed Reactor. Chem. Eng. Sci. 2019, 195, 541–552. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Parunin, P.D.; Okunev, A.G. Catalytic Process for Methane Production from Atmospheric Carbon Dioxide Utilizing Renewable Energy. Catal. Today 2017, 298, 117–123. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Lysikov, A.I.; Netskina, O.V.; Kuleshov, D.V.; Okunev, A.G. K2CO3-Containing Composite Sorbents Based on Thermally Modified Alumina: Synthesis, Properties, and Potential Application in a Direct Air Capture/Methanation Process. Ind. Eng. Chem. Res. 2020, 59, 7130–7139. [Google Scholar] [CrossRef]
- Tregambi, C.; Bareschino, P.; Hanak, D.; Montagnaro, F.; Pepe, F.; Mancusi, E. Modelling of an Integrated Process for Atmospheric Carbon Dioxide Capture and Methanation. J. Clean. Prod. 2022, 356, 131827. [Google Scholar] [CrossRef]
- Tregambi, C.; Bareschino, P.; Hanak, D.P.; Mancusi, E.; Montagnaro, F.; Pepe, F. Techno-Economic Assessment of a Synthetic Methane Production Process by Hydrogenation of Carbon Dioxide from Direct Air Capture. Int. J. Hydrogen Energy 2023, 48, 37594–37606. [Google Scholar] [CrossRef]
- Peters, R.; Baltruweit, M.; Grube, T.; Samsun, R.C.; Stolten, D. A Techno Economic Analysis of the Power to Gas Route. J. CO2 Util. 2019, 34, 616–634. [Google Scholar] [CrossRef]
- Harada, H.; Sinha, A.; Yajima, T.; Kawajiri, Y. Model-Based Techno–Economic Analysis of an Integrated Synthetic Natural Gas Production System with Direct Air Capture and Water Electrolysis. Carbon Capture Sci. Technol. 2024, 10, 100181. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; He, S.; Gao, L. Coal to Substitute Natural Gas Based on Combined Coal-Steam Gasification and One-Step Methanation. Appl. Energy 2019, 240, 851–859. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.-L.; Zhao, X.-Y.; Cao, J.-P. Methanation of Syngas from Biomass Gasification: An Overview. Int. J. Hydrogen Energy 2020, 45, 4223–4243. [Google Scholar] [CrossRef]
- Gómez, L.; Grasa, G.; Martínez, I.; Murillo, R. Performance Study of a Methanation Process for a Syngas Obtained from a Sorption Enhanced Gasification Process. Chem. Eng. Sci. 2023, 267, 118291. [Google Scholar] [CrossRef]
- Xing, W.; Liu, Y.; Zhang, W.; Sun, Y.; Kai, X.; Yang, T. Study on Methanation Performance of Biomass Gasification Syngas Based on a Ni/Al2O3 Monolithic Catalyst. ACS Omega 2020, 5, 28597–28605. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Chan, W.P.; Veksha, A.; Giannis, A.; Dou, X.; Wang, H.; Lisak, G.; Lim, T.-T. Thermodynamic Analyses of Synthetic Natural Gas Production via Municipal Solid Waste Gasification, High-Temperature Water Electrolysis and Methanation. Energy Convers. Manag. 2019, 202, 112160. [Google Scholar] [CrossRef]
- Duyar, M.S.; Treviño, M.A.A.; Farrauto, R.J. Dual Function Materials for CO2 Capture and Conversion Using Renewable H2. Appl. Catal. B Environ. 2015, 168–169, 370–376. [Google Scholar] [CrossRef]
- Tregambi, C.; Mancusi, E.; Solimene, R.; Pepe, F. Modeling of an Autothermal Process for Integrated Carbon Dioxide Capture and Methanation by Magnesium Looping (MgO/MgCO3) and Renewable Hydrogen. Ind. Eng. Chem. Res. 2023, 62, 22016–22027. [Google Scholar] [CrossRef]
- Omodolor, I.S.; Otor, H.O.; Andonegui, J.A.; Allen, B.J.; Alba-Rubio, A.C. Dual-Function Materials for CO2 Capture and Conversion: A Review. Ind. Eng. Chem. Res. 2020, 59, 17612–17631. [Google Scholar] [CrossRef]
- Melo Bravo, P.; Debecker, D.P. Combining CO2 Capture and Catalytic Conversion to Methane. Waste Dispos. Sustain. Energy 2019, 1, 53–65. [Google Scholar] [CrossRef]
- Fan, J.; Hong, H.; Jin, H. Biomass and Coal Co-Feed Power and SNG Polygeneration with Chemical Looping Combustion to Reduce Carbon Footprint for Sustainable Energy Development: Process Simulation and Thermodynamic Assessment. Renew. Energy 2018, 125, 260–269. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas–Biomass Oxycombustion Hybrid System: Energy Integration and Potential Applications. Appl. Energy 2016, 167, 221–229. [Google Scholar] [CrossRef]
- Kermani, A.A.; Houshfar, E. Design and Energy, Exergy, and Exergoeconomic Analyses of a Novel Biomass-Based Green Hydrogen and Power Generation System Integrated with Carbon Capture and Power-to-Gas. Int. J. Hydrogen Energy 2024, 52, 177–189. [Google Scholar] [CrossRef]
- Fleiß, B.; Bartik, A.; Priscak, J.; Benedikt, F.; Fuchs, J.; Müller, S.; Hofbauer, H. Experimental Demonstration of 80 KWTh Chemical Looping Combustion of Biogenic Feedstock Coupled with Direct CO2 Utilization by Exhaust Gas Methanation. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Escamilla, A.; Sánchez, D.; García-Rodríguez, L. Assessment of Power-to-Power Renewable Energy Storage Based on the Smart Integration of Hydrogen and Micro Gas Turbine Technologies. Int. J. Hydrogen Energy 2022, 47, 17505–17525. [Google Scholar] [CrossRef]
- Kotas, T.J. The Exergy Method of Thermal Plant Analysis; Paragon Publishing: Trowbridge, UK, 2012. [Google Scholar]
- Uebbing, J.; Rihko-Struckmann, L.K.; Sundmacher, K. Exergetic Assessment of CO2 Methanation Processes for the Chemical Storage of Renewable Energies. Appl. Energy 2019, 233–234, 271–282. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Standard Reference Database Number 69. In NIST Chemistry WebBook; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2023. [Google Scholar] [CrossRef]
- Syu, F.-S.; Chiueh, P.-T. Process Simulation of Rice Straw Torrefaction. Sustain. Environ. Res. 2012, 22, 177–183. [Google Scholar]
- Arteaga-Pérez, L.E.; Segura, C.; Espinoza, D.; Radovic, L.R.; Jiménez, R. Torrefaction of Pinus Radiata and Eucalyptus Globulus: A Combined Experimental and Modeling Approach to Process Synthesis. Energy Sustain. Dev. 2015, 29, 13–23. [Google Scholar] [CrossRef]
- Haseli, Y. Simplified Model of Torrefaction-Grinding Process Integrated with a Power Plant. Fuel Process. Technol. 2019, 188, 118–128. [Google Scholar] [CrossRef]
- Manouchehrinejad, M.; Mani, S. Process Simulation of an Integrated Biomass Torrefaction and Pelletization (IBTP) Plant to Produce Solid Biofuels. Energy Convers. Manag. X 2019, 1, 100008. [Google Scholar] [CrossRef]
- Manzolini, G.; MacChi, E.; Gazzani, M. CO2 Capture in Integrated Gasification Combined Cycle with SEWGS—Part B: Economic Assessment. Fuel 2013, 105, 220–227. [Google Scholar] [CrossRef]
- Grimm, A.; de Jong, W.A.; Kramer, G.J. Renewable Hydrogen Production: A Techno-Economic Comparison of Photoelectrochemical Cells and Photovoltaic-Electrolysis. Int. J. Hydrogen Energy 2020, 45, 22545–22555. [Google Scholar] [CrossRef]
- Doddapaneni, T.R.K.C.; Praveenkumar, R.; Tolvanen, H.; Rintala, J.; Konttinen, J. Techno-Economic Evaluation of Integrating Torrefaction with Anaerobic Digestion. Appl. Energy 2018, 213, 272–284. [Google Scholar] [CrossRef]
- Zhao, C.; Huang, J.; Xie, D.; Qiao, Y.; Xu, M. Thermodynamic and Techno-Economic Analysis of Hydrogen Production from Food Waste by Torrefaction Integrated with Steam Gasification. Energy Convers. Manag. 2024, 299, 117826. [Google Scholar] [CrossRef]
- Erlach, B. Biomass Upgrading Technologies for Carbon-Neutral and Carbon-Negative Electricity Generation: Techno-Economic Analysis of Hydrothermal Carbonization and Comparison with Wood Pelletizing, Torrefaction and Anaerobic Digestion. Ph.D. Thesis, Technische Universität, Berlin, Germany, 2014. [Google Scholar]
- Sayyaadi, H.; Mehrabipour, R. Efficiency Enhancement of a Gas Turbine Cycle Using an Optimized Tubular Recuperative Heat Exchanger. Energy 2012, 38, 362–375. [Google Scholar] [CrossRef]
- Hanak, D.P.; Jenkins, B.G.; Kruger, T.; Manovic, V. High-Efficiency Negative-Carbon Emission Power Generation from Integrated Solid-Oxide Fuel Cell and Calciner. Appl. Energy 2017, 205, 1189–1201. [Google Scholar] [CrossRef]
- Zhu, L.; He, Y.; Li, L.; Wu, P. Tech-Economic Assessment of Second-Generation CCS: Chemical Looping Combustion. Energy 2018, 144, 915–927. [Google Scholar] [CrossRef]
- Aminyavari, M.; Mamaghani, A.H.; Shirazi, A.; Najafi, B.; Rinaldi, F. Exergetic, Economic, and Environmental Evaluations and Multi-Objective Optimization of an Internal-Reforming SOFC-Gas Turbine Cycle Coupled with a Rankine Cycle. Appl. Therm. Eng. 2016, 108, 833–846. [Google Scholar] [CrossRef]
- Kreutz, T.; Williams, R.; Consonni, S.; Chiesa, P. Co-Production of Hydrogen, Electricity and CO2 from Coal with Commercially Ready Technology. Part B: Economic Analysis. Int. J. Hydrogen Energy 2005, 30, 769–784. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Mulder, M. Power-to-Gas in Electricity Markets Dominated by Renewables. Appl. Energy 2018, 232, 258–272. [Google Scholar] [CrossRef]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A Comparative Technoeconomic Analysis of Renewable Hydrogen Production Using Solar Energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef]
- Gorre, J.; Ruoss, F.; Karjunen, H.; Schaffert, J.; Tynjälä, T. Cost Benefits of Optimizing Hydrogen Storage and Methanation Capacities for Power-to-Gas Plants in Dynamic Operation. Appl. Energy 2020, 257, 113967. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Zauner, A. Report on the Costs Involved with PtG Technologies and Their Potentials across the EU; EU Horizon 2020 Project STORE&GO; European Union: Brussels, Belgium, 2018. [Google Scholar]
- Huld, T.; Müller, R.; Gambardella, A. A New Solar Radiation Database for Estimating PV Performance in Europe and Africa. Sol. Energy 2012, 86, 1803–1815. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/jrc/en/pvgis/downloads/sarah (accessed on 1 April 2024).
- Tregambi, C.; Bareschino, P.; Mancusi, E.; Pepe, F.; Montagnaro, F.; Solimene, R.; Salatino, P. Modelling of a Concentrated Solar Power—Photovoltaics Hybrid Plant for Carbon Dioxide Capture and Utilization via Calcium Looping and Methanation. Energy Convers. Manag. 2021, 230, 113792. [Google Scholar] [CrossRef]



| Component (Scaling Parameter) | Correlation | Ref. | Year | |
|---|---|---|---|---|
| PV | Photovoltaic field, installed (overall peak power, PPV [kW]) | [56] | – | |
| TORREFACTION | Feeding unit (biomass feed rate, FB [t h−1]) | [57] | 2018 | |
| Drying unit (biomass feed rate, FB [t h−1]) | [58] | 2002 | ||
| Reactor (dry biomass feed rate, FDB [t h−1]) | [58] | 2020 | ||
| Storage (torrefied biomass mass, MTBM [t]) | [59] | 2014 | ||
| Condenser (heat transfer surface, AC [m2]) | [60,61] | 2012 | ||
| CHEMICAL LOOPING | Reactors and oxygen carrier (input power, PCLC, input [MW]) | [62] | 2018 | |
| Cyclones (input power, PCLC, input [MW]) | [62] | 2018 | ||
| High-temperature heat exchangers (input power, PCLC, input [MW]) | [62] | 2018 | ||
| Oxygen carrier (input power, PCLC, input [MW]) | [62] | 2018 | ||
| Steam turbine (output power, PST [kW]) | [61,63] | 2016 | ||
| METHANATION | CO2 dryer and compressor, installed (compressor power, PC [kW]) | [64] | 2002 | |
| Packed bed reactors | see detailed methodology in ref. | [31] | – | |
| Catalyst | see detailed methodology in ref. | [31] | – | |
| Mid-temperature heat exchangers (heat transfer surface, AHX [m2]) | [61,63] | 2012 | ||
| Condenser (heat transfer surface, AC [m2]) | [60] | 2012 | ||
| H2 | Electrolyzer (peak power from PV field, PE [kW]) | [65,66] | – | |
| [Nm3]) | [67,68] | – |
| Symbol | Description | Value | Dimension |
|---|---|---|---|
| CF | Capacity factor of the integrated plant | 0.85 | – |
| ltcat | Catalyst lifetime | 5 | y |
| PC | CO2 compressor power | 1.2–1.6 | kW |
| ltEC | Electrolyzer lifetime | 10 | y |
| PoE | Energy price | 0.10; 0.12 *; 0.14 | € kWh−1 |
| FOM | O&M fixed costs | 1 | % |
| UHXs | Heat transfer coefficient in heat exchangers | 350 | W m−2 K−1 |
| PCLC,input | Input power of CLC (based on HHV of torrefied biomasses) | 62.4–81.5 | kW |
| γ | Integrated plant lifetime | 25 | y |
| ΔTHXs | Mean temperature difference for heat exchangers | 25 | °C |
| ltOC | Oxygen carrier lifetime | 5 | y |
| PPV | Peak power of photovoltaic field | 12–370 (torrefaction) 919–1463 (H2 prod.) | kWp |
| PST | Power output of steam turbine | 21.3–32.5 kW | kWe |
| PM | Power recovered from methanation | 25.1–30.6 | kWth |
| PTD | Power recovered from steam cooling and condensation after biomass torrefaction | 1.5–47.0 | kWth |
| PIR | Project interest rate | 4.75; 6.75 *; 8.75 | % y−1 |
| ηPV | PV modules efficiency | 0.20 | − |
| ηF | System losses of overall PV field | 0.14 | − |
| ηel | Thermal-to-electric conversion efficiency | 0.40 | − |
| WCH4,y | Yearly produced methane | 55.4–69.8 | t y−1 |
| Process Unit | Flow Direction | Energy Flow [kW] | Exergy Flow [kW] | Flow Composition | Mass Flow [kg h−1] |
|---|---|---|---|---|---|
| Torrefaction | In | 178.9 | 185.5 | Wet Biomass | 93.4 |
| Out (with; w/o torgas) | 167.4; 110.5 | 141.1; 83.6 | Torrefied Biomass | 10.0 | |
| Water (L) | 68.3 | ||||
| Torgas | 14.0 | ||||
| CLC | In | 63.6 | 65.5 | Torrefied Biomass | 10.0 |
| Out | 63.4 | 41.3 | Water (L) | 2.5 | |
| CO2 | 21.5 | ||||
| Electrolysis | In | 182.6 | 182.6 | Water (L) | 35.2 |
| Out | 155.3 | 133.6 | O2 | 31.3 | |
| H2 | 3.9 | ||||
| Methanation | In | 156.5 | 134.8 | CO2 | 21.5 |
| H2 | 3.9 | ||||
| Out | 149.3 | 130.7 | Methanation gas | 8.5 | |
| Water (L) | 17.4 |
| Efficiency | SBP | OS | GM | HH |
|---|---|---|---|---|
| Torrefaction w/o torgas | 45% | 68% | 57% | 34% |
| Torrefaction with torgas | 76% | 95% | 86% | 94% |
| CLC | 63% | 61% | 67% | 65% |
| Electrolysis | 73% | 73% | 73% | 73% |
| Methanation | 97% | 96% | 95% | 96% |
| Global w/o torgas | 51% | 60% | 57% | 45% |
| Global with torgas | 67% | 70% | 69% | 77% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutillo, E.A.; Tregambi, C.; Bareschino, P.; Mancusi, E.; Continillo, G.; Pepe, F. Energetic, Exergetic, and Techno-Economic Analysis of A Bioenergy with Carbon Capture and Utilization Process via Integrated Torrefaction–CLC–Methanation. Energies 2024, 17, 2690. https://doi.org/10.3390/en17112690
Cutillo EA, Tregambi C, Bareschino P, Mancusi E, Continillo G, Pepe F. Energetic, Exergetic, and Techno-Economic Analysis of A Bioenergy with Carbon Capture and Utilization Process via Integrated Torrefaction–CLC–Methanation. Energies. 2024; 17(11):2690. https://doi.org/10.3390/en17112690
Chicago/Turabian StyleCutillo, Enrico Alberto, Claudio Tregambi, Piero Bareschino, Erasmo Mancusi, Gaetano Continillo, and Francesco Pepe. 2024. "Energetic, Exergetic, and Techno-Economic Analysis of A Bioenergy with Carbon Capture and Utilization Process via Integrated Torrefaction–CLC–Methanation" Energies 17, no. 11: 2690. https://doi.org/10.3390/en17112690
APA StyleCutillo, E. A., Tregambi, C., Bareschino, P., Mancusi, E., Continillo, G., & Pepe, F. (2024). Energetic, Exergetic, and Techno-Economic Analysis of A Bioenergy with Carbon Capture and Utilization Process via Integrated Torrefaction–CLC–Methanation. Energies, 17(11), 2690. https://doi.org/10.3390/en17112690









