Research Progress of Cs-Based All-Inorganic Perovskite Solar Cells
Abstract
1. Introduction
2. Structure and Working Principle of ISPCs
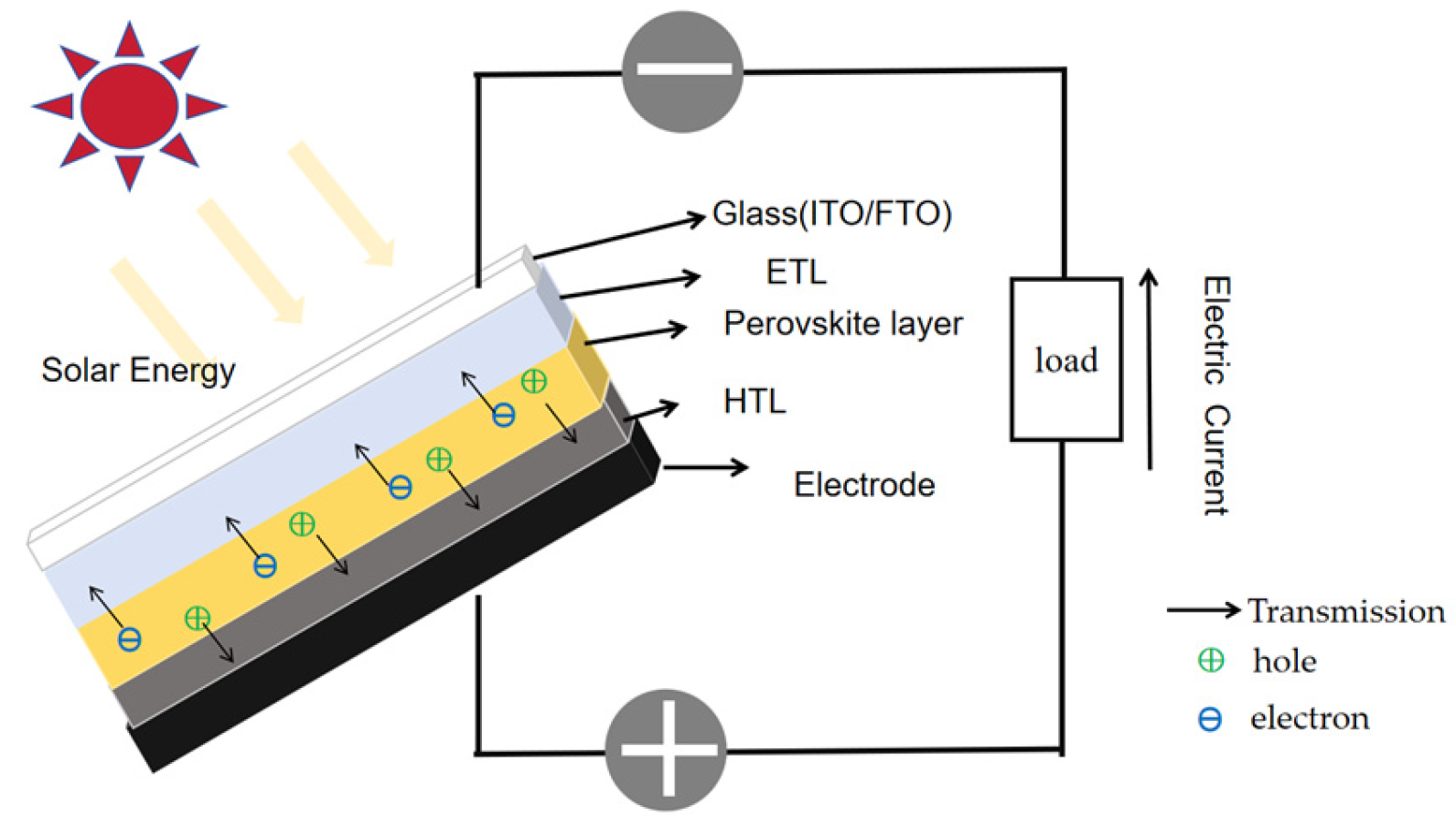
Structure of ISPCs
3. Preparation Method of Perovskite Thin Films
3.1. One-Step Method
3.2. Two-Step Method
3.3. Other Methods
4. Hole Transport Layer and Electrode Material
4.1. Hole Transport Layer
4.2. Electrode Material
5. Optimization of the Photovoltaic Performance of IPSCs
5.1. Doping Ions
5.1.1. Doping of A-Site Ions
5.1.2. Doping of B-Site Ions
5.2. Interface Modification
5.2.1. Weakening Charge Recombination
5.2.2. Optimize the Light Absorption Capacity and Charge Transfer Capability of Cells
6. Summary and Prospect
- (1)
- To address the issues of the organic hole transport layer being prone to moisture and the expensive and ion migration-prone nature of metal electrode materials, it can be resolved by using inorganic hole transport layers such as two-dimensional MeXene materials, PCBM, or researching carbon-based IPSCs without hole transport layers. This optimizes the energy level matching arrangement to enhance the charge transfer capability between the perovskite layer and the inorganic hole transport layer/carbon electrode, thereby improving the stability and optoelectronic performance of the device.
- (2)
- In response to the problem of CsPbI3 easily transforming from the α phase to the δ phase, by doping with small-radius elements and reducing the defect state density by passivating interface defects, the phase stability of CsPbI3 can be improved. In addition, increasing research on lead-free IPSCs is of great significance for environmental sustainability, and researching new lead-free perovskite solar cells is a necessary trend for future development.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Han, Q.; Bae, S.H.; Sun, P.; Hsieh, Y.T.; Yang, Y.M.; Rim, Y.S.; Zhao, H.; Chen, Q.; Shi, W.; Li, G.; et al. Single Crystal Formamidinium Lead Iodide (FAPbI3): Insight into the Structural, Optical, and Electrical Properties. Adv. Mater 2016, 28, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. All Inorganic Perovskite Solar Cells and Absorption Layer Band Gap Regulation. Master’s Thesis, Henan University, Zhengzhou, China, 2019. [Google Scholar]
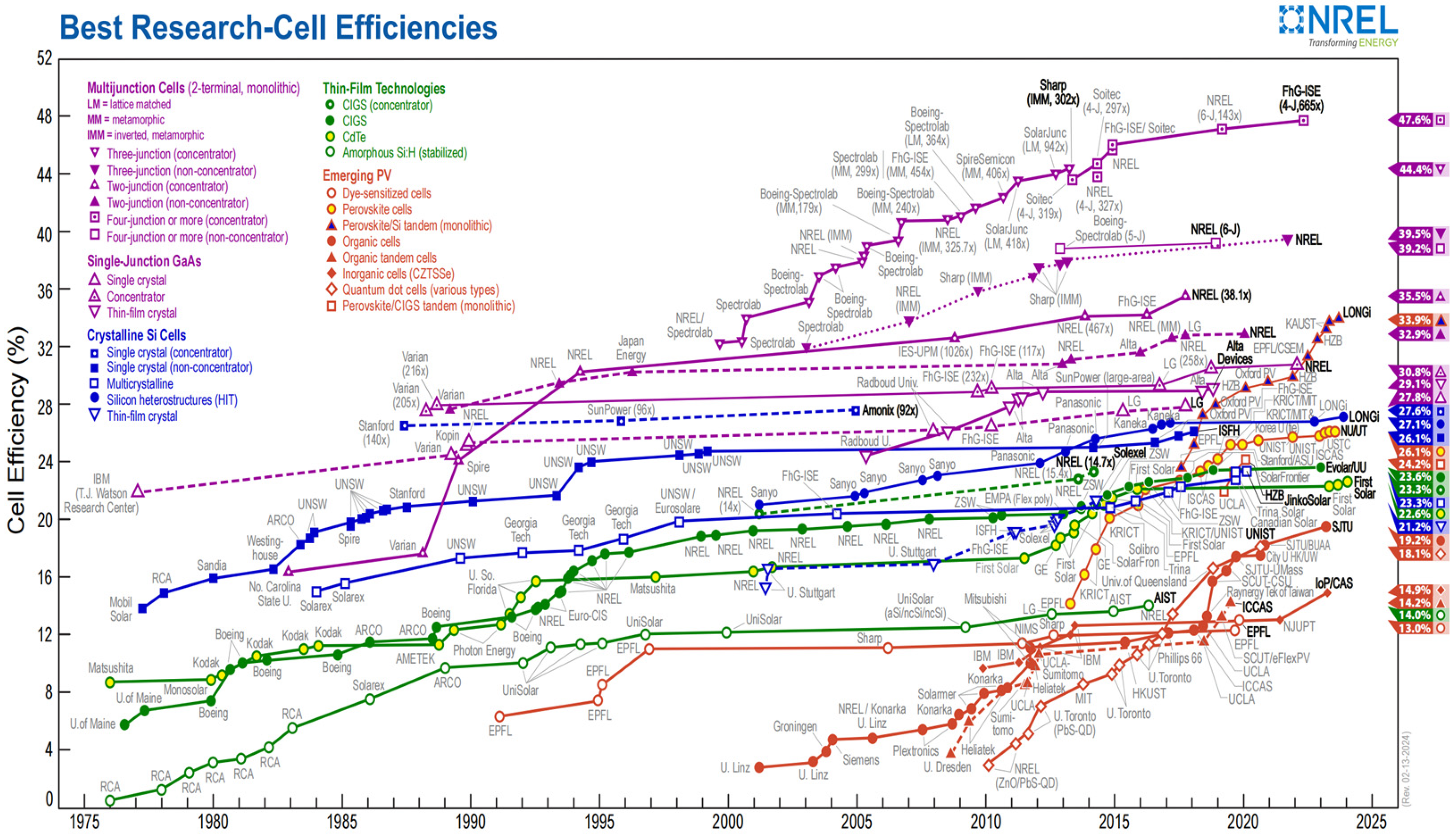
- National Renewable Energy Laboratory. Best Research-Cell Efficiency Chart; National Renewable Energy Laboratory: Golden, CO, USA, 2024. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 23 May 2024).
- Liang, Z.; Zhang, Y.; Xu, H.; Chen, W.; Liu, B.; Zhang, J.; Zhang, H.; Wang, Z.; Kang, D.-H.; Zeng, J.; et al. Homogenizing out-of-plane cation composition in perovskite solar cells. Nature 2023, 624, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant, J.R.; Haque, S.A. Light and oxygen induced degradation limits the operational stability of methylammonium lead triiodide perovskite solar cells. Energy Environ. Sci. 2016, 9, 1655–1660. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, W.-J.; Chiu, C.-C.; Juwita, R.; Tsai, H.-H.G. First-Principles Investigation of the Thermal Degradation Mechanisms of Methylammonium Lead Triiodide Perovskite. J. Phys. Chem. C 2020, 124, 14521–14530. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef]
- Akbulatov, A.F.; Luchkin, S.Y.; Frolova, L.A.; Dremova, N.N.; Gerasimov, K.L.; Zhidkov, I.S.; Anokhin, D.V.; Kurmaev, E.Z.; Stevenson, K.J.; Troshin, P.A. Probing the Intrinsic Thermal and Photochemical Stability of Hybrid and Inorganic Lead Halide Perovskites. J. Phys. Chem. Lett. 2017, 8, 1211–1218. [Google Scholar] [CrossRef]
- Imran, M.; Saleem, A.; Khan, N.A.; Kamboh, A.H. Enhanced efficiency and stability of perovskite solar cells by partial replacement of CH3NH3+ with inorganic Cs+ in CH3NH3PbI3 perovskite absorber layer. Phys. B Condens. Matter 2019, 572, 1–11. [Google Scholar] [CrossRef]
- Hsiao, Y.-W.; Wang, S.-Y.; Huang, C.-L.; Leu, C.-C.; Shih, C.-F. Resistive Switching Property of Organic–Inorganic Tri-Cation Lead Iodide Perovskite Memory Device. Nanomaterials 2020, 10, 1155. [Google Scholar] [CrossRef]
- Kulbak, M.; Cahen, D.; Hodes, G. How Important Is the Organic Part of Lead Halide Perovskite Photovoltaic Cells? Efficient CsPbBr3 Cells. J. Phys. Chem. Lett. 2015, 6, 2452–2456. [Google Scholar] [CrossRef] [PubMed]
- Kulbak, M.; Gupta, S.; Kedem, N.; Levine, I.; Bendikov, T.; Hodes, G.; Cahen, D. Cesium Enhances Long-Term Stability of Lead Bromide Perovskite-Based Solar Cells. J. Phys. Chem. Lett. 2015, 7, 167–172. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, G.; Jing, Y.; Zhang, H.; Wang, X.; Lu, Y.; Wu, J.; Lan, Z. n-type absorber by Cd2+ doping achieves high-performance carbon-based CsPbIBr2 perovskite solar cells. J. Colloid Interface Sci. 2022, 608, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, G.; Li, R.; Jing, Y.; Zhang, H.; Wang, X.; Du, Z.; Wu, J.; Lan, Z. PbS/CdS heterojunction thin layer affords high-performance carbon-based all-inorganic solar cells. Nano Energy 2022, 95, 106973. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, F.; Li, R.; Jing, Y.; Chen, Q.; Chen, X.; Deng, C.; Du, Z.; Sun, W.; Wu, J.; et al. Mxene regulates the stress of perovskite and improves interface contact for high-efficiency carbon-based all-inorganic solar cells. Chem. Eng. J. 2023, 461, 141895. [Google Scholar] [CrossRef]
- Gao, L.; Liu, F.; Li, R.; Yao, Q.; Jiang, D.; Zhong, C.; Lin, W.; Huang, M.; Wu, J.; Lan, Z. Multifunctional surface modification of carbon-based all-inorganic CsPbI2Br solar cells by 3-(trifluoromethyl) phenyltrimethyl ammonium iodide. J. Alloy. Compd. 2024, 989, 174374. [Google Scholar] [CrossRef]
- Chen, B.; Wang, P.; Ren, N.; Li, R.; Zhao, Y.; Zhang, X. Tin dioxide buffer layer-assisted efficiency and stability of wide-bandgap inverted perovskite solar cells. J. Semicond. 2022, 43, 052201. [Google Scholar] [CrossRef]
- Yue, M. Investigation on Light Management In Perovskite Solar Cells and Process Conditions of Inverted Cspbi3 Solar Cells. Master’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2022. [Google Scholar]
- Doherty, T.; Winchester, A.J.; Macpherson, S.; Johnstone, D.; Pareek, V.; Tennyson, B.; Kosar, S.; Kosasih, F.; Anaya, M.; Abdi-Jalebi, M.; et al. Performance-limiting nanoscale trap clusters at grain junctions in halide perovskites. Nature 2020, 580, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Hu, S.; Tang, J.; Xiao, Z. Material exploration via designing spatial arrangement of octahedral units: A case study of lead halide perovskites. Front. Optoelectron. 2021, 14, 252–259. [Google Scholar] [CrossRef]
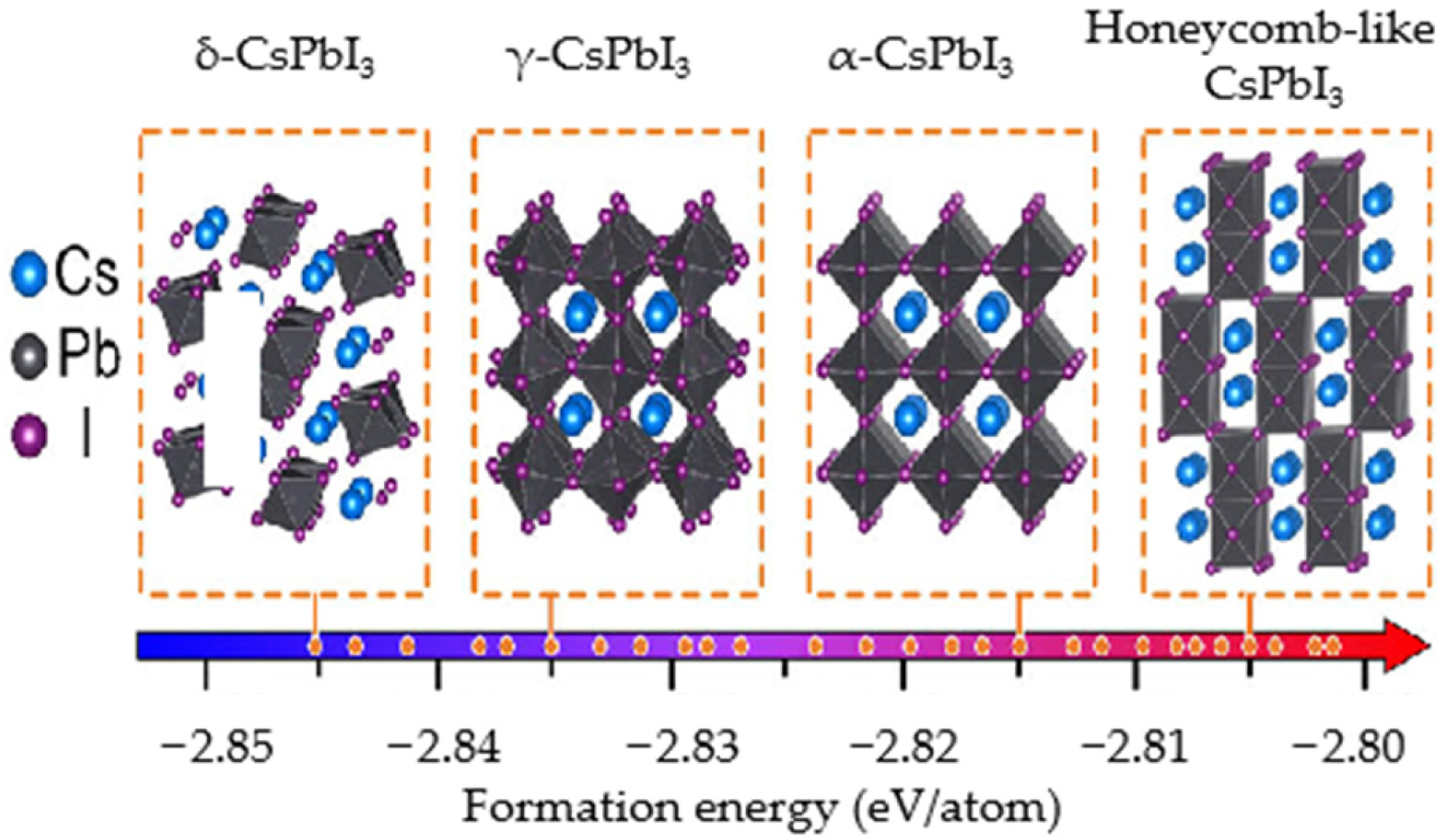
- Steele, J.A.; Jin, H.; Dovgaliuk, I.; Berger, R.F.; Braeckevelt, T.; Yuan, H.; Martin, C.; Solano, E.; Lejaeghere, K.; Rogge, S.M.J.; et al. Thermal unequilibrium of strained black CsPbI 3 thin films. Science 2019, 365, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yin, W.-J. Thermodynamic Stability Trend of Cubic Perovskites. J. Am. Chem. Soc. 2017, 139, 14905–14908. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Optimization of Light Absorption Layer for CsPbI3 All-Inorganic Perovskite Solar Cells Prepared by Low Temperature Solution Method. Master’s Thesis, Zhejiang Sci-Tech University, Hangzhou, China, 2023. [Google Scholar]
- Wali, Q.; Aamir, M.; Ullah, A.; Iftikhar, F.J.; Khan, M.E.; Akhtar, J.; Yang, S. Fundamentals of Hysteresis in Perovskite Solar Cells: From Structure-Property Relationship to Neoteric Breakthroughs. Chem. Rec. 2022, 22, e202100150. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhou, X.; Yang, R.; Yang, Z.; Yu, W.; Wang, X.; Li, C.; Liu, S.; Chang, R.P.H. Surface optimization to eliminate hysteresis for record efficiency planar perovskite solar cells. Energy Environ. Sci. 2016, 9, 3071–3078. [Google Scholar] [CrossRef]
- Mao, P.; Zhou, Q.; Jin, Z.; Li, H.; Wang, J. Efficiency-Enhanced Planar Perovskite Solar Cells via an Isopropanol/Ethanol Mixed Solvent Process. ACS Appl. Mater. Interfaces 2016, 8, 23837–23843. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Tsai, C.-M.; Jiang, Y.-H.; Gollavelli, G.; Mohanta, N.; Diau, E.W.-G.; Hsu, C.-S. Interfacial Engineering with Cross-Linkable Fullerene Derivatives for High-Performance Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 38530–38536. [Google Scholar] [CrossRef] [PubMed]
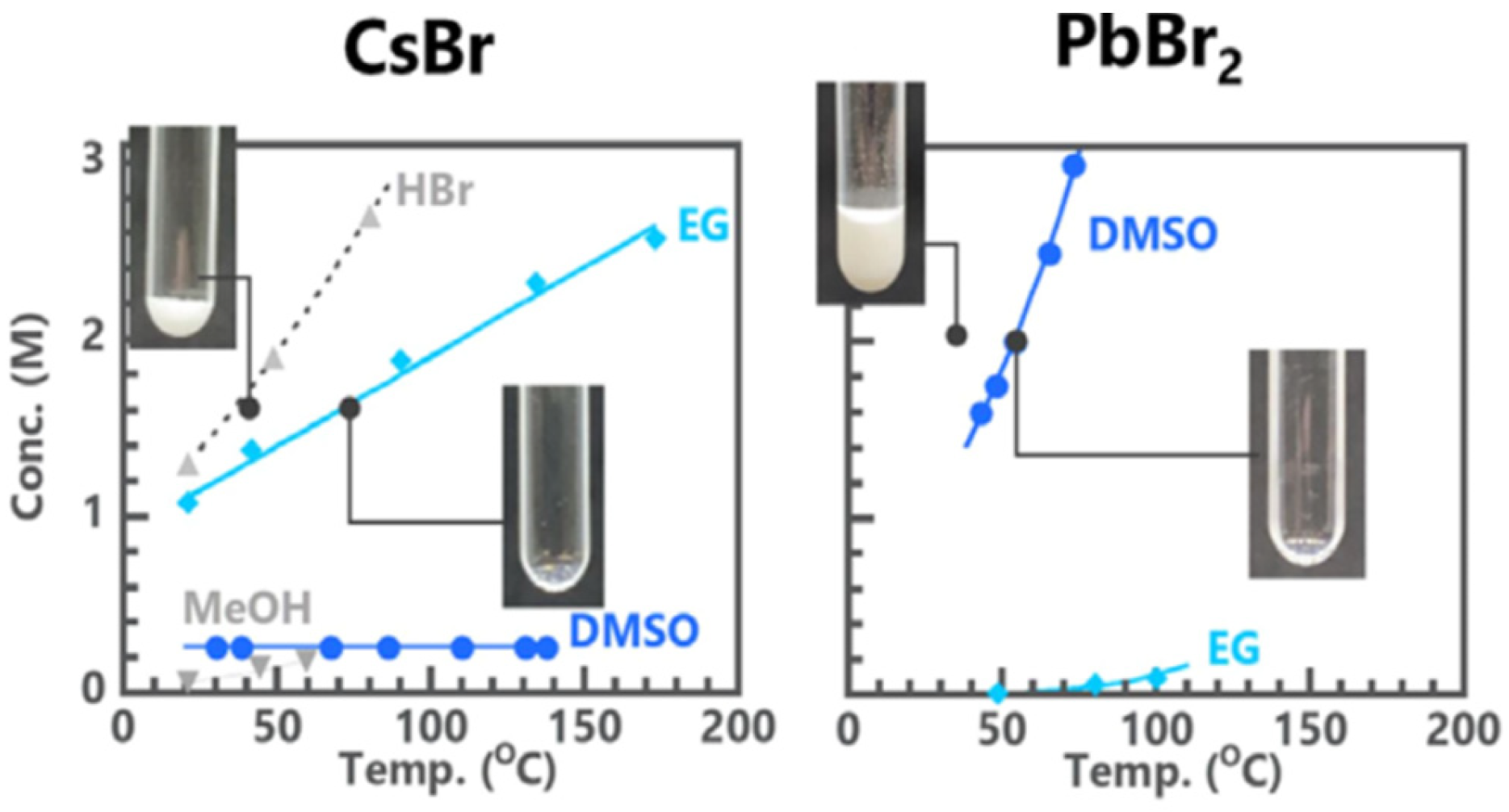
- Satoshi, W.; Taiki, H.; Masaru, I.; Yusuke, I.; Masashi, K.; Tetsuya, K. Single Crystallization of Cs4PbBr6 Perovskite from Su-persaturated Organic Solutions Optimized Through Solubility Studies. ACS Omega 2023, 8, 2455–2461. [Google Scholar]
- Zhang, X.; Fei, C.; Shen, L.; Baral, P.; Vijayaraghavan, S.N.; Yan, F.; Gong, X.; Wang, H. Manipulating Nucleation and Crystal Growth of Inorganic Perovskite Solar Cells Manipulating Nucleation and Crystal Growth of Inorganic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 38522–38529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Study on the Preparation and Photoelectric Performance of High-Efficiency Inorganic Perovskite Solar Cells. Master’s Thesis, Hubei University, Wuhan, China, 2018. [Google Scholar]
- Yang, J.; Yu, H.; Wu, S.; Cai, C.; Gao, J.; Lu, X.; Gao, X.; Shui, L.; Wu, S.; Liu, J.-M. A Mixed Antisolvent-Assisted Crystallization Strategy for Efficient All-Inorganic CsPbIBr2 Perovskite Solar Cells by a Low-Temperature Process. ACS Appl. Energy Mater. 2022, 5, 2881–2889. [Google Scholar] [CrossRef]
- McMeekin, D.P.; Mahesh, S.; Noel, N.K.; Klug, M.T.; Lim, J.; Warby, J.H.; Ball, J.M.; Herz, L.M.; Johnston, M.B.; Snaith, H.J. Solution-Processed All-Perovskite Multi-junction Solar Cells. Joule 2019, 3, 387–401. [Google Scholar] [CrossRef]
- Wan, X.; Yu, Z.; Tian, W.; Huang, F.; Jin, S.; Yang, X.; Cheng, Y.-B.; Hagfeldt, A.; Sun, L. Efficient and stable planar all-inorganic perovskite solar cells based on high-quality CsPbBr3 films with controllable morphology. J. Energy Chem. 2020, 46, 8–15. [Google Scholar] [CrossRef]
- Mali, S.S.; Patil, J.V.; Shao, J.-Y.; Zhong, Y.-W.; Rondiya, S.R.; Dzade, N.Y.; Hong, C.K. Phase-heterojunction all-inorganic perovskite solar cells surpassing 21.5% efficiency. Nat. Energy 2023, 8, 989–1001. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, T.; Zhang, B.; Wang, J.; Yu, X.; Xiao, Y.; Xiao, J.; Ku, Z.; Peng, Y. Defect Passivation and Fermi Level Modification for >10% Evaporated All-Inorganic CsPbBr3 Perovskite Solar Cells. ACS Appl. Energy Mater. 2022, 5, 8049–8056. [Google Scholar] [CrossRef]
- Noman, M.; Shahzaib, M.; Jan, S.T.; Shah, S.N.; Khan, A.D. 26.48% efficient and stable FAPbI3 perovskite solar cells employing SrCu2O2 as hole transport layer. RSC Adv. 2023, 13, 1892–1905. [Google Scholar] [CrossRef]
- Liu, W.; Yao, X.; He, B.; Sui, H.; Wei, M.; Chen, H.; Duan, J.; Tang, Q. A self-assembled CuS-MXene bridge for hole-boosting 10.51% efficiency all-inorganic tri-brominated perovskite solar cell. J. Mater. Chem. A 2023, 11, 20206–20214. [Google Scholar] [CrossRef]
- Tan, J.; Dou, J.; Tang, Q. A photovoltaic hydrovoltaic coupled carbon-based, all-inorganic CsPbBr3 perovskite solar cell. Chem. Commun. 2023, 59, 4608–4611. [Google Scholar] [CrossRef]
- Wei, H.; Xiao, J.; Yang, Y.; Lv, S.; Shi, J.; Xu, X.; Dong, J.; Luo, Y.; Li, D.; Meng, Q. Free-standing flexible carbon electrode for highly efficient hole-conductor-free perovskite solar cells. Carbon 2015, 93, 861–868. [Google Scholar] [CrossRef]
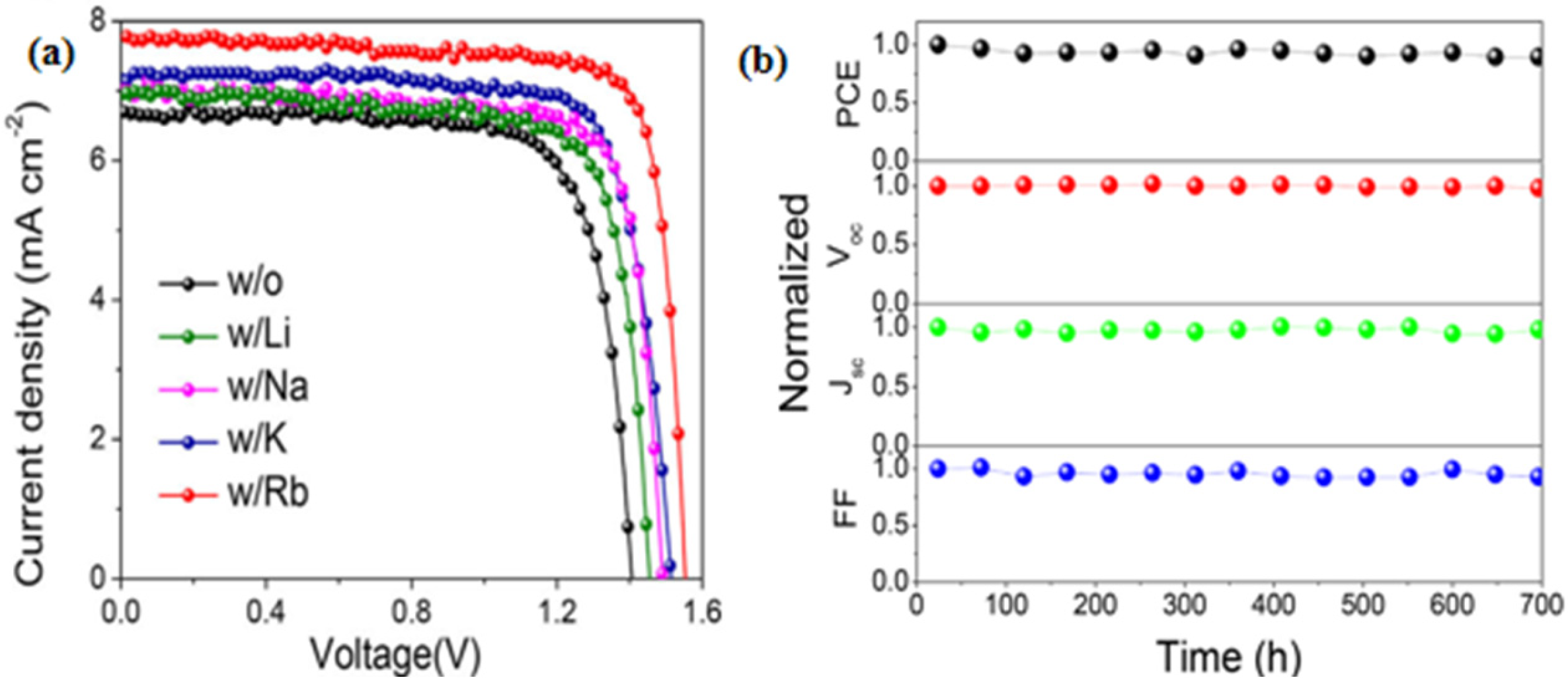
- Li, Y.; Duan, J.; Yuan, H.; Zhao, Y.; He, B.; Tang, Q. Lattice Modulation of Alkali Metal Cations Doped Cs1−xRxPbBr3 Halides for Inorganic Perovskite Solar Cells. Sol. RRL 2018, 2, 1800164. [Google Scholar] [CrossRef]
- Hua, J. Preparation of CsPbBr3 Perovskite Solar Cells Based on Vacuum Evaporation Method. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2020. [Google Scholar]
- Li, Q.; Zhang, P.; Yao, L.; Deng, L.; Ren, X.; Li, Y. Research progress of less-lead/lead-free perovskite solar cells. J. Synth. Cryst. 2022, 51, 551–558. [Google Scholar] [CrossRef]
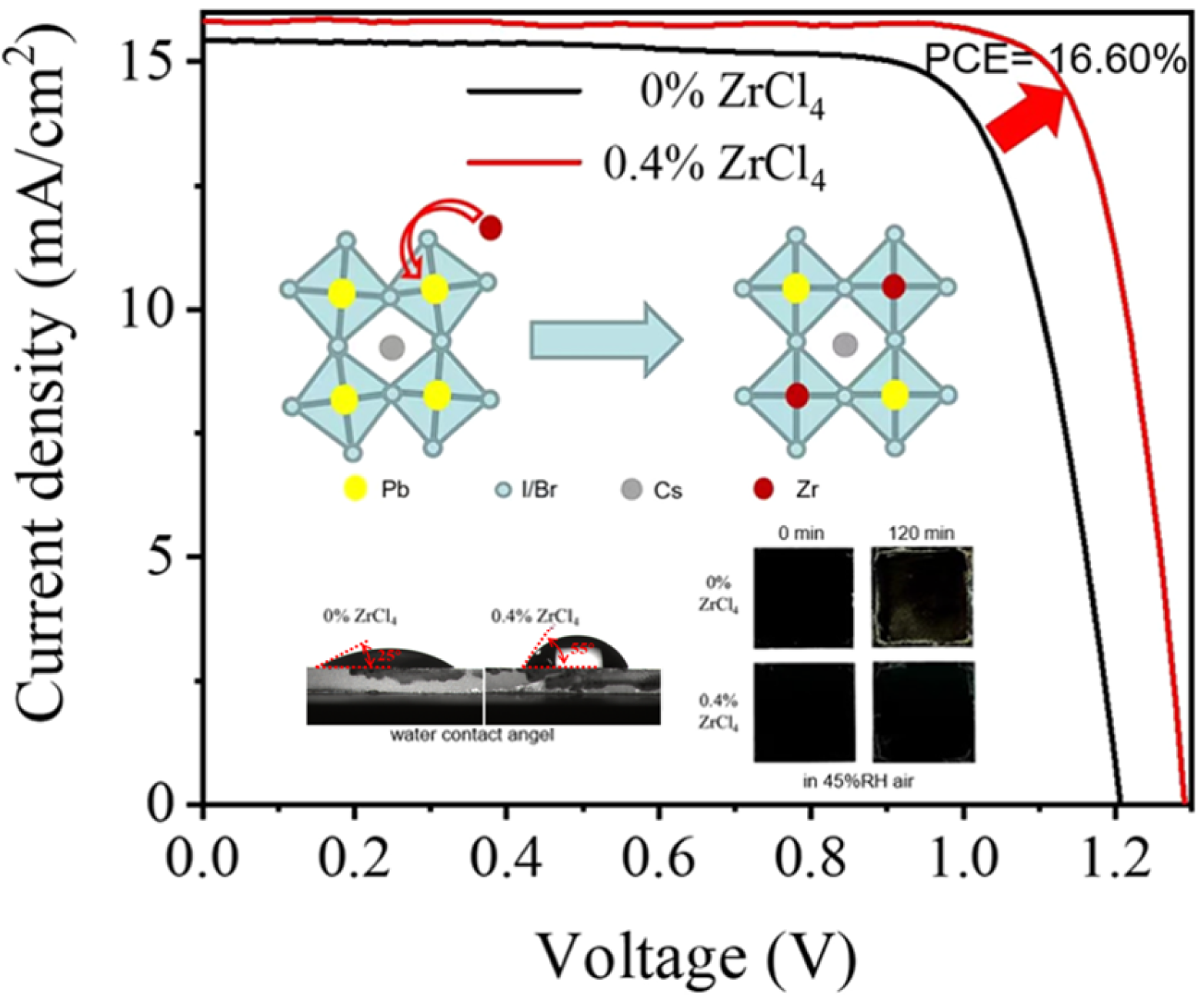
- Ma, P.; Bie, T.; Liu, Y.; Yang, L.; Bi, S.; Wang, Z.; Shao, M. Zirconium Doping to Enable High-Efficiency and Stable CsPbI2Br All-Inorganic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2024, 16, 1217–1224. [Google Scholar] [CrossRef]
- Abate, A. Perovskite Solar Cells Go Lead Free. Joule 2017, 1, 659–664. [Google Scholar] [CrossRef]
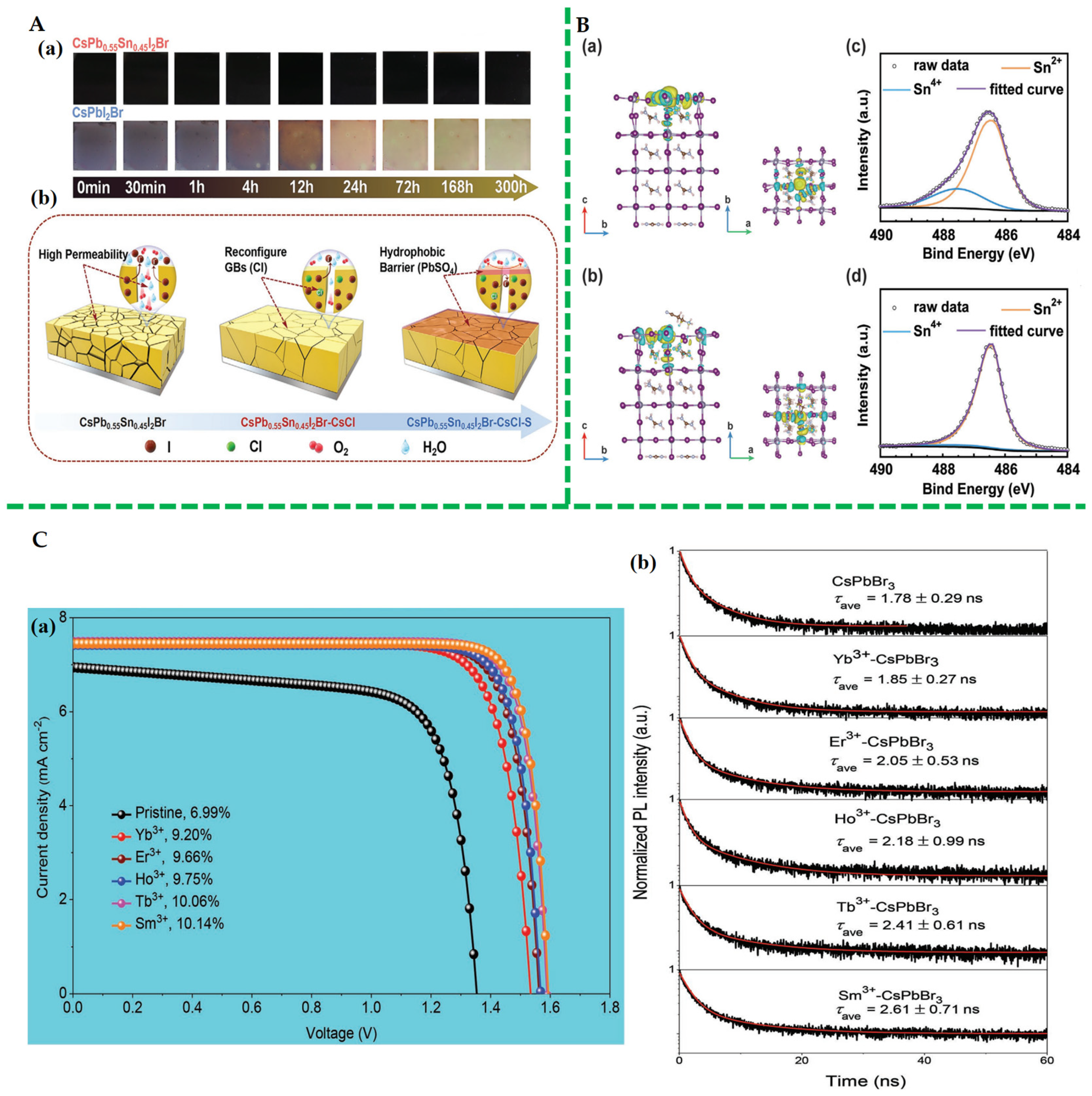
- Chen, G.; Li, P.; Xue, T.; Su, M.; Ma, J.; Zhang, Y.; Wu, T.; Han, L.; Aldamasy, M.; Li, M.; et al. Design of Low Bandgap CsPb1−XSnXI2Br Perovskite Solar Cells with Excellent Phase Stability. Small 2021, 17, 2101380. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Liu, Y.; Min, L.; Wang, L.; Yuan, Z.; Sun, H.; Huang, L.; Li, L.; Meng, X. Cyano-Coordinated Tin Halide Perovskites for Wearable Health Monitoring and Weak Light Imaging. Adv. Mater. 2024, e2400090. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiong, J.; Xing, Y.; Gan, X.; Zhu, W.; Xuan, R.; Liu, X.; Huang, L.; Zhu, Y.; Zhang, J. Reductive Sn2+ Compensator for Efficient and Stable Sn-Pb Mixed Perovskite Solar Cells. Adv. Sci. 2024, 2400962. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Gong, J.; Zhou, H.; Huang, X.; Liu, M.; Zhou, Y.; Yang, S. Surface Sn(IV) Hydrolysis Improves Inorganic Sn–Pb Perovskite Solar Cells. ACS Energy Lett. 2023, 8, 1035–1041. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; Yang, X.; Wang, Y.; He, B.; Tang, Q. Lanthanide Ions Doped CsPbBr3 Halides for Htm-Free 10.14% Efficiency Inorganic Perovskite Solar Cell with an Ultrahigh Open-Circuit Voltage of 1.594 V. Adv. Energy Mater. 2018, 8, 1802346. [Google Scholar] [CrossRef]
- Yang, X.; Luo, D.; Xiang, Y.; Zhao, L.; Anaya, M.; Shen, Y.; Wu, J.; Yang, W.; Chiang, Y.; Tu, Y.; et al. Buried Interfaces in Halide Perovskite Photovoltaics. Adv. Mater. 2021, 33, 2006435. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Krishna, A.; Xu, J.; Yao, J. Interface Engineering for Highly Efficient and Stable Perovskite Solar Cells. Adv. Opt. Mater. 2023, 12, 2301949. [Google Scholar] [CrossRef]
- Yu, B.; Shi, J.; Tan, S.; Cui, Y.; Zhao, W.; Wu, H.; Luo, Y.; Li, D.; Meng, Q. Efficient (>20%) and Stable All-Inorganic Cesium Lead Triiodide Solar Cell Enabled by Thiocyanate Molten Salts. Angew. Chem. Int. Ed. 2021, 60, 13436–13443. [Google Scholar] [CrossRef]
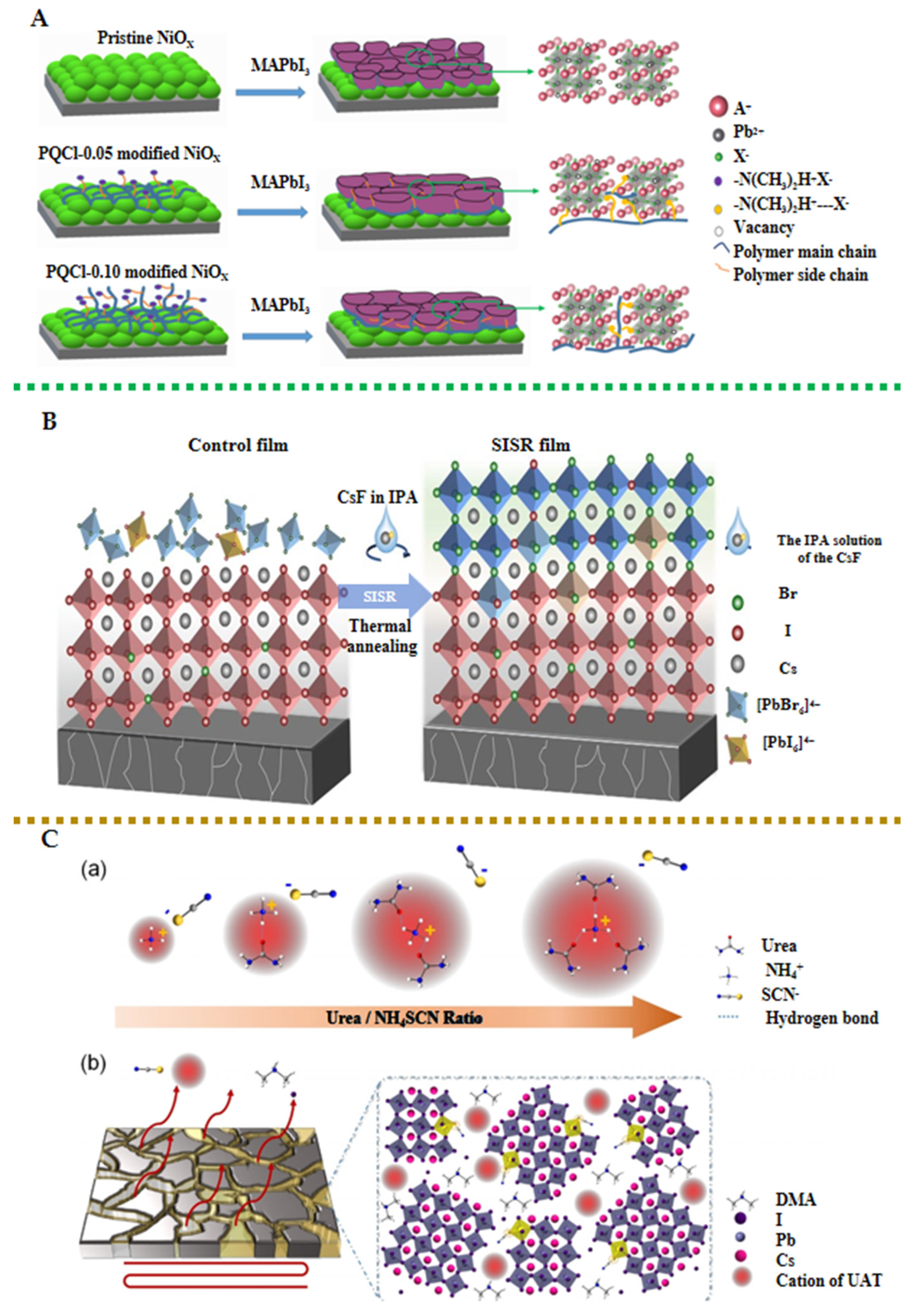
- Ho, I.H.; Huang, Y.J.; Cai, C.E.; Liu, B.T.; Wu, T.M.; Lee, R.H. Enhanced Photovoltaic Performance of Inverted Perovskite Solar Cells through Surface Modification of a NiOX-Based Hole-Transporting Layer with Quaternary Ammonium Halide-Containing Cellulose Derivatives. Polymers 2023, 15, 437. [Google Scholar] [CrossRef]
- Chu, X.; Ye, Q.; Wang, Z.; Zhang, C.; Ma, F.; Qu, Z.; Zhao, Y.; Yin, Z.; Deng, H.-X.; Zhang, X.; et al. Surface in situ reconstruction of inorganic perovskite films enabling long carrier lifetimes and solar cells with 21% efficiency. Nat. Energy 2023, 8, 372–380. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, R.; Kroll, M.; Hofstetter, Y.J.; Jia, X.; Becker-Koch, D.; Paulus, F.; Löffler, M.; Nehm, F.; Leo, K.; et al. Efficient Thermally Evaporated γ-CsPbI3 Perovskite Solar Cells. Adv. Energy Mater. 2021, 11, 2100299. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, M.; Sun, Y.; Yang, X.; Xun, W.; Li, M.; Chen, R.; Lin, Y.; Li, P.; Jiang, Q.-S. A buried interface modification strategy for enhancing the photovoltaic performance of NiOx-based inverted perovskite solar cells. Vacuum 2024, 222, 113057. [Google Scholar] [CrossRef]
- Liu, Y. Photoelecteic Properties of Inorganic Perovskite and Its Applications. Master’s Thesis, Qingdao University of Science and Technology, Tsingtao, China, 2023. [Google Scholar]
- Wang, Y.; Zhang, T.; Kan, M.; Li, Y.; Wang, T.; Zhao, Y. Efficient α-CsPbI3 Photovoltaics with Surface Terminated Organic Cations. Joule 2018, 2, 2065–2075. [Google Scholar] [CrossRef]
- Wu, S.; Yun, T.; Zheng, C.; Luo, X.; Qiu, P.; Yu, H.; Wang, Q.; Gao, J.; Lu, X.; Gao, X.; et al. Ionic Liquid Bridge Assisting Bifacial Defect Passivation for Efficient All-Inorganic Perovskite Cells with High Open-Circuit Voltage. ACS Appl. Mater. Interfaces 2024, 16, 7297–7309. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Q.; Zhang, H.; Xie, H.; Du, Y.; Liu, L.; Feng, X.; Najar, A.; Ren, X.; Liu, S. Managing Multiple Halide-Related Defects for Efficient and Stable Inorganic Perovskite Solar Cells. Angew. Chem. Int. Ed. 2023, 62, e202305815. [Google Scholar] [CrossRef]
| Operational | Device | Optimization Strategy | PCE (%) | Stability | Refs. |
|---|---|---|---|---|---|
| positive-type structure | FTO/TiO2/CsPbIBr2-1.0% Cd2+/Carbon | Cd2+ doping CsPbIBr2 thin film | 10.63 | 92% of the initial efficiency (25 °C, 30% RH, 40 day) | [16] |
| positive-type structure | FTO)/TiO2/CsPbI1.5Br1.5/PbS&R-CdS/Carbon | Adding a lead sulfide/cadmium sulfide heterojunction thin layer between CsPbI1.5Br1.5 and the carbon electrode | 13.65 | 90% of the initial efficiency (25 °C, 30% RH, 1200 h) 87% of the initial efficiency (N2, 85 °C, 400 h) | [17] |
| positive-type structure | FTO/MX-TiO2/CsPbI2Br/PCBM/carbon | Incorporating titanium dioxide NPs into CH3COO- functionalized MXene as the ETL | 15.48 | 91% of the initial efficiency (25 °C, 20–30% RH, 1250 h) 85% of the initial efficiency (N2, 85 °C, 400 h) | [18] |
| positive-type structure | FTO/SnO2/CsPbI2Br/TFPTAI/carbon | Modify the surface of CsPbI2Br with TFPTAI | 14.02 | 89% of the initial efficiency (25 °C, 15–25% RH, 700 h) | [19] |
| inverted-type structure | ITO/PTAA/Perovskite/PCBM/BCP/SnO2/Cu | Using ALD-prepared tin dioxide as an additional buffer layer on top of the BCP layer | 21.13 | 90% of the initial efficiency (25 °C, 20–40% RH, 600 h) | [20] |
| inverted-type structure | FTO/NiOx/CsPbI3/OAI/C60/BCP/Ag | The CsPbI3 thin film with a three-step annealing process and a maximum annealing temperature of 180 °C | 13.89 | 80% of the initial efficiency (N2, 25 day) | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Yang, L.; Zhang, X.; Wang, L.; Sun, W. Research Progress of Cs-Based All-Inorganic Perovskite Solar Cells. Energies 2024, 17, 2671. https://doi.org/10.3390/en17112671
Xu S, Yang L, Zhang X, Wang L, Sun W. Research Progress of Cs-Based All-Inorganic Perovskite Solar Cells. Energies. 2024; 17(11):2671. https://doi.org/10.3390/en17112671
Chicago/Turabian StyleXu, Shihui, Lin Yang, Xiaoping Zhang, Lisi Wang, and Wei Sun. 2024. "Research Progress of Cs-Based All-Inorganic Perovskite Solar Cells" Energies 17, no. 11: 2671. https://doi.org/10.3390/en17112671
APA StyleXu, S., Yang, L., Zhang, X., Wang, L., & Sun, W. (2024). Research Progress of Cs-Based All-Inorganic Perovskite Solar Cells. Energies, 17(11), 2671. https://doi.org/10.3390/en17112671





