Analysis of Breaking Characteristics of C4F7N/CO2 Mixture Gas in Circuit Breaker
Abstract
1. Introduction
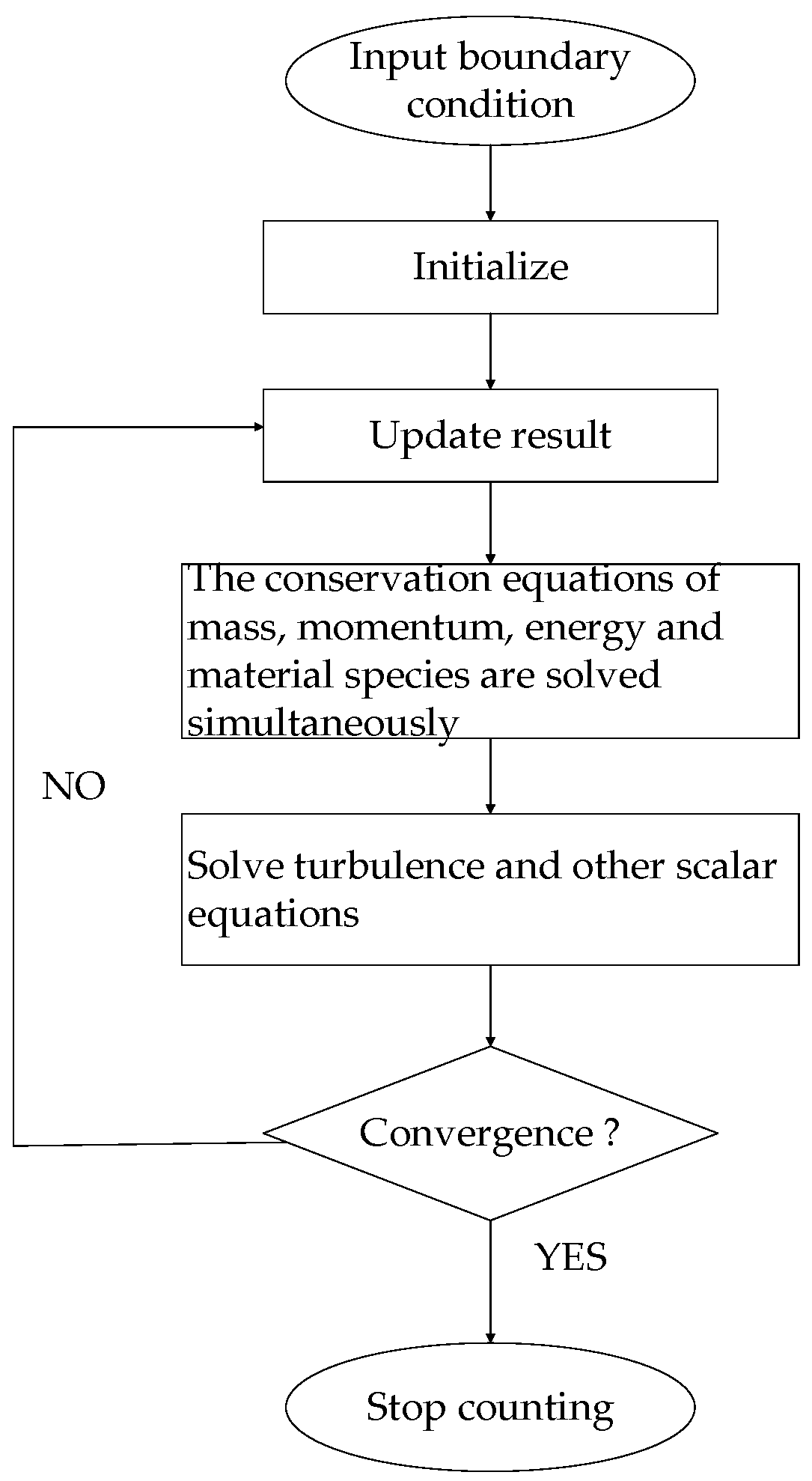
2. Mathematical Modeling of Electric Arc
2.1. Fundamental Equation Governing the System
2.2. Turbulence Model
2.3. Radiation Model
2.4. Geometric Model and Boundary Conditions
- (1)
- Symmetric boundary: the symmetry axis of the circuit breaker is set to the axis.
- (2)
- Pressure outlet: the circuit breaker has a pressure outlet, the pressure at the pressure outlet is set to the base pressure, the temperature is set to 293 K.
- (3)
- Solid boundary: calculate the heat flux at the solid boundary around the region where the heat is 0.
- (4)
- Dynamic mesh: in order to simplify the calculation, the method of moving static contacts is adopted.
- (5)
- Except for velocity: the radial component of the independent variable of the governing equation is set to 0.
- (6)
- User-defined scalar (UDS) to solve the current continuity equation: the end of the virtual electrode is set as the current density boundary, and the end of the static contact is zero potential.
3. Results and Discussion
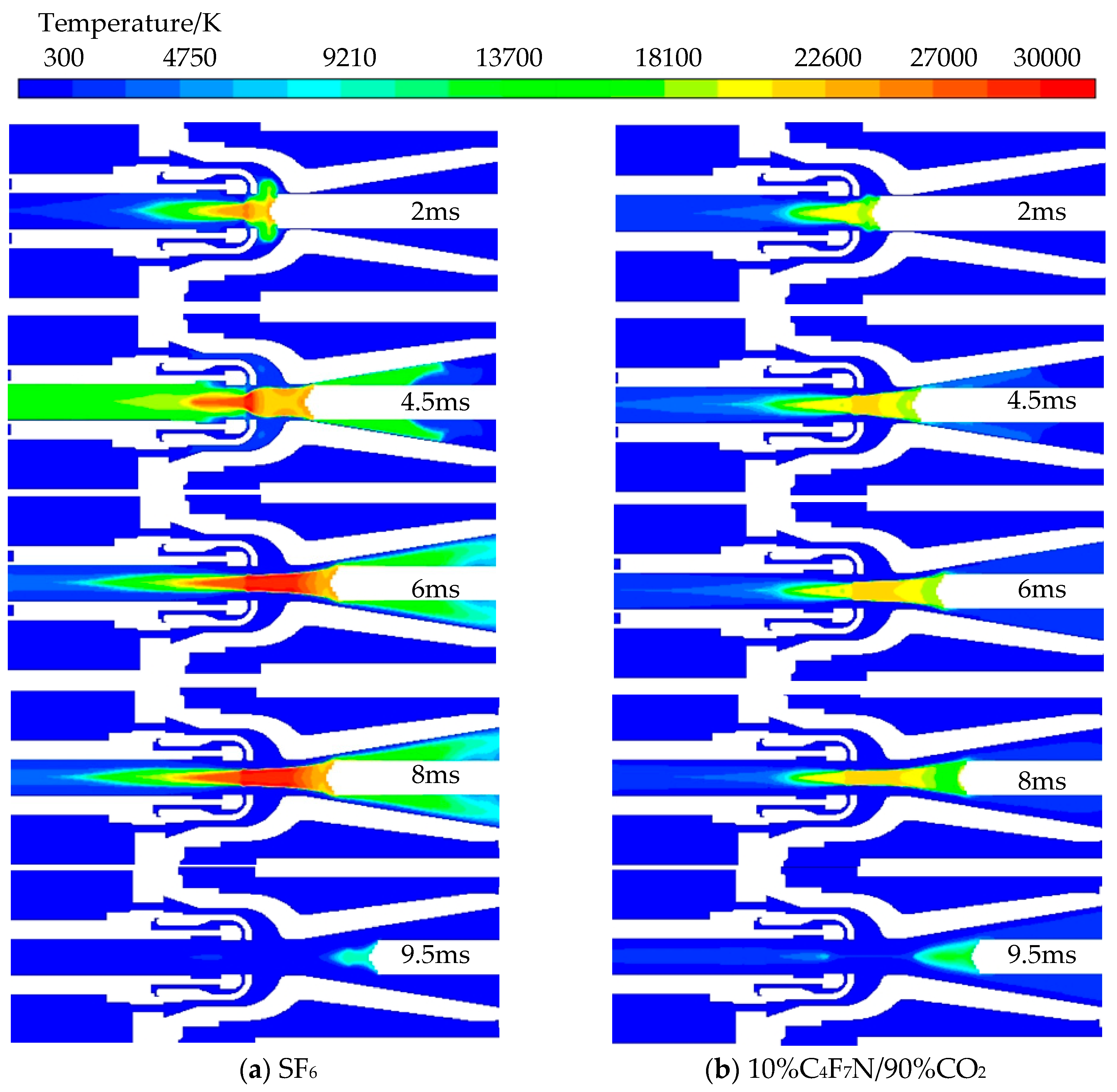
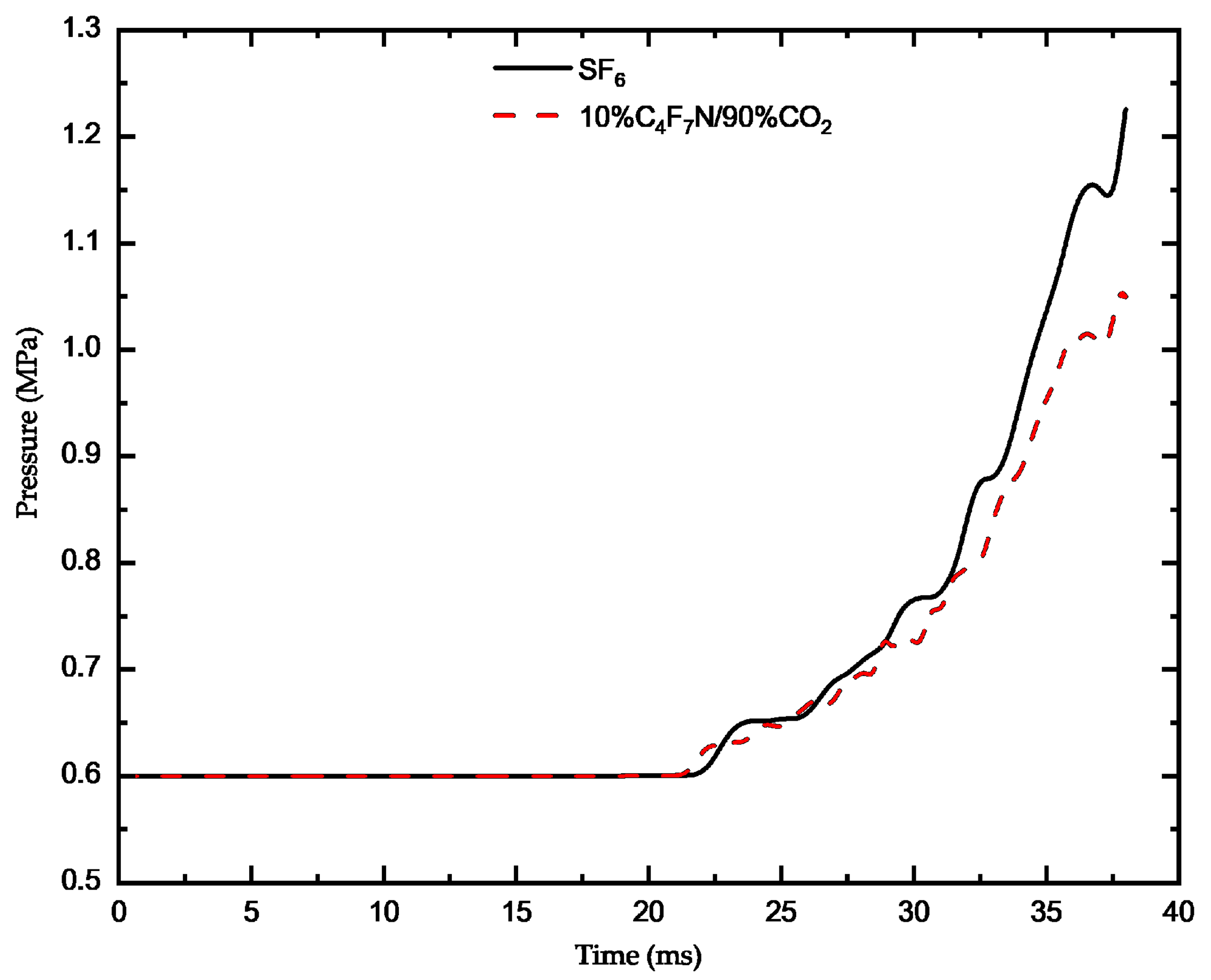
3.1. Analysis of Arc Dynamic Characteristics of C4F7N Mixed Gas
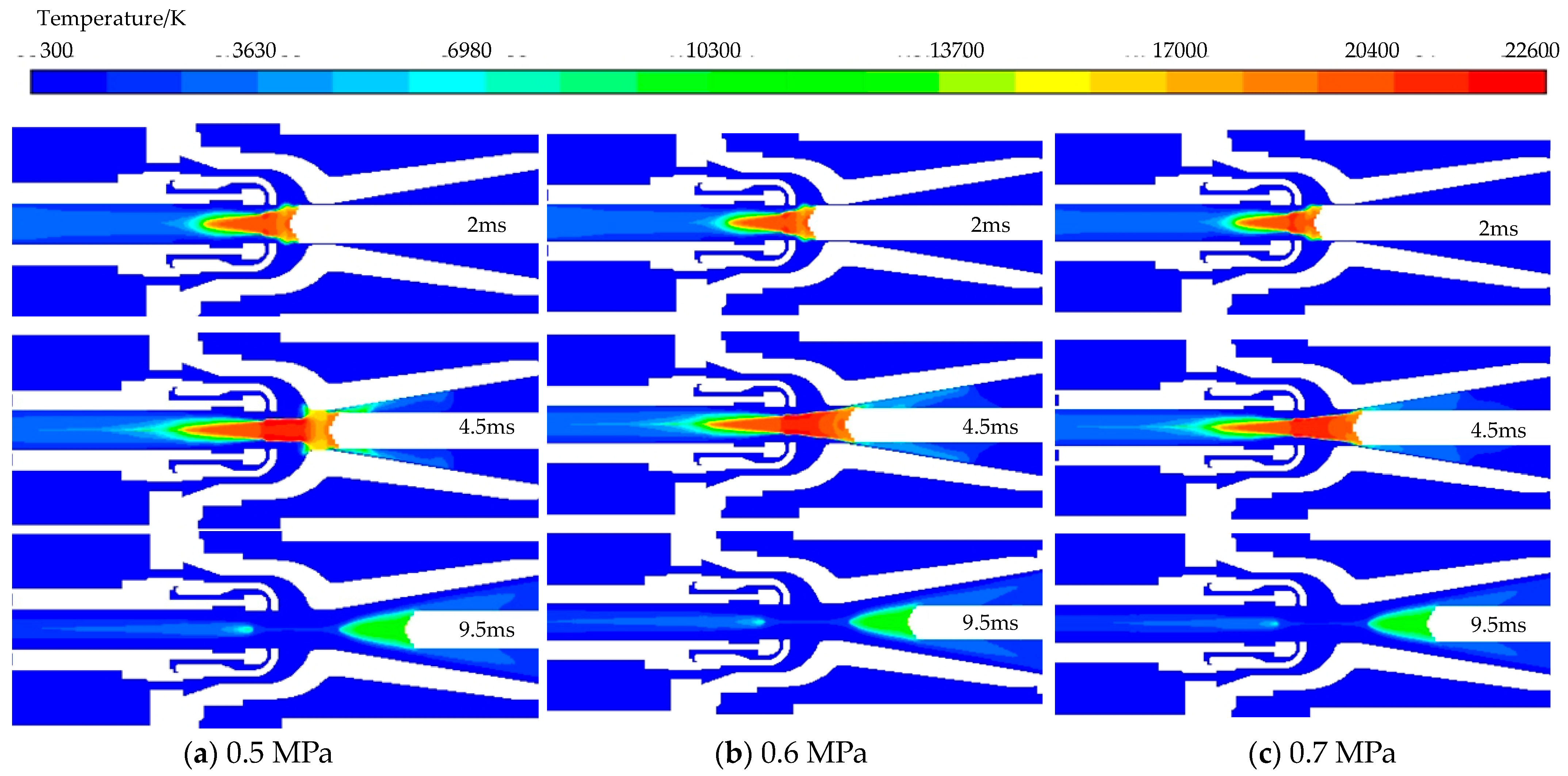
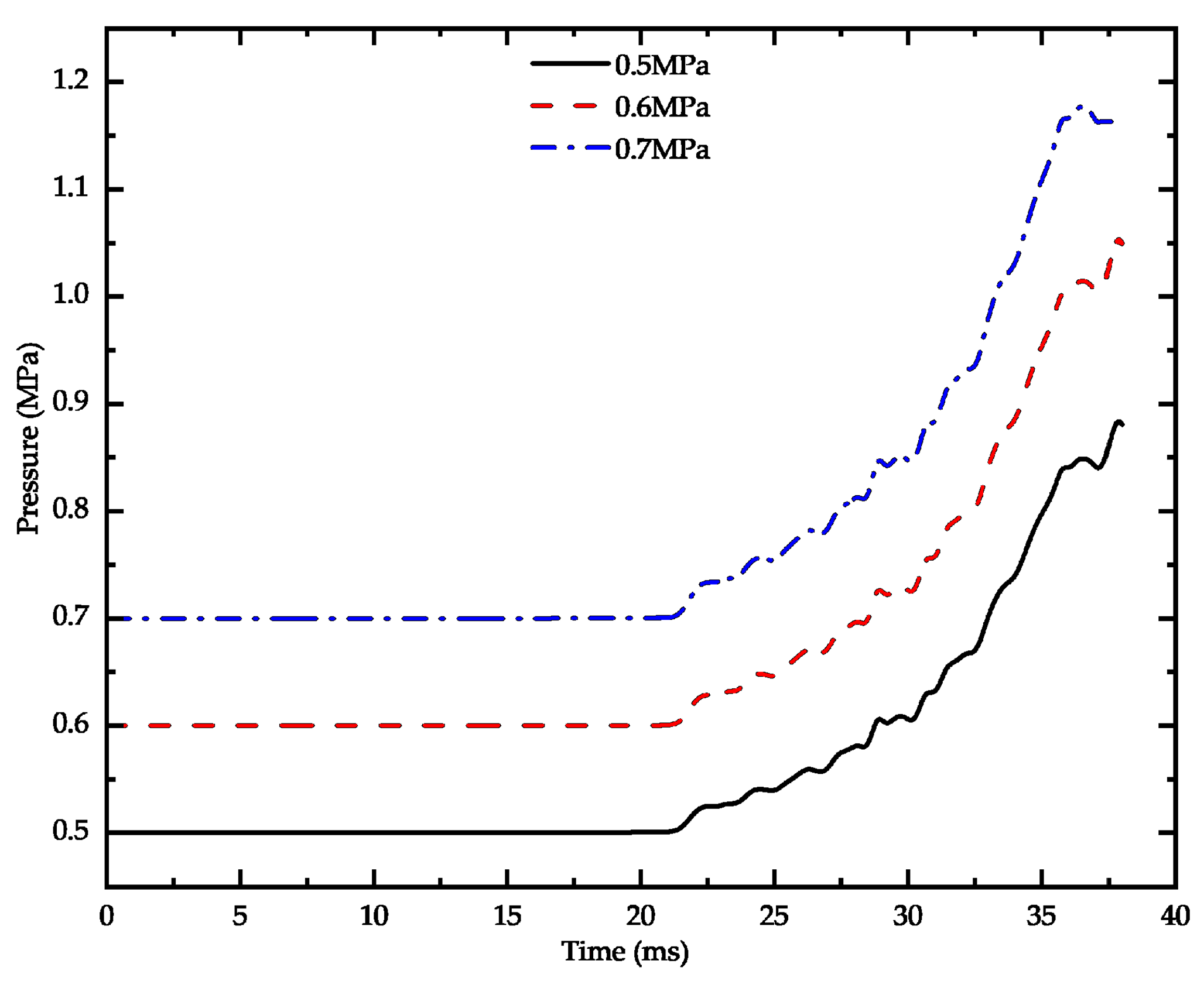
3.2. Influence of Inflation Pressure on Breaking Characteristics
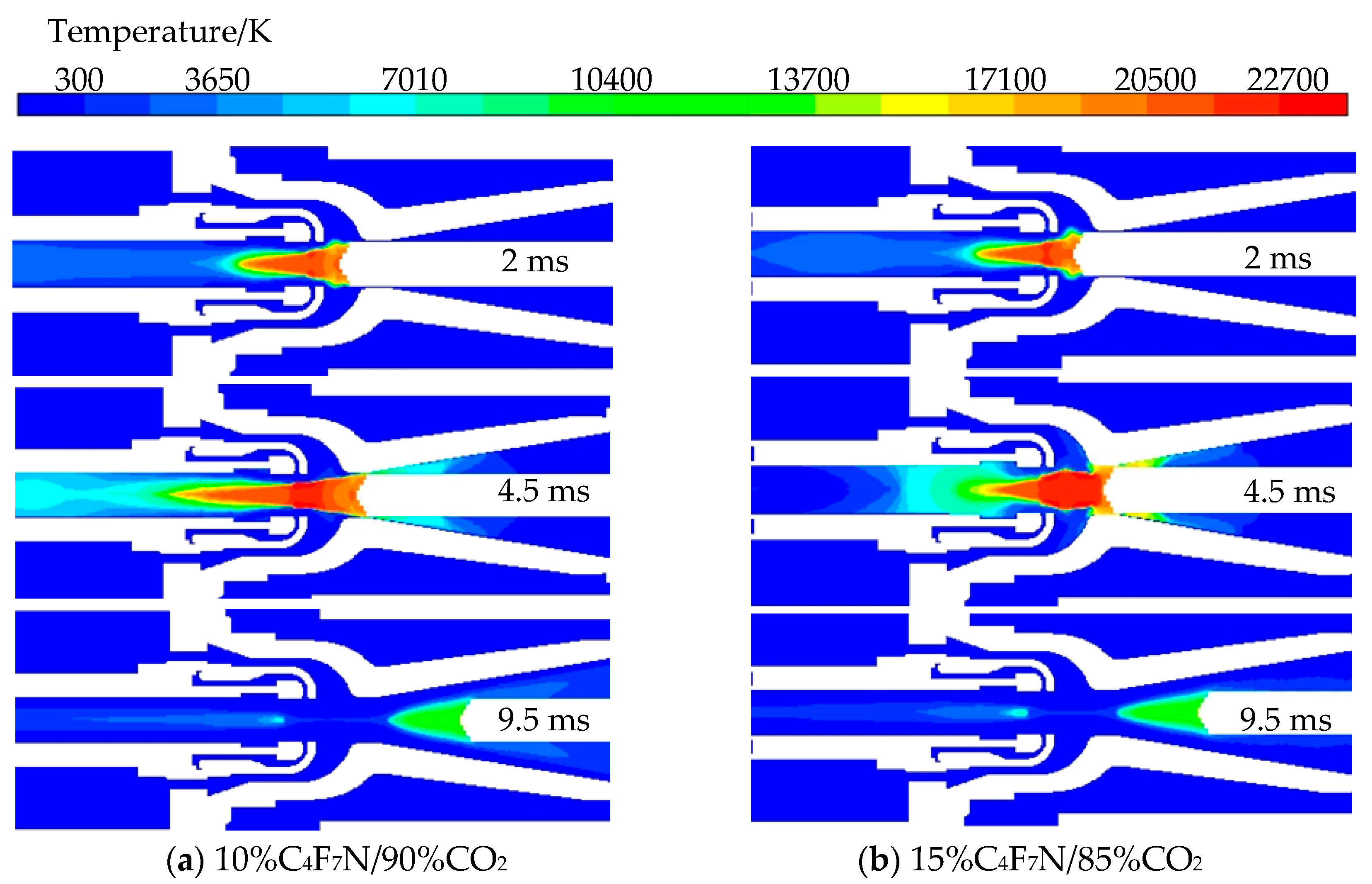
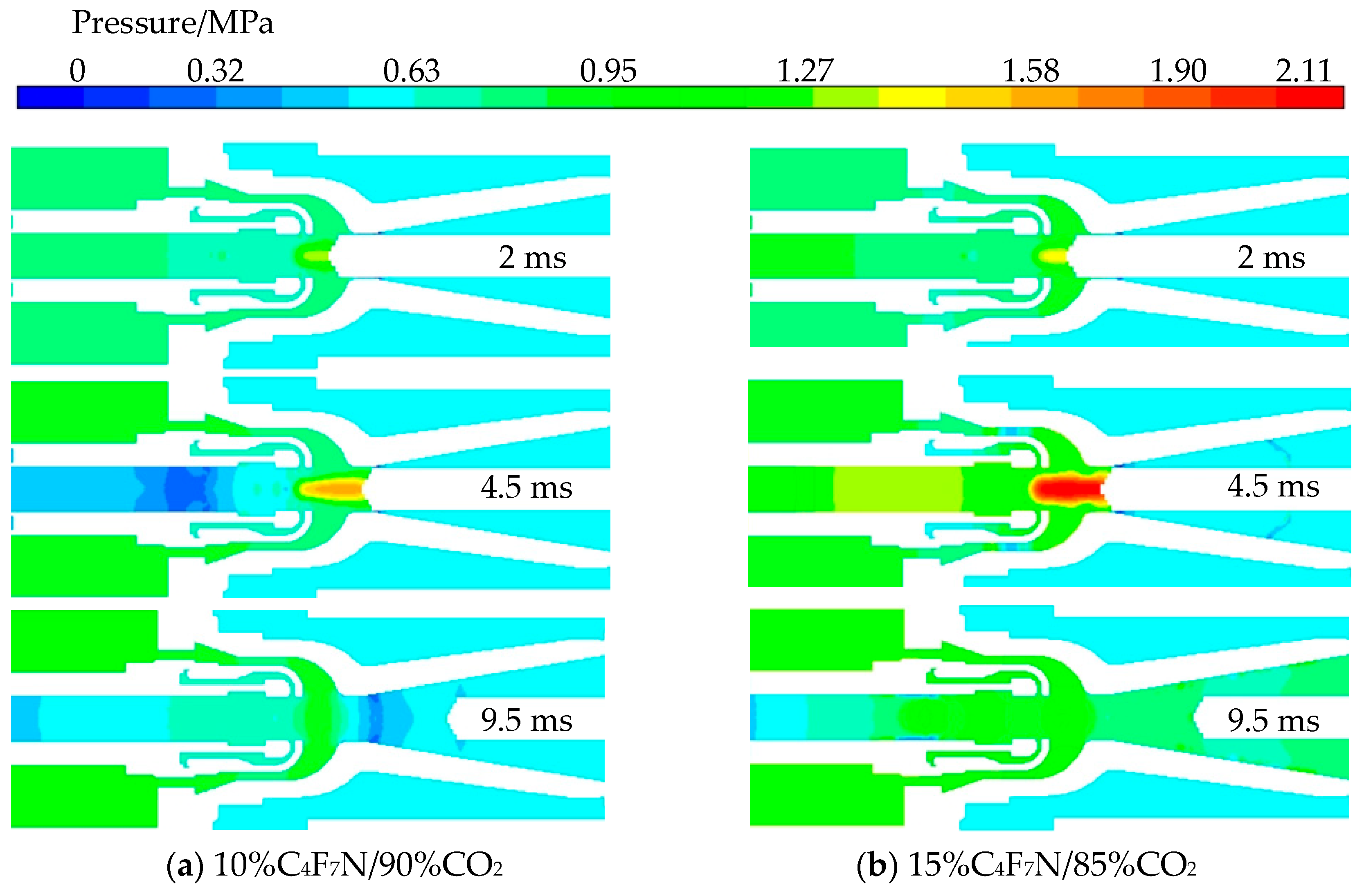
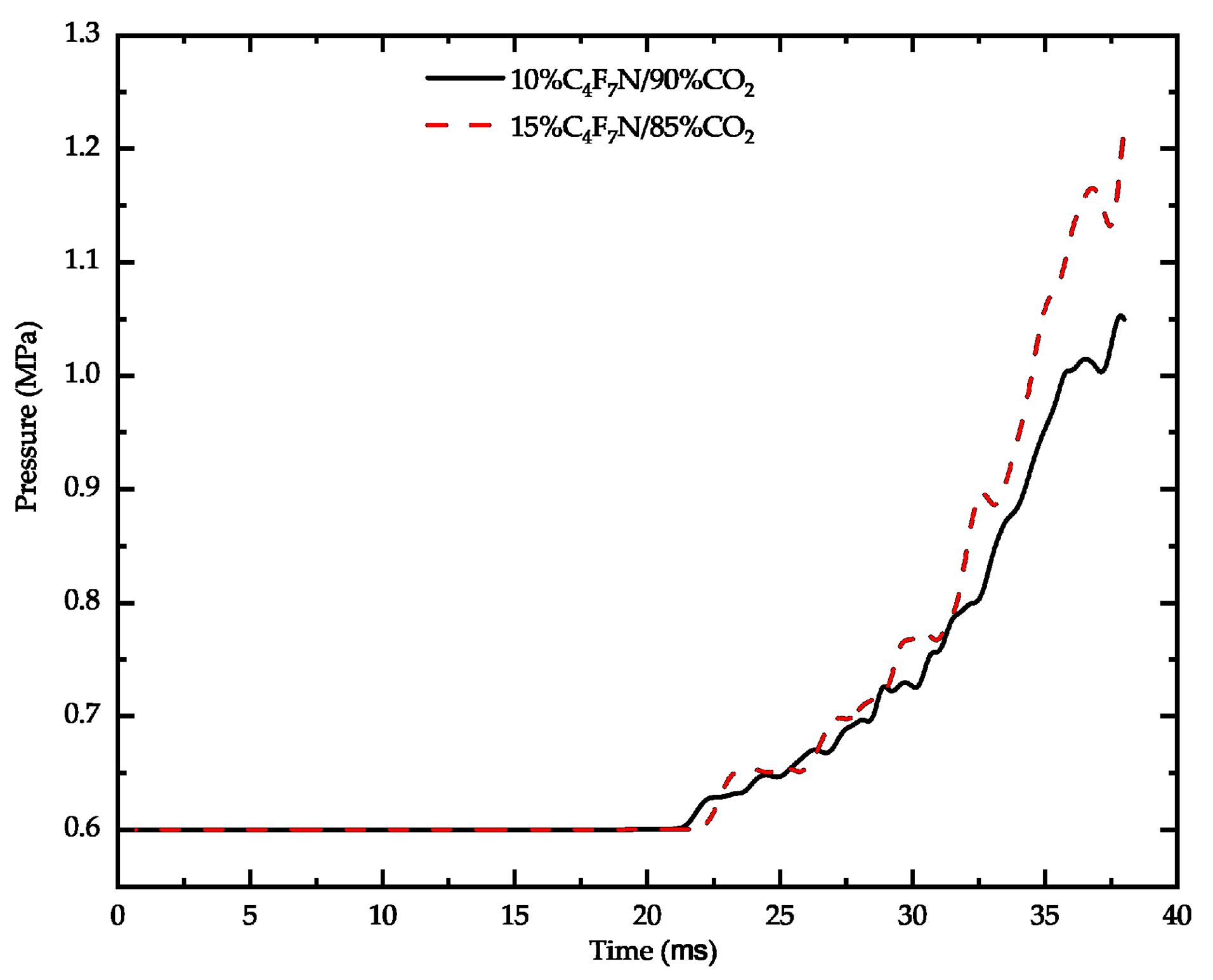
3.3. Influence of C4F7N Proportion on Breaking Characteristics
4. Conclusions
- Under the same conditions, the arc temperature of pure SF6 gas is higher, resulting in a farther axial diffusion and a stronger arc-extinguishing effect, making it less prone to re-ignition. During the process of arc combustion, the pressure in the high-pressure region of the arc core is lower compared to that of pure SF6 gas, leading to a smaller high-pressure area. Additionally, the pressure established in the compressor at the moment of arc combustion and extinction is also lower than that of pure SF6 gas.
- The cutting performance of the mixture can be enhanced by adjusting the inflation pressure, When the aeration pressure is greater than 0.6 MPa, improving the air pressure effect has little significance, with 0.6 MPa being identified as an optimal inflation pressure.
- Following an increase in the proportion of C4F7N, there is a rise in arc temperature during ignition and extinction, but a decrease when the current peak is reached. Moreover, there is a significant increase in the core pressure after elevating the proportion of C4F7N, leading to a greater pressure difference established by the compressor chamber. Nevertheless, due to minimal change in extinguishment temperature, a 10% proportion of C4F7N remains an appropriate choice.
- In this paper, a two-dimensional axisymmetric structure is used for calculation, which may be slightly different from the actual three-dimensional structure. In this study, the simulation method is used to conduct research. Subsequent experiments need to be conducted to explore the breaking characteristics of the C4F7N/CO2 mixture.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Götte, N.; Krampert, T.; Nikolic, P.G. Series connection of gas and vacuum circuit breakers as a hybrid circuit breaker in high-voltage applications. IEEE Trans. Plasma Sci. 2020, 48, 2577–2584. [Google Scholar] [CrossRef]
- Gui, Z.W.; Li, Y. Challenges and proposals on SF6 emission reduction approaches. Sci. Total Environ. 2024, 906, 167347. [Google Scholar]
- Loizou, L.; Han, Q.; Chen, L.J.; Liu, Q.; Waldron, M.; Wilson, G.; Bautista, R.F. Partial discharge characteristics of C3F7CN gas mixture using the UHF method. Energies 2022, 15, 7731. [Google Scholar] [CrossRef]
- Huo, P.; Jiang, X. Study on lightning impulse insulation characteristics of C4F7N/CO2 binary gas mixture. High Volt. Appar. 2021, 57, 1–5. [Google Scholar]
- Owens, J.; Xiao, A. Recent development of two alternative gases to SF6 for high voltage electrical power applications. Energies 2021, 14, 5051. [Google Scholar] [CrossRef]
- Zebouchi, N.; Li, H.L.; Haddad, M.A. Development of Future compact and eco-friendly HVDC gas-insulated systems: Shape optimization of a DC spacer model and novel materials investigation. Energies 2020, 13, 3288. [Google Scholar] [CrossRef]
- Narayanan, V.R.; Gnybida, M.; Rümpler, C. Transport and radiation properties of C4F7N-CO2 gas mixtures with added oxygen. J. Phys. D Appl. Phys. 2022, 55, 295502. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, K.L. Review of the decomposition characteristics of eco-friendly insulation gas. High Volt. 2021, 6, 733–749. [Google Scholar] [CrossRef]
- Carles, A.; Schlernitzauer, A.; Vignes, M.; Cros, G.; Magous, R.; Maurice, T.; Oiry, C. Heptafluoroisobutyronitrile (C4F7N), a gas used for insulating and arc quenching in electrical switchgear, is neurotoxic in the mouse brain. Toxicology 2022, 480, 153319. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Hao, M. Determination and assessment of a complete and self-consistent electron-neutral collision cross-section set for the C4F7N molecule. J. Phys. D Appl. Phys. 2023, 56, 134001. [Google Scholar] [CrossRef]
- Ovad, T.; Sapunar, M. Excitation and fragmentation of the dielectric gas C4F7N: Electrons vs photons. J. Phys. D Appl. Phys. 2023, 158, 014303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, X. Calculation of thermodynamic physical parameters of C4F7N/CO2 and C4F7N/N2 gas mixtures. High Volt Eng. 2020, 46, 250–256. [Google Scholar]
- Li, W.; Zhang, X.X. Research and application progress of environmental protection insulating gas C4F7N Part II: Material compatibility, safety and equipment developmen. Trans. China Electrotech. Soc. 2021, 36, 4567–4579. [Google Scholar]
- Chachereau, A.; Hsl, A. Electrical insulation properties of the perfluoronitrile C4F7N. J. Phys. D Appl. Phys. 2018, 51, 495201. [Google Scholar] [CrossRef]
- Zhang, X.X.; Chen, Q. Experimental study on power frequency breakdown characteristics of C4F7N/CO2 gas mixture under quasi-homogeneous electric field. IEEE Access 2019, 7, 19100–19108. [Google Scholar] [CrossRef]
- Xiong, J.Y.; Zhang, B.Y. Study on measurements of swarm parameters in SF6 alternative gases by pulsed townsend method. Proc. CSEE 2021, 41, 759–769. [Google Scholar]
- Zhang, T.; Zhou, W.; Zheng, Y.; Yu, J.H. Insulation properties of C4F7N/CO2 mixtures under non-uniform electric field. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1747–1754. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, W.; Yu, J.H.; Yu, Z.X. Insulation properties of C4F7N/CO2 mixtures under lightning impulse. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 181–188. [Google Scholar] [CrossRef]
- Wang, C.L.; Li, J.; Cao, Y.D. Study on arc-extinguishing performance of DC C4F7N/CO2 mixed gas. Earth Environ. Sci. 2021, 692, 032097. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, E.Y.; Li, Z.B.; Wang, Y.X.; Zhang, Z.G.; Zhang, R.; Yan, X.L. Research on arc extinguishing performance of C4F7N/CO2 gases with slight positive pressure. IEEE Trans. Plasma Sci. 2022, 50, 410–416. [Google Scholar] [CrossRef]
- Xiao, S.; Gao, B. The sensitivity of C4F7N to electric field and its influence to environment-friendly insulating gas mixture C4F7N/CO2. J. Phys. D Appl. Phys. 2018, 54, 055501. [Google Scholar] [CrossRef]
- Seeger, M. Perspectives on research on high voltage gas circuit breakers. Plasma Chem. Plasma Process. 2015, 35, 527–541. [Google Scholar] [CrossRef]
- Bian, C.X.; He, B.N. Research on interruption performance of environmentally friendly C4F7N mixed-gas-insulated switchgear. Energies 2022, 15, 6500. [Google Scholar] [CrossRef]
- Yan, J.D.; Nuttall, K.I.; Fang, M.T.C. A comparative study of turbulence models for SF6 arcs in a supersonic nozzle. J. Phys. D Appl. Phys. 1999, 32, 1401–1406. [Google Scholar] [CrossRef]
- Dixon, C.M.; Yan, J.D.; Fang, M.T.C. A comparison of three radiation models for the calculation of nozzle arcs. J. Phys. D Appl. Phys. 2004, 37, 3309–3318. [Google Scholar] [CrossRef]
- Song, Y.; Lin, X. Interruption performance of C4F7N/CO2 gas in high-voltage circuit breaker. High Volt Eng. 2023, 49, 971–981. [Google Scholar]



| Symbol | Interpretation and Unit |
|---|---|
| t | Time (seconds) |
| ρ | Density (kg/m3) |
| Velocity vector (m/s) | |
| p | Pressure (Pa) |
| τ | Pressure tensor (Pa) |
| Current density (A/m2) | |
| Magnetic induction intensity (T) | |
| k | Heat conductivity (w/(m·K)) |
| T | Temperature (K) |
| σ | Conductivity (S/m) |
| Electric field intensity (V/m) | |
| q | Net radiation loss per unit volume and time (w/m3) |
| φ | Electric potential (V) |
| μm | Magnetic conductivity (H/m) |
| Constant Parameter | Value |
|---|---|
| σk | 1 |
| σε | 1.3 |
| C | 0.09 |
| C1ε | 1.44 |
| C2e | 1.92 |
| Symbol | Interpretation and Unit |
|---|---|
| R83 | Radiation radius corresponding to 0.83 Tmax (m) |
| R4k | Radius corresponding to the isotherm at 4000 K (m) |
| qa | Volumetric radiation (w/m3) |
| q0 | the maximum volumetric radiation source for radiation re-absorption in the range of R83 to R4k (w/m3) |
| Q | Net radiation loss in the core area of the arc (w/m3) |
| PCT | Percentage of reabsorbed energy |
| Aeq | The equivalent area of the reabsorbed area (m2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, L.; Wang, W.; Geng, Z. Analysis of Breaking Characteristics of C4F7N/CO2 Mixture Gas in Circuit Breaker. Energies 2024, 17, 2638. https://doi.org/10.3390/en17112638
Li X, Liu L, Wang W, Geng Z. Analysis of Breaking Characteristics of C4F7N/CO2 Mixture Gas in Circuit Breaker. Energies. 2024; 17(11):2638. https://doi.org/10.3390/en17112638
Chicago/Turabian StyleLi, Xiaolong, Lei Liu, Wen Wang, and Zhenxin Geng. 2024. "Analysis of Breaking Characteristics of C4F7N/CO2 Mixture Gas in Circuit Breaker" Energies 17, no. 11: 2638. https://doi.org/10.3390/en17112638
APA StyleLi, X., Liu, L., Wang, W., & Geng, Z. (2024). Analysis of Breaking Characteristics of C4F7N/CO2 Mixture Gas in Circuit Breaker. Energies, 17(11), 2638. https://doi.org/10.3390/en17112638






