The Role of Amphiphilic Nanosilica Fluid in Reducing Viscosity in Heavy Oil
Abstract
1. Introduction
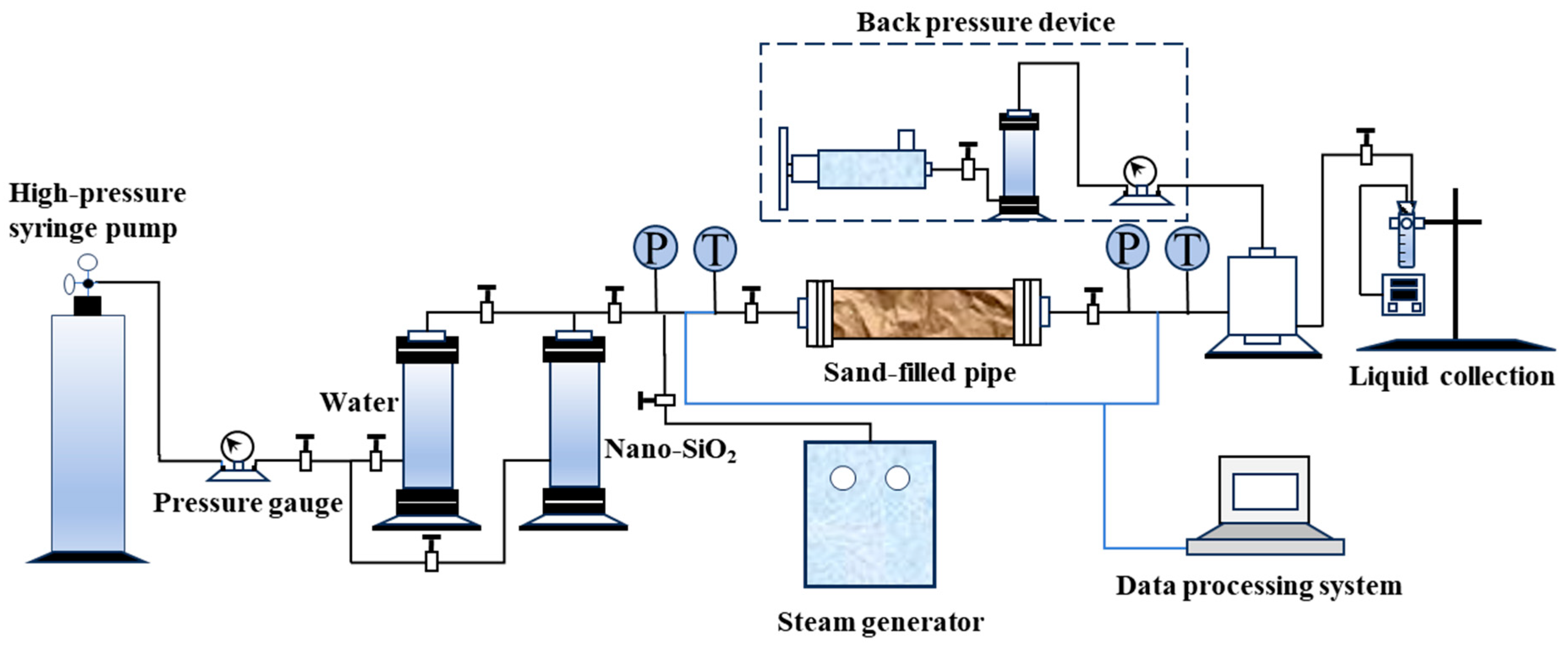
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Material Synthesis
2.2.2. Optimization of Synthesis Conditions
2.2.3. Determination of Viscosity Reduction Rate
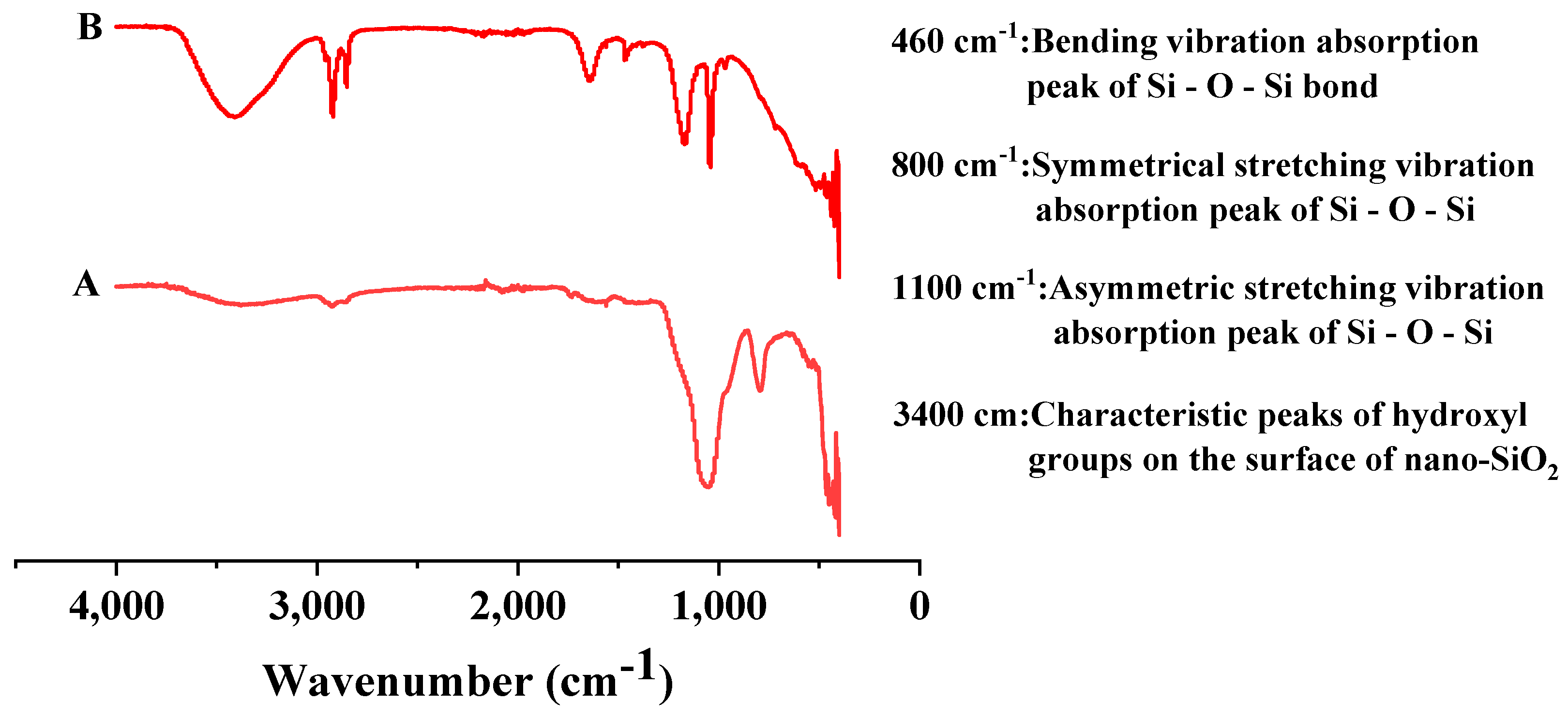
2.2.4. Infrared Spectroscopy Measurement (FTIR)
2.2.5. Transmission Electron Microscopy Measurements (TEM)
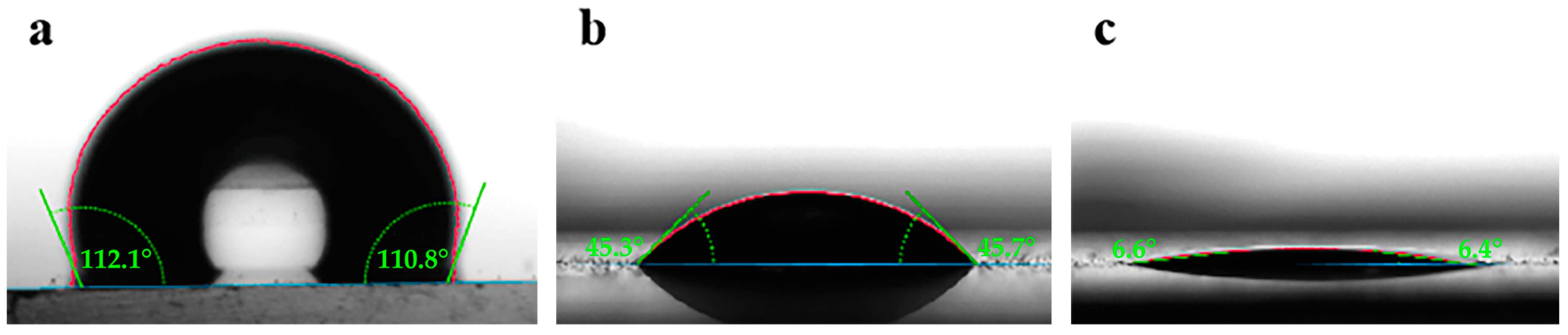
2.2.6. Contact Angle Measurement
2.2.7. Static Adsorption Experiment
2.2.8. Dynamic Adsorption Experiment
2.2.9. Evaluation Methods for Oil Displacement Performance
2.2.10. Long-Term Stability Testing
3. Results and Discussion
3.1. Nanomaterial Characterization
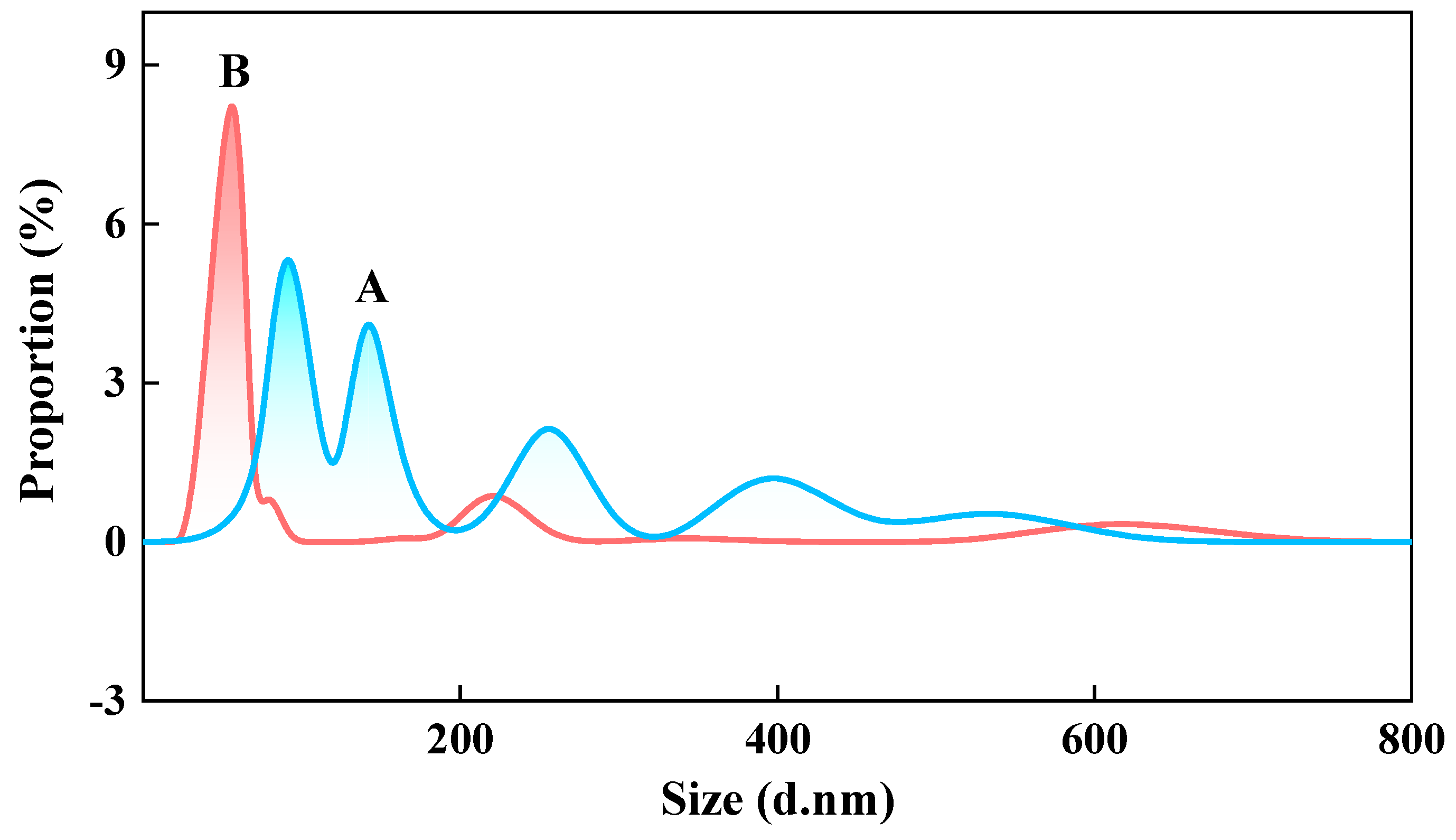
3.1.1. Particle Size Distribution
3.1.2. Material Characterization
3.2. Performance Evaluation
3.2.1. Wettability
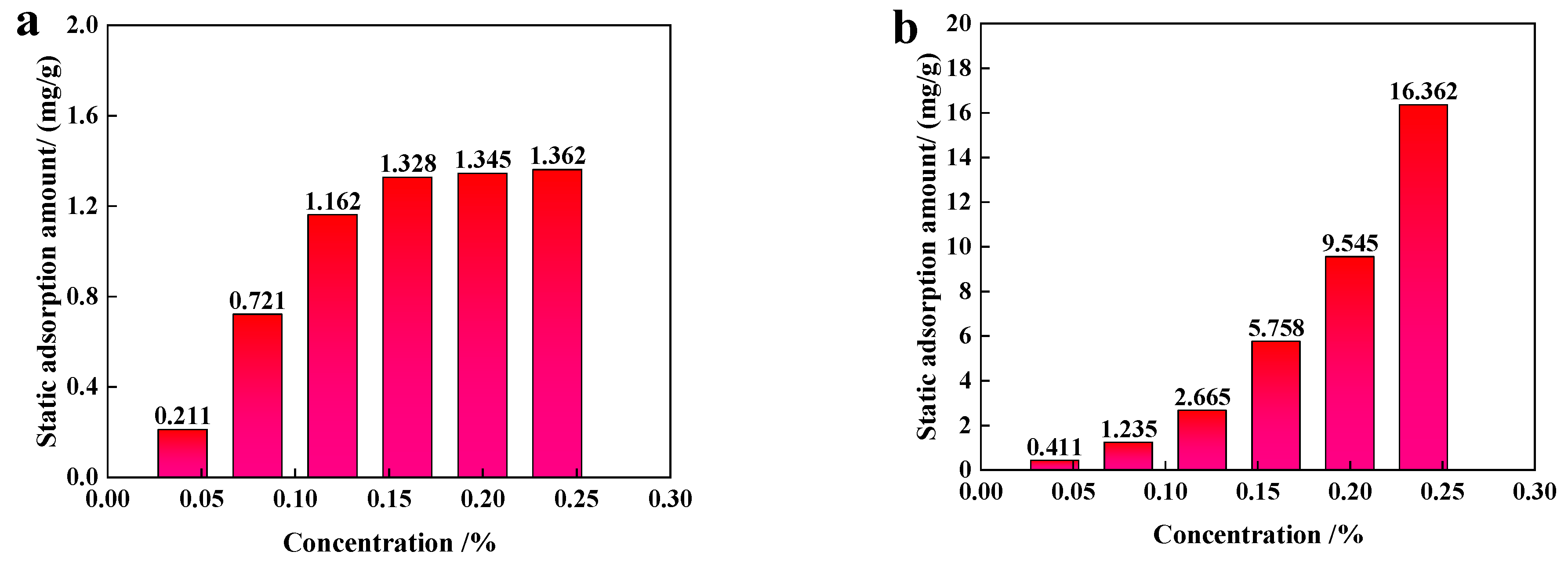
3.2.2. Static Adsorption Performance
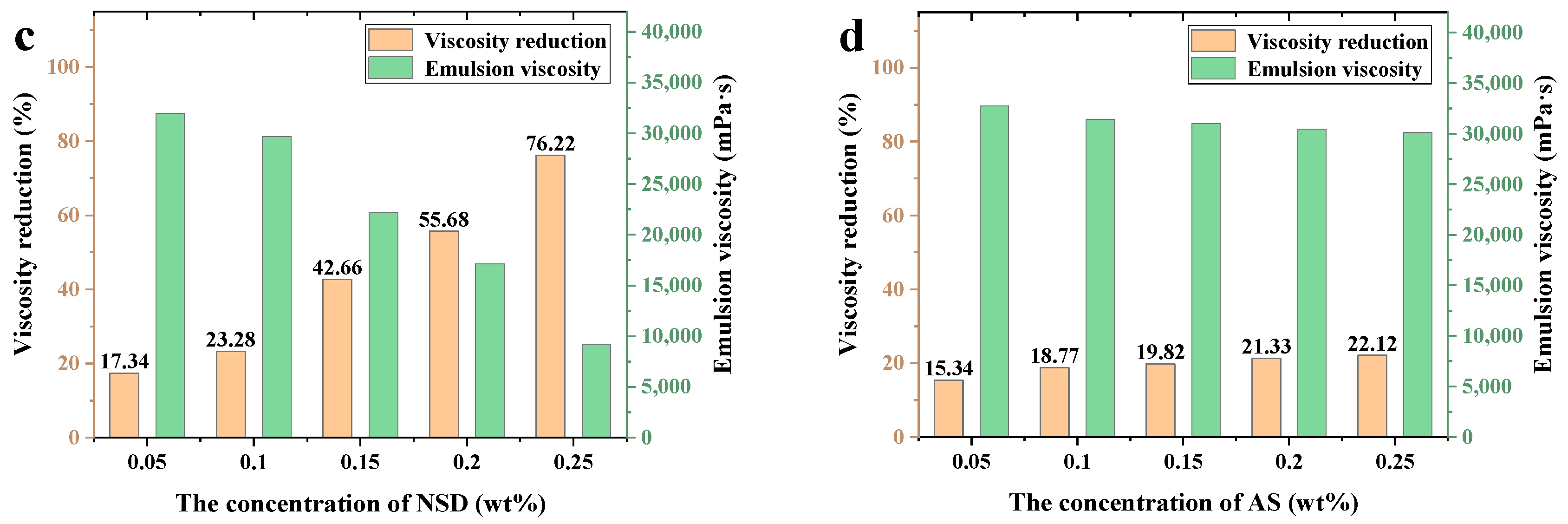
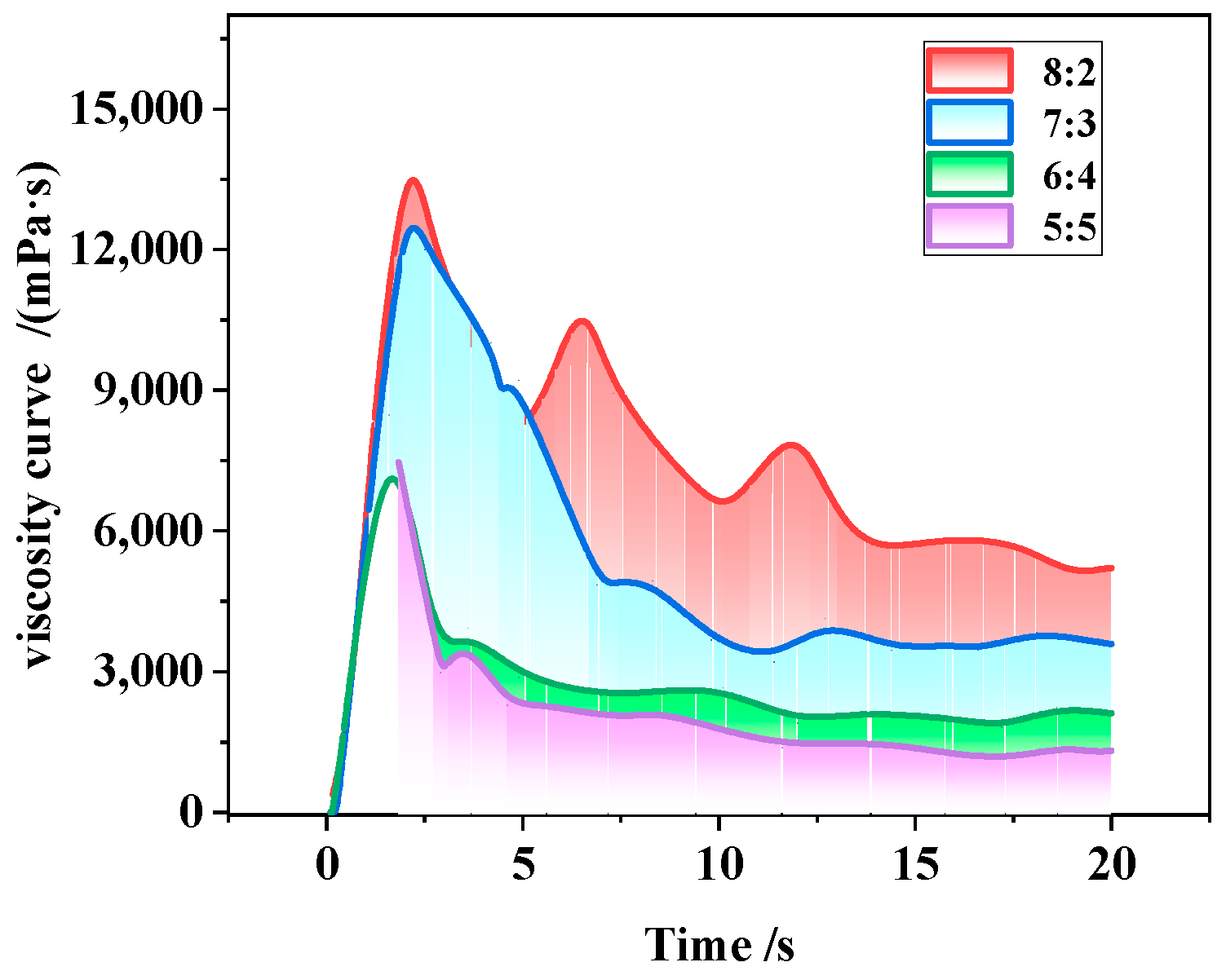
3.2.3. Viscosity-Reducing Performance
3.2.4. Moisture Content Performance
3.2.5. Interfacial Tension
3.2.6. Oil Recovery Efficiency
4. Conclusions
- Our experimental results show that at a concentration of 0.2 wt% and a salinity of 8829 mg/L, the viscosity reduction rates of thick oil (LD-1) before and after aging are 85.29% and 81.36%, respectively. The NSD material was stabilized for more than 90 days under different conditions. Compared with the AS viscosity reducer, 0.2 wt% NSD material has a 29.61% increase in viscosity reduction and better temperature resistance. Achieving higher viscosity reduction rates effectively improves the fluidity and recoverability of heavy oil in reservoirs, thus enhancing oilfield development and production efficiency.
- Contact angle experiments demonstrate that 0.2 wt% concentration of NSD changes the reservoir rock surface from oil-wet to water-wet; interface tension experiments show that the interfacial tension between 0.2 wt% NSD and thick oil is 0.076 mN/m.
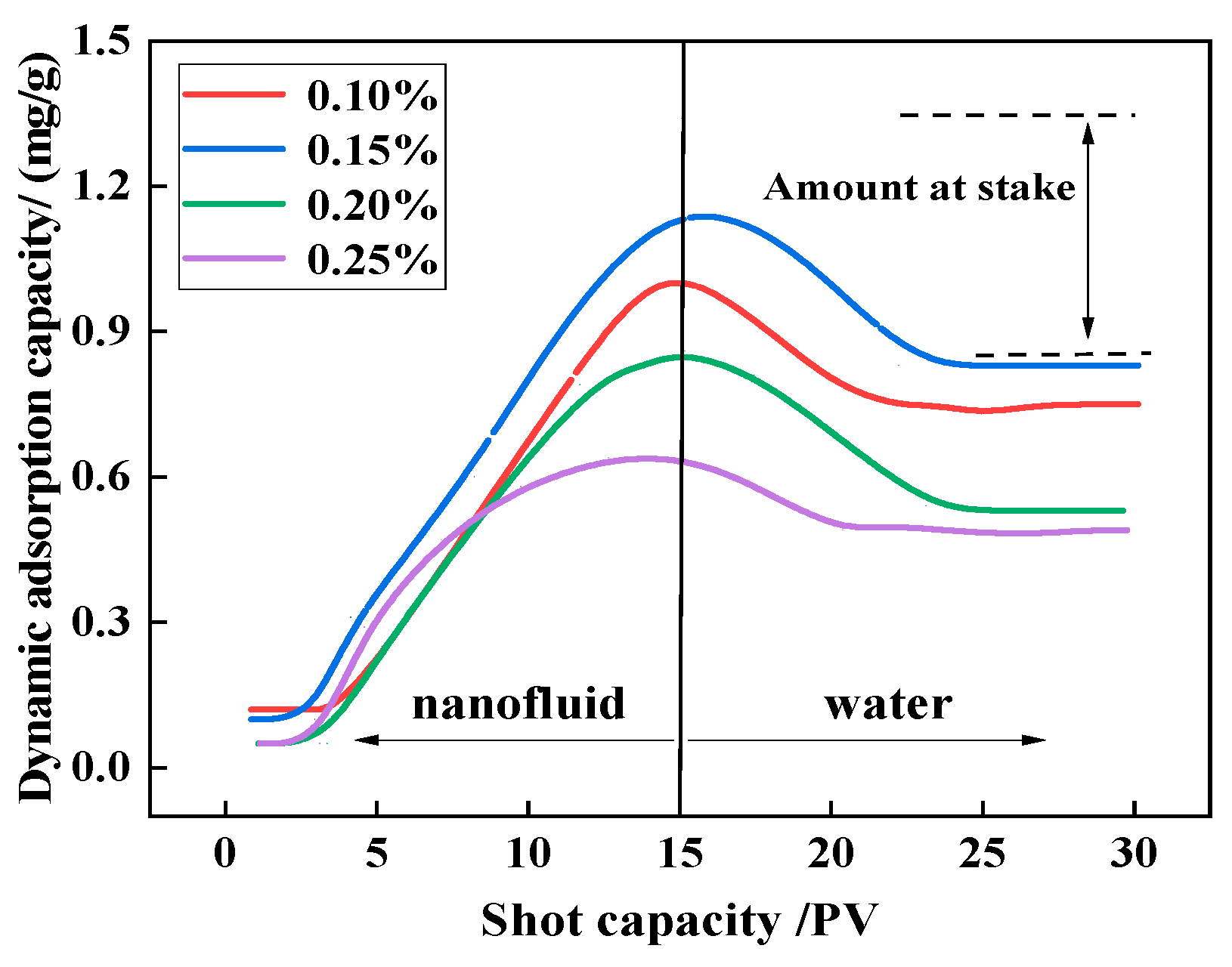
- The dynamic and static adsorption capacities of 0.2 wt% NSD were 1.328 mg/g-sand and 0.745 mg/g-sand, respectively, at a liquid–solid ratio of 10:1. The static adsorption loss of NAD was reduced by 8.2 mg/g-sand as compared with that of the AS viscosity reducer. This indicates a lower loss during reservoir flow.
- One-dimensional displacement experiments validated the oil displacement performance of NSD at different concentrations (0.1 wt%, 0.15%, 0.2 wt%, 0.25 wt%) at 250 °C and compared the oil recovery efficiency of 0.2 wt% NSD with different types of viscosity reducers. The experimental results show that the recovery rate increases with the concentration of NSD, and the recovery rate of thick oil with 0.2 wt% NSD at 250 °C can be increased by 22.8%. The study of nanoviscosity reduction drive systems can effectively improve the development of heavy oil.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negin, C.; Ali, S.; Xie, Q. Application of nanotechnology for enhancing oil recovery—A review. Petroleum 2016, 2, 324–333. [Google Scholar] [CrossRef]
- Orgaz, F.; Rawson, H. Characterization of various stages of the sol-gel process. J. Non-Cryst. Solids 1986, 82, 57–68. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Hart, A.; Lewis, C.; White, T.; Greaves, M.; Wood, J. Effect of cyclohexane as hydrogen-donor in ultradispersed catalytic upgrading of heavy oil. Fuel Process. Technol. 2015, 138, 724–733. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. Heavy crude oil upgrading using nanoparticles by applying electromagnetic technique. Fuel 2018, 232, 704–711. [Google Scholar] [CrossRef]
- Zabala, R.; Franco, C.A.; Cortés, F.B. Application of nanofluids for improving oil mobility in heavy oil and extra-heavy oil: A field test. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. SPE 2016: SPE-179677-MS. [Google Scholar]
- Manan, M.A.; Farad, S.; Piroozian, A.; Esmail, M.J.A. Effects of nanoparticle types on carbon dioxide foam flooding in enhanced oil recovery. Pet. Sci. Technol. 2015, 33, 1286–1294. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Shen, M.; Qi, D.; Xu, G.; Bao, M. Study on the application performance of surfactant compound system BS-9 in heavy oil emulsification and viscosity reduction. Oilfield Chem. 2010, 27, 81–83+25. [Google Scholar]
- Alsoraya, M.; Zhao, M.; Fan, D. Engineered nanomaterials for sustainable oil separation and recovery. ChemNanoMat 2020, 6, 1539–1552. [Google Scholar] [CrossRef]
- Shahrabadi, A.; Bagherzadeh, H.; Roostaie, A.; Golghanddashti, H. Experimental Investigation of HLP Nanofluid Potential to Enhance Oil Recovery: A Mechanistic Approach. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Li, C.; Li, Y.; Pu, H. Molecular simulation study of interfacial tension reduction and oil detachment in nanochannels by Surface-modified silica nanoparticles. Fuel 2021, 292, 120318. [Google Scholar] [CrossRef]
- Chen, L.; Huang, F.; Li, G.; Mao, Z.; Hu, Y.; Liu, L.; Zeng, H.; Xu, S. Experimental Study on Fiber Balls for Bridging in Fractured-Vuggy Reservoir. SPE J. 2023, 28, 1880–1894. [Google Scholar] [CrossRef]
- Chen, L.; Li, G.; Chen, Y.; Zeng, H.; Mao, Z.; Liu, L.; Wang, X.; Xu, S. Thixotropy research of laponite-hydrogel composites for water shutoff in horizontal wells. J. Pet. Sci. Eng. 2022, 208, 109600–109611. [Google Scholar] [CrossRef]
- Natalya, S.A.C.; Kadja, G.T.M.; Azhari, N.J.; Khalil, M.; Fajar, A.T. Two-dimensional (2D) nanomaterials for enhanced oil recovery (EOR): A review. FlatChem 2022, 34, 100383. [Google Scholar] [CrossRef]
- El-Masry, J.F.; Bou-Hamdan, K.F.; Abbas, A.H.; Martyushev, D.A. A Comprehensive Review on Utilizing Nanomaterials in Enhanced Oil Recovery Applications. Energies 2023, 16, 691. [Google Scholar] [CrossRef]
- Mao, J.; Li, J.; Li, Y.; Zhao, J. Research and progress of chemical flooding agents for ultra-heavy oil. Appl. Chem. Eng. 2016, 45, 1367–1371. [Google Scholar]
- Wang, D.; Zhang, J.; Lv, X.; He, C.; Li, Q.; Tan, Y. Evaluation of Gemini surfactant for viscosity reduction of heavy oil in offshore S oilfield. Editor. Dep. Pet. Geol. Recovery Effic. 2015, 22, 109–113. [Google Scholar]
- Guo, J.; Wang, H.; Chen, C.; Chen, Y.; Xie, X. Synthesis and evaluation of an oil-soluble viscosity reducer for heavy oil. Pet. Sci. 2010, 7, 536–540. [Google Scholar] [CrossRef]
- Subramanian, D.; Wu, K.; Firoozabadi, A. Ionic liquids as viscosity modifiers for heavy and extra-heavy crude oils. Fuel 2015, 143, 519–526. [Google Scholar] [CrossRef]
- Privman, V.; Goia, D.V.; Park, J.; Matijević, E. Mechanism of Formation of Monodispersed Colloids by Aggregation of Nanosize Precursors. J. Colloid Interface Sci. 1998, 213, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Ray, J.C.; Choi, J.S.; Ahn, W.S. Morphology control of MSU-1 silica particles. J. Non-Cryst. Solids 2008, 354, 1–9. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Almao, P.P. Nanoparticle technology for heavy oil in-situ upgrading and recovery enhancement: Opportunities and challenges. Appl. Energy 2014, 133, 374–387. [Google Scholar] [CrossRef]
- Huibers, B.M.J.; Pales, A.R.; Bai, L.; Li, C.; Mu, L.; Ladner, D.; Daigle, H.; Darnault, C.J. Wettability alteration of sandstones by silica nanoparticle dispersions in light and heavy crude oil. J. Nanoparticle Res. 2017, 19, 323. [Google Scholar] [CrossRef]
- Lashari, N.; Ganat, T. Synthesized graphene oxide and fumed aerosil 380 dispersion stability and characterization with partially hydrolyzed polyacrylamide. Chin. J. Chem. Eng. 2021, 34, 307–322. [Google Scholar] [CrossRef]
- Patel, H.; Shah, S.; Ahmed, R.; Ucan, S. Effects of nanoparticles and temperature on heavy oil viscosity. J. Pet. Sci. Eng. 2018, 167, 819–828. [Google Scholar] [CrossRef]
- Desouky, S.; Betiha, M.; Badawi, A.; Ghanem, A.; Khalil, S. Catalytic aquathermolysis of Egyptian heavy crude oil. Int. J. Chem. Mol. Eng. 2013, 7, 638–643. [Google Scholar]
- Aristizábal-Fontal, J.E.; Cortés, F.B.; Franco, C.A. Viscosity reduction of extra heavy crude oil by magnetite nanoparticle-based ferrofluids. Adsorpt. Sci. Technol. 2018, 36, 23–45. [Google Scholar] [CrossRef]
- Li, H.; Gao, H.; Zhao, X.; Xia, Z.; Yu, B.; Sun, D. Experimental study on viscosity reduction of heavy oil with water content by synergistic effect of microwave and nano-catalyst. J. Pet. Sci. Eng. 2022, 208, 109271. [Google Scholar] [CrossRef]
- Ahmadi, A.; Manshad, A.K.; Akbari, M.; Ali, J.A.; Jaf, P.T.; Abdulrahman, A.F. Nano-stabilized foam for enhanced oil recovery using green nanocomposites and anionic surfactants: An experimental study. Energy 2024, 290, 130201. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.; Zhong, X.; Zhang, S.; Pu, H.; Zhao, J.X. Development of silicon quantum dots based nano-fluid for enhanced oil recovery in tight Bakken cores. Fuel 2020, 277, 118203. [Google Scholar] [CrossRef]
- Dong, Z.; Qu, N.; Jiang, Q.; Zhang, T.; Han, Z.; Li, J.; Zhang, R.; Cheng, Z. Preparation of polystyrene and silane-modified nano-silica superhydrophobic and superoleophilic three-dimensional composite fiber membranes for efficient oil absorption and oil-water separation. J. Environ. Chem. Eng. 2024, 12, 112690. [Google Scholar] [CrossRef]
- Xue, Q.; Wu, W.; Wu, J.Q.; Zhang, Y.; Gao, X.; Lv, Z.; Lei, Y.; Huang, Y. Nano-Fe3O4/chitosan-based superhydrophobic coatings with magnetic oil-water separation and photothermal conversion properties. Colloids Surf. A Physicochem. Eng. Asp. 2024, 689, 133698. [Google Scholar] [CrossRef]
- Urazov, K.K.; Sviridenko, N.N. NiO based catalysts obtained “in-situ” for heavy crude oil upgrading: Effect of NiO precursor on the catalytic cracking products composition. J. Taiwan Inst. Chem. Eng. 2021, 127, 151–156. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Rakhmatullin, I.Z.; Sitnov, S.A.; Laikov, A.V.; Klochkov, V.V.; Vakhin, A.V. Influence of Co-based catalyst on subfractional composition of heavy oil asphaltenes during aquathermolysis. J. Pet. Sci. Eng. 2020, 186, 106721. [Google Scholar] [CrossRef]
- Temizel, C.; Canbaz, C.H.; Tran, M.; Abdelfatah, E.; Jia, B.; Putra, D.; Irani, M.; Alkouh, A. A comprehensive review heavy oil reservoirs, latest techniques, discoveries, technologies and applications in the oil and gas industry. In Proceedings of the SPE International Heavy Oil Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2018; OnePetro: Richardson, TX, USA, 2018. [Google Scholar]
- Tang, X.; Duan, W.; Xu, K.; Zheng, C. Three-dimensional network gel structure and viscosity reduction mechanism of heavy oil. Colloids and Surfaces, A. Physicochem. Eng. Asp. 2022, 653, 130060. [Google Scholar] [CrossRef]
- Chen, S.; Han, M.; AlSofi, A. Evaluation of Viscosity Reducers for Heavy Crude Oil Viscosity Reduction and Displacement. In Proceedings of the 83rd EAGE Annual Conference & Exhibition, Madrid, Spain, 6–9 June 2022; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2022; Volume 2022, pp. 1–5. [Google Scholar]
- Chen, S.; AlSofi, A.M.; Wang, J.; Alotaibi, M.B. A Polycyclic–Aromatic Hydrocarbon-Based Water-Soluble Formulation for Heavy Oil Viscosity Reduction and Oil Displacement. Energy Fuels 2023, 37, 11864–11880. [Google Scholar] [CrossRef]
- Xu, J.; Wang, N.; Xue, S.; Zhang, H.; Zhang, J.; Xia, S.; Han, Y. Insights into the mechanism during viscosity reduction process of heavy oil through molecule simulation. Fuel 2022, 310, 122270. [Google Scholar] [CrossRef]
- Majeed, E.M.; Naife, T.M. Enhancement viscosity reduction and API of Iraqi heavy crude oil by nanoparticles. In Proceedings of the AIP Conference Proceedings, Baghdad, Iraq, 24–25 November 2021; AIP Publishing: Melville, NY, USA, 2023; Volume 2651. [Google Scholar]
- Yu, J.; Quan, H.; Huang, Z.; Li, P.; Chang, S. Synthesis of a Heavy-Oil Viscosity Reducer Containing a Benzene Ring and Its Viscosity Reduction Mechanism. ChemistrySelect 2022, 7, e202102694. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. Metallic nanoparticles for enhanced heavy oil recovery: Promises and challenges. Energy Procedia 2015, 75, 2068–2073. [Google Scholar] [CrossRef]
- Luo, P.; Gu, Y. Effects of asphaltene content on the heavy oil viscosity at different temperatures. Fuel 2007, 86, 1069–1078. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Wang, Y.; Li, P. GC-MS used in study on the mechanism of the viscosity reduction of heavy oil through aquathermolysis catalyzed by aromatic sulfonic H3PMo12O40. Energy 2010, 35, 3454–3460. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Babadagli, T. Viscosity reduction of heavy oil/bitumen using micro-and nano-metal particles during aqueous and non-aqueous thermal applications. J. Pet. Sci. Eng. 2014, 119, 210–220. [Google Scholar] [CrossRef]
- Clark, P.D.; Dowling, N.I.; Hyne, J.B.; Lesage, K.L. The chemistry of organosulphur compound types occurring in heavy oils: 4. the high-temperature reaction of thiophene and tetrahydrothiophene with aqueous solutions of aluminium and first-row transition-metal cations. Fuel 1987, 66, 1353–1357. [Google Scholar] [CrossRef]
- Hasan, S.W.; Ghannam, M.T.; Esmail, N. Heavy crude oil viscosity reduction and rheology for pipeline transportation. Fuel 2010, 89, 1095–1100. [Google Scholar] [CrossRef]
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010, 3, 700–714. [Google Scholar] [CrossRef]
- Acres, R.G.; Ellis, A.V.; Alvino, J.; Lenahan, C.E.; Khodakov, D.A.; Metha, G.F.; Andersson, G.G. Molecular structure of 3-aminopropyltriethoxysilane layers formed on silanol-terminated silicon surfaces. J. Phys. Chem. C 2012, 116, 6289–6297. [Google Scholar] [CrossRef]
- Wang, F.; Han, B.; Zhang, L.; Xu, L.; Yu, H.; Shi, W. CO2 reforming with methane over small-sized Ni@ SiO2 catalysts with unique features of sintering-free and low carbon. Appl. Catal. B Environ. 2018, 235, 26–35. [Google Scholar] [CrossRef]
- Mai Nga, T.; Nguyen, N.H.; Tsubota, T.; Shinogi, Y.; Dultz, S.; Nguyen, M.N. Fern Dicranopteris linearis-derived biochars: Adjusting surface properties by direct processing of the silica phase. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123937. [Google Scholar]
- Sadatshojaei, E.; Jamialahmadi, M.; Esmaeilzadeh, F.; Wood, D.A.; Ghazanfari, M.H. The impacts of silica nanoparticles coupled with low-salinity water on wettability and interfacial tension: Experiments on a carbonate core. J. Dispers. Sci. Technol. 2019, 41, 1159–1173. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, L.; Tang, D.; Li, L.; Ge, J.; Zhang, G.; Pei, H. Effect of nano-SiO2 and surfactants on the oil-water interfacial properties. Colloid Polym. Sci. 2019, 297, 903–915. [Google Scholar] [CrossRef]
- Ahmed, A.; Mohd Saaid, I.; Pilus, R.M.; Abbas Ahmed, A.; Tunio, A.H.; Baig, M.K. Development of surface treated nanosilica for wettability alteration and interfacial tension reduction. J. Dispers. Sci. Technol. 2018, 39, 1469–1475. [Google Scholar] [CrossRef]
- Sofla SJ, D.; James, L.A.; Zhang, Y. Understanding the behavior of H+-protected silica nanoparticles at the oil-water interface for enhanced oil recovery (EOR) applications. J. Mol. Liq. 2019, 274, 98–114. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O. Metal oxide-based nanoparticles: Revealing their potential to en-hance oil recovery in different wettability systems. Appl. Nanosci. 2015, 5, 181–199. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z. Challenges and future of chemical assisted heavy oil recovery processes. Adv. Colloid Interface Sci. 2020, 275, 102081. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, W.; Dai, C.; Huang, Y.; Li, H.; Zhao, M.; He, L.; Jiao, B. Reducing surfactant adsorption on rock by silica nanoparticles for en-hanced oil recovery. J. Pet. Sci. Eng. 2017, 153, 283–287. [Google Scholar] [CrossRef]
- Mohammed, I.; Afagwu, C.C.; Adjei, S.; Kadafur, I.B.; Jamal, M.S.; Awotunde, A.A. A review on polymer, gas, surfactant and nanoparticle adsorption modeling in porous media. Oil Gas Sci. Technol. Rev. d’ifp Energ. Nouv. 2020, 75, 77. [Google Scholar] [CrossRef]
- Zhou, W.; Dong, M.; Liu, Q.; Xiao, H. Experimental investigation of surfactant adsorption on sand and oil-water interface in heavy oil/water/sand systems. In Proceedings of the PETSOC Canadian International Petroleum Conference, Calgary, AB, Canada, 7–9 June 2005. PETSOC-2005-192. [Google Scholar]
- Ameri, H.; Motahari, M.; Ghahramani, K.; Moghadasi, J. Viscosity reduction of heavy crude oils and synthetic oils with various types of rheological behavior by nano-silica fluids. Chem. Pap. 2022, 76, 6499–6515. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Li, X.; Zhang, Z.; Zhao, M.; Su, C. Preparation of silica-supported nanoFe/Ni alloy and its application in viscosity reduction of heavy oil. Micro Nano Lett. 2015, 10, 167–171. [Google Scholar] [CrossRef]
- Wang, C.; Gao, L.; Liu, M.; Xia, S.; Han, Y. Viscosity reduction mechanism of functionalized silica nanoparticles in heavy oil-water system. Fuel Process. Technol. 2022, 237, 107454. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Salem Ragab, A.M.; Hannora, A.E. A Comparative investigation of nano particle effects for improved oil recovery—Experimental work. In Proceedings of the SPE Kuwait Oil and Gas Show and Conference, Mishref, Kuwait, 11–14 October 2015. [Google Scholar]
- Dai, C.; Wang, S.; Li, Y.; Gao, M.; Liu, Y.; Sun, Y.; Zhao, M. The first study of surface modified silica nanoparticles in pressure-decreasing application. RSC Adv. 2015, 5, 61838–61845. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of nanoparticles in enhanced oil recovery: A critical review of recent progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Li, S.; Torsaeter, O. Enhancing oil recovery of low-permeability berea sandstone through optimised nanofluids concentration. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013. [Google Scholar]
- Skauge, T.; Spildo, K.; Skauge, A. Nano-sized particles for EOR. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Youssif, M.I.; El-Maghraby, R.M.; Saleh, S.M.; Elgibaly, A. Silica nanofluid flooding for enhanced oil recovery in sandstone rocks. Egypt. J. Pet. 2018, 27, 105–110. [Google Scholar] [CrossRef]




| Ion Type | Na+ | K+ | Mg2+ | Ca2+ | Cl− | SO42− | HCO3− | Total Salinity |
|---|---|---|---|---|---|---|---|---|
| Ion Content (mg/L) | 2448 | 231 | 672 | 832 | 4256 | 66 | 324 | 8829 |
| w (Saturated Hydrocarbons)/% | w (Aromatic Hydrocarbons)/% | w (Resin Content of Heavy Oil)/% | w (Asphaltene)/% |
|---|---|---|---|
| 28.14 | 37.53 | 19.79 | 14.54 |
| Materials | Concentration/% | Retention Time/d | Reduction Rate/% |
|---|---|---|---|
| NSD | 0.2 | 30 | 83.12 |
| 0.2 | 60 | 82.52 | |
| 0.2 | 90 | 80.12 |
| Test | NSD Concentration (wt%) | Steam Temperature (°C) | Saturated Oil (mL) | Oil Saturation (%) | Oil Recovery (%) | |||
|---|---|---|---|---|---|---|---|---|
| Steam Flooding | NSD Flooding | Poststeam Flooding | Additional Increment | |||||
| A | 0.1 | 250 | 50.4 | 80.6 | 31.8 | 36.3 | 37.2 | 5.4 |
| B | 0.15 | 50.8 | 80.9 | 32.0 | 44.2 | 47.9 | 15.9 | |
| C | 0.2 | 50.6 | 80.7 | 30.9 | 49.2 | 53.7 | 22.8 | |
| D | 0.25 | 50.5 | 80.6 | 32.3 | 50.3 | 55.2 | 22.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zheng, W.; Zhang, H.; Tang, C.; Zhang, J.; Yu, D.; Lu, X.; Li, G. The Role of Amphiphilic Nanosilica Fluid in Reducing Viscosity in Heavy Oil. Energies 2024, 17, 2625. https://doi.org/10.3390/en17112625
Wang Y, Zheng W, Zhang H, Tang C, Zhang J, Yu D, Lu X, Li G. The Role of Amphiphilic Nanosilica Fluid in Reducing Viscosity in Heavy Oil. Energies. 2024; 17(11):2625. https://doi.org/10.3390/en17112625
Chicago/Turabian StyleWang, Yuejie, Wei Zheng, Hongyou Zhang, Chenyang Tang, Jun Zhang, Dengfei Yu, Xuanfeng Lu, and Gang Li. 2024. "The Role of Amphiphilic Nanosilica Fluid in Reducing Viscosity in Heavy Oil" Energies 17, no. 11: 2625. https://doi.org/10.3390/en17112625
APA StyleWang, Y., Zheng, W., Zhang, H., Tang, C., Zhang, J., Yu, D., Lu, X., & Li, G. (2024). The Role of Amphiphilic Nanosilica Fluid in Reducing Viscosity in Heavy Oil. Energies, 17(11), 2625. https://doi.org/10.3390/en17112625





