Abstract
Ensuring a stable and cost-effective energy supply is a major challenge for the International Energy Agency (IEA). Additionally, the effectiveness of vermiculite and dolomite in mitigating the adverse effects of diesel oil, a petroleum-derived product, on plant growth and development, and on the biochemical activity of the soil, were assessed. Therefore, an attempt was made in the study to determine the energy properties of Zea mays, which is suitable for cultivation in contaminated areas. For these purposes, several parameters were analyzed in its biomass, including calorific value (Q), heating value (Hv), energy yield (Yep), ash content, and the presence of carbon (C), hydrogen (H), sulfur (S), nitrogen (N), and oxygen (O). Biochemical activity was measured through the evaluation of soil enzymes serving as indicators for the carbon (dehydrogenases, catalase, β-glucosidase), nitrogen (urease), sulfur (arylsulfatase), and phosphorus (acid and alkaline phosphatase) cycles. The plant greenness index was also determined. It has been demonstrated that diesel oil does not alter the calorific value of Zea mays biomass but significantly reduces the biomass quantity and destabilizes the biochemical properties of the soil. Zea mays contained an average of 6.84% ash, 49.88% C, 5.65% H, 0.17% S, 2.90% N, and 34.57% O. The calorific value of Zea mays ranged from 15.02 to 15.54 MJ kg−1 d.m. of plants, and the heating value ranged from 18.25 to 19.21 MJ kg−1 d.m. of plants. The biomass obtained from contaminated soil is recommended for energy purposes. The sorbents used—vermiculite and dolomite—proved to be less effective in the remediation of soil contaminated with diesel oil.
1. Introduction
Agricultural, forestry, and municipal waste by-products can be used to a significant extent for energy generation. The utilization of renewable energy sources has become a primary element of energy strategies. According to the International Energy Agency [1,2], low-emission fuels accounted for only 1% of global energy consumption in 2022. In the ‘net-zero emissions by 2050’ scenario, low-emission fuels are expected to account for approximately 2% of global electricity production by 2050. Therefore, to achieve these targets, rapid deployment of low-emission fuel production and distribution is necessary. It is forecasted that, by 2026 [1,3] clean energy sources will cover global electricity demand. Thus, doubling the global pace of progress in energy efficiency is a significant step towards achieving net-zero emissions. Therefore, it is worthwhile to seek energy crops, especially those resistant to extreme conditions [4,5]. This leads, on the one hand, to soil remediation and, on the other hand, to the acquisition of inexpensive energy [6,7].
Within the framework of the Net Zero Emissions by 2050 (NZE) scenario, more than 60% of the 100 exajoules (EJ) of global bioenergy supply in 2050 is anticipated to stem from agricultural residues, organic remnants, municipal waste, and forest residues, currently approximated at around 20% [2]. In a recent IEA special report on climate change, Gül et al. [8] outlined strategies for the global energy sector to attain net-zero emissions by 2050. The authors suggest focusing on the production of bioenergy from short-rotation crops cultivated on arable lands, pastures, and marginal lands unsuitable for food production. Such crops could contribute to about 50% of bioenergy production compared to the currently envisaged bioenergy crops and forest plantations. According to the authors, sustainable bioenergy utilization not only helps to avoid the adverse effects of increased deforestation but also mitigates competition for plants used for consumption purposes. This approach can also have yield benefits extending beyond the energy sector, such as health advantages arising from reduced air pollution, proper waste management, or the reduction of methane emissions from inefficient burning and decay of waste.
One of the challenges in agriculture is to achieve better yields and, consequently, greater biomass, even under unfavorable environmental conditions [9]. Maize, characterized by excellent photosynthetic efficiency (C4 plant), exhibits high tolerance to biotic stresses and thrives across a broad range of tropical, subtropical, and temperate zones [10,11,12,13]. In Kenya, maize is cultivated in the tropical lowlands (0–1000 m above sea level) and in the long-season mountainous areas (>1800 m above sea level) [10,12]. The growing interest in cultivating this crop stems from its versatile applications [14,15,16]. It is estimated that maize requires 1222 dm3 of water per kg of product, which compares favorably with other staple cereals. Therefore, it plays a crucial role in increasing yields worldwide [17].
Maize (Zea mays) is not a typical short-rotation crop, but it is frequently cultivated in a crop rotation system with other plants to minimize the risk of plant diseases, pest infestations, nutrient depletion [18], and pollution.
The continuing global demand for crude oil continues to contribute significantly to the accumulation of pollutants in the soil, prompting the ongoing search for effective remediation strategies. Phytoremediation, as one of the possible remediation techniques, has the potential to enhance the biomass available for bioenergy production. While plant yields may not be high, and plants exhibit low resistance to hydrocarbons, the latter possess the capability for their degradation. The degradation processes from petroleum-derived substances depend on the chemical structure and properties of hydrocarbons [19,20] and their accumulation in the environment [21]. Baghaie et al. [22] demonstrated that the intercropping system of corn and white clover contributes to diesel oil degradation in the soil. According to Borowik et al. [23], soil contamination with diesel fuel significantly inhibits the growth and development of maize, modifies its chemical composition, and reduces the bioconcentration index of nitrogen, phosphorus, calcium, and potassium. In soil sown with Dactylis glomerata, the degradation of diesel oil occurred through rapid degradation of xylene, ethylbenzene, toluene, naphthalene, and anthracene, as well as slower degradation of benzo(a)anthracene, benzo(b)fluoranthene, and indeno(1,2,3-cd)pyrene [19]. The significant role of plants in the phytoremediation of soils contaminated with petroleum-derived substances has been corroborated by Jing et al. [24] and Iqbal et al. [25]. Plants possessing the ability to facilitate the degradation of these pollutants or to participate in their absorption, translocation, and accumulation in the root zone are highly valuable. This is because petroleum hydrocarbons, upon migration to terrestrial environments, can alter the activity and efficiency of ecosystems [26]. Therefore, sound environmental practices in the production of bioenergy feedstocks are crucial. This challenge aligns with the principles of sustainable development, wherein it is anticipated that plant biomass should be extensively employed for heat generation or biofuel conversion on a large scale [27].
The biomass derived from maize is used in energy production, serving as one of the substrates in agricultural biogas plants [28,29]. It can be utilized in both direct combustion processes for solid feedstocks and for the production of liquid and gaseous biofuels [17,30,31]. Its rapid growth reflects its use as a forage, industrial, and energy crop in certain countries [30,31].
The fact that soil enzymes participate in the breakdown of cellulose, hemicellulose, and lignin into glucose and simple sugars, which can be processed in energy-generating processes, is crucial for understanding and optimizing energy production from biomass [32,33]. This enzymatic capability to effectively degrade complex organic polymers present in various types of plant biomass and organic residues facilitates the efficient utilization of these raw materials in the production of biofuels, providing a sustainable alternative to traditional energy sources [32,34]. Understanding the action of soil enzymes is therefore essential to optimizing biotechnological processes aimed at maximizing the energy potential contained in biomass. Research on soil enzymes is also of paramount importance in the context of increased activity or stability, which can contribute to enhancing biomass conversion processes and creating more efficient and sustainable solutions and modern technologies in the field of renewable energy production.
Soil enzymes respond rapidly to environmental changes, including contamination with diesel oil. Research by Borowik et al. [35], Borowik et al. [19], and Wyszkowska et al. [36] indicate that diesel oil stimulates the activity of soil enzymes. Similar results were reported by Goma-Tchimbakala et al. [37], who observed high activities of β-glucosidase, β-glucosaminidase, and acid phosphatase in soils contaminated with diesel oil and gasoline. According to Gospodarek et al. [38], this effect varies depending on the contaminant substance, the specific enzyme analyzed, and the duration of pollutant persistence in the soil. On the one hand, diesel oil enhances soil enzyme activity but, on the other hand, its constituent aliphatic hydrocarbons (about 75%) and aromatic hydrocarbons (25%) induce a negative response in plants at the seed germination stage by inhibiting this process, inducing oxidative stress and disrupting plant metabolic activity. This is a consequence of the accumulation of petroleum-derived compounds in plant lipoprotein membranes, covalent binding to proteins, DNA, and RNA [39,40], which ultimately leads to a reduction in maize yield and consequently its energy potential [41].
Enzyme activity, together with other biological and chemical properties, can provide insights into the response of soils to disturbances caused by pollution. Assessing their levels can aid in evaluating the condition of the soil and the potential for restoring equilibrium [35,36,38,42,43]. Yang et al. [43] suggest that enzymes from the oxidoreductase class are more sensitive to environmental changes than hydrolases. Hence, oxidoreductases are used as primary bioindicators, while hydrolases serve as auxiliary enzymes associated with the carbon, nitrogen, sulfur, and phosphorus cycles.
The above information formed the basis for conducting a pot experiment aimed at determining the energy properties of Zea mays, based on the heat of combustion and the content of ash, carbon, hydrogen, sulfur, nitrogen, and oxygen in the biomass of this plant. An additional objective was to determine the effectiveness of vermiculite and dolomite in the remediation of soil contaminated with diesel oil and the impact of these sorbents on the calorific value of maize and soil enzyme activity. The research hypothesis in the context of our scientific work anticipates that (a) diesel oil reduces the amount of energy obtained from plants by decreasing the biomass of maize, thereby additionally disrupting the biochemical balance of the soil; (b) vermiculite and dolomite are effective in alleviating the negative effects of diesel oil on the activity of soil enzymes, consequently alleviating the unfavorable influence of diesel oil on the biomass of Zea mays.
2. Materials and Methods
2.1. Soil
The soil material was obtained from arable land located in northeastern Poland (NE, Poland, 53.713° N, 20.432° E). This soil was formed from sands, which cover approximately 50% of the agricultural land in Poland [44]. According to the classification of the International Union of Soil Sciences [45], these soils were classified as Eutric Cambisols. The soil sampled from the topsoil layer (0–0.20 m) was air-dried and sieved through a 5–7 mm mesh to remove larger stones and plant residues. It was a loamy sand soil comprising the following granulometric composition: sand—75.68%; silt—23.08%; clay—1.24%. The soil had an organic carbon content (Corg) of 9.29 g, total nitrogen content (NTotal) of 1.22 g, and a pH in KCl of 4.2.
2.2. Zea mays
For the phytoremediation of soil contaminated with diesel, it was decided to use Zea mays, one of the most important crops worldwide with various applications in food production, animal feed, and biofuels [46,47]. Zea mays, characterized by high photosynthetic efficiency (C4) and rapid biomass growth, can be processed into ethanol, biodiesel, biogas, bio-oil, and chemical compounds that can replace or complement fossil fuels and petroleum-derived products. It can also contribute to carbon sequestration and enhance soil quality. It is therefore a crop that can support the sustainable development of energy and biomass, provided it is grown in an environmentally and economically sound manner [18,48]. An additional advantage in favor of using Zea mays in the research was its impressive dry biomass yield [49]. In our study, we used the maize variety DS1897B, produced by Pioneer, Poland, and purchased from FarmSYSTEM s.c., Bydgoszcz, Poland. The maize was grown in pots with a capacity of 3.5 dm3. Eight Zea mays seeds were sown in each pot and, after germination, five plants were retained per pot. The harvest of the above-ground parts and roots of Zea mays was carried out at the Biologische Bundesanstalt, Bundessortenamt, and Chemical (BBCH) stage 51.
2.3. Characteristics of Petroleum-Derived Substances
The EFECTA diesel oil is a new type of fuel for Diesel engines in Poland. Introduced by PKN Orlen in 2022, its main advantages are reduced combustion, engine cleaning, and reduced pollutant emissions. EFECTA 95 diesel oil is a premium fuel that meets the requirements of the PN-EN 590:2022-08 [50] standard and contains refining additives that improve its properties. The cetane number of the oil is at least 51, indicating better ignition and reduced engine noise. Optimum efficiency and fuel economy are determined by the oil density, which according to the manufacturer ranges from 820 to 845 kg·m−3. The minimum flash point of 55 °C suggests increased safety and reduced risk of ignition as well as engine operation. Diesel fuel 95 EFECTA contains a maximum of 10 mg of sulfur, 200 mg of water, and 24 mg of solid contaminants per kilogram. Additionally, it contains 7.0% polycyclic aromatic hydrocarbons and 7.0% fatty acid methyl esters (FAME). Detailed specifications are available at https://www.orlen.pl/pl/dla-biznesu/produkty/paliwa/oleje-napedowe/verva-on (accessed on 21 May 2024) [51].
2.4. Vermiculite Characteristics
Vermiculite is a clay mineral that expands upon heating [52,53]. It forms during the weathering or hydrothermal alteration of biotite or phlogopite [54]. Vermiculite is chemically inert, biologically stable, and non-combustible. It has a high sorption capacity and buffering properties [55,56,57,58].
Vermiculite is also employed as a filtering material in the food industry, a carrier in animal feeds, or a sorbent in environmental protection. Vermiculite’s ability to absorb and store a significant amount of water and cations, gradually releasing them, characterizes it as an excellent buffering material influencing the maintenance of optimal soil pH, aeration, and nutrient retention [55,56,57,58]. The vermiculite used in the experiment was purchased from Sobex company (Drezdenko, Poland). It was characterized by the following properties: pHKCl 3.79, hydrolytic acidity 66.00 mmol(+) kg−1 d.m. of soil, and sum of exchangeable base cations 576.00 mmol(+) kg−1 d.m. of soil.
2.5. Dolomite Characteristics
Ground dolomite is a sedimentary rock primarily composed of calcium and magnesium carbonates. It finds applications in the production of refractory materials, mineral fertilizers, cement, and ceramics. The characteristics of ground dolomite depend on its chemical composition, microstructure, texture, and porosity [59,60]. The dolomite used in the experiment was purchased from Biovita Sp. z o.o. (Tenczynek, Poland). It was characterized by the following properties: pHKCl 8.70, hydrolytic acidity 9.75 mmol(+) kg−1 d.m. of soil, and sum of exchangeable base cations 992.00 mmol(+) kg−1 d.m. of soil.
2.6. Research Design
The pot experiment was conducted in the vegetative hall of the University of Warmia and Mazury in Olsztyn (NE, Poland) with four replications.
The study was a two-factor experiment: factor 1—diesel oil dose (described in Section 2.3): 0 and 24 cm3 kg−1 d.m. of soil; factor 2—addition of sorbents: vermiculite and dolomite (described in Section 2.4 and Section 2.5) in amounts of 0 and 10 g kg−1 d.m. of soil. The experiment was conducted in polyethylene pots with dimensions of 14.5 cm (ϕ base diameter), 19.5 cm (ϕ top diameter), and 16.5 cm (height). A total of 24 pots were used in the experiment. Each pot contained 3.4 kg of soil.
The procedure for setting up and conducting the experiment was as follows: (1) preparation of approximately 3.4 kg of soil; (2) application of N, P, K, and Mg fertilizers to the soil at rates of 225, 50, 150 and 20 mg kg−1 dry soil, respectively, with nitrogen applied as N2H4CO, phosphorus as KH2PO4, potassium as KH2PO4 and KCl, and magnesium as MgSO4 × 7H2O; (3) application of diesel oil (DO) at rates of 0 and 24 cm3 kg−1 dry soil; (4) application of sorbents, vermiculite, and dolomite, at rates of 0 and 10 g kg−1 dry soil. The prepared soil was mixed with mineral fertilizers and, in relevant treatments, with diesel oil and sorbents. The entire mixture was packed into polyethylene pots with a capacity of 3.5 dm3. The soil moisture in the pots was adjusted to 60% of the maximum water-holding capacity and maintained at this level throughout the plant’s vegetative period (60 days). On the day of plant harvest (aerial parts and roots), soil samples were collected for biochemical and physicochemical analyses.
2.7. Determination of the Heat of Combustion and Elemental Composition of Zea mays Biomass
Samples of Zea mays biomass were prepared in accordance with the recommendations presented in the PN-EN 14780:2017 standard [61], as well as methods described in the publications by Wyszkowska et al. [9,62] and Kwiatkowski et al. [63]. The biomass was dried in a dryer at a temperature of 105 °C (BINDER FD 53, Tuttlingen, Germany). For analysis, 50 g of well-dried and ground biomass (Retsch SM 200, Haan, Germany) with a diameter of 0.5 mm was selected. The following parameters were chosen to determine the energy value of the biomass, which were determined according to the following standards:
- (1)
- The heat of combustion (Q) and calorific value (Hv)—PN-EN ISO 18125:2017 [64],
- (2)
- Energy content obtained from plant biomass (Yep),
- (3)
- Ash content—PN-EN ISO 18122:2016-01 [65],
- (4)
- Carbon (C), hydrogen (H), and nitrogen (N) content—PN-EN ISO 16948:2015-07 [66], sulfur (S)—PN-G-04584:2021 [67,68], and oxygen (O).
The following equipment was used for the above determinations: C-2000 calorimeter (IKA, WERKE, Wilmington, DE, USA), CHS 500 analyzer (ELTRA, Neuss, Germany), K-424 fermentation unit, and B-324 distillation unit (BUCHI, Labotechnik AG, Flawil, Switzerland).
2.8. Physicochemical and Chemical Soil Indicators
For physicochemical determinations, the soil was air-dried at room temperature. The scope of soil chemical properties included the determination of total nitrogen (Ntot) and organic carbon (Corg) content. Analyses were performed using the Vario Max CNS analyzer (Elementar, Jena, Germany). Soil particle size distribution was determined by the aerometric method, and pH in 1 mol KCl dm−3 was measured potentiometrically using the HI 2221 m (Hanna Instruments, Washington, UK). The analyses were conducted in triplicate. The determination of soil enzyme activities, including dehydrogenases (Deh), catalase (Cat), and β-glucosidase (Glu) as indicators of the C cycle, urease (Ure) related to the N cycle, arylsulfatase (Aryl) linked to the S cycle, and acid phosphatase (AcP) and alkaline phosphatase (AlP) associated with the P cycle, was performed on fresh soil samples directly after harvesting the experimental plots. The specific procedures for enzyme assays (buffers, incubation and reaction stoppage times, and temperature) were described in our previous publications [19,69,70]. The activity of Deh, Ure, Glu, Aryl, AcP, and AlP enzymes was determined using a Perkin-Elmer Lambda 25 spectrophotometer (Waltham MA, USA).
2.9. Calculations and Statistical Analyses
To evaluate the influence of diesel oil (DO), vermiculite (V), and dolomite (D) on the soil’s biochemical activity, as well as on the biomass of Zea mays aerial parts and roots, we calculated the indices of oil and sorbent impact using the formulas described in our previous research [69,71]. These data were presented on thermal maps using RStudio 2023.06.0 software [72] with the addition of R 4.2.2 [73] and the gplots library [74]. We also calculated the coefficients η2, which were presented on a pie chart created with the Circos 0.68 package [75]. Principal component analysis (PCA) was used to highlight the interdependencies between the data, including soil enzyme activity, the yield of aerial parts (Ya) and Zea mays roots (Yr), the energy obtained from plant biomass (Yep), combustion heat of plant biomass (Q), the calorific value of plant biomass (Hv), and plant greenness index (SPAD). A separate statistical analysis for each variable was conducted using the Tukey test with a significance level of p < 0.05 [76].
3. Results
3.1. Zea mays Biomass and Its Energetic Value
The Soil contamination of diesel oil negatively affected the growth and development of Zea mays (Table 1). The applied EFECTA diesel oil in the experiment resulted in a significant reduction in the aerial parts’ biomass yield (Ya) and root yield (Yr) of the plants, by 89% and 84%, respectively. The application of vermiculite to uncontaminated soil increased the aerial parts’ biomass yield of Zea mays by 3%, whereas dolomite did not significantly alter it. The effect of these sorbents in diesel oil-contaminated soil varied. Vermiculite mitigated the negative influence of the petroleum-derived product on the aerial parts’ biomass (by 13%), whereas dolomite exacerbated (by 29%) the adverse effects of diesel oil on Zea mays yield. Unfortunately, the sorbents applied not only significantly negatively exerted the growth of Zea mays roots in uncontaminated soil but also proved ineffective in mitigating the negative effect of diesel oil on root growth and development. These relationships were manifested in the ratio of aerial parts’ biomass to root yield, which was highest in both the uncontaminated and diesel oil-contaminated series in the dolomite-supplemented plots. The application of sorbents to uncontaminated soil increased the leaf greenness index by a range of 9% (vermiculite) to 18% (dolomite). Contrastingly, no significant changes were observed in soil contaminated with diesel oil.

Table 1.
The Biomass of Zea mays and Leaf Greenness Index.
Despite the negative effect of diesel oil on the growth and development of Zea mays, no drastic alterations induced by this petroleum-derived substance were observed in the heat of combustion and calorific value of Zea mays (Table 2). Nevertheless, the adverse effect of diesel oil on plant growth and development resulted in a significant reduction (by 89%) in the amount of energy from Zea mays biomass obtained from 1 kg of soil. The energy content of Zea mays biomass collected from 1 kg of soil varied and ranged from 0.028 to 0.369 MJ. In uncontaminated soils, sorbents altered the obtained energy from biomass, albeit to a limited extent. Vermiculite decreased it only by 3%, whereas dolomite increased it by 4%. In diesel-contaminated soil, vermiculite enhanced the energy obtained from biomass by 13%, whereas dolomite decreased it by 29%.

Table 2.
Combustion heat (Q), calorific value (Hv), and the amount of obtained energy (Yep) from Zea mays biomass.
Diesel fuel (Table 3) significantly increased the sulfur content in plant biomass by 254%, ash by 71%, and nitrogen by 66%. It did not significantly change the oxygen, carbon, and hydrogen content. Conversely, vermiculite decreased the sulfur content by 11% and nitrogen by 5% in biomass, with no effect on the other parameters investigated. Meanwhile, dolomite increased the nitrogen content in biomass by 31%, ash by 15%, and sulfur by 6%. Similar to diesel fuel, it did not significantly modify the hydrogen, carbon, and oxygen content.

Table 3.
Ash content and elemental composition of Zea mays biomass.
The calculated indices of the influence of diesel oil and sorbents confirm previous observations regarding their effect on both the yield of Zea mays and parameters allowing for the determination of its energy efficiency (Figure 1). Positive index values, indicating the mitigation of the adverse effects of soil contamination with diesel oil on the aerial parts’ biomass and the obtained energy value, were observed after the application of vermiculite. Therefore, the use of vermiculite contributes most significantly to the increased energy yield of Zea mays biomass.
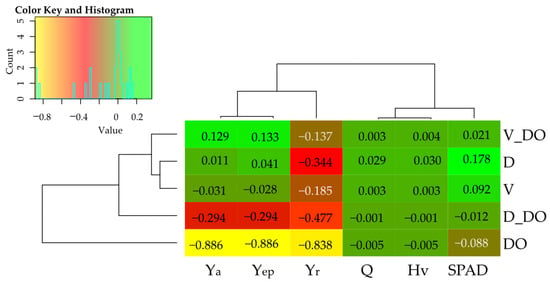
Figure 1.
Index of the influence of diesel oil (DO) and sorbents on the aerial parts yield (Ya), root yield (Yr), combustion heat (Q), calorific value (Hv), amount of obtained energy (Yep) from Zea mays biomass, and the greenness index (SPAD). Abbreviations are explained in Table 1.
Analyzing the influence indices of diesel oil and sorbents on the elemental composition and ash content in maize biomass (Figure 2), a significant increase in the ash, N, and S indices is observed after the application of diesel oil and dolomite. To a lesser extent, the indices were influenced by the application of vermiculite and the combined use of sorbents with the petroleum-derived substance. However, the addition of vermiculite to soil contaminated with diesel oil significantly reduced the content of N (by 4.5%) and S (by 11.7%).
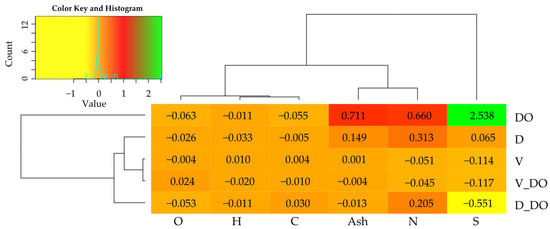
Figure 2.
The impact index of diesel oil (DO) and sorbents on the content of ash, C, H, S, N, and O in Zea mays biomass. Explanations are provided in Table 1.
3.2. Soil Enzyme Activity
The EFECTA 95 diesel oil significantly contributed to the increase in arylsulfatase activity (Aryl) by 241%, alkaline phosphatase activity (AlP) by 220%, catalase activity (Cat) by 186%, β-glucosidase activity (Glu) by 48%, dehydrogenase activity (Deh) by 43%, and urease activity (Ure) by 11%, while reducing the activity of acid phosphatase (PcA) by 10% (Figure 3). Supplementation of uncontaminated soil with vermiculite and dolomite stimulated the activity of Deh (by 39% and 107%, respectively), Cat (by 9% and 54%), Ure (by 14% and 296%), AlP (by 8% and 40%), and Aryl (by 19% and 141%). Vermiculite also additionally increased the activity of AcP by 9%, while dolomite increased Glu activity by 9%. Application of sorbents to soil under DO pressure resulted in a decrease in AlP and Glu activity and did not alter AcP activity. Both fertilizers stimulated the activity of Ure, Deh, Aryl, and Cat. Vermiculite, in particular, most stimulated Aryl activity (by 85%), followed by Deh (by 59%), Ure (by 29%), and Cat (by 10%), whereas dolomite stimulated Ure (by 319%), Deh (by 119%), Aryl (by 61%), and Cat (by 31%) activity.
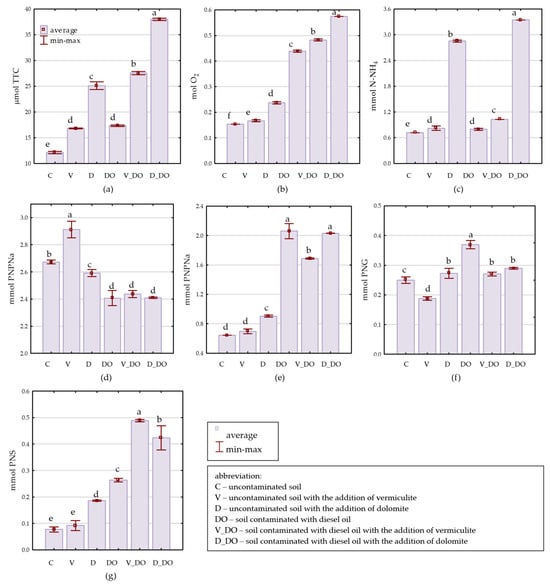
Figure 3.
Enzyme activity in 1 kg dm of soil × h−1; (a)—dehydrogenases, (b)—catalase, (c)—urease, (d)—acid phosphatase, (e)—alkaline phosphatase, (f)—β-glucosidase, and (g)—arylsulfatase. Homogeneous groups denoted with letters a–f were calculated separately for each enzyme.
The indices of the influence of DO and sorbents, calculated based on soil enzyme activity, revealed a positive effect of the petroleum-derived product on the activity of five out of the seven enzymes studied. This is indicated by the positive values of the indices presented in Figure 4. The influence index of vermiculite and dolomite in plots contaminated with DO took negative values for Glu and AlP and positive values for Deh, Cat, Aryl, and Ure, demonstrating that both sorbents reduced the activity of two enzymes and stimulated four in soil exposed to diesel oil.
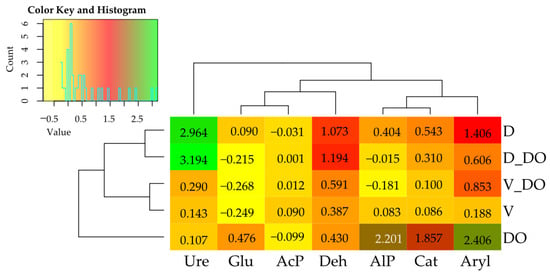
Figure 4.
Index of the influence of diesel oil and sorbents on the activity of soil enzymes. Deh—dehydrogenases; Cat—catalase; Ure—urease; AcP—acid phosphatase; AlP—alkaline phosphatase; Glu—β-glucosidase; Aryl—arylsulfatase. Abbreviations are explained in Table 1.
The diesel oil applied to the soil at a rate of 24 kg−1 significantly increased the organic carbon and total nitrogen content by 62% and 21%, respectively (Figure 5a). Both sorbents significantly enhanced the carbon content in both uncontaminated soil and soil contaminated with DO, with dolomite exerting a more pronounced effect. Less dramatic changes were observed in the nitrogen content of the soil under the influence of sorbents, with only dolomite significantly reducing it (by 11%) in uncontaminated soil. Changes in soil with DO under the influence of both preparations were statistically insignificant (Figure 5b). The consequence of changes in Corg and NTotal content in the soil observed under the influence of DO and sorbents was the carbon-to-nitrogen ratio (C:N), influencing the decomposition of organic matter. It was substantially higher in soil contaminated with DO. The highest C:N was observed in plots with DO and dolomite, as well as dolomite alone (Figure 5c). Both sorbents increased soil pH in both uncontaminated soil and soil contaminated with DO, with dolomite significantly elevating pH by 2.3 pH units in uncontaminated soil and by 1.6 pH units in soil contaminated with DO (Figure 5d).
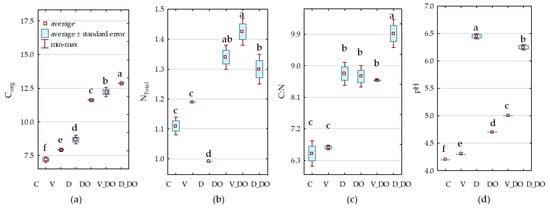
Figure 5.
Organic carbon content (a), total nitrogen content (b) in g kg−1 d.m. of soil, carbon-to-nitrogen ratio (c), and soil pH (d). Homogeneous groups denoted with letters a–f were calculated separately for C, N, C:N, and pH. Abbreviations are explained in Table 1.
3.3. Interactions between Zea mays Biomass and Energy Efficiency and Soil Enzymatic Activity
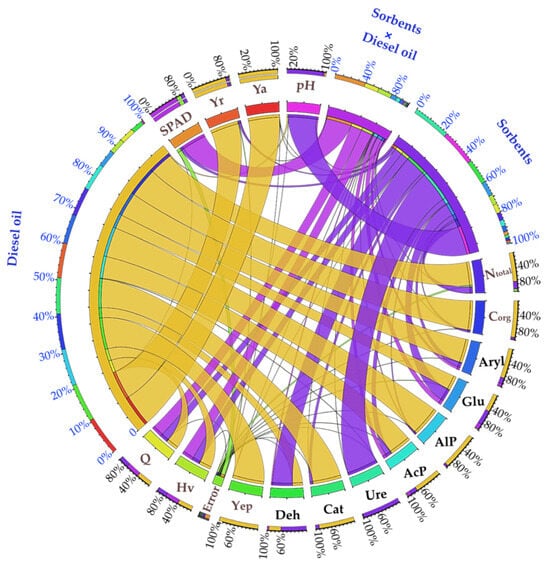
Analyzing the contribution of independent variables (η2) to the formation of dependent variables in soils, it was observed that diesel oil had a significantly greater effect on the dependent variables than the sorbents applied (Figure 6). Diesel oil influenced the aerial parts biomass growth and biomass energy efficiency (Yep) by over 99%, the organic carbon content in the soil by 93%, the growth of Zea mays roots by 90%, the total nitrogen content by 74%, and plant biomass heat of combustion (Q) and calorific value (Hv) by 38%. Similarly, the activity of soil enzymes was more strongly shaped by DO contamination, with 45% (Glu), 70% AcP, 76% Aryl, 91% Cat, and 94% (AlP). Only pH, as well as the activity of Ure and Deh, were more dependent on sorbents, accounting for 92%, 98%, and 65%, respectively.
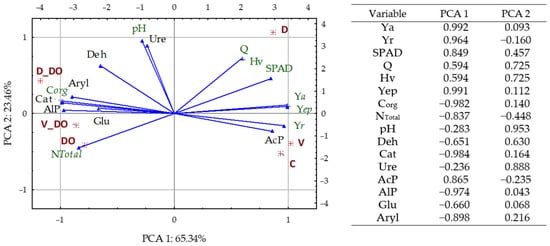
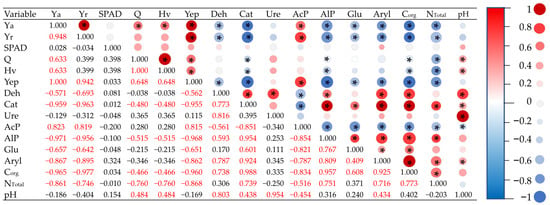
The heat of combustion (Q), the calorific value (Hv), and the amount of energy produced (Yep) from plant biomass are significantly positively correlated with the yield of Zea mays aerial parts (Figure 7). Plant yield (Ya and Yr) and Yep were significantly negatively correlated with the activity of all enzymes, except for AcP, which was positively correlated. In turn, Ure activity was not significantly correlated with any of the above parameters. Also, the Corg and NTotal content in the soil is significantly negatively correlated with Ya, Yr, Q, Hv, Yep, and AcP, and positively correlated with Cat, AlP, and Aryl. Additionally, the Corg content is significantly positively correlated with the Deh and Glu content.
The relationships between the studied variables are well described by the Principal Component Analysis (PCA) plot (Figure 8). The first principal component is strongly positively correlated with Ya, Yr, Yep, Q, Hv, and AcP, while negatively correlated with Corg, Cat, AlP, Aryl, NTotal, Glu, and Deh. On the other hand, the second principal component is most strongly positively correlated with Q, Hv, pH, Ure, and Deh. The distribution of the vectors representing the original variables and the distribution of the cases clearly indicate that soil contamination with diesel oil significantly increased the activity of all enzymes except AcP. It also shows that the energy value obtained from Zea mays biomass is significantly positively correlated, not only with the amount of biomass obtained but also with the activity of AcP, and negatively correlated with the content of Corg and NTotal in the soil.
4. Discussion
4.1. The Biomass of Zea mays and Its Energy Value
Carbon-rich biomass is becoming an increasingly important resource in the context of biotechnological processing and biofuel production [77,78]. Current scientific research focuses on finding methods for efficient biomass utilization and developing technologies that could contribute to increasing the share of renewable energy sources [78,79,80]. According to Severes et al. [77] and Vuppaladadiyam et al. [81], determining the contents of carbon, hydrogen, oxygen, and ash in corn biomass is crucial for determining its calorific value. In the context of harmful gas emissions released into the atmosphere during biomass combustion, the sulfur and nitrogen contents are also key. In our studies, the carbon content ranged from 47.85% to 51.12%, hydrogen from 5.54% to 5.79%, and oxygen from 31.98% to 36.04%. According to Allende et al. [82] when assessing the suitability of biomass for energy generation, the nitrogen and sulfur content must also be considered as potential of greenhouse gas emissions. In our studies, the nitrogen content ranged from 1.94% to 4.09%, and the sulfur content from 0.08% to 0.33%. Another significant parameter describing the suitability of biomass for energy purposes is the ash content, which can be a limiting factor in the feasibility of biomass use in thermochemical conversion [82]. The ash content of Zea mays biomass in our studies ranged from 4.97% to 8.51%, which is consistent with the findings of Melikoglu et al. [79]. The mentioned ash contents do not limit the quality of the tested biomass as an energy fuel source. The calorific value of Zea mays biomass obtained from contaminated soil was 15.043 MJ kg−1 and from uncontaminated soil 15.115 MJ kg−1. These values are comparable to those obtained by other researchers (Table 4).

Table 4.
Heating values of different plant species.
The calorific value of maize biomass is mainly influenced by carbon, hydrogen, and oxygen [93]. Carbon influences the calorific value through its high energy content, combustion heat, ratio to other elements, chemical structure of the biomass, and combustion efficiency [94]. Wojcieszek et al. [95] reported that the heat of combustion of fresh maize cob kernels ranges from 7.62 to 10.79 MJ kg−1, while that of seasoned kernels varies from 16.19 to 16.53 MJ kg−1. High-quality maize biomass contains 47–54% carbon [96]. Despite the lower content of hydrogen compared to carbon in biomass, its impact on the calorific value is significant [97]. Its decarbonization potential positions it as one of the most promising energy carriers, crucial for the transition to a low-emission energy system [98]. Sulaiman et al. [96] classify plants with high energy potential as those with biomass containing approximately 40–44% oxygen. Nitrogen (0.1–0.5%) and sulfur (about 0.1%) are much lower. Another important factor affecting the calorific value of biomass is the ash content [99]. High ash content reduces the calorific value of biomass. Morissette et al. [100] found that an ash content of 5.88% leads to incomplete combustion of biomass. The response of plants to soil contamination by petroleum-derived substances is complex. It depends on various factors, such as plant species, the type of petroleum-derived substance, the degree of soil contamination, environmental conditions (e.g., temperature, salinity, pH), nutrient availability, and soil oxygenation [101,102,103]. The beneficial effect of plants on soil remediation is due to from their ability to absorb and degrade petroleum contaminants. Plants grown in such areas may exhibit reduced yields, and their tissues may accumulate pollutants. This process is known as phytoremediation, which uses the physiological processes of plants to detoxify the environment or improve the safety of the food chain through the phytostabilization of toxic elements [101].
In our study, soil contamination with diesel oil adversely affected the growth and development of Zea mays. The EFECTA diesel oil used in the experiment resulted in a significant reduction in yield, both in aerial and root parts. These contaminants disrupt the physiological processes of plants at all stages of their development [103]. According to Kvesitadze et al. [101], the penetration of pollutants into the plant depends on the thickness of the cuticle and its wax layer. They are transported through the conducting tissue, i.e., phloem, and then undergo enzymatic transformations. Hussein et al. [103] suggest that this is related to the properties of hydrocarbons, which can inhibit water and oxygen uptake. Sequestration of small hydrocarbon molecules in cell membranes leads to a reduction in their integrity and even cell death.
To mitigate the effects of soil contamination and restore the soil’s productivity, we applied vermiculite and ground dolomite in our study as sorbents that could potentially limit the negative impact of the petroleum-derived product on plant growth and development. The interaction of these sorbents in diesel oil-contaminated soil was variable. Vermiculite, as a mineral with high sorption capacity [54,57], mitigated the adverse effects of the petroleum-derived product on the aboveground biomass. This could contribute to creating more favorable conditions for plant growth and influence the increased availability of nutrients. According to studies by Vasilyeva et al. [59], Marwa et al. [52], Balidakis et al. [57], Szadkowski et al. [55], and Jafari et al. [56], vermiculite may enhance soil aeration and contribute to improved water retention, which is crucial for root growth and development.
The application of dolomite to uncontaminated soil did not significantly alter the aerial parts biomass yield of plants, but it increased the negative effect of diesel oil (DO) on the yield of Zea mays. According to Huang et al. [104], von Wilpert and Lukes [105], and Wu et al. [106], soil dolomite can have both beneficial and adverse effects on the growth of crops and their biomass. This is mainly determined by the quantity added to the soil [107]. The application of dolomite contributes to changes in environmental pH [104,106]. The results of authors such as Ptáček et al. [108], Wu et al. [106], Zhang et al. [109], and Filep et al. [110] indicate that the use of dolomite affects an increase in CO2 emissions in the soil and faster mineralization of organic matter. Another possible explanation for these changes is the increased biological activity resulting from an increase in soil pH [107]. However, excessive amounts of dolomite applied to the soil may lead to a disturbance in the proportions of different ions in the soil solution, which, in turn, can negatively affect the availability of other nutrients. In our study, the dolomite applied raised the soil pH from 4.2 to 6.5, significantly reducing soil acidification and thereby influencing the intensification of biochemical processes. This effect did not translate into plant biomass, probably due to the inefficient soil deacidification by dolomite in the early stages of plant development, as dolomite is a mineral.
4.2. Soil Biochemical Activity
The assessment of soil pollution risk and the implementation of remediation measures require monitoring, verification of the degree of soil function destabilization, and the process of their regeneration [42,111]. According to Polyak et al. [111], Borowik et al. [35], Adipah [112], and Macci et al. [113], soil pollution with petroleum-derived products initially leads to an increase in soil enzymatic activity, followed by a decrease. Unfortunately, soil restoration may take several years. In our 60-day study, soil contamination with diesel oil 95 EFECTA significantly increased enzyme activity, especially Aryl, AlP, Cat, Glu, Deh, and Ure. According to Dindar et al. [114], the increase in enzymatic activity may be due to the biodegradation of readily degradable hydrocarbons. Long-term changes in soil biochemical activity can probably be explained by the fact that residues of petroleum-derived products in the soil consist mainly of compounds that are resistant to biodegradation. Heavy oil fractions block enzyme activity by coating the surfaces of organic-mineral particles and cells, preventing soluble substrates from reaching enzyme molecules [42,115]. Different results were reported by Gospodarek et al. [38], who observed that soil contamination with diesel oil strongly inhibited dehydrogenase and urease activity, with little effect on acid and alkaline phosphatase activity. These results are difficult to dispute, as the degree of biodegradation activity of petroleum-derived substances depends, among other factors, on microbial activity, nutrient availability, oxygen availability, temperature, pH, salinity, as well as the type and degree of pollution and time [36,115,116,117,118].
In our study, the application of sorbents to the soil under the diesel oil (DO) pressure resulted in a reduction of the activity of two investigated soil enzymes (alkaline phosphatase—AlP, β-glucosidase—Glu) and did not alter the activity of one—acid phosphatase (AcP). Both amendments stimulated the activity of four enzymes tested (urease—Ure, dehydrogenases—Deh, arylsulfatase—Aryl, and catalase—Cat). Sorbents, especially dolomite, contributed significantly to the changes in the soil pH. The increased soil pH influenced the improvement of its biological activity, as highlighted by Mahmud and Chong [107]. Higher soil pH is responsible for breaking ionic and hydrogen bonds in the active center of dehydrogenases, an enzyme that indicates the intensity of respiratory metabolism in soil microorganisms [27,36,119]. According to Nannipieri et al. [120], Mierzwa-Hersztek et al. [121], and Piotrowska-Długosz et al. [122], dehydrogenases are enzymes considered as indicators of soil biochemical and microbiological activity. They catalyze the removal of hydrogen atoms from organic compounds, thereby accelerating the oxidation of organic matter. Changes in the soil environment also significantly affected the activity of arylsulfatase, an enzyme that plays a crucial role in sulfur transformations in soil ecosystems. Arylsulfatase catalyzes the hydrolysis of aromatic sulfate esters into phenols and inorganic sulfates. It is often used as an indicator of soil fertility and the intensity of soil-forming processes [123].
The hydrogen ion potential (pH) plays a crucial role in the biodegradation of petroleum hydrocarbons [124,125]. According to Kanungo et al. [126], complex petroleum hydrocarbons are decomposed by both aerobic and anaerobic bacteria, utilizing electron acceptors such as nitrates, sulfates, or carbon dioxide. In this way, they use carbon as an energy source. The authors emphasize that the degradation of petroleum products involves several enzymes, such as oxidoreductases and hydrolases, with the final product being acetyl-CoA [127].
The soil contamination with diesel oil not only significantly destabilized plant growth and development and the enzymatic properties of the soil but also affected the content of organic carbon and total nitrogen. In our study, both uncontaminated soil and soil contaminated with diesel oil, and sorbents significantly increased the carbon content and raised the soil pH. Larger changes occurred after the application of dolomite than vermiculite. Additionally, dolomite significantly reduced the nitrogen content in uncontaminated soil and, in soil contaminated with diesel oil, the changes under the influence of both sorbents were statistically insignificant. According to Wu et al. [106], soil pH is positively correlated with the Corg content. A positive correlation between these parameters was also observed in our study. Furthermore, the content of Corg and NTotal in the soil was significantly negatively correlated with Ya, Yr, Q, Hv, Yep, and AcP, and positively correlated with Cat, AlP, and Aryl. One of the mechanisms responsible for increased soil organic carbon content is the increased hydrophilicity of organic matter molecules with increasing in pH [106,110]. According to Shaaban et al. [124], the addition of dolomite to the soil can also lead to high mineralization of organic nitrogen and, probably in our study, such a phenomenon caused the reduction of the NTotal content by dolomite.
5. Conclusions
The analysis of the heat of combustion of Zea mays biomass and its ash, carbon, hydrogen, oxygen, sulfur, and nitrogen content allowed the energy potential of this raw material to be determined. The results indicate that it is a promising energy source that can be effectively utilized in energy processes. Diesel oil increased the enzymatic activity of the soil, but the results obtained from the biomass yield of Zea mays and the amount of energy obtained prove that it had a negative effect on the growth and development of this plant. Therefore, it destabilized the soil quality. Hence, the quantification of soil quality based solely on the measurement of enzyme activity can therefore be very misleading. The cultivation of Zea mays on soil contaminated with petroleum-derived products is justified, and the obtained biomass can be used for energy purposes, as the heat of combustion and the calorific value of this plant do not lose their quality. Among the sorbents applied, vermiculite proved to be more useful in the remediation of soil contaminated with diesel oil. Hence, vermiculite should be recommended for cultivation aimed at achieving the appropriate biomass quantity of maize in soils contaminated with diesel oil Nevertheless, considering the need for sustainable technologies in the context of the development of renewable energy sources, efforts should be made to develop methods to reduce the sensitivity of Zea mays to the effects of diesel oil. Such an approach can contribute to a balance between energy efficiency and environmental protection.
Author Contributions
Conceptualization and experimental design and methodology, J.W., A.B., M.Z. and J.K.; investigation, J.W., A.B. and M.Z.; statistical analyses, J.W., A.B. and M.Z.; writing—review and editing, J.W., A.B. and M.Z.; supervision, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Warmia and Mazury in Olsztyn, Faculty of Agriculture and Forestry, Department of Soil Science and Microbiology (grant No. 30.610.006-110) and project financially supported by the Minister of Education and Science in the range of the program entitled Funded by the Minister of Science under the Regional Initiative of Excellence Program.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IEA 50: Bioenergy. Available online: https://www.iea.org/energy-system/renewables/bioenergy (accessed on 8 February 2024).
- Gül, T.; Cozzi, L. Petr Havlik What Does Net-Zero Emissions by 2050 Mean for Bioenergy and Land Use?—Analysis. Available online: https://www.iea.org/articles/what-does-net-zero-emissions-by-2050-mean-for-bioenergy-and-land-use (accessed on 8 February 2024).
- Tracking Clean Energy Progress 2023—Analysis. Available online: https://www.iea.org/reports/tracking-clean-energy-progress-2023 (accessed on 8 February 2024).
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing Climate-Resilient Crops: Improving Plant Tolerance to Stress Combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Králík, T.; Knápek, J.; Vávrová, K.; Outrata, D.; Romportl, D.; Horák, M.; Jandera, J. Ecosystem Services and Economic Competitiveness of Perennial Energy Crops in the Modelling of Biomass Potential—A Case Study of the Czech Republic. Renew. Sustain. Energy Rev. 2023, 173, 113120. [Google Scholar] [CrossRef]
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-Assisted Remediation of Hydrocarbons in Water and Soil: Application, Mechanisms, Challenges and Opportunities. Chemosphere 2020, 247, 125932. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.M.; Khan, A.H.; Nicholls, C.; Sohoo, I.; Otterpohl, R. Using Landfill Sites and Marginal Lands for Socio-Economically Sustainable Biomass Production through Cultivation of Non-Food Energy Crops: An Analysis Focused on South Asia and Europe. Sustainability 2023, 15, 4923. [Google Scholar] [CrossRef]
- Günal, A.Ç.; Tunca, S.K.; Arslan, P.; Gül, G.; Dinçel, A.S. How Does Sublethal Permethrin Effect Non-Target Aquatic Organisms? Environ. Sci. Pollut. Res. 2021, 28, 52405–52417. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Sensitivity of Zea mays and Soil Microorganisms to the Toxic Effect of Chromium (VI). Int. J. Mol. Sci. 2023, 24, 178. [Google Scholar] [CrossRef] [PubMed]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Cairns, J.E.; Zaidi, P.H.; Beyene, Y.; Makumbi, D.; Gowda, M.; Magorokosho, C.; Zaman-Allah, M.; Olsen, M.; Das, A.; et al. Beat the Stress: Breeding for Climate Resilience in Maize for the Tropical Rainfed Environments. Theor. Appl. Genet. 2021, 134, 1729–1752. [Google Scholar] [CrossRef]
- Bellon, M.R.; Hodson, D.; Bergvinson, D.; Beck, D.; Martinez-Romero, E.; Montoya, Y. Targeting Agricultural Research to Benefit Poor Farmers: Relating Poverty Mapping to Maize Environments in Mexico. Food Policy 2005, 30, 476–492. [Google Scholar] [CrossRef]
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the Global Number and Distribution of Maize and Wheat Farms. Glob. Food Secur. 2021, 30, 100558. [Google Scholar] [CrossRef]
- Pechanova, O.; Takáč, T.; Šamaj, J.; Pechan, T. Maize Proteomics: An Insight into the Biology of an Important Cereal Crop. Proteomics 2013, 13, 637–662. [Google Scholar] [CrossRef] [PubMed]
- Bonner, I.J.; Cafferty, K.G.; Muth, D.J.; Tomer, M.D.; James, D.E.; Porter, S.A.; Karlen, D.L. Opportunities for Energy Crop Production Based on Subfield Scale Distribution of Profitability. Energies 2014, 7, 6509–6526. [Google Scholar] [CrossRef]
- Mattioni, B.; Kessler-Mathieu, M.; Wang, D.; Tilley, M. Ancient Grains: A Key Solution to Address Climate Change and Food Security. In Sustainable Agricultural Practices and Product Design; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2023; Volume 1449, pp. 51–75. [Google Scholar]
- Migut, D.; Buczek, J.; Jańczak-Pieniążek, M.; Szpunar-Krok, E. Industrial and energy use of maize plants. Pol. J. Sustain. Dev. 2021, 25, 57–64. [Google Scholar] [CrossRef]
- Abreu, M.; Silva, L.; Ribeiro, B.; Ferreira, A.; Alves, L.; Paixão, S.M.; Gouveia, L.; Moura, P.; Carvalheiro, F.; Duarte, L.C.; et al. Low Indirect Land Use Change (ILUC) Energy Crops to Bioenergy and Biofuels—A Review. Energies 2022, 15, 4348. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. The Role of Dactylis Glomerata and Diesel Oil in the Formation of Microbiome and Soil Enzyme Activity. Sensors 2020, 20, 3362. [Google Scholar] [CrossRef] [PubMed]
- Hawrot-Paw, M.; Koniuszy, A.; Zając, G.; Szyszlak-Bargłowicz, J. Ecotoxicity of Soil Contaminated with Diesel Fuel and Biodiesel. Sci. Rep. 2020, 10, 16436. [Google Scholar] [CrossRef]
- Hidalgo, K.J.; Sierra-Garcia, I.N.; Dellagnezze, B.M.; de Oliveira, V.M. Metagenomic Insights Into the Mechanisms for Biodegradation of Polycyclic Aromatic Hydrocarbons in the Oil Supply Chain. Front. Microbiol. 2020, 11, 561506. [Google Scholar] [CrossRef] [PubMed]
- Baghaie, A.; Jabari, A.; Sattari, R. The Effect of Corn and White Clover Intercropping on Biodegradation of Diesel Oil in Arsenic Contaminated Soil in the Presence of Piriformospora Indica. J. Hum. Environ. Health Promot. 2020, 6, 53–59. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J. Remediation of Soil Contaminated with Diesel Oil. J. Elem. 2018, 23, 767–788. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, J.; Cheng, Z.; Zhai, W.; Liu, D.; Zhou, Z.; Wang, P. The Influence of Oxytetracycline on the Degradation and Enantioselectivity of the Chiral Pesticide Beta-Cypermethrin in Soil. Environ. Pollut. 2019, 255, 113215. [Google Scholar] [CrossRef]
- Iqbal, A.; Mukherjee, M.; Rashid, J.; Khan, S.A.; Ali, M.A.; Arshad, M. Development of Plant-Microbe Phytoremediation System for Petroleum Hydrocarbon Degradation: An Insight from Alkb Gene Expression and Phytotoxicity Analysis. Sci. Total Environ. 2019, 671, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt. Materials 2022, 15, 5738. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Calorific Value of Zea mays Biomass Derived from Soil Contaminated with Chromium (VI) Disrupting the Soil’s Biochemical Properties. Energies 2023, 16, 3788. [Google Scholar] [CrossRef]
- Kulichkova, G.; Ivanova, T.; Köttner, M.; Volodko, O.; Spivak, S.; Tsygankov, S.; Blume, Y. Plant Feedstocks and Their Biogas Production Potentials. Open Agric. J. 2020, 14, 219–234. [Google Scholar] [CrossRef]
- Chomczyńska, M.; Pawłowska, M.; Szczepaniak, O.; Duma, E. Biogas Generation from Maize and Cocksfoot Growing in Degraded Soil Enriched with New Zeolite Substrate. Energies 2022, 15, 377. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a Sustainable Bioeconomy: An Overview of World Biomass Production and Utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Dahiya, A. Bioenergy: Biomass to Biofuels and Waste to Energy; Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-12-815498-4. [Google Scholar]
- Margida, M.G.; Lashermes, G.; Moorhead, D.L. Estimating Relative Cellulolytic and Ligninolytic Enzyme Activities as Functions of Lignin and Cellulose Content in Decomposing Plant Litter. Soil Biol. Biochem. 2020, 141, 107689. [Google Scholar] [CrossRef]
- Silva, J.P.; Ticona, A.R.P.; Hamann, P.R.V.; Quirino, B.F.; Noronha, E.F. Deconstruction of Lignin: From Enzymes to Microorganisms. Molecules 2021, 26, 2299. [Google Scholar] [CrossRef] [PubMed]
- Błońska, E.; Piaszczyk, W.; Staszel, K.; Lasota, J. Enzymatic Activity of Soils and Soil Organic Matter Stabilization as an Effect of Components Released from the Decomposition of Litter. Appl. Soil Ecol. 2021, 157, 103723. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. Implications of Soil Pollution with Diesel Oil and BP Petroleum with ACTIVE Technology for Soil Health. Int. J. Environ. Res. Public Health 2019, 16, 2474. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The Usability of Sorbents in Restoring Enzymatic Activity in Soils Polluted with Petroleum-Derived Products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef] [PubMed]
- Goma-Tchimbakala, E.J.C.D.; Pietrini, I.; Goma-Tchimbakala, J.; Corgnati, S.P. Use of Shotgun Metagenomics to Assess the Microbial Diversity and Hydrocarbons Degrading Functions of Auto-Mechanic Workshops Soils Polluted with Gasoline and Diesel Fuel. Microorganisms 2023, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Gospodarek, J.; Rusin, M.; Barczyk, G.; Nadgórska-Socha, A. The Effect of Petroleum-Derived Substances and Their Bioremediation on Soil Enzymatic Activity and Soil Invertebrates. Agronomy 2021, 11, 80. [Google Scholar] [CrossRef]
- Bao, H.; Wang, J.; Zhang, H.; Li, J.; Li, H.; Wu, F. Effects of Biochar and Organic Substrates on Biodegradation of Polycyclic Aromatic Hydrocarbons and Microbial Community Structure in PAHs-Contaminated Soils. J. Hazard. Mater. 2020, 385, 121595. [Google Scholar] [CrossRef] [PubMed]
- Mukome, F.N.D.; Buelow, M.C.; Shang, J.; Peng, J.; Rodriguez, M.; Mackay, D.M.; Pignatello, J.J.; Sihota, N.; Hoelen, T.P.; Parikh, S.J. Biochar Amendment as a Remediation Strategy for Surface Soils Impacted by Crude Oil. Environ. Pollut. 2020, 265, 115006. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Ejaz, M.; Cheema, S.A.; Khan, M.I.; Zhao, B.; Liqun, C.; Salim, M.A.; Naveed, M.; Khan, N.; Núñez-Delgado, A.; et al. Phytotoxicity of Petroleum Hydrocarbons: Sources, Impacts and Remediation Strategies. Environ. Res. 2021, 197, 111031. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, M.-S.; Kim, J.-G.; Kim, S.-O. Use of Soil Enzymes as Indicators for Contaminated Soil Monitoring and Sustainable Management. Sustainability 2020, 12, 8209. [Google Scholar] [CrossRef]
- Yang, J.; Yang, F.; Yang, Y.; Xing, G.; Deng, C.; Shen, Y.; Luo, L.; Li, B.; Yuan, H. A Proposal of “Core Enzyme” Bioindicator in Long-Term Pb-Zn Ore Pollution Areas Based on Topsoil Property Analysis. Environ. Pollut. 2016, 213, 760–769. [Google Scholar] [CrossRef]
- Usowicz, B.; Lipiec, J. Spatial Variability of Soil Properties and Cereal Yield in a Cultivated Field on Sandy Soil. Soil Tillage Res. 2017, 174, 241–250. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Report No 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Yesmin, M.N.; Azad, M.A.K.; Kamuruzzaman, M.; Ali, S. The Potentiality of Bioethanol Production from Corn (Zea mays L.) as a Renewable Source. J. Ecobiotechnol. 2020, 12, 1–4. [Google Scholar] [CrossRef]
- Morales-Máximo, C.N.; López-Sosa, L.B.; Rutiaga-Quiñones, J.G.; Corral-Huacuz, J.C.; Aguilera-Mandujano, A.; Pintor-Ibarra, L.F.; López-Miranda, A.; Delgado-Domínguez, S.N.; Rodríguez-Magallón, M.D.C.; Morales-Máximo, M. Characterization of Agricultural Residues of Zea mays for Their Application as Solid Biofuel: Case Study in San Francisco Pichátaro, Michoacán, Mexico. Energies 2022, 15, 6870. [Google Scholar] [CrossRef]
- Grippi, D.; Clemente, R.; Bernal, M.P. Chemical and Bioenergetic Characterization of Biofuels from Plant Biomass: Perspectives for Southern Europe. Appl. Sci. 2020, 10, 3571. [Google Scholar] [CrossRef]
- Singamsetti, A.; Shahi, J.P.; Zaidi, P.H.; Seetharam, K.; Vinayan, M.T.; Kumar, M.; Singla, S.; Shikha, K.; Madankar, K. Genotype × Environment Interaction and Selection of Maize (Zea mays L.) Hybrids across Moisture Regimes. Field Crops Res. 2021, 270, 108224. [Google Scholar] [CrossRef]
- PN-EN 590:2022-08; Paliwa Do Pojazdów Samochodowych—Oleje Napędowe—Wymagania i Metody Badań. Fuels for Motor Vehicles—Diesel Oils—Requirements and Test Methods. International Standard Confirmed. International Organization for Standardization: Geneva, Switzerland, 2022.
- VERVA ON—Paliwo Premium Diesel—ORLEN. Available online: https://www.orlen.pl/pl/dla-biznesu/produkty/paliwa/oleje-napedowe/verva-on (accessed on 8 February 2024).
- Marwa, E.M.M.; Meharg, A.A.; Rice, C.M. The Effect of Heating Temperature on the Properties of Vermiculites from Tanzania with Respect to Potential Agronomic Applications. Appl. Clay Sci. 2009, 43, 376–382. [Google Scholar] [CrossRef]
- Petersen, R.R.; Christensen, J.F.S.; Jørgensen, N.T.; Gustafson, S.; Lindbjerg, L.A.; Yue, Y. Preparation and Thermal Properties of Commercial Vermiculite Bonded with Potassium Silicate. Thermochim. Acta 2021, 699, 178926. [Google Scholar] [CrossRef]
- Ma, T.; Sun, H.; Peng, T.; Zhang, Q. Transformation Process from Phlogopite to Vermiculite under Hydrothermal Conditions. Appl. Clay Sci. 2021, 208, 106094. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rybiński, P.; Żukowski, W.; Zaborski, M. Characterization of Ethylene–Propylene Composites Filled with Perlite and Vermiculite Minerals: Mechanical, Barrier, and Flammability Properties. Materials 2020, 13, 585. [Google Scholar] [CrossRef]
- Jafari, F.; Khademi, H.; Shahrokh, V.; Cano, A.F.; Acosta, J.A.; Khormali, F. Biological Weathering of Phlogopite during Enriched Vermicomposting. Pedosphere 2021, 31, 440–451. [Google Scholar] [CrossRef]
- Balidakis, A.; Matsi, T.; Karagianni, A.-G.; Ipsilantis, I. Sewage Sludge Treated with Bentonite, Vermiculite or Biochar Can Improve Soil Properties and Enhance Growth of Grasses. Soil Use Manag. 2023, 39, 1403–1421. [Google Scholar] [CrossRef]
- Vasilyeva, G.; Mikhedova, E.; Zinnatshina, L.; Strijakova, E.; Akhmetov, L.; Sushkova, S.; Ortega-Calvo, J.-J. Use of Natural Sorbents for Accelerated Bioremediation of Grey Forest Soil Contaminated with Crude Oil. Sci. Total Environ. 2022, 850, 157952. [Google Scholar] [CrossRef]
- Cai, W.K.; Liu, J.H.; Zhou, C.H.; Keeling, J.; Glasmacher, U.A. Structure, Genesis and Resources Efficiency of Dolomite: New Insights and Remaining Enigmas. Chem. Geol. 2021, 573, 120191. [Google Scholar] [CrossRef]
- Guo, P.; Wen, H.; Li, C.; He, H.; Sánchez-Román, M. Lacustrine Dolomite in Deep Time: What Really Matters in Early Dolomite Formation and Accumulation? Earth-Sci. Rev. 2023, 246, 104575. [Google Scholar] [CrossRef]
- PN-EN 14780:2017; Solid Biofuels—Sample Preparation. Polish Standardization Committee: Warsaw, Poland, 2020.
- Wyszkowska, J.; Boros-Lajszner, E.; Kucharski, J. Calorific Value of Festuca Rubra Biomass in the Phytostabilization of Soil Contaminated with Nickel, Cobalt and Cadmium Which Disrupt the Microbiological and Biochemical Properties of Soil. Energies 2022, 15, 3445. [Google Scholar] [CrossRef]
- Kwiatkowski, J.; Graban, Ł.; Stolarski, M.J. The Quality of Virginia Fanpetals Biomass as an Energy Source, Depending on the Type of Propagating Material and Plantation Age. Energies 2024, 17, 218. [Google Scholar] [CrossRef]
- PN-EN ISO 18125:2017-07; Solid Biofuels—Determination of Calorific Value. Polish Standardization Committee: Warsaw, Poland, 2017.
- PN-EN ISO 18122:2016-01; Solid Biofuels—Determination of Ash Content. Polish Standardization Committee: Warsaw, Poland, 2016.
- PN-EN ISO 16948:2015-07; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen. Polish Standardization Committee: Warsaw, Poland, 2015.
- PN-EN ISO 16994:2016-10; Solid Biofuels—Determination of Total Content of Sulfur and Chlorine. Polish Standard-Ization Committee: Warsaw, Poland, 2016.
- PN-G-04584:2001; Solid Fuels—Determination of Total and Ash Sulfur Content with Automatic Analyzers. Polish Standardization Committee: Warsaw, Poland, 2006.
- Wyszkowska, J.; Borowik, A.; Kucharski, J. The Role of Grass Compost and Zea mays in Alleviating Toxic Effects of Tetracycline on the Soil Bacteria Community. Int. J. Environ. Res. Public Health 2022, 19, 7357. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of Aerobic Microorganisms and Soil Enzyme Response to Soil Contamination with Ekodiesel Ultra Fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Mitigation of the Adverse Impact of Copper, Nickel, and Zinc on Soil Microorganisms and Enzymes by Mineral Sorbents. Materials 2022, 15, 5198. [Google Scholar] [CrossRef]
- Citing RStudio. Available online: https://support.posit.co/hc/en-us/articles/206212048-Citing-RStudio (accessed on 8 December 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org (accessed on 8 February 2024).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data. 2022. Available online: https://rdrr.io/cran/gplots/ (accessed on 8 February 2024).
- Krzywinski, M.; Schein, J.; Birol, İ.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Tibco Software Inc. Statistica, Version 13; Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA, 2021; Available online: http://statistica.io (accessed on 10 July 2023).
- Esteves, B.; Sen, U.; Pereira, H. Influence of Chemical Composition on Heating Value of Biomass: A Review and Bibliometric Analysis. Energies 2023, 16, 4226. [Google Scholar] [CrossRef]
- Puri, L.; Hu, Y.; Naterer, G. Critical Review of the Role of Ash Content and Composition in Biomass Pyrolysis. Front. Fuels 2024, 2, 1378361. [Google Scholar] [CrossRef]
- Melikoglu, M.; Ozdemir, M.; Ates, M. Pyrolysis Kinetics, Physicochemical Characteristics and Thermal Decomposition Behavior of Agricultural Wastes Using Thermogravimetric Analysis. Energy Nexus 2023, 11, 100231. [Google Scholar] [CrossRef]
- Shahbeik, H.; Kazemi Shariat Panahi, H.; Dehhaghi, M.; Guillemin, G.J.; Fallahi, A.; Hosseinzadeh-Bandbafha, H.; Amiri, H.; Rehan, M.; Raikwar, D.; Latine, H.; et al. Biomass to Biofuels Using Hydrothermal Liquefaction: A Comprehensive Review. Renew. Sustain. Energy Rev. 2024, 189, 113976. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Varsha Vuppaladadiyam, S.S.; Sikarwar, V.S.; Ahmad, E.; Pant, K.K.; Murugavel, S.; Pandey, A.; Bhattacharya, S.; Sarmah, A.; Leu, S.-Y. A Critical Review on Biomass Pyrolysis: Reaction Mechanisms, Process Modeling and Potential Challenges. J. Energy Inst. 2023, 108, 101236. [Google Scholar] [CrossRef]
- Allende, S.; Brodie, G.; Jacob, M.V. Breakdown of Biomass for Energy Applications Using Microwave Pyrolysis: A Technological Review. Environ. Res. 2023, 226, 115619. [Google Scholar] [CrossRef] [PubMed]
- Boros-Lajszner, E.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Energetic Value of Elymus elongatus L. and Zea mays L. Grown on Soil Polluted with Ni2+, Co2+, Cd2+, and Sensitivity of Rhizospheric Bacteria to Heavy Metals. Energies 2021, 14, 4903. [Google Scholar] [CrossRef]
- Sobol, Ł.; Wolski, K.; Radkowski, A.; Piwowarczyk, E.; Jurkowski, M.; Bujak, H.; Dyjakon, A. Determination of Energy Parameters and Their Variability between Varieties of Fodder and Turf Grasses. Sustainability 2022, 14, 11369. [Google Scholar] [CrossRef]
- Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes. Energies 2021, 14, 1669. [Google Scholar] [CrossRef]
- Cî Cîrlig, N.; Țîței, V.; Iurcu-Străistaru, E.; Guțu, A.; Cozari, S.; Teleuță, A.; Gudîma, A.; Nazar, B.; Covalciuc, D. Some Physiological Features and the Productivity of the Energy Crops Miscanthus X Giganteus and Sorghum Almum under the Conditions of the Republic of Moldova. Symp. Agric. Food Eng. 2022, 65, 103–108. [Google Scholar]
- Stolarski, M.; Krzyżaniak, M.; Śnieg, M.; Słomińska, E.; Piórkowski, M.; Filipkowski, R. Thermophysical and Chemical Properties of Perennial Energy Crops Depending on Harvest Period. Int. Agrophysics 2014, 28, 201–211. [Google Scholar] [CrossRef]
- Papamatthaiakis, N.; Laine, A.; Haapala, A.; Ikonen, R.; Kuittinen, S.; Pappinen, A.; Kolström, M.; Mola-Yudego, B. New Energy Crop Alternatives for Northern Europe: Yield, Chemical and Physical Properties of Giant Knotweed (Fallopia Sachalinensis Var. ‘Igniscum’) and Virginia Mallow (Sida Hermaphrodita). Fuel 2021, 304, 121349. [Google Scholar] [CrossRef]
- Bilgili, M.E. Exploitable Potential of Biomass Energy in Electrical Energy Production in the Mediterranean Region of Turkey. J. Agric. Sci. 2022, 28, 666–676. [Google Scholar] [CrossRef]
- Chandel, R.; Narang, M.K.; Thakur, S.S.; Chandel, R.; Narang, M.K.; Thakur, S.S. Scaling Mechanization and Profitability in Maize Cultivation through Innovative Maize Planters along with Agroforestry Approach: Sustainable and Climate Smart Approach to Diversify Rice Based Cereal Systems in Various Regions. In New Prospects of Maize; IntechOpen: London, UK, 2023; ISBN 978-1-83768-632-2. [Google Scholar]
- Shakouri, M.; Exstrom, C.L.; Ramanathan, S.; Suraneni, P.; Vaux, J.S. Pretreatment of Corn Stover Ash to Improve Its Effectiveness as a Supplementary Cementitious Material in Concrete. Cem. Concr. Compos. 2020, 112, 103658. [Google Scholar] [CrossRef]
- Pinto, C.W.; Barth, G.; Molin, R.; Silva, D.A.D.; Pauletti, V. Characterization of oat biomass for energy production. Rev. Caatinga 2021, 34, 537–547. [Google Scholar] [CrossRef]
- Qu, J.; Xue, J.; Sun, M.; Li, K.; Wang, J.; Zhang, G.; Wang, L.; Jiang, Z.; Zhang, Y. Superefficient Non-Radical Degradation of Benzo[a]Pyrene in Soil by Fe-Biochar Composites Activating Persulfate. Chem. Eng. J. 2024, 481, 148585. [Google Scholar] [CrossRef]
- Korpaniuk, T.; Ishchenko, Y.; Koval, N. Backgrounds for Improving Resource Management of Agricultural Enterprises Based on Economic Diagnostics of Biofuel Consumption. J. Soc. Sci. Res. 2019, 5, 367–380. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Przybył, J.; Czajkowski, Ł.; Majka, J.; Pawłowski, A. Effects of Harvest Maturity on the Chemical and Energetic Properties of Corn Stover Biomass Combustion. Materials 2022, 15, 2831. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, M.A.; Adetifa, B.O.; Adekomaya, S.; Lawal, N.S.; Adama, O. Experimental Characterization of Maize Cob and Stalk-Based Pellets for Energy Use. Eng. J. 2019, 23, 117–128. [Google Scholar] [CrossRef]
- Yahya, A.M.; Adeleke, A.A.; Nzerem, P.; Ikubanni, P.P.; Ayuba, S.; Rasheed, H.A.; Gimba, A.; Okafor, I.; Okolie, J.A.; Paramasivam, P. Comprehensive Characterization of Some Selected Biomass for Bioenergy Production. ACS Omega 2023, 8, 43771–43791. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, M.; Bartolucci, L.; Cordiner, S.; D’Andrea, G.; De Maina, E.; Mulone, V. Biomass to H2: Evaluation of the Impact of PV and TES Power Supply on the Performance of an Integrated Bio-Thermo-Chemical Upgrading Process for Wet Residual Biomass. Energies 2023, 16, 2966. [Google Scholar] [CrossRef]
- Galhano dos Santos, R.; Bordado, J.C.; Mateus, M.M. Estimation of HHV of Lignocellulosic Biomass towards Hierarchical Cluster Analysis by Euclidean’s Distance Method. Fuel 2018, 221, 72–77. [Google Scholar] [CrossRef]
- Morissette, R.; Savoie, P.; Villeneuve, J. Combustion of Corn Stover Bales in a Small 146-kW Boiler. Energies 2011, 4, 1102–1111. [Google Scholar] [CrossRef]
- Kvesitadze, G.; Khatisashvili, G.; Sadunishvili, T.; Kvesitadze, E. Plants for Remediation: Uptake, Translocation and Transformation of Organic Pollutants. In Plants, Pollutants and Remediation; Öztürk, M., Ashraf, M., Aksoy, A., Ahmad, M.S.A., Hakeem, K.R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 241–308. ISBN 978-94-017-7194-8. [Google Scholar]
- Bakina, L.G.; Polyak, Y.M.; Gerasimov, A.O.; Mayachkina, N.V.; Chugunova, M.V.; Khomyakov, Y.V.; Vertebny, V.A. Mutual Effects of Crude Oil and Plants in Contaminated Soil: A Field Study. Environ. Geochem. Health 2022, 44, 69–82. [Google Scholar] [CrossRef]
- Hussein, Z.; Hamido, N.; Hegazy, A.; El-Dessouky, M.; Mohamed, N.; Safwat, G. Phytoremediation of Crude Petroleum Oil Pollution: A Review. Egypt. J. Bot. 2022, 62, 611–640. [Google Scholar] [CrossRef]
- Huang, J.; Fisher, P.R.; Argo, W.R. Container Substrate-pH Response to Differing Limestone Type and Particle Size. HortScience 2007, 42, 1268–1273. [Google Scholar] [CrossRef]
- von Wilpert, K.; Lukes, M. Ecochemical Effects of Phonolite Rock Powder, Dolomite and Potassium Sulfate in a Spruce Stand on an Acidified Glacial Loam. Nutr. Cycl. Agroecosyst. 2003, 65, 115–127. [Google Scholar] [CrossRef]
- Wu, H.; Hu, J.; Shaaban, M.; Xu, P.; Zhao, J.; Hu, R. The Effect of Dolomite Amendment on Soil Organic Carbon Mineralization Is Determined by the Dolomite Size. Ecol. Process. 2021, 10, 8. [Google Scholar] [CrossRef]
- Mahmud, M.S.; Chong, K.P. Effects of Liming on Soil Properties and Its Roles in Increasing the Productivity and Profitability of the Oil Palm Industry in Malaysia. Agriculture 2022, 12, 322. [Google Scholar] [CrossRef]
- Ptáček, P.; Šoukal, F.; Opravil, T. Thermal Decomposition of Ferroan Dolomite: A Comparative Study in Nitrogen, Carbon Dioxide, Air and Oxygen. Solid State Sci. 2021, 122, 106778. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, Z.; Wang, G.; Lou, G.; Liu, X. Kinetic Analyses of Coke Combustion and Thermal Decompositions of Limestone and Dolomite Based on the Sintering Atmosphere. Fuel 2021, 289, 119870. [Google Scholar] [CrossRef]
- Filep, T.; Kincses, I.; Nagy, P. Dissolved Organic Carbon (Doc) and Dissolved Organic Nitrogen (Don) Content of an Arenosol as Affected by Liming in a Pot Experiment. Arch. Agron. Soil Sci. 2003, 49, 111–117. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Bakina, L.G.; Mayachkina, N.V.; Chugunova, M.V.; Bityutskii, N.P.; Yakkonen, K.L.; Shavarda, A.L. Long-Term Effects of Oil Contamination on Soil Quality and Metabolic Function. Environ. Geochem. Health 2023, 46, 13. [Google Scholar] [CrossRef] [PubMed]
- Adipah, S. Introduction of Petroleum Hydrocarbons Contaminants and Its Human Effects. J. Environ. Sci. Public Health 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Macci, C.; Peruzzi, E.; Doni, S.; Masciandaro, G. Monitoring of a Long Term Phytoremediation Process of a Soil Contaminated by Heavy Metals and Hydrocarbons in Tuscany. Environ. Sci. Pollut. Res. 2020, 27, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Dindar, E.; Topaç Şağban, F.O.; Başkaya, H.S. Variations of Soil Enzyme Activities in Petroleum-Hydrocarbon Contaminated Soil. Int. Biodeterior. Biodegrad. 2015, 105, 268–275. [Google Scholar] [CrossRef]
- Hewelke, E.; Szatyłowicz, J.; Hewelke, P.; Gnatowski, T.; Aghalarov, R. The Impact of Diesel Oil Pollution on the Hydrophobicity and CO2 Efflux of Forest Soils. Water. Air. Soil Pollut. 2018, 229, 51. [Google Scholar] [CrossRef]
- Jakubauskaite, V.; Zukauskaite, A.; Kryzevicius, Z.; Khan, M.J.H. Model-Centric Optimisation of Biochemical Remediation of Petroleum Hydrocarbon Contaminated Soil. Soil Use Manag. 2024, 40, e12983. [Google Scholar] [CrossRef]
- Kim, P.-G.; Tarafdar, A.; Kwon, J.-H. Effect of Soil pH on the Sorption Capacity of Soil Organic Matter for Polycyclic Aromatic Hydrocarbons in Unsaturated Soils. Pedosphere 2023, 33, 365–371. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Kucharski, J.; Wałdowska, E. The Influence of Diesel Oil Contamination on Soil Enzymes Activity. Rostl. Vyroba 2002, 48, 58–62. [Google Scholar] [CrossRef]
- Moeskops, B.; Sukristiyonubowo; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil Microbial Communities and Activities under Intensive Organic and Conventional Vegetable Farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil Enzyme Activity: A Brief History and Biochemistry as a Basis for Appropriate Interpretations and Meta-Analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Wolny-Koładka, K.; Gondek, K.; Gałązka, A.; Gawryjołek, K. Effect of Coapplication of Biochar and Nutrients on Microbiocenotic Composition, Dehydrogenase Activity Index and Chemical Properties of Sandy Soil. Waste Biomass Valorization 2020, 11, 3911–3923. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Długosz, J.; Kalisz, B.; Gąsiorek, M. Soil Microbial and Enzymatic Properties in Luvisols as Affected by Different Types of Agricultural Land-Use Systems and Soil Depth. Agronomy 2024, 14, 83. [Google Scholar] [CrossRef]
- Yu, M.; Wu, M.; Secundo, F.; Liu, Z. Detection, Production, Modification, and Application of Arylsulfatases. Biotechnol. Adv. 2023, 67, 108207. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Wu, L.; Peng, Q.; van Zwieten, L.; Chhajro, M.A.; Wu, Y.; Lin, S.; Ahmed, M.M.; Khalid, M.S.; Abid, M.; et al. Influence of Ameliorating Soil Acidity with Dolomite on the Priming of Soil C Content and CO2 Emission. Environ. Sci. Pollut. Res. 2017, 24, 9241–9250. [Google Scholar] [CrossRef]
- Zainab, R.; Hasnain, M.; Ali, F.; Dias, D.A.; El-Keblawy, A.; Abideen, Z. Exploring the Bioremediation Capability of Petroleum-Contaminated Soils for Enhanced Environmental Sustainability and Minimization of Ecotoxicological Concerns. Environ. Sci. Pollut. Res. 2023, 30, 104933–104957. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, J.; Sahoo, T.; Bal, M.; Behera, I.D. Performance of Bioremediation Strategy in Waste Lubricating Oil Pollutants: A Review. Geomicrobiol. J. 2024, 41, 360–373. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. A New Look on Factors Affecting Microbial Degradation of Petroleum Hydrocarbon Pollutants. Int. Biodeterior. Biodegrad. 2017, 120, 71–83. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).