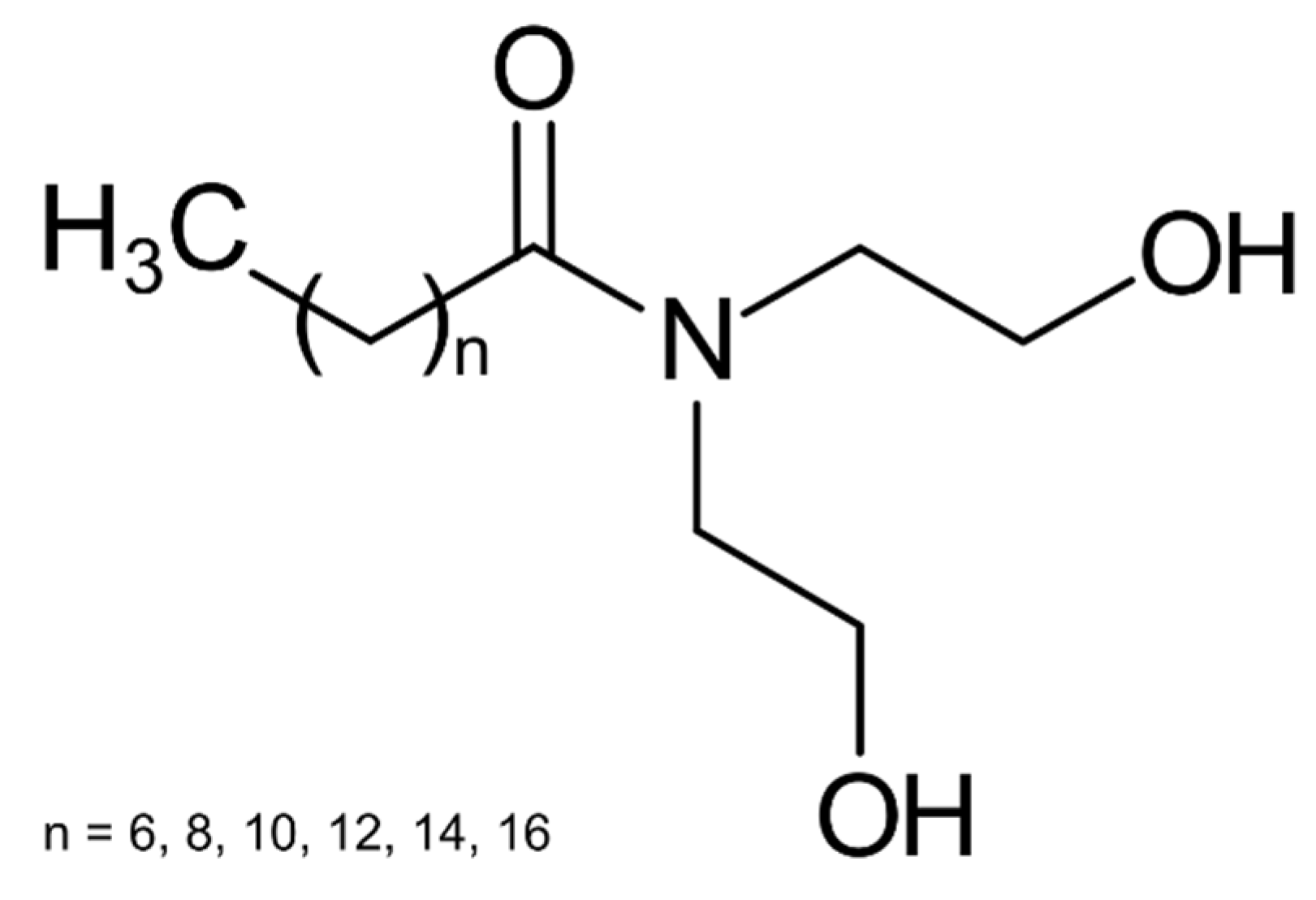
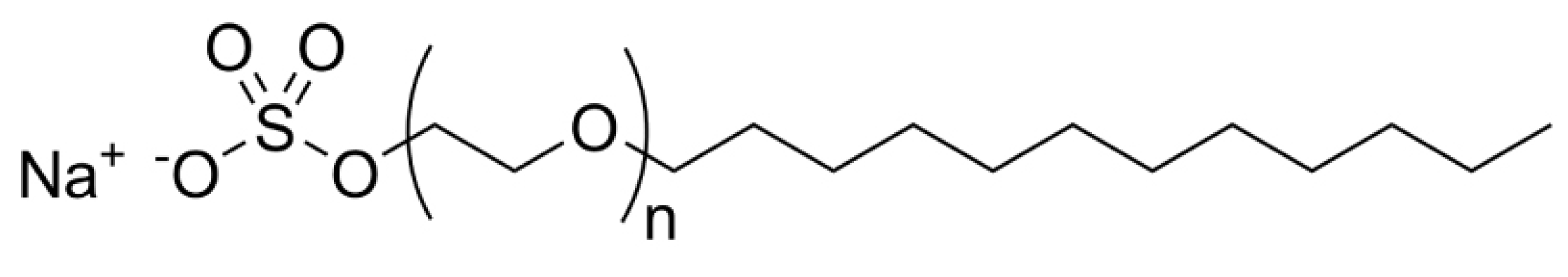
For the tests, the surfactant mixtures were dissolved in synthetic brine, and the oil was added to the cylinder of the instrument. After tempering, the apparatus was used to homogenize the phases. The results were determined 60 min after stirring.
3.1. Phase Behavior Test Results
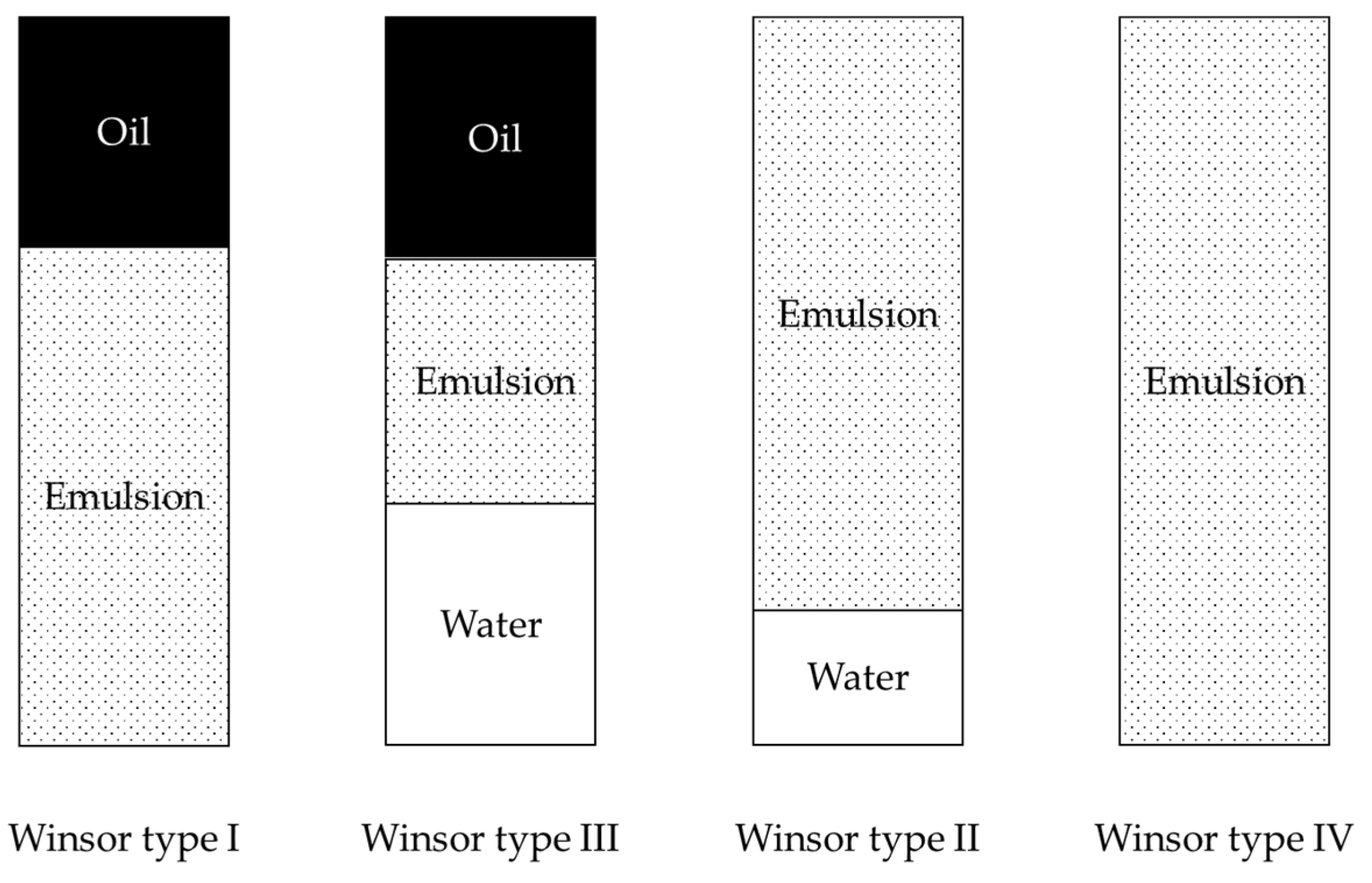
For the phase behavior tests, homogenization of the oily and aqueous phases was performed with an automatic device at 80 °C. The quality of the emulsion was characterized according to the Winsor theory after 1 h following the end of stirring. In the case of surfactant selection, mainly the Winsor type-III emulsion is acceptable. The results for surfactant S1 are summarized in
Table 6.
Using surfactant S1, in the case of a short stirring time and a low stirring speed, complete separation of the phases occurred after 1 h following the cessation of stirring (5 cases). Winsor type-III emulsions were observed at four mixing times, and Winsor type-II emulsions were stored for 1 h after the cessation of mixing. At a stirring speed of 1500 rpm and a stirring time of 10 min, a Winsor type-IV complete emulsion was observed after 1 h of storage.
The phase behavior test results are shown in
Table 7 for S2.
For surfactant S2, there was no emulsion phase after 1 h at stirring speeds of 500 and 750 rpm. At 1000 rpm, a stirring time of 3 min was required for the emulsion phase to remain after one hour. With a longer mixing time and/or faster mixing, a Winsor II emulsion was observed after 1 h of sedimentation.
The results in the case of surfactant SM1 are summarized in
Table 8.
For the surfactant composition SM1, the minimum stirring speed for the emulsion phase remaining after one hour was 750 rpm. The minimum stirring time was 30 s at 750 rpm and 15 s at 1000 rpm. After one hour, the emulsion phase was Winsor type II in all cases.
The phase behavior test results are shown in
Table 9 for SM2.
In the case of the surfactant composition SM2, even the slowest stirring speed (500 rpm) was sufficient to form the emulsion phase remaining after one hour. In this case, the minimum mixing time was 5 min. At 750 rpm, 15 s was sufficient to allow the emulsion phase to form after 1 h. Winsor type-II emulsions were observed in all cases.
The results in the case of surfactant SM3 are summarized in
Table 10.
In the case of the surfactant composition SM3, the emulsion phase remaining after 1 h of storage was Winsor type II in each case. This required a stirring time of 3 min at 500 rpm and 30 s at 750 rpm.
For each surfactant and surfactant mixture, stirring speeds of 1250 and 1500 rpm were sufficient to form an emulsion phase that remained after one hour of settling. Stirring at 500 and 750 rpm and a stirring time of less than 15 s were not sufficient for any of the samples tested to form an emulsion phase remaining after one hour of storage.
3.2. Emulsification Effect
In the experimental study, certain parameters were held constant, including the test temperature, the ratio of the aqueous and oily phases, and the test duration. The temperature was maintained at 80 °C, while the phase ratio was maintained at 50–50 V/V%. Following the mixing process, a 1 h duration was designated for the test (separation) phase. Throughout this period, the samples underwent no agitation, allowing the phases to naturally separate at 80 °C. Stirring was performed at varying speeds between 500 and 1500 RPM, with stirring times ranging from 5 to 600 s.
The assessment of the emulsifying effect was based on the proportion of the emulsified phase measured after 1 h following the cessation of mixing, expressed in V/V% [
4]. This quantified emulsification effect enabled the objective measurement of surfactant efficiency in emulsion preparation. The results of the emulsifying effect experiments using surfactant S2 are shown in
Table 11.
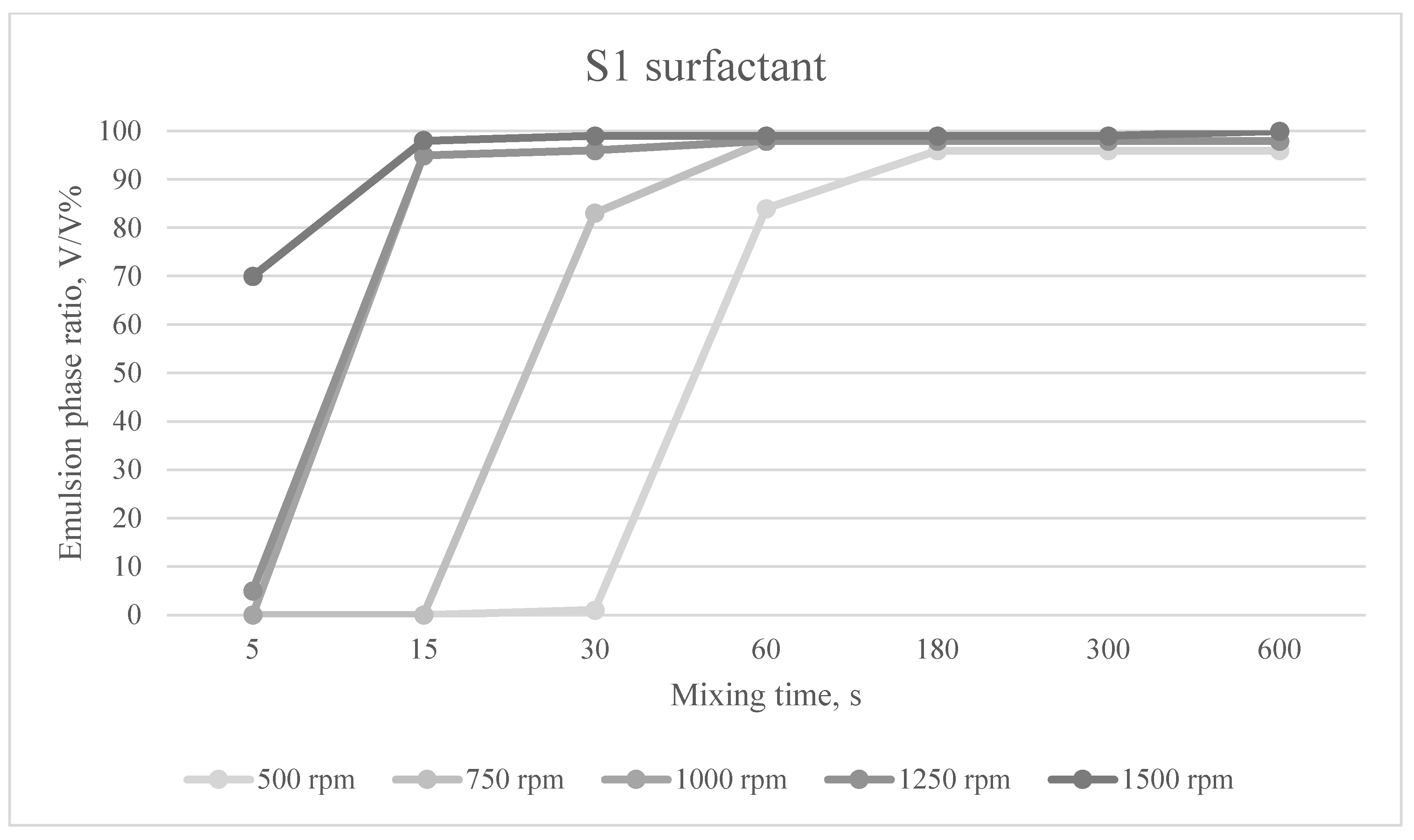
The emulsion phases formed at different mixing times and mixing speeds for the S1 surfactant are shown in
Figure 4.
When using surfactant S1, the emulsifying effect varied between 96 and 100% if a Winsor I or Winsor II emulsion was observed after one hour. If there was a Winsor II or Winsor III emulsion after one hour, the emulsifying effect ranged from 1 to 84%.
The results of the emulsifying effect tests with surfactant S2 are summarized in
Table 12.
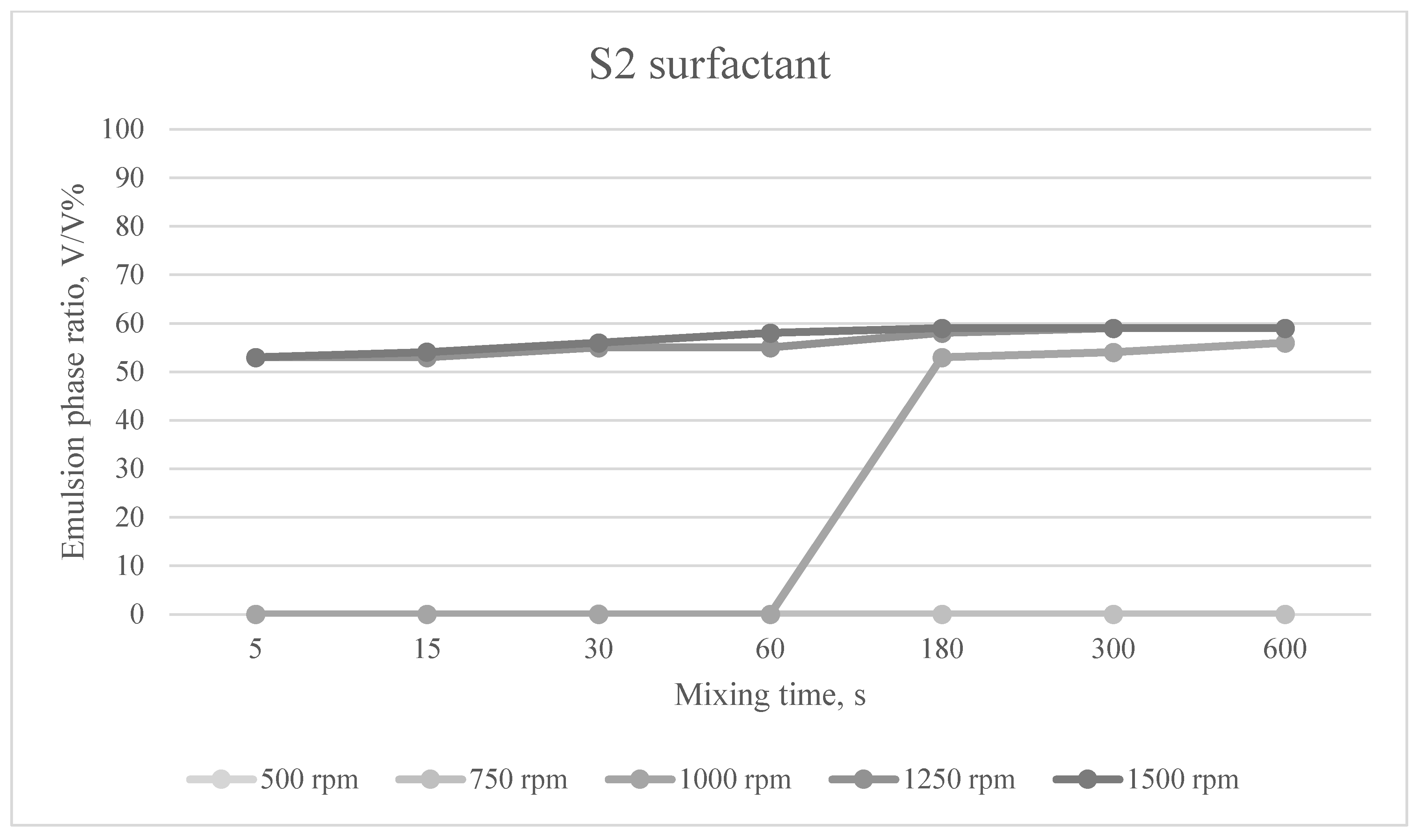
The emulsion phases formed at different mixing times and mixing speeds for the S2 surfactant are shown in
Figure 5.
For surfactant S2, the emulsifying effect varied between 53 and 59% depending on the mixing parameters. As the mixing speed and/or time increased, the emulsifying effect increased.
The results of the emulsifying effect tests with the SM1 surfactant are summarized in
Table 13.
The emulsion phases formed at different mixing times and mixing speeds for the SM1 surfactant package are shown in
Figure 6.
In the case of the surfactant SM1, the emulsifying effect varied between 53 and 59%, similar to that of the surfactant S2, but a shorter mixing time and/or a slower mixing speed were sufficient for the emulsifier to remain after one hour.
The results of the emulsifying effect tests with the surfactant SM2 are summarized in
Table 14.
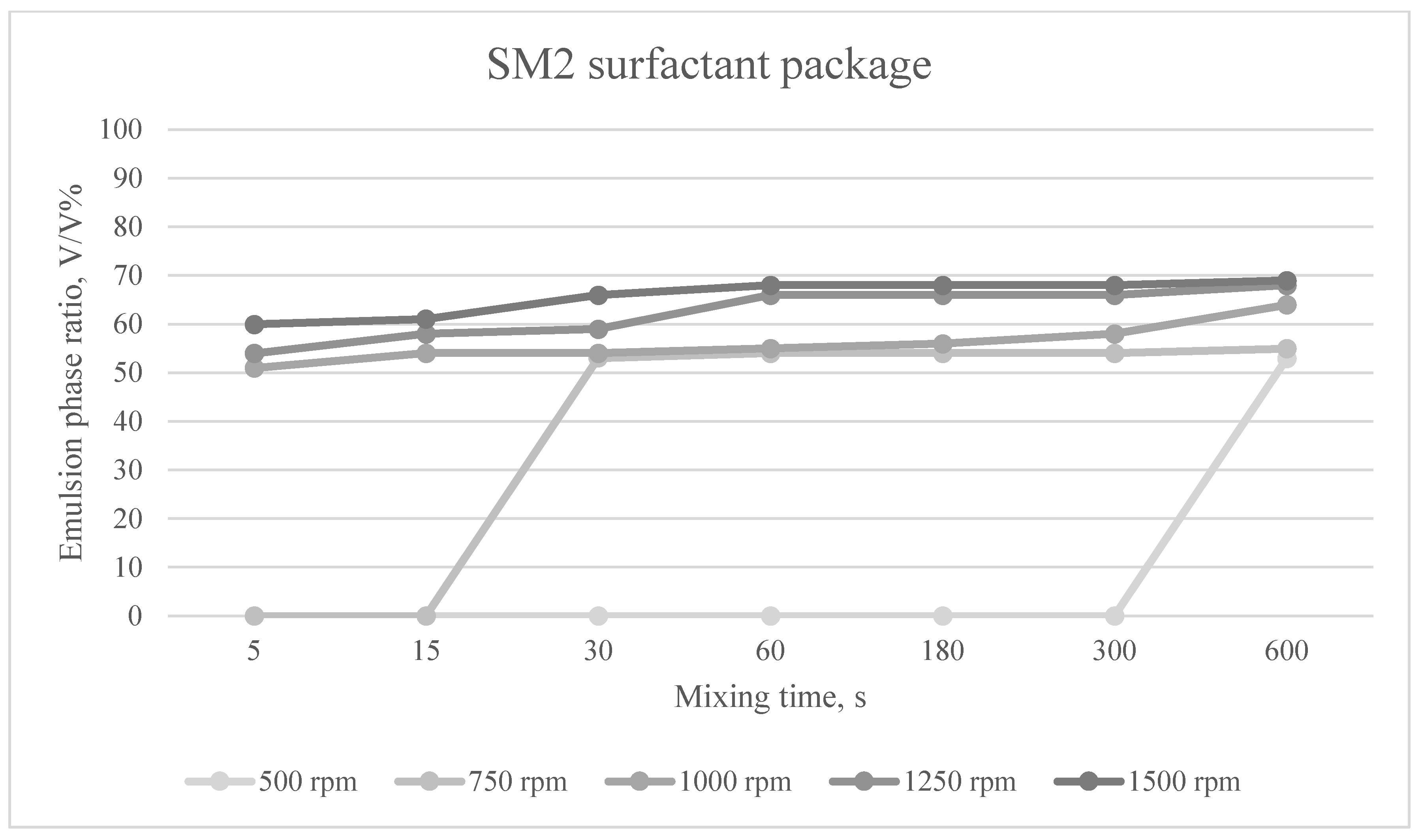
The emulsion phases formed at different mixing times and mixing speeds for the SM2 surfactant package are shown in
Figure 7.
In the case of the surfactant composition SM2, the emulsifying effect increased compared to that of the surfactant composition SM1. The emulsifying effect ranged from 51 to 69%.
The results of the emulsifying effect tests with the SM3 surfactant are summarized in
Table 15.
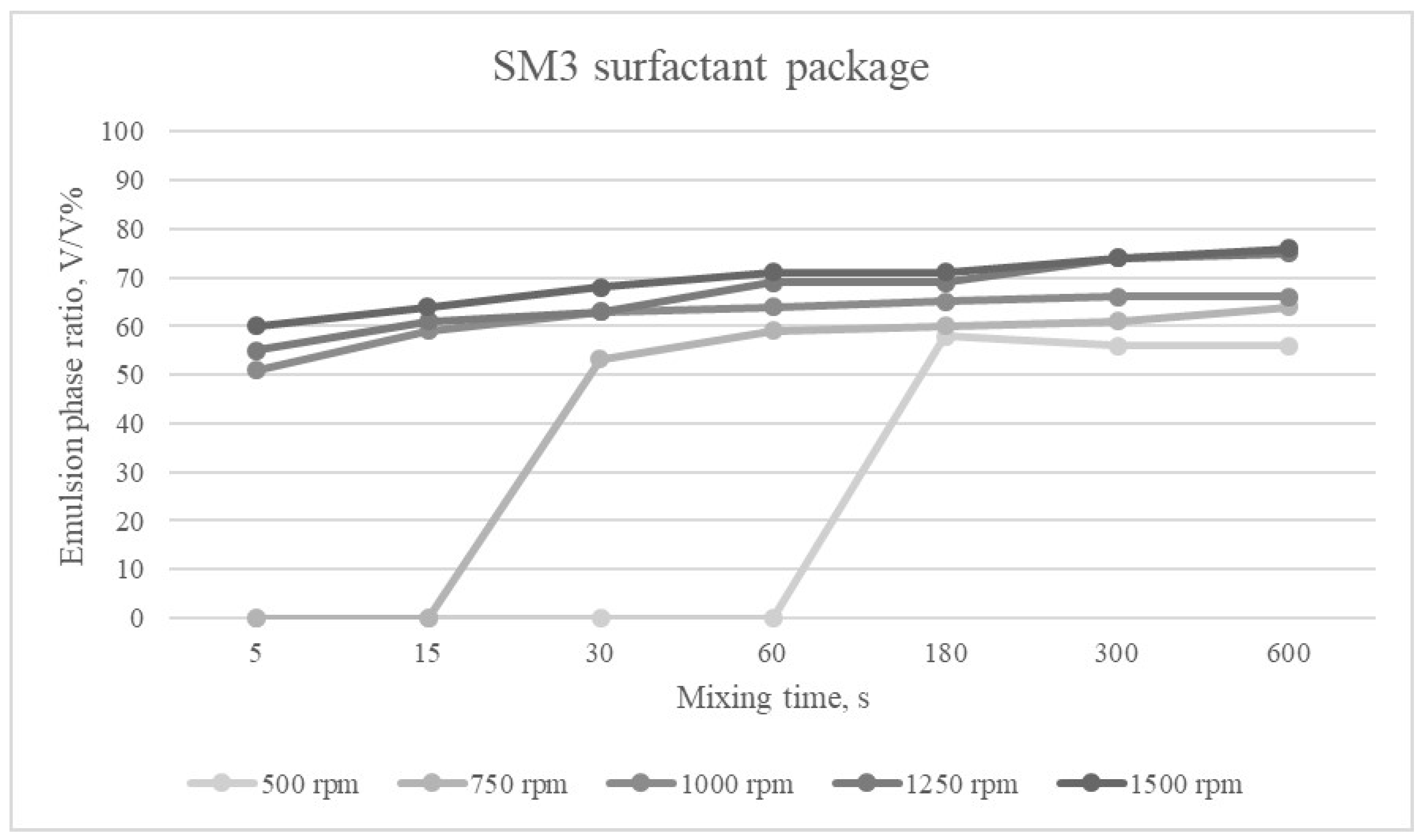
The emulsion phases formed at different mixing times and mixing speeds for the SM3 surfactant package are shown in
Figure 8.
Among the surfactant compositions, SM3 had the greatest emulsifying effect. Its value varied between 51 and 76% depending on the mixing parameters.
When the emulsifying effect was 0%, the mechanical energy generated by mixing was not enough to homogenize the phases sufficiently, and due to the large size of the dispersed droplets, the phase separation process took less than one hour. In one case, a Winsor IV type emulsion was formed using the S1 surfactant at a stirring speed of 1500 rpm and a stirring time of 10 min. In this case, the mechanical energy invested by mixing the phases was large enough to allow the dispersed droplets to fragment and remain stable after one hour of storage.
The highest emulsifying effect was achieved with the surfactant S1. In the case of surfactant compositions, increasing the proportion of surfactant S1 increased the emulsifying effect and decreased the minimum mixing time/mixing required to form an emulsion remaining after one hour of storage.
When two surfactant compositions were used, the test sample had an emulsifying effect of 5 s mixing time with a mixing speed of 1000 rpm, although the surfactants alone did not emulsify under similar test parameters.