Abstract
Effective decarbonization of the power generation sector requires a multi-pronged approach, including the implementation of CO2 capture and storage (CCS) technologies. The Graz cycle features oxy-combustion CO2 capture in a power production scheme which can result in higher thermal efficiencies than that of a combined cycle. However, the auxiliary consumption required by the air separation unit to provide pure O2 results in a significant energy penalty relative to an unabated plant. In order to mitigate this penalty, the present study explores the possibility of chemical looping combustion (CLC) as an alternative means to supply oxygen for conversion of the fuel. For a midscale power plant, despite reducing the levelized cost of electricity (LCOE) by approximately 12.6% at a CO2 tax of EUR 100/ton and a natural gas price of EUR 6.5/GJ and eliminating the energy penalty of CCS relative to an unabated combined cycle, the cost reductions of CLC in the Graz cycle were not compelling relative to commercially available post-combustion CO2 capture with amines. Although the central assumptions yielded a 3% lower cost for the Graz-CLC cycle, an uncertainty quantification study revealed an 85.3% overlap in the interquartile LCOE range with that of the amine benchmark, indicating that the potential economic benefit is small compared to the uncertainty of the assessment. Thus, this study indicates that the potential of CLC in gas-fired power production is limited, even when considering highly efficient advanced configurations like the Graz cycle.
1. Introduction
Carbon capture and storage (CCS) strategies are expected to play a critical role in achieving the climate change mitigation targets established by the IPCC [1] and agreed upon during COP28 [2]. The “State of the Climate in 2017” report by the National Oceanic and Atmospheric Administration (NOAA) states that in 2017, the dominant greenhouse gases released into the Earth’s atmosphere (carbon dioxide, methane, and nitrous oxide) reached new record levels and that the ten warmest years on record have occurred since 1998, with the four warmest years occurring since 2014 [3]. While the development and deployment of CCS technologies have progressed since COP21, large-scale adoption and ongoing research and development efforts remain critical to achieving global climate goals. The pace and extent of these developments will be influenced by policy measures, financial incentives, and future technological advancements. In an effort to mitigate the environmental impact of carbon dioxide (CO2) emissions from power generation in plants which operate with fossil fuels as primary energy feedstocks, a range of technological solutions are available based on different working principles and currently at different stages of development. Captured CO2 is stored in geological caverns where it can be used for enhanced oil and gas recovery [4] or used as feedstock in the production of synthetic fuels or methanol [5]. Despite widespread agreement on its importance and its relatively high technical readiness, CCS has not yet been implemented on a scale that matches the level of ambition set forth a decade ago [6].
CCS entails the removal of CO2 from the power generation cycle before it is emitted to the atmosphere. CCS options vary in terms of the point of the process at which removal takes place and relative to the nature of the technology employed to accomplish this [7], with the increased capital costs and associated energy penalty being the critical points for implementation. Post-combustion CO2 capture [8] is based on CO2 elimination from an exhaust gas stream by means of absorption, adsorption, or membrane units once the combustion of the fuel has taken place. Post-combustion is best suited to avoid emissions from low-carbon intensity fuels such as in natural gas combined cycles (NGCC), where the CO2 is found diluted and at low pressures, with an energy penalty in the range of 8 percentage points relative to unabated plants [9]. Post-combustion capture from coal at a 90% rate presents energy penalties above 10 percentage points [10]. For solid fossil fuels, pre-combustion CO2 capture [11] based on chemical absorption appears as an effective means to reduce this energy penalty in Integrated Gasification Combined Cycles (IGCC), while some studies indicate that this avenue achieves energy penalties comparable to post-combustion CO2 capture in natural gas-fired plants [12]. Alternatively, oxy-combustion capture [13] entails performing the conversion of the fuel in a nitrogen-free environment where the working fluid of the power cycle consists of the combustion products (H2O and CO2). This requires the delivery of purified O2 from an air separation unit (ASU) to the combustion chamber of the cycle, with and associated auxiliary power consumption and a partial recirculation of products to control the firing temperature. Additionally, the use of a mixture of H2O and CO2 implies a modification of the pressure ratio of the gas turbine and coolant flow performance [14]. The inherent advantage of this capture technology is that a relatively purified CO2 stream is obtained after heat recovery and water condensation.
Amongst the oxy-combustion capture technologies for power generation available, one of the most prominent options is the Graz cycle [15]. It constitutes an advanced power generation design originally conceived as a high-temperature steam cycle fueled by H2 and O2 [16], in which the Brayton and Rankine cycles are integrated to maximize synergies. This plant was adapted to carry out the combustion of natural gas and operate with a mixture of H2O and CO2 in which, after condensation of steam, the CO2 is separated and compressed in an intercooled compressor for storage, leading to CO2 mitigation costs as low as USD 17.5/ton [17]. In this design, a closed vacuum steam cycle is implemented to maximize performance by retrieving the condensation enthalpy of water [18]. This cycle can achieve notably high thermal efficiencies and therefore reduce the energy penalty associated with CO2 capture; a recent study by Mitterrutzner et al. [19] indicates a thermal efficiency of 53.1% operating at nominal design conditions, although the energy intensive O2 production system imposes a large auxiliary consumption of around 10% of the fuel heat input. Syngas from oxy-blown gasification of coal or biomass can also be employed as fuel in this cycle, thus sharing a common ASU.
Finally, chemical looping combustion (CLC) [20] appears as a thermochemical process for inherent CO2 capture presenting a lower degree of technological maturity. The oxidation of the gaseous fuel is achieved indirectly employing an intermediate substance that serves as oxygen carrier between two distinct reactors, (1) an air reactor, in which the oxygen carrier is oxidized, and (2) a fuel reactor, where the oxygen carrier is reduced with the fuel producing a reduction gas outlet (H2O and CO2), thereby isolating the combustion products from the air. In principle, CLC could potentially avoid the energy penalty of CO2 capture while achieving 100% CO2 avoidance. Nevertheless, given the oxygen carrier, valves, and filter material limitations, the operating temperature must be around ~300 °C lower than conventional combustion chambers, thereby limiting its thermodynamics performance. A critical aspect of CLC design is the selection of the oxygen carrier material [21]; numerous studies have been conducted in the past [22] with metal oxides such as Ni-, Cu- and Fe-based materials, indicating that Ni has the highest prospects for achieving high operation temperatures [23]. Further efforts with CLC aim to develop materials capable of processing solid fuels [24] or alternatively carrying out a reforming reaction of the fuel for H2 generation [25,26]. Lastly, there is an interesting potential in the integration of CLC in chemical production processes of commodities which require an oxidation step of reactants [27,28,29]. With regards to CLC reactor technologies, several novel pathways are under development beyond dual interconnected fluidized beds [30], such as internally circulating reactors (ICRs) [31] and gas switching technology (GST) [32], which aim to simplify the operation and scale-up of chemical looping applications for pressurized operation.
The CLC working principle offers an interesting opportunity as it fulfills the function of an ASU and therefore presents an integration potential with the Graz cycle previously described, which could circumvent the major drawback of a large auxiliary consumption for O2 production in a natural gas-fired plant. A knowledge gap in the literature exists regarding this possibility. Therefore, the objective of this work is to evaluate the techno-economic potential of integrating CLC in the Graz cycle for midscale power production capacities, under the assumption that sufficient technological development has taken place so that CLC reactors and gas turbines operating with a mixture of CO2 and H2O are de-risked and readily deployable. This approach aims to quantify in economic terms the benefit of further R&D investments to mature and commercialize these CCS technologies in power generation applications using natural gas as fuel. The following section provides a description of the novel scheme proposed, the conventional Graz power cycle, where O2 is produced in an ASU, together with several reference benchmark power plants with and without CO2 capture: a combined cycle (CC) employing an industrial gas turbine, a 2 × aeroderivative gas turbine operating in open cycle, and a CC plant with post-combustion CO2 capture with amines. Key performance indicators based on “4E” analysis (energy, environmental, exergy, and economic analysis) are defined. According to those metrics with detailed sensitivity studies of the economic assumptions, results are subsequently provided to ultimately derive the main conclusions of the study.
2. Methodology
In this section, a description of the power cycles, their topology, and the corresponding modeling assumptions is provided. Key performance indicators in terms of energy, environment, exergy, and economics are defined.
2.1. Plant Description
This study presents three reference power generation plants (one incorporating post-combustion CCS) and two advanced power plants with oxy-combustion CO2 capture and inherent carbon capture through CLC for midscale power generation capacities. Plant models were carried out with UniSim Design® [33] using Peng–Robinson as the fluid package for estimation of thermodynamic properties, except for streams containing only water or steam, for which the ASME steam tables have been employed. The main modelling assumptions for the performance of the advanced power plants with oxy-combustion CO2 capture are provided in Table A1 in Appendix A. The conditions and composition of the natural gas fuel employed in all plant models are given in Table A2 in Appendix A.
2.1.1. Benchmarks
Benchmarks were developed based on vendor data [34] for industrial heavy duty gas turbines. To specify the model where information is not available, values from [35] were used for several technology components.
Combined Cycle (CC)
The reference combined cycle features a General Electric 9E.04 gas turbine with a nominal power output of 145 MW operating at a pressure ratio of 13.3 and reaching an exhaust temperature of 542 °C. The bottoming cycle consists of a two-pressure level heat recovery steam generator (HSRG), steam turbine, and condenser for an overall thermal efficiency of 55.0%, producing a net power output of 215 MW. The gas turbine model was built considering a simplified blade cooling model, calibrating the turbomachinery efficiencies and combustor firing temperature to reach the open-cycle efficiency and the net power output specified in the brochure datasheets. The steam turbine efficiencies were assumed from [35], considering an MITA of 10 °C in the evaporators and an approach of 5 °C, while maximum steam temperature was taken as 520 °C. The combined cycle configuration is briefly depicted in Figure 1. The stream summary for this plant is presented in Table A3 in Appendix A.
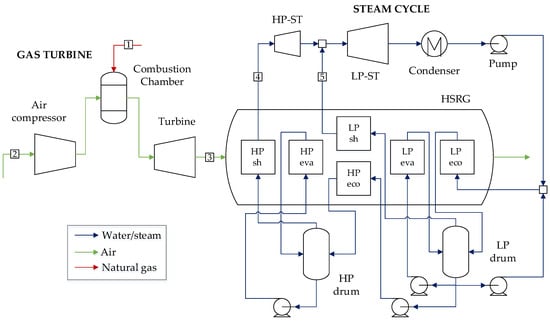
Figure 1.
CC plant configuration based on GE 9E.04 gas turbine.
Open Cycle (OC)
Alternatively to power generation in a combined cycle, which presents a high capital expenditure, midscale power generation may benefit from aeroderivative gas turbines operating in open cycle, since they present a higher efficiency relative to the combined cycle as well as a smaller plant footprint. For this reason, a configuration consisting of two General Electric LMS-100s is considered. These aeroderivative gas turbines achieve a power output of 117 MW with an efficiency of 44.3%. They operate at a pressure ratio of 42, substantially higher relative to industrial gas turbines, with a lower exhaust temperature (432 °C). An intercooler is used during the air compression step to enhance efficiency. The aeroderivative GE LMS-100 is a three-spool turbomachine with a high-pressure (HPT) and a low-pressure turbine (LPT) to mechanically drive a high-pressure (HPC) and low-pressure compressor (LPC), respectively, and a final power generation turbine (PGT) coupled to a generator, as depicted in Figure 2. The stream summary for this power cycle is presented in Table A3 in Appendix A.
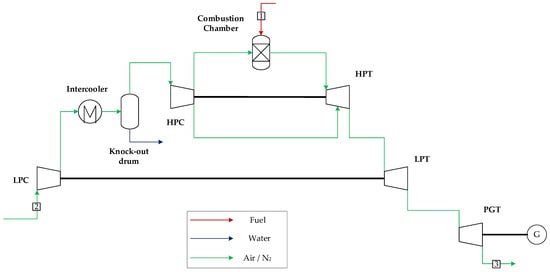
Figure 2.
OC gas turbine (GE LMS-100).
Post-Combustion Capture (PCC)
Another reference power plant consisting of a GE 9E.04 combined cycle with post-combustion CCS based on CO2 capture with methyl-ethanolamine (MEA) is evaluated. The absorption section was modeled according to [35] and is depicted in Figure 3, resulting in an amine regeneration duty of 3.96 MJth/kg of CO2 captured. Cool-down with the recirculating water of the exhaust gases is required prior to the absorption column as well as a blower to overcome the pressure losses of the absorber. The CO2 captured is dehydrated and compressed in a four-stage inter-cooled compressor to 150 bar. A block flow diagram of the process is depicted in Figure 2. The stream summary for this plant in shown in Table A3 in Appendix A.

Figure 3.
Block flow diagram of the PCC plant.
2.1.2. Graz Cycle with ASU (GASU)
The Graz cycle with pressurized oxygen delivery from an ASU is depicted in Figure 4. O2 is provided at 40 bar and 98%mol. purity by means of a pumped liquid oxygen (PLOX) ASU, modeled according to prior work [36]. The resulting specific power consumption for this configuration and delivery conditions is 341.1 kWh/ton of O2. The Graz cycle is modeled with similar assumptions and topology as those reflected in [18]. Natural gas (NG) fuel is combusted in the chamber with O2 and the recycle stream to reach a combustor outlet temperature (COT) of 1400 °C. At TITISO of 1300 °C (turbine inlet temperature after mixing the hot gas with cooling flows) has been assumed. Steam from the HP steam turbine (T-101) outlet is used as coolant. After expansion in the gas turbine (T-102), hot gases are routed to an HSRG (E-100), which raises the HP superheated steam at 180 bar conducted to T-101. The gas turbine pressure ratio is set to 40 [18], given that the temperature drop of a water/CO2 mixture upon expansion is smaller relative to air for an equivalent pressure ratio. After heat recovery, the H2O/CO2 mixture is partially recycled to the combustor chamber after an intercooled re-compression to control the combustor outlet temperature. This intercooler (E-101), in which HP steam is raised, is required because the resulting adiabatic temperature rise would be too large in a single stage. Thus, the outlet temperature after the second stage is limited to 550 °C. The portion that is not recycled is routed to a sequence of two stages of compression and heat recovery whereby steam at a vacuum pressure (VP) of 0.65 bar is raised by condensing water from the stream mixture of H2O and CO2. After a final heat rejection to ambient temperature in C-101, CO2 with a small fraction of water, nitrogen, and argon (the latter two originating from the O2 stream) is obtained. It is further compressed in an intercooled compressor and dehydrated to finally deliver the CO2 stream at 150 bar for transportation and storage. The VP steam at superheated conditions is routed to a vacuum steam turbine (T-100) to generate additional power. After expansion to 0.04 bar, it is condensed and then pumped again to repeat the cycle. The stream summary for this plant can be found in Table A4 in Appendix A.
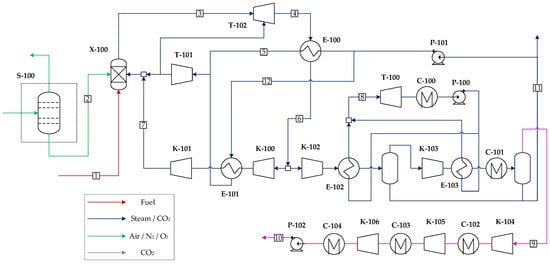
Figure 4.
GASU power cycle. Equipment labels correspond to components referred to in the exergy analysis.
2.1.3. Graz Cycle Integrating CLC (GCLC)
It is noted that the ASU auxiliary consumption significantly curtails the net thermal efficiency of the Graz cycle and presents a high capital investment. It is therefore proposed to employ CLC as an alternative pathway to combust the fuel, integrating a secondary power cycle in the air side, as depicted in Figure 5. Air is compressed in a two-stage intercooled compressor up to the CLC operating pressure (18 bar) and preheated in a recuperator. The flow rate of air is stoichiometrically selected to combust the natural gas fuel in the reduction reactor. In the air reactor, a N2 outlet stream is obtained, which is expanded in a turbine and then routed to the recuperator hot side to preheat incoming air to the CLC. Remnant heat from this stream and heat prior to the intercooler are used to economize water. On the reduction side, combustion products are expanded in the gas turbine and then routed to the HSRG, where HP steam is raised. Subsequently, a portion of the flow after heat recovery is recompressed and recycled to the CLC reduction reactor to control the operating temperature, while the remaining fraction is further cooled and condensed generating vacuum pressure steam (0.65 bar) for the steam cycle. After water knock out, the CO2 stream is further compressed in an intercooled compressor, dehydrated, and sent to storage pressure. It is relevant to point out that the CLC must operate at a lower temperature than conventional combustion chambers of gas turbines due to material limitations of the oxygen carrier and the valves and filters required to prevent ingress of solid particles in the turbomachinery components. This temperature limitation implies a reduction in the pressure ratio to achieve similar turbine outlet temperatures as in the Graz-CLC case from which to raise steam. An optimization for a fixed CLC operating temperature leads to a pressure ratio of 18 for a maximum thermal efficiency. Consequently, the recycle compressor does not require an intercooling stage, potentially reducing the capital costs of the cycle. The stream summary for this plant can be found in Table A5 in Appendix A.
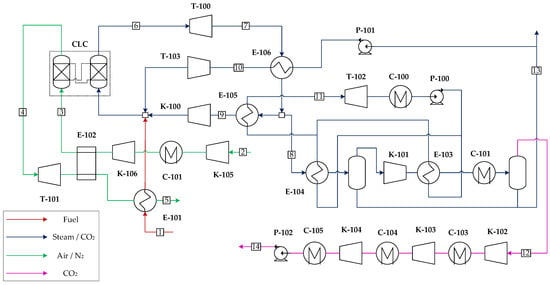
Figure 5.
Simplified GCLC power scheme. Equipment labels correspond to the components referred to in the exergy analysis.
Regarding CLC performance, there are numerous technologies that could eventually be developed to a deployment level but to date have not been proven at the scale of the plants proposed. In this assessment, it is assumed that several technological breakthroughs are reached in order to investigate the potential economic benefits if the technology was currently available in the market. Therefore, it is assumed that the CLC system can operate at the required pressure ratio, with a reactor outlet temperature of 1100 °C, achieving full fuel conversion and a reduced mixing between outlet streams such that the retrieved CO2 stream does not require further purification [37]. Therefore, this component is modeled as a black box where mass and energy balances are performed according to the schematic shown in Figure 6. Given the relatively low turbine inlet temperatures (TIT), blade refrigeration was not considered because it will have a minor impact on performance.

Figure 6.
Schematic of the black-box CLC model employed in this study.
Since the turbomachinery components for the Graz cycle power plants are not yet developed, the GASU and GCLC plants were sized by fixing the thermal heat input in order to achieve similar net power outputs to the OC case.
2.2. Key Performance Indicators
The modeled plants are compared through a 4E energy, environmental, exergy, and economic analysis. The key performance indicators of each area are defined in the following sections.
2.2.1. Energy and Environmental Analysis
The most relevant metric of a power plant performance from an energy perspective is the net thermal efficiency, Equation (1), resulting from the relation between the net power output and the total heat rate to the plant, which is the product of the fuel heating value and the flow rate. Similarly, the gross thermal efficiency is based on the gross power output (where auxiliary consumption of units such as CO2 compression and ASU are not discounted) and the fuel heat input in Equation (2).
Several indicators characterize the performance of the plants from an environmental perspective. The capture rate (Equation (3)) is the relation between the CO2 that is routed for transport and storage with respect to the total amount that is generated in the combustion of the fuel. The specific emissions (Equation (4)) account for the amount of CO2 released to the atmosphere (due to incomplete capture) relative to the net power generation of the plant. On the other hand, the CO2 avoided (Equation (5)) is the relationship between the specific emissions which have been prevented when implementing CCS relative to an unabated reference plant and the specific emissions incurred by that reference plant. Since CCS typically results in an energy penalty relative to an unabated plant, the avoided CO2 results are lower than the capture rate. In the present evaluation, the unabated combined cycle configuration is taken as the reference plant for the calculation of this metric.
Finally, the specific primary energy cost of the CO2 avoided or SPECCA (Equation (6)) represents the amount of the fuel heating value that is required to prevent the emissions of a mass unit of CO2 relative to the reference plant.
2.2.2. Exergy Analysis
Any thermal or chemical process is a complex system composed of several subsystems which interact between each other and with the environment across their boundaries. There can be energy and mass interactions. On the other hand, energy interactions can be in the form of heat and work. In order to consistently compare the relative performance of each of the subsystems based on the different types of interactions that occur between them, a function of state known as exergy is used, which corresponds to the maximum useful work that could be obtained from a given system relative to an environment at , and known composition. The exergy balance to a subsystem , exchanging heat and work with the surroundings with material inlet and outlet streams, is shown in Equation (7). Under stationary conditions, there is no accumulation of exergy in the system, and if there is no change of volume (rigid system), the balance is simplified, requiring only the knowledge of the heat and work flows across it as well as the specific exergy flows of the material streams. The exergy flow of a given stream ei in Equation (8) contains a physical exergy contribution term , which accounts for the maximal specific work that can be obtained theoretically from that stream because of its thermomechanical (pressure and temperature) imbalance with the environment at and as well as a chemical exergy term, which accounts for chemical imbalance (chemical composition) with a reference environment.
The application of the balance results in the determination of the exergy destruction in every subsystem, which represents the amount of exergy that has been wasted (i.e., no longer useful work can be obtained) due to the irreversible nature of the process and is therefore always greater than zero. In order to perform the exergy analysis of the power plant configurations, the exergy flow of all material and energy streams involved in the process is calculated with a dedicated tool [38]. Balances are carried out with SySBaC [39] system balance calculator, a code in the Scilab and Excel platforms which enables us to categorize and group components to perform the balance based on the specified flows of a transported magnitude. These balances allow us to define a rational exergy efficiency of the compound system as the relation between import cost exergy and useful export exergy, as shown in Equation (10).
2.2.3. Economic Analysis
The foreseen advantages of novel plants integrating CCS in terms of environmental and thermal performance must be translated into an economic value function. For that purpose, an estimation of the capital and operational costs and a cash flow analysis must be conducted to consistently compare different technology options. The Standardized Economic Assessment (SEA) tool developed by the authors of [40] is employed for that task. Turton correlations [41] allow us to determine the bare erected costs (BECs) of individual equipment items. Alternatively, capacity–cost scaling correlations for the analogous scope of the plant can be used to determine capital costs. This approach was used to estimate the capital cost of the Graz cycle from [13] or the HSRG and MEA absorption units from the reference plants [35]. The cost estimation basis selected was in 2020 EUR for a plant located in Western Europe. The base economic assumptions for the evaluation are summarized in Table 1. A 10% process contingency was assumed for the Graz power cycle and CLC unit, given that these technologies are not currently commercial, to reflect this added uncertainty in the cost estimate. SEA tool files for all the plants evaluated can be found in [42].

Table 1.
Economic assumptions.
The economic value function employed to compare the different proposed plants is the levelized cost of electricity (LCOE), which incorporates the cost contribution of capital and fixed and variable operating costs (which include fuel and CO2 emissions tax) and is defined as the selling price of electricity at which the net present value, Equation (12), i.e., summation of annualized discounted cash flows (Equation (13)), is zero at the end of the project lifetime:
As an alternative economic metric for the plants with CCS, the cost of CO2 avoidance in Equation (14) represents the additional cost per unit of captured CO2 required for integrating CCS when the LCOE is evaluated with no CO2 tax for emissions.
3. Results
Results are provided in three sections. Energy and environmental performance metrics, directly accessible from the plant models, are first provided. Then, the exergy analysis and balances with the distribution of losses and exergy diagrams are given. Finally, the economic performance for the base economic assumptions are shown, as well as a sensitivity and uncertainty quantification study to the main economic assumptions.
3.1. Energy and Environmental Analysis
The power breakdown and the plant performance according to the previously defined metrics are provided in Table 2. It can be seen that the energy penalty resulting from integrating carbon capture and storage with post-combustion CO2 capture (PCC) results in 7.4 percentage points, which is primarily the consequence of LP steam extraction from the bottoming cycle to regenerate the absorbent and, secondly, the exhaust gas blower power consumption. This is consistent with results obtained for large-scale combined cycles [9]. The GASU scheme results in an energy penalty of 2.6 percentage points, which is consistent with reported values [18]. Notably, the gross energy efficiency of the GASU plant is significantly higher (10.6 percentage points) than that of the CC configuration. The model developed considers inherently safer O2 pressurization of the combustion chamber pressure through liquid pumps in the ASU, leading to an auxiliary consumption equivalent to 10.0% of the fuel heat input, while the CO2 capture section accounts for 2.9%. On the other hand, the GCLC scheme achieves a 1.9% higher net thermal efficiency than the CC benchmark. However, the gross electrical efficiency for this scheme is 6.1 percentage points lower for the GCLC compared to the GASU plant because the firing temperature of the gas turbine was reduced to 1100 °C due to the material limitations of the CLC system. This is a key result: despite a significantly lower TIT, avoidance of ASU auxiliary consumption results in an improved energy performance relative to the CC reference plant.

Table 2.
Energy and environmental performance of the different plants.
In terms of CO2 emissions, the detrimental effect of open-cycle gas turbine operation (OC) is observed, presenting approximately 24.2% higher specific emissions. Note that, although the PCC reached a capture rate above 90%, the avoidance is lower because more fuel has to be burned to reach the same power output due to a lower . Complete capture is achieved in the GASU plant as there are no sources of emissions, whereas the undesired mixing of CLC reactor outlet streams causes a small reduction in the capture rate. Since the thermal efficiency of the GCLC is higher than the CC benchmark, the CO2 avoidance is practically the same as the capture rate. In terms of the SPECCA index, it is seen that the GASU plant reduces the primary energy cost by 2.16 MJ/kg of CO2 relative to the PCC plant, whereas the GCLC achieves negative values for SPECCA as a result of its enhanced thermal performance.
3.2. Exergy Analysis
An exergy analysis of the plants with CCS based on the Graz cycle was conducted in order to better understand the performance of these schemes; the SySBaC files can be downloaded from [43]. An exergy study of the reference plants is of less interest as these are well-known configurations for power generation and such an analysis is shown in prior work for large-scale combined cycles [44]. Figure 7 shows the exergy distribution of the GASU and GCLC schemes based on equipment categories (compressors, heat recovery, heat rejection, turbines, fuel conversion, mixers and pumps, CO2 compression, exergy loss, and useful effect). It can be seen that the major exergy destruction contributors are the combustion chamber and CLC reactor (fuel conversion item). This is the case because chemical degradation imposes a large exergy loss due to its highly irreversible nature relative to thermomechanical processes. The useful effect denotes the exergy efficiency defined in Equation (10), revealing that replacing the ASU with the CLC system results in a 4.2% higher exergy efficiency. This results from the fact that the ASU for O2 production is a net power consumer (accounting for 6.5% of the total exergy provided by the fuel), whereas the CLC and associated air/N2 recuperated cycle is a net power producer. Exergy losses are somewhat larger for the GCLC because of the air exhaust heat content, as it cannot be entirely retrieved in the economizer units.
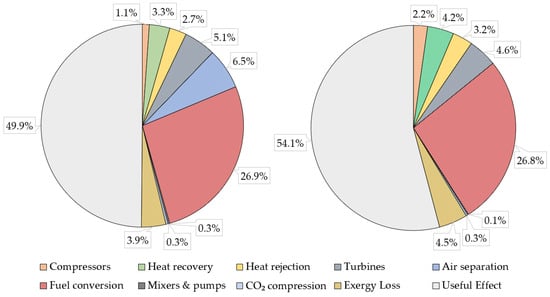
Figure 7.
Exergy distribution for the GASU (left) and GCLC (right) plants.
Figure 8 shows the rational exergy efficiency the main plant components according to the equipment labels shown in Figure 4 and Figure 5 for the GASU and GCLC, respectively. It is seen that the components which present the lowest exergy utilization ε are those in which chemical reactions occur (CLC, combustion chamber) and the ultimate heat rejection equipment (condenser of the VP steam cycle: C-100, where a large heat rejection to ambient takes place with a low exergy content). On the other hand, in those components where there is only physical exergy exchange, the exergy utilization is close to 100% in most cases because the total exergy flow is mostly chemical exergy. For the GASU plant, the ASU (S-100), with no chemical reactions but changes in composition due to distillation columns, also presents low performance, as a large exergy input in the form of electrical power is consumed to generate pressurized and purified O2 for the power cycle and because purified N2 is not considered as a valuable output from the unit.

Figure 8.
Exergy utilization rate per plant subsystem for the GASU (above) and GCLC (below) schemes.
In order to map the flows of exergy across the two power plants integrating advanced CCS technologies, an exergy flow diagram is provided in Figure 9 and Figure 10 for the GASU and GCLC plants, respectively, with the total exergy flows and exergy destruction taking place in each equipment unit, provided in MW.

Figure 9.
Exergy flow diagram for the GASU plant. Values are in MW; red values indicate exergy destruction in the component.
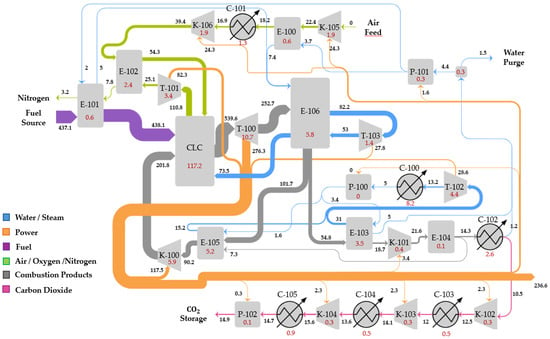
Figure 10.
Exergy flow diagram of the GCLC plant. Values are in MW; red values indicate exergy destruction in the component.
3.3. Economic Analysis
The specific capital investment and LCOE are first shown for the base case economic assumptions. A sensitivity analysis of key economic variables, preserving all the others at constant values, is then performed to evaluate their relative influence on the LCOE. Finally, an uncertainty quantification study is carried out to evaluate if there is statistical significance for the potential cost reductions achieved with the advanced CCS concepts.
3.3.1. Specific Investment and LCOE
Figure 11 shows the specific capital costs (total overnight costs) per kW of electricity produced for each of the configurations based on the plant sections: O2 production, power generation, and CO2 capture. It is observed that the integration of CCS imposes a substantial additional capital requirement, which is largest for the PCC plant. The CCS scope for this configuration entails a direct contact heat exchanger, a blower to overcome the pressure losses of the absorber, the absorption and stripper column with heat exchangers, and the CO2 compressor. On the other hand, for the plants employing a Graz cycle, this scope is only limited to several compression stages with intercooling of the CO2 product and a supercritical CO2 pump. However, the cost contribution of the O2 production section is significant (CLC and air power unit for the GCLC case and the ASU for the GASU plant), ultimately leading to a specific capital investment (TOC), which is 74.7, 132.8, and 98.8% higher for the PCC, GASU, and GCLC plants, respectively, relative to the unabated CC benchmark. The OC scheme, comprising two aeroderivative gas turbines, presents a specific TOC reduction of 14.6% as the costly units of the bottoming cycle are avoided.
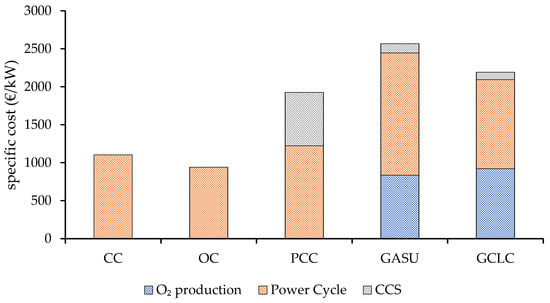
Figure 11.
Specific capital investment for the different power plants.
The LCOE results of the cash flow analysis conducted in accordance with the assumptions reflected in Table 1 are provided in Figure 12, where the different cost contributions as well as the COCA for the plants with CCS are shown (note that for COCA calculation, no CO2 tax is considered). Notably, the cost of the CC configuration is primarily driven by the fuel and the CO2 taxes, whereas the plants with CCS present a comparatively larger capital cost contribution. The LCOE of the PCC plant is EUR 9.8/MWh (−9.9%) lower than the unabated CC. The conventional Graz cycle shows a higher cost than the PCC, providing EUR 5.0/MWh (−5.0%) savings relative to CC. The GCLC plant reached EUR 12.4/MWh (−12.6%) cost reduction relative to this benchmark. Finally, the OC case showed an increase of EUR 17.0/MWh (+17.3%) despite its lower specific capital costs because of its lower efficiency, which leads to increased fuel consumption and CO2 intensity.
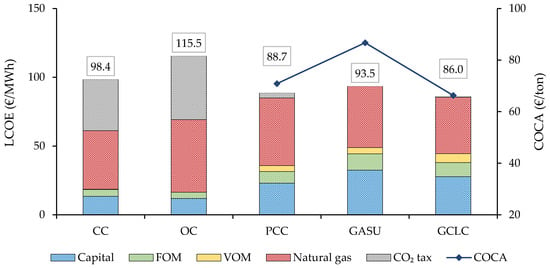
Figure 12.
LCOE for the different power plants (bars—left axis). COCA for plants with CCS (line—right axis).
When looking at the COCA, the GASU plant shows a EUR 15.9/ton (+22.4%) higher avoidance cost than the PCC, while the GCLC scheme only shows a mild avoidance cost reduction of EUR 4.6/ton (−6.5%) relative to PCC plant. In order for the CCS plants to be economically attractive relative to the CC, a minimum CO2 price of EUR 70.9, 86.7, and 66.3/ton is required for the PCC, GASU, and GCLC plants, respectively.
These results highlight that the improved energy performance of the Graz cycle plants is practically offset by the higher capital investment required. The economic results for the GASU plant presented in this study are aligned with the values reported in the IEAGHG report [13]; the economies of scale benefit the plants presented in this report as they are designed for 1000 MW output, leading to lower specific costs, which is offset by a higher natural gas price employed (EUR 8/GJ), ultimately achieving a similar LCOE.
The key assumption that defines the thermal performance of the GCLC power cycle is the achievable temperature in the reactor system. Ni-based oxygen carriers present attractive prospects for power generation since they reach operating temperatures in the range of 900 to 1100 °C, showing agglomeration and de-fluidization above the upper temperature limit [45]. Figure 13 shows the effect of increasing the CLC operating temperature to up to 1200 °C, if the material limitations were overcome. If such an optimistic hypothesis were realized, the GCLC would achieve an LCOE cost reduction of EUR 16.5/MWh (−16.8%) and EUR 6.7/MWh (−7.6%) relative to the unabated CC and the PCC benchmarks, respectively. These gains remain minor when taking into perspective the technology breakthroughs required and uncertainty associated to the novel scheme.
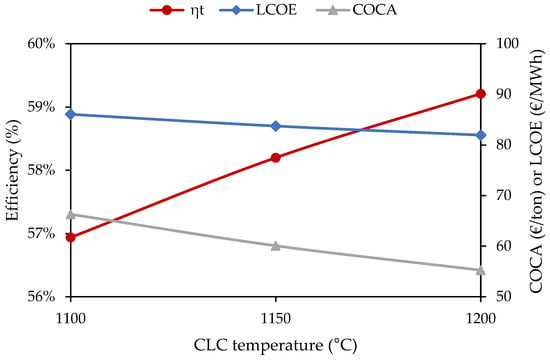
Figure 13.
Effect of increasing CLC operating temperature on thermal efficiency (ηt), LCOE, and COCA for the GCLC power cycle.
3.3.2. Sensitivity Analysis
Individual sensitivity analyses of key economic variables were carried out in order to evaluate the relative effect when the remaining assumptions are kept at the base case values, and these are shown in Figure 14. The economic variables that were investigated were the price of natural gas, CO2 tax, plant capacity factor (CF), discount rate, CO2 transport and storage costs, and a capital cost factor for the total investment of the PCC, GASU, and GCLC plants, since the not-yet-commercial technologies present the largest uncertainty in the estimate. The results indicate that the most influential variable on the LCOE is the primary energy cost of natural gas, showing a steeper line for those plants with lower thermal efficiency. For high natural gas prices, the thermal efficiency advantage of the GCLC increases its economic potential relative to the PCC plant. Increasing CO2 taxes has a very detrimental effect on the economic performance of those plants without CCS (CC, OC), while the influence on the PCC is greater than the GASU and GCLC plants due to the lower capture rate achieved. It is noteworthy to highlight that the cost factor sensitivity affects the GASU plant most significantly because it presents the highest specific investment, whereas the PCC and GCLC show a similar dependence. In the subsequent analysis, the cost uncertainty contribution of the CLC and Graz power cycle, as well as the PCC CO2 capture plant components, will be evaluated separately.

Figure 14.
Sensitivity analyses for the different plants.
3.3.3. Uncertainty Quantification
In the prior section, the effect of key cost drivers of the plants is assessed, but it is not suitable to quantify the uncertainty related to a cost estimate when the economic variables are not known with precision. For this reason, an uncertainty quantification study using the SEA tool application [46] is performed by introducing a normal distribution for economic variable assumptions instead of fixed value, the bounds of which are known with a certain confidence level. The input parameters for the economic variable distributions, with an assumed 99% confidence (meaning that the actual value of the variable will be found between low and high bounds with a 99% probability) are provided in Table 3. Thus, this evaluation will clarify whether the base case economic results regarding the potential of the novel CCS technologies is conclusive.

Table 3.
Input economic variable distribution parameters (assumed).
The histograms of the LCOE results for 1000 experimental runs are presented in Figure 15. The box plot shown in Figure 16 presents the first, second (median), and third quartile, providing a measure of the dispersion of LCOE for each of the configurations. The average mean is represented with a cross. Numerical values are provided for each case with the labels shown to the left of each plot. The whiskers extend from the edges of the box and represent the minimum and maximum values of the dataset, excluding outliers, which are those values larger than the quartile limit plus 1.5 times the interquartile range (IQR). The IQR is the difference between the limits of the first and third quartiles (box).

Figure 15.
LCOE distribution after 1000 runs for the different power plants based on economic variable distributions shown in Table 3. The bin size has been set to EUR 2/GJ.
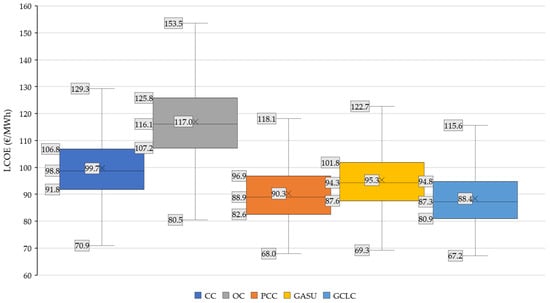
Figure 16.
Box plot for the power plant configurations.
Considering the IQR for the CC plant, the results indicate that there is a 34.0% overlap between that benchmark and the PCC plant, while for the GASU configuration this results in as high as 66.7% of the values. For the GCLC plant, there is only a 20.0% overlap in the LCOE results. If this comparison is established between the PCC and the Graz cycle plants, the overlap for the GASU plant is of 65.0%, while the GCLC shares 85.3% of the IQR of the values. This highlights that the expected cost reductions of the advanced CCS options relative to post-combustion capture are relatively small, as anticipated by the prior LCOE assessment for base case economic assumptions. Interestingly, the IQR (and similarly with the differences between maximum and minimum values) for unabated plants (EUR 15–18.6/MWh) are larger relative to the plants with CCS (EUR 13.9–14.3/MWh), underlining that the capital cost uncertainty introduced by the novel plants with reduced CO2 footprint is practically offset by operational cost of CO2 emissions taxes, which, together with the natural gas price, are the economic variables that are most influential in LCOE (see Figure 12).
4. Summary and Conclusions
A techno-economic assessment of five different power plants was conducted. Three reference plants, including a combined cycle using a GE 9E.04 industrial gas turbine (CC), open cycle with aeroderivative GE LMS-100 gas turbines (OC), and post-combustion CO2 capture with MEA absorption integrated in the combined cycle (PCC), were evaluated. Two advanced power plants incorporating CCS were studied: a Graz power cycle with O2 delivered from an ASU for oxy-combustion of the fuel (GASU) and a Graz cycle integrating chemical looping combustion to eliminate the auxiliary consumption of the ASU (GCLC). Exergy analysis of these two advanced plants with CCS was also performed. The main findings of the study are summarized as follows:
- The energy penalty of the PCC plant resulted in 7.4 percentage points. The GASU plant reduces this energy penalty to 2.6%. On the other hand, the GCLC model presented a thermal efficiency 1.9% above the unabated CC scheme despite operating at a lower TIT of 1100 °C due to CLC material limitations, thus eliminating the energy penalty of CO2 capture for midscale production capacities.
- In terms of CO2 emissions, the PCC presents an avoidance of 89.8%, relative to 100% for the GASU scheme. The GCLC yielded an avoidance rate of 98.6% (due to mixing of oxidation and reduction reactor outlet streams leading to some CO2 emissions). The higher thermal efficiency of the GCLC concept relative to the CC benchmark led to a negative SPECCA of −0.61 MJ/ton. Finally, the OC configuration results in 24.2% higher specific emissions than the CC (372.8 kg/MWh) due to the lower thermal efficiency of open-cycle operation.
- From an exergy analysis perspective, it was seen that the exergy destruction resulting from the ASU was entirely avoided with the integration of CLC. The largest source of exergy destruction was, unsurprisingly, the CLC reactor where the fuel is degraded, representing approximately 26.8% of the total exergy input for the GCLC case. The GCLC achieved an exergy efficiency of 54.1%, that is, a 4.2% higher exergy utilization of the fuel than that of the GASU plant.
- The subsequent economic assessment showed that at a CO2 tax of EUR 100/ton and a natural gas price of EUR 6.5/GJ, the GCLC scheme achieved a cost reduction of 12.6% relative to the CC, achieving a COCA of EUR 66.3/ton, while the cost reduction for PCC and GASU resulted in 9.9% and 5.0%, respectively. The OC scheme presented the highest levelized cost (17.3% higher than the CC) due to larger specific fuel consumption and emissions despite the lowest capital investment. Optimistic assumptions for a CLC operating temperature of 1200 °C led to a marginal cost reduction of 7.6% for the GCLC plant with respect to the PCC benchmark. A further uncertainty quantification study defining normal distributions for fuel price, CO2 tax, capacity factor, and capital cost of the most uncertain plant components showed that the IQR overlap between PCC and the GCLC was as high as 85.3%.
The results presented in this study reveal that the integration of two CO2 capture technologies, CLC and the Graz cycle, potentially achieves very attractive results in terms of energy performance and CO2 footprint for midscale power generation applications. However, due to an increased capital investment, these results do not translate into a significant economic benefit relative to post-combustion CO2 capture, which presents a higher technology readiness level and lower commercial uncertainty as the power generation components remain unchanged. Moreover, the evaluation was conducted assuming a foreseen performance of the CLC technology and Graz cycle plant, which have not been yet deployed nor demonstrated at the scale investigated. Therefore, this study indicates the relatively small economic incentive to invest in developing such technologies for CO2 capture in natural gas-fueled plants. It is recommended, therefore, to direct research and development efforts regarding CLC to CCS integration in solid-fuel power plants, where a larger amount of CO2 from a carbon-intensive fuel can be captured for a given amount of air heating or to applications where it can be employed as a process intensification means to replace air separation units in the production of chemicals such as sulfuric acid, nitric acid, or ethylene oxide. Future studies will focus on evaluating the economic potential of such integrations.
Author Contributions
Conceptualization: C.A.d.P.; Formal Analysis: C.A.d.P., S.C. and A.N.-C.; Methodology: C.A.d.P., A.N.-C. and S.S.-O.; Investigation: C.A.d.P., A.N.-C. and S.S.-O.; Visualization: A.N.-C., C.A.d.P. and Á.J.Á.; Software: C.A.d.P., A.N.-C. and Á.J.Á.; Writing—Original Draft: C.A.d.P., S.S.-O. and S.C. Writing—Review & Editing: C.A.d.P.; Supervision: Á.J.Á. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the European Union, NextGenerationeEU, Ministerio de Universidades Grant RD 289/2021 (UP2021-035). 

Data Availability Statement
SEA Files: https://bit.ly/4cM1Vlp (accessed on 1 April 2024); SySBaC Files: https://bit.ly/3PVPBW5 (accessed on 1 April 2024).
Acknowledgments
The authors would like to acknowledge Honeywell for the free academic license of the software UniSim Design R481.
Conflicts of Interest
The authors declare no conflict of interest.
Acronyms
| ACF | Annualized cash flows |
| ASME | American Society of Mechanical Engineers |
| ASU | Air separation units |
| BEC | Bare erected cost |
| CCS | Carbon capture and storage |
| CC | Combined cycle |
| CF | Capacity factor |
| Ch | Chemical |
| CLC | Chemical looping combustion |
| COT | Combustor outlet temperature |
| COCA | Cost of CO2 avoided |
| EPC | Engineering, procurement, and construction |
| Eva | Evaporator |
| Eco | Economizer |
| FOM | Fixed operating and maintenance costs |
| GHG | Greenhouse gas |
| HP | High pressure |
| HPC | High-pressure compressor |
| HPT | High-pressure turbine |
| HRSG | Heat recovery steam generator |
| IGCC | Integrated gasification combined cycle |
| IP | Intermediate pressure |
| IPCC | Intergovernmental Panel on Climate Change |
| IQR | Interquartile range |
| LCOE | Levelized cost of electricity |
| LHV | Lower heating value |
| LP | Low pressure |
| LPC | Low-pressure compressor |
| LPT | Low-pressure turbine |
| MEA | Methyl-ethanol amine |
| MITA | Minimal internal temperature approach |
| NG | Natural gas |
| NPV | Net present value |
| OC | Owners costs/open cycle |
| PC | Process contingency |
| PT | Project contingency |
| Sh | Superheater |
| SEA | Standardized economic assessment |
| SPECCA | Specific primary energy consumption of CO2 avoided |
| TIT | Turbine inlet temperature |
| TOT | Turbine outlet temperature |
| TOC | Total overnight cost |
| T&S | Transport and storage |
| VOM | Variable operating and maintenance cost |
List of Symbols
| CO2 avoidance (%) | |
| Exergy (J) | |
| CO2 capture (%) | |
| Specific emissions (kg/MWh) | |
| Flow of exergy (W) | |
| Exergy flow (J/mol) | |
| Electricity generation (MWh) | |
| Exergy destruction (J) | |
| Discount rate (%) | |
| Mass flow (kg/s) | |
| Plant lifetime (years) | |
| Pressure (bar) | |
| Time (s) | |
| Temperature (K) | |
| Volume (m3) | |
| Heat (J) | |
| Work (J) | |
| Exergy efficiency | |
| Exergy utilization | |
| Thermal efficiency (%) |
Appendix A

Table A1.
Modeling assumptions for the GASU and GCLC power plants.
Table A1.
Modeling assumptions for the GASU and GCLC power plants.
| Parameter | Value | Unit |
|---|---|---|
| Net power output | ~236 | MW |
| Combustor outlet temperature (COT)—GASU | 1400 | °C |
| Turbine inlet temperature (TITISO)—GASU | 1300 | °C |
| CLC reactor operating temperature—GCLC | 1100 | °C |
| Maximum steam temperature | 565 | °C |
| Pressure drop in HSRG | 3 | kPa |
| Pressure drop in combustion chamber/CLC | 50 | kPa |
| Recuperator/intercooler pressure drop | 10 | kPa |
| Inlet pressure of high-pressure steam turbine | 180.0 | bar |
| Inlet pressure of low-pressure steam turbine | 0.65 | bar |
| Condensation pressure | 0.04 | bar |
| MITA in vacuum HSRG | 2 | °C |
| MITA in HSRG | 10 | °C |
| MITA in Recuperator—GCLC | 30 | °C |
| Polytropic efficiency of power cycle compressors | 90.0 | % |
| Polytropic efficiency of gas turbine | 87.0 | % |
| Polytropic efficiency of HP steam turbine | 90.0 | % |
| Polytropic efficiency of LP steam turbine | 85.0 | % |
| Polytropic efficiency of CO2 compressors | 85.0 | % |
| Isentropic efficiency of pumps | 85.0 | % |
| CO2 delivery pressure | 150 | bar |

Table A2.
Natural gas conditions and composition.
Table A2.
Natural gas conditions and composition.
| Parameter | Value | Units |
|---|---|---|
| Temperature | 25 | °C |
| Pressure | 80 | bar |
| LHV | 46,500.3 | kJ/kg |
| Species | % mol | |
| N2 | 0.89 | |
| C1 | 89.00 | |
| C2 | 7.00 | |
| C3 | 1.11 | |
| CO2 | 2.00 | |

Table A3.
Stream summary of the CC, OC and PCC plants.
Table A3.
Stream summary of the CC, OC and PCC plants.
| Stream | T (°C) | P (bar) | m (kg/s) | N2 | O2 | Ar | CO2 | H2O |
|---|---|---|---|---|---|---|---|---|
| CC (Figure 1) | ||||||||
| 1 | 25.0 | 80.0 | 8.4 | - | ||||
| 2 | 15.0 | 1.0 | 409.7 | 77.30 | 20.73 | 0.92 | 0.04 | 1.01 |
| 3 | 542.0 | 1.0 | 418.1 | 74.75 | 13.43 | 0.89 | 3.48 | 7.45 |
| 4 | 520.0 | 64.0 | 52.2 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 |
| 5 | 200.0 | 4.5 | 12.0 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 |
| OC (Figure 2) | ||||||||
| 1 | 25.0 | 80.0 | 5.7 | - | ||||
| 2 | 15.0 | 1.0 | 227.5 | 77.30 | 20.73 | 0.92 | 0.04 | 1.01 |
| 3 | 432.0 | 1.0 | 232.6 | 74.50 | 11.95 | 0.89 | 4.21 | 8.42 |
| PCC (Figure 3) | ||||||||
| 1 | 25.0 | 80.0 | 8.4 | - | ||||
| 2 | 15.0 | 1.0 | 409.7 | 77.30 | 20.73 | 0.92 | 0.04 | 1.01 |
| 3 | 542.0 | 1.0 | 418.1 | 74.75 | 13.43 | 0.89 | 3.48 | 7.45 |
| 4 | 50.1 | 1.1 | 417.6 | 74.86 | 13.44 | 0.90 | 3.49 | 7.31 |
| 5 | 38.2 | 1.0 | 408.1 | 78.02 | 14.10 | 0.93 | 0.34 | 6.60 |
| 6 | 141.1 | 2.6 | 37.3 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 |
| 7 | 25.0 | 150.0 | 20.8 | 0.00 | 0.00 | 0.00 | 100.00 | 0.00 |

Table A4.
Stream summary of GASU plant.
Table A4.
Stream summary of GASU plant.
| Stream | Property | Composition (%mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (MW) | (MW) | . (MW) | (°C) | (bar) | (kg/s) | Ar | CO2 | H2O | N2 | O2 | |
| 1 | 474.4 | 5.2 | 469.2 | 15.0 | 80.0 | 9.7 | - | ||||
| 2 | 14.2 | 10.0 | 4.2 | 19.1 | 40.4 | 36.8 | 2.0 | 0.0 | 0.0 | 0.0 | 98.0 |
| 3 | 576.9 | 544.6 | 32.3 | 1400.3 | 40.0 | 210.7 | 0.5 | 11.6 | 87.8 | 0.1 | 0.0 |
| 4 | 233.3 | 199.5 | 33.8 | 592.3 | 1.0 | 231.9 | 0.4 | 10.4 | 89.1 | 0.1 | 0.0 |
| 5 | 116.8 | 112.0 | 4.8 | 565.0 | 180.0 | 70.7 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| 6 | 134.4 | 100.6 | 33.8 | 172.0 | 1.0 | 231.9 | 0.4 | 10.4 | 89.1 | 0.1 | 0.0 |
| 7 | 154.6 | 137.9 | 16.7 | 550.0 | 40.0 | 114.7 | 0.4 | 10.4 | 89.1 | 0.1 | 0.0 |
| 8 | 50.9 | 45.3 | 5.7 | 167.9 | 0.7 | 83.2 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| 9 | 12.3 | 1.3 | 10.9 | 25.0 | 2.5 | 26.9 | 3.7 | 94.2 | 1.3 | 0.8 | 0.0 |
| 10 | 16.7 | 5.8 | 11.0 | 42.0 | 150.0 | 26.7 | 3.8 | 95.4 | 0.0 | 0.8 | 0.0 |
| 11 | 8.4 | 2.2 | 6.1 | 76.3 | 1.0 | 90.3 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| 12 | 2.0 | 0.8 | 1.1 | 79.9 | 210.0 | 16.4 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |

Table A5.
Stream summary of GCLC plant.
Table A5.
Stream summary of GCLC plant.
| Stream | Property | Composition (%mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (MW) | (MW) | (MW) | T (°C) | P (bar) | m (kg/s) | Ar | CO2 | H2O | N2 | O2 | |
| 1 | 437.1 | 4.8 | 432.3 | 15.0 | 80.0 | 8.9 | - | ||||
| 2 | 0.0 | 0.0 | 0.0 | 15.0 | 1.0 | 143.7 | 0.92 | 0.04 | 1.01 | 77.30 | 20.73 |
| 3 | 54.3 | 54.2 | 0.0 | 386.8 | 18.0 | 143.2 | 0.93 | 0.04 | 0.42 | 77.76 | 20.86 |
| 4 | 110.8 | 108.6 | 2.2 | 1100.0 | 18.0 | 110.9 | 1.65 | 0.37 | 1.20 | 96.78 | 0.00 |
| 5 | 3.2 | 1.0 | 2.2 | 90.0 | 1.0 | 110.9 | 1.65 | 0.37 | 1.20 | 96.78 | 0.00 |
| 6 | 539.6 | 499.0 | 40.7 | 1099.9 | 18.0 | 259.5 | 0.01 | 12.42 | 87.02 | 0.55 | 0.01 |
| 7 | 252.7 | 212.0 | 40.6 | 582.5 | 1.0 | 259.5 | 0.01 | 12.42 | 87.02 | 0.55 | 0.01 |
| 8 | 54.8 | 40.6 | 14.2 | 227.0 | 1.0 | 90.9 | 0.01 | 12.42 | 87.02 | 0.55 | 0.01 |
| 9 | 90.2 | 63.8 | 26.4 | 110.0 | 1.0 | 168.6 | 0.01 | 12.42 | 87.02 | 0.55 | 0.01 |
| 10 | 82.2 | 78.8 | 3.3 | 565.0 | 180.0 | 49.7 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 |
| 11 | 46.2 | 41.3 | 4.9 | 204.3 | 0.7 | 72.4 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 |
| 12 | 6.0 | 1.4 | 4.5 | 71.3 | 1.0 | 66.8 | 0.00 | 0.03 | 99.97 | 0.00 | 0.00 |
| 13 | 10.5 | 0.8 | 9.7 | 25.0 | 1.8 | 24.1 | 0.04 | 93.95 | 1.78 | 4.16 | 0.07 |
| 14 | 14.9 | 5.2 | 9.7 | 43.1 | 150.0 | 24.0 | 0.04 | 95.65 | 0.00 | 4.24 | 0.07 |
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R. Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Available online: https://climate.ec.europa.eu/eu-action/international-action-climate-change/global-climate-action_en (accessed on 1 April 2024).
- Hartfield, G.; Blunden, J.; Arndt, D.S. State of the Climate in 2017. Bull. Am. Meteorol. Soc. 2018, 99, Si–S310. [Google Scholar] [CrossRef]
- Liu, S.; Ren, B.; Li, H.; Yang, Y.; Wang, Z.; Wang, B.; Xu, J.; Agarwal, R. CO2 storage with enhanced gas recovery (CSEGR): A review of experimental and numerical studies. Pet. Sci. 2022, 19, 594–607. [Google Scholar] [CrossRef]
- Del Pozo, C.A.; Cloete, S.; Álvaro, Á.J. Techno-economic assessment of long-term methanol production from natural gas and renewables. Energy Convers. Manag. 2022, 266, 115785. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Khan, M.N.; Cloete, S.; Amini, S. Efficiency Improvement of Chemical Looping Combustion Combined Cycle Power Plants. Energy Technol. 2019, 7, 1900567. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Jansen, D.; Gazzani, M.; Manzolini, G.; Dijk, E.V.; Carbo, M. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef]
- Romano, M.C.; Chiesa, P.; Lozza, G. Pre-combustion CO2 capture from natural gas power plants, with ATR and MDEA processes. Int. J. Greenh. Gas Control 2010, 4, 785–797. [Google Scholar] [CrossRef]
- Mancuso, L.; Ferrari, N.; Chiesa, P.; Maartelli, E.; Romano, M. Oxy-Combustion Turbine Power Plants; IEAGHG: Cheltenham, UK, 2015. [Google Scholar]
- Jonsson, M.; Bolland, O.; Bücker, D.; Rost, M. Gas turbine cooling model for evaluation of novel cycles. Proc. ECOS 2005, 6, 641–650. [Google Scholar]
- Wimmer, K.; Sanz, W. Optimization and comparison of the two promising oxy-combustion cycles NET Power cycle and Graz Cycle. Int. J. Greenh. Gas Control 2020, 99, 103055. [Google Scholar] [CrossRef]
- Jericha, H. Efficient steam cycles with internal combustion of hydrogen and stoichiometric oxygen for turbines and piston engines. Int. J. Hydrogen Energy 1987, 12, 345–354. [Google Scholar] [CrossRef]
- Sanz, W.; Jericha, H.; Moser, M.; Heitmeir, F. Thermodynamic and economic investigation of an improved Graz cycle power plant for CO2 capture. J. Eng. Gas Turbines Power 2005, 127, 765–772. [Google Scholar] [CrossRef]
- Heitmeir, F.; Sanz, W.; Göttlich, E.; Jericha, H. The Graz cycle–a zero emission power plant of highest efficiency. In Proceedings of the XXXV Kraftwerkstechnisches Kolloquium, Dresden, Germany, 10–11 October 2003. [Google Scholar]
- Mitterrutzner, B.; Sanz, W.; Nord, L.O. A part-load analysis and control strategies for the Graz Cycle. Int. J. Greenh. Gas Control 2022, 113, 103521. [Google Scholar] [CrossRef]
- Abuelgasim, S.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2021, 764, 142892. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; He, Y.; Li, L.; Wu, P. Tech-economic assessment of second-generation CCS: Chemical looping combustion. Energy 2018, 144, 915–927. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; García-Labiano, F.; de Diego, F.L.; Gayán, P.; Celaya, J. Mapping of the range of operational conditions for Cu-, Fe-, and Ni-based oxygen carriers in chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 533–549. [Google Scholar] [CrossRef]
- Ishida, M.; Yamamoto, M.; Ohba, T. Experimental results of chemical-looping combustion with NiO/NiAl2O4 particle circulation at 1200 C. Energy Convers. Manag. 2002, 43, 1469–1478. [Google Scholar] [CrossRef]
- Ströhle, J.; Orth, M.; Epple, B. Chemical looping combustion of hard coal in a 1MWth pilot plant using ilmenite as oxygen carrier. Appl. Energy 2015, 157, 288–294. [Google Scholar] [CrossRef]
- Chen, J.; Duan, L.; Ma, Y.; Jiang, Y.; Huang, A.; Zhu, H.; Jiao, H.; Li, M.; Hu, Y.; Zhou, H.; et al. Recent progress in calcium looping integrated with chemical looping combustion (CaL-CLC) using bifunctional CaO/CuO composites for CO2 capture: A state-of-the-art review. Fuel 2023, 334, 126630. [Google Scholar] [CrossRef]
- Nazir, S.M.; Cloete, J.H.; Cloete, S.; Amini, S. Efficient hydrogen production with CO2 capture using gas switching reforming. Energy 2019, 185, 372–385. [Google Scholar] [CrossRef]
- Ruan, C.; Wang, X.; Wang, C.; Zheng, L.; Li, L.; Lin, J.; Liu, X.; Li, F.; Wang, X. Selective catalytic oxidation of ammonia to nitric oxide via chemical looping. Nat. Commun. 2022, 13, 718. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Imtiaz, Q.; Donat, F.; Müller, C.R.; Li, F. Chemical looping beyond combustion–a perspective. Energy Environ. Sci. 2020, 13, 772–804. [Google Scholar] [CrossRef]
- García-Labiano, F.; de Diego, L.F.; Cabello, A.; Gayán, P.; Abad, A.; Adánez, J.; Sprachmann, G. Sulphuric acid production via Chemical Looping Combustion of elemental sulphur. Appl. Energy 2016, 178, 736–745. [Google Scholar] [CrossRef]
- Cabello, A.; Abad, A.; de las Obras Loscertales, M.; Bartocci, P.; García-Labiano, F.; de Diego, L.F. Exploring design options for pressurized chemical looping combustion of natural gas. Fuel 2023, 342, 126983. [Google Scholar] [CrossRef]
- Osman, M.; Zaabout, A.; Cloete, S.; Amini, S. Experimental demonstration of pressurized chemical looping combustion in an internally circulating reactor for power production with integrated CO2 capture. Chem. Eng. J. 2020, 401, 125974. [Google Scholar] [CrossRef]
- Zaabout, A.; Cloete, S.; Johansen, S.T.; van Sint Annaland, M.; Gallucci, F.; Amini, S. Experimental Demonstration of a Novel Gas Switching Combustion Reactor for Power Production with Integrated CO2 Capture. Ind. Eng. Chem. Res. 2013, 52, 14241–14250. [Google Scholar] [CrossRef]
- Anonymous. 2020. Available online: https://www.honeywellprocess.com/en-US/online_campaigns/unisim-design/Pages/index.html (accessed on 1 April 2024).
- Available online: https://www.ge.com/gas-power/products/gas-turbines (accessed on 21 January 2024).
- Anantharaman, R.; Bolland, O.; Booth, N.; Van Dorst, E.; Fernandez, E.S.; Franco, F.; Macchi, E.; Manzolini, G.; Nikolic, D.; Pfeffer, A.; et al. Cesar Deliverable D2.4.3. European Best Practice Guidelines for Assessment of CO2 Capture Technologies; Zenodo: Geneva, Switzerland, 2018. [Google Scholar]
- Del Pozo, C.A.; Álvaro, Á.J.; Casano, J.J.R.; Cloete, S. Exergoeconomic assessment of air separation units for pressurized O2 production incorporating two-phase expanders. Cryogenics 2022, 124, 103477. [Google Scholar] [CrossRef]
- Posch, S.; Haider, M. Optimization of CO2 compression and purification units (CO2CPU) for CCS power plants. Fuel 2012, 101, 254–263. [Google Scholar] [CrossRef]
- Del Pozo, C.A.; Álvaro, Á.J.; Martín, J.R.; Paniagua, I.L.; Orgaz, S.S.; Fernández, C.G.; Carlier, R.N. Exergy Calculation Modelling Tool for Mixtures in Power Generation: Application to WGS and ASU units of an IGCC Power Plant with Pre-combustion CO2 Capture. In Proceedings of the XI Congreso Nacional y II Internacional de Ingeniería Termodinámica (11-CNIT), Albacete, Spain, 12–28 June 2019. [Google Scholar]
- Álvaro, Á.J.; del Pozo, C.A. System Balance Calculator (SysBaC); Departamento de Ingeniería Energética (ETSII-UPM): Madrid, Spain, 2023. [Google Scholar]
- Del Pozo, C.A.; Cloete, S.; Álvaro, Á.J. Standard Economic Assessment (SEA) Tool. Available online: https://bit.ly/3hyF1TT (accessed on 1 April 2024).
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis and Design of Chemical Processes; Pearson Education: London, UK, 2008. [Google Scholar]
- Del Pozo, C.A.; Orgaz, S.S.; Calvo, A.N.; Álvaro, Á.J.; Cloete, S. Integration of CLC in the Graz Cycle—SEA Files. 2024. Available online: https://bit.ly/SEAGCLC (accessed on 1 April 2024).
- Del Pozo, C.A.; Orgaz, S.S.; Calvo, A.N.; Álvaro, Á.J.; Cloete, S. Integration of CLC in the Graz Cycle—SySBaC Files. 2024. Available online: https://bit.ly/ExGCLC (accessed on 1 April 2024).
- Del Pozo, C.A.; Álvaro, Á.J.; Cloete, S.; del Pozo Martín de Hijas, G.; Jose, A. The Potential of Chemically Recuperated Power Cycles in Markets with High Shares of Variable Renewables. Energies 2023, 16, 7046. [Google Scholar] [CrossRef]
- Nandy, A.; Loha, C.; Gu, S.; Sarkar, P.; Karmakar, M.K.; Chatterjee, P.K. Present status and overview of Chemical Looping Combustion technology. Renew. Sustain. Energy Rev. 2016, 59, 597–619. [Google Scholar] [CrossRef]
- Cloete, S.; del Pozo, C.A.; Cloete, J.H.; Álvaro, Á. The future of fuels: Uncertainty quantification study of mid-century ammonia and methanol production costs. Energy Convers. Manag. 2023, 297, 117701. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).