Thermal Performance of Lignocellulose’s By-Product Wallboards with Bio-Based Microencapsulated Phase Change Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Technological Offer to Produce Board Samples
- Weighing of lignocellulosic raw material with electronic balance Kern EMB 600-2 (to the nearest of 0.01 g).
- Preparation of binder.
- 3.
- The binder is mixed with the raw material in a mixing tank, pouring the raw material and stirring with electric hand mixer, and the binder mixture is slowly and evenly added. The mixing process is carried out for another 120 s after adding all the binder.
- 4.
- Being evenly leveled, the mixed mass is formed into a matrix.
- 5.
- The board is pressed into the matrix to 25 mm thickness.
- 6.
- According to the data sheet of the binder manufacturer, the samples must be kept under pressure in the matrix for a minimum of 12 h.
- 7.
- The obtained board is kept in laboratory conditions for 10–14 days, then removed from the matrix and sawed according to the standards of the test to be performed.
2.3. Components of the Raw Material of the Boards
2.4. Thermal Conductivity
2.5. Heat Capacity
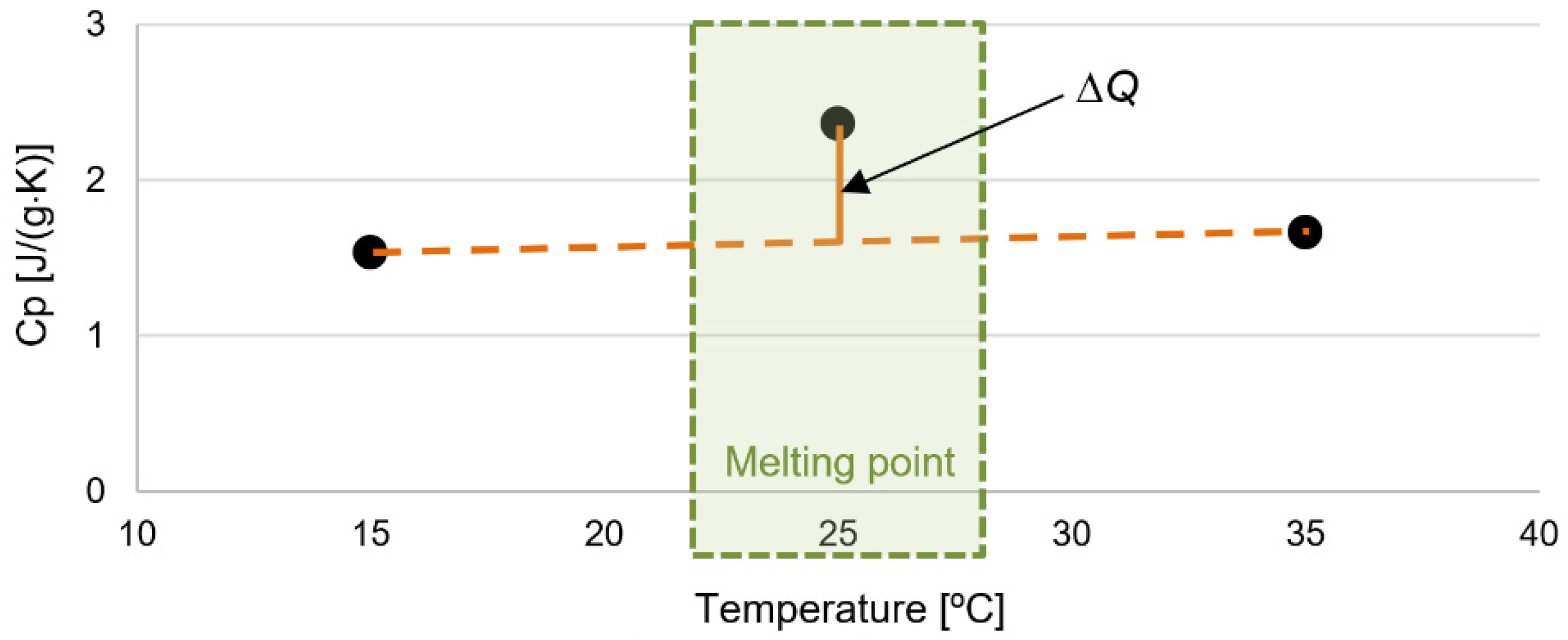
2.6. Latent Heat
3. Results
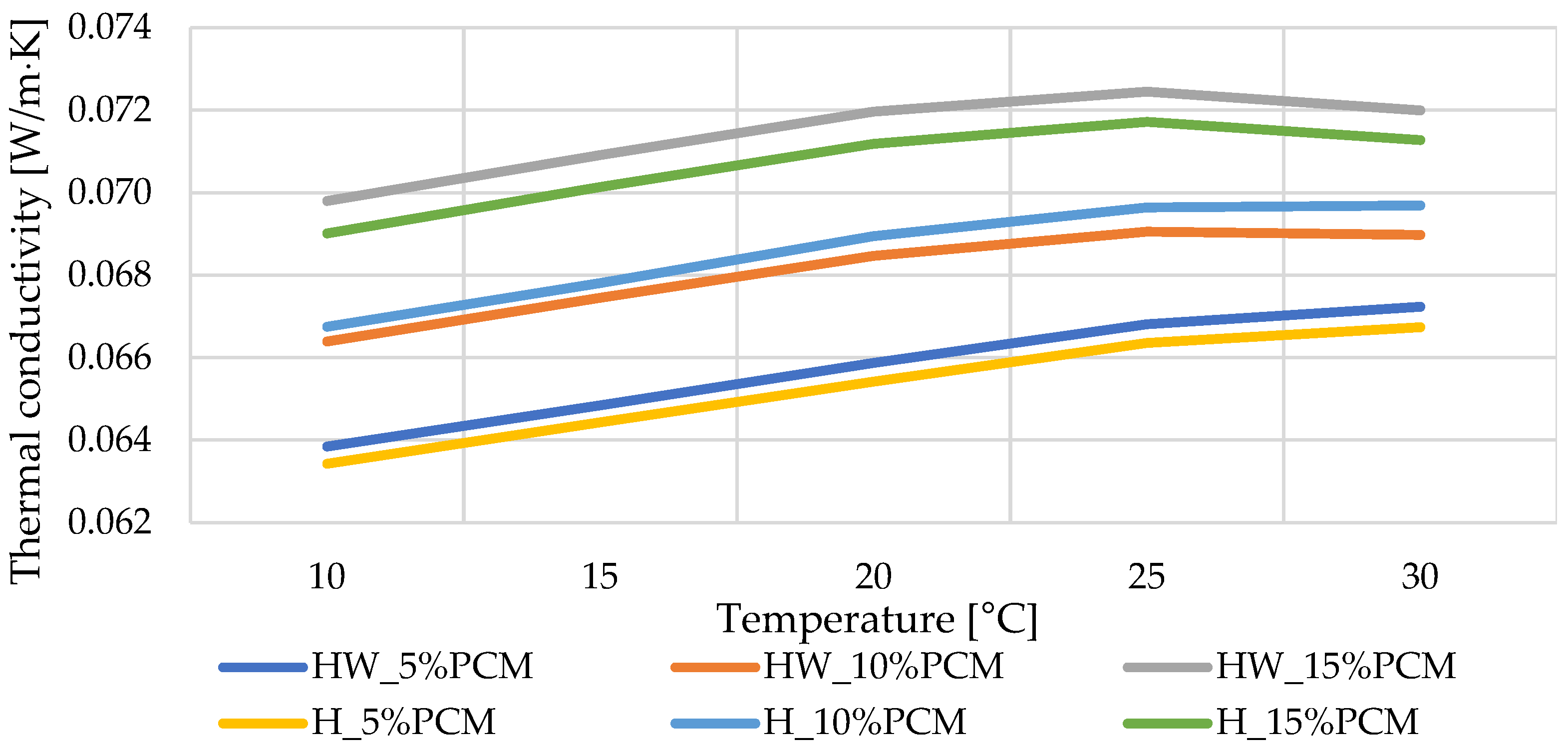
3.1. Thermal Conductivity
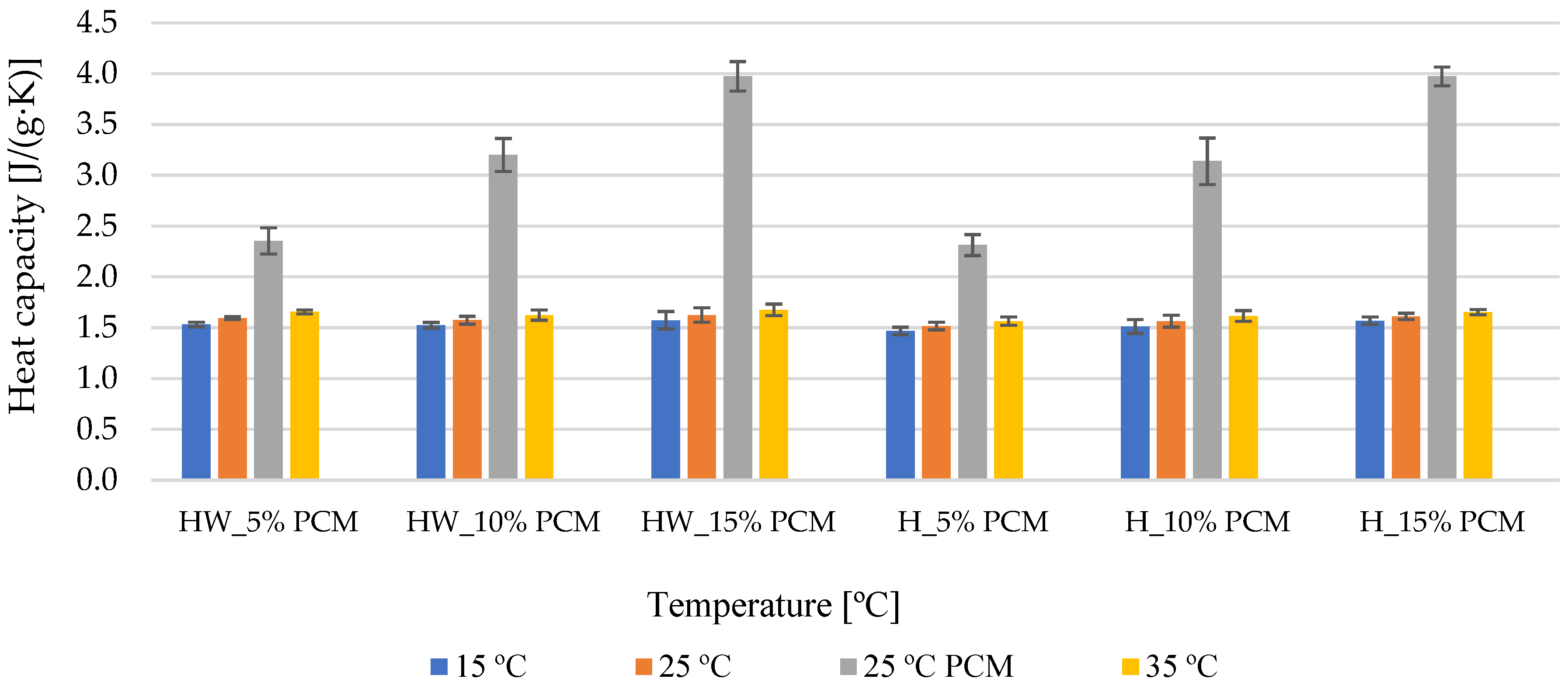
3.2. Heat Capacity
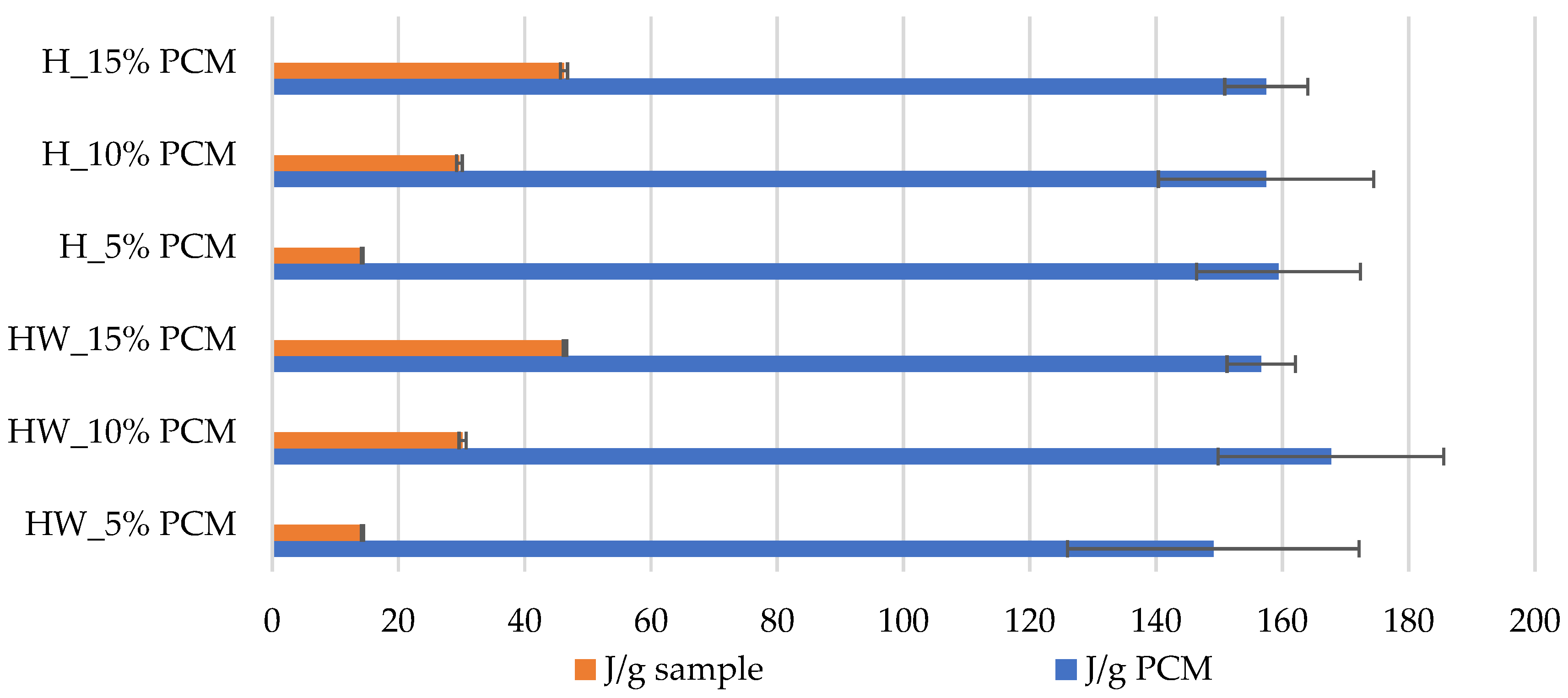
3.3. Latent Heat
4. Discussion
- A microencapsulated bio-based phase change material was chosen and employed to enable precise quantification of the incorporated phase change amount, eliminating the risk of loss when the room temperature rises between 23 °C and 28 °C. Increasing the quantity of PCM in a sample leads to an increase in the materials’ thermal conductivity, changing the function of the material.
- However, since this material is intended not only to exhibit high thermal conductivity but also to possess a significant heat capacity, it was crucial to achieve a balance. The goal was to ensure that the material does not absorb excessive heat, allowing it to correlate with room temperature. It should also function as a thermal capacity material when the room temperature rises between 23 °C and 28 °C. If the thermal conductivity coefficient is much lower, it is likely that this material, with included PCM, would not effectively function as a heat storage material, impeding the transfer of heat to the capsules.
- The incorporation of phase change microcapsules proved to be a transformative addition, resulting in a substantial increase in the heat capacity of the board, reaching an impressive enhancement of up to 147%. This boost in heat storage capacity holds promising implications for the overall energy performance of the material, offering potential benefits for various applications that require efficient heat management.
- As the content of phase change material (PCM) increases, a noticeable reduction in thermal conductivity is observed. Remarkably, samples lacking PCM exhibited the most favorable thermal conductivity, approximately 0.062 W/(m·K). This finding is pivotal to understanding the influence of PCM inclusion on the materials’ heat transfer properties, providing valuable insight for material selection and design in specific thermal applications.
- Furthermore, the specific heat capacity of samples incorporating 15% encapsulated phase change material displayed a remarkable increase, surging by 2.5-times to reach 3.97 J/(g·K). This substantial improvement in specific heat capacity bestows upon the material the capability to significantly mitigate indoor temperature fluctuations. This attribute is particularly valuable in scenarios involving direct solar radiation, where the material can effectively absorb and release heat, contributing to the creation of more stable and comfortable indoor environments.
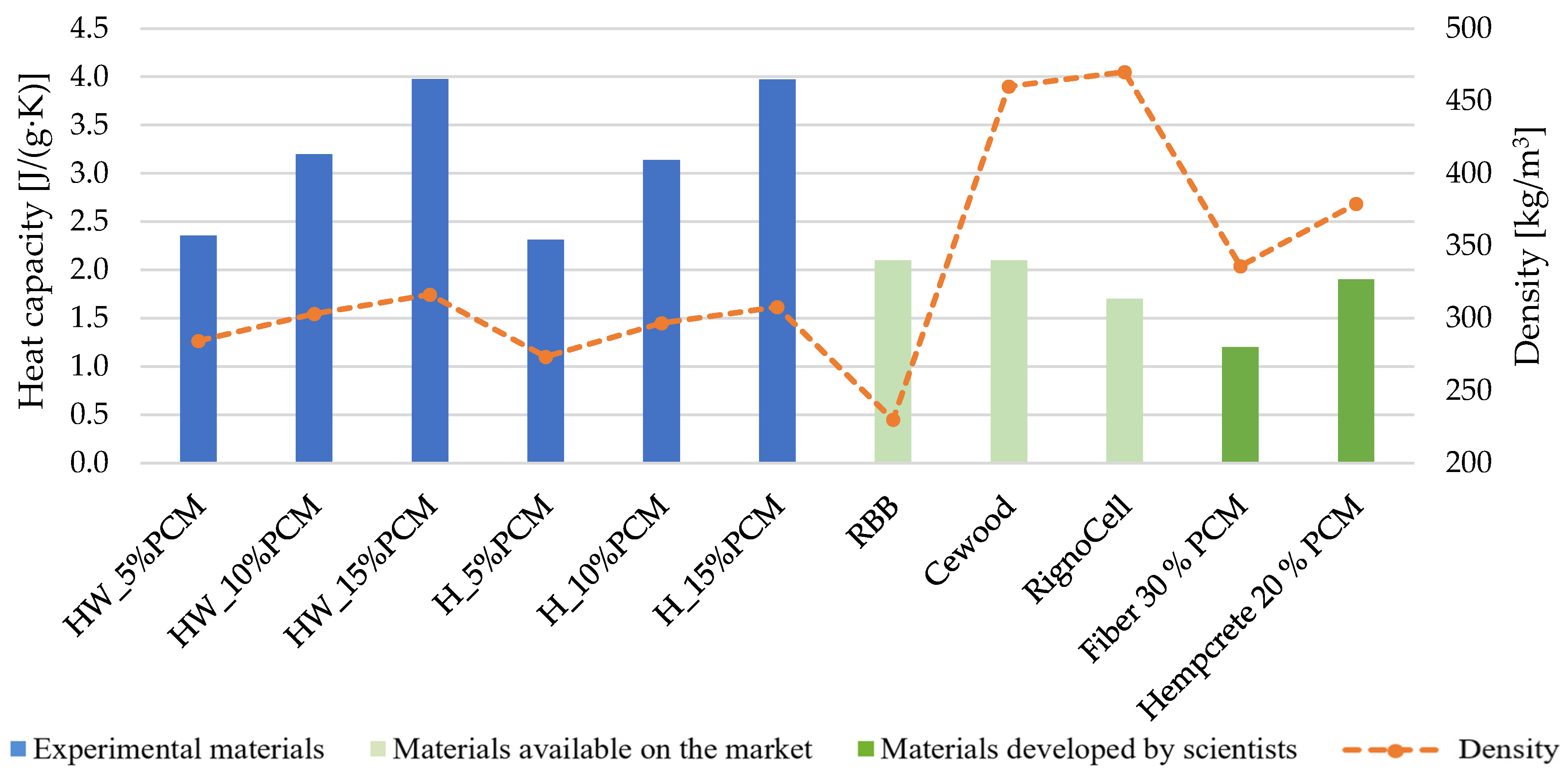
- At the moment, it is difficult to find lignocellulosic-based thermal insulation materials with PCM on the market. However, for comparison purposes, RBB wood fiber with a density of 230 kg/m3 and a heat capacity of 2.1 J/(g·K) is chosen, along with a comparable cedar planer cement board with a density of 450 kg/m3. Additionally, RignoCell steam-exploded hemp fiberboard, having a similar density but a heat capacity percentile of 1.70 J/(g·K), is also selected. Compared to materials developed by scientists with 20 to 30% added PCM (1.2–1.9 J/(g·K)), our material achieves a 2.6-times better result.
- Adding 5% PCM to HW and H samples yields an average result that is 19% higher than the results of commercially available materials. Furthermore, the experimental materials produced a result that is 51% higher than that achieved by material results developed by other researchers. This difference is likely attributed to the lower PCM content, greater material thickness and higher density of the dispersion used in the studies of those researchers.
- In addition to the findings, our rigorous calculations of latent heat of fusion yielded results with minimal dispersion, signifying the reliability and robustness of our experimental approach. The consistency in our results establishes confidence in the characteristic values of latent heat, which consistently hover around 160 J/g. This congruence with existing literature data for the specific PCM type employed in our study validates the accuracy and relevance of our research outcomes.
- The material was tested in fire tests, revealing its combustibility. However, in comparison to similar materials, it achieved Class B–D ratings (with A being the highest). This indicates that additional fire protection measures should be considered in the future.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Energy Technology Perspective, Transition to Sustainable Buildings: Strategies and Opportunities to 2050. 2013. Available online: https://www.iea.org/etp/buildings/ (accessed on 15 February 2023).
- Haghighat, F. Applying Energy Storage in Ultra-low Energy Buildings—Final Report; European Centre for Electoral Support: Brussels, Belgium, 2014. [Google Scholar]
- Khan, R.J.; Bhuiyan, M.D.Z.H.; Ahmed, D.H. Investigation of heat transfer of a building wall in the presence of phase change material (PCM). Energy Built Environ. 2020, 1, 199–206. [Google Scholar] [CrossRef]
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Páez, D.A.; Martínez, M.H.; Bermúdez, L.A.C. Possibilities for the Development of Acoustic-Mechanical Systems based on Colombian Typical Fibres. In Proceedings of the of ICSV23, Athens, Greece, 10–14 July 2016. [Google Scholar]
- Herrera, M.; Martínez, A.; Mosquera, G.; Flórez, R.E.R. System and Software Development to Measure Cylindrical Pipes based on Acoustic Reflectometry. Tecciencia 2013, 8, 83–92. [Google Scholar] [CrossRef]
- Raluca, I.; Tamas-Gavrea, T.; Daniela, D.M.; Claudiu, A. Physical and Mechanical Property Characterization of Hemp Shive Reinforced Gypsum Composite Board. Adv. Eng. Forum 2017, 2, 262–271. [Google Scholar]
- Kūliņš, L.; Griņevičs, G.; Laiveniece, L.; Spulle, U. Līmēto Materiālu Ražošana; Talsu Tipogrāfija: Jelgava, Latvia, 2019; p. 122.
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2005; pp. 246–255. [Google Scholar]
- Ryms, M.; Klugmann-Radziemska, E. Possibilities and benefits of a new method of modifying conventional building materials with phase-change materials (PCMs). Constr. Build. Mater. 2019, 211, 1013–1024. [Google Scholar] [CrossRef]
- Whiffen, T.; Russell-Smith, G.; Riffat, S. Active thermal mass enhancement using phase change materials. Energy Build. 2016, 111, 1–11. [Google Scholar] [CrossRef]
- Pomianowski, M.Z.; Heiselberg, P.; Jensen, R.L.; Cheng, R.; Zhang, Y. A new experimental method to determine specific heat capacity of inhomogeneous concrete material with incorporated microencapsulated-PCM. Cem. Concr. Res. 2014, 55, 22–34. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Zhu, N.; Ma, Z.J.; Wang, S.W. Dynamic characteristics and energy performance of buildings using phase change materials: A review. Energy Convers. Manag. 2009, 50, 3169–3181. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Wang, X.; Lu, E.; Lin, W.; Liu, T.; Shi, Z.; Tang, R.; Wang, C. Heat storage performance of the binary systems neopentyl glycol/pentaerythritol and neopentyl glycol/trihydroxy methylaminomethane as solid–solid phase change materials. Energy Convers. Manag. 2000, 41, 129–134. [Google Scholar] [CrossRef]
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. A review on phase change materials for thermal energy storage in buildings: Heating and hybrid applications. J. Energy Storage 2021, 33, 101913. [Google Scholar] [CrossRef]
- Adham, M.; Elnokaly, M.A.; Aly, A.M.M. Empirical investigation to explore potential gains from the amalgamation of phase changing materials (PCMs) and wood shavings. Energy Built Environ. 2021, 2, 315–326. [Google Scholar]
- Castell, A.; Medrano, M.; Castellón, C.; Cabeza, L.F. Experimental results and simulation models for the use of PCM in buildings. In Proceedings of the 6th Workshop IEA/ECES IA, Sevilla, Spain, November 2009. [Google Scholar]
- Hauer, A.; Mehling, H.; Schossig, P.; Yamaha, M.; Cabeza, L.F.; Martin, V.; Setterwall, F. International Energy Agency Implementing Agreement on Energy Conservation through Energy Storage; Final Report; European Centre for Electoral Support: Brussels, Belgium, 2005. [Google Scholar]
- Krancane, L.; Vanaga, R.; Blumberga, A. Modeling of building envelope’s thermal properties by applying phase change materials. Energy Procedia 2016, 95, 175–180. [Google Scholar] [CrossRef]
- Ryms, M.; Januszewicz, K.; Kazimierski, P.; Łuczak, J.; Klugmann-Radziemska, E.; Lewandowski, W.M. Post-Pyrolytic Carbon as a Phase Change Materials (PCMs) Carrier for Application in Building Materials. Materials 2020, 13, 1268. [Google Scholar] [CrossRef]
- Madad, A.; Mouhib, T.; Mouhsen, A. Phase Change Materials for Building Applications: A Thorough Review and New Perspectives. Buildings 2018, 8, 63. [Google Scholar] [CrossRef]
- ZRao, Z.; Wang, S.; Zhang, Z. Energy saving latent heat storage and environmental friendly humidity-controlled materials for indoor climate. Renew. Sustain. Energy Rev. 2012, 16, 3136–3145. [Google Scholar]
- Cao, L.; Su, D.; Tang, Y.; Fang, G.; Tang, F. Properties evaluation and applications of thermal energy storage materials in buildings. Renew. Sustain. Energy Rev. 2015, 48, 500–522. [Google Scholar] [CrossRef]
- Memon, S.A. Phase change materials integrated in building walls: A state of the art review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Hendinata, L.K.; Siddiq, N.A.; Utami, S.S.; Fikri, A.I.R.; Suprapto, M.A.; Prilia, R. Delignified wood biocomposites as sustainable and transparent materials for passive cooling applications. Wood Mater. Sci. Eng. 2023, 1–11. [Google Scholar] [CrossRef]
- Mathis, D.; Blanchet, P.; Landry, V.; Lagière, P. Impregnation of Wood with Microencapsulated Bio-Based Phase Change Materials for High Thermal Mass Engineered Wood Flooring. Appl. Sci. 2018, 8, 2696. [Google Scholar] [CrossRef]
- Schossig, P.; Henning, H.M.; Gschwander, S.; Haussmann, T. Micro-encapsulated phase-change materials integrated into construction materials. Sol. Energy Mater. Sol. Cells 2005, 89, 297–306. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Z.; Fan, S.; Li, J. Thermal performance of a lightweight building with phase change material under a humid subtropical climate. Energy Built Environ. 2022, 3, 73–85. [Google Scholar] [CrossRef]
- Laasri, I.A.; Es-Sakali, N.; Outzourhit, A.; Mghazli, M.O. Evaluation of phase change materials for a light-weight building in Morocco: Effect of building’s volume, window orientation & infiltration. Energy Built Environ. 2023. [Google Scholar] [CrossRef]
- Agrawal, U.S.; Wanjari, S.P. Light-weight and high thermal insulation building material; a comparative study between cenosphere & fly ash. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Lai, C.-M.; Chen, R.; Lin, C.-Y. Heat transfer and thermal storage behaviour of gypsum boards incorporating micro-encapsulated PCM. Energy Build. 2010, 42, 1259–1266. [Google Scholar] [CrossRef]
- Mathis, D.; Blanchet, P.; Lagière, P.; Landry, V. Performance of Wood-Based Panels Integrated with a Bio-Based Phase Change Material: A Full-Scale Experiment in a Cold Climate with Timber-Frame Huts. Energies 2018, 11, 3093. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sanjayan, J.; Wang, X. Experimental Research on Using Form-stable PCM-Integrated Cementitious Composite for Reducing Overheating in Buildings. Buildings 2019, 9, 57. [Google Scholar] [CrossRef]
- Castell, A.; Martorell, I.; Medrano, M.; Pérez, G.; Cabeza, L. Experimental study of using PCM in brick constructive solutions for passive cooling. Energy Build. 2010, 42, 534–540. [Google Scholar] [CrossRef]
- Bravo, J.P.; Venegas, T.; Correa, E.; Álamos, A.; Sepúlveda, F.; Vasco, D.A.; Barreneche, C. Experimental and Computational Study of the Implementation of mPCM-Modified Gypsum Boards in a Test Enclosure. Buildings 2020, 10, 15. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Q.; Luo, L.; Fan, Y.; Wang, Q.; Jia, G. Review Research Progress on the Phase Change Materials for Cold Thermal Energy Storage. Energies 2021, 14, 1–46. [Google Scholar] [CrossRef]
- Voronin, D.V.; Ivanov, E.; Gushchin, P.; Fakhrullin, R.; Vinokurov, V. Clay Composites for Thermal Energy Storage: A Review. Molecules 2020, 25, 1504. [Google Scholar] [CrossRef]
- Podara, V.C.; Kartsonakis, I.A.; Charitidis, C.A. Review Towards Phase Change Materials for Thermal Energy Storage: Classification, Improvements and Applications in the Building Sector. Appl. Sci. 2021, 11, 1490. [Google Scholar] [CrossRef]
- Sam, M.N.; Caggiano, A.; Mankel, C.; Koenders, E. A Comparative Study on the Thermal Energy Storage Performance of Bio-Based and Paraffin-Based PCMs Using DSC Procedures. Materials 2020, 13, 1705. [Google Scholar]
- Jeong, S.G.; Chung, O.; Yu, S.; Kim, S.; Kim, S. Improvement of the thermal properties of Bio-based PCM using exfoliated graphite nanoplateles. Sol. Energy Mater. Sol. Cells 2013, 117, 87–92. [Google Scholar] [CrossRef]
- Nazari, M.; Jebrane, M.; Terziev, N. Bio-Based Phase Change Materials Incorporated in Lignocellulose Matrix for Energy Storage in Buildings—A Review. Energies 2020, 13, 3065. [Google Scholar] [CrossRef]
- Yen, T.; Chen, H.; Huang, Y.; Ko, C. The dividing strength of lightweight aggregate concrete and the packing strength of light-weight aggregate. J. Chin. Inst. Eng. 1998, 21, 611–618. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A.; Alkan, C. Preparation, characterization and thermal properties of lauric acid/expanded perlite as novel form-stable composite phase change material. Chem. Eng. J. 2009, 155, 899–904. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Xiao, W.; Zeng, R.; Zhang, Q.; Di, H. Review on thermal performance of phase change energy storage building envelope. Sci. Bull. 2009, 54, 920–928. [Google Scholar] [CrossRef]
- Laghari, A.H.M.S.; Rashid, Y. Micro-Encapsulated Phase Change Materials: A Review of Encapsulation, Safety and Thermal Characteristics. Sustainability 2016, 8, 1046. [Google Scholar]
- Okogeri, O.; Stathopoulos, V.N. What about greener phase change materials? A review on biobased phase change materials for thermal energy storage applications. Int. J. Thermofluids 2021, 10, 100081. [Google Scholar] [CrossRef]
- Chalkia, V.; Tachos, N.; Pandis, P.K.; Giannakas, A.; Koukou, M.K.; Vrachopoulos, M.G.; Coelho, L.; Ladavos, A.; Stathopoulos, V.N. Influence of organic phase change materials on the physical and mechanical properties of HDPE and PP polymers. RSC Adv. 2018, 8, 27438–27447. [Google Scholar] [CrossRef] [PubMed]
- Socaciu, L.; Plesa, A.; Unguresan, P.; Giurgiu, O. Review on phase change materials for building applications. Leonardo Electron. J. Pract. Technol. 2014, 25, 179–194. [Google Scholar]
- Mousa, M.; Bayomy, A.; Saghir, M. Phase change materials effect on the thermal radius and energy storage capacity of energy piles: Experimental and numerical study. Int. J. Thermofluids 2021, 10, 100094. [Google Scholar] [CrossRef]
- Pignata, A.; Minuto, F.D.; Lanzini, A.; Papurello, D. A feasibility study of a tube bundle exchanger with phase change materials: A case study. J. Build. Eng. 2023, 78, 107622. [Google Scholar] [CrossRef]
- Kosny, N.S.J. DHFMA Method for Dynamic Thermal Property Measurement of PCM-integrated Building Materials. Curr. Sustain./Renew. Energy Rep. 2015, 2, 41–46. [Google Scholar]
- Bittle, R.R.; Taylor, R.E. Thermal Conductivity; Ashworth, R., Smith, D.R., Eds.; Plenum: New York, NY, USA, 1984; pp. 379–390. [Google Scholar]
- Kirilovs, E.; Zotova, I.; Gendelis, S.; Jörg-Gusovius, H.; Kukle, S.; Stramkale, V. Experimental Study of Using Micro-Encapsulated Phase-Change Material Integrated into Hemp Shive Wallboard. Buildings 2020, 10, 228. [Google Scholar] [CrossRef]
- Vitola, L.; Gendelis, S.; Sinka, M.; Pundiene, I.; Bajare, D. Assessment of Plant Origin By-Products as Lightweight Aggregates for Bio-Composite Bounded by Starch Binder. Energies 2022, 15, 5330. [Google Scholar] [CrossRef]
- Marani, A.; Madhkhan, M. Thermal performance of concrete sandwich panels incorporating phase change materials: An experimental study. J. Mater. Res. Technol. 2021, 12, 760–775. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, M.; Liu, L.; Huan, C.; Zhao, Y.; Qi, C.; Song, K.-I. Experimental study on thermal and mechanical properties of cemented paste backfill with phase change material. J. Mater. Res. Technol. 2019, 9, 2164–2175. [Google Scholar] [CrossRef]
- Zhu, L.; Ding, W.; Dang, F.; Sang, G.; Xue, Y.; Wang, Q. Combined experimental and theoretical investigation of pore characteristics effect on thermal conductivity of light-weight aggregate concrete including microencapsulated PCM. J. Mater. Res. Technol. 2023, 26, 587–602. [Google Scholar] [CrossRef]
- Jin, X.; Xu, X.; Zhang, X.; Yin, Y. Determination of the PCM melting temperature range using DSC. Thermochim. Acta 2014, 595, 17–21. [Google Scholar] [CrossRef]
- Bumanis, G.; Bajare, D. PCM Modified Gypsum Hempcrete with Increased Heat Capacity for Nearly Zero Energy Buildings. Environ. Clim. Technol. 2022, 26, 524–534. [Google Scholar] [CrossRef]
- Kośny, J.; Yarbrough, D.W.; Miller, W.A.; Kenneth, E. Analysis of the Dynamic Thermal Performance of Fiberous Insulations Containing Phase Change Materials; DWY Final Report; Oklahoma State University: Stillwater, OK, USA, 2009. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotova, I.; Gendelis, S.; Kirilovs, E.; Štefanec, D. Thermal Performance of Lignocellulose’s By-Product Wallboards with Bio-Based Microencapsulated Phase Change Materials. Energies 2024, 17, 257. https://doi.org/10.3390/en17010257
Zotova I, Gendelis S, Kirilovs E, Štefanec D. Thermal Performance of Lignocellulose’s By-Product Wallboards with Bio-Based Microencapsulated Phase Change Materials. Energies. 2024; 17(1):257. https://doi.org/10.3390/en17010257
Chicago/Turabian StyleZotova, Inga, Staņislavs Gendelis, Edgars Kirilovs, and Dejan Štefanec. 2024. "Thermal Performance of Lignocellulose’s By-Product Wallboards with Bio-Based Microencapsulated Phase Change Materials" Energies 17, no. 1: 257. https://doi.org/10.3390/en17010257
APA StyleZotova, I., Gendelis, S., Kirilovs, E., & Štefanec, D. (2024). Thermal Performance of Lignocellulose’s By-Product Wallboards with Bio-Based Microencapsulated Phase Change Materials. Energies, 17(1), 257. https://doi.org/10.3390/en17010257








