Emissions from Light-Duty Vehicles—From Statistics to Emission Regulations and Vehicle Testing in the European Union
Abstract
1. Vehicles around the World
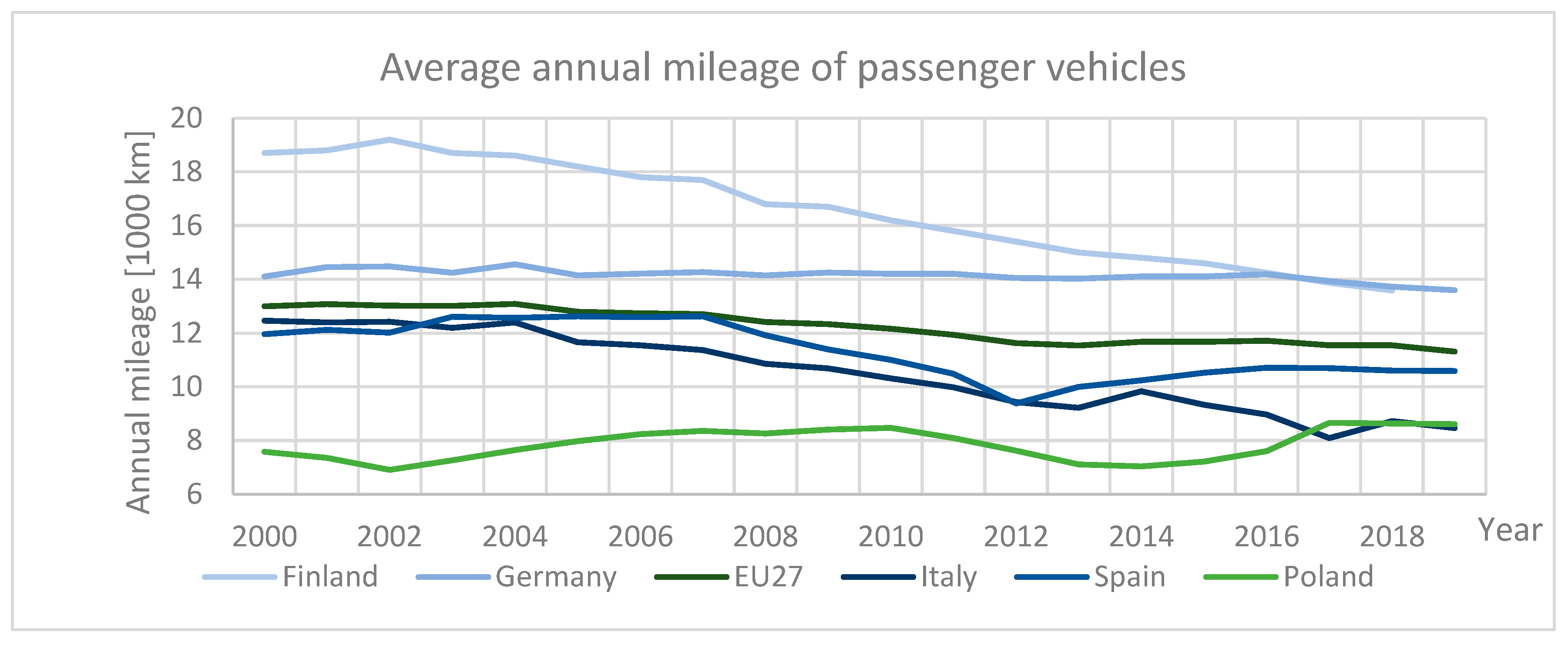
1.1. Statistical Information on the Automotive Industry
1.2. Vehicle Categories
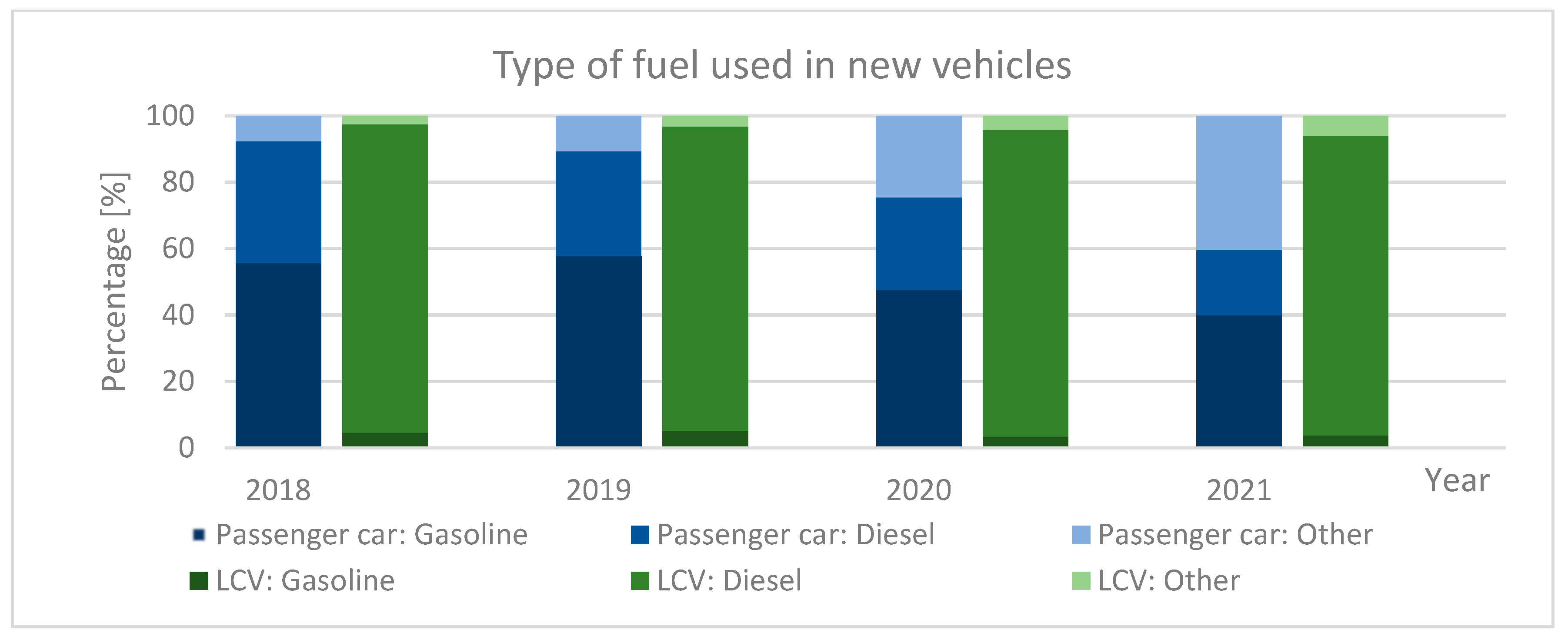
1.3. Types of Fuel Used
2. Vehicle Emissions
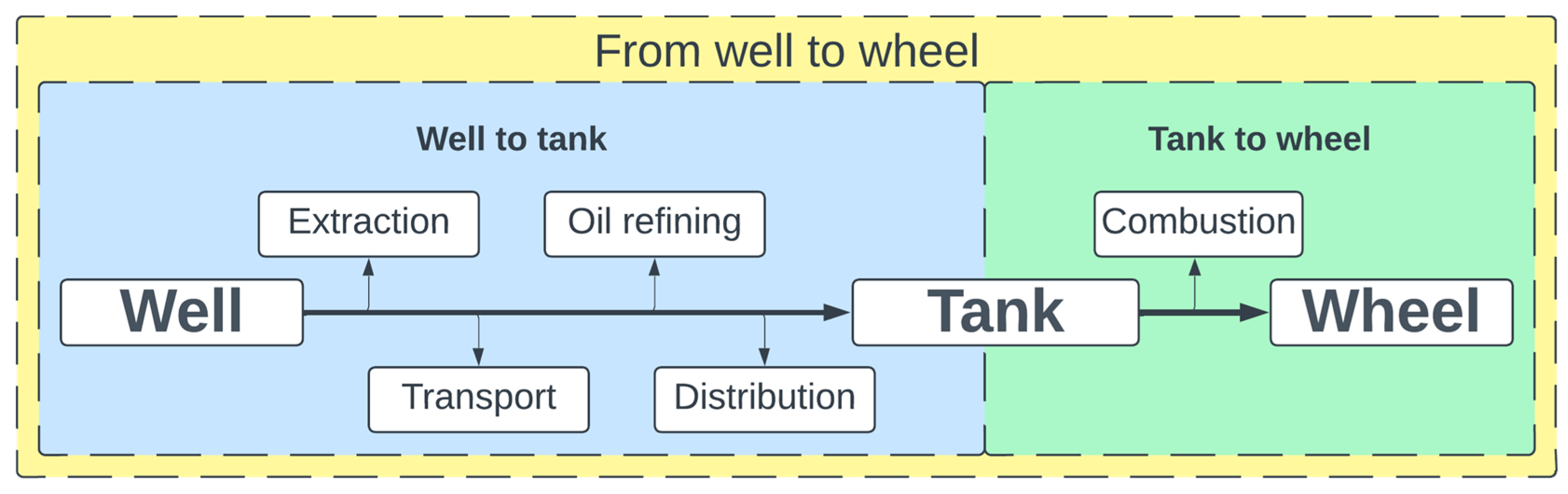
2.1. Emission and Low-Stack Emission
2.2. Selected Legal Regulations
2.3. Regulated Emission—European Emission Standard
2.4. Nonexhaust and Unregulated Emissions
2.5. Impact of Nonregulated Emission on Health
2.6. Micro-Contaminants and Their Classification
3. Vehicle Exhaust
3.1. General Characteristics of Vehicle Exhaust
| Fuel a | CO | CO2 | HC | NOx _ | PM |
|---|---|---|---|---|---|
| [g/km] | [g/km] | [g/km] | [g/km] | [mg/km] | |
| Zhang et al., 2023 [84] | |||||
| Gasoline | 0.84 | 270.28 | 0.24 | 0.04 | |
| Gasoline (Hybrid) | 0.28 | 98.72 | 0.004 | 0.0009 | |
| Šarkan et al., 2022 [86] | |||||
| LPG | 1.90 | 213.97 | 0.0043 | 0.0513 | |
| Gasoline | 1.93 | 217.69 | 0.0040 | 0.0311 | |
| Asoaf et al., 2022 [83] b,c | |||||
| Gasoline | 0.81 | 0.19 | 0.01 | ||
| CNG | 0.76 | 1.79 | 0.01 | ||
| Diesel | 3.58 | 0.27 | 0.80 | ||
| Hakkarainen et al., 2020 [85] | |||||
| CNG | 0.201 | 0.48 | |||
| Gasoline (E10) | 0.031 | 1.35 | |||
| Diesel (B7) | 0.463 | 0.64 | |||
| Ntziachristos and Samaras 2019 [82] d,e | |||||
| LPG | 0.62 | 173.88 | 0.056 | 1.1 | |
| CNG | 0.616 | 171.71 | 0.056 | 1.1 | |
| Gasoline | 0.62 | 221.83 | 0.061 | 1.6 | |
| Park et al., 2019 [81] | |||||
| LPG | 0.82 | 270.5 | 0.001 | ||
| Gasoline | 0.42 | 265.0 | 0.007 | ||
| Diesel | 0.03 | 273.0 | 0.657 | ||
| Hesteberg et al., 2008 [80] | |||||
| CNG | 0.42 | 0.59 | 0.18 | 10 | |
| Diesel | 0.70 | 0.12 | 0.81 | 80 | |
3.2. Microcontaminants Emission from Vehicles
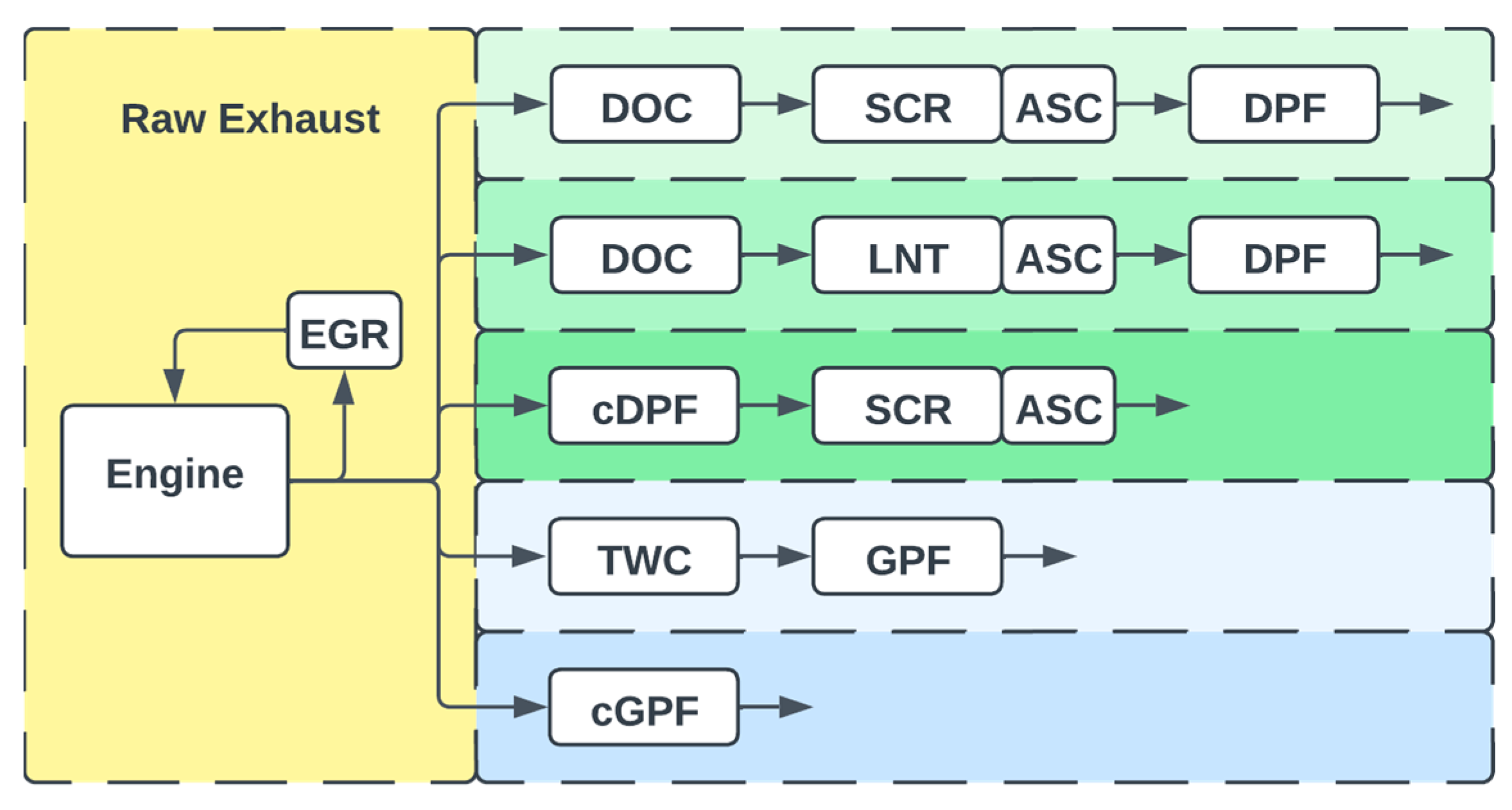
3.3. Comparison of Exhaust-Gas Treatment Systems
4. Factors Affecting Emissions from Vehicles with Gasoline Engines
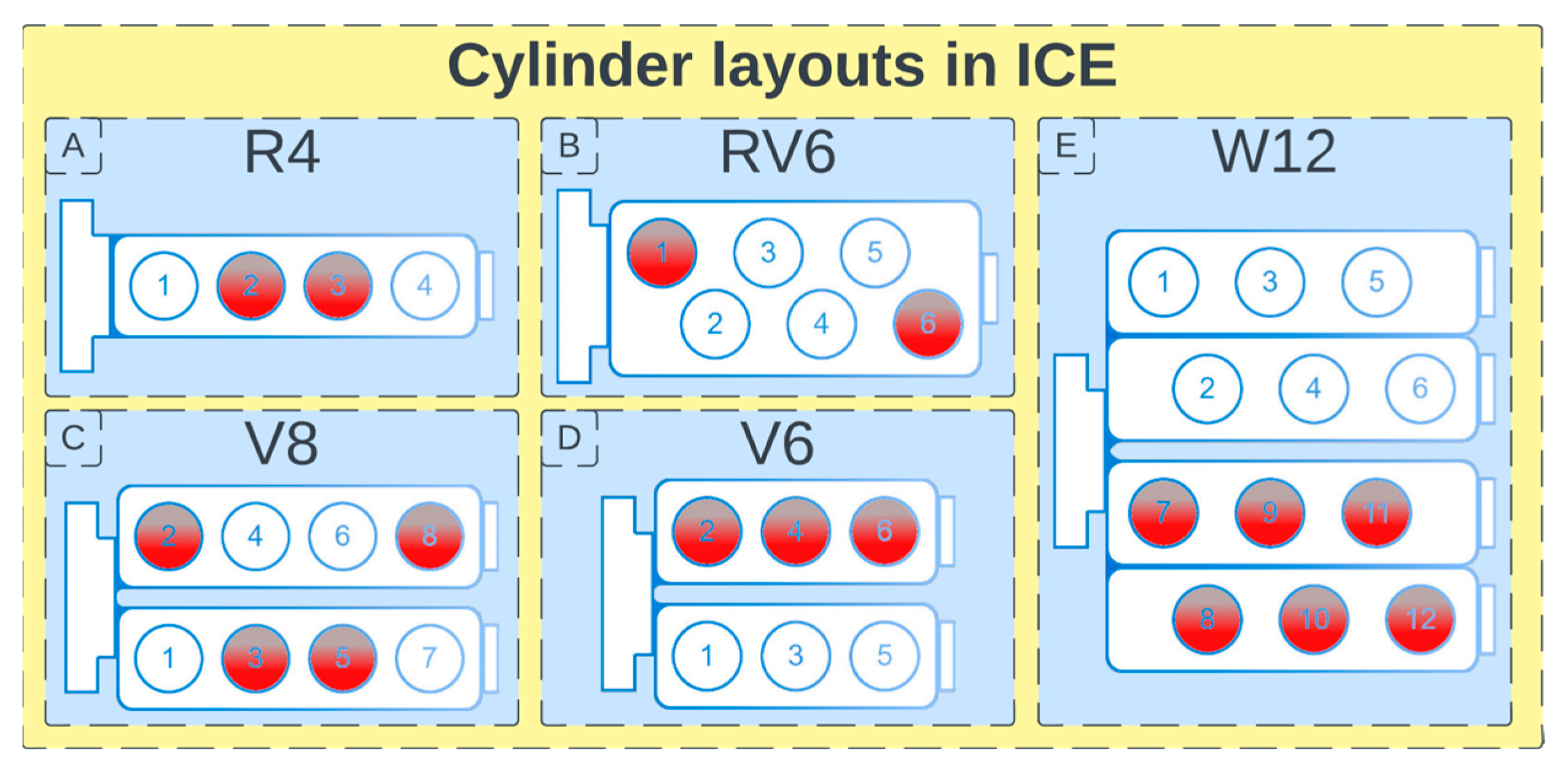
4.1. Downsizing and Rightsizing
4.2. Octane Number
4.3. Physicochemical Composition of Fuel
4.4. Engine Oil
4.5. Wet Piston Effect
4.6. Fuel-to-Air Ratio
4.7. Engine Operating Parameters
4.8. Exhaust-Gas Recirculation and Combustion Air
4.9. Cold Start and Weather Conditions
4.10. Start–Stop Systems
4.11. Other Systems
4.12. Driving Style
5. Vehicle-Testing Procedure
5.1. First Emission Tests
5.2. Coastdown
5.3. Chassis Dynamometer Test
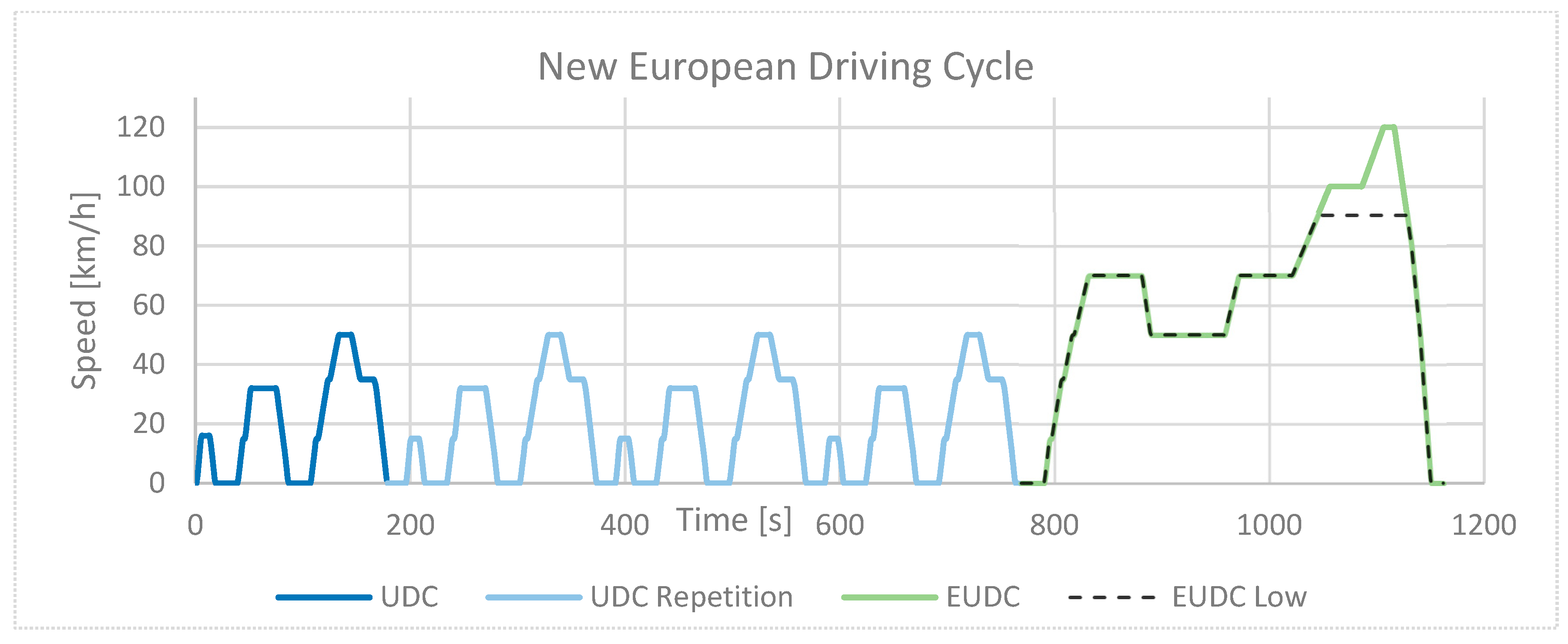
5.4. European Driving Cycles
- Type I: a cold-start emission test;
- Type II: emissions at idle; the test is carried out immediately after the end of the EUDC;
- Type III: checks crankcase emissions when idling and at 50 km/h;
- Type IV: test for the loss of hydrocarbons as a result of their evaporation from the fuel system for SI vehicles;
- Type V: test to verify the durability of pollution-control devices;
- Type VI: carbon monoxide and hydrocarbon emissions test at −7 °C;
- Type VII: fuel consumption and range test;
- Type VIII: environmental tests;
- Type IX: noise-level test.
5.5. Comparison of Dyno Chassis Tests
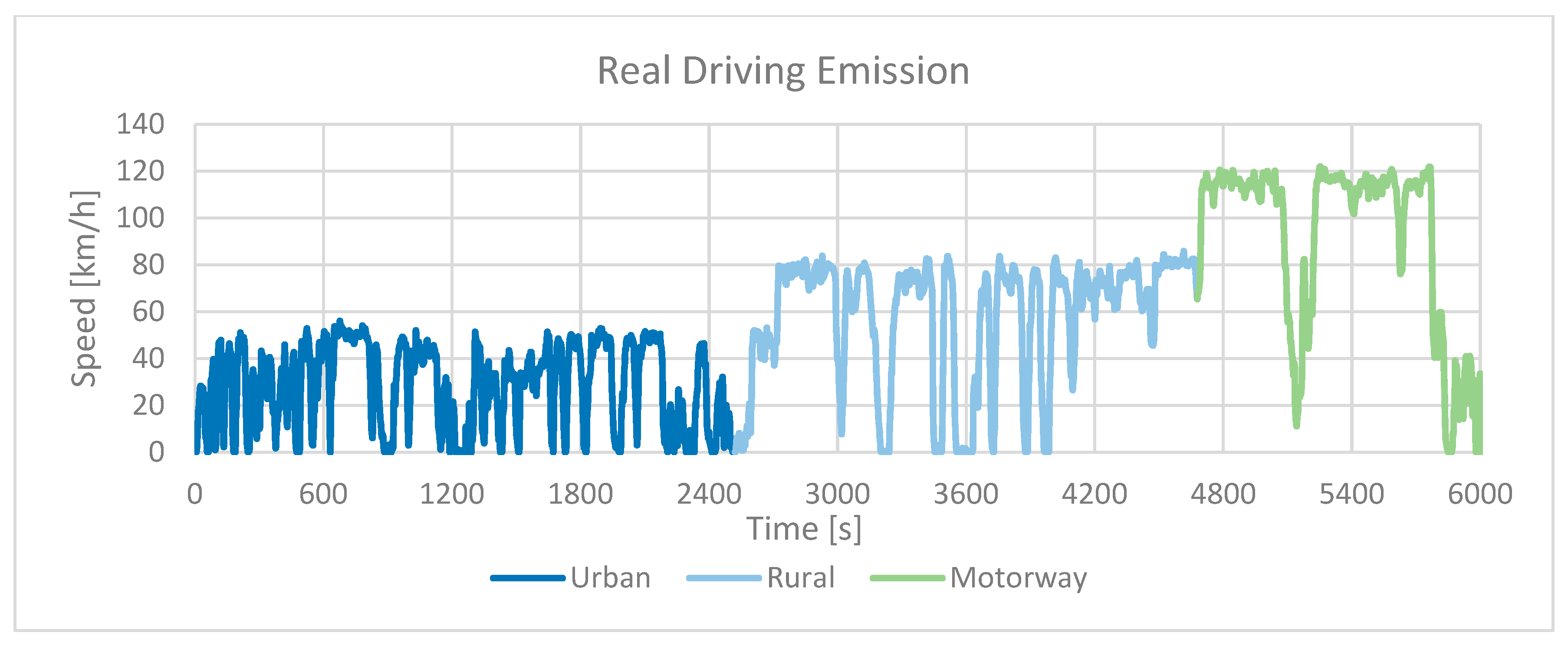
5.6. Real Driving Emissions
6. Current Challenges in Vehicle Emission
7. Future Trends
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| AKI | Antiknock Index | GC | Gas Chromatography | PWR | Power-to-weight ratio |
| ASC | Ammonia Slip Catalyst | GPF | Gasoline Particulate Filter | QCL | Quantum Cascade Laser |
| BAT | Best Available Techniques | HC | Hydrocarbons | RDE | Real Driving Emissions |
| CA | Crank Angle | IC | Ion Chromatography | RON | Research Octane Number |
| CAV | Connected and Autonomous Vehicles | ICE | Internal combustion engine | RPM | Revolutions Per Minute |
| CI | Compressed Ignition | ISC-FCM | In-service Conformity–Fuel- Consumption Measurement | PGMs | Platinum group metals |
| CNG | Compressed Natural Gas | LDV | Light-duty vehicles | PM | Particulate Matter |
| DI | Direct Injection | LEV | Low-emission vehicle | SCR | Selective Catalytic Reduction |
| DIPE | Diisopropyl Ether | LNT | Lean Nitrogen Trap | SI | Spark Ignition |
| DOC | Diesel Oxidation Catalytic Converter | LPG | Liquid Petroleum Gas | TAEE | Ethyl Tert-Amyl Ether |
| DPF | Diesel Particulate Filter | MCs | Microcontaminants | TAME | Methyl Tert-Amyl Ether |
| EGR | Exhaust-Gas Recirculation | MeOH | Methanol | TEQ | Toxic Equivalency Factor |
| EOBD II | Engine Onboard Diagnostic type II | MON | Motor Octane Number | TTW | Tank to wheel |
| ETBE | Ethyl Tert-Butyl Ether | MP-AES | Microwave Plasma–Atomic Emission Spectroscopy | TWC | Three-way catalytic converter |
| EtOH | Ethanol | MS | Mass Spectrometry | UDC | Urban Driving Cycle |
| EU | European Union | MTBE | Methyl Tert-Butyl Ether | UV | Ultraviolet |
| EUDC | Extraurban Driving Cycle | NEDC | New European Driving Cycle | WLTC | Worldwide Harmonised Light-Duty Vehicle Test Cycle |
| EVs | Electric vehicle | NMHC | Nonmethane Hydrocarbons | WLTP | Worldwide Harmonised Light-Duty Vehicle Test Procedure |
| FID | Flame Ionization Detector | NTE | Not to exceed | WTT | Well to tank |
| FT-IR | Fourier Transform Infrared Spectroscopy | ON | Octane Number | WTW | Well to wheel |
| FTP | Federal Test Procedure | PAHs | Polycyclic Aromatic Hydrocarbons | λ | Air–fuel ratio |
| LCV | Light commercial vehicles | PN | Particle Number |
References
- Rae, J.B.; Binder, A.K. Automotive industry—Przemysł samochodowy. Encycl. Br. 2023. Available online: https://www.britannica.com/technology/automotive-industry (accessed on 1 March 2023).
- ACEA. The Automobile Industry—Pocket Guide 2022/2023; ACEA: Belgium, Brussels, 2022; Volume 23, pp. 1–106. [Google Scholar]
- European Comission’s Directorate-General for Mobility and Transport. EU Transport in Figures—Statistical Pocketbook 2022; European Union: Belgium, Brussels, 2022; ISBN 9789276536994. [Google Scholar]
- Odyssee-Mure. Sectoral Profile—Transport. 2021. Available online: https://www.odyssee-mure.eu/publications/efficiency-by-sector/transport/transport-eu.pdf (accessed on 1 April 2023).
- Ministry of Interior and Administration. Ustawa z Dnia 20 Czerwca 1993 r—Prawo o Ruchu Drogowym; Ministry of Interior and Administration: Warsaw, Poland, 1993.
- Parlament Europejski i Rada. Dyrektywa 2007/46/WE z Dnia 5 Września 2007r—Ustanawiająca Ramy dla Homologacji Pojazdów Silnikowych i ich Przyczep Oraz Układów, Części i Oddzielnych Zespołów Technicznych Przeznaczonych do Tych Pojazdów. 2007. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/PDF/?uri=CELEX:02007L0046-20180331 (accessed on 1 March 2023).
- EU; European Parliament. Council of the European Union Regulation (EC) No 715/2007 of the European Parliament and of the Council. Off. J. Eur. Communities 2006, 2006, 1–54. [Google Scholar]
- Merkisz, J.; Pielecha, J.; Radzimirski, S. Emisja Zanieczyszczeń Motoryzacyjnych w Swietle Nowych Przepisów Unii Europejskiej; Wydawnictwa Komunikacji i Łączności Sp. z o.o.: Warszawa, Poland, 2012; ISBN 978-83-206-1831-0. [Google Scholar]
- European Commission; Council of the European Union. COMMISSION REGULATION (EU) 2017/1151; European Union: Belgium, Brussels, 2017; Volume 10, pp. 1–21. [Google Scholar]
- ICCT Europe, European Vehicle Market Statistics. Pocketb; International Council on Clean Transportation: Berlin, Germany, 2019; Volume 23, pp. 1–105.
- Kowalczyk, G. Tak może wyglądać podatek od spalinówe wynikający z KPO. Sprawdzamy, jak wylicza się go w innych państwach. Bus. Insid. 2022, VII, 1–2. Available online: https://businessinsider.com.pl/technologie/motoryzacja/jak-moze-wygladac-polski-podatek-od-samochodow-spalinowych-sprawdzamy-jak-wyliczaja/1c5lvdd (accessed on 7 March 2023).
- Daniel, A. Your Guide to Owning a Car in Japan. Available online: https://www.borderlink.co.jp/magazine/1938/ (accessed on 7 March 2023).
- European Comission. ROZPORZĄDZENIE PARLAMENTU EUROPEJSKIEGO I RADY (UE) 2016/1628 z dnia 14 września 2016 r. 2016. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/?uri=CELEX%3A32016R1628 (accessed on 7 March 2023).
- Merkisz, J.; Pielecha, J.; Radzimirski, S. New Trends in Emission Control in the European Union. Springer Int. Publ. 2014, 4, 175. [Google Scholar] [CrossRef]
- Ho, C.S.; Peng, J.; Yun, U.; Zhang, Q.; Mao, H. Impacts of methanol fuel on vehicular emissions: A review. Front. Environ. Sci. Eng. 2022, 16, 121. [Google Scholar] [CrossRef]
- Skoda Co to są Samochody Hybrydowe? 2021. Available online: www.skoda-auto.pl (accessed on 7 March 2023).
- Environmental Protection Agency Fuel Economy. 2023. Available online: https://www.fueleconomy.gov/ (accessed on 14 March 2023).
- International Energy Agency. Fuel economy in the European Union. In Global Fuel Economy Initiative 2021; IEA: Paris, France, 2022. [Google Scholar]
- European Commission. DIRECTIVE 2010/75/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control; European Union: Belgium, Brussels, 2010. [Google Scholar]
- European Commission. Stepping up Europe’s 2030 Climate Ambition; European Union: Belgium, Brussels, 2020; pp. 1–26. [Google Scholar]
- Ministry of Environment. Ustawa z Dnia 27 Kwietnia 2001 r. Prawo Ochrony środowiska; Ministry of Environmet: Warsaw, Poland, 2001. [Google Scholar]
- Merkisz, J.; Pielecha, J. Emisja cząstek stałych ze źródeł motoryzacyjnych. In Wydaw. Politech. Poznańskiej; 2014; Volume 1, pp. 1–20. ISBN 978-83-7775-325-5. [Google Scholar]
- Environmental Protection Agency Introduction to Source Classification Codes and their Use for EIS Submissions. Available online: https://sor-scc-api.epa.gov/sccwebservices/sccsearch/docs/SCC-IntroToSCCs_2021.pdf (accessed on 14 March 2023).
- Pełka, G.; Ciapała, B.; Kaczmarczyk, M.; Będkowska, A.; Malik, D.; Luboń, W.; Podlweska, E.; Kaczmarczyk, M. Niska Emisja—Efektywność Energetyczna w Gminach i Samorządach—Monografia; Globenergia sp. z o.o.: Kraków, Poland, 2017. [Google Scholar]
- Sejmik Województwa Małopolskiego. Uchwała Nr XXXIX/612/09 z Dnia 21 Grudnia 2009 r; Sejmik Województwa Małopolskiego: Krakow, Poland, 2009. [Google Scholar]
- Rada Miasta Krakowa. Uchwała nr C/2707/22 z dnia 23 Listopada 2022; Rada Miasta Krakowa: Kraków, Poland, 2022. [Google Scholar]
- Najwyższa Izba Kontroli. Informacja o wynikach kontroli: OCHRONA POWIETRZA PRZED ZANIECZYSZCZENIAMI; Supreme Audit Office: Warsaw, Poland, 2014; pp. 1–280. Available online: https://www.nik.gov.pl/kontrole/wyniki-kontroli-nik/pobierz,lkr~p_17_078_201709271210561506514256~02,typ,kk.pdf (accessed on 21 March 2023).
- Sejmik Województwa Małopolskiego. Uchwała Nr XXV/373/20 z dnia 28 września 2020 r. w sprawie Programu ochrony powietrza dla województwa małopolskiego; Regional Council of Małopolska: Krakow, Poland, 2020; pp. 1–393. Available online: http://edziennik.malopolska.uw.gov.pl/WDU_K/2020/6337/akt.pdf (accessed on 21 March 2023).
- CLARS. Urban Access Regulations in Europe. Available online: https://urbanaccessregulations.eu/ (accessed on 21 March 2023).
- Giechaskiel, B.; Riccobono, F.; Vlachos, T.; Mendoza-Villafuerte, P.; Suarez-Bertoa, R.; Fontaras, G.; Bonnel, P.; Weiss, M. Vehicle Emission Factors of Solid Nanoparticles in the Laboratory and on the Road Using Portable Emission Measurement Systems (PEMS). Front. Environ. Sci. 2015, 3, 82. [Google Scholar] [CrossRef]
- Tomašek, I.; Horwell, C.J.; Bisig, C.; Damby, D.E.; Comte, P.; Czerwinski, J.; Petri-Fink, A.; Clift, M.J.D.; Drasler, B.; Rothen-Rutishauser, B. Respiratory hazard assessment of combined exposure to complete gasoline exhaust and respirable volcanic ash in a multicellular human lung model at the air-liquid interface. Environ. Pollut. 2018, 238, 977–987. [Google Scholar] [CrossRef]
- European Commission. Well-to-Wheels Analyses. EU Science Hub, 2016. Available online: https://joint-research-centre.ec.europa.eu/welcome-jec-website/jec-activities/well-wheels-analyses_en (accessed on 1 March 2023).
- European Comission. REGULATION (EU) 2019/631 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 17 April 2019; European Union: Belgium, Brussels, 2019; pp. 1–41. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0631 (accessed on 28 March 2023).
- European Commission. COMMISSION REGULATION (EU) 2018/1832 of 5 November 2018; European Union: Belgium, Brussels, 2018. [Google Scholar]
- Brzeżański, M.; Rodak, Ł. Influence of the method of creating a hydrogen-air mixture on the emission of nitrogen oxides in a spark-ignition engine. Combust. Engines 2019, 178, 224–227. [Google Scholar] [CrossRef]
- Ministry of Environment. ROZPORZĄDZENIE MINISTRA GOSPODARKI 1 z dnia 9 października 2015 r. w sprawie wymagań jakościowych dla paliw ciekłych; Ministry of Environment: Warsaw, Poland, 2015. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20150001680/O/D20151680.pdf (accessed on 28 March 2023).
- Giechaskiel, B.; Lähde, T.; Clairotte, M.; Valverde, V.; Pavlovic, J.; Suarez-Bertoa, R.; Grigoratos, T.; Zardini, A.A.; Perujo, A.; Marotta, A.; et al. Post Euro 6/VI activities focusing on particle number. SAE World Congr. Exp. 2019. [Google Scholar] [CrossRef]
- Di Iorio, S.; Catapano, F.; Sementa, P.; Vaglieco, B.M.; Nicol, G.; Sgroi, M.F. Sub-23 nm Particle Emissions from Gasoline Direct Injection Vehicles and Engines: Sampling and Measure. SAE Tech. Pap. 2020, 0396, 1–9. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Mamakos, A.; Woodburn, J.; Szczotka, A.; Bielaczyc, P. Evaluation of a 10 nm Particle Number Portable Emissions Measurement System (PEMS). Sensors 2019, 19, 5531. [Google Scholar] [CrossRef] [PubMed]
- Lähde, T.; Giechaskiel, B.; Martini, G.; Woodburn, J.; Bielaczyc, P.; Schreiber, D.; Huber, M.; Dimopoulos Eggenschwiler, P.; Fittavolini, C.; Florio, S.; et al. Reproducibility of the 10-nm Solid Particle Number Methodology for Light-Duty Vehicles Exhaust Measurements. Atmosphere 2022, 13, 872. [Google Scholar] [CrossRef]
- European Commission Council Regulation of the European parliament and of the council emissions and Battery durability (euro 7). Off. J. Eur. Union 2022, 0365, 1–66.
- Giechaskiel, B.; Melas, A.; Martini, G.; Dilara, P. Overview of vehicle exhaust particle number regulations. Processes 2021, 9, 2216. [Google Scholar] [CrossRef]
- European Comission Commission proposes new Euro 7 standards to reduce pollutant emissions from vehicles and improve air quality. Press Corner 2022, 6495, 10–12.
- Michalik, M.; Brzezański, M.; Wilczyńska-Michalik, W.; Fisior, K.; Klimas, B.; Samek, L.; Pietras, B. Characterisation of solid particles emitted from diesel and petrol engines as a contribution to the determination of the origin of carbonaceous particles in urban aerosol. IOP Conf. Ser. Mater. Sci. Eng. 2016, 148, 012079. [Google Scholar] [CrossRef]
- Pacura, W.; Szramowiat-Sala, K.; Macherzyński, M.; Gołaś, J.; Bielaczyc, P. Analysis of Micro-Contaminants in Solid Particles from Direct Injection Gasoline Vehicles. Energies 2022, 15, 5732. [Google Scholar] [CrossRef]
- Keyte, I.J.; Harrison, R.M.; Lammel, G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons—A review. Chem. Soc. Rev. 2013, 42, 9333. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, L.; Huang, Q.; Zhang, W.; Duan, S.; Gao, H.; Wang, W. PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) and nitrated-PAHs (NPAHs) emitted by gasoline vehicles: Characterization and health risk assessment. Sci. Total Environ. 2020, 727, 138631. [Google Scholar] [CrossRef]
- Raza, M.; Chen, L.; Leach, F.; Ding, S. A Review of particulate number (PN) emissions from gasoline direct injection (gdi) engines and their control techniques. Energies 2018, 11, 1417. [Google Scholar] [CrossRef]
- Szramowiat, K.; Woodburn, J.; Pacura, W.; Berent, K.; Bielaczyc, P.; Gołaś, J. Engine-generated solid particles—A case study. Combust. Engines 2018, 174, 33–39. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Storch, M.; Melder, J.; Richter, S.; Gaiser, N.; Schlichting, S.; Naumann, C.; Schünemann, E.; Aigner, M.; Patrick, O.; et al. Soot formation of renewable gasoline: From fuel chemistry to particulate emissions from engines. Fuel 2023, 348, 128109. [Google Scholar] [CrossRef]
- Idzior, M. Aging of engine oils and their influence on the wear of an internal combustion engine. Combust. Engines 2021, 185, 15–20. [Google Scholar] [CrossRef]
- Harrison, R.M.; Allan, J.; Carruthers, D.; Heal, M.R.; Lewis, A.C.; Marner, B.; Murrells, T.; Williams, A. Non-exhaust vehicle emissions of particulate matter and VOC from road traffic: A review. Atmos. Environ. 2021, 262, 118592. [Google Scholar] [CrossRef]
- Air Quality Expert Group. Non-exhaust emissions from road traffic; Department for Environment Food & Rural Affairs: London, UK, 2019; pp. 1–93. Available online: https://uk-air.defra.gov.uk/assets/documents/reports/cat09/1907101151_20190709_Non_Exhaust_Emissions_typeset_Final.pdf (accessed on 4 April 2023).
- European Environment Agency. National Emissions Reported to the Convention on Long-Range Transboundary Air Pollution (LRTAP Convention). 2022. Available online: https://www.eea.europa.eu/data-and-maps/data/national-emissions-reported-to-the-convention-on-long-range-transboundary-air-pollution-lrtap-convention-16/folder_contents (accessed on 1 March 2023).
- Bandowe, B.A.M.; Meusel, H. Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment—A review. Sci. Total Environ. 2017, 581–582, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Cochran, R.E.; Dongari, N.; Jeong, H.; Beránek, J.; Haddadi, S.; Shipp, J.; Kubátová, A. Determination of polycyclic aromatic hydrocarbons and their oxy-, nitro-, and hydroxy-oxidation products. Anal. Chim. Acta 2012, 740, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.; Granner, D.; Mayes, B.; Rodwell, V. Biologia Harpera, 7th ed.; PZWL: Warsaw, Poland, 2018; ISBN 978-83-200-5410-1. [Google Scholar]
- Bielański, A. Podstawy Chemii Nieorganicznej. Wydaw. Nauk. PWN 2010, 1, 1–546. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). ToxFAQs; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023. Available online: https://www.atsdr.cdc.gov/az/a.html (accessed on 4 April 2023).
- Hao, X.; Zhang, X.; Cao, X.; Shen, X.; Shi, J.; Yao, Z. Characterization and carcinogenic risk assessment of polycyclic aromatic and nitro-polycyclic aromatic hydrocarbons in exhaust emission from gasoline passenger cars using on-road measurements in Beijing, China. Sci. Total Environ. 2018, 645, 347–355. [Google Scholar] [CrossRef]
- Borillo, G.C.; Tadano, Y.S.; Godoi, A.F.L.; Pauliquevis, T.; Sarmiento, H.; Rempel, D.; Yamamoto, C.I.; Marchi, M.R.R.; Potgieter-Vermaak, S.; Godoi, R.H.M. Polycyclic Aromatic Hydrocarbons (PAHs) and nitrated analogs associated to particulate matter emission from a Euro V-SCR engine fuelled with diesel/biodiesel blends. Sci. Total Environ. 2018, 644, 675–682. [Google Scholar] [CrossRef]
- Santa Cruz Biotechnology Inc. 1,4-naphthoquinone toxicity—Safety datasheet; SCBT: Santa Cruz, CA, USA, 2023; pp. 1–13. Available online: https://datasheets.scbt.com/sc-237768.pdf (accessed on 4 April 2023).
- Li, X.; Zheng, Y.; Guan, C.; Cheung, C.S.; Huang, Z. Effect of biodiesel on PAH, OPAH, and NPAH emissions from a direct injection diesel engine. Environ. Sci. Pollut. Res. 2018, 25, 34131–34138. [Google Scholar] [CrossRef]
- Hamra, G.B.; Laden, F.; Cohen, A.J.; Raaschou-Nielsen, O.; Brauer, M.; Loomis, D. Lung Cancer and Exposure to Nitrogen Dioxide and Traffic: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2015, 123, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Makhniashvili, I. Nitrowe Pochodne Wielopierœcieniowych Wêglowodorów Aromatycznych w Œrodowisku. 2003; pp. 17–20. Available online: http://yadda.icm.edu.pl/baztech/element/bwmeta1.element.baztech-d695c81d-dc3d-4200-bc24-8a91b8ab1244 (accessed on 4 April 2023).
- Tokar, E.J.; Boyd, W.A.; Freedman, J.H.; Waalkes, M.P. Toxic Effects of Metals. In Casarett and Doull’s Toxicology: The Basic Science of Poisons, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2012. [Google Scholar]
- Treichel, J.L.; Henry, M.M.; Skumatz, C.M.B.; Eells, J.T.; Burke, J.M. Formate, the toxic metabolite of methanol, in cultured ocular cells. Neurotoxicology 2003, 24, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Phosphate toxicity: New insights into an old problem. Clin. Sci. 2011, 120, 91–97. [Google Scholar] [CrossRef] [PubMed]
- California Air Resource Board. Sulphates and Health; California Air Resource Board: Sacramento, CA, USA, 2023. Available online: https://ww2.arb.ca.gov/resources/sulfate-and-health (accessed on 4 April 2023).
- Pauling, L.; Pauling, P. Chemia; Wydawnictwo Naukowe PWN: Warsaw, Poland, 1998; Volume 5, pp. 20–370. ISBN 9788301122676. [Google Scholar]
- Lenntech Periodic Table—Health Effect of Elements. Available online: https://www.lenntech.com/periodic/elements/zr.htm (accessed on 4 April 2023).
- New Jersey Department of Health. Zirconium. In Hazardous Substance Fact Sheet; New Jersey Department of Health: Trenton, NJ, USA, 2008. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/2047.pdf (accessed on 4 April 2023).
- Levin, R.; Schwartz, J. A better cost:benefit analysis yields better and fairer results: EPA’s lead and copper rule revision. Environ. Res. 2023, 229, 115738. [Google Scholar] [CrossRef] [PubMed]
- New York State Water Resources Institute. Micropollutants & Emerging Contaminants; Cornell College of Agricultrue and Life Sciences: Ithaca, NY, USA, 2023; Available online: https://cals.cornell.edu/water-resources-institute/research-themes/legacy-emerging-contaminants (accessed on 11 April 2023).
- Bisig, C.; Comte, P.; Güdel, M.; Czerwinski, J.; Mayer, A.; Müller, L.; Petri-Fink, A.; Rothen-Rutishauser, B. Assessment of lung cell toxicity of various gasoline engine exhausts using a versatile in vitro exposure system. Environ. Pollut. 2018, 235, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Überall, A.; Otte, R.; Eilts, P.; Krahl, J. A literature research about particle emissions from engines with direct gasoline injection and the potential to reduce these emissions. Fuel 2015, 147, 203–207. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, Z.; Qin, Y.; Peng, J.; Li, M.; Lei, J.; Wu, Y.; Min, H.; Shijin, S. The impact of fuel compositions on the particulate emissions of direct injection gasoline engine. Fuel 2016, 166, 543–552. [Google Scholar] [CrossRef]
- Zając, P. Silniki pojazdów samochodowych. In Technik pojazdów samochodowych; Wydawnictwa Komunikacji i Łączności WKŁ: Sulejówek, Poland, 2011; Volume 1, pp. 1–399. ISBN 978-83-206-1783-2. [Google Scholar]
- Al-Arkawazi, S.A.F. Analyzing and predicting the relation between air–fuel ratio (AFR), lambda (λ) and the exhaust emissions percentages and values of gasoline-fueled vehicles using versatile and portable emissions measurement system tool. SN Appl. Sci. 2019, 1, 1370. [Google Scholar] [CrossRef]
- Hesterberg, T.W.; Lapin, C.A.; Bunn, W.B. A Comparison of Emissions from Vehicles Fueled with Diesel or Compressed Natural Gas. Environ. Sci. Technol. 2008, 42, 6437–6445. [Google Scholar] [CrossRef]
- Park, G.; Mun, S.; Hong, H.; Chung, T.; Jung, S.; Kim, S.; Seo, S.; Kim, J.; Lee, J.; Kim, K.; et al. Characterization of emission factors concerning gasoline, LPG, and diesel vehicles via transient chassis-dynamometer tests. Appl. Sci. 2019, 9, 1573. [Google Scholar] [CrossRef]
- Ntziachristos, L.; Samaras, Z. Exhaust emissions from road transport. In EMEP/EEA air pollutant emission inventory guidebook 2019; Publications Office of the European Union Location: Luxembourg, Luxembourg, 2019. [Google Scholar] [CrossRef]
- Aosaf, M.R.; Wang, Y.; Du, K. Comparison of the emission factors of air pollutants from gasoline, CNG, LPG and diesel fueled vehicles at idle speed. Environ. Pollut. 2022, 305, 119296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Y.; Feng, T.; Chen, Y. Comparative Analysis of Emission Characteristics of In-Use China II-V Gasoline, Hybrid, Diesel-Fueled Vehicles. Atmosphere 2023, 14, 272. [Google Scholar] [CrossRef]
- Hakkarainen, H.; Aakko-Saksa, P.; Sainio, M.; Ihantola, T.; Rönkkö, T.J.; Koponen, P.; Rönkkö, T.; Jalava, P.I. Toxicological evaluation of exhaust emissions from light-duty vehicles using different fuel alternatives in sub-freezing conditions. Part. Fibre Toxicol. 2020, 17, 17. [Google Scholar] [CrossRef]
- Šarkan, B.; Jaśkiewicz, M.; Kubiak, P.; Tarnapowicz, D.; Loman, M. Exhaust Emissions Measurement of a Vehicle with Retrofitted LPG System. Energies 2022, 15, 1184. [Google Scholar] [CrossRef]
- Owczuk, M.; Matuszewska, A.; Wojs, M.K.; Orliński, P. Wpływ rodzaju paliwa stosowanego w silniku o zapłonie iskrowym na skład spalin. Przem. Chem. 2018, 1, 124–129. [Google Scholar] [CrossRef]
- HT Auto Desk Is CNG the right fuel for your car? Hindustan Times 2021, X, 1.
- Lin, Y.-C.; Li, Y.-C.; Shangdiar, S.; Chou, F.-C.; Sheu, Y.-T.; Cheng, P.-C. Assessment of PM2.5 and PAH content in PM2.5 emitted from mobile source gasoline-fueled vehicles in concomitant with the vehicle model and mileages. Chemosphere 2019, 226, 502–508. [Google Scholar] [CrossRef]
- Muñoz, M.; Haag, R.; Honegger, P.; Zeyer, K.; Mohn, J.; Comte, P.; Czerwinski, J.; Heeb, N.V. Co-formation and co-release of genotoxic PAHs, alkyl-PAHs and soot nanoparticles from gasoline direct injection vehicles. Atmos. Environ. 2018, 178, 242–254. [Google Scholar] [CrossRef]
- Jakober, C.A.; Robert, M.A.; Riddle, S.G.; Destaillats, H.; Charles, M.J.; Green, P.G.; Kleeman, M.J. Carbonyl Emissions from Gasoline and Diesel Motor Vehicles. Environ. Sci. Technol. 2008, 42, 4697–4703. [Google Scholar] [CrossRef]
- Riddle, S.G.; Jakober, C.A.; Robert, M.A.; Cahill, T.M.; Charles, M.J.; Kleeman, M.J. Large PAHs detected in fine particulate matter emitted from light-duty gasoline vehicles. Atmos. Environ. 2007, 41, 8658–8668. [Google Scholar] [CrossRef]
- Oda, J.; Maeda, I.; Mori, T.; Yasuhara, A.; Saito, Y. The Relative Proportions of Polycyclic Aromatic Hydrocarbons and Oxygenated Derivatives in Accumulated Organic Particulates as Affected by Air Pollution Sources. Environ. Technol. 1998, 19, 961–976. [Google Scholar] [CrossRef]
- ECOpoint Inc. Emission Standards: China. DieselNet Technol. Guid. 2023, 5, 1–4. Available online: https://dieselnet.com/standards/cn/ld.php (accessed on 1 March 2023).
- Kumar, V.; Kothiyal, N.C.; Saruchi; Mehra, R.; Parkash, A.; Sinha, R.R.; Tayagi, S.K.; Gaba, R. Determination of some carcinogenic PAHs with toxic equivalency factor along roadside soil within a fast developing northern city of India. J. Earth Syst. Sci. 2014, 123, 479–489. [Google Scholar] [CrossRef]
- World Health Organisation. Health Effects of Particulate Matter; World Health Organisation: Geneva, Switzerland, 2013. [Google Scholar]
- Pacura, W.; Szramowiat, K.; Berent, K.; Sławek, A.; Gołaś, J. The possibilities of GPF Surface modification in the aspect of micro-contaminants removal. Energy Rep. 2022, 8, 9261–9269. [Google Scholar] [CrossRef]
- Zvirin, Y.; Gutman, M.; Tartakovsky, L. Fuel Effects on Emissions. Handb. Air Pollut. Intern. Combust. Engines 1998, 16, 547–651. [Google Scholar] [CrossRef]
- Maricq, M.M. Engine, aftertreatment, fuel quality and non-tailpipe achievements to lower gasoline vehicle PM emissions: Literature review and future prospects. Sci. Total Environ. 2023, 866, 161225. [Google Scholar] [CrossRef] [PubMed]
- Lattimore, T.; Herreros, J.M.; Xu, H.; Shuai, S. Investigation of compression ratio and fuel effect on combustion and PM emissions in a DISI engine. Fuel 2016, 169, 68–78. [Google Scholar] [CrossRef]
- Majewski, W.A.; Khair, M.K. Diesel Emissions and Their Control; SAE International: Warrendale, PA, USA, 2006. [Google Scholar]
- Kamela, W.; Wojs, M.K.; Orliński, P. Calculation Method for Assessing the Storage Capacity of Nitrogen Compounds in LNT Reactors. Energies 2022, 15, 7819. [Google Scholar] [CrossRef]
- Vrabie, V.; Scarpete, D.; Zbarcea, O. The new exhaust aftertreatment system for reducing nox emissions OF diesel engines: Lean nox trap (LNT). A study. Trans Motauto World 2016, 1, 35–38. [Google Scholar]
- Wang, Y.; Raman, S.; Grizzle, J.W. Dynamic modeling of a lean NOx trap for lean burn engine control. In Proceedings of the 1999 American Control Conference (Cat. No. 99CH36251), San Diego, CA, USA, 2–4 June 1999; IEEE: New York, NY, USA, 1999; Volume 2, pp. 1208–1212. [Google Scholar]
- Körfer, T.; Schnorbus, T.; Holderbaum, B.; Wittka, T. Advanced, combined exhaust aftertreatment systems for light-duty diesel engines to meet next emission regulations. Intern. Combust. Engines Performance Fuel Econ. Emiss. 2013, 205–217. [Google Scholar] [CrossRef]
- Ren, Y.; Lou, D.; Tan, P.; Zhang, Y.; Sun, X. Emission reduction characteristics of after-treatment system on natural gas engine: Effects of platinum group metal loadings and ratios. J. Clean. Prod. 2021, 298, 126833. [Google Scholar] [CrossRef]
- Cooper, J.; Beecham, J. A study of platinum group metals in three-way autocatalysts. Platin. Met. Rev. 2013, 57, 281–288. [Google Scholar] [CrossRef]
- Yakoumis, I.; Moschovi, A.M.; Giannopoulou, I.; Panias, D. Real life experimental determination of platinum group metals content in automotive catalytic converters. IOP Conf. Ser. Mater. Sci. Eng. 2018, 329, 1–13. [Google Scholar] [CrossRef]
- Kim, B.S.; Jeong, H.; Bae, J.; Kim, P.S.; Kim, C.H.; Lee, H. Lean NOx trap catalysts with high low-temperature activity and hydrothermal stability. Appl. Catal. B Environ. 2020, 270, 118871. [Google Scholar] [CrossRef]
- Clairotte, M.; Adam, T.W.; Zardini, A.A.; Manfredi, U.; Martini, G.; Krasenbrink, A.; Vicet, A.; Tournié, E.; Astorga, C. Effects of low temperature on the cold start gaseous emissions from light duty vehicles fuelled by ethanol-blended gasoline. Appl. Energy 2013, 102, 44–54. [Google Scholar] [CrossRef]
- Barreau, M.; Courtois, X.; Can, F. Selective catalytic reduction of NO at low temperature using a (ethanol+ammonia) mixture over a Ag/Al2O3 + WO3/Cex-ZryO2 dual-bed catalytic system: Reactivity insight of WO3/Cex-ZryO2. Catal. Today 2020, 355, 375–384. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Lee, S.; Choi, H.; Min, K. Characteristics of NOx emission of light-duty diesel vehicle with LNT and SCR system by season and RDE phase. Sci. Total Environ. 2021, 782, 146750. [Google Scholar] [CrossRef]
- Shelef, M.; McCabe, R. Twenty-five years after introduction of automotive catalysts: What next? Catal. Today 2000, 62, 35–50. [Google Scholar] [CrossRef]
- Czerwinski, J.; Comte, P.; Heeb, N.; Mayer, A.; Hensel, V. Nanoparticle Emissions of DI Gasoline Cars with/without GPF. SAE Tech. Pap. 2017, 1004, 1–12. [Google Scholar] [CrossRef]
- Van Nieuwstadt, M.; Ulrey, J. Control Strategies for Gasoline Particulate Filters. SAE Tech. Pap. Ser. 2017, 1, 1–5. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology, Properties Behavior and Measurement of Airborne Particles; John Wiley & Sons, Inc: Hoboken, NJ, USA, 1999; pp. 1–448. ISBN 978-1-119-49404-1. [Google Scholar]
- AVL. AVL Powertrain Engineering Techday # 4. Gasoline Particle Filter Development and Calibration. AVL Experience on GPF System. 2017. Available online: https://www.avl.com/documents/5490654/6605734/AVL+PTE+Techday+%234_02_GPF+Development+and+Calibration_GWolbank.pdf (accessed on 1 March 2023).
- Coulet, B.; Rose, D.; Boger, T.; Glasson, T. Gasoline Engines with Particulate Filters Experiences with Accumulation of Ash and Impact on Filter Performance. MTZ Worldw. 2019, 80, 42–47. [Google Scholar] [CrossRef]
- Rubino, L.; Piotr Oles, J.; La Rocca, A. Evaluating Performance of Uncoated GPF in Real World Driving Using Experimental Results and CFD modelling. SAE Tech. Pap. Ser. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Rubino, L.; Thier, D.; Schumann, T.; Guettler, S.; Russ, G. Fundamental Study of GPF Performance on Soot and Ash Accumulation over Artemis Urban and Motorway Cycles—Comparison of Engine Bench Results with GPF Durability Study on Road. SAE Tech. Pap. Ser. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Joshi, A.; Johnson, T.V. Gasoline Particulate Filters—A Review. Emiss. Control Sci. Technol. 2018, 4, 219–239. [Google Scholar] [CrossRef]
- Majewski, W.A. Gasoline particulate filters. Ecopoint Inc. Revis. 2019, 5, 1–23. [Google Scholar]
- Van Nieuwstadt, M.; Shah, A.; Serban, E.; Martin, D. Regeneration Strategies for Gasoline Particulate Filters; SAE International: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Chan, T.W.; Saffaripour, M.; Liu, F.; Hendren, J.; Thomson, K.A.; Kubsh, J.; Brezny, R.; Rideout, G. Characterization of Real-Time Particle Emissions from a Gasoline Direct Injection Vehicle Equipped with a Catalyzed Gasoline Particulate Filter During Filter Regeneration. Emiss. Control Sci. Technol. 2016, 2, 75–88. [Google Scholar] [CrossRef]
- Morgan, C. Platinum Group Metal and Washcoat Chemistry Effects on Coated Gasoline Particulate Filter Design. Johns. Matthey Technol. Rev. 2015, 59, 188–192. [Google Scholar] [CrossRef]
- Arunachalam, H.; Pozzato, G.; Hoffman, M.A.; Onori, S. Modeling the thermal and soot oxidation dynamics inside a ceria-coated gasoline particulate filter. Control Eng. Pract. 2020, 94, 104199. [Google Scholar] [CrossRef]
- Caillaud, S.; Courtois, O.; Delvigne, T.; Hennebert, B. Contribution of Lubricant Additives to Ash Generation on a Close-Coupled GPF. SAE Int. J. Adv. Curr. Pract. Mobil. 2020, 3, 477–484. [Google Scholar] [CrossRef]
- Zuo, Q.; Xie, Y.; Zhu, G.; Wei, K.; Zhang, B.; Chen, W.; Tang, Y.; Wang, Z. Investigations on a new C-GPFs with electric heating for enhancing the integrated regeneration performance under critical parameters. Energy 2021, 225, 120265. [Google Scholar] [CrossRef]
- Jo, S.; Cha, J.; Park, S. Exhaust emission characteristics of stoichiometric combustion applying to diesel particulate filter(DPF) and three-way catalytic converter(TWC). Energy 2022, 254, 124196. [Google Scholar] [CrossRef]
- Galassi, M.C.; Martini, G. Durability Demonstration Procedures of Emission Control Devices for Euro 6 Vehicles. JRC Sci. Policy Rep. 2014, 1, 1–64. [Google Scholar] [CrossRef]
- Pielecha, I.; Cieślik, W.; Borowski, P.; Czajka, J.; Bueschke, W. Reduction of the number of cylinders in internal combustion engines—Contemporary trends in downsizing. Combust. Engines 2014, 159, 12–25. [Google Scholar] [CrossRef]
- Sroka, Z.J. Work Cycle of Internal Combustion Engine Due to Rightsizing. IntechOpen 2021, 1, 1–21. [Google Scholar] [CrossRef]
- Society of Automotive Engineers of Japan Inc. Gasoline engines—2017 Yearbook. J. Soc. Automot. Eng. Jpn. 2017, 71, 1–11. [Google Scholar]
- Brzeżański, M.; Śliwiński, K. Downsizing—A new direction of automobile engine development. Combust. Engines 2004, 119, 3–11. [Google Scholar] [CrossRef]
- Ortiz-Soto, E.; Younkins, M. Advanced Cylinder Deactivation with Miller Cycle. MTZ Worldw. 2019, 80, 58–63. [Google Scholar] [CrossRef]
- Patil, C.; Varade, S.; Wadkar, S. A Review of Engine Downsizing and its Effects. Int. J. Curr. Eng. Technol. 2017, 7, 319–324. [Google Scholar]
- Bueschke, W.; Czajka, J.; Pielecha, I.; Cieślik, W.; Borowski, P.; Wisłocki, K. Doładowanie zakresowe nowoczesnych silników spalinowych. Logistyka 2014, 3, 849–857. [Google Scholar]
- Bor, M.; Idzior, M.; Karpiuk, W.; Smolec, R. Possibilities of development of internal combustion engines, including downsizing and rightsizing strategy. AUTOBUSY—Tech. Eksploat. Syst. Transp. 2019, 24, 155–160. [Google Scholar] [CrossRef]
- SROKA, Z.; DWORACZYŃSKI, M. Assessment of thermodynamic cycle of internal combustion engine in terms of rightsizing. Combust. Engines 2019, 178, 182–186. [Google Scholar] [CrossRef]
- ASTM D2699-23; American Society for Testing and Materials ASTM D2699-23 Standard Test Method for Research Octane Number of Spark-Ignition Engine Fuel. ASTM: West Conshohocken, PA, USA, 2023; pp. 1–48. [CrossRef]
- ASTM D2700-23; Standard Test Method for Motor Octane Number of Spark-Ignition Engine Fuel. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023; Volume 05.05, pp. 1–59. [CrossRef]
- Rosenblatt, D.; Karman, D. GDI Engines and Alcohol Fuels. IEA-AMF Reports. 2020; pp. 1–38. Available online: https://www.iea-amf.org/app/webroot/files/file/Annex%20Reports/AMF_Annex_54.pdf (accessed on 25 April 2023).
- Sayin, C.; Kilicaslan, I.; Canakci, M.; Ozsezen, N. An experimental study of the effect of octane number higher than engine requirement on the engine performance and emissions. Appl. Therm. Eng. 2005, 25, 1315–1324. [Google Scholar] [CrossRef]
- Karavalakis, G.; Short, D.; Vu, D.; Russell, R.; Hajbabaei, M.; Asa-Awuku, A.; Durbin, T.D. Evaluating the effects of aromatics content in gasoline on gaseous and particulate matter emissions from SI-PFI and SIDI vehicles. Environ. Sci. Technol. 2015, 49, 7021–7031. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, K.; Jetter, J.J. Impact of gasoline composition on particulate matter emissions from a direct-injection gasoline engine: Applicability of the particulate matter index. Int. J. Engine Res. 2014, 15, 298–306. [Google Scholar] [CrossRef]
- Hutchison, B.R.M.; Wallace, J.S. Influence of fuel volatility on particulate matter emissions from a production DISI engine. Fuel 2021, 303, 121206. [Google Scholar] [CrossRef]
- Therasme, O.; Volk, T.A.; Eisenbies, M.H.; Amidon, T.E.; Fortier, M.O. Life cycle greenhouse gas emissions of ethanol produced via fermentation of sugars derived from shrub willow (Salix ssp.) hot water extraction in the Northeast United States. Biotechnol. Biofuels 2021, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, C.; Durbin, T.D.; Johnson, K.C.; Karavalakis, G. The effect of ethanol and iso-butanol blends on polycyclic aromatic hydrocarbon (PAH) emissions from PFI and GDI vehicles. Atmos. Pollut. Res. 2020, 11, 2056–2067. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Liu, D. Effects of oxygenated biofuel additives on soot formation: A comprehensive review of laboratory-scale studies. Fuel 2022, 313, 122635. [Google Scholar] [CrossRef]
- Kruczyński, S.; Gis, W.; Orliński, P.; Sikora, M. Influence of the use of ethanol fuel on selected parameters of the gasoline engine. IOP Conf. Ser. Mater. Sci. Eng. 2018, 421, 042041. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, S.; Undavalli, V.K.; Khandelwal, B.; Kumar, S. Unregulated emissions from oxygenated fuels. In Advancement in Oxygenated Fuels for Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2023; pp. 221–240. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, S.; Awadh, B.; Khandelwal, B. Applicability of aromatic selection towards newer formulated fuels for regulated and unregulated emissions reduction in CI engine. Fuel Process. Technol. 2020, 209, 106548. [Google Scholar] [CrossRef]
- Rodriguez, F.; Dornoff, J. Beyond NOx: Emission of unregulated pollutants from a modern gasoline car. Int. Counc. Clean Transp. 2019, 1–37. Available online: https://theicct.org/wp-content/uploads/2021/06/NOx_Pollutants_LDV_FV_20190503_0.pdf (accessed on 2 May 2023).
- Magnusson, R.; Nilsson, C.; Andersson, B. Emissions of Aldehydes and Ketones from a Two-Stroke Engine Using Ethanol and Ethanol-Blended Gasoline as Fuel. Environ. Sci. Technol. 2002, 36, 1656–1664. [Google Scholar] [CrossRef]
- Zervas, E.; Montagne, X.; Lahaye, J. C1—C5 organic acid emissions from an SI engine: Influence of fuel and air/fuel equivalence ratio. Environ. Sci. Technol. 2001, 35, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Bock, N.R.H.; Baum, M.M.; Moss, J.A.; Castonguay, A.E.; Jocic, S.; Northrop, W.F. Dicarboxylic acid emissions from a GDI engine equipped with a catalytic gasoline particulate filter. Fuel 2020, 275, 117940. [Google Scholar] [CrossRef]
- Leach, F.; Chapman, E.; Jetter, J.J.; Rubino, L.; Christensen, E.D.; St. John, P.C.; Fioroni, G.M.; McCormick, R.L. A Review and Perspective on Particulate Matter Indices Linking Fuel Composition to Particulate Emissions from Gasoline Engines. SAE Int. J. Fuels Lubr. 2021, 15, 4–15. [Google Scholar] [CrossRef]
- Crawford, R.; Lyons, J. An Improved Index for Particulate Matter Emissions (PME) CRC Report No. RW-107-2. Coordinating Research Council. 2021; pp. 1–122. Available online: https://crcao.org/wp-content/uploads/2021/03/CRC_RW107-2_2021.03.26.pdf (accessed on 2 May 2023).
- Pirjola, L.; Karjalainen, P.; Heikkilä, J.; Saari, S.; Tzamkiozis, T.; Ntziachristos, L.; Kulmala, K.; Keskinen, J.; Rönkkö, T. Effects of fresh lubricant oils on particle emissions emitted by a modern gasoline direct injection passenger car. Environ. Sci. Technol. 2015, 49, 3644–3652. [Google Scholar] [CrossRef] [PubMed]
- Piekoszewski, W.; Tuszyński, W. Właściwości przeciwzużyciowe, przeciwzatarciowe i trwałość zmęczeniowa węzła tarcia jako efekt rodzaju i stężenia dodatków smarnościowych w oleju. Tribologia 2003, 187, 203–220. [Google Scholar]
- West, B.; Sluder, C.S. Lubricating Oil Consumption on the Standard Road Cycle. SAE Tech. Pap. 2013, 0884, 1–8. [Google Scholar] [CrossRef]
- Young, J. Chapter 8 Corrosion by Sulfur. In Corrosion Series; Elsevier: Amsterdam, The Netherlands, 2008; pp. 361–396. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Sjöberg, M.; Vuilleumier, D.; Ding, C.; Liu, F.; Li, X. Impact of coolant temperature on piston wall-wetting and smoke generation in a stratified-charge DISI engine operated on E30 fuel. Proc. Combust. Inst. 2019, 37, 4955–4963. [Google Scholar] [CrossRef]
- Wu, C.; Chen, R.; Pu, J.; Lin, T. The influence of air—Fuel ratio on engine performance and pollutant emission of an SI engine using ethanol—Gasoline-blended fuels. Atmos. Environ. 2004, 38, 7093–7100. [Google Scholar] [CrossRef]
- Price, P.; Twiney, B.; Stone, R.; Kar, K.; Walmsley, H. Particulate and Hydrocarbon Emissions from a Spray Guided Direct Injection Spark Ignition Engine with Oxygenate Fuel Blends. SAE Int. 2007, 0472, 776–790. [Google Scholar] [CrossRef]
- Sharma, N.; Agarwal, A.K. Gasoline Direct Injection Engines and Particulate Emissions. In Air Pollution and Control; Springer: Singapore, 2018; pp. 87–105. [Google Scholar] [CrossRef]
- Qin, J.; Li, X.; Pei, Y. Effects of Combustion Parameters and Lubricating Oil on Particulate Matter Emissions from a Turbo-Charged GDI Engine Fueled with Methanol/Gasoline Blends. SAE Tech. Pap. 2014, 2841, 1–12. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, T.; Fu, J.; Dai, H.; Liu, J. Experimental study on the effects of injection parameters and exhaust gas recirculation on combustion, emission and performance of Atkinson cycle gasoline direct-injection engine. Energy 2022, 238, 121784. [Google Scholar] [CrossRef]
- Merkisz, J.; Pielecha, J. Nanoparticle Emissions from Combustion Engines; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Sher, E. Handbook of Air Pollution From Internal Combustion Engines; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 9780126398557. [Google Scholar]
- Cieslik, W.; Borowski, P.; Pielecha, I.; Bueschke, W.; Czajka, J.; Wisłocki, K. Systemy recyrkulacji spalin we wspó ł czesnych konstrukcjach silnikowych. Logistyka 2014, 3, 1118–1127. [Google Scholar]
- Brzeżański, M. Emisja Toksycznych Składników Spalin w Fazie Nagrzewania Sie Silnika o Zapłonie Iskrowym z Zastosowaniem Akumulatora Ciepła; Seria Mechanika – Wydawnictwo Politechniki Poznańskiej: Krakow, Poland, 2006; Volume 1, pp. 1–150. ISBN 0860-097X. [Google Scholar]
- Pielecha, J.; Skobiej, K.; Gis, M.; Gis, W. Particle Number Emission from Vehicles of Various Drives in the RDE Tests. Energies 2022, 15, 6471. [Google Scholar] [CrossRef]
- Laskowski, P.; Zasina, D.; Zimakowska-Laskowska, M.; Orliński, P. Modelling Hydrocarbons Cold-Start Emission from Passenger Cars. Adv. Sci. Technol. Res. J. 2021, 15, 117–125. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Valverde, V.; Kontses, A.; Suarez-Bertoa, R.; Selleri, T.; Melas, A.; Otura, M.; Ferrarese, C.; Martini, G.; Balazs, A.; et al. Effect of extreme temperatures and driving conditions on gaseous pollutants of a euro 6d-temp gasoline vehicle. Atmosphere 2021, 12, 1011. [Google Scholar] [CrossRef]
- Engelmann, D.; Zimmerli, Y.; Czerwinski, J.; Bonsack, P. Real driving emissions in extended driving conditions. Energies 2021, 14, 7310. [Google Scholar] [CrossRef]
- Brzeżański, M.; Rybarz, M. ZAGADNIENIA TWORZENIA SIĘ KONDENSATU W UKŁADZIE WYLOTOWYM SILNIKA SPALINOWEGO. Czas. Tech. Mech.—Wydaw. Politech. Krak. 2008, 8-M, 209–218. [Google Scholar]
- Fonseca, N.; Casanova, J.; Valdés, M. Influence of the stop/start system on CO2 emissions of a diesel vehicle in urban traffic. Transp. Res. Part D Transp. Environ. 2011, 16, 194–200. [Google Scholar] [CrossRef]
- Hoffman, J.; Neumann, T.; Jeworutzken, K.; Schutz, M. Electrification Start/Stop System in the Porsche Panamera Development Objectives. Electrification 2010, 112, 4–11. [Google Scholar]
- Silva, S.F.; Eckert, J.J.; Silva, F.L.; de Alkmin Silva, L.C.; Dedini, F.G. Modelagem e simulação de um sistema start/stop para redução de consumo de combustível e emissões de gases poluentes. Bulcher Eng. Proc. 2021, 8, 486–498. [Google Scholar] [CrossRef]
- Storey, J.M.; Moses-DeBusk, M.; Huff, S.; Thomas, J.; Eibl, M.; Li, F. Characterization of GDI PM during Vehicle Start-Stop Operation. SAE Int. Powertrains Fuels Lubr. Meet. 2019, 50, 1–9. [Google Scholar] [CrossRef]
- Lutwyche, G. Material Innovations That Are Making Vehicles Lighter; Grainger & Worrall: Bridgnorth, UK, 2021; Volume XI, pp. 1–3. [Google Scholar]
- Chau, K.T. Pure electric vehicles. In Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 655–684. [Google Scholar] [CrossRef]
- Piechna, J. A Review of Active Aerodynamic Systems for Road Vehicles. Energies 2021, 14, 7887. [Google Scholar] [CrossRef]
- Sivak, M.; Schoettle, B. Eco-driving: Strategic, tactical, and operational decisions of the driver that influence vehicle fuel economy. Transp. Policy 2012, 22, 96–99. [Google Scholar] [CrossRef]
- Alessandrini, A.; Cattivera, A.; Filippi, F.; Ortenzi, F. Driving Style Influence on Car CO2 Emissions; U.S. Environmental Protection Agency: Washington, DC, USA, 2012; Volume 8, pp. 1–6. Available online: https://www3.epa.gov/ttn/chief/conference/ei20/session8/acattivera.pdf (accessed on 16 May 2023).
- Huang, Y.; Ng, E.C.Y.; Zhou, J.L.; Surawski, N.C.; Chan, E.F.C.; Hong, G. Eco-driving technology for sustainable road transport: A review. Renew. Sustain. Energy Rev. 2018, 93, 596–609. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Welstand, J.S. Vehicle emissions and air quality: The early years (1940s–1950s). Atmosphere 2021, 12, 1354. [Google Scholar] [CrossRef]
- Magill, P.; Hutchinson, D.; Stormes, J. Hydrocarbon Constituents of Automobile Exhaust Gases. In Proceedings of the Second National Air Pollution Symposium, Menlo Park, CA, USA, 5–6 May 1952. [Google Scholar]
- Ligterink, N.; Mensch, P.; Cuelenaere, R.; Hausberger, S.; Leitner, D.; Silberholz, G. Correction algorithms for WLTP chassis dynamometer and coast-down testing. Earth Life Soc. Sci. 2015, R10955, 1–73. [Google Scholar]
- Šarkan, B.; Skrúcaný, T.; Semanová, Š.; Madleňák, R.; Kuranc, A.; Sejkorová, M.; Caban, J. Vehicle Coast-Down Method As a Tool for Calculating Total Resistance for the Purposes of Type-Approval Fuel Consumption. Sci. J. Silesian Univ. Technol. Ser. Transp. 2018, 98, 161–172. [Google Scholar] [CrossRef]
- European Comission. DYREKTYWA 98/69/WE PARLAMENTU EUROPEJSKIEGO I RADY z dnia 13 października 1998 r. odnosząca się do środków mających zapobiegać zanieczyszczeniu powietrza przez emisje z pojazdów silnikowych i zmieniająca dyrektywę Rady 70/220/EWG. Dz. URZĘDOWY WSPÓLNOT Eur. Eur. Union Belg. Bruss. 1999, L350/1, 1–56. Available online: https://sip.lex.pl/akty-prawne/dzienniki-UE/dyrektywa-98-69-we-odnoszaca-sie-do-srodkow-majacych-zapobiegac-67455602 (accessed on 23 May 2023).
- European Comission. Regulation (EU) No 168/2013 of The European Parliament and of the Council of 15 January 2013 on the approval and market surveillance of two- or three-wheel vehicles and quadricycles. Off. J. Eur. Union 2013, 60/52, 1–77. [Google Scholar]
- UNECE. Concerning the Adoption of Uniform Technical Prescriptions for Wheeled Vehicles, Equipment and Parts which can be fitted and/or be used on Wheeled Vehicles and the Conditions for Reciprocal Recognition of Approvals Granted on the Basis of these Prescriptions; UN Digital Library: New York, NY, USA, 2013; Volume 101, pp. 1–100. Available online: https://unece.org/fileadmin/DAM/trans/main/wp29/wp29regs/2015/R101r3e.pdf (accessed on 23 May 2023).
- Barlow, T.; Latham, S.; Mccrae, I.; Boulter, P. A Reference Book of Driving Cycles for Use in the Measurement of Road Vehicle Emissions; TRL: Wokingham, UK, 2009; Volume 4, pp. 1–280. [Google Scholar]
- Dornoff, J.; Tietge, U.; Mock, P. ON THE WAY TO “REAL-WORLD” CO2 VALUES: THE EUROPEAN PASSENGER CAR MARKET IN ITS FIRST YEAR AFTER INTRODUCING THE WLTP. ICCT White Paper. 2020. Available online: https://theicct.org/publication/on-the-way-to-real-world-co2-values-the-european-passenger-car-market-in-its-first-year-after-introducing-the-wltp/ (accessed on 23 May 2023).
- Tsiakmakis, S.; Fontaras, G.; Cubito, C.; Pavlovic, J.; Anagnostopoulos, K.; Ciuffo, B. From NEDC to WLTP: Effect on the Type-approval CO2 Emissions of Light-Duty Vehicles; Publications Office of the European Union: Belgium, Brussels, 2017; 50p. [Google Scholar] [CrossRef]
- Fontaras, G.; Zacharof, N.G.; Ciuffo, B. Fuel consumption and CO2 emissions from passenger cars in Europe—Laboratory versus real-world emissions. Prog. Energy Combust. Sci. 2017, 60, 97–131. [Google Scholar] [CrossRef]
- Galindo, E.; Blanco, D.; Brace, C.J.; CHappell, E.; Burke, R. Chassis Dynamometer Testing: Addressing the Challenges of New Global Legislation; SAE International: Warrendale, PA, USA, 2017; ISBN 978-0768082784. [Google Scholar]
- European Federation for Transport and Environment. WHO Adds Pressure for Stricter Euro-5 Standards. 2006. Available online: https://www.transportenvironment.org/discover/who-adds-pressure-stricter-euro-5-standards/ (accessed on 30 May 2023).
- European Commission and Council of the European Union Commission Regulation (EU) 2016/427 amending Regulation (EC) No 692/2008 as regards emissions from light passenger and commercial vehicles (Euro 6) (Text with EEA relevance). Off. J. Eur. Union 2016, 82, 1–98.
- European Comission Commission Regulation (EU) 2016/646 of 20 April 2016 amending Regulation (EC) No 692/2008 as regards emissions from light passenger and commercial vehicles (Euro 6). Off. J. Eur. Union 2016, 109, 1–22.
- Andrych-Zalewska, M.; Chlopek, Z.; Merkisz, J.; Pielecha, J. Comparison of Gasoline Engine Exhaust Emissions of a Passenger Car through the WLTC and RDE Type Approval Tests. Energies 2022, 15, 8157. [Google Scholar] [CrossRef]
- Weiss, M.; Paffumi, E.; Clairotte, M.; Drossinos, Y.; Vlachos, T.; Joint Research Centre (European Commission). Including Cold-Start Emissions in the Real-Driving Emissions (RDE) Test Procedure Effects; Publications Office of the European Union: Belgium, Brussels, 2017. [Google Scholar] [CrossRef]
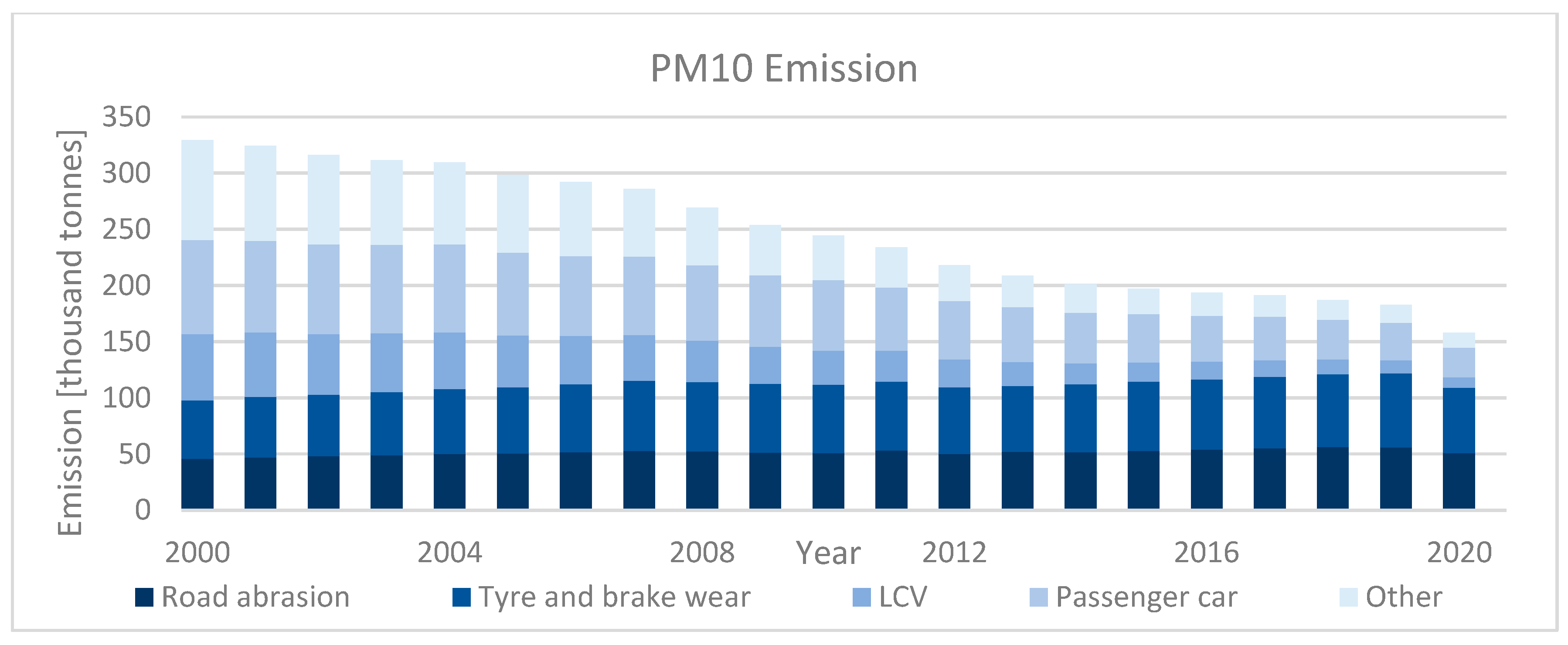
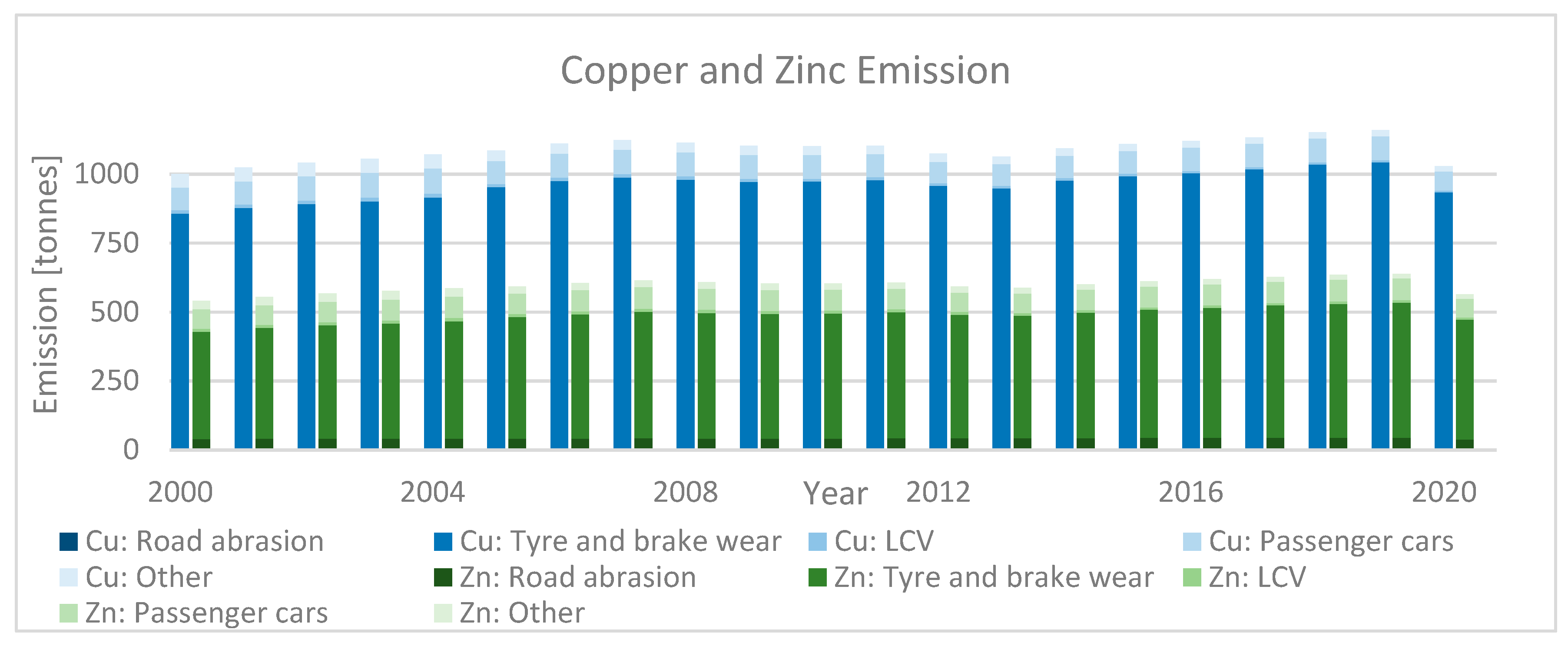
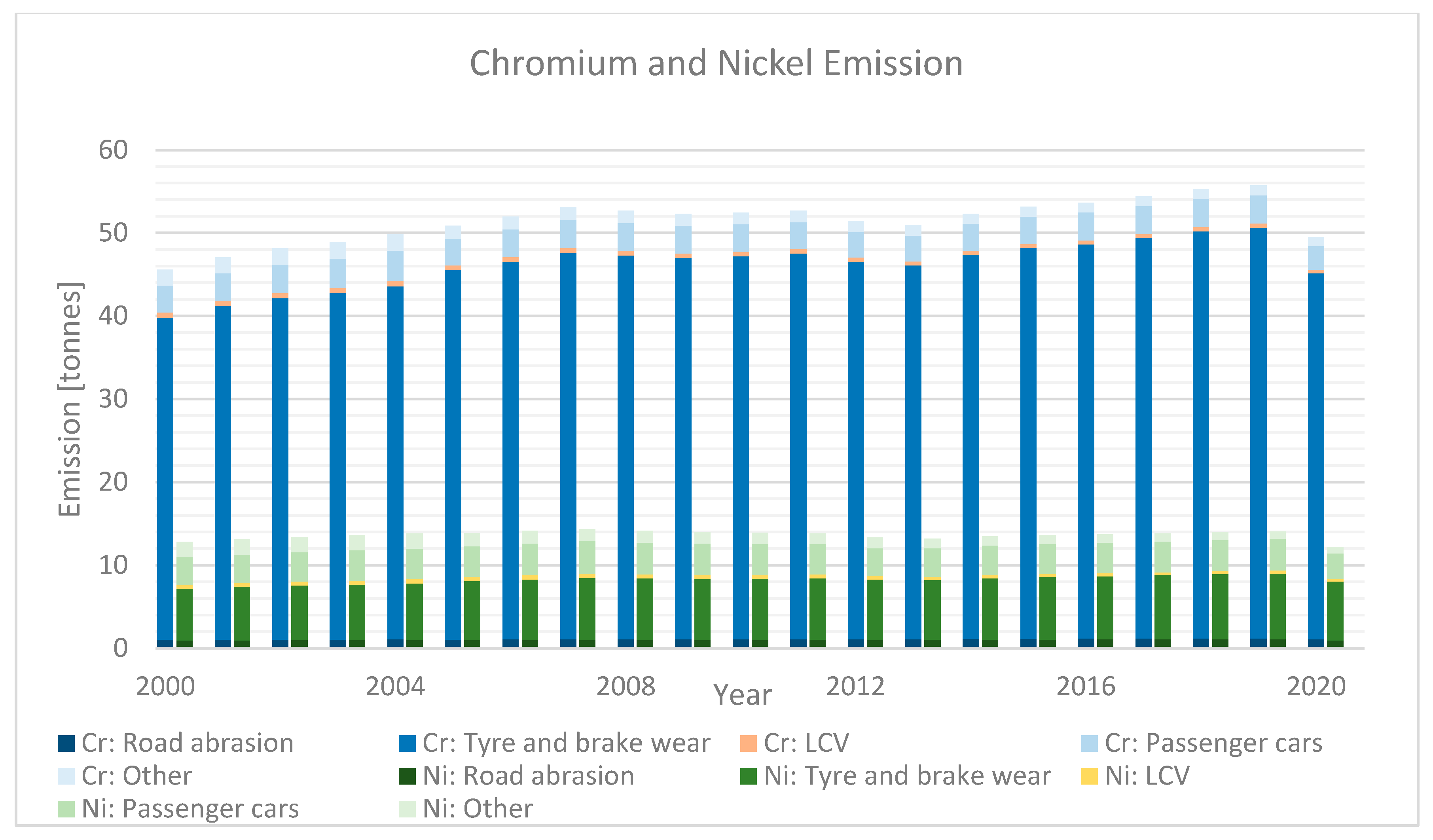
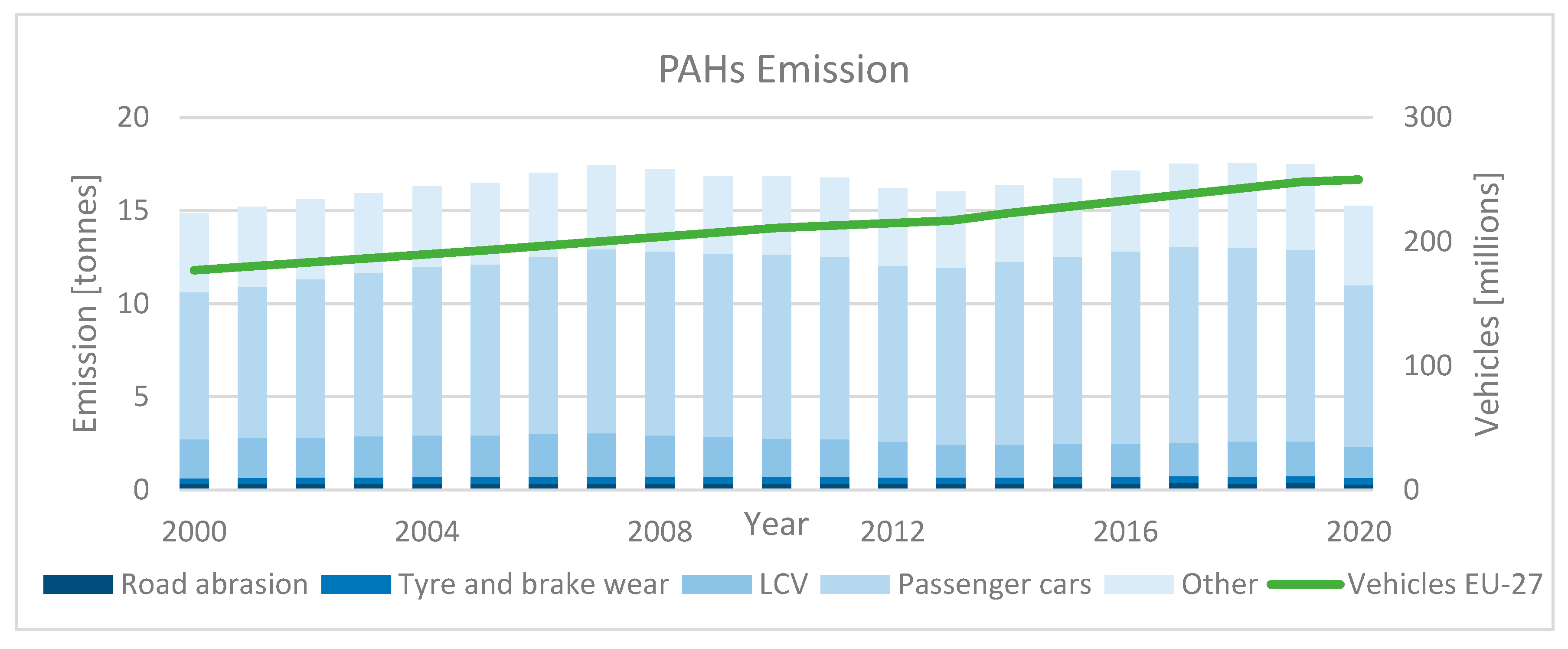

| Category | Description | |
|---|---|---|
| M | Motor vehicles intended for the transport of persons and luggage with at least four wheels. | |
| M1 | With up to 8 seats in addition to the driver’s seat | |
| M2 | With more than 8 seats, weighing less than 5 tonnes | |
| M3 | With more than 8 seats, weighing more than 5 tonnes | |
| N | Motor vehicles designed to transport cargo | |
| N1 | Weighing less than 3.5 tonnes | |
| N2 | Over 3.5 tonnes and under 12 tonnes | |
| N3 | Weighing more than 12 tonnes | |
| O | Trailers designed to transport cargo, people, or residential purposes | |
| O1 | Weighing less than 750 kg | |
| O2 | Weighing more than 750 kg, less than 3.5 tonnes | |
| O3 | Weighing more than 3.5 tonnes, less than 10 tonnes | |
| O4 | Weighing more than 10 tonnes | |
| L | Two- or three-wheeled vehicles and some four-wheeled vehicles | |
| T | Agricultural tractors | |
| G | Off-road vehicles | |
| R | Agricultural trailers | |
| CO | HC | NMHC | NOx | HC+ NOx | PM | PN | CO2 (a) | |
|---|---|---|---|---|---|---|---|---|
| [g/km] | [g/km] | [g/km] | [g/km] | [g/km] | [g/km] | [#/km] | [g/km] | |
| SI | 1.0 | 0.10 | 0.068 | 0.060 | - | 0.0045 | 6 × 1011 (b) | 95 |
| CI | 0.5 | - | - | 0.080 | 0.170 | 0.0045 | 6 × 1011 | 95 |
| Name | Possible Harmful Effects | Source |
|---|---|---|
| PAHs | PAHs affect the reproductive system and make it difficult to maintain a pregnancy. The offspring can also experience the same effects while showing much more frequent defects at birth and lower body weight. These compounds are carcinogenic. | [59,60,61] |
| Oxidized PAHs | ||
| 1,4-Naphthoquinone | Toxic by inhalation, irritating the eyes, respiratory system, and skin. Toxic to aquatic organisms. | [62,63] |
| Nitro-PAH | ||
| 6-Nitro chrysene | It has a hundred times greater carcinogenic potential than chrysene. The presence of the NO2− group destroys and hinders DNA repair. | [59,63,64,65] |
| Anions | ||
| [F ] Fluorides | Fluoride may cause irritation to the respiratory system, skin, and eyes. | [66] |
| [Cl−] Chlorides | The presence of chlorides may lead to irritation of the eyes and respiratory system. | [59] |
| [Br−] Bromides | Bromine compounds can cause restlessness, confusion, stupor, nausea, vomiting, and skin conditions such as angiomas and rashes (bromism). | [59] |
| [HCOO−] Formate | Formates can cause hypoxia at the cellular level and metabolic acidosis. | [67] |
| [NO2−] Nitrites | Excess can cause low blood pressure, accelerated pulse, headaches, stomach cramps, vomiting, and thyroid damage. | [59] |
| [NO3−] Nitrates | Excess can cause low blood pressure, accelerated pulse, headaches, stomach cramps, vomiting, and thyroid damage. | [59] |
| [PO4 3−] Phosphates | Due to the high retention in the body, phosphates can lead to cell and tissue damage and negatively affect the kidneys, and circulatory and reproductive systems. | [68] |
| [SO4 2−] Sulphur | Sulphates can aggravate asthma by limiting lung function and have a negative effect on the heart. | [69] |
| Metals | ||
| [Al] Aluminium | Excess can lead to respiratory problems and neurological diseases. | [59,66] |
| [Ba] Bar | Consuming barium-contaminated water can cause digestive problems, muscle weakness, and kidney damage. | [59] |
| [Bi] Bismuth | Excess bismuth can cause damage to the kidneys, brain, and bone tissue. | [59] |
| [Ca] Calcium | Responsible for the carbonate hardness of water; in combination with sulphate, it forms gypsum. | [70] |
| [Co] Cobalt | In high concentrations, it is harmful to the respiratory and haematological systems. It exhibits carcinogenic properties. | [59,66] |
| [Cr] Chromium | May be carcinogenic and cause ulceration of the respiratory tract and skin. | [66] |
| [Cu] Copper | It can be accumulated by plants. In high doses, it can cause vomiting, diarrhoea, and abdominal pain. Continued exposure may cause kidney and liver damage. | [59,66] |
| [Fe] Iron | Excess iron can cause abdominal pain, diarrhoea, vomiting, metabolic acidosis, liver damage, and cardiac collapse. Iron poisoning can be fatal in children. | [66] |
| [K] Potassium | Forms strongly basic potassium hydroxide | [70] |
| [Mg] Magnesium | Responsible for the carbonate hardness of water; magnesium oxide and sulphate have laxative properties | [66,70] |
| [Mn] Manganese | Excess manganese negatively affects the nervous system, can cause personality changes, movement and reproductive system disorders, and is irritating to the lungs. | [59,66] |
| [Na] Sodium | Forms strongly alkaline sodium hydroxide, excess leads to hypernatremia. | [70] |
| [Nb] Niobium | As dust can irritate the eyes and skin, niobium nitrate can cause permanent lung damage. | [71] |
| [Ni] Nickel | It causes an allergic skin reaction in 10–20% of the population. Inhalation of nickel may cause reduced lung capacity and bronchitis. | [59,66] |
| [P] Phosphorus | Phosphorus compounds can cause nausea, abdominal pain and drowsiness, and long exposure can cause osteoporosis and kidney damage. | [59,71] |
| [S] Sulphur | Sulphur compounds can damage the circulatory and immune systems, cause skin and eye irritation, and have a negative effect on the lungs | [71] |
| [Sb] Antimony | Inhalation of antimony-containing dust can cause abdominal pain, vomiting, diarrhoea, lung irritation, and stomach ulcers. | [59] |
| [Si] Silicon | Inhalation of silicon-containing dust may cause respiratory irritation. | [59] |
| [Ti] Titan | Titanium can accumulate in the lungs, reducing their capacity; titanium dioxide can cause oxidative DNA damage. | [59] |
| [Zn] Zinc | Excess can cause stomach cramps, nausea, and vomiting. Long exposure can cause anaemia. | [59,66] |
| [Zr] Zircon | May be irritating to eyes, skin, and lungs. Prolonged exposure may cause allergic reactions and cocci. | [72] |
| Microcontaminants | Organic | Inorganic |
|---|---|---|
| Example | Polycyclic aromatic hydrocarbons and their derivatives. | Elements, metals, and salts. |
| Removal | Oxidation—also catalytic | None a creation of ash deposits |
| Extraction method | In organic solvents, e.g., cyclohexane | In acid solutions, e.g., methylsulphonic acid, HNO3 |
| Analytical techniques | GC-MS | IC, MP-AES |
| Test Object | Emission Standard of the Test Object | Sample Source | Identified Microcontaminants |
|---|---|---|---|
| Pacura et al., 2022 [45] | |||
| DISI vehicles from 2017 and 2019 | Euro 6b–Euro 6d-TEMP | PM from WLTC tests | PAHs, PAHs derivatives, metals, anions +TEQ |
| Zhao et al., 2020 [47] | |||
| PFI and DISI vehicles from 2000–2018 | China 1–China 5 | PM from UDC tests | PAHs, PAHs derivatives +TEQ |
| Lin et al., 2019 [89] | |||
| Gasoline LDV from 2004–2015 | Euro 3–Euro 6 | PM from NEDC tests | PAHs |
| Muñoz et al., 2018 [90] | |||
| DISI vehicles | Euro 3–Euro 6 | PM from WLTC tests | PAHs, alkyl-PAHs, soot +TEQ |
| Jakober et al., 2008 [91] | |||
| Gasoline LDV from 1991–2002 | PM and gas-phase carbonyls from FTP tests | Aldehydes, ketones, dicarbonyls | |
| Riddle et al., 2007 [92] | |||
| Gasoline LDV from 1991–2002 | PM from FTP tests | PAHs | |
| Oda et al., 1998 [93] | |||
| Gasoline LDV (pre–1998) | PM scrapped from exhaust pipe | PAHs, PAHs derivatives | |
| Unit | RON | MON | |
|---|---|---|---|
| Standard | – | ASTM D2699 | ASTM D 2700 |
| Engine rotation | rpm | 600 ± 6 | 900 ± 9 |
| Opening the intake valve | °CA | 10 | |
| Closing the intake valve | 214 ± 2.5 | ||
| Ignition angle | 347 | 334 | |
| Opening the outlet valve | 500 | ||
| Closing the discharge valve | 735 ± 2.5 | ||
| oil temperature | °C | 57 ± 8 | |
| Air temperature | 52 ± 1 | 38 ± 2.8 | |
| Air humidity | gH2O/kg air | 3.56–7.12 | |
| Additive | MeOH | EtOH | MTBE | ETBE | TAME | TAEE | DIPE |
|---|---|---|---|---|---|---|---|
| RON | 131 | 128 | 119 | 114 | 113 | 112 | 105 |
| MON | 102 | 103 | 101 | 99 | 100 | 93 | 98 |
| EGR [%] | NOx [g/h] | CO [g/h] | HC [g/h] |
|---|---|---|---|
| 0 | 115 | 21 | 5 |
| 10 | 80 | 21 | 5 |
| 20 | 35 | 25 | 6 |
| 40 | 6 | 120 | 18 |
| Selected Parameters of Driving Cycles | |||||
|---|---|---|---|---|---|
| Test | NEDC | WLTC 3b | WLTC 3a | WLTC 2 | WTLC 1 |
| Parameters of the Tested Vehicle | |||||
| Power to weight (W/kg) | – | >34 | >34 | 22–34 | ≤22 |
| Max. speed of vehicle (km/h) | – | ≥120 | <120 | – | – |
| Test Parameters | |||||
| Time (s) | 1180 | 1800 | 1800 | 1800 | 1611 |
| Distance (km) | 10.93 | 23.27 | 23.19 | 22.65 | 11.43 |
| Average speed with stops (km/h) | 33.35 | 46.53 | 46.40 | 45.31 | 25.56 |
| Average speed without stops (km/h) | 43.10 | 51.35 | 51.17 | 50.00 | 32.17 |
| Maximum speed (km/h) | 120.0 | 131.3 | 131.3 | 123.1 | 64.4 |
| Maximum acceleration (m × s−2) | 1.04 | 1.58 | 1.58 | 0.96 | 0.76 |
| Stop duration (%) | 22.6 | 13.4 | 13.4 | 13.3 | 22.1 |
| Total stop time (s) | 267 | 242 | 242 | 240 | 356 |
| Road Requirements | ||
|---|---|---|
| Duration | 90–120 min | |
| Distance | Urban | >16 km each |
| Rural | ||
| Motorway | ||
| Trip composition | Urban | 29–44% of the total distance |
| Rural | 23–43% of the total distance | |
| Motorway | ||
| Average speed | Urban | 15–40 km/h |
| Rural | 60–90 km/h | |
| Motorway | >90 km/h >100 km/h for min. 5 min | |
| Boundary Conditions of A Correct Test | ||
| Payload | ≤90% max. weight | |
| Altitude | Moderate a | 0–700 m above sea level |
| Extended b | 700–1300 m above sea level | |
| Altitude difference | <100 m (start–finish) | |
| Cumulative altitude gain | 1200 m/100 km | |
| Ambient temperature | Moderate a | 0–30 °C |
| Extended b | −7–0 °C; 30–35 °C | |
| Use of auxiliary systems | Used as in real life | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacura, W.; Szramowiat-Sala, K.; Gołaś, J. Emissions from Light-Duty Vehicles—From Statistics to Emission Regulations and Vehicle Testing in the European Union. Energies 2024, 17, 209. https://doi.org/10.3390/en17010209
Pacura W, Szramowiat-Sala K, Gołaś J. Emissions from Light-Duty Vehicles—From Statistics to Emission Regulations and Vehicle Testing in the European Union. Energies. 2024; 17(1):209. https://doi.org/10.3390/en17010209
Chicago/Turabian StylePacura, Wiktor, Katarzyna Szramowiat-Sala, and Janusz Gołaś. 2024. "Emissions from Light-Duty Vehicles—From Statistics to Emission Regulations and Vehicle Testing in the European Union" Energies 17, no. 1: 209. https://doi.org/10.3390/en17010209
APA StylePacura, W., Szramowiat-Sala, K., & Gołaś, J. (2024). Emissions from Light-Duty Vehicles—From Statistics to Emission Regulations and Vehicle Testing in the European Union. Energies, 17(1), 209. https://doi.org/10.3390/en17010209









