Hydrogen Production from Supercritical Water Gasification of Model Compounds of Crude Glycerol from Biodiesel Industries
Abstract
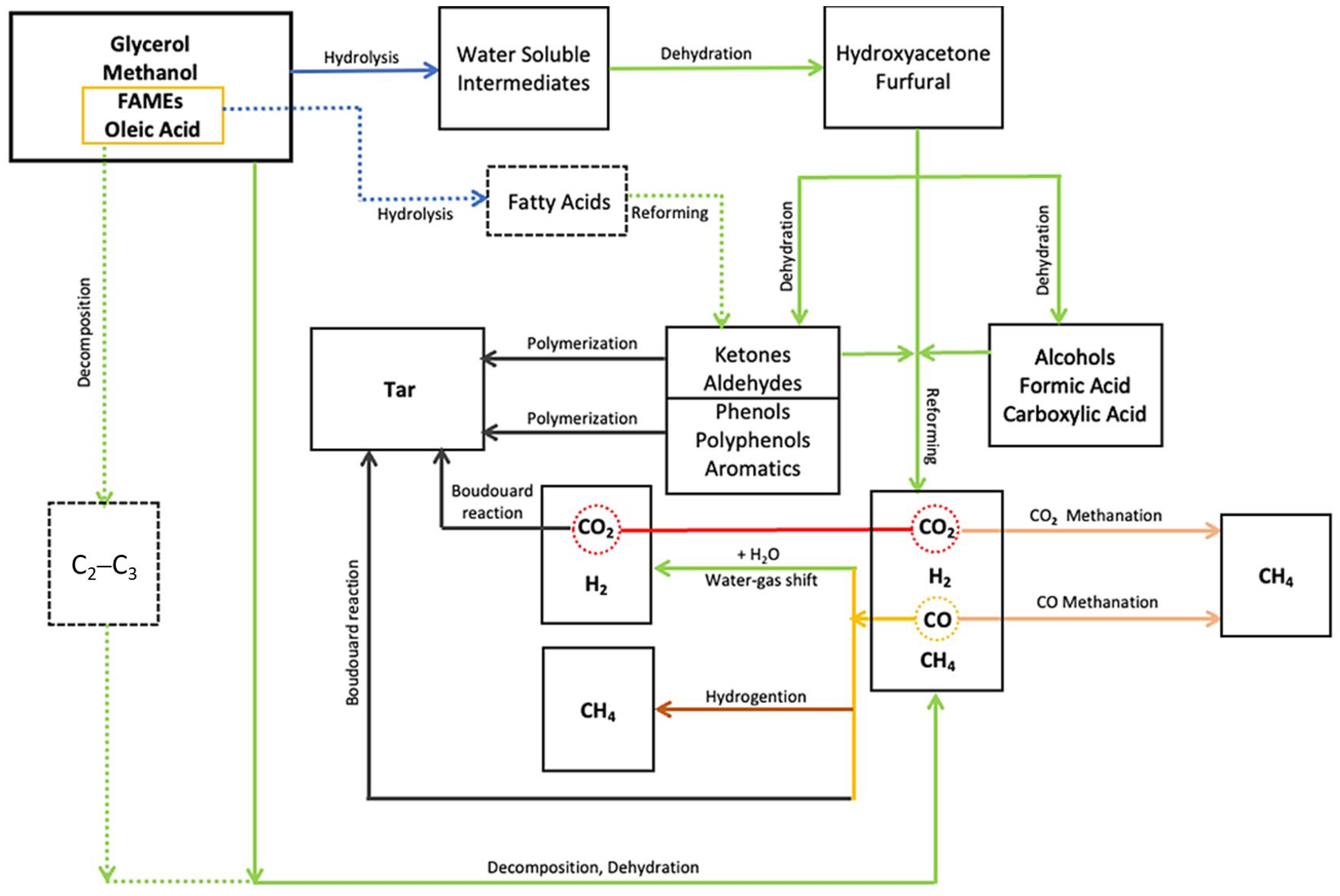
1. Introduction
2. Materials and Methods
2.1. Feedstock
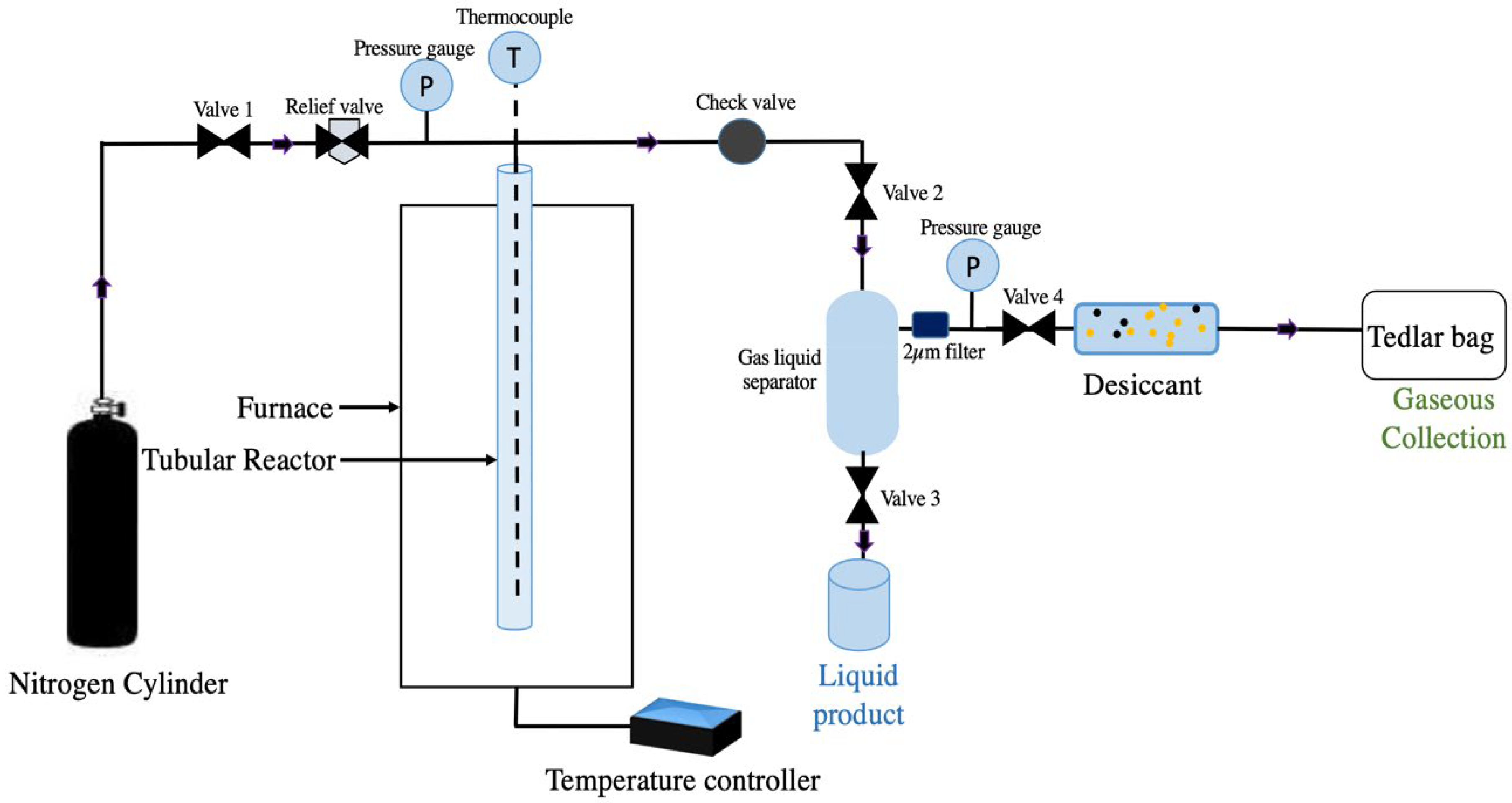
2.2. Supercritical Water Gasification Experiments
2.3. Feedstock and Product Characterization
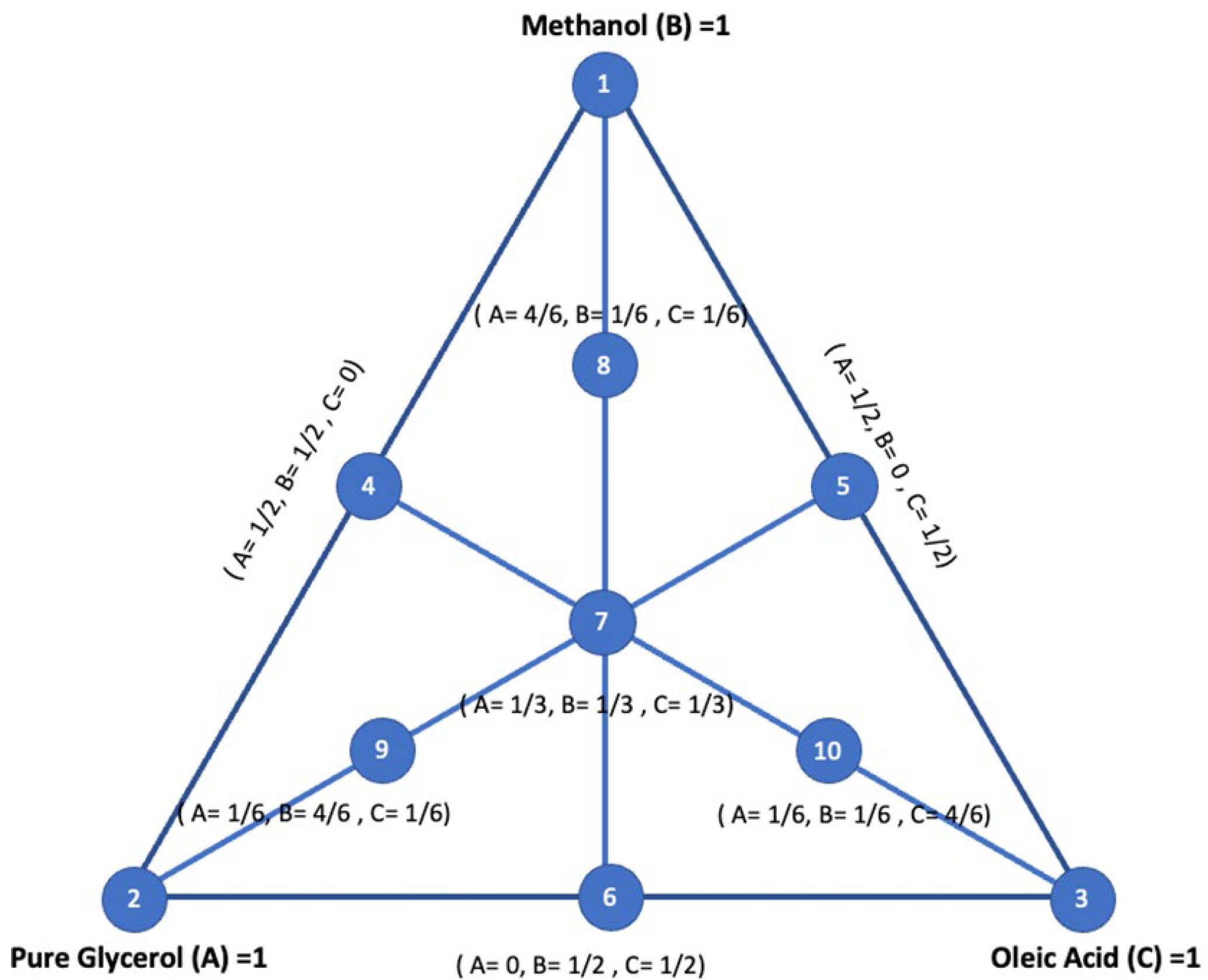
2.4. Design of Experiments
3. Results and Discussion
3.1. Feedstock Characterization
3.2. SCWG of Model Compounds of Crude Glycerol
3.3. SCWG of a Three-Component Mixture of Model Compounds of Glycerol
3.4. Validity of Model Equation with Simulated Crude Glycerol and Crude Glycerol
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rapier, R. Fossil Fuels Still Supply 84 Percent of World Energy—And Other Eye Openers from BP’s Annual Review, Forbes. Available online: https://www.forbes.com/sites/rrapier/2020/06/20/bp-review-new-highs-in-global-energy-consumption-and-carbon-emissions-in-2019/?sh=8dd42c166a16 (accessed on 2 March 2023).
- Nanda, S.; Reddy, S.N.; Mitra, S.K.; Kozinski, J.A. The progressive routes for carbon capture and sequestration. Energy Sci. Eng. 2016, 4, 99–122. [Google Scholar] [CrossRef]
- Gaur, A.; Dwivedi, G.; Baredar, P.; Jain, S. Influence of blending additives in biodiesel on physiochemical properties, engine performance, and emission characteristics. Fuel 2022, 321, 124072. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Statista. Supply of Biodiesel Worldwide in 2021 with a Forecast from 2025 to 2045. Available online: https://www.statista.com/statistics/1364388/global-biodiesel-supply/ (accessed on 2 March 2023).
- Statista. Biodiesel Production in Canada from 2012 to 2021. Available online: https://www.statista.com/statistics/485400/total-biodiesel-canadian-production/ (accessed on 2 March 2023).
- Chozhavendhan, S.; Singh, M.V.P.; Fransila, B.; Kumar, R.P.; Devi, G.K. A review on influencing parameters of biodiesel production and purification processes. Curr. Res. Green Sustain. Chem. 2020, 1–2, 1–6. [Google Scholar] [CrossRef]
- Ningaraju, C.; Yatish, K.V.; Mithun, P.R.; Sakar, M.; Balakrishna, G. Simultaneous refining of biodiesel-derived crude glycerol and synthesis of value-added powdered catalysts for biodiesel production: A green chemistry approach for sustainable biodiesel industries. J. Clean. Prod. 2022, 363, 132448. [Google Scholar]
- Vivek, N.; Sindhu, R.; Madhavan, A.; Anju, A.J.; Castro, E.; Faraco, V.; Pandey, A.; Binod, P. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate—Metabolic aspects, challenges and possibilities: An overview. Bioresour. Technol. 2017, 239, 507–517. [Google Scholar] [CrossRef]
- He, Q.S.; McNutt, J.; Yang, J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew. Sustain. Energy Rev. 2017, 71, 63–76. [Google Scholar] [CrossRef]
- Ortiz, F.J.G.; Ollero, P.; Serrera, A.; Sanz, A. Thermodynamic study of the supercritical water reforming of glycerol. Int. J. Hydrogen Energy 2011, 36, 8994–9013. [Google Scholar] [CrossRef]
- van Bennekom, J.G.; Venderbosch, R.H.; Assink, D.; Heeres, H.J. Reforming of methanol and glycerol in supercritical water. J. Supercrit. Fluids 2011, 58, 99–113. [Google Scholar] [CrossRef]
- Guo, S.; Guo, L.; Cao, C.; Yin, J.; Lu, Y.; Zhang, X. Hydrogen production from glycerol by supercritical water gasification in a continuous flow tubular reactor. Int. J. Hydrogen Energy 2012, 37, 5559–5568. [Google Scholar] [CrossRef]
- Guo, S.; Guo, L.; Yin, J.; Jin, H. Supercritical water gasification of glycerol: Intermediates and kinetics. J. Supercrit. Fluids 2013, 78, 95–102. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Marx, D.B.; Sun, R. Optimization of hydrogen production from supercritical water gasification of crude glycerol—Byproduct of biodiesel production. Int. J. Energy Res. 2013, 37, 1600–1609. [Google Scholar] [CrossRef]
- Tapah, B.F.; Santos, R.C.D.; Leeke, G.A. Processing of glycerol under sub and supercritical water conditions. Renew. Energy 2014, 62, 353–361. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109546. [Google Scholar] [CrossRef]
- Okolie, J.A.; Rana, R.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass: A state-of-the-art review of process parameters, reaction mechanisms and catalysis. Sustain. Energy Fuels 2019, 3, 578–598. [Google Scholar] [CrossRef]
- Ferreira-Pinto, L.; Parizi, M.P.S.; de Araújo, P.C.C.; Zanette, A.F.; Cardozo-Filho, L. Experimental basic factors in the production of H2 via supercritical water gasification. Int. J. Hydrogen Energy 2019, 44, 25365–25383. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Global Hydrogen Review 2021. Available online: www.iea.org (accessed on 2 March 2023).
- Xu, D.; Wang, S.; Hu, X.; Chen, C.; Zhang, Q.; Gong, Y. Catalytic gasification of glycine and glycerol in supercritical water. Int. J. Hydrogen Energy 2009, 34, 5357–5364. [Google Scholar] [CrossRef]
- Yan, M.; Liu, Y.; Song, Y.; Xu, A.; Zhu, G.; Jiang, J.; Hantoko, D. Comprehensive Experimental Study on Energy Conversion of household kitchen waste via integrated hydrothermal carbonization and supercritical water gasification. Energy 2022, 242, 123054. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Yu, C.; Jiang, J.; Guo, M.; Hantoko, D.; Yan, M. A two-step process for energy-efficient conversion of food waste via supercritical water gasification: Process design, products analysis, and electricity evaluation. Sci. Total Environ. 2021, 752, 142331. [Google Scholar] [CrossRef]
- Supamathanon, N.; Boonserm, K.; Osakoo, N.; Wittayakun, J.; Prayoonpokarach, S.; Chanlek, N.; Dungkaew, W. Potassium supported on zeolite-geopolymer hybrid materials as a new solid base catalyst for transesterification of soybean oil. Renew. Energy 2023, 202, 1460–1469. [Google Scholar] [CrossRef]
- Youssef, E.A.; Nakhla, G.; Charpentier, P.A. Oleic acid gasification over supported metal catalysts in supercritical water: Hydrogen production and product distribution. Int. J. Hydrogen Energy 2011, 36, 4830–4842. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Kramlich, J.C.; Novosselov, I.V. Gasification pathways and reaction mechanisms of primary alcohols in supercritical water. ACS Sustain. Chem. Eng. 2020, 8, 4598–4605. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, R.; Jin, H.; Lian, X.; Guo, L.; Huang, J. Supercritical water gasification of glycerol and glucose in different reactors: The effect of metal wall. Int. J. Hydrogen Energy 2016, 41, 16002–16008. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Kozinski, J.A. Supercritical water gasification of glycerol and methanol mixtures as model waste residues from biodiesel refinery. Chem. Eng. Res. Des. 2016, 113, 17–27. [Google Scholar] [CrossRef]
- Correa, C.R.; Kruse, A. Supercritical water gasification of biomass for hydrogen production—Review. J. Supercrit. Fluids 2018, 133, 573–590. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Noncatalytic gasification of lignin in supercritical water using a batch reactor for hydrogen production: An Experimental and Modeling Study. Energy Fuels 2015, 29, 1776–1784. [Google Scholar] [CrossRef]
- Rana, R.; Nanda, S.; Kozinski, J.A.; Dalai, A.K. Investigating the applicability of Athabasca bitumen as a feedstock for hydrogen production through catalytic supercritical water gasification. J. Environ. Chem. Eng. 2018, 6, 182–189. [Google Scholar] [CrossRef]
- Sarker, T.R.; Nanda, S.; Dalai, A.K. Hydrogen-rich gas production from hydrothermal gasification of fuel pellets obtained from co-pelletization of agricultural residues. Int. J. Hydrogen Energy. 2022. [Google Scholar] [CrossRef]
- Dang, T.N.; Sahraei, O.A.; Olivier, A.; Iliuta, M.C. Effect of impurities on glycerol steam reforming over Ni-promoted metallurgical waste driven catalyst. Int. J. Hydrogen Energy 2022, 47, 4614–4630. [Google Scholar] [CrossRef]
- Gong, M.; Nanda, S.; Hunter, H.N.; Zhu, W.; Dalai, A.K.; Kozinski, J.A. Lewis acid catalyzed gasification of humic acid in supercritical water. Catal. Today 2017, 291, 13–23. [Google Scholar] [CrossRef]
- Zhiyong, Y.; Xiuyi, T. Hydrogen generation from oily wastewater via supercritical water gasification (SCWG). J. Ind. Eng. Chem. 2015, 23, 44–49. [Google Scholar] [CrossRef]
- Khorasani, R.; Khodaparasti, M.S.; Tavakoli, O. Hydrogen production from dairy wastewater using catalytic supercritical water gasification: Mechanism and reaction pathway. Int. J. Hydrogen Energy 2021, 46, 22368–22384. [Google Scholar] [CrossRef]
- Chen, J.; Fu, L.; Tian, M.; Kang, S.; Jiaqiang, E. Comparison and synergistic effect analysis on supercritical water gasification of waste thermoplastic plastics based on orthogonal experiments. Energy 2022, 261, 125104. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, L.; Jin, H.; Huang, J.; Li, S.; Lian, X. Effects of reaction time and catalyst on gasification of glucose in supercritical water: Detailed reaction pathway and mechanisms. Int. J. Hydrogen Energy 2016, 41, 6630–6639. [Google Scholar] [CrossRef]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of supercritical water gasification with lignocellulosic real biomass as the feedstocks: Process parameters, biomass composition, Catalyst Development, reactor design and its challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of timothy grass as an energy crop in the presence of alkali carbonate and hydroxide catalysts. Biomass Bioenergy 2016, 95, 378–387. [Google Scholar] [CrossRef]
- Zhang, H.W.; Zhu, W.; Gong, M.; Fan, Y. Influence of alkali catalysts on the supercritical water gasification of cyanobacteria. Fresenius Environ. Bull. 2016, 25, 2970–2976. [Google Scholar]
- Cengiz, N.Ü.; Yıldız, G.; Sert, M.; Gökkaya, D.S.; Sağlam, M.; Yüksel, M.; Ballice, L. Hydrothermal gasification of a biodiesel by-product crude glycerol in the presence of phosphate based catalysts. Int. J. Hydrogen Energy 2015, 40, 14806–14815. [Google Scholar] [CrossRef]
- Madenoğlu, T.G.; Sağlam, M.; Yüksel, M.; Ballice, L. Hydrothermal gasification of biomass model compounds (cellulose and lignin alkali) and model mixtures. J. Supercrit. Fluids 2016, 115, 79–85. [Google Scholar] [CrossRef]
- Štefanko, D.; Rusková, R.; Jelemenský, Ľ. Kinetic models of simple alcohols SCWG. Chem. Papers 2019, 74, 333–347. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Xing, X.; Wang, H.; Zeng, Y.; Xu, C.C. Supercritical water gasification of biomass model compounds: A Review. Renew. Sustain. Energy Rev. 2020, 118, 109529. [Google Scholar] [CrossRef]
- Wei, N.; Xu, D.; Hao, B.; Guo, S.; Guo, Y.; Wang, S. Chemical reactions of organic compounds in supercritical water gasification and oxidation. Water Res. 2021, 190, 116634. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Feng, H.; Wei, W.; Wang, G.; Sun, J.; Jin, H.; Guo, L. Study on the detailed reaction pathway and catalytic mechanism of a Ni/ZrO2 catalyst for supercritical water gasification of diesel oil. Fuel 2022, 312, 122849. [Google Scholar] [CrossRef]
- Bian, C.; Zhang, R.; Dong, L.; Bai, B.; Li, W.; Jin, H.; Cao, C. Hydrogen/methane production from supercritical water gasification of lignite coal with plastic waste blends. Energy Fuels 2020, 34, 11165–11174. [Google Scholar] [CrossRef]
- Sebastiani, D.; Parrinello, M. Ab-initio study of NMR chemical shifts of water under normal and supercritical conditions. ChemPhysChem 2002, 3, 675. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.R.A.; Prins, W.; van Swaaij, W.P.M.; van de Beld, B.; Elliott, D.C.; Neuenschwander, G.G.; Kruse, A.; et al. Biomass gasification in near- and super-critical water: Status and prospects. Biomass Bioenergy 2005, 29, 269–292. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of water for chemical reactions in high-temperature water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef]
- Jarana, M.B.G.; Sánchez-Oneto, J.; Portela, J.R.; Nebot Sanz, E.; de la Ossa, E.J.M. Supercritical water gasification of industrial organic wastes. J. Supercrit. Fluids 2008, 46, 329–334. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Cui, D.; Bai, J.; Wang, Z.; Xu, F.; Wang, Z. Advances in supercritical water gasification of lignocellulosic biomass for hydrogen production. JAAP 2023, 170, 105934. [Google Scholar] [CrossRef]
- Liao, Z.; Wang, C.; Tang, X.; Wang, Y.; Lin, Z.; Ren, P.; Wang, L.; Xu, H.; Yang, J.; Cai, J. Product characterization of hydrothermal liquefaction and supercritical water gasification of water hyacinth. Environ. Eng. Sci. 2022, 39, 268–286. [Google Scholar] [CrossRef]
- Surjosatyo, A.; Anggriawan, M.B.; Hermawan, A.A.; Dafiqurrohman, H. Comparison between secondary thermal cracking methods and venturi scrubber filtering in order to reduce tar in biomass gasification. Energy Proc. 2019, 158, 749–754. [Google Scholar] [CrossRef]

| Crude Glycerol | Simulated Crude Glycerol | Pure Glycerol | Methanol | Oleic Acid | |
|---|---|---|---|---|---|
| Glycerol (wt%) | 41.9 | 44.7 | >99 | - | - |
| Methanol (wt%) | 20.2 | 21.5 | - | >99 | - |
| Oleic acid (wt%) | 31.7 | 33.8 | - | - | >99 |
| Ash (wt%) | 4.5 | - | - | - | - |
| Moisture (wt%) | 1.7 | - | - | - | - |
| Carbon (wt%) | 48.7 | 48.1 | 38.4 | 32.6 | 77.8 |
| Hydrogen (wt%) | 9.8 | 9.7 | 9.1 | 11.8 | 12.7 |
| Nitrogen (wt%) | 0.4 | 0.4 | 0.5 | 0.2 | 0.4 |
| Sulfur (wt%) | - | - | 0.1 | - | 0.1 |
| Oxygen (wt%) | 36.6 | 41.8 | 51.9 | 55.4 | 9.1 |
| pH value | 8.5 | 7.8 | 7.0 | 8.1 | 9.2 |
| Element | Concentration (ppm) |
|---|---|
| K | 5473 |
| Na | 117 |
| Si | 11.1 |
| Ca | 9.8 |
| Fe | 4.7 |
| S | 3.4 |
| P | 2.7 |
| Mg | 1.9 |
| Cs | 1.7 |
| Li | 1.3 |
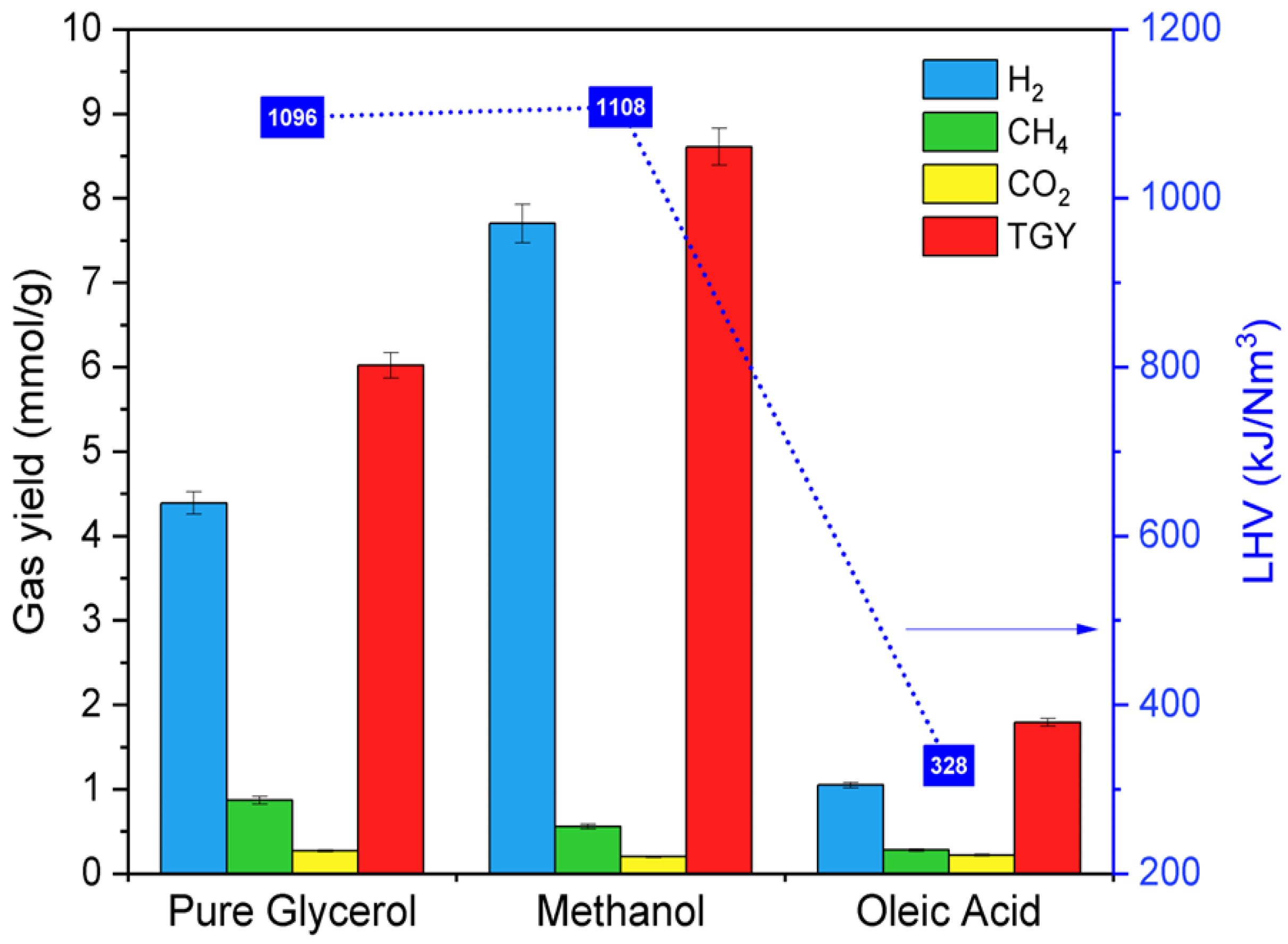
| Model Compound | Total Gas Yield (mmol/g) | LHV (kJ/Nm3) | HGE (%) | HS (%) |
|---|---|---|---|---|
| Pure glycerol | 6.02 | 1096 | 16.7 | 72.9 |
| Methanol | 8.61 | 1108 | 15.7 | 89.4 |
| Oleic acid | 1.79 | 328 | 3.7 | 58.7 |
| Model Compound | Feedstock Concentration (wt%) | H2 Yield (mmol/g) | CH4 Yield (mmol/g) | CO2 Yield (mmol/g) | Total Gas Yield (mmol/g) | LHV (kJ/Nm3) |
|---|---|---|---|---|---|---|
| Pure glycerol | 10 | 4.39 | 0.87 | 0.27 | 6.02 | 1096 |
| 20 | 4.11 | 1.01 | 0.21 | 5.83 | 1125 | |
| 30 | 3.91 | 1.09 | 0.17 | 5.67 | 1132 | |
| Methanol | 10 | 7.70 | 0.56 | 0.20 | 8.61 | 1108 |
| 20 | 6.97 | 0.68 | 0.16 | 8.02 | 1133 | |
| 30 | 6.61 | 0.79 | 0.12 | 7.77 | 1158 | |
| Oleic acid | 10 | 1.05 | 0.28 | 0.22 | 1.79 | 328 |
| 20 | 0.98 | 0.31 | 0.19 | 1.66 | 332 | |
| 30 | 0.91 | 0.34 | 0.16 | 1.60 | 341 |
| Experimental Run | Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 | Response 4 |
|---|---|---|---|---|---|---|---|
| Pure Glycerol (A) | Methanol (B) | Oleic Acid (C) | H2 Yield (Y1) (mmol/g) | CH4 Yield (Y2) (mmol/g) | Total Gas Yield (Y3) (mmol/g) | CO2 Yield (Y4) (mmol/g) | |
| 1 | 0 | 0 | 1 | 1.05 | 0.28 | 1.79 | 0.22 |
| 2 | 0 | 0 | 1 | 1.05 | 0.29 | 1.80 | 0.22 |
| 3 | 0 | 0.5 | 0.5 | 6.32 | 0.51 | 7.87 | 0.57 |
| 4 | 0.167 | 0.167 | 0.667 | 3.66 | 0.53 | 5.13 | 0.62 |
| 5 | 0 | 1 | 0 | 7.70 | 0.56 | 8.61 | 0.20 |
| 6 | 0 | 1 | 0 | 7.69 | 0.56 | 8.60 | 0.19 |
| 7 | 0.167 | 0.667 | 0.167 | 7.06 | 0.58 | 8.37 | 0.59 |
| 8 | 0.333 | 0.333 | 0.333 | 5.37 | 0.59 | 8.36 | 1.22 |
| 9 | 0.5 | 0 | 0.5 | 2.82 | 0.72 | 4.44 | 0.57 |
| 10 | 0.5 | 0.5 | 0 | 6.88 | 0.75 | 8.39 | 0.38 |
| 11 | 0.5 | 0.5 | 0 | 6.89 | 0.75 | 8.40 | 0.38 |
| 12 | 0.667 | 0.167 | 0.167 | 4.93 | 0.84 | 7.50 | 0.82 |
| 13 | 1 | 0 | 0 | 4.40 | 0.87 | 6.10 | 0.27 |
| 14 | 1 | 0 | 0 | 4.39 | 0.87 | 6.02 | 0.27 |
| H2 Yield | CH4 Yield | CO2 Yield | Total Gas Yield | |
|---|---|---|---|---|
| Model | ||||
| Sum of squares | 66.2 | 0.5 | 0.7 | 75.4 |
| Mean square error | 11.0 | 0.09 | 0.12 | 15.1 |
| Degree of freedom | 6 | 5 | 6 | 5 |
| F-value | 5191.5 | 101.6 | 27.0 | 94.6 |
| p-value | <0.0001 | <0.0001 | 0.0002 | <0.0001 |
| Residual | ||||
| Sum of squares | 0.02 | 0.007 | 0.03 | 1.27 |
| Mean square error | 0.002 | 0.001 | 0.004 | 0.16 |
| Degree of freedom | 7 | 8 | 7 | 8 |
| Model prediction | ||||
| R2 | 0.9998 | 0.9845 | 0.9586 | 0.9834 |
| Adjusted R2 | 0.9996 | 0.9748 | 0.9231 | 0.9730 |
| Predicted R2 | 0.9972 | 0.8327 | 0.5389 | 0.9517 |
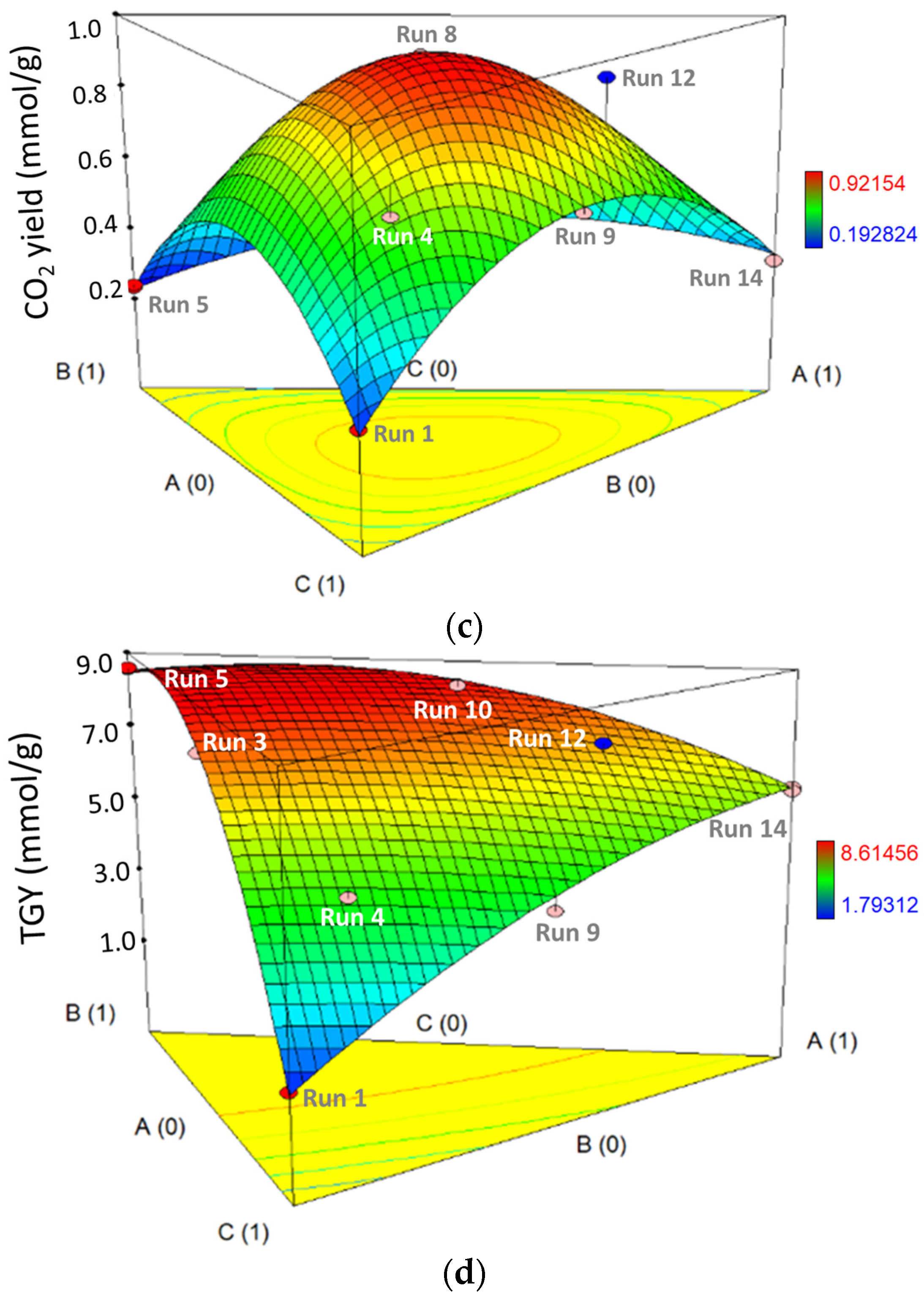
| Response | H2 Yield | CH4 Yield | CO2 Yield | Total Gas Yield |
|---|---|---|---|---|
| AB | <0.0001 | - | 0.0325 | 0.0056 |
| BC | <0.0001 | 0.0433 | 0.0026 | <0.0001 |
| AC | 0.0565 | 0.0021 | 0.0023 | 0.0543 |
| ABC | 0.0009 | 0.1054 | 0.0030 | - |
| Response | Predicted Values (P) (mmol/g) | Experimental Yields from Simulated Crude Glycerol (ESCG) (mmol/g) | Error Percentage (e%SCG) | Experimental Yields from Crude Glycerol (ERCG) (mmol/g) | Error Percentage (e%RCG) | Difference (d%RCG-SCG) |
|---|---|---|---|---|---|---|
| H2 yield | 4.68 | 4.69 | 0.14 | 4.70 | 0.42 | 0.28 |
| CH4 yield | 0.68 | 0.68 | 1.0 | 0.67 | –1.17 | –2.15 |
| CO2 yield | 0.90 | 0.92 | 2.24 | 0.92 | 2.35 | 0.11 |
| Total gas yield | 6.97 | 7.21 | 3.36 | 7.23 | 3.56 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandelwal, K.; Boahene, P.; Nanda, S.; Dalai, A.K. Hydrogen Production from Supercritical Water Gasification of Model Compounds of Crude Glycerol from Biodiesel Industries. Energies 2023, 16, 3746. https://doi.org/10.3390/en16093746
Khandelwal K, Boahene P, Nanda S, Dalai AK. Hydrogen Production from Supercritical Water Gasification of Model Compounds of Crude Glycerol from Biodiesel Industries. Energies. 2023; 16(9):3746. https://doi.org/10.3390/en16093746
Chicago/Turabian StyleKhandelwal, Kapil, Philip Boahene, Sonil Nanda, and Ajay K. Dalai. 2023. "Hydrogen Production from Supercritical Water Gasification of Model Compounds of Crude Glycerol from Biodiesel Industries" Energies 16, no. 9: 3746. https://doi.org/10.3390/en16093746
APA StyleKhandelwal, K., Boahene, P., Nanda, S., & Dalai, A. K. (2023). Hydrogen Production from Supercritical Water Gasification of Model Compounds of Crude Glycerol from Biodiesel Industries. Energies, 16(9), 3746. https://doi.org/10.3390/en16093746







