1. Introduction
The use of fossil fuels has brought mankind great technological advances in a short period of time [
1]. However, this has caused problems such as climate change and air pollution by releasing greenhouse gases and reactive trace species into the atmosphere. As mitigation measures have been taken to prevent climate change and air pollution caused by fossil fuel consumption, an energy transition is expected to lead to decarbonization or the creation of a carbon-neutral society. Since clean technologies that do not emit greenhouse gases or air pollutants, such as hydrogen technology, are expected to play an important role in this energy transition [
1,
2]. The transportation sector, including road vehicles, aviation, and shipping, is the source of 16% of greenhouse gas emissions [
3]. Particularly, the combustion of fossil fuels such as gasoline and diesel in automobile engines is a major source of greenhouse gases and air pollutants. Therefore, clean technologies are also expected to be used in automobiles.
Battery electric vehicles (BEVs) and fuel cell vehicles (FCVs) that use motors instead of internal combustion engines are considered new clean technologies for achieving zero carbon emissions. Alternatively, for cases where high power output is required and the torque of the motor is reduced, hydrogen internal combustion engine vehicles (H2-ICEVs) are being developed, in which hydrogen is burned in the engine to maintain a constant torque even under high power conditions with no emission of carbonaceous emissions [
4].
Hydrogen-powered vehicles with spark-ignition (SI) engines are being developed for commercial use owing to their high suitability for using hydrogen as a sole fuel [
3], although some drawbacks still exist [
5]. However, the low cetane number and high auto-ignition temperature of hydrogen make its use as a sole fuel in compression ignition (CI) engines difficult. Therefore, hydrogen has been used as an additive with diesel to facilitate mixed combustion in CI engines [
4].
Many reports have presented the effects of hydrogen addition to CI engines using engine dynamometers [
3,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23]. A typical trend of the effects of hydrogen addition to diesel on CI engine emissions exhibited improved engine performance and increased engine temperature, which resulted in reductions of CO, PM, and CO
2 emissions. However, it was also shown that the effect of the hydrogen addition on total hydrocarbon (THC) and NO
x was more complex, and the hydrogen addition resulted in an increase or reduction of total hydro THC and NO
x depending on the engine load. Studies on engine dynamometers have been performed with a constant load. During driving in the actual situation, engine load varies frequently, and how hydrogen addition changed the emissions during such actual running has not been investigated.
In this study, hydrogen was added to the intake air of a four-cylinder CI engine equipped on a diesel truck that was running in a practical test-driving mode and three different constant speed test-driving modes on a chassis dynamometer at the Low Emission Vehicle Facility in the National Institute for Environmental Studies (NIES) and its effects on the engine’s fuel consumption and exhaust gas composition were investigated. Similar benefits of hydrogen addition to the previous studies conducted using engine dynamometers were observed, which included and reduced diesel consumption and reduced CO2, CO, and PM emissions. However, an increase in NOx emission and total energy consumption was shown as a disadvantage to be considered.
2. Experimental Method
The experiments were conducted on a diesel truck manufactured in 1991, of 2440 kg vehicle weight and 4605 kg gross vehicle weight, with a mileage of 230,000 km, equipped with a direct-injection CI engine with a displacement of 3600 cc, to which the 1989 emission regulations in Japan had been adapted.
Table 1 shows technical specifications of the test engine.
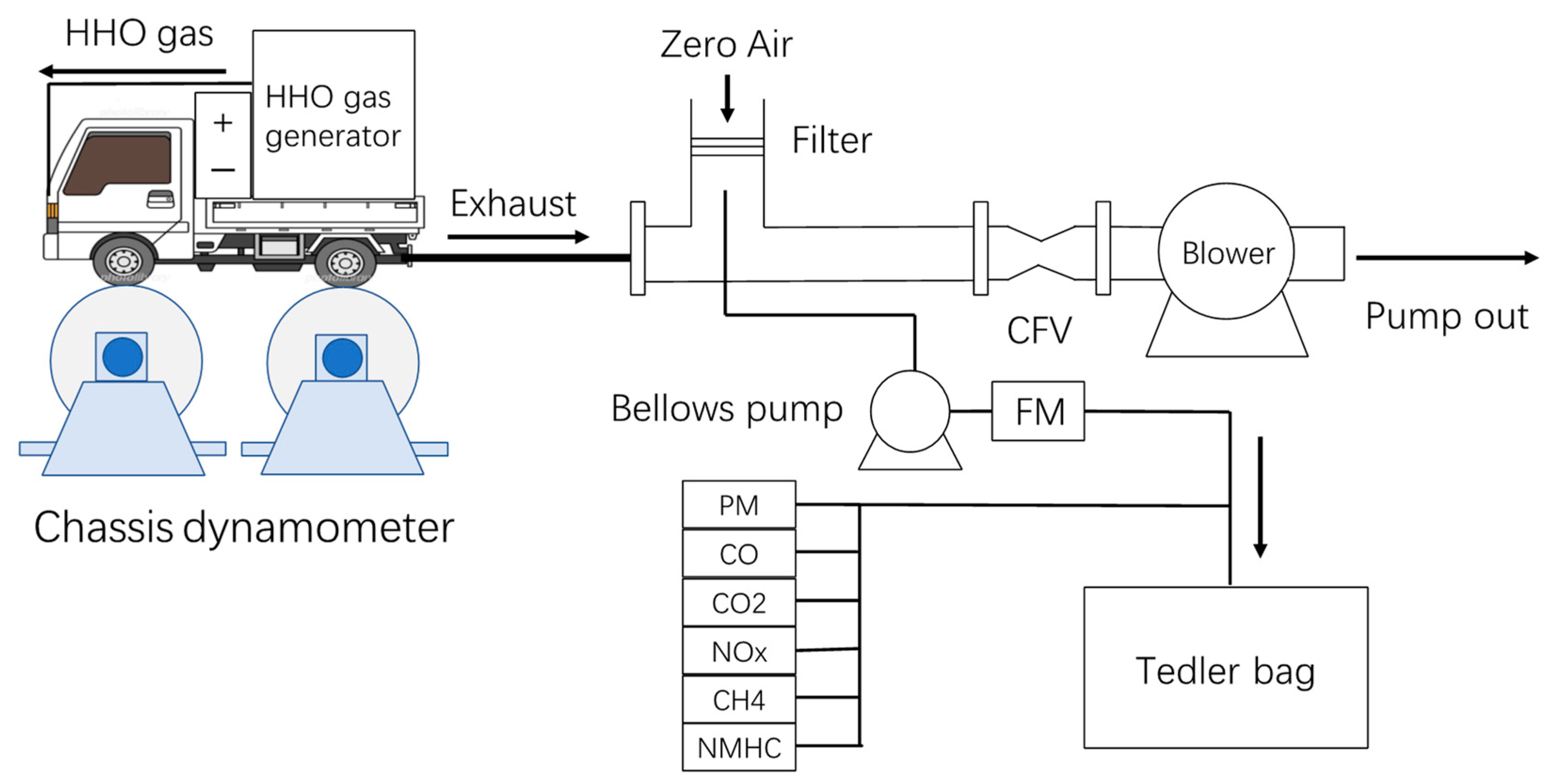
For the driving test, the truck was installed on a chassis dynamometer at the National Institute for Environmental Studies [
24], as shown in
Figure 1.
Hydrogen was generated by electrolysis of water using an oxyhydrogen (HHO) generator (Enehelper EH-4000, Notoice Ltd. Amagasaki, Hyogo, Japan. maximum total gas production: 67 L/min) installed on the cargo bed. The produced gas contained hydrogen and oxygen at a 2:1 ratio and a few water vapors. As produced, hydrogen gas was then mixed with intake air and placed into the engine’s combustion chamber at a constant flow rate (0, 3, 30, 60, and 67 L/min). To avoid gas backflow, safety and metering devices such as a gas-only flow meter, flow control valve, pressurizer, and backflow prevention valve were installed between the HHO generator and the air intake manifold. Since hydrogen was produced through electrolysis, which electrolyzes water and does not separate gases, it contained not only hydrogen, oxygen, and water vapor but also substances in a special state that may change combustion reactions [
25]. Although there are some differences in the absolute amount of pollutant production between the use of hydrogen and HHO as a second fuel, the relative effects of addition show similar trends [
26]. Therefore, in this study, we approximately treated HHO gas as a mixture of hydrogen and oxygen in a 2:1 ratio.
Test run experiments were performed as shown in
Table 2, with the following four test driving modes: JE 05 driving cycle [
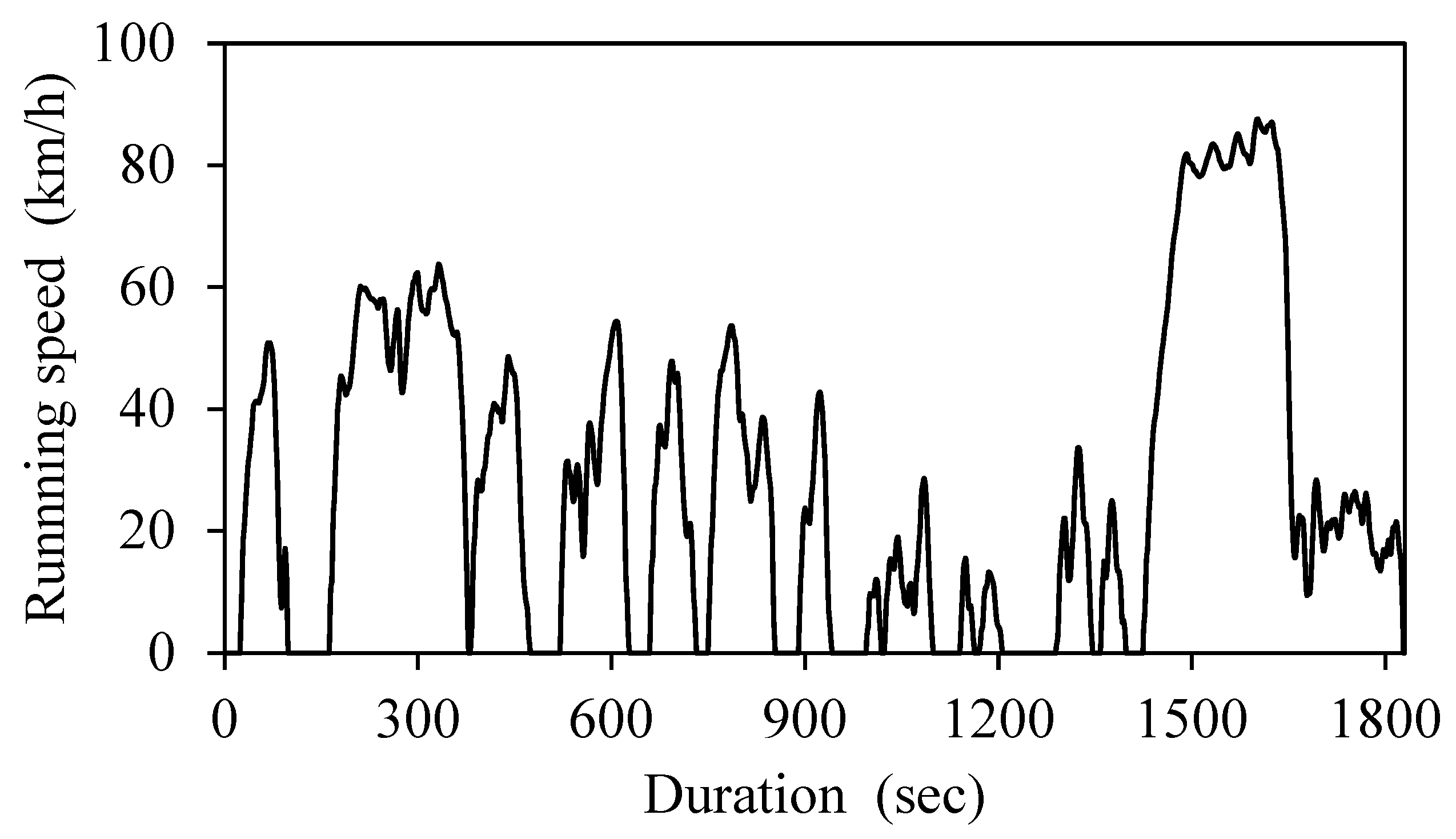
27] constant speed 40 km/h, constant speed 60 km/h, and constant speed 80 km/h, where the truck was driven by a programmed robot (ADS-1100, HORIBA). The JE 05 driving cycle is the emission test cycle for heavy vehicles, including diesel trucks simulating driving on a city road and a highway, which consists of many accelerations and decelerations that attempt to simulate driving on a city road, whose maximum speed, average speed, and duration are 88 km/h, 27 km/h and approximately 30 min, respectively (
Figure 2).
The tailpipe of the vehicle was connected to a constant volume sampler (CVS; CVS-7200, HORIBA) via an introduction tube and dilution tunnel, and the exhaust gas was diluted with zero air generated by a dilution air purifier (DAR-2200, HORIBA) [
26]. The total CVS flow rate was 3.5–20 m
3 min
−1, which varied according to the critical flow venturi (CFV), as shown in
Figure 1. The exhaust gas dilution factor was varied with each running cycle and was determined by the ratio of CO
2 concentrations in the tailpipe and CVS. Total emissions of CO, CO
2, NOx, CH
4, and THC during every test run were measured with a MEXA-7200 (HORIBA), and those of PM were measured by gravimetric analysis after being collected on a filter at a constant flow rate.
Diluted exhaust gas was also collected continuously in a Tedlar bag having 150 L of volume through a poly-tetra-fluoro-ethylene (PTFE) bellows pump during a test run at a constant flow rate depending on the driving cycle. A part of collected air was then introduced into sorbent-filled cartridges at a constant flow rate of 50 sccm for 3 min. The hydrocarbons (HCs) captured in the cartridges were desorbed using thermal desorption system (Unity2, Markes International Ltd., Bridgend, UK) at 623 K and introduced to a gas chromatograph with a flame ionization detector (GC-FID; HP 6890, Agilent Technology, USA) equipped with dual columns (Agilent J&W HP-1 and Agilent J&W GS-Gaspro, Agilent Technology, USA) [
28]. In the analysis, the GC column was set at 313 K for 5 min at first, then the temperature was increased at a rate of 5 K min
−1 up to 413 K in 20 min and maintained for 5 min. Retention times and conversion factors between the GC-FID peak area and VOC concentration for each species were obtained by calibrating them against a standard gas that includes various kinds of VOCs (PAMS-J58, Sumito Seika Chemicals, Japan) [
28].
Furthermore, the change in combustion temperature with the addition of hydrogen was evaluated using the KUCRS combustion model generated by the KUCRS software [
29] with some modifications [
30]. In the calculations, a simplified reaction simulation was performed with n-cetane alone as fuel at an initial temperature of 1000 K, an initial pressure of 30 atm, and constant volume conditions using the Cantera program [
31]. The combustion temperature was calculated as an adiabatic flame temperature after ignition. Because the present model does not include the NO
x formation mechanism, the amount of NO
x formed was not quantitatively evaluated; instead, relative change in the predicted combustion temperature in the hydrogen addition was calculated as a measure for the NO
x formation. The results for the fuel-rich condition with an equivalence ratio of 10 are presented to emphasize that the addition of hydrogen affects combustion temperature even under oxygen-deficient conditions. The correlation between the amount of hydrogen added and combustion temperature was found to show the same trend for different equivalent ratios. Calculations were performed for hydrogen addition volume fractions of 0, 0.001, 0.01, and 0.02; for simplicity, initial air CO
2 concentration and reduced fuel consumption due to hydrogen addition were not considered.
Hydrogen energy share (
HES) was used as an indicator of hydrogenation and calculated as follows:
where
M is the fuel consumption per run experiment (kg/run), and
LHV is the lower heating value (
LHV) [
3]. In this study,
LHVs of 43 and 121 MJ/kg were used for diesel and hydrogen, respectively [
32].
3. Results and Discussion
The fuel consumption and total emissions of each component in each test are shown in
Table 3. In all tests, with the addition of hydrogen, a decrease in fuel consumption and a fluctuation in emissions of each component were observed.
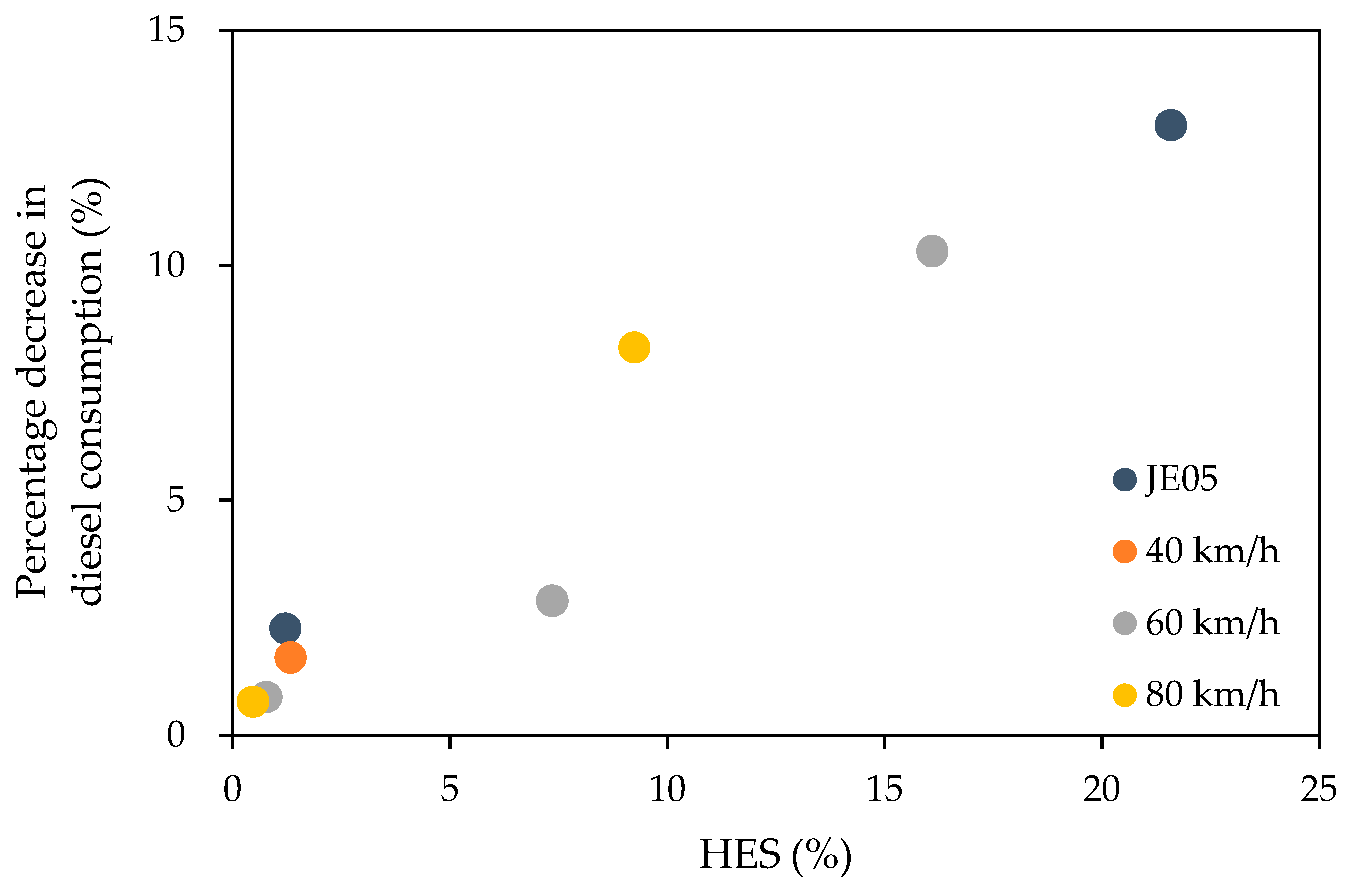
Figure 3 shows the reduction in fuel consumption corresponding to the total amount of hydrogen added.
From
Figure 3, in general, an increase in HES reduced the fuel consumption. For JE 05 driving cycle, fuel consumption decreased by 2.3% from 1.38 L without hydrogen to 1.35 L with a hydrogen flow rate of 2 L/min. For hydrogen flow at 40 L/min, fuel consumption decreased by 13% from 1.38 L to 1.20 L. At a constant speed of 40 km/h, fuel consumption decreased by 1.6% from 0.61 L without hydrogen to 0.60 L with a hydrogen flow rate of 2 L/min. At a constant speed of 60 km/h, the fuel consumption decreased by 0.8% from 1.06 L without hydrogen to 1.05 L with a hydrogen flow rate of 2 L/min and 10.3% to 0.95 L at a hydrogen flow rate of 45 L/min. At a constant speed of 80 km/h, the consumption decreased by 0.7% from 1.74 L without hydrogen to 1.73 L with a hydrogen flow rate of 2 L/min and by 8.3% to 1.60 L at a hydrogen flow rate of 40 L/min.
On comparing the constant speed modes, a tendency for a greater reduction in fuel consumption relative to HES at 80 km/h than at speeds below 80 km/h was observed. The JE 05 driving cycle did not show the lowest reduction in fuel consumption relative to HES, despite the lowest average speed of 27.4 km/h. The reduction in fuel consumption relative to HES was found to be slightly dependent on the driving mode, but additional experiments are required to clarify the extent of the dependence. Although the reduction in diesel consumption corresponding to an increase in HES was slightly different, they showed almost linear dependence where the percentage decrease in diesel consumption is approximately 0.6 × HES. This relationship suggests that HES is useful to roughly predict the reduction in diesel consumption.
CO
2 was the most substantial emission component. CO
2 was found to be reduced in all the driving tests having hydrogen-added fuel. In each test run, the maximum reduction in CO
2 occurred at the maximum hydrogen flow rate of 40 L/min, and 45 L/min at 60 km/h constant speed. The reduction in CO
2 was mainly due to an increase in the hydrogen ratio in the fuel mixture and a decrease in the consumption of diesel.
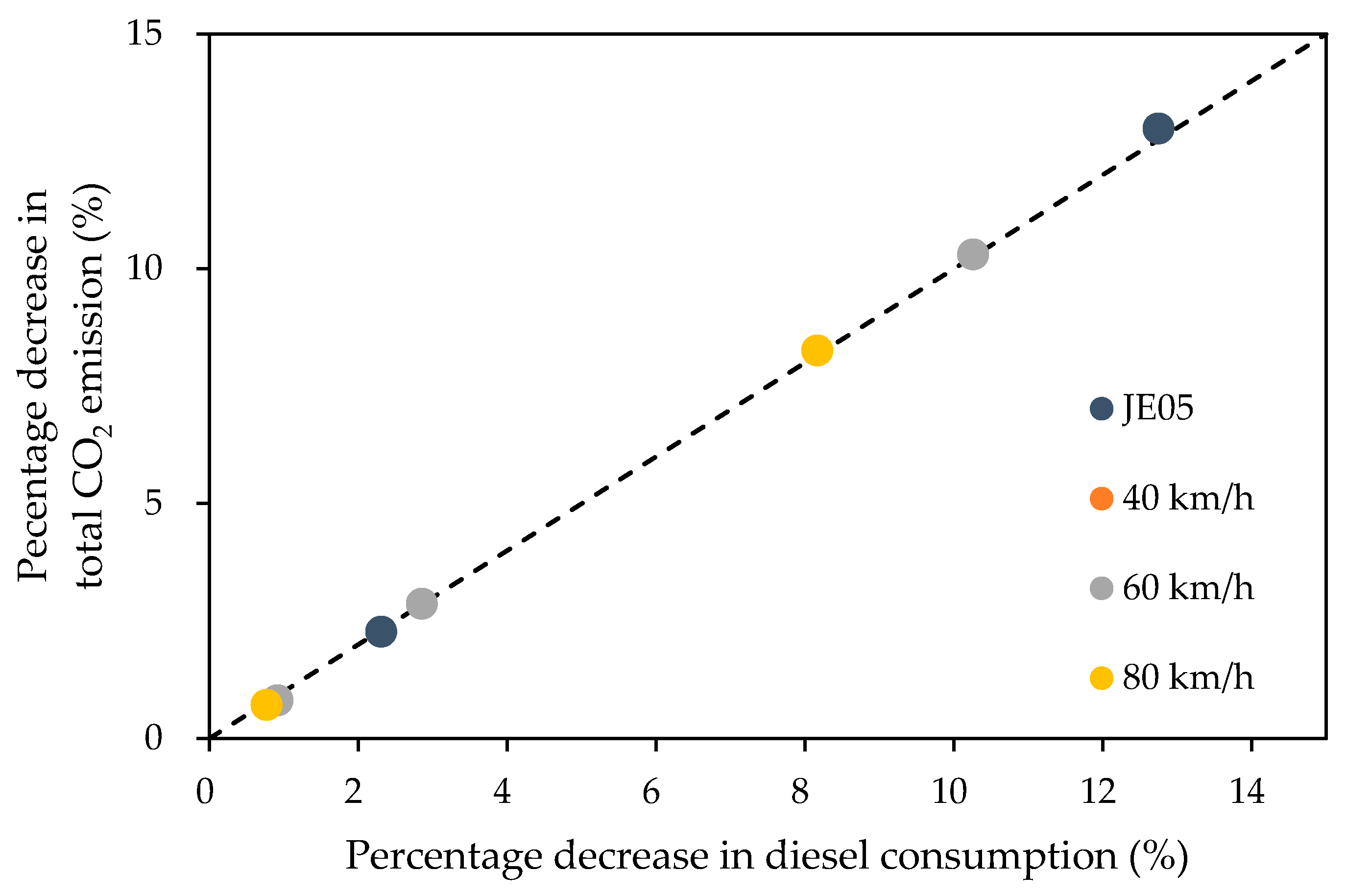
Figure 4 shows the relationship of the percentage reduction in fuel consumption to the percentage reduction in CO
2 emissions.
The percentage reduction in total CO
2 emissions due to the addition of hydrogen exhibited a very good agreement with the percentage reduction in fuel consumption. This indicates that most of the fuel consumed was converted to CO
2, and improving diesel consumption is directly linked with a reduction in CO
2 emission, and that reduction of CO
2 emission can be estimated from HES as approximately 0.6 × HES using the relationship shown in
Figure 3.
The change in NO
x emissions with the addition of hydrogen is shown in
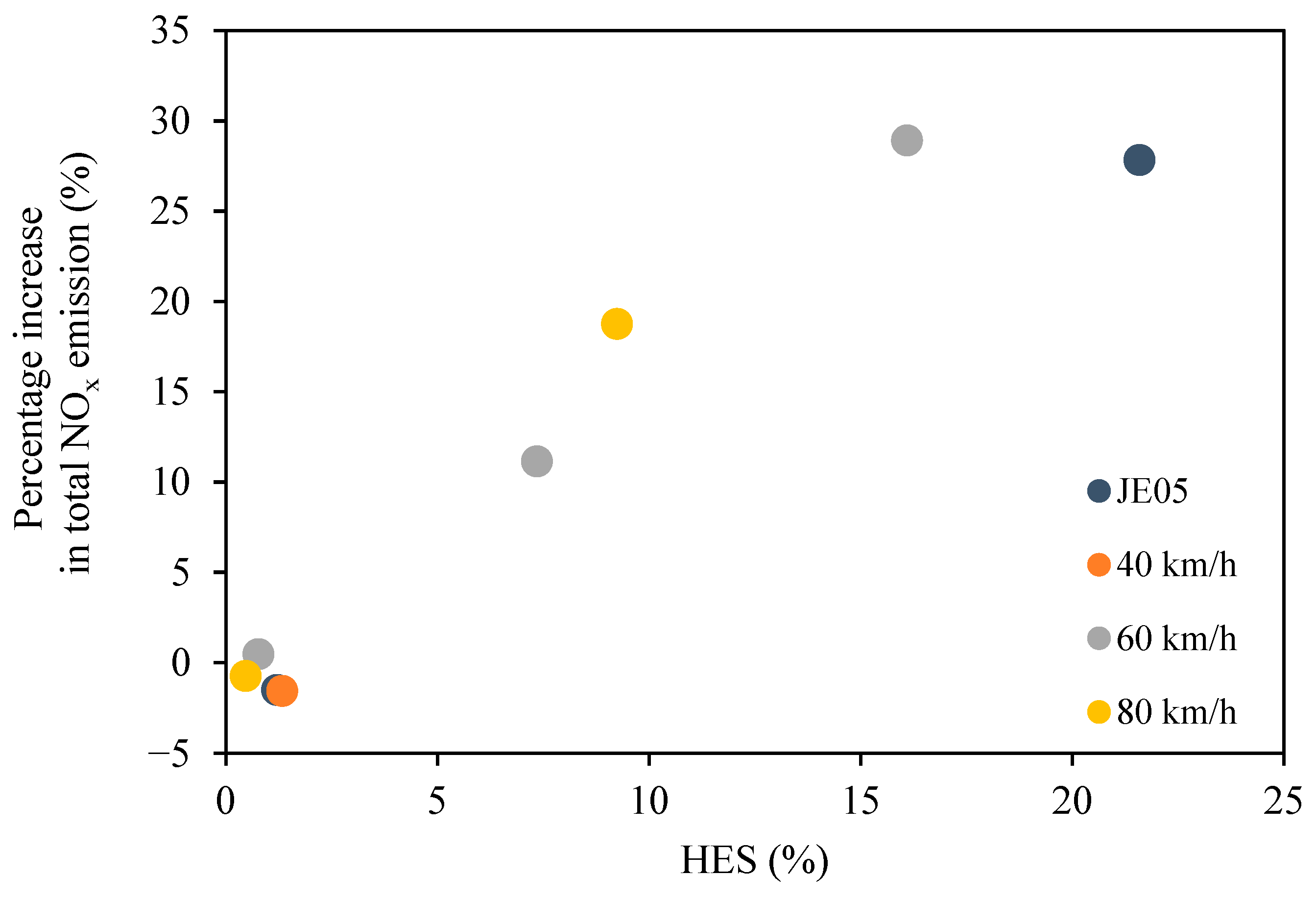
Figure 5.
At hydrogen addition at a rate of 2 L/min (HES~1%), NOx emissions decreased by less than 2% for JE 05 driving cycle, constant speed 40 km/h and 80 km/h. On the other hand, NOx emissions began to increase as hydrogen flow increased. The increase in NOx emissions with HES tended to increase with increasing average speed during the test runs; however, the differences between the driving modes were not pronounced. At a constant speed of 80 km/h, where the effect of hydrogen was greatest, the percentage increase of NOx was 20% at 10% HES, almost twice that of the HES. The effect of hydrogen was smallest in JE05, the percentage increase of NOx was 1.5 times that of the HES.
Since NO
x production enhances with increasing combustion temperature, in the present experiment, the addition of hydrogen was considered to have increased the combustion temperature. The kinetic simulations under the fuel-rich condition were used to estimate the change in combustion temperature with the addition of hydrogen to n-cetane combustion.
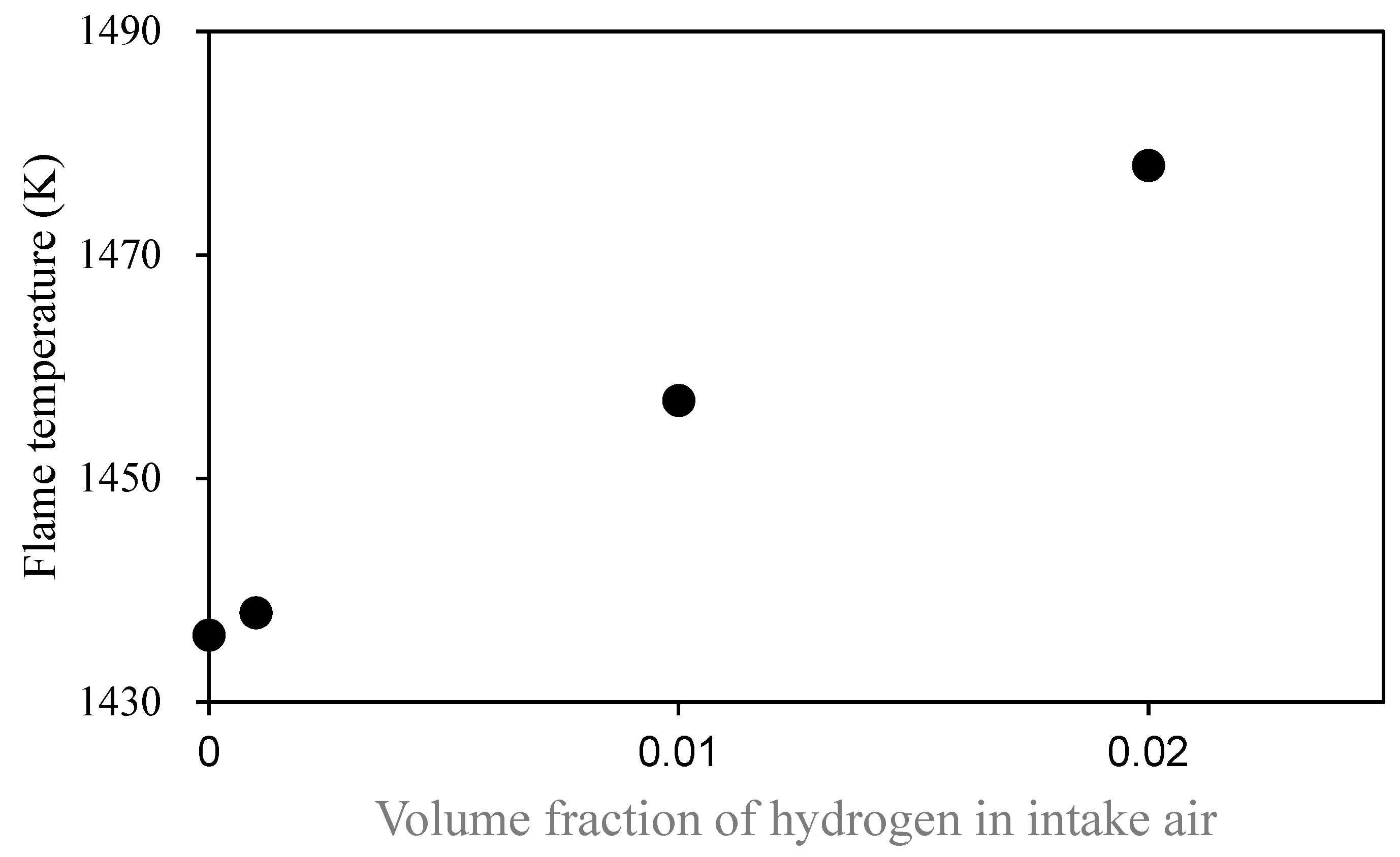
Figure 6 shows the change in combustion temperature predicted by the model calculations.
Since the calculation conditions of the model were different from those in real, these absolute values of the predicted temperatures cannot be applied to the experiments. An increase in combustion temperature was relatively predicted as the hydrogen addition increased, as shown in
Figure 6. The same trend was also observed in simulations under stoichiometric and lean conditions. Talibi et al. reported that hydrogenation increases NO
x emissions only when the combustion temperature exceeds a threshold temperature and decreases when the temperature is below the threshold [
13]. The small amount of hydrogen added (HES~1%) resulted in a decrease in NO
x as the temperature did not exceed the threshold value, whereas the condition with sufficient hydrogen added resulted in an increase in NO
x as the temperature rose above the threshold value.
The percentage reduction in CO and PM relative to the percentage reduction in diesel consumption due to the addition of hydrogen is shown in
Figure 7.
Almost all running tests demonstrated reduced CO and PM emissions with the addition of hydrogen. As CO and PM are derived from diesel, it is assumed that the addition of hydrogen reduced diesel combustion consumption, and therefore emissions were reduced accordingly. With the hydrogen addition at a rate of 2 L/min (HES~1%), the rate of reduction in CO and PM emissions was consistent with the rate of reduction in diesel consumption. Furthermore, as the hydrogen addition increased, both the emissions of CO and PM were reduced by more than the reduction in diesel consumption. This can be explained by considering that the addition of hydrogen raises the combustion temperature, increasing the degree of complete combustion and reducing CO and PM emissions [
13].
Since hydrocarbons also originate from diesel combustion, such as CO and PM, THC emissions also changed with the addition of hydrogen. The relationship between THC emissions and the reduction in diesel consumption due to the addition of hydrogen gas for each driving test is shown in
Figure 8.
With the addition of a large quantity of hydrogen (≥40 L/min, ≥10% HES), a reduction in THC emissions was observed. For JE 05 driving cycle, the rate of reduction in THC emissions was greater than that in diesel consumption alone. For running tests at higher average speeds other than JE 05 driving cycle, although THC emissions were reduced, the rate of reduction was smaller than those in diesel consumption alone. On the contrary, for the addition of a smaller quantity of hydrogen (<40 L/min, <10% HES), THC emissions increased.
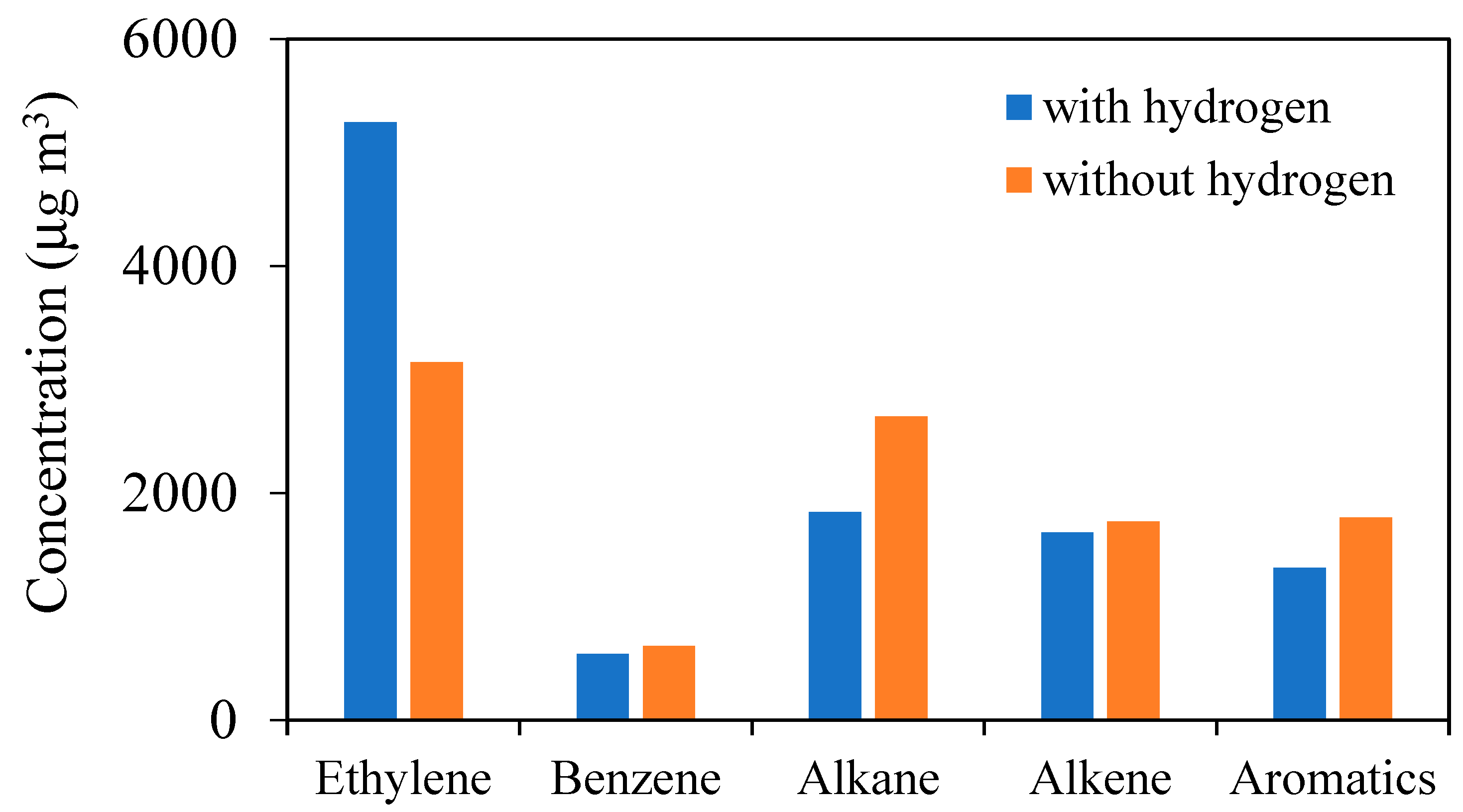
Figure 9 shows the change in HC components with hydrogen addition (2 L/min) at the constant speed of 40 km/h in a running test, as measured by the GC-FID.
Except for ethylene, a reduction in measured VOCs was observed after the addition of hydrogen. This is mainly due to the reduction in diesel consumption after hydrogen addition. Although not all VOCs were analyzed, it was assumed that the increase in combustion temperature due to hydrogen addition accelerated the decomposition of the VOCs with a higher carbon number, resulting in a simultaneous increase in ethylene emissions. Since a large amount of acetylene is emitted as an exhaust gas component from diesel vehicles, besides ethylene, acetylene emissions may also have increased. This promotion of VOC decomposition reactions is also thought to have suppressed PM formation besides contributing to the degree of complete combustion.
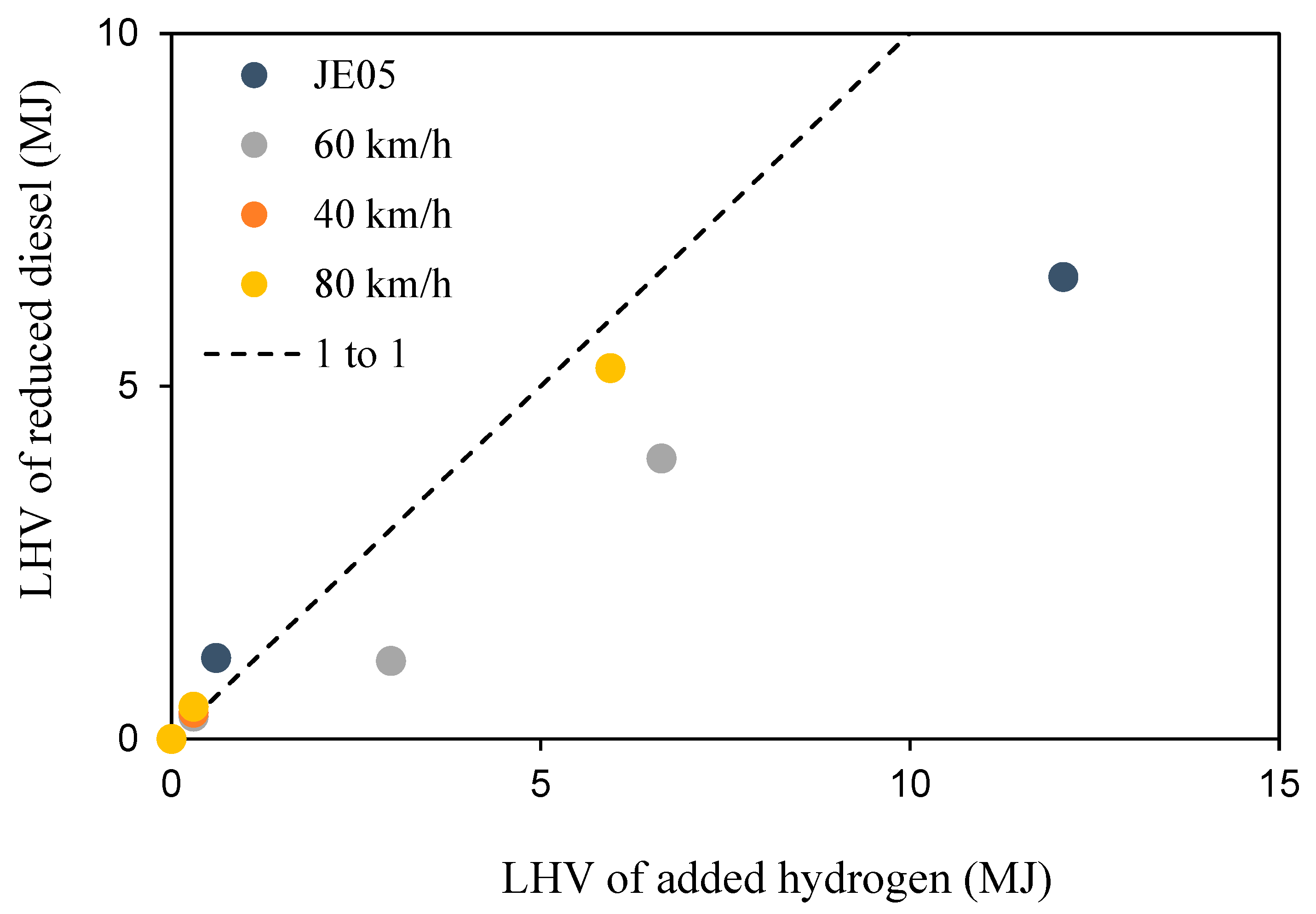
Finally, the energy balance of hydrogen addition was checked. A comparison of the LHV of the added hydrogen and that of the diesel suppressed by hydrogen addition is shown in
Figure 10.
For small hydrogen additions (<40 L/min, <10% HES), the LHV of the added hydrogen has shown to be consistent with the LHV of the reduced diesel fuel. On the other hand, for large hydrogen additions (≥40 L/min, ≥10% HES), the LHV of the added hydrogen was greater than the LHV of the reduced diesel. For JE05, only 50% of the energy of added hydrogen was used to power the truck. Presumably, some of the energy gained from combustion was used to increase the combustion temperature, which, in turn, increased the total energy.
4. Conclusions
In this study, hydrogen was added to the intake air of a diesel truck having a four-cylinder engine during running tests on a chassis dynamometer to investigate the effect of hydrogen addition on the engine’s fuel consumption and exhaust gas composition. The observed effects of hydrogen addition in JE05 actual driving mode and constant speed driving modes were similar and corresponded to the previous studies conducted using an engine dynamometer. The benefits included a reduction in diesel consumption and a reduction in CO2, CO, and PM emissions.
The reduction in diesel consumption and the increase in HES showed almost linear dependence, where the percentage decrease in diesel consumption is approximately 0.6 × HES. The percentage reduction of CO2 showed a one-to-one relationship to the reduction in diesel consumption, i.e., approximately 0.6 × HES. The reduction in emissions of CO, PM, and HCs (except for ethylene) has a one-to-one or larger correlation with the reduction of diesel consumption, under the conditions of this study. This could be explained by an increase in the combustion temperature with increasing hydrogen addition.
On the other hand, as an apparent disadvantage, it was observed that NOx emissions increased in this study owing to the increase in combustion temperature. The effect of hydrogen was smallest in JE05 and largest at a constant speed of 80 km/h, the percentage increase of NOx was 1.5~2.0 times that of the HES It was also observed that THC emissions increased because of a substantial increase in ethylene emissions when hydrogen addition was not sufficient. The requirement of total LHV was more when hydrogen was added than for diesel alone. For JE05, only 50% of the energy of added hydrogen was used to power the truck. This also could be because energy is partly being used for increasing the combustion temperature.
A major advantage of hydrogen-mixed combustion diesel vehicles is that a hydrogen tank could be installed in vehicles equipped with CI engines. Hydrogen additions to engines could be an inexpensive and simple low-carbon strategy using existing CI engines with little or no modification on engines. However, as mentioned above, an increase in NOx emission and total energy consumption exists as a major disadvantage of hydrogen addition. How to deal with those disadvantages is the next issue to be considered.