Investigation of Optimal Temperature for Thermal Catalytic Conversion of Marine Biomass for Recovery of Higher-Added-Value Energy Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Characterisation
2.2. Thermal and Chemical Analysis at the Small Scale (TGA-FTIR-GC/MS)
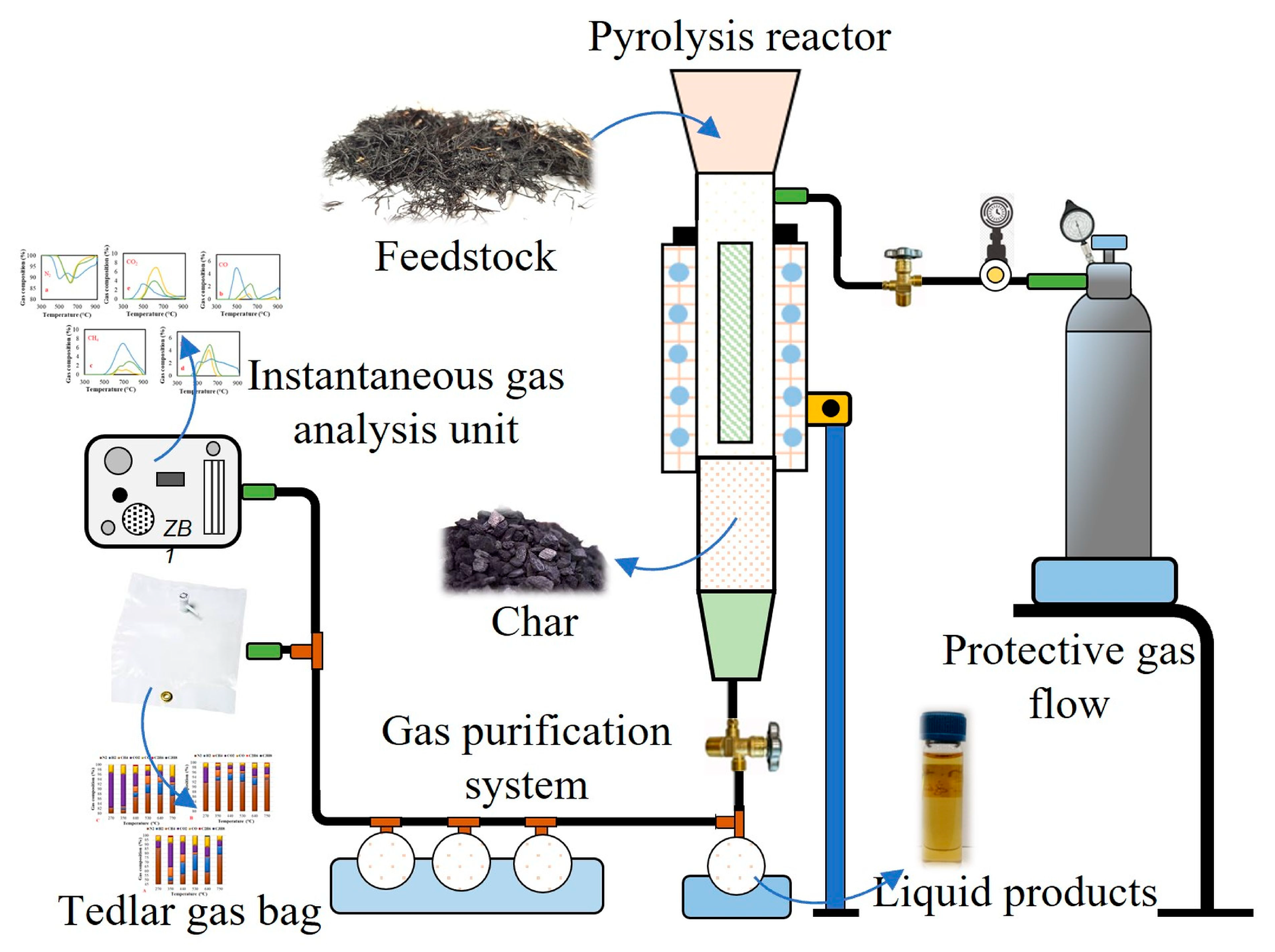
2.3. Pyrolysis Experiments at the Laboratory Scale Bench
3. Results
3.1. Feedstock Characterisation
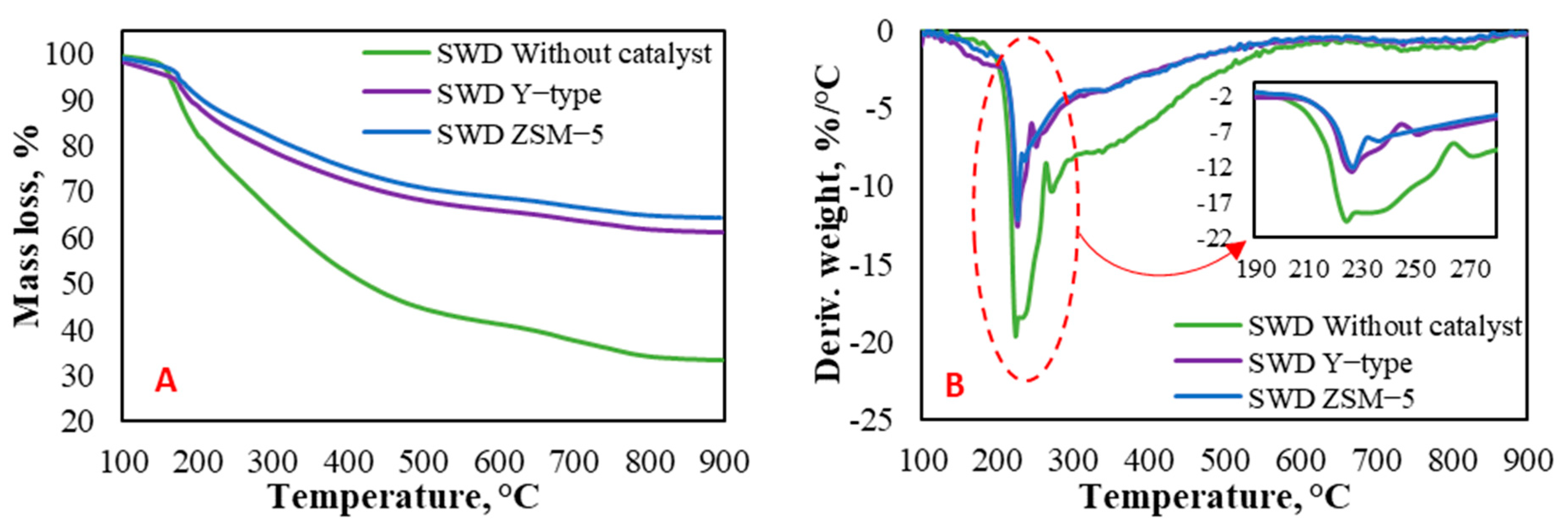
3.2. TGA-DTG-FTIR Analysis of the Seaweed Sample
3.3. Pyrolysis at Laboratory Scale Experimental Bench
3.3.1. Pyrolysis Temperature Influence Analysis for Gas Composition during the Whole Process
3.3.2. Pyrolysis Gaseous Product Analysis by the GC/MS
3.3.3. Liquid Product Analysis by the GC/MS
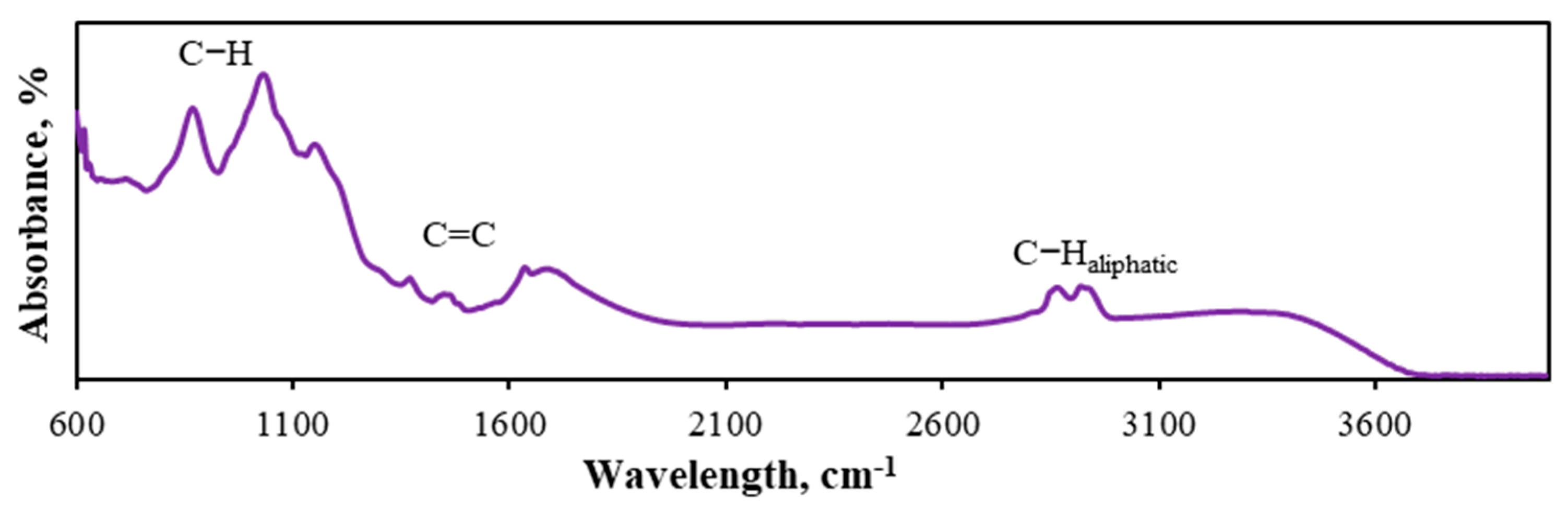
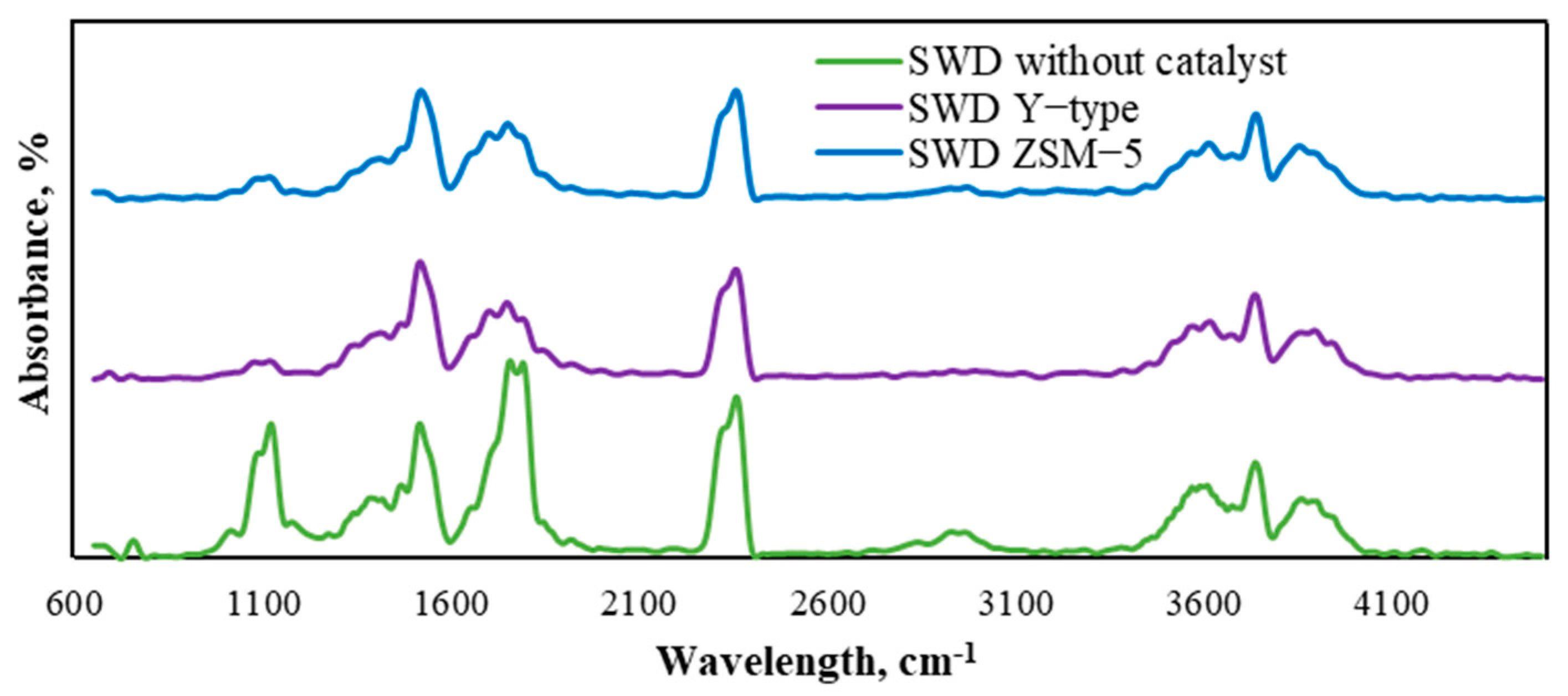
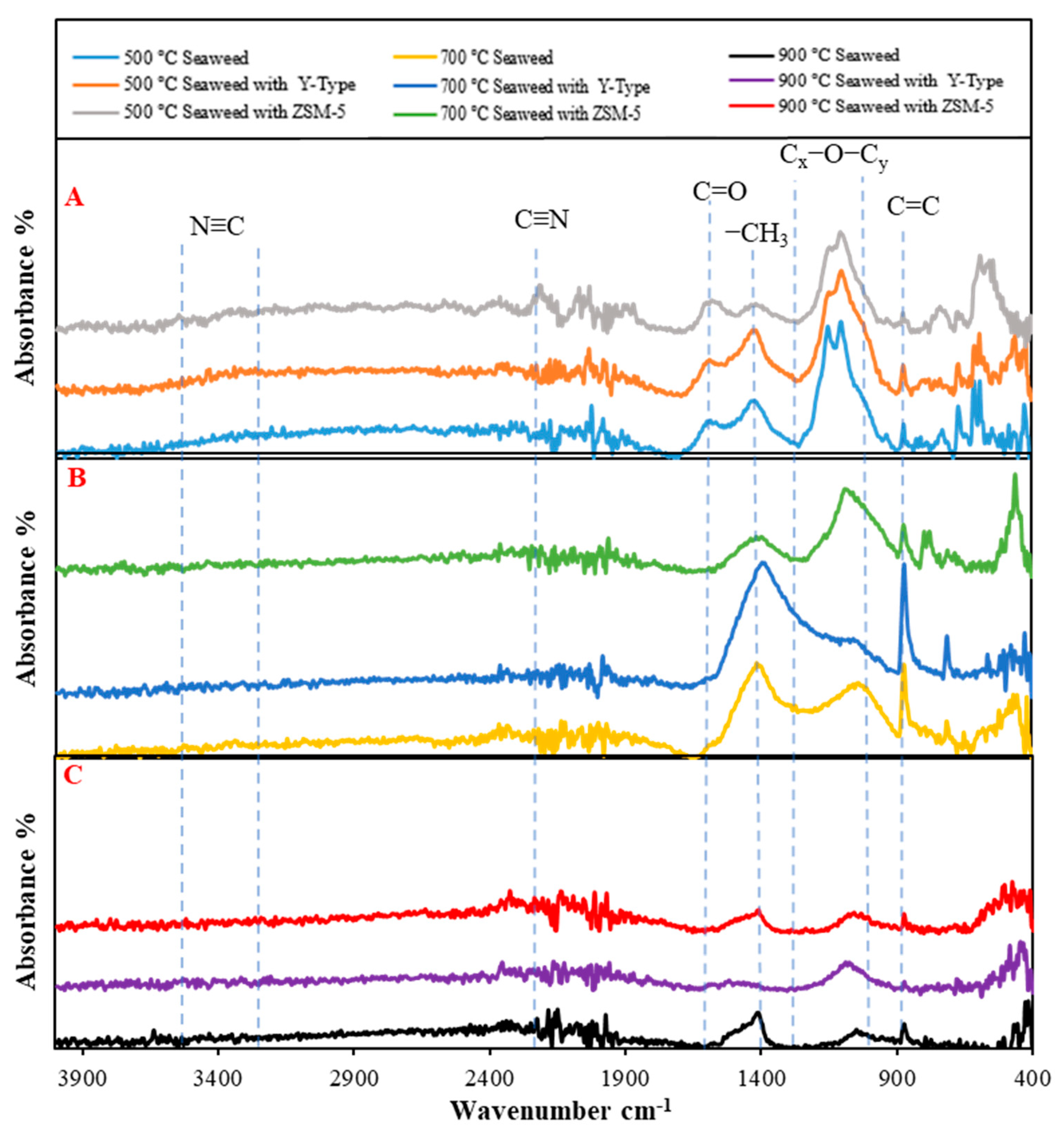
3.3.4. Solid Product Analysis by the FTIR
3.3.5. The Yields of the Recovered Pyrolysis Products
3.3.6. A Comparison between Temperature and Catalyst Influence for the Product Formation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kober, T.; Schiffer, H.W.; Densing, M.; Panos, E. Global Energy Perspectives to 2060—WEC’s World Energy Scenarios 2019. Energy Strateg. Rev. 2020, 31, 100523. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union Regulation (EU) 2019/943 of the European Parliament and of the Council of 5 June 2019 on the Internal Market for Electricity. Off. J. Eur. Union 2019, 62, 54–191.
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Kwon, E.E.; Nadagouda, M.N.; Aminabhavi, T.M. Biomass Utilization and Production of Biofuels from Carbon Neutral Materials. Environ. Pollut. 2021, 276, 116731. [Google Scholar] [CrossRef]
- Cao, B.; Xia, Z.; Wang, S.; Abomohra, A.E.F.; Cai, N.; Hu, Y.; Yuan, C.; Qian, L.; Liu, L.; Liu, X.; et al. A Study on Catalytic Co-Pyrolysis of Cellulose with Seaweeds Polysaccharides over ZSM-5: Towards High-Quality Biofuel Production. J. Anal. Appl. Pyrolysis 2018, 134, 526–535. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Wang, S.; Xu, L.; Barati, B.; Cao, B.; Wang, H.; Xie, K.; Wang, Q. Catalytic Fast Hydropyrolysis of Seaweed Biomass with Different Zeolite Catalysts to Produce High-Grade Bio-Oil. Process Saf. Environ. Prot. 2021, 146, 69–76. [Google Scholar] [CrossRef]
- Atangana Njock, P.G.; Zhou, A.; Yin, Z.; Shen, S.L. Integrated Risk Assessment Approach for Eutrophication in Coastal Waters: Case of Baltic Sea. J. Clean. Prod. 2023, 387, 135673. [Google Scholar] [CrossRef]
- Vigouroux, G.; Kari, E.; Beltrán-Abaunza, J.M.; Uotila, P.; Yuan, D.; Destouni, G. Trend Correlations for Coastal Eutrophication and Its Main Local and Whole-Sea Drivers—Application to the Baltic Sea. Sci. Total Environ. 2021, 779, 146367. [Google Scholar] [CrossRef]
- Kotta, J.; Futter, M.; Kaasik, A.; Liversage, K.; Rätsep, M.; Barboza, F.R.; Bergström, L.; Bergström, P.; Bobsien, I.; Díaz, E.; et al. Cleaning up Seas Using Blue Growth Initiatives: Mussel Farming for Eutrophication Control in the Baltic Sea. Sci. Total Environ. 2020, 709, 136144. [Google Scholar] [CrossRef]
- Weinberger, F.; Paalme, T.; Wikström, S.A. Seaweed Resources of the Baltic Sea, Kattegat and German and Danish North Sea Coasts. Bot. Mar. 2020, 63, 61–72. [Google Scholar] [CrossRef]
- Lowery, C.M.; Leckie, R.M.; Bryant, R.; Elderbak, K.; Parker, A.; Polyak, D.E.; Schmidt, M.; Snoeyenbos-West, O.; Sterzinar, E. The Late Cretaceous Western Interior Seaway as a Model for Oxygenation Change in Epicontinental Restricted Basins. Earth-Sci. Rev. 2018, 177, 545–564. [Google Scholar] [CrossRef]
- Syrpas, M.; Bukauskaitė, J.; Paškauskas, R.; Bašinskienė, L.; Venskutonis, P.R. Recovery of Lipophilic Products from Wild Cyanobacteria (Aphanizomenon Flos-Aquae) Isolated from the Curonian Lagoon by Means of Supercritical Carbon Dioxide Extraction. Algal Res. 2018, 35, 10–21. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Afflerbach, J.C.; Frazier, M.; Halpern, B.S. Blue Growth Potential to Mitigate Climate Change through Seaweed Offsetting. Curr. Biol. 2019, 29, 3087–3093.e3. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.B.; Shelamoff, V.; Layton, C. Seaweed Ecosystems May Not Mitigate CO2emissions. ICES J. Mar. Sci. 2022, 79, 585–592. [Google Scholar] [CrossRef]
- Jiang, D.; Xia, Z.; Wang, S.; Li, H.; Gong, X.; Yuan, C.; El-Fatah Abomohra, A.; Cao, B.; Hu, X.; He, Z.; et al. Mechanism Research on Catalytic Pyrolysis of Sulfated Polysaccharide Using ZSM-5 Catalysts by Py-GC/MS and Density Functional Theory Studies. J. Anal. Appl. Pyrolysis 2019, 143, 104680. [Google Scholar] [CrossRef]
- Yuan, C.; Jiang, D.; Wang, S.; Barati, B.; Gong, X.; Cao, B.; Zhang, R.P.; Zhang, C.; Odey, E.A. Study on Catalytic Pyrolysis Mechanism of Seaweed Polysaccharide Monomer. Combust. Flame 2020, 218, 1–11. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Chen, Y.; Xia, M.; Chen, X.; Chen, H. Transformation of Nitrogen and Evolution of N-Containing Species during Algae Pyrolysis. Environ. Sci. Technol. 2017, 51, 6570–6579. [Google Scholar] [CrossRef]
- Ye, N.; Li, D.; Chen, L.; Zhang, X.; Xu, D. Comparative Studies of the Pyrolytic and Kinetic Characteristics of Maize Straw and the Seaweed Ulva Pertusa. PLoS ONE 2010, 5, e12641. [Google Scholar] [CrossRef]
- Aboulkas, A.; Hammani, H.; El Achaby, M.; Bilal, E.; Barakat, A.; El harfi, K. Valorization of Algal Waste via Pyrolysis in a Fixed-Bed Reactor: Production and Characterization of Bio-Oil and Bio-Char. Bioresour. Technol. 2017, 243, 400–408. [Google Scholar] [CrossRef]
- Nawaz, A.; Kumar, P. Thermocatalytic Pyrolysis of Sesbania bispinosa Biomass over Y-Zeolite Catalyst towards Clean Fuel and Valuable Chemicals. Energy 2023, 263, 125684. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. Influence of Carbon Black Filler on Pyrolysis Kinetic Behaviour and TG-FTIR-GC–MS Analysis of Glass Fibre Reinforced Polymer Composites. Energy 2021, 233, 121167. [Google Scholar] [CrossRef]
- Gözke, G.; Açıkalın, K. Pyrolysis Characteristics and Kinetics of Sour Cherry Stalk and Flesh via Thermogravimetric Analysis Using Isoconversional Methods. J. Therm. Anal. Calorim. 2021, 146, 893–910. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Beerlandt, J.; Dewil, R. Thermogravimetric Pyrolysis of Waste Polyethylene-Terephthalate and Polystyrene: A Critical Assessment of Kinetics Modelling. Resour. Conserv. Recycl. 2011, 55, 772–781. [Google Scholar] [CrossRef]
- Torres-Sciancalepore, R.; Asensio, D.; Nassini, D.; Fernandez, A.; Rodriguez, R.; Fouga, G.; Mazza, G. Assessment of the Behavior of Rosa Rubiginosa Seed Waste during Slow Pyrolysis Process towards Complete Recovery: Kinetic Modeling and Product Analysis. Energy Convers. Manag. 2022, 272, 116340. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.M.; Wang, N.; Yu, L.J.; Li, Z.; He, P.M. Research on Pyrolysis Characteristics of Seaweed. Energy Fuels 2007, 21, 3723–3729. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Stasiulaitiene, I.; Zakarauskas, K.; Striūgas, N. Pyrolysis of All Layers of Surgical Mask Waste as a Mixture and Its Life-Cycle Assessment. Sustain. Prod. Consum. 2022, 32, 519–531. [Google Scholar] [CrossRef]
- ASTM E870-82; Standard Test Methods for Analysis of Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2011. [CrossRef]
- Premarathna, A.D.; Tuvikene, R.; Fernando, P.H.P.; Adhikari, R.; Perera, M.C.N.; Ranahewa, T.H.; Howlader, M.M.; Wangchuk, P.; Jayasooriya, A.P.; Rajapakse, R.P.V.J. Comparative Analysis of Proximate Compositions, Mineral and Functional Chemical Groups of 15 Different Seaweed Species. Sci. Rep. 2022, 12, 19610. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, W.; Xu, W.; Liu, Y. Removal of Elemental Mercury by Bio-Chars Derived from Seaweed Impregnated with Potassium Iodine. Chem. Eng. J. 2018, 339, 468–478. [Google Scholar] [CrossRef]
- Meenakshi, S.; Umayaparvathi, S.; Arumugam, M.; Balasubramanian, T. In Vitro Antioxidant Properties and FTIR Analysis of Two Seaweeds of Gulf of Mannar. Asian Pac. J. Trop. Biomed. 2011, 1, S66–S70. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.P. The Effect of the Biomass Components Lignin, Cellulose and Hemicellulose on TGA and Fixed Bed Pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Waters, C.L.; Janupala, R.R.; Mallinson, R.G.; Lobban, L.L. Staged Thermal Fractionation for Segregation of Lignin and Cellulose Pyrolysis Products: An Experimental Study of Residence Time and Temperature Effects. J. Anal. Appl. Pyrolysis 2017, 126, 380–389. [Google Scholar] [CrossRef]
- Yousef, S.; Kiminaitė, I.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. Recovery of Phenol and Acetic Acid from Glass Fibre Reinforced Thermoplastic Resin Using Catalytic Pyrolysis Process on ZSM-5 Zeolite Catalyst and Its Kinetic Behaviour. Thermochim. Acta 2022, 715, 179293. [Google Scholar] [CrossRef]
- Khan, N.; Sudhakar, K.; Mamat, R. Thermogravimetric Analysis of Marine Macroalgae Waste Biomass as Bio-Renewable Fuel. J. Chem. 2022, 2022, 9. [Google Scholar] [CrossRef]
- Sanchez-Silva, L.; López-González, D.; Villaseñor, J.; Sánchez, P.; Valverde, J.L. Thermogravimetric-Mass Spectrometric Analysis of Lignocellulosic and Marine Biomass Pyrolysis. Bioresour. Technol. 2012, 109, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Gani, A.; Naruse, I. Effect of Cellulose and Lignin Content on Pyrolysis and Combustion Characteristics for Several Types of Biomass. Renew. Energy 2007, 32, 649–661. [Google Scholar] [CrossRef]
- Kumar, A.; Jindal, M.; Rawat, S.; Kumar, J.; Sripadi, P.; Yang, B.; Thallada, B. Upgradation of Sugarcane Bagasse Lignin: Fractionation to Cyclic Alcohols Production. Catal. Today 2023, 408, 182–193. [Google Scholar] [CrossRef]
- Eimontas, J.; Striūgas, N.; Abdelnaby, M.A.; Yousef, S. Catalytic Pyrolysis Kinetic Behavior and TG-FTIR-GC–MS Analysis of Metallized Food Packaging Plastics with Different Concentrations of ZSM-5 Zeolite Catalyst. Polymers 2021, 13, 702. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Hu, Y.M.; Xu, S.N.; He, Z.X.; Ji, H.S. Study on the Synergistic Co-Pyrolysis Behaviors of Mixed Rice Husk and Two Types of Seaweed by a Combined TG-FTIR Technique. J. Anal. Appl. Pyrolysis 2015, 114, 109–118. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, E.; Chen, A. Volatile Production from Pyrolysis of Cellulose, Hemicellulose and Lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Eimontas, J.; Striūgas, N.; Zakarauskas, K.; Navickas, K.; Venslauskas, K. Synergetic Approach for Energy Recovery from Coastal Wastes Based on Combination of Biological and Thermal Treatment. Environ. Technol. 2021, 43, 2755–2770. [Google Scholar] [CrossRef] [PubMed]
- Acomb, J.C.; Wu, C.; Williams, P.T. The Use of Different Metal Catalysts for the Simultaneous Production of Carbon Nanotubes and Hydrogen from Pyrolysis of Plastic Feedstocks. Appl. Catal. B Environ. 2016, 180, 497–510. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, N.; Xie, L.; Li, A. Study of the Fast Pyrolysis of Oilfield Sludge with Solid Heat Carrier in a Rotary Kiln for Pyrolytic Oil Production. J. Anal. Appl. Pyrolysis 2014, 105, 183–190. [Google Scholar] [CrossRef]
- Su, B.; Han, W.; He, H.; Jin, H.; Yang, S.; Zhang, X. Mechanism and Experimental Validation of a Thermochemical Energy Conversion Process by Utilization of Biogas Chemical Energy. Energy Convers. Manag. 2020, 214, 112827. [Google Scholar] [CrossRef]
- Ng, H.M.; Saidi, N.M.; Omar, F.S.; Ramesh, K.; Ramesh, S.; Bashir, S. Thermogravimetric Analysis of Polymers. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Lin, H.; Wang, S.; Zhang, L.; Ru, B.; Zhou, J.; Luo, Z. Structural Evolution of Chars from Biomass Components Pyrolysis in a Xenon Lamp Radiation Reactor. Chin. J. Chem. Eng. 2017, 25, 232–237. [Google Scholar] [CrossRef]
- Xin, S.; Yang, H.; Chen, Y.; Yang, M.; Chen, L.; Wang, X.; Chen, H. Chemical Structure Evolution of Char during the Pyrolysis of Cellulose. J. Anal. Appl. Pyrolysis 2015, 116, 263–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Chen, C.; Wang, Y.; Wang, W.; Li, A. Liquid Oils Produced from Pyrolysis of Plastic Wastes with Heat Carrier in Rotary Kiln. Fuel Process. Technol. 2020, 206, 106455. [Google Scholar] [CrossRef]
- Eimontas, J.; Yousef, S.; Striūgas, N.; Abdelnaby, M.A. Catalytic Pyrolysis Kinetic Behaviour and TG-FTIR-GC–MS Analysis of Waste Fishing Nets over ZSM-5 Zeolite Catalyst for Caprolactam Recovery. Renew. Energy 2021, 179, 1385–1403. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; Da Silva, J.C.G.; De Sena, R.F.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Thermal Investigation of Plastic Solid Waste Pyrolysis via the Deconvolution Technique Using the Asymmetric Double Sigmoidal Function: Determination of the Kinetic Triplet, Thermodynamic Parameters, Thermal Lifetime and Pyrolytic Oil Composition for Clean. Energy Convers. Manag. 2019, 200, 112031. [Google Scholar] [CrossRef]
- Miao, P.; Li, K.; Fan, J.; Xu, N.; Zhu, X.; Li, C. Efficient Ring-Opening Reaction of Tetralin over Nanosized ZSM-5 Zeolite: Effect of SiO2/Al2O3 Ratio and Reaction Condition. Energy Fuels 2019, 33, 9480–9490. [Google Scholar] [CrossRef]
- Chen, L.; Cui, W.; Li, J.; Wang, H.; Dong, X.; Chen, P.; Zhou, Y.; Dong, F. The High Selectivity for Benzoic Acid Formation on Ca2Sb2O7 Enables Efficient and Stable Toluene Mineralization. Appl. Catal. B Environ. 2020, 271, 118948. [Google Scholar] [CrossRef]
- Bento, C.; Gonçalves, A.C.; Jesus, F.; Simões, M.; Silva, L.R. Phenolic Compounds: Sources, Properties and Applications. In Bioactive Compounds: Sources, Properties and Applications; Nova Science Pub Inc.: Hauppauge, NY, USA, 2017; ISBN 9781536124248. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015. [CrossRef]
- Novais, C.; Pereira, C.; Molina, A.K.; Liberal, Â.; Dias, M.I.; Añibarro-Ortega, M.; Alves, M.J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Barros, L. Bioactive and Nutritional Potential of Medicinal and Aromatic Plant (Map) Seasoning Mixtures. Molecules 2021, 26, 1587. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, Y.; Tamura, M.; Miyaoka, S.; Kumagai, S.; Tanji, M.; Nakagawa, Y.; Yoshioka, T.; Tomishige, K. Low-Temperature Catalytic Upgrading of Waste Polyolefinic Plastics into Liquid Fuels and Waxes. Appl. Catal. B Environ. 2021, 285, 119805. [Google Scholar] [CrossRef]
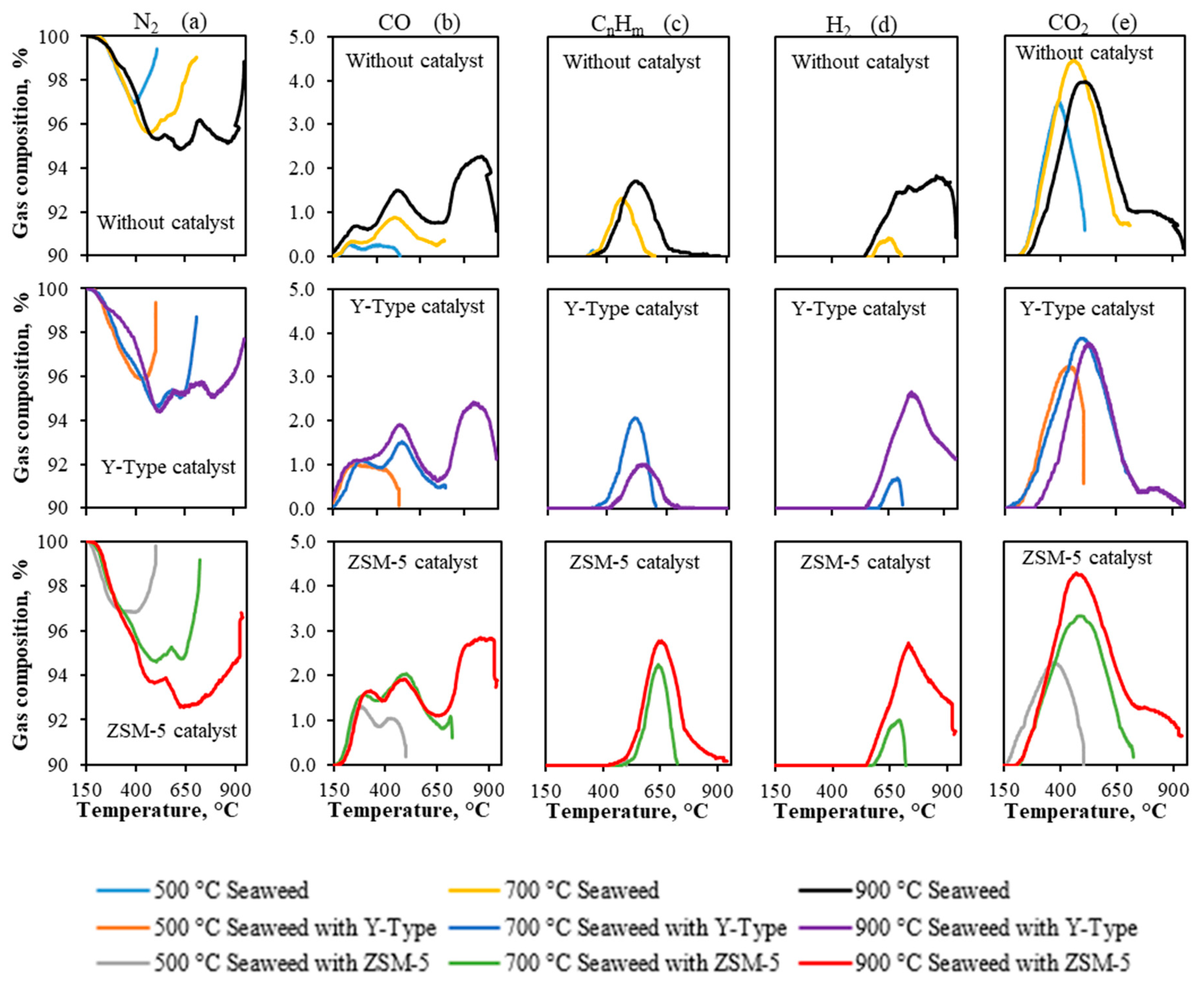
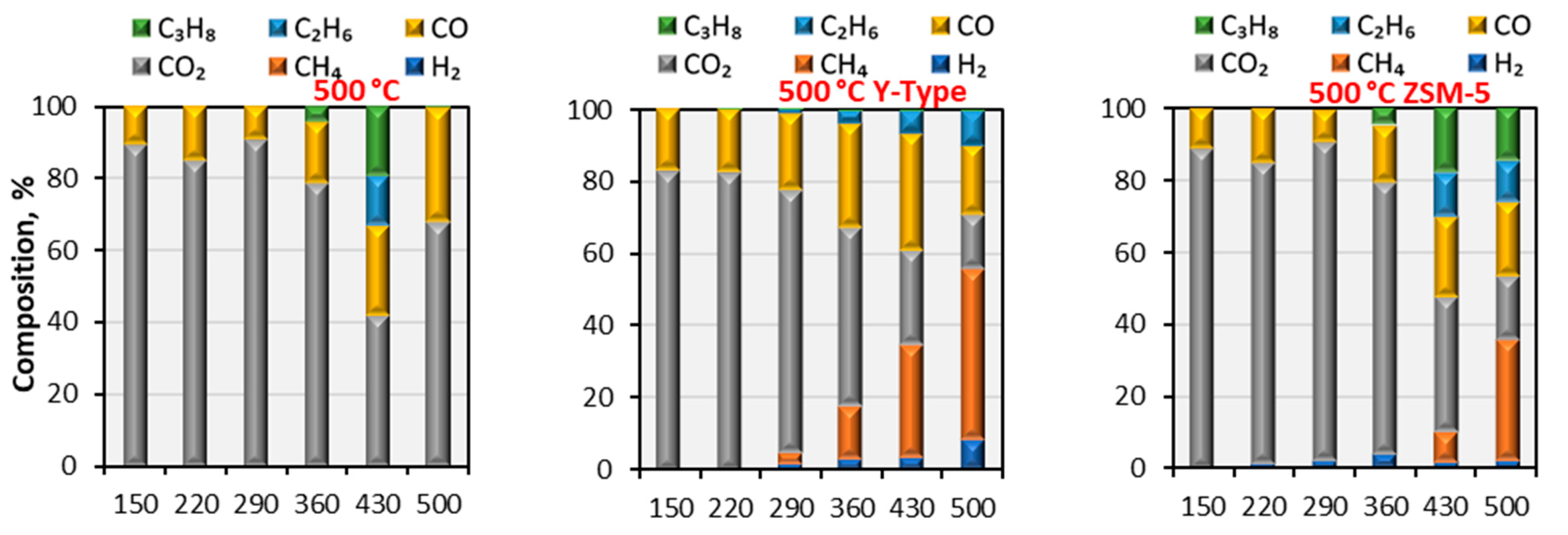
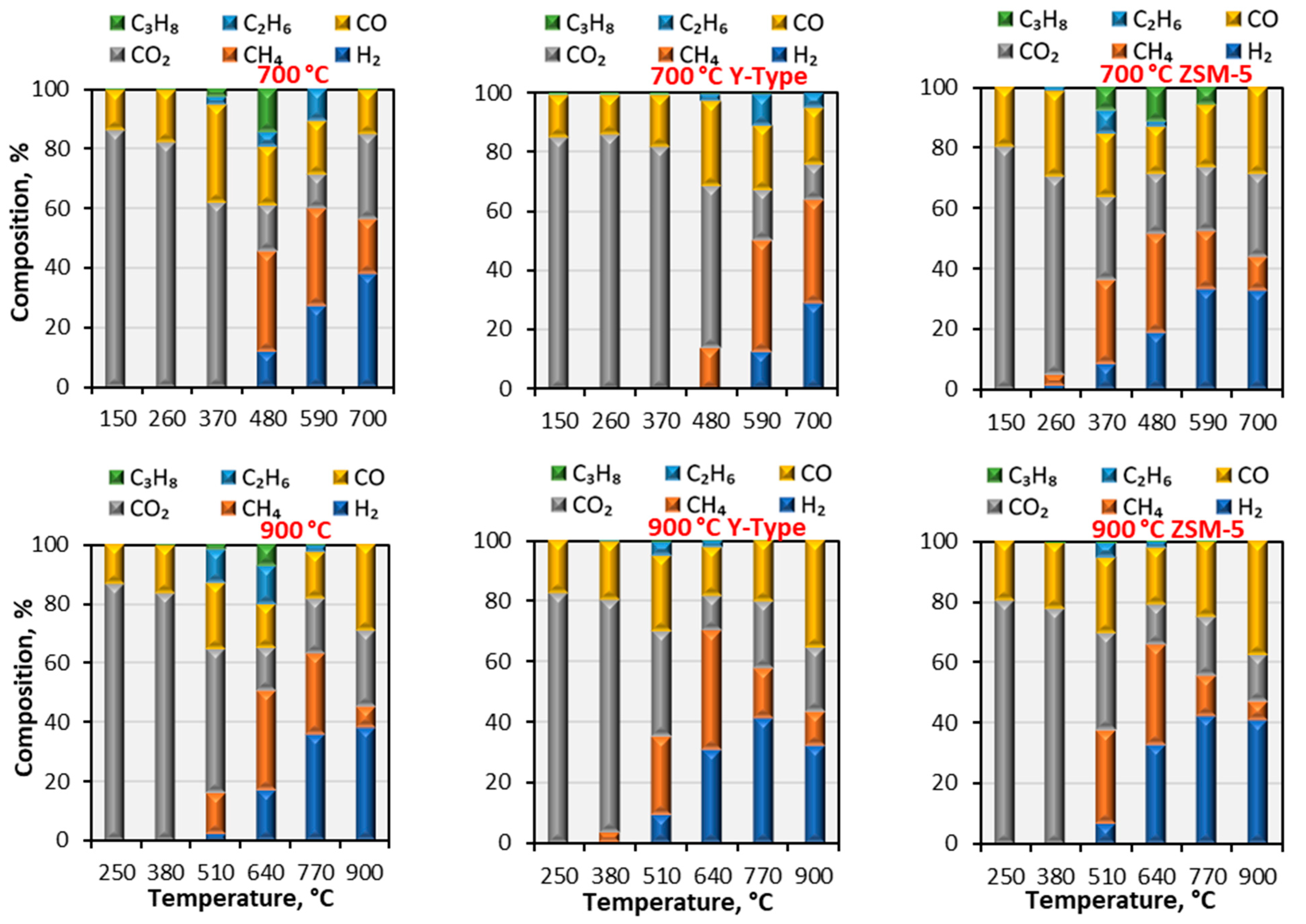
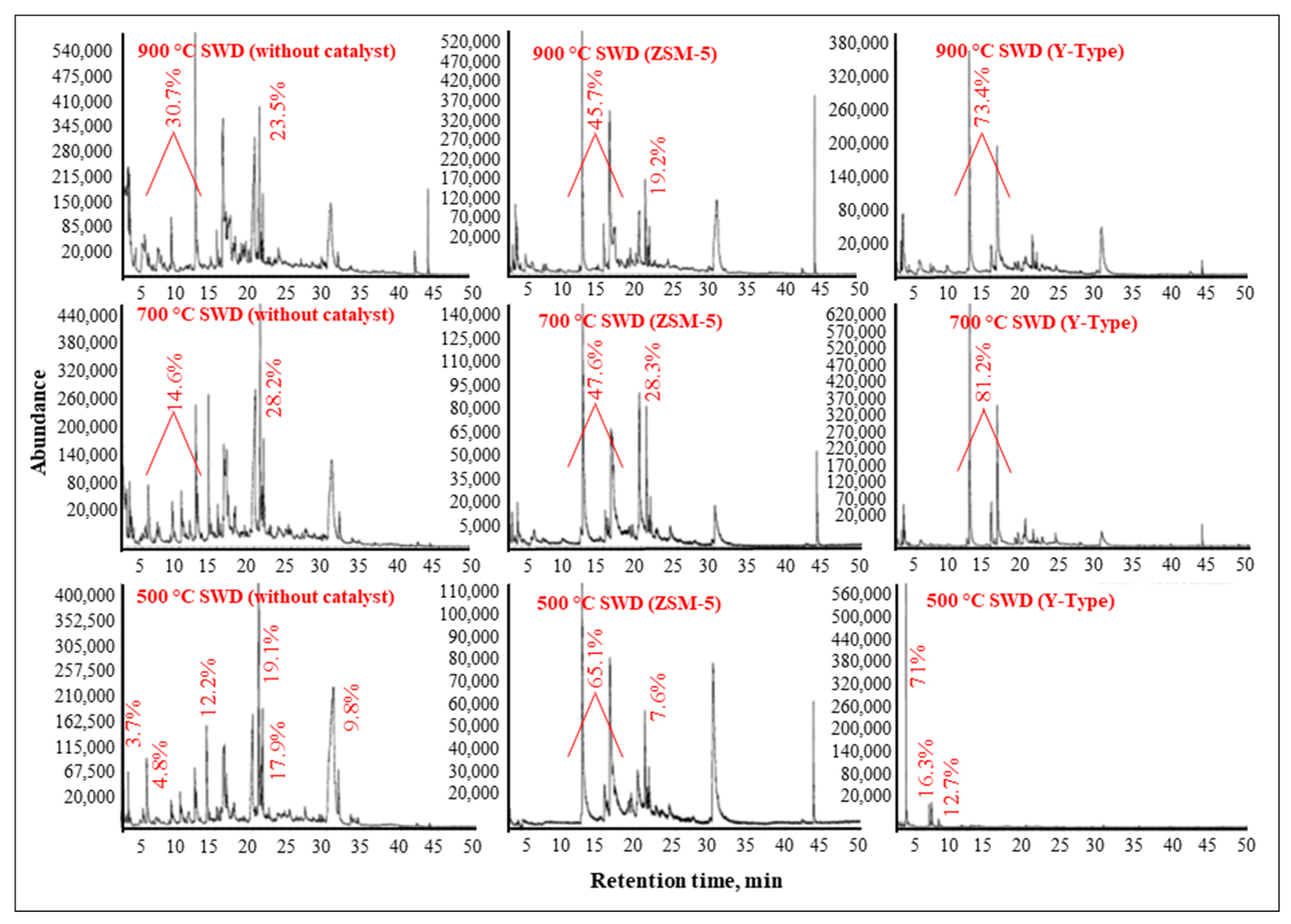
| ZSM-5 | Y-Type |
|---|---|
| SiO2/Al2O3 | SiO2/Al2O3 |
| Molar ratio—38 | Molar ratio—>5 |
| Specific surface area—≥250 m2/g | Specific surface area—≥578 m2/g |
| Pore volume (≥0.25 mL/g) | Unit cell size (<2.453 nm) |
| Dimension (Φ2 × 2–10 mm) | Particle size distribution D50 (<6 µm) |
| Bulk density (0.72 kg/L) | Bulk density (0.68 kg/L) |
| Crushing strength (≥98 N/cm2) | Crushing strength (≥142 N/cm2) |
| Parameter, d.b. | Seaweed |
|---|---|
| Carbon, wt.% | 46.93 ± 0.05 |
| Hydrogen, wt.% | 4.73 ± 0.06 |
| Nitrogen, wt.% | 4.13 ± 0.14 |
| Oxygen, wt.% (diff.) | 30.16 ± 0.51 |
| Chloride, wt.% | 0.05 ± 0.01 |
| Sulphur, wt.% | 5.13 ± 0.23 |
| Volatiles, wt.% | 58.30 ± 0.19 |
| Ashes, wt.% | 8.87 ± 0.04 |
| Moisture, wt.% | 0.55 ± 0.01 |
| Fixed carbon, wt.% | 32.23 ± 0.15 |
| LHV, MJ/kg | 16.51 ± 0.07 |
| Sample | Liquids, wt.% | Gasses, wt.% | Solids, wt.% |
|---|---|---|---|
| Seaweed 500 °C | 20.22 | 32.51 | 47.27 |
| Seaweed 700 °C | 19.33 | 42.45 | 38.22 |
| Seaweed 900 °C | 18.27 | 47.44 | 34.30 |
| Seaweed 500 °C ZSM-5 | 24.04 | 34.66 | 41.30 |
| Seaweed 700 °C ZSM-5 | 21.97 | 39.72 | 38.31 |
| Seaweed 900 °C ZSM-5 | 19.81 | 45.55 | 34.64 |
| Seaweed 500 °C Y-Type | 25.87 | 29.62 | 44.51 |
| Seaweed 700 °C Y-Type | 22.21 | 39.19 | 38.60 |
| Seaweed 900 °C Y-Type | 20.53 | 44.22 | 35.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eimontas, J.; Jančauskas, A.; Zakarauskas, K.; Striūgas, N.; Vorotinskienė, L. Investigation of Optimal Temperature for Thermal Catalytic Conversion of Marine Biomass for Recovery of Higher-Added-Value Energy Products. Energies 2023, 16, 3457. https://doi.org/10.3390/en16083457
Eimontas J, Jančauskas A, Zakarauskas K, Striūgas N, Vorotinskienė L. Investigation of Optimal Temperature for Thermal Catalytic Conversion of Marine Biomass for Recovery of Higher-Added-Value Energy Products. Energies. 2023; 16(8):3457. https://doi.org/10.3390/en16083457
Chicago/Turabian StyleEimontas, Justas, Adolfas Jančauskas, Kęstutis Zakarauskas, Nerijus Striūgas, and Lina Vorotinskienė. 2023. "Investigation of Optimal Temperature for Thermal Catalytic Conversion of Marine Biomass for Recovery of Higher-Added-Value Energy Products" Energies 16, no. 8: 3457. https://doi.org/10.3390/en16083457
APA StyleEimontas, J., Jančauskas, A., Zakarauskas, K., Striūgas, N., & Vorotinskienė, L. (2023). Investigation of Optimal Temperature for Thermal Catalytic Conversion of Marine Biomass for Recovery of Higher-Added-Value Energy Products. Energies, 16(8), 3457. https://doi.org/10.3390/en16083457








