Oxidation Kinetics of Neat Methyl Oleate and as a Blend with Solketal
Abstract
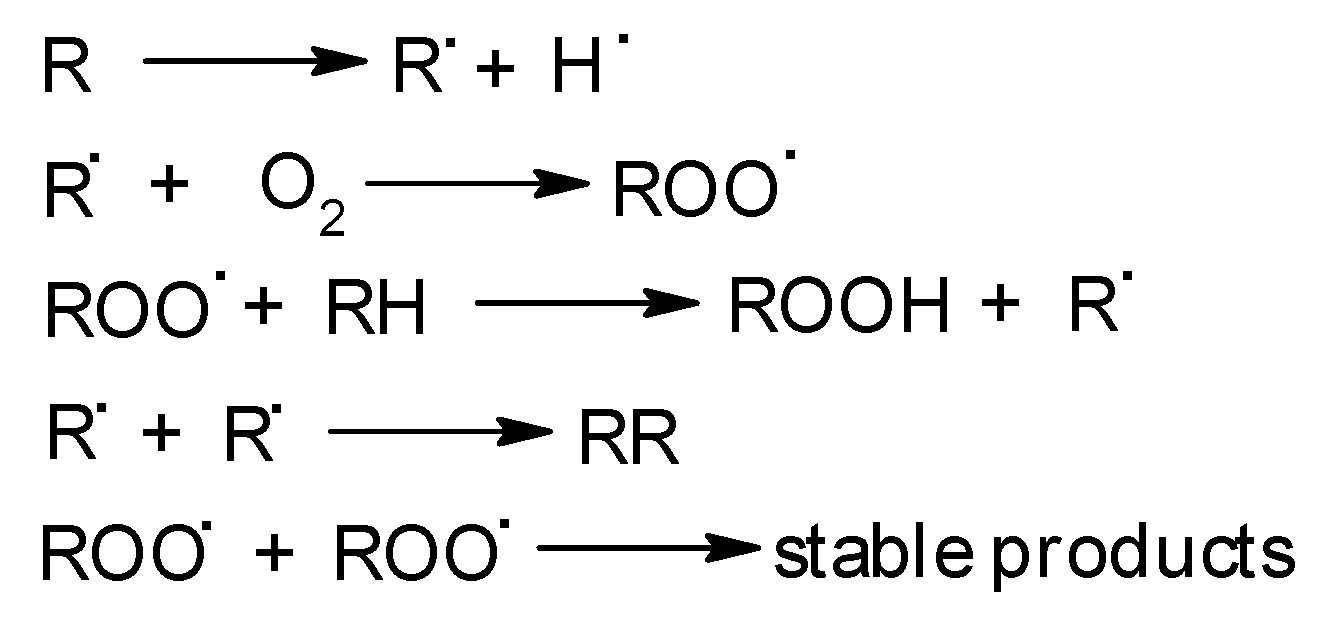
1. Introduction
2. Materials and Methods
2.1. Chemicals
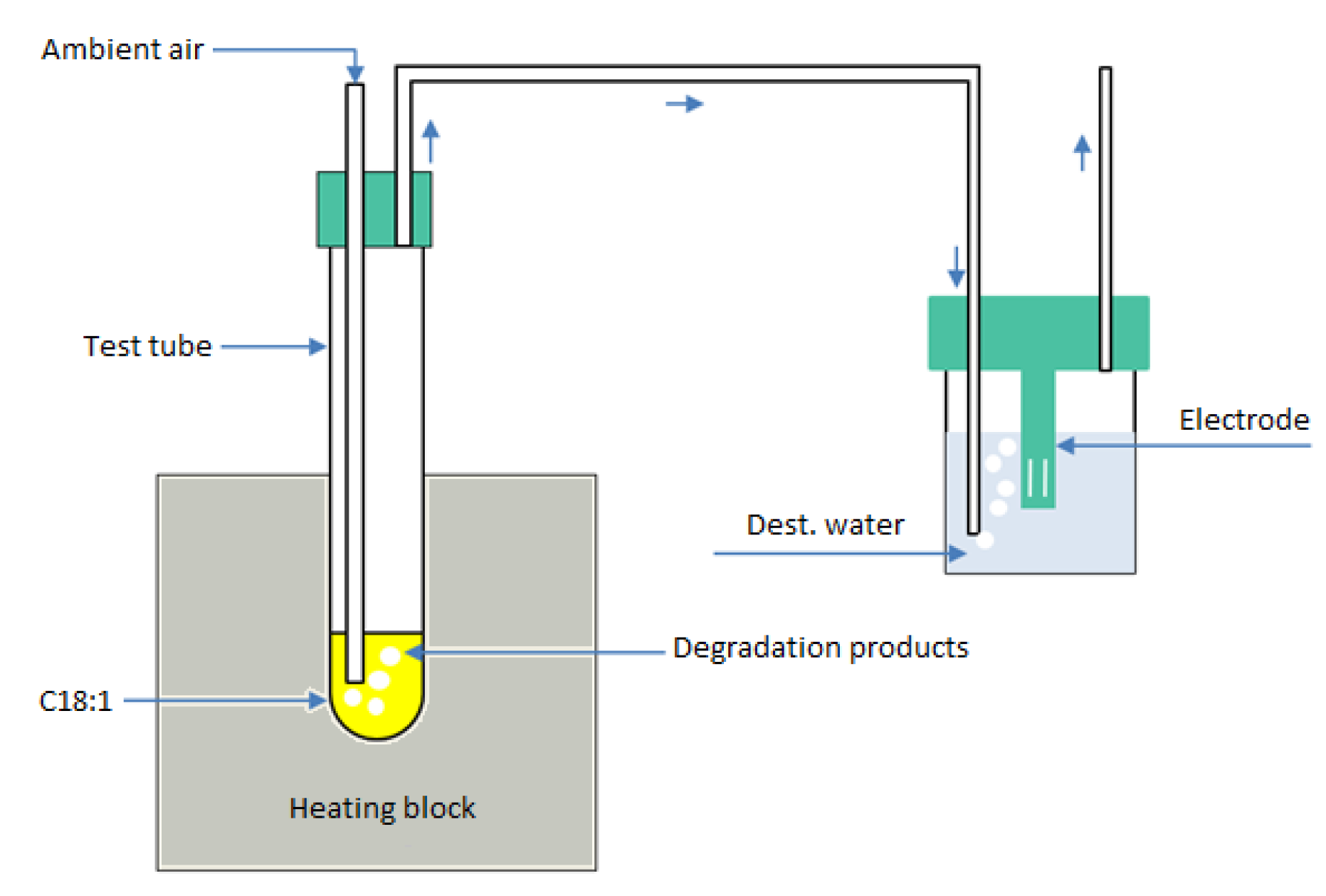
2.2. Fuel Aging
2.3. Kinematic Viscosity
2.4. Acid Value
2.5. Peroxide Value
2.6. Gas Chromatography with Mass Spectrometry (GC-MS)
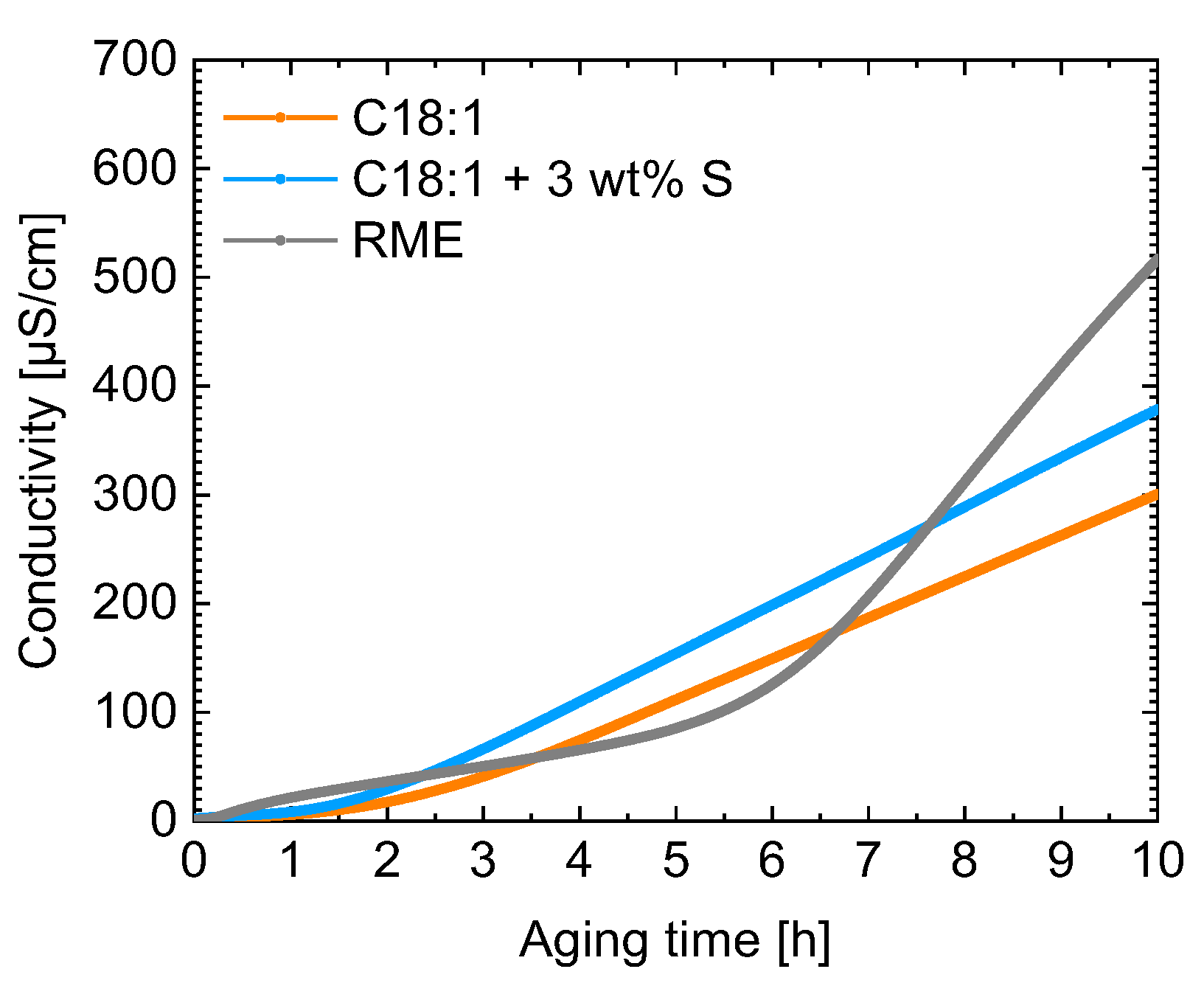
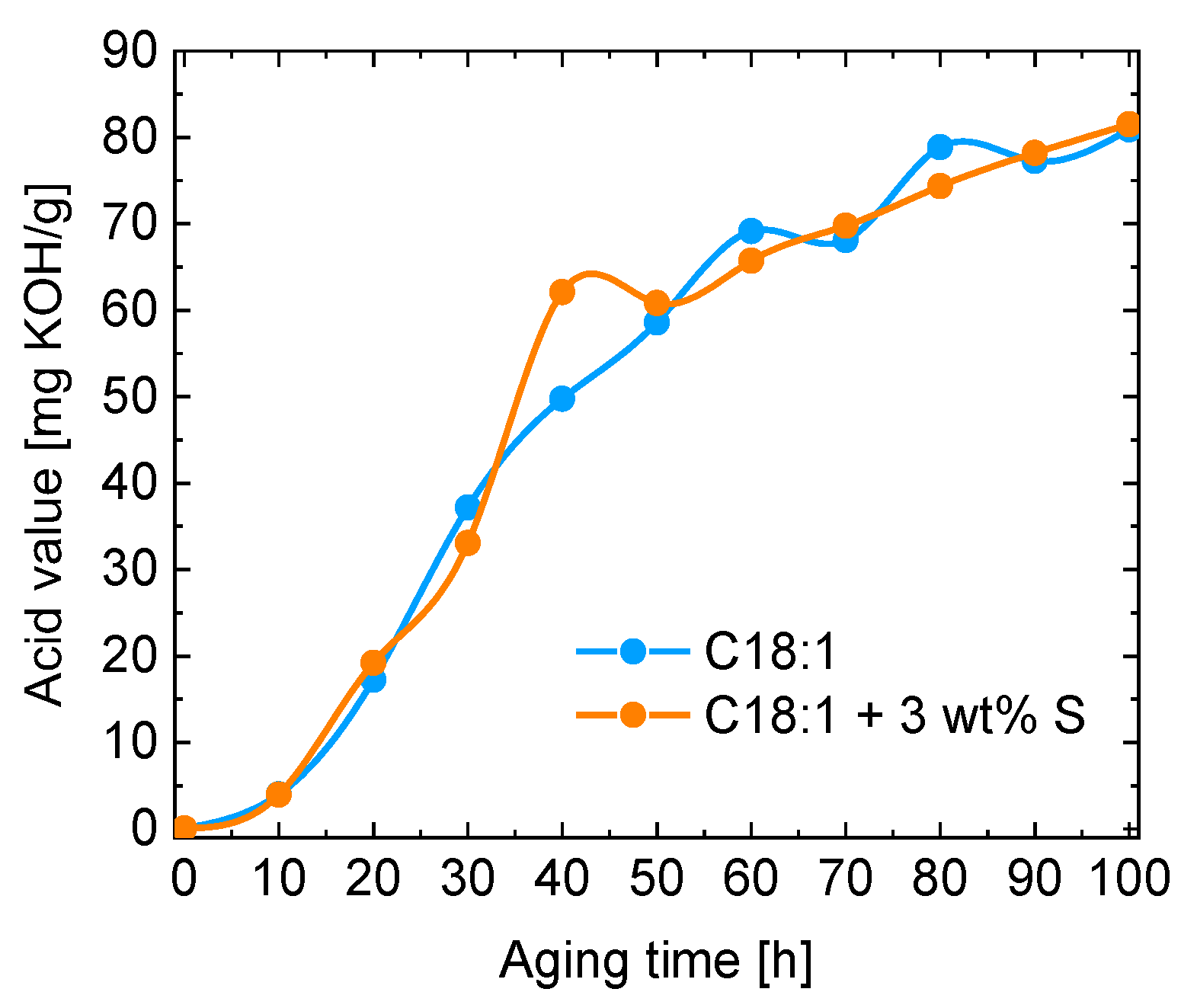
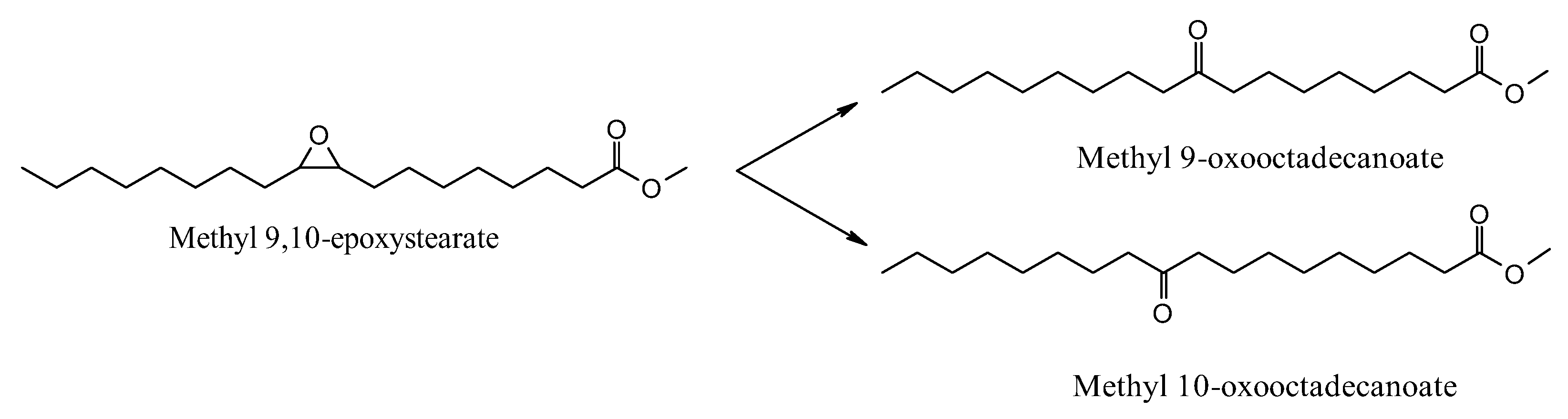
3. Results and Discussion
4. Conclusions
5. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.L.; McCormick, R.L. Spectroscopic Study of Biodiesel Degradation Pathways; SAE Technical Paper; SAE: Warrendale, PA, USA, 2006. [Google Scholar]
- Tuerck, J.; Singer, A.; Lichtinger, A.; Almaddad, M.; Türck, R.; Jakob, M.; Garbe, T.; Ruck, W.; Krahl, J. Solketal as a renewable fuel component in ternary blends with biodiesel and diesel fuel or HVO and the impact on physical and chemical properties. Fuel 2022, 310, 122463. [Google Scholar] [CrossRef]
- Hamacher, D.; Schrader, W. Investigating Molecular Transformation Processes of Biodiesel Components During Long-Term Storage Via High-Resolution Mass Spectrometry. ChemSusChem 2022, 15, e202200456. [Google Scholar] [CrossRef] [PubMed]
- Krahl, J.; Petchatnikov, M.; Schmidt, L.; Munack, A.; Bünger, J. Spektroskopische Untersuchungen zur Ergründung der Wechselwirkungen zwischen Biodiesel und Dieselkraftstoff bei Blends; Braunschweig und Coburg: Braunschweig, Germany, 2009. [Google Scholar]
- Singer, A.; Schröder, O.; Pabst, C.; Munack, A.; Bünger, J.; Ruck, W.; Krahl, J. Aging studies of biodiesel and HVO and their testing as neat fuel and blends for exhaust emissions in heavy-duty engines and passenger cars. Fuel 2015, 153, 595–603. [Google Scholar] [CrossRef]
- Frankel, E. Lipid Oxidation; The Oily Press: Dundee, Scotland, 1998; Volume 13. [Google Scholar]
- Kim, H.J.; Min, D.B. 11. Chemistry of Lipid Oxidation. Food Lipids Chem. Nutr. Biotechnol. 2008, 1, 299. [Google Scholar]
- Stuhr, R.; Bayer, P.; von Wangelin, A.J. The Diverse Modes of Oxygen Reactivity in Life & Chemistry. ChemSusChem 2022, 15, e202201323. [Google Scholar]
- Schaich, K.M. Lipid oxidation: Theoretical aspects. In Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2005; Volume 1, pp. 273–303. [Google Scholar]
- Min, D.; Boff, J. Chemistry and reaction of singlet oxygen in foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef]
- Knothe, G. Some aspects of biodiesel oxidative stability. Fuel Process. Technol. 2007, 88, 669–677. [Google Scholar] [CrossRef]
- Regensburger, J. Spektroskopische Untersuchungen zur Singulett-Sauerstoff-Lumineszenz in Biomolekülen, Bakterien und Zellen; Universität Regensburg: Regensburg, Germany, 2010. [Google Scholar]
- Bär, F.; Knorr, M.; Schröder, O.; Hopf, H.; Garbe, T.; Krahl, J. Rancimat vs. rapid small scale oxidation test (RSSOT) correlation analysis, based on a comprehensive study of literature. Fuel 2021, 291, 120160. [Google Scholar] [CrossRef]
- Romanov, A.N.; Rufov, Y.N.; Korchak, V.N. Thermal generation of singlet oxygen (1ΔgO2) on ZSM-5 zeolite. Mendeleev Commun. 2000, 10, 116–117. [Google Scholar] [CrossRef]
- Kumar, N. Oxidative stability of biodiesel: Causes, effects and prevention. Fuel 2017, 190, 328–350. [Google Scholar] [CrossRef]
- Flitsch, S.; Neu, P.M.; Schober, S.; Kienzl, N.; Ullmann, J.R.; Mittelbach, M. Quantitation of aging products formed in biodiesel during the rancimat accelerated oxidation test. Energy Fuels 2014, 28, 5849–5856. [Google Scholar] [CrossRef]
- Xin, J.; Imahara, H.; Saka, S. Kinetics on the oxidation of biodiesel stabilized with antioxidant. Fuel 2009, 88, 282–286. [Google Scholar] [CrossRef]
- Domingos, A.K.; Saad, E.B.; Vechiatto, W.W.; Wilhelm, H.M.; Ramos, L.P. The influence of BHA, BHT and TBHQ on the oxidation stability of soybean oil ethyl esters (biodiesel). J. Braz. Chem. Soc. 2007, 18, 416–423. [Google Scholar] [CrossRef]
- Türck, J. Kraftstoffe für die Mobilität von morgen. In Proceedings of the Kraftstoffe für die Mobilität von Morgen, 4. Tagung der Fuels Joint Research Group, Dresden, Germany, 10–11 June 2021; Volume 30, pp. 470–477. [Google Scholar]
- Mota, C.J.; da Silva, C.X.; Jr, N.R.; Costa, J.; da Silva, F. Glycerin Derivatives as Fuel Additives: The Addition of Glycerol/Acetone Ketal (Solketal) in Gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- Glassman, I. Soot formation in combustion processes. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1989; Volume 22, pp. 295–311. [Google Scholar]
- Bowden, J.; Johnston, A.; Russell, J. Octane-cetane relationship. Southwest Research In San Antonio TX Belvoir Fuels and Lubricants Research; Ft. Belvoir: Fairfax County, VA, USA, 1974. [Google Scholar]
- Samoilov, V.; Borisov, R.; Stolonogova, T.; Zarezin, D.; Maximov, A.; Bermeshev, M.; Chernysheva, E.; Kapustin, V. Glycerol to renewable fuel oxygenates. Part II: Gasoline-blending characteristics of glycerol and glycol derivatives with C3-C4 alkyl (idene) substituents. Fuel 2020, 280, 118585. [Google Scholar] [CrossRef]
- Moser, B.R. Comparative oxidative stability of fatty acid alkyl esters by accelerated methods. J. Am. Oil Chem. Soc. 2009, 86, 699–706. [Google Scholar] [CrossRef]
- Sabudak, T.; Yildiz, M. Biodiesel production from waste frying oils and its quality control. Waste Manag. 2010, 30, 799–803. [Google Scholar] [CrossRef] [PubMed]
- ISO 3960:2012; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. ISO: Geneva, Switzerland, 2012.
- Ball, J.C.; Anderson, J.E.; Wallington, T.J. Depolymerization of polyester polymers from the oxidation of soybean biodiesel. Energy Fuels 2018, 32, 12587–12596. [Google Scholar] [CrossRef]
- Canakci, M.; Monyem, A.; Van Gerpen, J. Accelerated oxidation processes in biodiesel. Trans. ASAE 1999, 42, 1565. [Google Scholar] [CrossRef]
- Thompson, J.; Peterson, C.; Reece, D.; Beck, S. Two-year storage study with methyl and ethyl esters of rapeseed. Trans. ASAE 1998, 41, 931. [Google Scholar] [CrossRef]
- Bouaid, A.; Martinez, M.; Aracil, J. Production of biodiesel from bioethanol and Brassica carinata oil: Oxidation stability study. Bioresour. Technol. 2009, 100, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Marmesat, S.; Dobarganes, M.C.; Márquez-Ruiz, G.; Velasco, J. Evaporative light scattering detector in normal-phase high-performance liquid chromatography determination of FAME oxidation products. J. Chromatogr. A 2012, 1254, 62–70. [Google Scholar] [CrossRef]
- Velasco, J.; Marmesat, S.; Bordeaux, O.; Márquez-Ruiz, G.; Dobarganes, C. Formation and evolution of monoepoxy fatty acids in thermoxidized olive and sunflower oils and quantitation in used frying oils from restaurants and fried-food outlets. J. Agric. Food Chem. 2004, 52, 4438–4443. [Google Scholar] [CrossRef] [PubMed]
- Wolff, R.L. Heat-induced geometrical isomerization of α-linolenic acid: Effect of temperature and heating time on the appearance of individual isomers. J. Am. Oil Chem. Soc. 1993, 70, 425–430. [Google Scholar] [CrossRef]
- Cheng, N.; Zhang, J.; Yin, J.; Li, S. Computational and experimental research on mechanism of cis/trans isomerization of oleic acid. Heliyon 2018, 4, e00768. [Google Scholar] [CrossRef]
- Emken, E. cis and trans Analysis of fatty esters by gas chromatography: Octadecenoate and octadecadienoate isomers. Lipids 1972, 7, 459–466. [Google Scholar] [CrossRef]
- Moser, B.R.; Cermak, S.C.; Doll, K.M.; Kenar, J.A.; Sharma, B.K. A review of fatty epoxide ring opening reactions: Chemistry, recent advances, and applications. J. Am. Oil Chem. Soc. 2022, 99, 801–842. [Google Scholar] [CrossRef]
- Frankel, E. Chemistry of free radical and singlet oxidation of lipids. Prog. Lipid Res. 1984, 23, 197–221. [Google Scholar] [CrossRef]
- Cravador, A.; Krief, A. Unusual reactivity of selenoboranes towards epoxides: New selective routes to b-hydroxyselenides and allylalcohols. Tetrahedron Lett. 1981, 22, 2491–2494. [Google Scholar] [CrossRef]
- Wei, Y.; Li, G.; Lv, Q.; Cheng, C.; Guo, H. Epoxidation of methyl oleate and unsaturated fatty acid methyl esters obtained from vegetable source over Ti-containing silica catalysts. Ind. Eng. Chem. Res. 2018, 57, 16284–16294. [Google Scholar] [CrossRef]
- Parker, R.-E.; Isaacs, N. Mechanisms of epoxide reactions. Chem. Rev. 1959, 59, 737–799. [Google Scholar] [CrossRef]
- Saurabh, T.; Patnaik, M.; Bhagt, S.; Renge, V. Epoxidation of vegetable oils: A review. Int. J. Adv. Eng. Technol 2011, 2, 491–501. [Google Scholar]
- Marteau, C.; Ruyffelaere, F.; Aubry, J.-M.; Penverne, C.; Favier, D.; Nardello-Rataj, V. Oxidative degradation of fragrant aldehydes. Autoxidation by molecular oxygen. Tetrahedron 2013, 69, 2268–2275. [Google Scholar] [CrossRef]
- Bravo, A.; Bjorsvik, H.-R.; Fontana, F.; Minisci, F.; Serri, A. Radical versus “oxenoid” oxygen insertion mechanism in the oxidation of alkanes and alcohols by aromatic peracids. New synthetic developments. J. Org. Chem. 1996, 61, 9409–9416. [Google Scholar] [CrossRef]
- Parker, W.E.; Ricciuti, C.; Ogg, C.; Swern, D. Preparation, Characterization and Polarographic Behavior of Longchain Aliphatic Peracids2. J. Am. Chem. Soc. 1955, 77, 4037–4041. [Google Scholar] [CrossRef]
- Greenspan, F.P. The convenient preparation of per-acids. J. Am. Chem. Soc. 1946, 68, 907. [Google Scholar] [CrossRef]
- Thornton, M.J.; Alleman, T.L.; Luecke, J.; McCormick, R.L. Impacts of biodiesel fuel blends oil dilution on light-duty diesel engine operation. SAE Int. J. Fuels Lubr. 2009, 2, 781–788. [Google Scholar] [CrossRef]
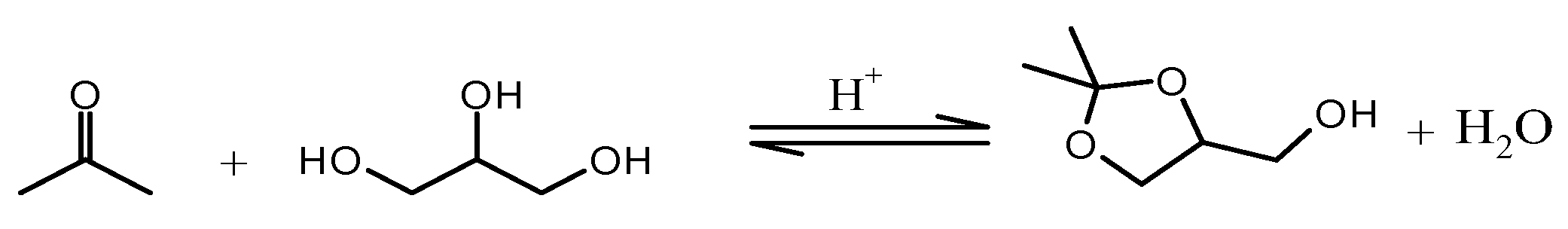
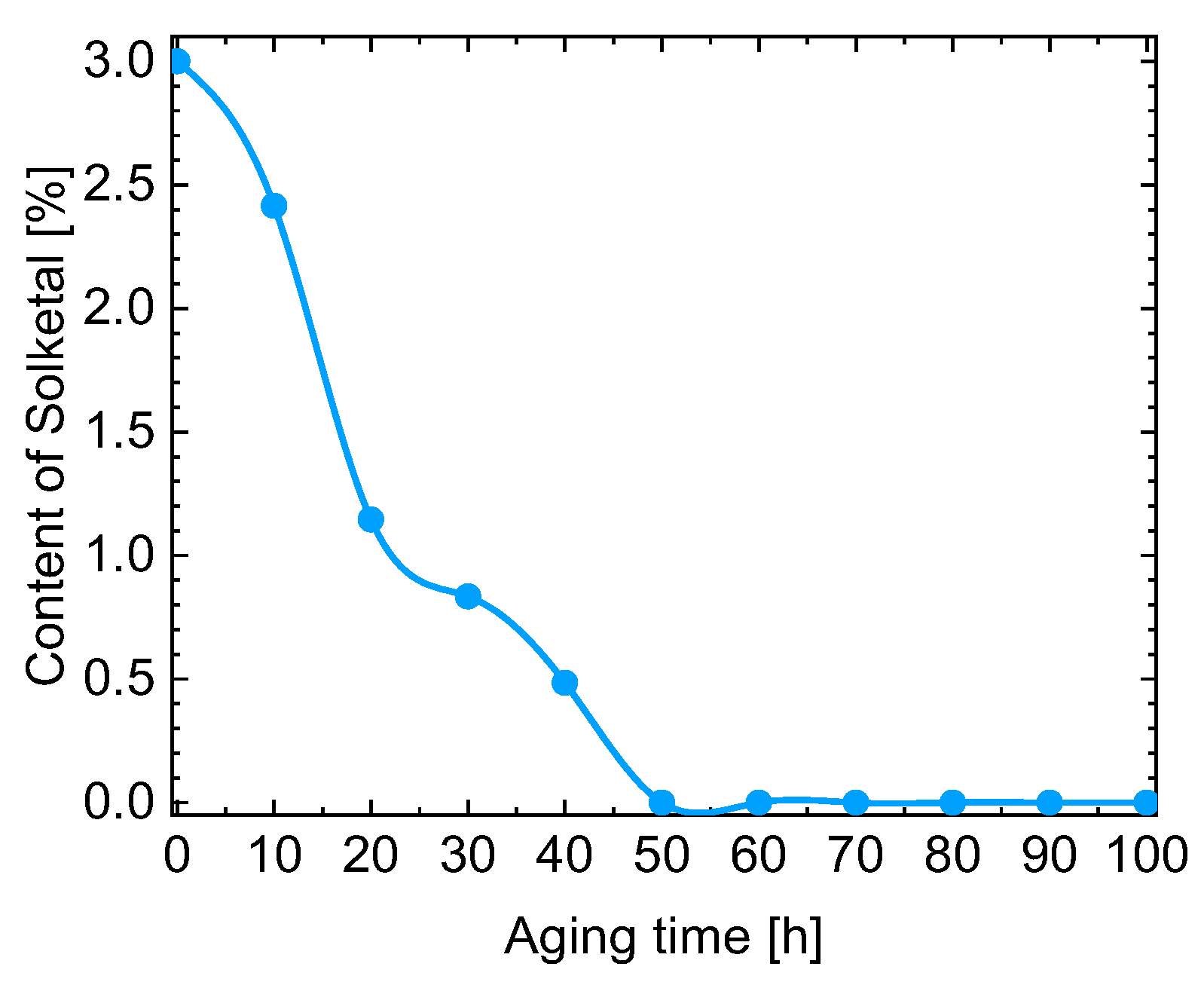
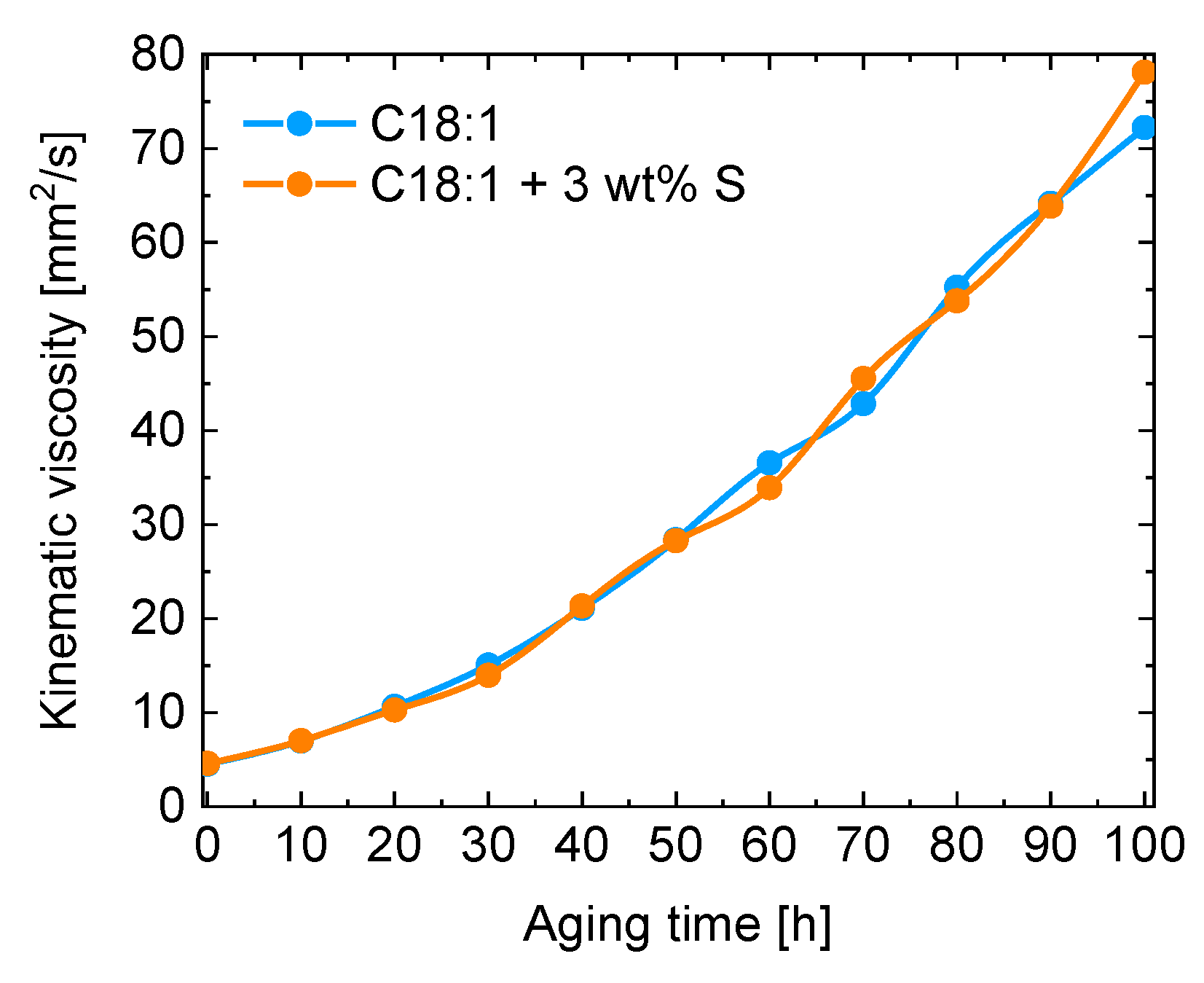
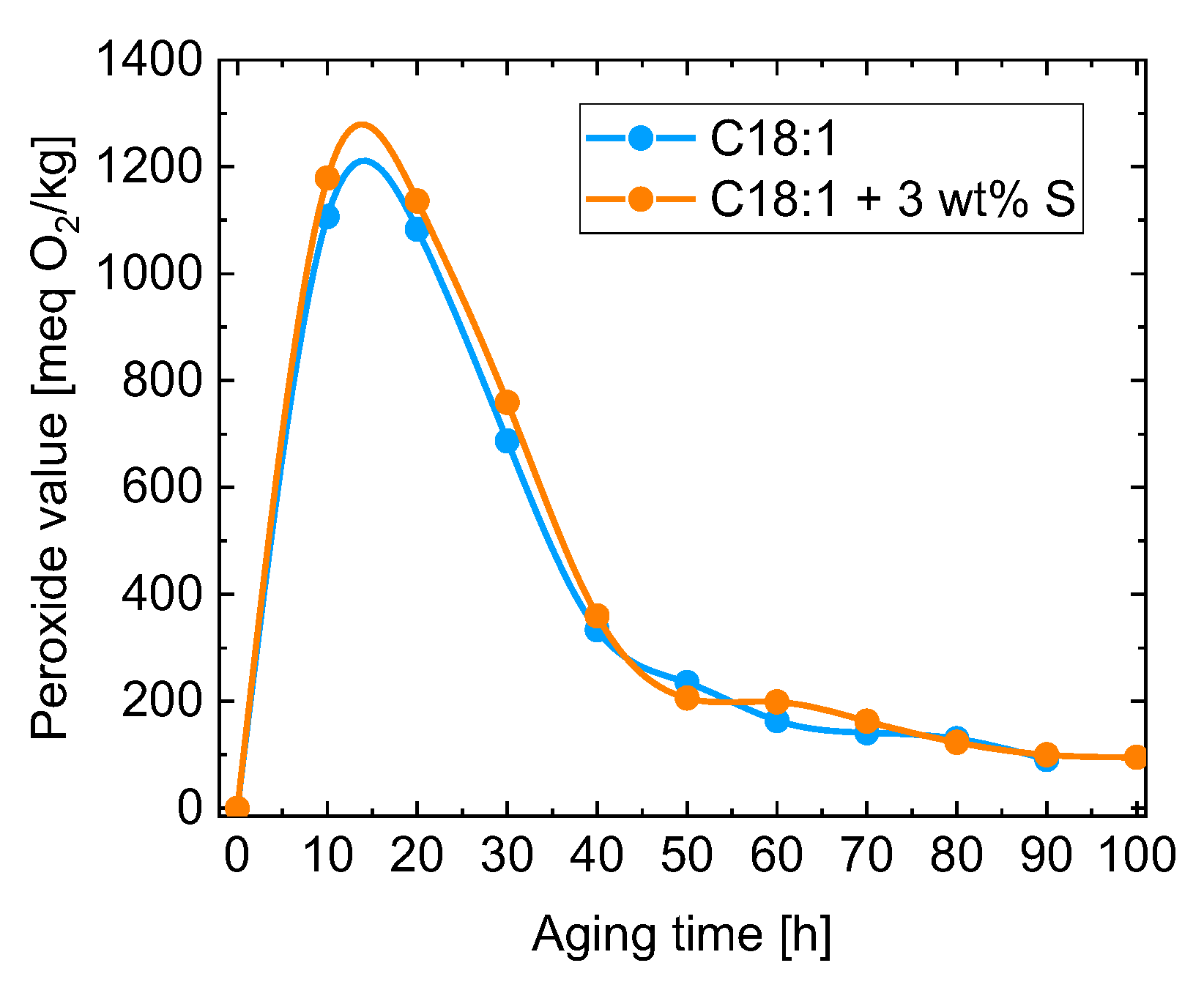
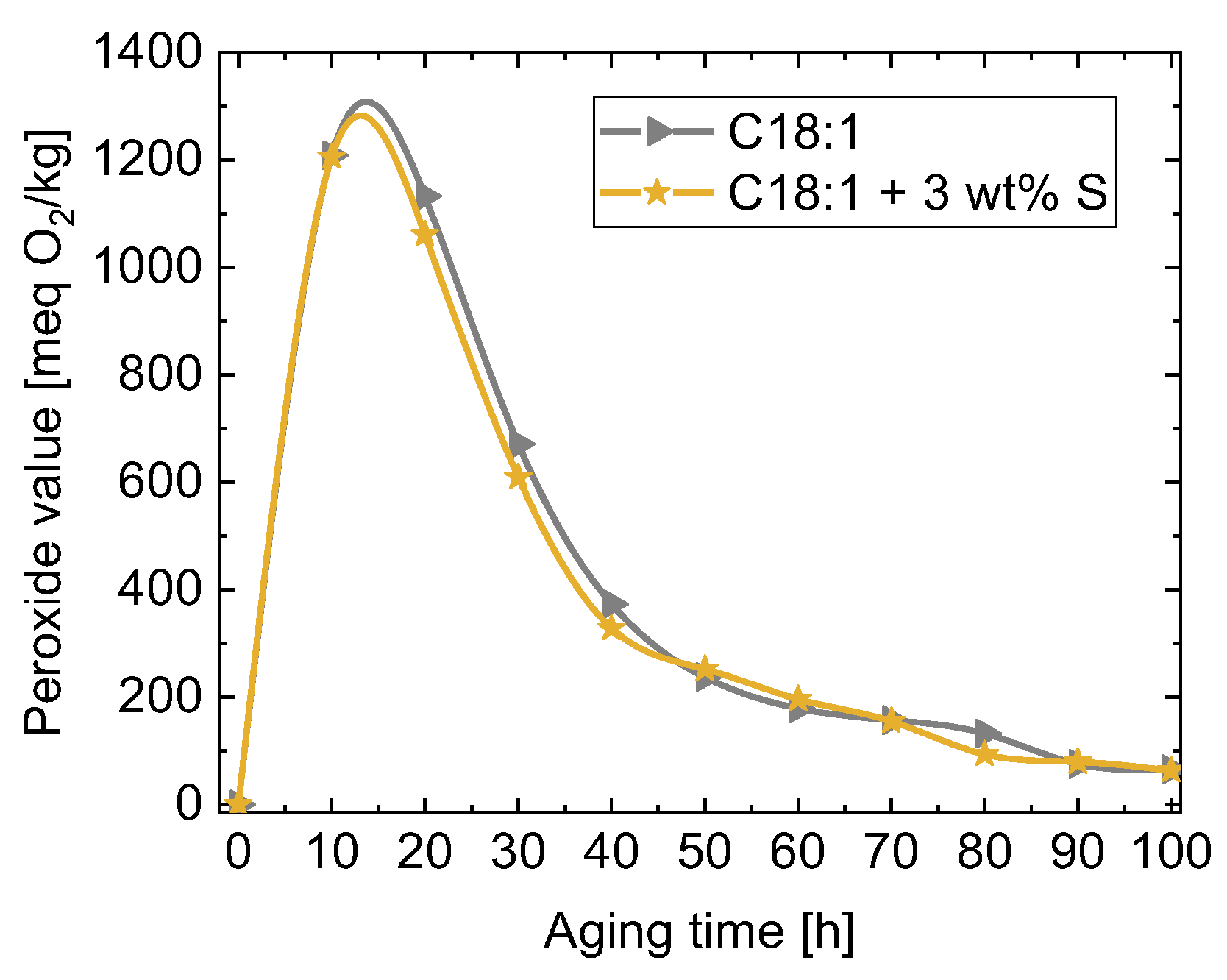
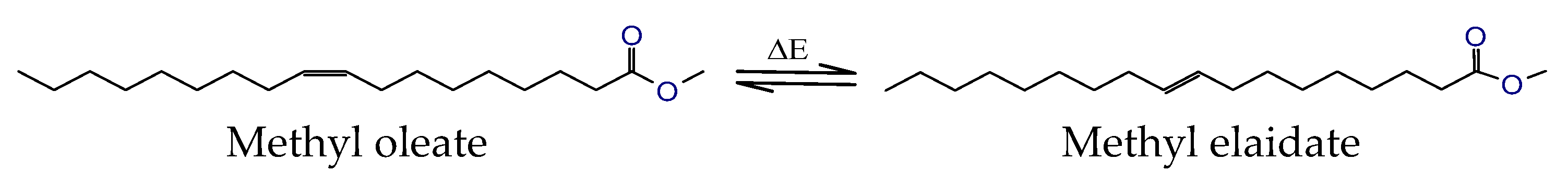
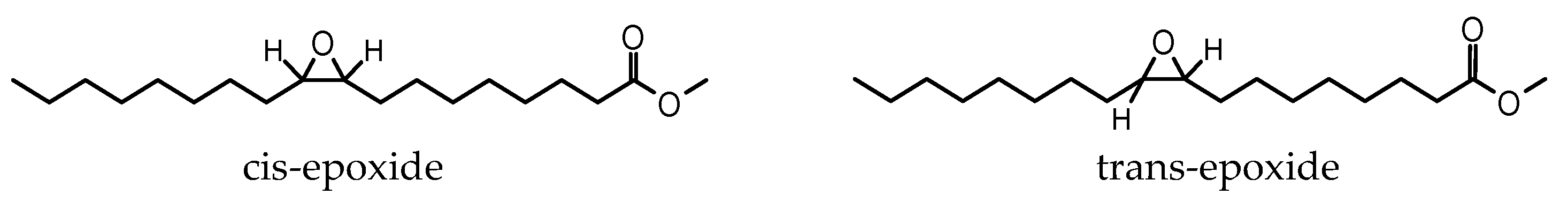
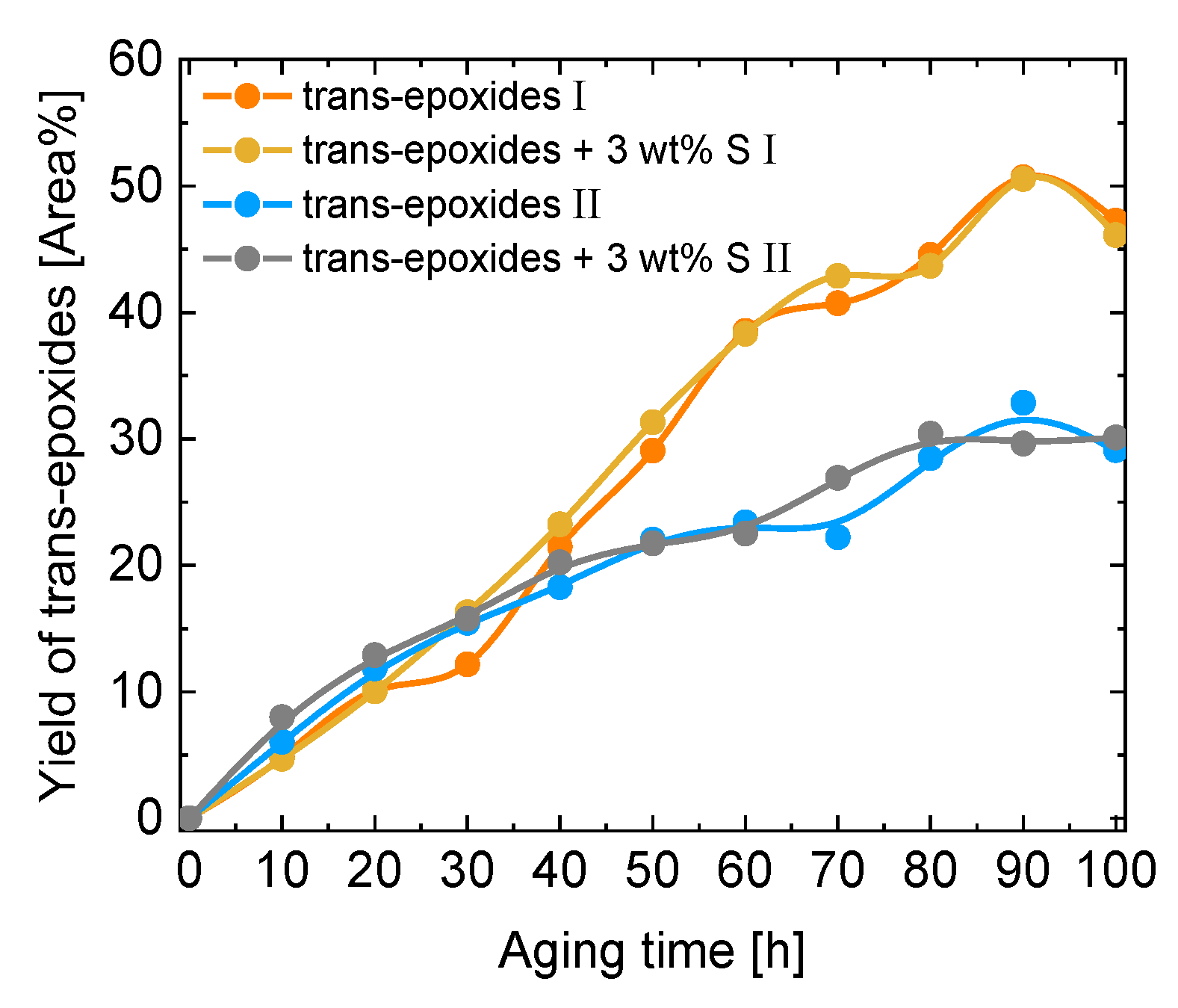
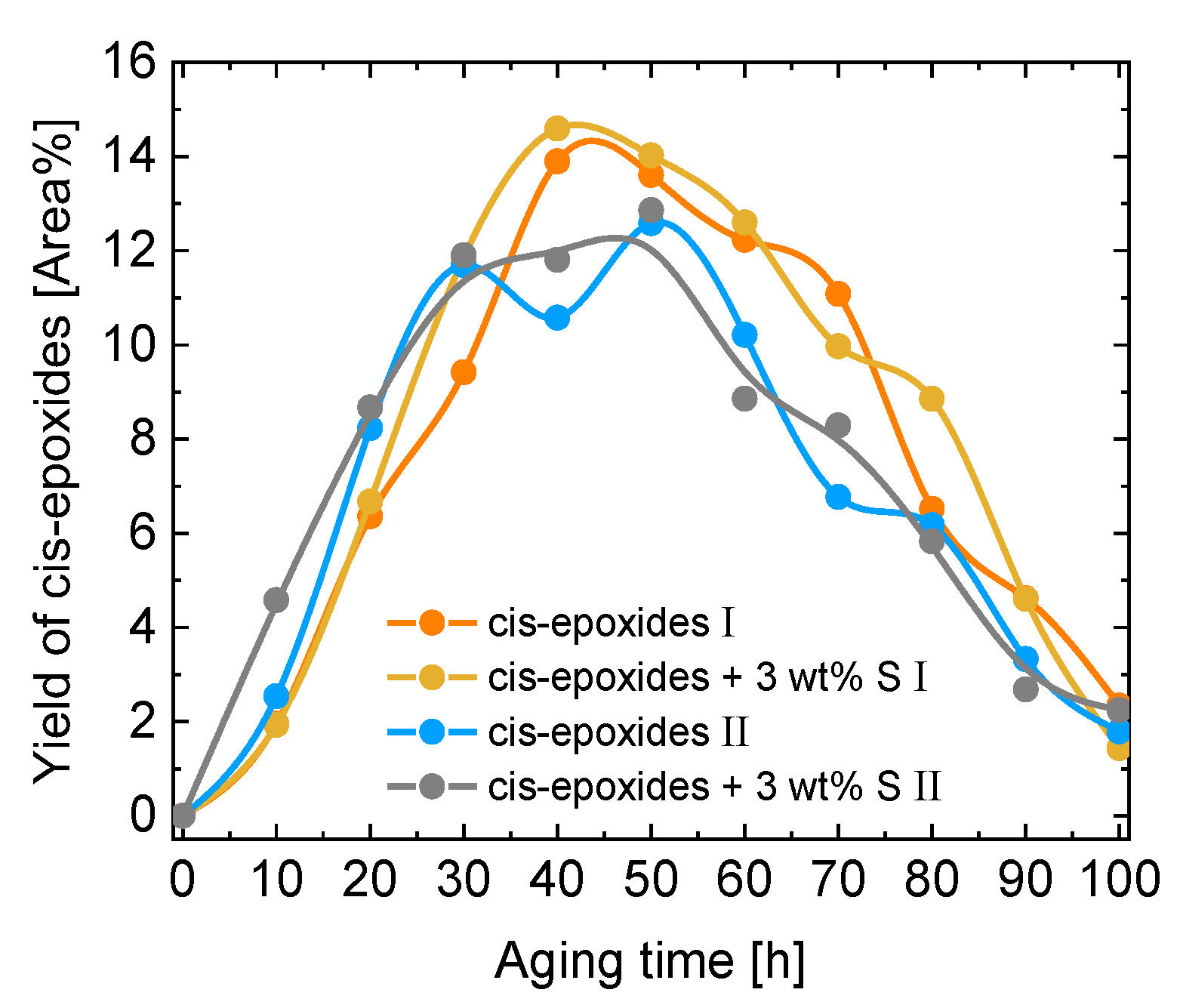

| Kin. Viscosity [mm2/s] | |
|---|---|
| Methyl oleate | 4.5 |
| Solketal | 5.1 |
| GC: | |||||
| Temperature Program | Column | ||||
| Start temp.: 60 °C for 3 min | Dimension: 30.0 m × 0.25 mm × 0.20 µm | ||||
| Heating rate: 10.0 K/min | Type: Phenomenex ZB-FAME | ||||
| Finale temp.: 250 °C for 10 min | Max. temp.: 280 °C | ||||
| Total run time: 32 min | |||||
| Injector temp.: 250 °C | |||||
| Pressure [kPa] | Total Flow [mL/min] | Split Ratio | Column Flow [mL/min] | Injection Volume [µL] | |
| 72.9 | 64.5 | 1:50 | 1.21 | 1.0 | |
| MS: | |||||
| Ion Source Temperature | 200 °C | ||||
| Interface Temperature | 250 °C | ||||
| Ionization mode | Electron Ionization | ||||
| Scan mode | Total Ion Count | ||||
| m/z | 20.00–600.00 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türck, J.; Schmitt, F.; Anthofer, L.; Lichtinger, A.; Türck, R.; Ruck, W.; Krahl, J. Oxidation Kinetics of Neat Methyl Oleate and as a Blend with Solketal. Energies 2023, 16, 3253. https://doi.org/10.3390/en16073253
Türck J, Schmitt F, Anthofer L, Lichtinger A, Türck R, Ruck W, Krahl J. Oxidation Kinetics of Neat Methyl Oleate and as a Blend with Solketal. Energies. 2023; 16(7):3253. https://doi.org/10.3390/en16073253
Chicago/Turabian StyleTürck, Julian, Fabian Schmitt, Lukas Anthofer, Anne Lichtinger, Ralf Türck, Wolfgang Ruck, and Jürgen Krahl. 2023. "Oxidation Kinetics of Neat Methyl Oleate and as a Blend with Solketal" Energies 16, no. 7: 3253. https://doi.org/10.3390/en16073253
APA StyleTürck, J., Schmitt, F., Anthofer, L., Lichtinger, A., Türck, R., Ruck, W., & Krahl, J. (2023). Oxidation Kinetics of Neat Methyl Oleate and as a Blend with Solketal. Energies, 16(7), 3253. https://doi.org/10.3390/en16073253







