Anaerobic Membrane Bioreactors (AnMBRs) for Wastewater Treatment: Recovery of Nutrients and Energy, and Management of Fouling
Abstract
1. Introduction
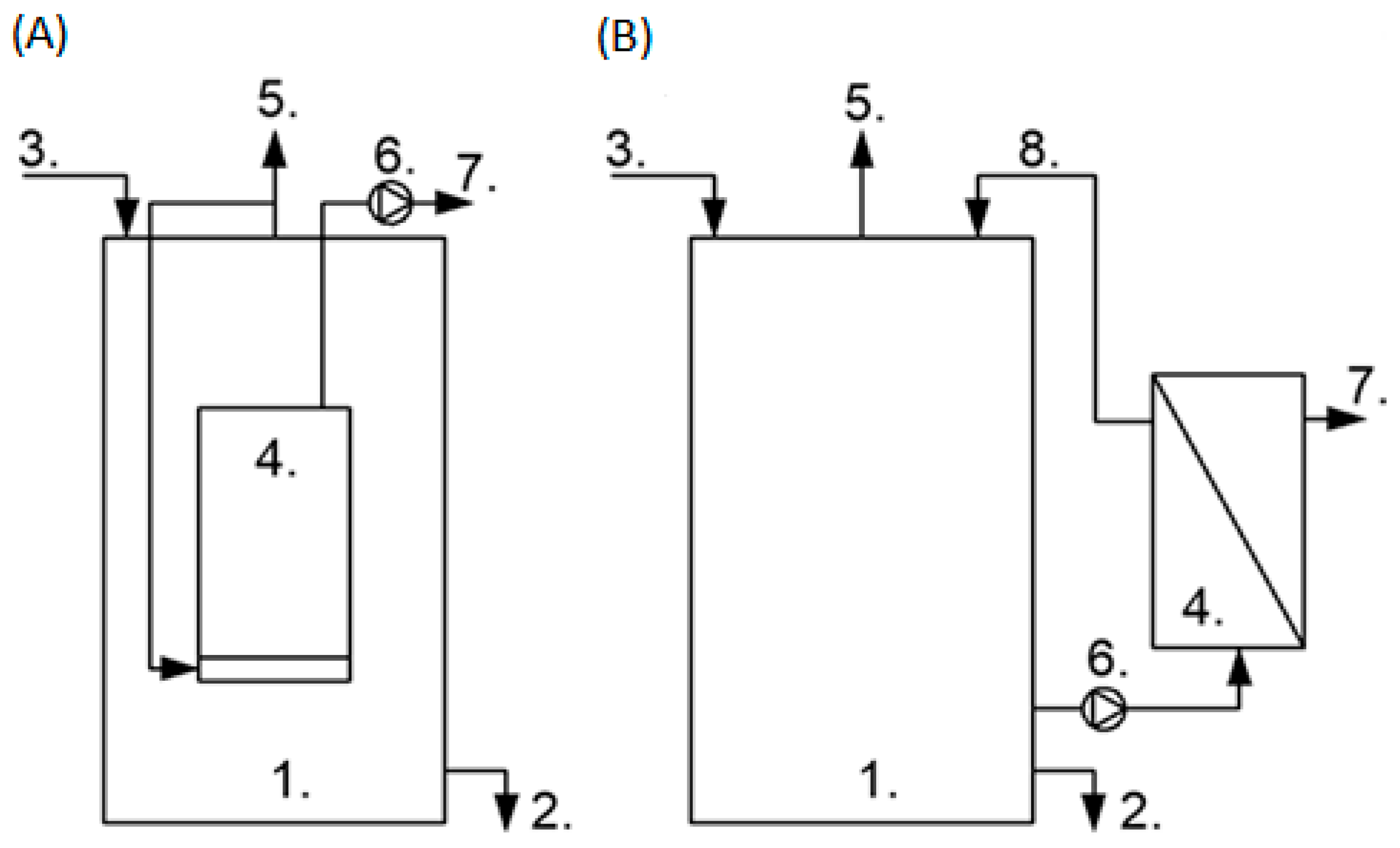
2. Anaerobic Membrane Bioreactors—Wastewater Treatment and Biogas Production
2.1. Industrial Wastewater
| Type of Reactor | Type of Wastewater | Operating Conditions | COD Removal (%) | Biogas Production (L CH4/g CODremoved) | Reference |
|---|---|---|---|---|---|
| Submerged AnMBR | Kraft evaporator condensate | OLR 1–7 kg COD/(m3·d) Temperature 55 ± 1 °C | 85–97 | 0.35 ± 0.10 | [26] |
| Submerged AnMBR | Meat-processing wastewater | OLR 0.4–3.2 kg COD/(m3·d) | 88–95 | 0.13–0.18 | [19] |
| Submerged AnMBR | Coffee processing wastewater with waste activated sludge | OLR 0.87–9.16 kg COD/(m3·d) | 92 ± 3 | 0.28 | [21] |
| HRT 10 d | |||||
| MLSS 50 g/L | |||||
| External AnMBR | Pharmaceutical wastewater | Flux 8.4 L/(m2·h) | 78 | 0.60 | [24] |
| OLR 2.5 g COD/(L·d) | |||||
| HRT 1.7–5, 3–3.5 d | |||||
| SRT 120–450 d | |||||
| Temperature 35–37 °C | |||||
| Submerged AnMBR | Paper mill wastewater | Flux 7.2 L/(m2·h) | 98 | - | [22] |
| OLR 7.0 kg COD/(m3·d) | |||||
| HRT 35 h | |||||
| SRT 40 d | |||||
| MLSS 12.9 g/L | |||||
| Temperature 21 °C | |||||
| AnMBR | Brewery wastewater | Flux 8.64 ± 0.69 L/(m2·h) | 99 | 0.53 ± 0.02 | [20] |
| OLR 3.5–11.5 kg COD/(m3·d) | |||||
| HRT 44 h | |||||
| MLSS 2.8 g/L | |||||
| Temperature 35 °C | |||||
| Submerged AnMBR | Textile wastewater | Flux 1.8–14.4 L/(m2·h) | 90 | - | [23] |
| HRT 24 h | |||||
| pH 6.8–7.2 | |||||
| Temperature 35 °C | |||||
| External AnMBR | Bamboo wastewater | Flux 33.4–16.2 L/(m2·h) | 89.1 ± 3.1 | 10.30 ± 0.80 L/d | [27] |
| OLR 6 kg COD/(m3·d) | |||||
| HRT 3 d | |||||
| MLSS 16 g/L | |||||
| Temperature 32 ± 2 °C | |||||
| Submerged AnMBR | Tannery wastewater | OLR 6 kg COD/(m3·d) | 90 | 0.16 L biogas/g CODrem | [28] |
2.2. Municipal Wastewater
| Type of Reactor | Operating Conditions | COD Removal (%) | Biogas Production | Reference |
|---|---|---|---|---|
| Anaerobic fluidized membrane bioreactor | Flux 4.1–7.5 L/(m2·h) | 94% (COD) 98% (BOD) | n.m. | [30] |
| HRT 4.6–6.8 h | ||||
| Temperature 8–30 °C | ||||
| External AnMBR | Flux 6–14 L/(m2·h) | >90% (COD) | n.m. | [2] |
| OLR 0.4–3.0 kg COD/(m3·d) | ||||
| HRT 2.2–33 h | ||||
| SRT > 6.2 d | ||||
| Temperature 17.1–35 °C | ||||
| External AnMBR | Flux 10–14 L/(m2·h) | 87 ± 1% (COD) | 0.18–0.23 Nm3 CH4/kg CODrem | [31] |
| VLR 2–2.5 kg COD/(m3·d) | ||||
| HRT 7 h | ||||
| Temperature 18 ± 2 °C | ||||
| Submerged AnMBR | Flux 10–14 L/(m2·h) | 90% (COD) | 19.10 ± 0.84 mg CH4/L | [34] |
| VLR 1.6–2.0 kg COD/(m3·d) | ||||
| HRT 12.8–14.2 h | ||||
| Temperature 18 ± 2 °C | ||||
| Submerged AnMBR | Flux 2.75–17.83 L/(m2·h) | >90% (COD) >95% (BOD) | 0.25–0.27 L biogas/g CODrem; 75–81% methane in the biogas | [35] |
| HRT 6 h | ||||
| Temperature 25 °C | ||||
| Submerged AnMBR | Flux 6 L/(m2·h) | 87% (COD) | 0.12 L CH4/g CODrem | [36] |
| OLR 3.0 kg COD/(m3·d) | ||||
| HRT 2.2 h | ||||
| MLSS 10.9 g/L | ||||
| Temperature 35 °C |
3. Wastewater Treatment with AnMBRs—Recovery of Energy
3.1. Methane Production and Recovery
3.2. Ethanol Production
3.3. Hydrogen Production
3.4. Bioelectrochemical Processes Using Microbial Fuel Cells
4. Wastewater Treatment with the AnMBR—Recovery of Nutrients and Water and Removal of Nutrients
5. Fouling Control
6. Future Perspectives of AnMBRs
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Shin, C.; Bae, J. Current status of the pilot-scale anaerobic membrane bioreactor treatments of domestic wastewaters: A critical review. Bioresour. Technol. 2018, 247, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chang, S.; Guo, Q.; Hong, Y.; Wu, P. Brewery wastewater treatment using an anaerobic membrane bioreactor. Biochem. Eng. J. 2016, 105, 321–331. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.S.; Chae, S.R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef]
- Song, X.; Luo, W.; Hai, F.I.; Price, W.E.; Guo, W.; Ngo, H.H.; Nghiem, L.D. Resource recovery from wastewater by anaerobic membrane bioreactors: Opportunities and challenges. Bioresour. Technol. 2018, 270, 669–677. [Google Scholar] [CrossRef]
- Dereli, R.K.; Ersahin, M.E.; Ozgun, H.; Ozturk, I.; Jeison, D.; van der Zee, F.; van Lier, J.B. Potentials of anaerobic membrane bioreactors to overcome treatment limitations induced by industrial wastewaters. Bioresour. Technol. 2012, 122, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Stadler, L.B.; Love, N.G.; Skerlos, S.J.; Raskin, L. Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: A critical review. Bioresour. Technol. 2012, 122, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Yang, S.; Li, Y.Y.; Wen, W.; Wang, X.C.; Chen, R. Application of anaerobic membrane bioreactors to municipal wastewater treatment at ambient temperature: A review of achievements, challenges, and perspectives. Bioresour. Technol. 2018, 267, 756–768. [Google Scholar] [CrossRef]
- Aslam, A.; Khan, S.J.; Shahzad, H.M.A. Anaerobic membrane bioreactors (AnMBRs) for municipal wastewater treatment- potential benefits, constraints, and future perspectives: An updated review. Sci. Total Environ. 2022, 802, 149612. [Google Scholar] [CrossRef]
- Vinardell, S.; Astals, S.; Peces, M.; Cardete, M.A.; Fernandez, I.; Mata-Alvarez, J.; Dosta, J. Advances in anaerobic membrane bioreactor technology for municipal wastewater treatment: A 2020 updated review. Renew. Sustain. Energy Rev. 2020, 130, 109936. [Google Scholar] [CrossRef]
- Crone, B.C.; Garland, J.L.; Sorial, G.A.; Vane, L.M. Significance of dissolved methane in effluents of anaerobically treated low strength wastewater and potential for recovery as an energy product: A review. Water Res. 2016, 104, 520–531. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.C.; Ngo, H.H.; Sun, Q.; Yang, Y. Anaerobic dynamic membrane bioreactor (AnDMBR) for wastewater treatment: A review. Bioresour. Technol. 2018, 247, 1107–1118. [Google Scholar] [CrossRef]
- McCarty, P.L.; Kim, J.; Shin, C.; Lee, P.H.; Bae, J. Anaerobic fluidized bed membrane bioreactors for the treatment of domestic wastewater. In Anaerobic Biotechnology: Environmental Protection and Resource Recovery; World Scientific: Singapore, 2015; pp. 211–242. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; McDonald, J.A.; Khan, S.J.; Le-Clech, P. Removal of trace organics by anaerobic membrane bioreactors. Water Res. 2014, 49, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.B.; Gonzalez-Gil, L.; Londoño, Y.A.; Zaiat, M.; Carballa, M.; Lema, J.M. Acidogenesis is a key step in the anaerobic biotransformation of organic micropollutants. J. Hazard. Mater. 2020, 389, 121888. [Google Scholar] [CrossRef]
- Mahmoud, I.; Gao, W.J.; Liao, B.Q.; Cumin, J.; Dagnew, M.; Hong, Y. Development of a high-rate submerged anaerobic membrane bioreactor. Environ. Technol. 2018, 39, 640–650. [Google Scholar] [CrossRef]
- Spagni, A.; Casu, S.; Crispino, N.; Farina, R.; Mattioli, D. Filterability in a submerged anaerobic membrane bioreactor. Desalination 2010, 250, 787–792. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Visvanathan, C.; Abeynayaka, A. Effect of organic loading rate on VFA production, organic matter removal and microbial activity of a two-stage thermophilic anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 5353–5360. [Google Scholar] [CrossRef]
- Galib, M.; Elbeshbishy, E.; Reid, R.; Hussain, A.; Lee, H.S. Energy-positive food wastewater treatment using an anaerobic membrane bioreactor (AnMBR). J. Environ. Manag. 2016, 182, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Guo, W.; Ngo, H.H.; Lee, D.J.; Tung, K.L.; Jin, P.; Wang, J.; Wu, Y. Challenges in biogas production from anaerobic membrane bioreactors. Renew. Energy 2016, 98, 120–134. [Google Scholar] [CrossRef]
- Chen, R.; Wen, W.; Jiang, H.; Lei, Z.; Li, M.; Li, Y.Y. Energy recovery potential of thermophilic high-solids co-digestion of coffee processing wastewater and waste activated sludge by anaerobic membrane bioreactor. Bioresour. Technol. 2019, 274, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Erkan, H.S.; Engin, G.O. The investigation of paper mill industry wastewater treatment and activated sludge properties in a submerged membrane bioreactor. Water Sci. Technol. 2017, 76, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Baêta, B.; Ramos, R.; Lima, D.; Aquino, S. Use of submerged anaerobic membrane bioreactor (SAMBR) containing powdered activated carbon (PAC) for the treatment of textile effluents. Water Sci. Technol. 2012, 65, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Svojitka, J.; Dvořák, L.; Studer, M.; Straub, J.O.; Frömelt, H.; Wintgens, T. Performance of an anaerobic membrane bioreactor for pharmaceutical wastewater treatment. Bioresour. Technol. 2017, 229, 180–189. [Google Scholar] [CrossRef]
- Saha, S.; Hussain, A.; Lee, J.; Lee, E.; Lee, H.S. An integrated leachate bed reactor—Anaerobic membrane bioreactor system (LBR-AnMBR) for food waste stabilization and biogas recovery. Chemosphere 2023, 311, 137054. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.Q.; Xie, K.; Lin, H.J.; Bertoldo, D. Treatment of kraft evaporator condensate using a thermophilic submerged anaerobic membrane bioreactor. Water Sci. Technol. 2010, 61, 2177–2183. [Google Scholar] [CrossRef]
- Xia, T.; Gao, X.; Wang, C.; Xu, X.; Zhu, L. An enhanced anaerobic membrane bioreactor treating bamboo industry wastewater by bamboo charcoal addition: Performance and microbial community analysis. Bioresour. Technol. 2016, 220, 26–33. [Google Scholar] [CrossRef]
- Hu, A.Y.; Stuckey, D.C. Treatment of dilute wastewaters using a novel submerged anaerobic membrane bioreactor. J. Environ. Eng. 2006, 132, 190–198. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, C.; Zhang, J.; Han, S.; Peng, Y.; Song, X. Impacts of lipids on the performance of anaerobic membrane bioreactors for food wastewater treatment. J. Membr. Sci. 2023, 666, 121104. [Google Scholar] [CrossRef]
- Shin, C.; McCarty, P.L.; Kim, J.; Bae, J. Pilot-scale temperate-climate treatment of domestic wastewater with a staged anaerobic fluidized membrane bioreactor (SAF-MBR). Bioresour. Technol. 2014, 159, 95–103. [Google Scholar] [CrossRef]
- Gouveia, J.; Plaza, F.; Garralon, G.; Fdz-Polanco, F.; Peña, M. Long-term operation of a pilot scale anaerobic membrane bioreactor (AnMBR) for the treatment of municipal wastewater under psychrophilic conditions. Bioresour. Technol. 2015, 185, 225–233. [Google Scholar] [CrossRef]
- Ho, J.; Sung, S. Methanogenic activities in anaerobic membrane bioreactors (AnMBR) treating synthetic municipal wastewater. Bioresour. Technol. 2010, 101, 2191–2196. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, Y.; Cao, C.; Zhang, J.; Ng, J.W.; Tang, C. Performance of a submerged anaerobic membrane bioreactor with forward osmosis membrane for low-strength wastewater treatment. Water Res. 2014, 50, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, J.; Plaza, F.; Garralon, G.; Fdz-Polanco, F.; Peña, M. A novel configuration for an anaerobic submerged membrane bioreactor (AnSMBR). Long-term treatment of municipal wastewater under psychrophilic conditions. Bioresour. Technol. 2015, 198, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Wu, J.; Rong, C.; Wang, T.; Li, L.; Luo, Z.; Ji, J.; Hanaoka, T.; Sakemi, S.; Ito, M.; et al. Large pilot-scale submerged anaerobic membrane bioreactor for the treatment of municipal wastewater and biogas production at 25 °C. Bioresour. Technol. 2021, 319, 124123. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wang, Z.; Miao, Y.; Wu, Z. A pilot-scale anaerobic membrane bioreactor under short hydraulic retention time for municipal wastewater treatment: Performance and microbial community identification. J. Water Reuse Desalin. 2018, 8, 58–67. [Google Scholar] [CrossRef]
- Chen, C.; Guo, W.; Ngo, H.H.; Chang, S.W.; Nguyen, D.D.; Nguyen, P.D.; Bui, X.T.; Wu, Y. Impact of reactor configurations on the performance of a granular anaerobic membrane bioreactor for municipal wastewater treatment. Int. Biodeter. Biodegr. 2017, 121, 131–138. [Google Scholar] [CrossRef]
- Meabe, E.; Déléris, S.; Soroa, S.; Sancho, L. Performance of anaerobic membrane bioreactor for sewage sludge treatment: Mesophilic and thermophilic processes. J. Membr. Sci. 2013, 446, 26–33. [Google Scholar] [CrossRef]
- He, Y.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Recent advances in membrane technologies for biorefining and bioenergy production. Biotechnol. Adv. 2012, 30, 817–858. [Google Scholar] [CrossRef]
- Mei, X.; Wang, Z.; Miao, Y.; Wu, Z. Recover energy from domestic wastewater using anaerobic membrane bioreactor: Operating parameters optimization and energy balance analysis. Energy 2016, 98, 146–154. [Google Scholar] [CrossRef]
- Matsunaga, K.; Kindaichi, T.; Ozaki, N.; Ohashi, A.; Nakahara, Y.; Sasakawa, M. Development of anammox reactor equipped with a degassing membrane to improve biomass retention. Water Sci. Technol. 2012, 66, 451–456. [Google Scholar] [CrossRef]
- Gimenez, J.B.; Martí, N.; Ferrer, J.; Seco, A. Methane recovery efficiency in a submerged anaerobic membrane bioreactor (SAnMBR) treating sulphate-rich urban wastewater: Evaluation of methane losses with the effluent. Bioresour. Technol. 2012, 118, 67–72. [Google Scholar] [CrossRef]
- Yeo, H.; An, J.; Reid, R.; Rittmann, B.E.; Lee, H.S. Contribution of liquid/gas mass-transfer limitations to dissolved methane oversaturation in anaerobic treatment of dilute wastewater. Environ. Sci. Technol. 2015, 49, 10366–10372. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Yin, H.; Dang, Z.; Liu, Y. Dissolved methane: A hurdle for anaerobic treatment of municipal wastewater. Environ. Sci. Technol. 2014, 48, 889–890. [Google Scholar] [CrossRef]
- Yue, X.; Koh, Y.K.K.; Ng, H.Y. Effects of dissolved organic matters (DOMs) on membrane fouling in anaerobic ceramic membrane bioreactors (AnCMBRs) treating domestic wastewater. Water Res. 2015, 86, 96–107. [Google Scholar] [CrossRef]
- Omar, H.; Rohani, S. Treatment of landfill waste, leachate and landfill gas: A review. Front. Chem. Sci. Eng. 2015, 9, 15–32. [Google Scholar] [CrossRef]
- Smith, A.L.; Stadler, L.B.; Cao, L.; Love, N.G.; Raskin, L.; Skerlos, S.J. Navigating wastewater energy recovery strategies: A life cycle comparison of anaerobic membrane bioreactor and conventional treatment systems with anaerobic digestion. Environ. Sci. Technol. 2014, 48, 5972–5981. [Google Scholar] [CrossRef] [PubMed]
- Hatamoto, M.; Yamamoto, H.; Kindaichi, T.; Ozaki, N.; Ohashi, A. Biological oxidation of dissolved methane in effluents from anaerobic reactors using a down-flow hanging sponge reactor. Water Res. 2010, 44, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Cookney, J.; Mcleod, A.; Mathioudakis, V.; Ncube, P.; Soares, A.; Jefferson, B.; McAdam, E.J. Dissolved methane recovery from anaerobic effluents using hollow fibre membrane contactors. J. Membr. Sci. 2016, 502, 141–150. [Google Scholar] [CrossRef]
- Kalakech, C.; Sohaib, Q.; Lesage, G.; Mericq, J.P. Progress and challenges in recovering dissolved methane from anaerobic bioreactor permeate using membrane contactors: A comprehensive review. J. Water Process Eng. 2022, 50, 103218. [Google Scholar] [CrossRef]
- Ylitervo, P.; Akinbomi, J.; Taherzadeh, M.J. Membrane bioreactors’ potential for ethanol and biogas production: A review. Environ. Technol. 2013, 34, 1711–1723. [Google Scholar] [CrossRef]
- Dereli, R.; Urban, D.; Heffernan, B.; Jordan, J.; Ewing, J.; Rosenberger, G.; Dunaev, T. Performance evaluation of a pilot-scale anaerobic membrane bioreactor (AnMBR) treating ethanol thin stillage. Environ. Technol. 2012, 33, 1511–1516. [Google Scholar] [CrossRef]
- Jain, A.; Chaurasia, S.P. Bioethanol production in membrane bioreactor (MBR) system: A review. Int. J. Environ. Res. Dev. 2014, 4, 387–394. [Google Scholar]
- Lee, D.Y.; Li, Y.Y.; Oh, Y.K.; Kim, M.S.; Noike, T. Effect of iron concentration on continuous H2 production using membrane bioreactor. Int. J. Hydrogen Energy 2009, 34, 1244–1252. [Google Scholar] [CrossRef]
- Shen, L.; Bagley, D.M.; Liss, S.N. Effect of organic loading rate on fermentative hydrogen production from continuous stirred tank and membrane bioreactors. Int. J. Hydrog. Energy 2009, 34, 3689–3696. [Google Scholar] [CrossRef]
- Buitrón, G.; Muñoz-Páez, K.M.; Hernández-Mendoza, C.E. Biohydrogen production using a granular sludge membrane bioreactor. Fuel 2019, 241, 954–961. [Google Scholar] [CrossRef]
- Lee, D.Y.; Li, Y.Y.; Noike, T. Influence of solids retention time on continuous H2 production using membrane bioreactor. Int. J. Hydrog. Energy 2010, 35, 52–60. [Google Scholar] [CrossRef]
- Kotay, S.M.; Das, D. Microbial hydrogen production from sewage sludge bioaugmented with a constructed microbial consortium. Int. J. Hydrog. Energy 2010, 35, 10653–10659. [Google Scholar] [CrossRef]
- El-Qelish, M.; Hassan, G.K.; Leaper, S.; Dessì, P.; Abdel-Karim, A. Membrane-based technologies for biohydrogen production: A review. J. Environ. Manag. 2022, 316, 115239. [Google Scholar] [CrossRef]
- Tian, Y.; Li, H.; Li, L.; Su, X.; Lu, Y.; Zuo, W.; Zhang, J. In-situ integration of microbial fuel cell with hollow-fiber membrane bioreactor for wastewater treatment and membrane fouling mitigation. Biosens. Bioelectron. 2015, 64, 189–195. [Google Scholar] [CrossRef]
- Su, X.; Tian, Y.; Sun, Z.; Lu, Y.; Li, Z. Performance of a combined system of microbial fuel cell and membrane bioreactor: Wastewater treatment, sludge reduction, energy recovery and membrane fouling. Biosens. Bioelectron. 2013, 49, 92–98. [Google Scholar] [CrossRef]
- Li, T.; Cai, Y.; Yang, X.L.; Wu, Y.; Yang, Y.L.; Song, H.L. Microbial Fuel Cell-Membrane Bioreactor integrated system for wastewater treatment and bioelectricity production: Overview. J. Environ. Eng. 2020, 146, 04019092. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, H.; Wang, J.; Cheng, B.; Yang, G.; Gao, F. Impacts of energy distribution and electric field on membrane fouling control in microbial fuel cell-membrane bioreactor (MFC-MBR) coupling system. Bioresour. Technol. 2018, 269, 339–345. [Google Scholar] [CrossRef]
- Li, H.; Zuo, W.; Tian, Y.; Zhang, J.; Di, S.; Li, L.; Su, X.Y. Simultaneous nitrification and denitrification in a novel membrane bioelectrochemical reactor with low membrane fouling tendency. Environ. Sci. Pollut. Res. 2016, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ahn, Y.; Logan, B.E. A two-stage microbial fuel cell and anaerobic fluidized bed membrane bioreactor (MFC-AFMBR) system for effective domestic wastewater treatment. Environ. Sci. Technol. 2014, 48, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ji, C.; Wang, K.; Le-Clech, P. Assessment of an anaerobic membrane bio-electrochemical reactor (AnMBER) for wastewater treatment and energy recovery. J. Membr. Sci. 2014, 450, 242–248. [Google Scholar] [CrossRef]
- Lee, Y.; Oa, S. High speed municipal sewage treatment in microbial fuel cell integrated with anaerobic membrane filtration system. Water Sci. Technol. 2014, 69, 2548–2553. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Ren, Y.; Wang, X. Preparation of conductive microfiltration membrane and its performance in a coupled configuration of membrane bioreactor with microbial fuel cell. RSC Adv. 2017, 7, 20824–20832. [Google Scholar] [CrossRef]
- Deng, L.J.; Guo, W.S.; Ngo, H.H.; Zhang, H.W.; Wang, J.; Li, J.X.; Xia, S.Q.; Wu, Y. Biofouling and control approaches in membrane bioreactors. Bioresour. Technol. 2016, 221, 656–665. [Google Scholar] [CrossRef]
- Jensen, P.; Yap, S.; Boyle-Gotla, A.; Janoschka, J.; Carney, C.; Pidou, M.; Batstone, D. Anaerobic membrane bioreactors enable high rate treatment of slaughterhouse wastewater. Biochem. Eng. J. 2015, 97, 132–141. [Google Scholar] [CrossRef]
- Dai, W.; Xu, X.; Liu, B.; Yang, F. Toward energy-neutral wastewater treatment: A membrane combined process of anaerobic digestion and nitritation–anammox for biogas recovery and nitrogen removal. Chem. Eng. J. 2015, 279, 725–734. [Google Scholar] [CrossRef]
- Jacob, P.; Phungsai, P.; Fukushi, K.; Visvanathan, C. Direct contact membrane distillation for anaerobic effluent treatment. J. Membr. Sci. 2015, 475, 330–339. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Huang, F.; Zhang, J. Removal of phosphorus from anaerobic membrane bioreactor effluent by ion exchange resin. Sep. Sci. Technol. 2016, 51, 2833–2843. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Dhar, B.R.; Elbeshbishy, E.; Lee, H.S. Ammonium nitrogen removal from the permeates of anaerobic membrane bioreactors: Economic regeneration of exhausted zeolite. Environ. Technol. 2014, 35, 2008–2017. [Google Scholar] [CrossRef]
- Gonzalez, E.; Diaz, O.; Ruigomez, I.; de Vera, C.R.; Rodriguez-Gomez, L.E.; Rodriguez-Sevilla, J.; Vera, L. Photosynthetic bacteria-based membrane bioreactor as post-treatment of an anaerobic membrane bioreactor effluent. Bioresour. Technol. 2017, 239, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.H.; Ye, Y.; Guo, W.; Du, B.; Wei, D.; Wei, Q.; Liu, Y. Nutrient recovery in anaerobic membrane bioreactors. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–307. [Google Scholar] [CrossRef]
- Heronemus, E.; Gamage, K.H.; Hettiarachchi, G.M.; Parameswaran, P. Efficient recovery of phosphorus and sulfur from Anaerobic Membrane Bioreactor (AnMBR) permeate using chemical addition of iron and evaluation of its nutrient availability for plant uptake. Sci. Total Environ. 2021, 783, 146850. [Google Scholar] [CrossRef]
- Viruela, A.; Murgui, M.; Gómez-Gil, T.; Durán, F.; Robles, Á.; Ruano, M.V.; Ferrer, J.; Seco, A. Water resource recovery by means of microalgae cultivation in outdoor photobioreactors using the effluent from an anaerobic membrane bioreactor fed with pre-treated sewage. Bioresour. Technol. 2016, 218, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Camejo, J.; Barat, R.; Aguado, D.; Ferrer, J. Continuous 3-year outdoor operation of a flat-panel membrane photobioreactor to treat effluent from an anaerobic membrane bioreactor. Water Res. 2020, 169, 115238. [Google Scholar] [CrossRef]
- Zielinska, M.; Mikucka, W. Membrane filtration for valorization of digestate from the anaerobic treatment of distillery stillage. Desalin. Water Treat. 2021, 215, 60–68. [Google Scholar] [CrossRef]
- Zielińska, M.; Rusanowska, P.; Zieliński, M.; Dudek, M.; Kazimierowicz, J.; Quattrocelli, P.; Dębowski, M. Liquid fraction of digestate pretreated with membrane filtration for cultivation of Chlorella vulgaris. Waste Manag. 2022, 146, 1–10. [Google Scholar] [CrossRef]
- Chang, H.; Kwon, D.; Kim, J. Rejections and membrane fouling of submerged direct contact hollow-fiber membrane distillation as post-treatment for anaerobic fluidized bed bioreactor treating domestic sewage. Chemosphere 2022, 296, 133964. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Duong, H.C.; Chen, S.S.; Nguyen, H.T.; Ngo, H.H.; Guo, W.; Le, H.Q.; Duong, C.C.; Le, A.H.; Bui, X.T. Water and nutrient recovery by a novel moving sponge–Anaerobic osmotic membrane bioreactor–Membrane distillation (AnOMBR-MD) closed-loop system. Bioresour. Technol. 2020, 312, 123573. [Google Scholar] [CrossRef]
- Kim, H.C.; Shin, J.; Won, S.; Lee, J.Y.; Maeng, S.K.; Song, K.G. Membrane distillation combined with an anaerobic moving bed biofilm reactor for treating municipal wastewater. Water Res. 2015, 71, 97–106. [Google Scholar] [CrossRef]
- Shi, M.; Xiao, M.; Feng, L.; Tu, T.; He, Q.; Yan, S. Water and green ammonia recovery from anaerobic digestion effluent by two-stage membrane distillation. J. Water Process Eng. 2022, 49, 102949. [Google Scholar] [CrossRef]
- Seraj, S.; Mohammadi, T.; Tofighy, M.A. Graphene-based membranes for membrane distillation applications: A review. J. Environ. Chem. Eng. 2022, 10, 107974. [Google Scholar] [CrossRef]
- Lotti, T.; Burzi, O.; Scaglione, D.; Ramos, C.A.; Ficara, E.; Pérez, J.; Carrera, J. Two-stage granular sludge partial nitritation/anammox process for the treatment of digestate from the anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag. 2019, 100, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, C.; Guzmán-Fierro, V.; Giustinianovich, E.; Alejo-Alvarez, L.; Behar, J.; Pereira, L.; Campos, V.; Fernández, K.; Roeckel, M. NOB suppression and adaptation strategies in the partial nitrification—Anammox process for a poultry manure anaerobic digester. Process Biochem. 2017, 58, 258–265. [Google Scholar] [CrossRef]
- Li, Z.Y.; Xu, X.D.; Xu, X.C.; Yang, F.L.; Zhang, S.S. Sustainable operation of submerged Anammox membrane bioreactor with recycling biogas sparging for alleviating membrane fouling. Chemosphere 2015, 140, 106–113. [Google Scholar] [CrossRef]
- Lee, J.; Alrashed, W.; Engel, K.; Yoo, K.; Neufeld, J.D.; Lee, H.S. Methane-based denitrification kinetics and syntrophy in a mem-brane biofilm reactor at low methane pressure. Sci. Total Environ. 2019, 695, 133818. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, Y.; Ai, J.; Li, Y.; Xing, Y.; Li, J. Research on microbial structures, functions and metabolic pathways in an advanced denitrification system coupled with aerobic methane oxidation based on metagenomics. Bioresour. Technol. 2021, 332, 125047. [Google Scholar] [CrossRef]
- Cao, Q.; Li, X.; Xie, Z.; Li, C.; Huang, S.; Zhu, B.; Li, D.; Liu, X. Compartmentation of microbial communities in structure and function for methane oxidation coupled to nitrification–denitrification. Bioresour. Technol. 2021, 341, 125761. [Google Scholar] [CrossRef]
- Martin, I.; Pidou, M.; Soares, A.; Judd, S.; Jefferson, B. Modelling the energy demands of aerobic and anaerobic membrane bioreactors for wastewater treatment. Environ. Technol. 2011, 32, 921–932. [Google Scholar] [CrossRef]
- Seib, M.D.; Berg, K.J.; Zitomer, D.H. Low energy anaerobic membrane bioreactor for municipal wastewater treatment. J. Membr. Sci. 2016, 514, 450–457. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, P.; Ye, L.; Chen, H.; Xu, X.; Zhu, L. Biological performance and fouling mitigation in the biochar-amended anaerobic membrane bioreactor (AnMBR) treating pharmaceutical wastewater. Bioresour. Technol. 2020, 302, 122805. [Google Scholar] [CrossRef]
- Lei, Z.; Ma, Y.; Wang, J.; Wang, X.C.; Li, Q.; Chen, R. Biochar addition supports high digestion performance and low membrane fouling rate in an anaerobic membrane bioreactor under low temperatures. Bioresour. Technol. 2021, 330, 124966. [Google Scholar] [CrossRef]
- Sohn, W.; Guo, W.; Ngo, H.H.; Deng, L.; Cheng, D.; Zhang, X. A review on membrane fouling control in anaerobic membrane bioreactors by adding performance enhancers. J. Water Process Eng. 2021, 40, 101867. [Google Scholar] [CrossRef]
- Robles, A.; Ruano, M.V.; García-Usach, F.; Ferrer, J. Sub-critical filtration conditions of commercial hollow-fibre membranes in a submerged anaerobic MBR (HF-SAnMBR) system: The effect of gas sparging intensity. Bioresour. Technol. 2012, 114, 247–254. [Google Scholar] [CrossRef]
- Juntawang, C.; Rongsayamanont, C.; Khan, E. Entrapped cells-based-anaerobic membrane bioreactor treating domestic wastewater: Performances, fouling, and bacterial community structure. Chemosphere 2017, 187, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Dolina, J.; Dlask, O.; Lederer, T.; Dvořák, L. Mitigation of membrane biofouling through surface modification with different forms of nanosilver. Chem. Eng. J. 2015, 275, 125–133. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Tao, Y.; Ozgun, H.; Spanjers, H.; van Lier, J.B. Characteristics and role of dynamic membrane layer in anaerobic membrane bioreactors. Biotechnol. Bioeng. 2016, 113, 761–771. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, M.; Ojo, A. Anaerobic Membrane Bioreactors (AnMBRs) for Wastewater Treatment: Recovery of Nutrients and Energy, and Management of Fouling. Energies 2023, 16, 2829. https://doi.org/10.3390/en16062829
Zielińska M, Ojo A. Anaerobic Membrane Bioreactors (AnMBRs) for Wastewater Treatment: Recovery of Nutrients and Energy, and Management of Fouling. Energies. 2023; 16(6):2829. https://doi.org/10.3390/en16062829
Chicago/Turabian StyleZielińska, Magdalena, and Adenike Ojo. 2023. "Anaerobic Membrane Bioreactors (AnMBRs) for Wastewater Treatment: Recovery of Nutrients and Energy, and Management of Fouling" Energies 16, no. 6: 2829. https://doi.org/10.3390/en16062829
APA StyleZielińska, M., & Ojo, A. (2023). Anaerobic Membrane Bioreactors (AnMBRs) for Wastewater Treatment: Recovery of Nutrients and Energy, and Management of Fouling. Energies, 16(6), 2829. https://doi.org/10.3390/en16062829






